Abstract
In this work, Fe-20Cr-20Mn-0.75N (wt.%) high-nitrogen stainless steel (HNSS) was studied using scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), and electrochemical testing. The corrosion behaviors of Fe-20Cr-20Mn-0.75N HNSS with different concentrations of NaCl were studied. The composition of a passive film on Fe-20Cr-20Mn-0.75N HNSS was analyzed using X-ray photoelectron spectroscopy (XPS) at an applied potential of 0.2VSCE. The results showed that, with the increase in Cl− concentration, the corrosion tendency and corrosion rate of Fe-20Cr-20Mn-0.75N HNSS get higher. In the solution of a low Cl− concentration, the fraction of Fe and Cr oxides in the passive film is higher, and the passive film is thicker and more stable. By increasing the stability of the passive film and preventing its rupture, the elevated NH4+ concentration can enhance the corrosion resistance of Fe-20Cr-20Mn-0.75N HNSS in a NaCl solution.
1. Introduction
High-nitrogen austenitic stainless steel (HNSS) has considerable hardness, strength, and wear resistance compared to traditional metallic materials [1,2,3]. In addition, HNSS exhibits remarkable corrosion resistance, particularly in acidic and chloride-rich solutions, illustrating its wide applicability in various fields such as the marine, transport, and biomedical industries [4,5]. However, because of the corrosive effects of Cl− in seawater, the service life of austenitic stainless steel (ASS) is significantly reduced, and the material surface is damaged from localized corrosion [6,7]. In contrast, the increasing nitrogen content in HNSS could enhance the passivation capacity of metallic materials, forming more stable and dense passive films with a reduction in the corrosion rate [8,9].
Cl− is one of the major corrosion contributors of ASS in a marine environment. In the accelerating corrosion of ASS, there are various mechanisms at play, including the small size, high diffusion rate, and strong acidic anionic properties, leading to the acceleration of the hydrolysis of corrosion products by penetrating the passive film [10,11]. Thus, a localized acidification of the sample, such as pitting, crevice, and stress cracking corrosion, appears in ASS [12,13,14]. Furthermore, Cl− reacts with Fe and/or Cr elements, forming soluble chlorides on the surface of ASS, while also disrupting the formation and stability of the passive film [15,16]. With the attribution of N3− ions, the ammonium ions form on the surface of the passive film, increasing the local pH of the film and promoting repassivation. Additionally, the synergistic effect of nitrogen and molybdenum atoms also plays a significant role in the above process [17].
In practical marine engineering applications, the variability of Cl− solutions across different geographical regions has a significant impact on the service life of austenitic stainless steel. Magdy et al. [18] conducted a study on the corrosion behavior of austenitic stainless steel with varying Cl− concentrations, indicating that the pitting potential of the samples leans towards a more negative (active) direction and a reduction in the corrosion resistance with increasing Cl− concentration. Studies in the existing literature have pointed out that an increase in nitrogen enhances the corrosion resistance of HNSS compared with nitrogen-free ASS. However, there is a lack of corresponding studies on the corrosion sensitivity of HNSS immersed in different Cl− concentrations. Research on the corrosion resistance capacity of HNSS materials used in various environmental conditions will improve their performance, ensuring their long-term reliability in specific applications. Additionally, understanding the impact of varying Cl− concentrations on HNSS can stimulate the alloy design of HNSS to enhance its corrosion resistance.
The current study primarily utilized electrochemical methods to investigate the corrosion behavior of Fe-20Cr-20Mn-0.75N HNSS in solutions with different Cl− concentrations, including 0.05, 0.1, 0.3, and 0.6 mol/L NaCl. The corroded morphologies and elemental distribution of the HNSS were characterized using scanning electron microscopy (SEM, JEOL, JSM-6480, Takeno, Japan) and energy dispersive spectroscopy (EDS, X-Max 20, Oxford, UK), respectively. The potentiostatic polarization (i-t) experiments were conducted at various applied potentials, along with the corresponding Mott–Schottky (M-S) curve analysis, to calculate donor and acceptor densities. The point defect model (PDM) was employed to determine the oxygen vacancy diffusion coefficient, allowing for an in-depth investigation of the performance of passive films. X-ray photoelectron spectroscopy (XPS) testing was conducted on the passive film generated at a constant potential of 0.2 VSCE to analyze the composition of the passive film for exploring the protective mechanism on the substrate.
