Abstract
The corrosion and wear resistance of aluminum-bronze coatings deposited by thermal flame spraying were investigated after a 10 h heat treatment at 500 °C in a nitrogen atmosphere. The coatings were characterized by SEM, EDS, XRD, and XRF. Corrosion resistance was evaluated by Tafel and EIS tests, while wear resistance was assessed using a ball-on-disc test. Results showed the heat treatment compacted the coating microstructure, increased oxide content, and improved splat bonding through diffusion mechanisms. This led to enhanced corrosion resistance, evidenced by reduced corrosion current density in electrochemical tests, resulting from densification impeding electrolyte penetration. Heat treatment also increased wear resistance, as indicated by lower wear rates in ball-on-disc tests, attributable to increased hardness, reduced friction coefficients, and annealing-induced microstructural changes. Overall, the heat treatment optimizes the anticorrosive and tribological properties of thermally sprayed aluminum-bronze coatings via favorable alterations in composition, morphology, and physical characteristics. The research provides a new understanding of how thermal spray parameters and post-deposition heat treatment can be utilized to enhance the corrosion and wear resistance of aluminum-bronze coatings for marine applications.
1. Introduction
The deterioration and wear of structural components in marine environments is an ongoing challenge for the naval, automotive, and aeronautical industries. Operating in aquatic settings leads to a high rate of material loss in these systems. To address this issue, there is a need to improve protection and repair processes for components exposed to these demanding conditions. Thermal spraying techniques, such as thermal projection by combustion, have undergone rapid development in recent years as methods to recover parts affected by corrosion and wear [1]. These coating methods aim to extend the service life of components, improve safety, and reduce costs associated with frequent replacements. Aluminum-bronze coatings have proven highly effective for recovering naval components affected by wear and corrosion [2]. Developing optimized thermal spraying parameters to deposit aluminum-bronze coatings with superior wear and corrosion resistance properties would further improve the durability and lifespan of naval components. This could lead to reduced manufacturing difficulties and replacement costs over time. Further research into novel thermal spraying methods and coating materials is essential to continue enhancing the recovery of worn parts across industries. Aluminum is a major alloying element in aluminum bronzes, which are copper-aluminum alloys. Aluminum improves the corrosion resistance, strength, hardness, and density of the alloy compared to pure copper through solid solution strengthening and the formation of a protective oxide layer. However, aluminum reduces machinability. The amount of aluminum added, typically between 5% and 11%, is controlled to optimize properties for the intended application. Other alloying elements like iron, nickel, and manganese may also be included [3].
Aluminum-bronze coatings exhibit favorable tribological properties and high tensile strength comparable to steel alloys [3]. These alloys demonstrate high strength. They are often used in applications requiring enhanced corrosion resistance, such as aircraft landing gear bushings and underwater ship propeller fasteners [4,5]. The tribological behavior of aluminum-bronze coatings is highly dependent on aluminum content. Extensive research has been conducted on the wear mechanisms and friction characteristics of aluminum-bronze alloys under various conditions. In a study by Sullivan et al. [6], the wear behavior of aluminum bronzes sliding against steel counter faces under boundary lubrication was investigated. The authors developed a model describing aluminum bronze wear rate as a function of aluminum content, load, and roughness under boundary lubrication.
Aluminum-bronze alloys with less than 8 wt% aluminum have a single-phase microstructure. This is a solid solution of aluminum dissolved in copper. For aluminum concentrations between 8 and 12 wt%, a second β phase precipitates above 565 °C. Upon cooling, this β phase either transforms to α + γ2 via a eutectoid reaction or is retained as β′ depending on the cooling rate. After aluminum, iron is the most common alloying element in aluminum bronzes, typically comprising 0.5–1 wt%. Iron combines with aluminum and nickel to form complex intermetallic κ phases in these alloys. The distinct phase constituents present in aluminum-bronze alloys contribute to their exceptional strength and corrosion resistance [6,7]. The precipitation of finely dispersed secondary phase particles as a result of excess iron content enhances the strength of the alloy [6]. The addition of nickel also induces the formation of precipitates, the composition and morphology of which are contingent upon the relative proportions of nickel and iron present [8].
Thermal flame spraying enables the fabrication of coatings with tailored properties on surfaces, irrespective of their internal microstructure. This technique serves as an efficacious approach for generating aluminum-bronze coatings across diverse applications in the naval sector [9]. Recent studies have demonstrated the efficacy of thermal spray coatings in improving the wear and corrosion resistance of worn components. These coatings, applied through flame projection, can enhance dimensional accuracy by rebuilding worn surfaces. Despite adhering to coating suppliers’ application guidelines, prior investigations have failed to optimize Cu-Al coating performance with respect to adhesion, mechanical attributes, and wear resistance [10]. In order to optimize the results, the coatings were deposited under varying conditions of working distance and substrate roughness. The effects of different physical and chemical pretreatment methods on the substrate surface were also investigated [7]. Furthermore, studies have been conducted to assess the impacts of feed gas pressures on the performance characteristics of aluminum bronze coatings, including tribological and anticorrosive properties [11,12]. Aluminum-bronze coatings demonstrate favorable wear and corrosion resistance characteristics owing to the formation of a thin copper oxide layer on the surface upon exposure to air. These coatings are advantageous for conserving and reinstating the dimensions of components functioning under severe operating conditions [13]. Post-deposition heat treatments such as solution annealing and aging have been utilized to enhance the properties of thermally sprayed coatings. Sudharshan Phani et al. demonstrated that solution annealing followed by aging of aluminum bronze coatings resulted in increased hardness and wear resistance compared to the as-sprayed condition [12].