2. Experimental Materials and Methods
2.1. Material and Solution Preparation
The material used in the experiment was Fe-20Cr20Mn-0.75N HNSS, and its chemical composition is shown in Table 1. The samples were cut into dimensions of 10 mm × 10 mm × 2 mm using wire cutting, sealed with epoxy resin, and exposed for further testing on a 10 × 10 cm surface. Before the electrochemical testing, the samples were wet-grounded using 600#, 1000#, 1500#, and 2000# sandpaper and polished with 2.5 μm diamond paste. Then, they were ultrasonically cleaned in water and ethanol by drying with cold air. Simultaneously, the different concentrations (0.05, 0.1, 0.3, and 0.6 mol/L) of NaCl solutions were prepared to investigate the Cl− effect on corrosion behavior.

Table 1.
Element composition of Fe-20Cr20Mn-0.75N HNSS (wt.%).
2.2. Corrosion Measurements
The electrochemical tests were conducted using a Gamry 1010E (Gamry Instruments, Inc., Philadelphia, PA, USA) electrochemical workstation with a traditional three-electrode system. In this setup, the working electrodes were the test sample, platinum electrode served as the auxiliary electrode, and saturated calomel electrode (SCE) was employed as the reference electrode. The open circuit potential (OCP) experiment was conducted for 3600 s before cyclic potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) measurements to ensure that the system reached a steady state. The potential scanning range, for cyclic potentiodynamic polarization measurements, was set to −0.5 to 1.2 VSCE, with a scanning rate of 0.5 mV/s. The reverse scan was initiated after the current density was 2 mA/cm2, and the final scanning potential was set at −0.3 VSCE. The EIS measurements were conducted at OCP, with a frequency range spanning from 104 to 10−2 Hz and an amplitude of 10 mV. Additionally, the potentiostatic polarization experiments were conducted for 1 h at four different potentials of 0.2 VSCE. The Mott–Schottky measurements were then immediately performed on the samples, and the test potential ranged from 0 to 0.9 VSCE, with a frequency of 1000 Hz, an amplitude of 10 mV, and continuous steps of 30 mV.
The corrosion morphologies of the samples were observed using a SEM. X-ray Photoelectron Spectroscopy (XPS, ESCALAB 250Xi T, ThermoFisher Scientific, Waltham, MA, USA) testing was performed on the passive film of the sample surface after 0.2 VSCE for 1 h. All the above experiments were conducted at 25 ± 1 °C, and each measurement was repeated three times to ensure the accuracy of the experiments.
3. Results and Discussion
3.1. OCP Measurements
The corrosion-potential-versus-time curves of Fe-20Cr-20Mn-0.75N HNSS in four different NaCl concentrations are shown in Figure 1. The corrosion potential rapidly increases until stabilization after 600 s, indicating a conversion of the passive film formed in air to the thermodynamically stable passive film in solution [19,20]. In addition, the stable corrosion potential values of the samples in 0.05 mol/L, 0.1 mol/L, 0.3 mol/L, and 0.6 mol/L NaCl solutions are −0.135 VSCE, −0.141 VSCE, −0.146 VSCE, and −0.152 VSCE, respectively. Thus, the corrosion potential of HNSS gradually decreases with increasing Cl− concentration, demonstrating an increased thermodynamic stability [21].