Zhang et al. investigated the influence of heat treatment on the microstructure, phase composition, mechanical properties, and wear behavior of arc-sprayed FeCrAl/Al coatings. The as-sprayed coating exhibited a dense, layered structure composed of alternating FeCrAl and Al splats with some porosity and oxides. Heat treatment led to the formation of FeAl intermetallic compounds, with Fe2Al5 initially forming at 500 °C and further intermetallic phases like Fe3Al and FeAl developing at higher temperatures up to 700 °C. The precipitation of these intermetallic phases influenced the coating microhardness, with a peak hardness achieved at 600 °C due to precipitation hardening effects. Compared to the annealed coatings, the as-sprayed coating displayed the lowest wear rate and coefficient of friction, attributed to its alternating hard FeCrAl and ductile Al structure. While heat treatment increased hardness, the precipitation of brittle FeAl intermetallics deteriorated the wear resistance at higher temperatures. The coating annealed at 700 °C exhibited improved wear properties over other annealed coatings due to the formation of Fe3Al phases and iron oxide lubricating films [14].
Kudła et al. (2023) conducted an experimental study investigating the fabrication of aluminum bronze coatings on a steel substrate using low-pressure cold spraying (LPCS) followed by post-deposition heat treatment. The authors found that the LPCS process produced dense, well-bonded aluminum bronze coatings with favorable mechanical properties. Subsequent heat treatment of the as-sprayed LPCS coatings was shown to further enhance selected coating properties. Overall, this study demonstrated that LPCS combined with post-deposition heat treatment can be an effective approach for fabricating aluminum bronze coatings on steel with tailored microstructures and mechanical performance [15].
The mechanical properties of aluminum-bronzes depend, to a large extent, on the aluminum content and the heat treatment applied [16]; Sidhu, Prakash, and Agrawal investigated the effect of heat treatment on the microstructure and properties of plasma-sprayed aluminum bronze coatings [17]. The coatings were produced using an Ar-25% H2 gas mixture and underwent heat treatment at various temperatures from 300 to 800 °C. The as-sprayed coatings exhibited a lamellar structure. Heat treatment led to diffusion between the lamellar boundaries, forming a homogenous structure and reducing porosity. Hardness and wear resistance increased after heat treatment up to 500 °C due to the formation of stable phases. However, at higher temperatures, from 600 to 800 °C, hardness decreased due to coarsening of precipitates and the partial decomposition of hard phases. The study showed that heat treatment at 500 °C for 1 h produced optimal properties for the aluminum bronze coatings [18].
An investigation of nanocrystalline copper-alumina coatings heat-treated at 300, 500, and 950 °C was conducted. Results showed the coatings heat-treated at 500 °C to have high density and excellent adhesion to the copper substrate. Additionally, these coatings exhibited a pattern of rings corresponding to the copper-aluminum oxide compound CuAlO2 while maintaining a fine-grained structure [12]. Despite the widespread use of aluminum-bronze coatings deposited by thermal spraying, there is a paucity of studies investigating the effects of post-deposition heat treatment on the mechanical and functional properties of such coatings [19]. While heat treatment has been shown to enhance the strength and adhesion of bulk aluminum-bronze alloys [3], its influence on thermally-sprayed aluminum-bronze coatings remains largely unexplored. The current literature contains no reports systematically examining how heat treatment impacts the tribological and corrosion performance of flame-sprayed aluminum-bronze coatings. This study seeks to address this gap by elucidating the effects of heat treatment temperature on the anticorrosive and tribological properties of aluminum-bronze coatings deposited via flame spraying. The results will provide new insights into optimizing the heat treatment of thermally-sprayed aluminum-bronze coatings for improved functionality.
2. Materials and Methods
An Aluminum-bronze coating made from Commercial PROXON®21071 (Castolin Eutectic, Menomonee, WI, USA) was applied. The coating has the following chemical composition: Aluminum: 10%, Silicon: Up to 0.6%, Iron: 2%, and Copper: Balance. Aluminum-bronze coatings were deposited on substrates via thermal flame spraying using TeroDyn® System 2000 equipment (Castolin Eutectic, Menomonee, WI, USA). Surface preparation involved abrasive sanding to 100 grit SiC paper, Al2O3 grit blasting (100 μm, 689.5 kPa, 30 s), and compressed air cleaning. Flame spray parameters are listed in Table 1. Substrate roughness averaged 30 μm.

Table 1.
Gas pressure applied during the fabrication of CuAl-1 and CuAl-2 coatings.
In order to evaluate and enhance the structural, electrochemical, and mechanical properties of the coatings, CuAl-1 and CuAl-2 samples underwent a 10-h heat treatment at 500 °C within a controlled nitrogen atmosphere Lindberg/Blue electric resistance furnace. This approach aimed to minimize oxidation of the coating-substrate system constituents. The heating process employed a constant ramp rate of 5 °C/min to reach the annealing temperature, followed by furnace cooling to ambient temperature.
The crystal structure was analyzed by X-ray diffraction using Cu Kα radiation (λ = 1.5406 Å) generated at 40 kV and 30 mA. Data were collected on a Panalytical X’Pert Pro diffractometer in Bragg-Brentano geometry over the 2θ range of 10–60° with a step size of 0.05° (Panalytical, Almelo, The Netherlands). Crystallite sizes were determined from X-ray peak broadening using the Williamson-Hall Equation (1).
Βcor cos (θ) = K λ/D + 4 ε sin (θ)
The morphology and chemical composition of the coatings were analyzed using scanning electron microscopy (SEM) and X-ray energy-dispersive spectroscopy (EDS). A Jeol JSM-7400F (JEOL, Akishima, Tokyo, Japan) operated at 30 kV in a high vacuum was employed for SEM imaging. Chemical analysis was performed using an EDAX APOLLO probe on a Hitachi SU1510 (Hitachi High-Technologies, Tokyo, Japan) EDS system.