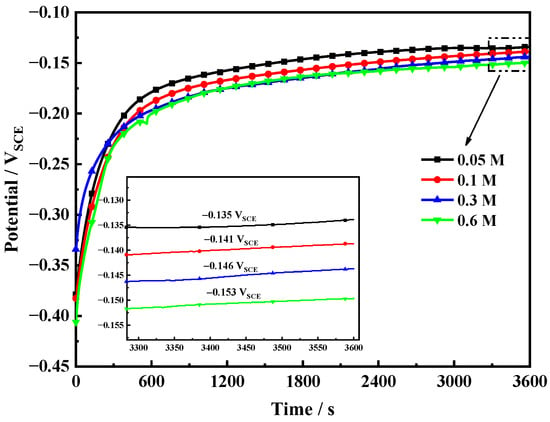
Figure 1.
Open circuit potential (OCP) curves of HNSS in tested solutions.
3.2. Electrochemical Impedance Spectroscopy (EIS)
Figure 2 depicts the EIS results of Fe-20Cr-20Mn-0.75N HNSS immersed in NaCl solutions with varying Cl− concentrations. In Figure 2a, the Nyquist plot shows a single capacitive arc covering a range of Cl− concentrations, exhibiting similarly corrosion mechanisms [22,23]. Thus, the corrosion process is determined by the diffusion of ions within the passive film [24,25,26]. The smaller radius of the impedance arc indicates the poorer protection of the passive film on the substrate. Furthermore, the results indicate that the protective ability of the passive film on the Fe-20Cr-20Mn-0.75N HNSS substrate gradually decreases with increasing Cl− concentration. The Bode plots (Figure 2b) illustrate that the maximum phase angles of the Fe-20Cr-20Mn-0.75N HNSS are relatively close to −80° in 0.05 mol/L, 0.1 mol/L, and 0.3 mol/L NaCl solutions [27]. Compared to the 0.05, 0.1, and 0.3 mol/L NaCl solutions, the phase angle is lower in the 0.6 mol/L NaCl solution, being around 75°. This phenomenon indicates a significant reduction in the passive ability of the HNSS. Furthermore, the magnitude |Z| at low frequencies decreases with Cl− concentration, indicating poorer surface corrosion resistance with Cl− [28,29,30]. The EIS data were fitted using the equivalent circuit shown in Figure 2c, and the impedance formula for the CPE is given by Equation (1) [31]:
where A is a constant, j2 = –1, ω represents the angular frequency. CPE corresponds to resistance, impedance, and capacitance when n = 0, 0.5, and 1, respectively. The results of the EIS fitting are presented in Table 2. The results indicate that as the NaCl concentration increases from 0.05 mol/L to 0.6 mol/L, the Rf value decreases from 5.88 × 105 Ω·cm2 to 2.17 × 105 Ω·cm2. This finding suggests that Cl− concentration results in a less stable passive film with inferior protective characteristics.
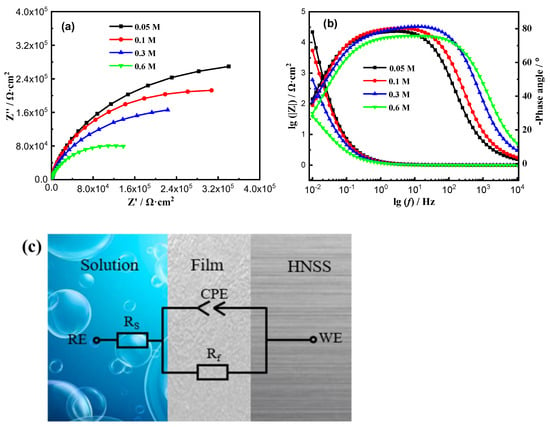
Figure 2.
EIS analysis of Fe-20Cr-20Mn-0.75N at different Cl− concentrations: (a) Nyquist plots, (b) Bode plots, (c) Equivalent circuit, where Rs represents the solution resistance, CPE is a constant phase element, and Rf is the polarization resistance of the passive film.

Table 2.
Equivalent circuit parameters of Fe-20Cr-20Mn-0.75N HNSS at different Cl− concentrations.
3.3. Potentiodynamic Cyclic Polarization Curve
Figure 3 shows the potentiodynamic cyclic polarization curves of Fe-20Cr-20Mn-0.75N HNSS at different Cl− concentrations. The corrosion trends of Fe-20Cr-20Mn-0.75N HNSS at different Cl− concentrations are generally consistent, exhibiting passivation behaviors with noticeable hysteresis loops. As the Cl− concentration in the solution increases, the passivation region decreases with a reduced stability of the passive film. This observation is consistent with the patterns identified in the EIS analysis. Furthermore, the corrosion potential gradually decreases with increased corrosion tendency. In particular, the corrosion current density is highest in the 0.6 mol/L NaCl solution, indicating the poorest corrosion resistance of the Fe-20Cr-20Mn-0.75N (Table 3). The above results show that the penetration ability of Cl− on the passivation film increases with concentration, resulting in a decrease in the corrosion resistance of Fe-20Cr-20Mn-0.75N [20,32].
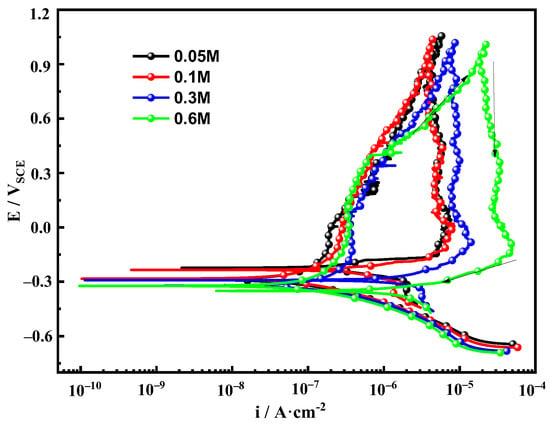
Figure 3.
Cyclic polarization curves of Fe-20Cr-20Mn-0.75N in different Cl− concentrations.

Table 3.
Fitting parameters of cyclic polarization curve.
3.4. Corrosion Morphologies
Figure 4 shows the morphological characteristics of Fe-20Cr-20Mn-0.75N HNSS after cyclic polarization at different concentrations of Cl−. The results indicate that with increasing Cl− concentration, the pitting on the sample surface transitions from a small and sparse status at low concentrations (0.05 mol/L) to become numerous at high concentrations (0.6 mol/L). According to the elemental composition of the matrix and pitting obtained through energy dispersive X-ray spectroscopy (EDS), the Fe content in the pits is higher, while the Cr and Mo components are relatively lower, and the content of Mn remains basically unchanged, as shown in Table 4. Meanwhile, the surface of the HNSS in higher concentrations of Cr and Mo elements has no pronounced corrosion. This phenomenon indicates that the dissolution of Fe elements is beneficial for the local corrosion behavior, while Cr and Mo elements facilitate the development of the passive film. Thus, the lower content of the Cr and Mo elements in the corrosion pits leads to the formation of a passive film that is more susceptible to Cl− infiltration, compared to the non-corroded regions on the matrix surface. Furthermore, the combination of Cl− and soluble halides accelerates the anodic dissolution of the material and increases the corrosion rate of Fe-20Cr-20Mn-0.75N HNSS [33].
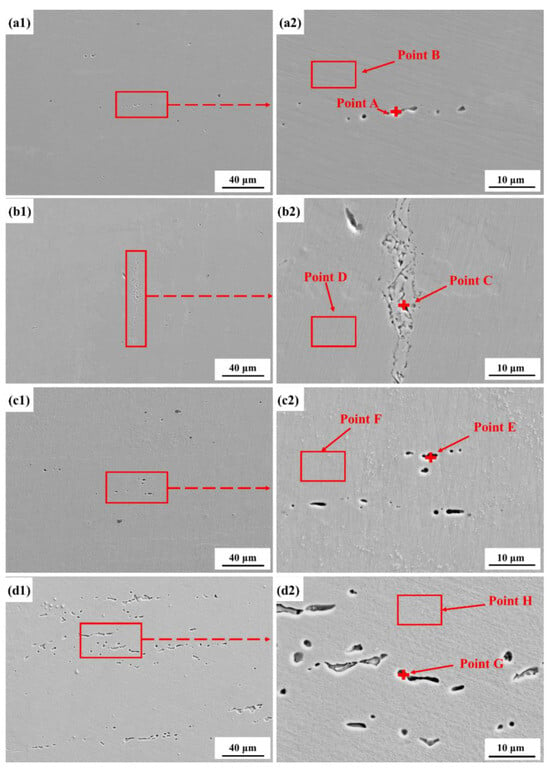
Figure 4.