The thickness of the coating cross-section was measured using Quartz PCI image analysis software version 11 (Quartz Image Corporation, Vancouver, BC, Canada). Ten thickness measurements were acquired at four different continuous regions across the coating. The obtained thickness values ranged from 127 to 299 μm. Microhardness measurements were performed using a Vickers indenter with a 10 g load for 30 s. Ball-on-disc wear testing was conducted to determine the wear resistance of the films. The tests utilized a stationary 6 mm diameter stainless steel ball sliding on the film-coated discs in ambient conditions under a 4 N load for 10 s, with a wear track radius of 6.8 mm. The maximum Hertzian contact pressure was calculated as 989 MPa at the start, assuming elastic properties of E = 200 GPa and ν = 0.30 for the SS 440 ball, and E = 190 GPa and ν = 0.275 for the copper—Aluminum coating from nanoindentation. The wear tracks and debris were examined post-test. The wear rate (K) was determined from the worn volume (V), applied load (W), and sliding distance (X) using Equation (2) [16].
K = V/W·X
The porosity of the coatings was determined by analyzing scanning electron microscopy (SEM) images of cross-sectional specimens at magnifications of 300× and 250× using ImageJ image analysis software version 1.54h (National Institutes of Health, Bethesda, MD, USA). The SEM micrographs were converted to grayscale, and the contrast was optimized. ImageJ was then used to quantify the total area and porous area in the images. The percentage porosity was calculated by dividing the porous area by the total area. This grayscale analysis method allowed the software to automatically determine the percentage porosity from the cross-sectional SEM images.
The corrosion and electrochemical behavior of the coatings were characterized using an equivalent circuit model, as depicted in Figure 1. This equivalent circuit model consists of two parallel circuits describing the behavior of the porous coating. The first parallel circuit contains CPE1, modeling the constant phase element representing the dielectric properties of the coating surface, and Rpo, representing the pore resistance through the coating. The second parallel circuit contains CPE2, modeling the constant phase element of the double layer at the substrate-electrolyte interface, and Rcor, representing the charge transfer resistance at the substrate-coating interface. Rsoln symbolizes the solution resistance. Using this equivalent circuit model allows for an improved understanding of the electrochemical properties of the coatings under investigation. The model aptly describes the electrochemical characteristics of the porous coating and substrate interface.
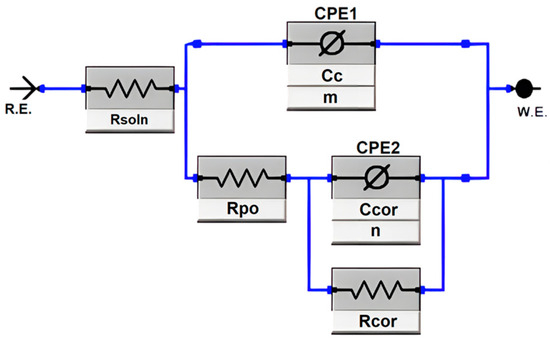
Figure 1.
Equivalent circuit for aluminum bronze plating [20].
The corrosion and electrochemical behavior of unheated-treated and heat-treated aluminum-bronzes were examined using a three-electrode configuration with a Gamry Reference 600 potentiostat (Gamry Instruments, Philadelphia, PA, USA) at ambient temperature. The exposed substrate surface area was constrained to 0.196 cm2. For corrosion testing, the substrates functioned as the working electrodes, while a platinum rod and saturated calomel electrode acted as the counter and reference electrodes, respectively. The electrolyte solution contained 3.5 wt% NaCl. Prior to each corrosion test, the open circuit potential was permitted to stabilize for 45 min in the test solution. Subsequently, potetiodynamic polarization or electrochemical impedance spectroscopy was conducted. For polarization tests, the potential range swept from −0.4 V to 0.5 V vs. the open circuit potential at a scan rate of 1 mV/s. Corrosion current density (Icorr) was determined by linear fit and Tafel extrapolation of the cathodic region of the polarization curve [9]. The electrochemical behavior of the coatings was examined through analysis of the electrochemical impedance spectroscopy (EIS) data. The EIS results were fitted to an equivalent circuit model previously utilized by Dermaj et al. [13] and Rahmouni et al. [21] for studying the corrosion processes of bronze in a 3% NaCl solution.
3. Results and Discussion
X-ray diffraction (XRD) analysis was conducted on CuAl-1 and CuAl-2 coatings, both with and without heat treatment. As shown in Figure 2a, the XRD spectra revealed the presence of AlCu3 phases characteristic of the Cu-Al alloy along with substrate phases in all coating samples. The coatings possess a face-centered cubic (FCC) structure, with aluminum atoms occupying the vertices of the cube and copper atoms situated on the faces. The centers of the cube faces are elucidated in the structural characterization section. Alterations in peak intensity, broadening, and shifting were also observed. Annealing-induced diffusion prompted the right-shifting of coating peaks. Figure 2b details a peak of the α phase for the coating applied at 345 KPa O2 and 69 KPa C2H2, corresponding to the (002) planes of the FCC structure at a 2θ angle of 50.5°. Heat treatment modified the peak intensity and width. The peak also shifted to lower 2θ values, indicating expanded crystal lattices from bronze to aluminum. Reduced peak broadening denotes increased crystallite size [12].
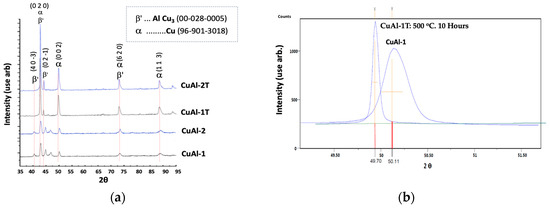
Figure 2.
The XRD results demonstrate the crystallographic structure of the CuAl coatings before and after heat treatment. (a) shows the XRD patterns obtained for the Al bronze coatings without and with heat treatment for the CuAl-1 and CuAl-2 samples. The XRD pattern reveals the face-centered cubic (FCC) structure of the coatings. (b) provides a detailed image of the (002) diffraction peak at 2θ = 50.5° corresponding to the FCC structure, shown for the CuAl-1 sample deposited at 345 KPa O2 and 69 KPa C2H2 pressures, without and with heat treatment.
Figure 3 shows the change in crystallite size in the coating, with and without heat treatment. The heat-treated coating increases the crystallite size. This increase can be attributed to the diffusion of atoms within the coating. Other possible factors include the reduction of grain boundaries and the occurrence of recrystallization and grain growth [12].
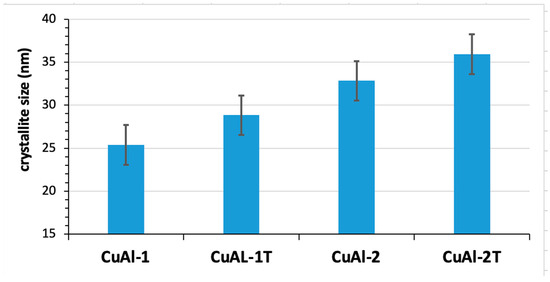
Figure 3.
Calculation of the average size of the crystallites in the CuAl-1, CuAl-1T and CuAl-2, CuAl-2T applications, before and after heat treatment.
Figure 4 presents the scanning electron microscopy (SEM) morphology of the CuAl-1 aluminum-bronze coating deposited under 345 kPa oxygen and 69 kPa acetylene, both before and after heat treatment. The pre-treated coating exhibits a compact microstructure characterized by isolated porosity, voids between splat layers, and oxide inclusions. This morphology is attributed to the impact and plastic deformation of high-velocity particles during the deposition process, leading to their subsequent entrapment by additional particles [22]. The coatings exhibited no deterioration, cracking, or delamination at the coating-substrate interface after heat treatment at 500 °C.
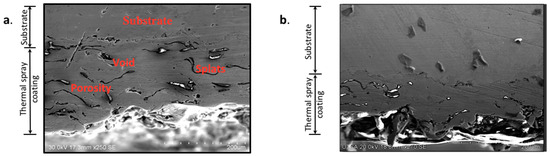
Figure 4.
SEM micrograph of CuAl-1 application. (a) without heat treatment at morphology in cross-section of the coatings at ×250. (b) With heat treatment in cross section and micrograph at ×270.
The elemental mapping obtained via energy-dispersive X-ray spectroscopy (EDS) indicates the coating is composed of Cu, Al, Fe, O, and Zn (Figure 5). Zn appears to diffuse from the substrate into the coating during heat treatment, precipitating more heavily at the coating-substrate interface. An increase in oxygen concentration within the coating is also observed, likely due to the penetration of oxygen during heat treatment and subsequent oxidation reactions. The diffusion kinetics of the constituent elements and their reactions with infiltrating oxygen species govern the oxidation behavior. Prolonged annealing times increase oxide content through the growth and coalescence of oxide phases, which ultimately impedes continued oxygen diffusion and reduces the oxidation rate. Aluminum segregates to the splat boundaries and forms a thin interfacial layer at the coating-substrate interface.
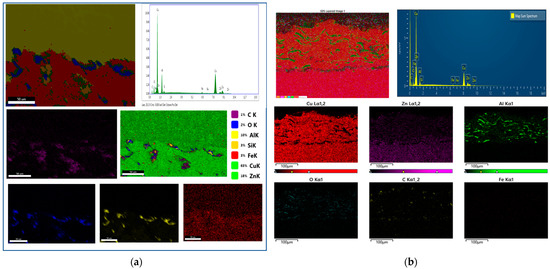
Figure 5.
Chemical mapping of the coatings of aluminum-bronze. (a) Without heat treatment: EDS map of the relative concentration of the main elements present in the Surface (b) with heat treatment: EDS elemental composition maps of CuAl-1T (345 KPa of oxygen and 69 KPa of acetylene) cross section.
Aluminum exhibits a high reactivity with oxygen, readily forming aluminum oxide precipitates or interfacial oxide layers upon exposure [23,24]. These oxide species can impart functional properties to aluminum coatings. The oxide layer formed at the aluminum-coating interface facilitates chemical bonding between the substrate and coating, enhancing overall adhesion within the system [23,24]. Studies have demonstrated that aluminum oxide is able to infiltrate the pores during heat treatment [25]. Furthermore, aluminum oxide surrounding the boundaries of the splats can serve as both a thermal barrier and a corrosion barrier [22,26].
Figure 6 presents a comparison of the microhardness profiles for CuAl-1 and CuAl-2 coatings with and without heat treatment. CuAl-1 exhibited a 20% decrease in hardness after heat treatment. This reduction can be attributed to the high porosity present within the coating microstructure, likely formed during its fabrication. This porosity hindered the diffusion processes that typically enhance hardness during heat treatment. Additionally, the increased crystallite size within the coating further compromised its mechanical properties. Conversely, CuAl-2 demonstrated an 11% increase in hardness upon heat treatment. This positive response is likely due to a combination of synergistic effects triggered by the treatment, including:
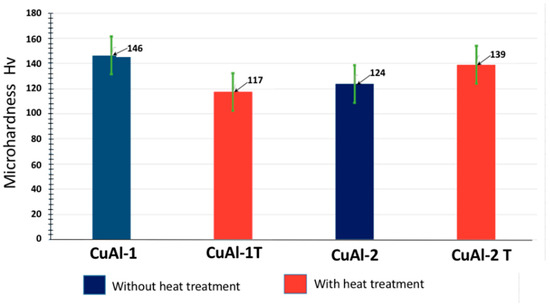
Figure 6.
The graph depicts the mean Vickers microhardness values of the coating with and without heat treatment.
- Enhanced metallurgical bonding between splats within the coating, leading to increased cohesion.
- Precipitation of strengthening phases further reinforces the microstructure.
- Coalescence of oxides, potentially influencing the overall hardness profile [20].
Heat treatment was effective in reducing porosity in the coating, as shown in Figure 7. Untreated coatings exhibited an average porosity of CuAl-1 4.6% and CuAl-2 4.1% as measured by quantitative image analysis. Following heat treatment at 500 °C for 10 h, the porosity decreased significantly to 2.8% ± 0.5% and 2.7% ± 0.5% (reductions of 1.8% and 1.4%, respectively). The small standard deviations indicate the high precision of the image analysis measurements. These data demonstrate that heat treatment under the specified conditions induces substantial porosity reduction in the coating, likely through sintering and densification mechanisms. Further study on the microstructural evolution could provide insight into the pore closure process.