Corrosion morphologies of Fe-20Cr-20Mn-0.75N HNSS after cyclic polarization in different Cl− concentrations: 0.05 mol/L (a1,a2), 0.1 mol/L (b1,b2), 0.3 mol/L (c1,c2), 0.6 mol/L (d1,d2).

Table 4.
EDS analysis of the element composition of point/region markers in Figure 4.
3.5. Potentiostatic Polarization Analysis
To further investigate the impact of Cl− concentration on the growth of passive films on Fe-20Cr-20Mn-0.75N HNSS, a potentiostatic polarization test was conducted with an applied passivation potential of 0.2 VSCE. The obtained curves, which also depict time and passivation current density, are shown in Figure 5a. The results indicate that with an increase in Cl− concentration, the passivation current density rises from 3.6 × 10−7 (0.05 mol/L) to 6.28 × 10−7 (0.6 mol/L). Additionally, with the increase in Cl− concentration, the current density exhibits partially metastable pitting peaks, and the occurrence time of these peaks advances. Figure 5b illustrates the double-logarithmic curves of current density versus time for Fe-20Cr-20Mn-0.75N HNSS at different Cl− concentrations. These curves can be used to assess the rates and kinetic characteristics of electrochemical reactions, as expressed in Equation (2) [34]:
where i represents the corrosion current density, A is a constant, k is the slope of the double-logarithmic curve, and t stands for the testing time. Figure 5b depicts the fitted slope values of the double-logarithmic curves for the potentiostatic polarization of the investigated samples in varying Cl− concentrations. Higher k values and low current densities indicate higher passive film formation rates. Conversely, when k is lower, the film formation rate becomes lower [35,36]. At Cl− concentrations of 0.05, 0.1, and 0.3 mol/L, the k values are approximately −0.7, indicating similar film formation rates at these potentials. This suggests that the passivation capability of the material remains relatively unchanged. As the Cl− concentration increases to 0.6 mol/L, the k value approaches −0.5, which indicates a lower passive film formation rate and reduced stability.
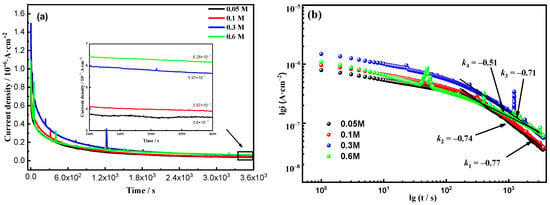
Figure 5.
Time-dependent current density (a) and double log (b) plots of Fe-20Cr-20Mn-0.75N at a passivation potential of 0.2 VSCE in different Cl− concentrations.
3.6. Mott–Schottky Analysis
In order to study the semiconductor characteristics of the passive film on the Fe-20Cr-20Mn-0.75N high-nitrogen steel [37], Mott–Schottky curves were measured at an applied potential of 0.2 VSCE using different concentrations of Cl−, as shown in Figure 6. The passive film on the surface of Fe-20Cr-20Mn-0.75N HNSS presents semiconductor p-n properties [38] in four different Cl− concentrations. As can be seen from Figure 6a, within the range of 0 VSCE to 0.4 VSCE (Region I), the curve shows an upward trend with an increasing slope, indicating that the passive film exhibits an n-type semiconductor behavior. The previous research [39] suggested that when the number of electrons in the passive film conduction band is greater than the number of holes in the valence band, the passive film is considered an n-type semiconductor (for example, Fe2O3, MoO3, etc.). Within the range of 0.4 VSCE to 0.8 VSCE (Region II), the curve tends to decrease, and at this time, the passive film exhibits a p-type semiconductor behavior. When there is a deficiency of metal ions or an excess of cation vacancies (for example, Cr2O3, MoO2, etc.), the passive film shows a p-type behavior [40]. Figure 6b shows the donor density (ND) and acceptor density (NA) measured for the Fe-20Cr-20Mn-0.75N high-nitrogen steel in different NaCl solutions at 0.2 VSCE. As the Cl− concentration increases, the carrier density gradually increases. In the 0.6 mol/L NaCl solution, the ND increases to 4.37 × 102⁰ cm⁻3, and the NA increases to 4.27 × 102⁰ cm⁻3. The ion movement within the passive film is the most intense, the protectiveness of the passive film on the matrix is the worst, and the corrosion resistance is the poorest.