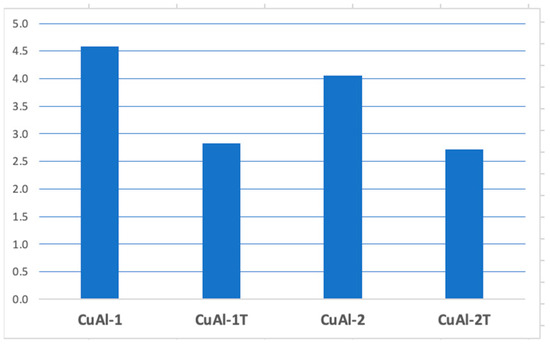
Figure 7.
Graph of the averages of the porosity of the coating without heat treatment and with heat treatment.
The aluminum-bronze coatings displayed low coefficients of friction, indicating potential suitability as coatings where strong metal-metal frictional properties are desired under both dry and lubricated conditions. Figure 8 illustrates the coefficients of friction (COF) for the aluminum-bronze coatings with and without heat treatment. The CuAl-1 coating exhibited an increased COF associated with reduced hardness, while the CuAl-2 coatings showed substantially decreased friction coefficients, likely due to the development of a more compact structure and elevated hardness during annealing. However, heat treatment significantly reduced the COF of the CuAl-2 coating.
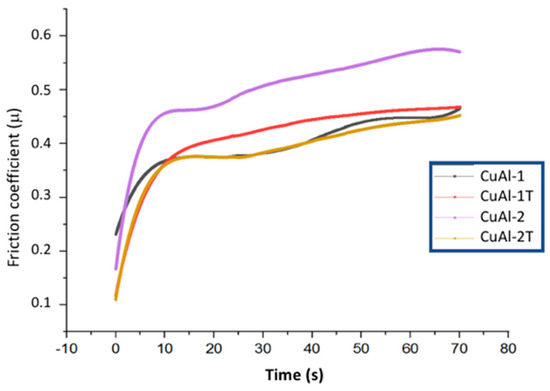
Figure 8.
Values of the coefficient of friction (COF) for the CuAl-1 and CuAl-2 aluminum-bronze coatings without and with heat treatment at 500 °C for 10 h.
Figure 9 presents scanning electron microscopy (SEM) micrographs of the wear track on the CuAl-2T sample. The micrographs reveal a diverse wear morphology, encompassing smooth and adhesive wear, plowing and rutting, and abrasive wear. Additionally, regions exhibiting polished texture, surface plastic deformation, and material transport are observed [22,27]. The mechanism of adhesive wear begins in the early stages of the Pin-on-disk test, when the surfaces make contact, forming cold weld joints between the contacting asperities of the tribological pair. Then, the deformation of the edges begins until there is an increase in the contact area between them. This is known as the running-in period, or transient state, which is characterized by very significant changes in the coefficient of friction. This behavior was explained by Tabor [28], after a reduction, as the cycles increase, in the deformation of the asperities that are in contact, causing an increase in the contact area between them while allowing other asperities to come into contact and adhesive.

Figure 9.
SEM micrograph of the representative areas of the wear track by the pin-on-disk test for the CuAl-2T at ×550: (a) without heat treatment: (b) with heat treatment.
The wear resistance of CuAl-2T and CuAl-1T was notably improved by heat treatment, as evidenced by the substantial reduction in wear rates shown in Figure 10. This enhancement in wear resistance can be attributed to a combination of metallurgical factors, including increased hardness due to precipitation strengthening, reduced coefficient of friction due to the formation of lubricious surface oxides, and microstructural changes such as grain refinement and dispersion of hard particles. Further investigation is required to elucidate the relative contributions of each mechanism to the overall improvement in wear performance of the heat-treated alloys.
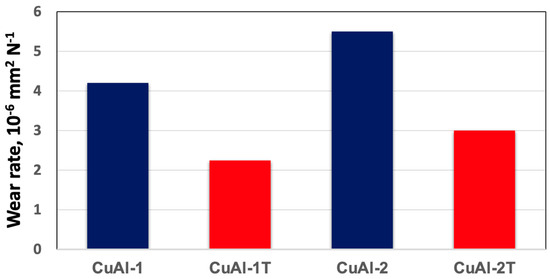
Figure 10.
Wear rates of coatings Deposited Using 440 stainless steel Pin.
Energy dispersive X-ray spectroscopy (EDS) microanalysis of the coating revealed traces of nickel adhered to the surface (Figure 11), suggesting abrasive wear of the stainless-steel pin with transfer of pin material to the coating. Elevated oxygen levels were also observed, indicative of enhanced oxide formation during testing that further promoted oxidative wear mechanisms.
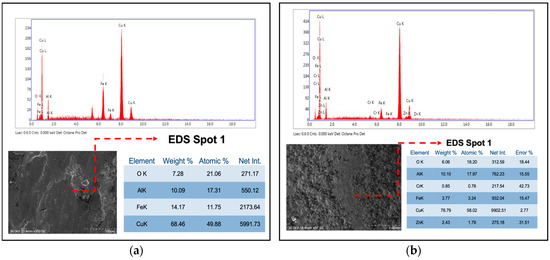
Figure 11.
EDS chemical microanalysis of CuAl-2 samples: (a) sample without heat treatment (EDS Spot 1); (b) sample with heat treatment (EDS Spot 1).
Electrochemical impedance spectroscopy (EIS) was utilized to evaluate the corrosion resistance of treated and untreated CuAl-1 and CuAl-2 aluminum bronze coatings. Impedance data were obtained by recording the impedance modulus (Zmod) and phase angle over a range of frequencies. These data are displayed in the form of Bode plots, as shown in Figure 12.
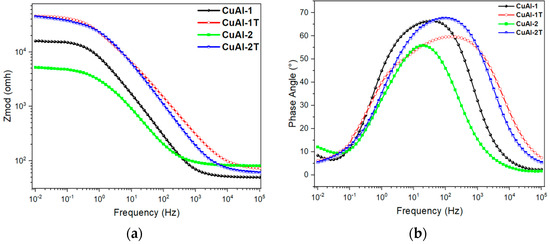
Figure 12.
Bode plots of the electrochemical impedance spectra for aluminum-bronze coatings. (a) Impedance modulus, Zmod, and (b) phase angle.
The phase angle plots in Figure 12b reveal two-time constants at high and medium frequencies for both CuAl-1 and CuAl-1T coatings, indicative of their dielectric properties. The time constants shift to higher frequencies for CuAl-1T versus CuAl-1, suggesting the dielectric response is influenced by electrolyte exposure and surface treatment. Furthermore, the decreased peak height for CuAl-1T indicates a less capacitive, more resistive response. However, the broader frequency range of the CuAl-1T time constant compared to CuAl-1 arises from the competing electrochemical processes at the coating-substrate interface at low frequencies and passivation layer formation at the coating-electrolyte interface at high frequencies, attributed to Al2O3 and CuO.
In contrast, the CuAl-2 coating exhibited a time constant over a range of medium frequencies, albeit with a lower intensity compared to CuAl-2T. This indicates a less capacitive and more resistive response for CuAl-2. The CuAl-2T coating displayed a time constant over a broader frequency range of 10 to 104 Hz, with greater intensity relative to the untreated CuAl-2 coating. These results suggest CuAl-2T has an enhanced capacitive response attributed to the coating-electrolyte interface versus CuAl-2.
Impedance spectroscopy revealed that thermal treatment of CuAl coatings increased the polarization resistance (Rp), indicating enhanced corrosion protection. Rp values for CuAl-1 and CuAl-2 coatings increased 3-fold and 5-fold, respectively, after thermal treatment. The CuAl-2T coating exhibited the highest Rp and corrosion resistance. Fitting the data to an equivalent circuit model showed that the constant phase elements (CPEs) representing the coatings had exponents (n, m) near 1, suggestive of a highly capacitive response. However, the CPE representing the substrate-coating interface had an exponent near 0, indicating a more resistive response. This implies that the electrolyte penetrates the coating, reaching the substrate and lowering corrosion protection. In summary, thermal treatment improved the corrosion resistance of Cu-Al coatings, with the heat-treated CuAl-2 coating providing the best protection. The results of the impedance fitting from the electric circuit model are shown in Table 2.

Table 2.
Results of fit of impedances for Cu-Al coatings.
Figure 13a,b shows the SEM micrograph of the CuAl-1T coating following the TAFEL test. The micrograph reveals a layer of corrosion products adhering to the coating surface, formed during exposure to the 3.5 wt% NaCl solution. Notably, precipitation of electrolyte crystals is observed, particularly within the porous cavities on the surface. Additionally, a homogeneous and dense protective patina, characteristic of bronzes, has formed [21], which, to the naked eye, is green in color and looks like fungi produced by moisture [28,29]. The corrosion mechanism is associated with the passage of the electrolyte through the coating defects. However, these displacements are delayed for these annealed coatings because the splats have greater adherence and less porosity. Figure 13c,d presents the morphology and elemental composition of the corrosion film formed on the coating surface, as revealed by SEM and EDS analysis. Following TAFEL electrochemical tests, the aluminum-bronze substrate opposite the coated surface exhibited a thin layer of salt crystals and corrosion products, primarily identified as cuprous chloride (CuCl) using EDS. Notably, a significant oxygen presence was observed, congruent with the increased formation of copper and aluminum oxides during annealing, suggesting a substantial contribution to enhanced corrosion resistance [10].
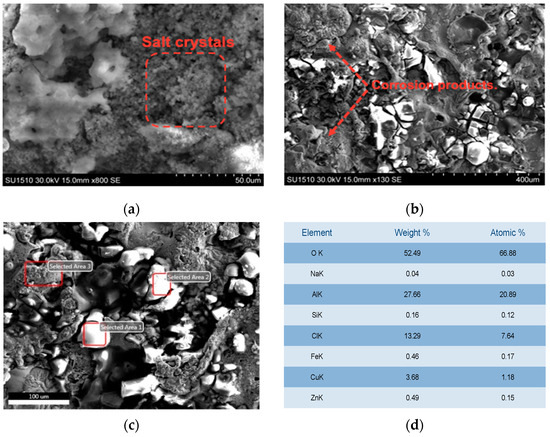
Figure 13.
Micrographics of the surface of the coating CuAl-1 after the impedance test: (a) SEM images, detail of salt crystals on the surface of CuAL-1 after impedance corrosion test; (b) detail of corrosion products on CuAL-1; (c) SEM image, detail of morphology of CuAL-1T after impedance corrosion test; (d) Table of EDX results measured on spot 1.
Microscopic observation of the corroded region of the CuAl-2T-coated sample revealed the presence of corrosion products without evidence of coating cracks or delamination (Figure 14). This observation indicates that the coating provided protection against cracking or delamination during the corrosion process.
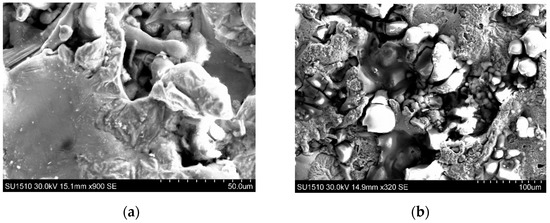
Figure 14.
SEM image of the corroded area of the sample CuAl-2T: (a,b).
Figure 15 shows the chemical analysis of the corroded surface, which agrees well with the results previously presented from TAFEL, where the corrosion products are associated with copper and aluminum oxides and cuprous chloride.