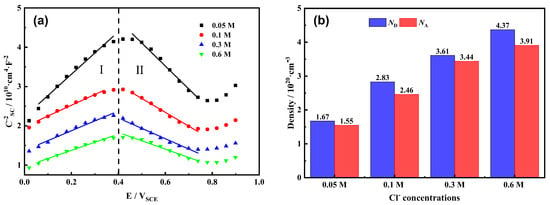
Figure 6.
Fe-20Cr-20Mn-0.75N in different NaCl solutions at applied potential of 0.2 VSCE: (a) Mott–Schottky curves; (b) carrier density.
3.7. Point Defect Model (PDM) of Fe-20Cr-20Mn-0.75N
Based on the study of the properties of the passive film on Fe-20Cr-20Mn-0.75N HNSS, a model was designed in the current work according to the point defect model (PDM) [41], as shown in Figure 7. In this model, m represents the metal atoms of HNSS, MM represents the matrix cations, and and represent the anion and cation vacancies, respectively. The matrix/passive film interface tends to produce , which is then annihilated at the passive film/solution interface. Metal-ion defects are generated at the passive film/solution interface and disappear at the matrix/passive film interface [30]. According to the PDM theory [31], or cation interstitials (such as Cr2+, Cr3+, and Fe2+) crossing the passive film are crucial for the film’s growth process. The dominant defects in the passive film are considered to be either oxygen vacancies or cation interstitials, which act as electron donors. Since it is not possible to separate the contributions of oxygen vacancies and cation interstitials to the measured diffusion coefficient values based on PDM, the diffusion coefficient (D0) is considered to be the combined diffusion coefficient of and .
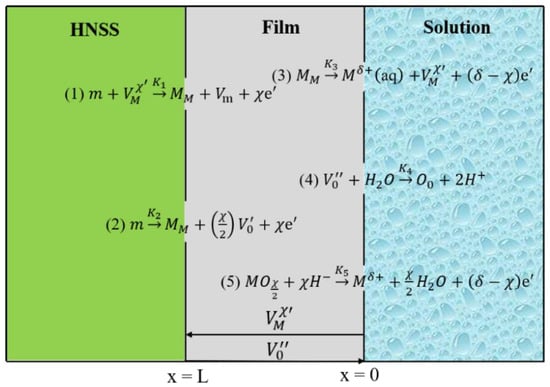
Figure 7.
Point defect model (PDM) of Fe-20Cr-20Mn-0.75N.
According to the PDM, the relationship between the applied potential and the donor density is given by Equation (3):
where ρ1, ρ2, and ρ3 are constants. As can be seen in Figure 7, the donor density within the passive film increases with the increase in the film’s forming potential. The defects in the passive film of Fe-20Cr-20Mn-0.75N HNSS are mainly defects. In Equation (3), ρ2 can be expressed by Equation (4):
Therefore, the diffusion coefficient D0 can be expressed as follows:
where R is the gas constant (8.314 J·mol−1·K−1), T is the temperature (K), F is the Faraday constant (96,500 C/mol), J0 = −iss/2e, iss is the stable current density under applied potential, and ωL is the electric field strength within the film (1 × 106 V/cm).