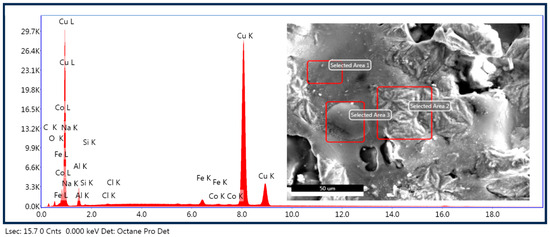
Figure 15.
EDS results of the corroded area of the sample CuAl-2T.
Potentiodynamic polarization curves were obtained for untreated and thermally treated aluminum bronze coatings (Figure 16). The electrochemical parameters derived from these curves are presented in Table 3. The CuAl-1T and CuAl-2T coatings exhibited current densities of 27.4 and 28.16 μA/cm2, respectively, indicating that the corrosion resistance of the coatings is significantly influenced by thermal treatment. Compared to the untreated coating, the CuAl-1T and CuAl-2T treated coatings reduced the current density by factors of 2 and 3, respectively, demonstrating a protective effect on the substrate. A similar trend was observed for the corrosion rate. Polarization resistance values were calculated using the Stern-Geary equation and followed trends similar to those obtained by electrochemical impedance spectroscopy.
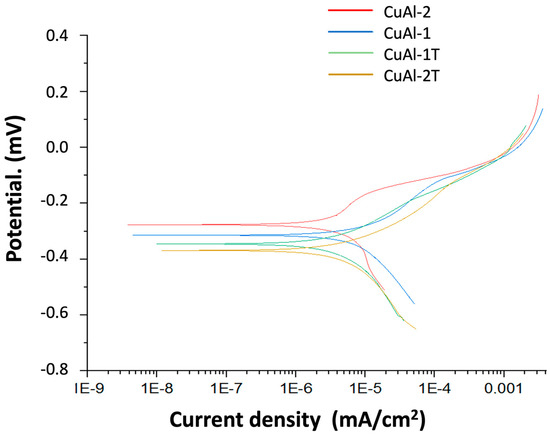
Figure 16.
Potentiodynamic polarization curves of CuAl-1, CuAl-1T, CuAl-2 and CuAl-2T.

Table 3.
Results of the Fit for Potentiodynamic Polarization Curves of Aluminum Bronze Coatings.
It is established that defects and pores in coatings facilitate electrolyte access to the coating-substrate interface, resulting in electrolyte accumulation and localized corrosion [30]. Heat treatment of the coatings improved corrosion resistance due to microstructural changes and the formation of a protective aluminum and copper oxide layer. This oxide layer decreases the corrosion rate, as evidenced by potentiodynamic polarization results. In a 3.5 wt% NaCl aqueous environment, corrosion of aluminum-bronze coatings can involve several reactions. Aluminum in the coating can oxidize to Al3+ ions, releasing three electrons. Concurrently, oxygen reduction occurs in water, generating hydroxide ions. Formed Al3+ ions may then react with chloride ions to produce aluminum chloride. Similarly, copper oxidation yields Cu2+ ions and two electrons. The resulting Cu2+ ions can subsequently react with chloride ions to form copper chloride.
4. Conclusions
Heat treatment of the CuAl-1 and CuAl-2 coatings allowed improving the resistance to corrosion. This was explained by the significant effect of diffusion mechanisms on the microstructure; that is, a more compact structure was achieved, with greater adherence between the coating and the substrate, better union between splats, and increased oxide content. On the other hand, the heat treatment on sample CuAl-2 caused a significant decrease in its wear rates. This can be attributed to increased hardness, reduced coefficient of friction, and structural improvement.
However, the good corrosion resistance of these coatings is associated with a thin passive oxide film consisting of Cu2O and Al2O3 and a possible reduction in porosity that was favored by the heat treatments. The pores or defects in these coatings act as “roads”, or trajectories, through which the corrosive electrolyte can flow towards the splats of the coating and even reach the substrate itself, promoting active or anodic zones. In this way, the corrosion process begins, where the substrate can be dissolved, resulting in loss of adherence of the coating-substrate system and pore-clogging by some of the corrosion products formed.
Author Contributions
O.P. and J.O. conceived and designed the experiments; J.A.M. performed the experiments; F.V. reviewed, edited, and provided analysis and interpretation of EIS measurements; and O.P., J.O., F.V. and J.A.M. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Departamento Administrativo de Ciencia, Tecnología e Innovación: Colciencias, convocatoria doctorados nacionales No. 727 de 2015.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors wish to acknowledge the technological support provided by Universidad Nacional de Colombia, Grupo de Investigación en Corrosión, Tribología y Energía; Centro de Investigación de Materiales Avanzados CIMA and Research Associate Kleberg Advanced Microscopy Center, University of Texas at San Antonio. The authors would like to thank Josefina Arellano Jimenez (Kleber Advanced Microscopy Center, University of Texas at San Antonio) for her technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pawlowski, L. The Science and Engineering of Thermal Spray Coatings; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Sidhu, T.S.; Prakash, S.; Agrawal, R.D. Studies on the properties of high velocity oxy-fuel thermal spray coatings for higher temperature applications. Mater. Sci. Eng. A 2006, 430, 27–34. [Google Scholar] [CrossRef]
- Davis, J.R. (Ed.) Aluminum and Aluminum Alloys, ASM Specialty Handbook; ASM International: Materials Park, OH, USA, 2001. [Google Scholar]
- Arpat, E.; Ürgen, M. Production of free standing Cu-Al intermetallics by cathodic arc plasma treatment. Intermetallics 2011, 19, 1817–1822. [Google Scholar] [CrossRef]
- Féron, D. Corrosion Behaviour and Protection of Copper and Aluminum Alloys in Seawater, 1st ed.; Woodhead: Cornwall, UK, 2007; pp. 127–140. [Google Scholar]
- Sullivan, J.L.; Wong, L.F. Wear of aluminium bronze on steel under conditions of boundary lubrication. Tribol. Int. 1985, 18, 275–281. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Yan, F. Load-dependent tribocorrosion behaviour of nickel-aluminium bronze in artificial seawater. Corros. Sci. 2018, 131, 252–263. [Google Scholar] [CrossRef]
- Al-Hashem, A.; Riad, W. The role of microstructure of nickel–aluminium–bronze alloy on its cavitation corrosion behavior in natural seawater. Mater. Charact. 2002, 48, 37–41. [Google Scholar] [CrossRef]
- Dimaté, L.M. Resistencia a la Corrosión en Recubrimientos Comerciales Metaceram 25050 y Proxon 21071 Producidos con el Sistema de Proyección Térmica por Llama; Universidad Nacional de Colombia: Bogotá, Colombia, 2011. [Google Scholar]
- Hanke, S.; Fischer, A.; Beyer, M.; dos Santos, J. Cavitation erosion of NiAl-bronze layers generated by friction surfacing. Wear 2011, 273, 32–37. [Google Scholar] [CrossRef]
- Li, Y.; Ngai, T.L.; Xia, W. Mechanical, friction and wear behaviors of a novel high-strength wear-resisting aluminum bronze. Wear 1996, 197, 130–136. [Google Scholar] [CrossRef]
- Sudharshan Phani, P.; Vishnukanthan, V.; Sundararajan, G. Effect of heat treatment on properties of cold sprayed nanocrystalline copper alumina coatings. Acta Mater. 2007, 55, 4741–4751. [Google Scholar] [CrossRef]
- Dermaj, A.; Hajjaji, N.; Joiret, S.; Rahmouni, K.; Srhiri, A.; Takenouti, H.; Vivier, V. Electrochemical and spectroscopic evidences of corrosion inhibition of bronze by a triazole derivative. Electrochim. Acta 2007, 52, 4654–4662. [Google Scholar] [CrossRef]
- Ndumia, J.N.; Kang, M.; Lin, J.; Liu, J.; Li, H. Influence of Heat Treatment on the Microstructure and Wear Properties of Arc-Sprayed FeCrAl/Al Coating. Coatings 2022, 12, 374. [Google Scholar] [CrossRef]
- Winnicki, M.; Baszczuk, A.; Gibas, A.; Jasiorski, M. Experimental study on aluminium bronze coatings fabricated by low pressure cold spraying and subsequent heat treatment. Surf. Coat. Technol. 2023, 456, 129260. [Google Scholar] [CrossRef]
- Cenoz, I. Metallography of aluminium bronze alloy as cast in permanent iron die. Metall. Mater. Eng. 2010, 16, 115–122. [Google Scholar]
- Sidhu, B.S.; Prakash, S.; Agrawal, R.S. Hot corrosion studies of HVOF sprayed Cr3C2-NiCr coating on nickel-based superalloys in an actual industrial environment of a coal fired boiler. Surf. Coat. Technol. 2005, 198, 107–117. [Google Scholar]
- Sidhu, T.S.; Prakash, S.; Agrawal, R.D. Effect of heat treatment on the structure and properties of plasma sprayed aluminum bronze coatings. Surf. Coat. Technol. 2005, 200, 2754–2762. [Google Scholar]
- Newaz, G.M.; Chen, X.; Lewis, G. Influence of Coating Thickness on the Performance of Aluminium Bronze Coatings. Surf. Eng. 2005, 21, 38–44. [Google Scholar]
- Chen, Y.; Qi, D.M.; Wang, H.P.; Xu, Z.; Yi, C.X.; Zhang, Z. Corrosion behavior of aluminum bronze under thin electrolyte layers containing artificial seawater. Int. J. Electrochem. Sci. 2015, 10, 9056–9072. [Google Scholar] [CrossRef]
- Rahmouni, K.; Keddam, M.; Srhiri, A.; Takenouti, H. Corrosion of copper in 3% NaCl solution polluted by sulphide ions. Corros. Sci. 2005, 47, 3249–3266. [Google Scholar] [CrossRef]
- Li, W.Y.; Li, C.J.; Liao, H.; Coddet, C. Effect of heat treatment on the microstructure and microhardness of cold-sprayed tin bronze coating. Appl. Surf. Sci. 2007, 253, 5967–5971. [Google Scholar] [CrossRef]
- Spadaro, C.; Dispenza, C.; Sunseri, C. The influence of the nature of the surface oxide on the adhesive fracture energy of aluminium-bonded joints as measured by T-peel tests. Int. J. Adhes. Adhes. 2008, 28, 211–221. [Google Scholar] [CrossRef]
- Jones, F.; Lee, H. Adhesion mechanisms in aluminum coatings. Coat. Technol. 2018, 22, 127–134. [Google Scholar]
- Smith, J. Observation of aluminum oxide infiltrating pores during thermal spraying. Surf. Coat. Technol. 2019, 350, 20–25. [Google Scholar]
- Lee, C. Aluminum oxide as a thermal and corrosion barrier in thermal spray coatings. Corros. Sci. 2020, 163, 108131. [Google Scholar]
- Cárdenas-Feria, D.C.; Herrera-Quintero, L.K.; Olaya-Florez, J.J. Resistencia al desgaste de recubrimientos de bronce al aluminio producidos con técnica de proyección térmica. Ing. Mec. 2015, 18, 173–180. [Google Scholar]
- Rodriguez, R.M.P.; Paredes, R.S.; Wido, S.H.; Calixto, A. Comparison of aluminum coatings deposited by flame spray and by electric arc spray. Surf. Coat. Technol. 2007, 202, 172–179. [Google Scholar] [CrossRef]
- Wharton, J.A.; Stokes, K.R. The Influence of Nickel-aluminium Bronze Microstructure and Crevice Solution on the Nitiation of Crevice Corrosion. Electrochim. Acta 2008, 53, 2463–2473. [Google Scholar] [CrossRef]
- Smith, J. Corrosion of Aluminum Bronze Coatings. J. Corros. Sci. 2021, 15, 123–145. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).