The point defect diffusion coefficient D0 (Equation (5)) obtained for the passive film is shown in Table 5. Under the same applied potential, the diffusion coefficient D0 of increases with increasing Cl− concentration; moreover, as the applied potential increases, the diffusion coefficient D0 gradually increases within the same Cl− concentration, indicating that the protectiveness of the passive film on the substrate deteriorates. This is mainly because the decrease in the diffusion coefficient leads to a reduction in the applied potential, which, in turn, reduces the ability of Fe0 in the passive film to transform into higher valent iron oxides, rendering the passive film highly unstable. This instability allows aggressive ions to more easily replace the oxygen vacancies within the film, thus diminishing the performance of the passive film [42]. Macdonald et al. [43] found that the steady-state current under passive conditions is independent of the applied potential, which is consistent with the passive film being an interstitial metal conductor or an oxygen vacancy conductor, with interstitial metals being the most likely defects. This model is also consistent with the n-type characteristics of the passive films.

Table 5.
The calculated diffusion coefficient D0.
3.8. XPS of the Passive Film
Through the analysis of passive films formed on Fe-20Cr-20Mn-0.75N HNSS in tested NaCl solutions, this investigation utilized fine XPS to quantify the different valence states of elements, as shown in Figure 8. The peaks of Fe 2p3/2 spectra corresponding to Fe0, Fe3O4, Fe2O3, and FeOOH [44] indicate a diminished content of iron oxides within the passive film as the chloride ion concentration increased, which led to the reduced thickness and protectiveness of the film. As shown in Figure 9, the peaks of Cr 2p3/2 spectra exhibited a decreasing ratio of Cr2O3 to Cr(OH)3 with increasing Cl− concentrations, suggesting a compromised stability of the passive film [45]. The Mo 3d5/2 spectra demonstrated a decrease in the peak intensity of MoO3, implying that higher Cl− concentrations result in less Mo oxide content within the passive film and a weakened resistance to chloride ion corrosion. The N 1s spectra showed alterations in the peaks of NH3 and NH4+, further highlighting the inferior protective properties of the passivation film under elevated chloride ion levels. Additionally, the O 1s spectra displayed a decline in the O2−/OH− peak intensity ratio concomitant with increasing Cl− concentrations, correlating with an enhanced risk of corrosion [46,47]. This showed that the augmentation of Cl− concentration substantially impacted the composition and stability of the passive film, thereby deteriorating its protective efficacy over the substrate [48,49,50].
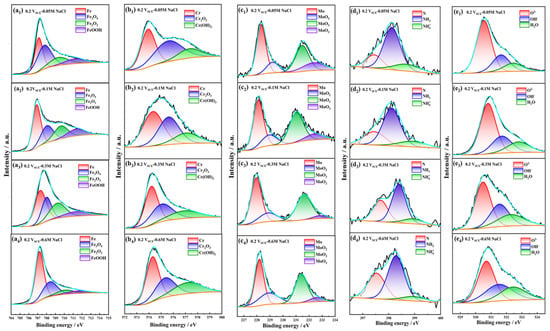
Figure 8.
The XPS of Fe 2p3/2 (a1–a4), Cr 2p3/2 (b1–b4), Mo 3d5/2 (c1–c4), N 1s (d1–d4), O 1s (e1–e4). The blue lines in the picture is the fitting curve.
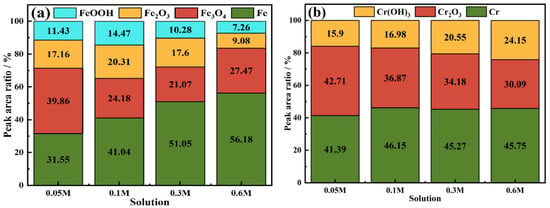
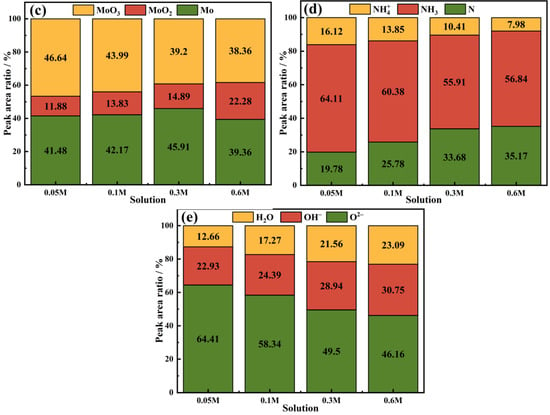
Figure 9.
Peak area percentage content of (a) Fe 2p3/2, (b) Cr 2p3/2, (c) Mo 3d5/2, (d) N 1s, and (e) O 1s spectra of Fe-20Cr-20Mn-0.75N passive films in different NaCl solutions.
4. Conclusions
- The corrosion tendency and corrosion rate of Fe-20Cr-20Mn-0.75N HNSS increased with increasing Cl− concentration.
- Fe-20Cr-20Mn-0.75N HNSS showed passive behavior in different concentrations of NaCl solutions. The anodic region of the polarization curves showed activation–passivation–activation behaviors, and all of them had wide passive regions, but the passive zones fluctuated due to the increase in the concentration, the poor corrosion environment, and the unstable corrosion. In 0.6 mol/L NaCl solution, Fe-20Cr-20Mn-0.75N HNSS had the highest corrosion current density, largest passive film dissolution rate, and lowest corrosion resistance.
- With the increase in Cl− concentration, the stable corrosion current density increased; at this time, the passive film’s resistance to Cl− ’s erosion ability became poor, and Fe-20Cr-20Mn-0.75N HNSS’s corrosion resistance decreased.
- In the solution with a low Cl− concentration, the passive film was thicker, and the surface was more stable. And the high content of NH4+ inhibited the rupture of the passive film; hence, the passive film was more dense, and the corrosion resistance increased.
Author Contributions
Data curation, W.Z., F.G. and C.L.; Writing—original draft, F.G., H.Z. and Z.L.; Writing—review and editing, F.G., H.Y. and Y.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Undergraduate Innovation and Entrepreneurship Training Program of Jiangsu University of Science and Technology (No. 202310289057Z) and National Key Research and Development Program of China (No. 2021YFB3401100).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Author Fengxin Gao was employed by the company GRG Metrology & Test Group Co. Authors Chengtao Li and Zhong Liu were employed by the company Suzhou Nuclear Power Research Institute. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ilevbare, G.O.; Burstein, G.T. The inhibition of pitting corrosion of stainless steels by chromate and molybdate ions. Corros. Sci. 2003, 45, 1545–1569. [Google Scholar] [CrossRef]
- Bairi, L.R.; Ningshen, S.; Mudali, U.K.; Raj, B. Microstructural analysis and corrosion behaviour of D9 stainless steel-zirconium metal waste form alloys. Corros. Sci. 2010, 52, 2291–2302. [Google Scholar] [CrossRef]
- Hu, S.F.; Sun, Z.Z.; Shen, F.H.; Deng, J.; Yang, W.P.; Yang, H.K. Carbon fiber breakage mechanism in aluminum (Al)/carbon fibers (CFs) composite sheet during accumulative roll bonding (ARB) process. J. Wuhan Univ. Technol. 2024, 39, 167–173. [Google Scholar] [CrossRef]
- Chen, S.S.; Yao, Z.F.; Guan, Y.B.; Yang, H.; Shahzad, M.B.; Wu, Y.Z.; Zhang, B.C.; Shen, L.; Yang, K. High nitrogen stainless steel drug-eluting stent-Assessment of pharmacokinetics and preclinical safety in vivo. Bioact. Mater. 2020, 5, 779–786. [Google Scholar] [CrossRef]
- Zhou, R.; Northwood, D.O.; Liu, C. On nitrogen diffusion during solution treatment in a high nitrogen austenitic stainless steel. J. Mater. Res. Technol. 2020, 9, 2331–2337. [Google Scholar] [CrossRef]
- Fossati, A.; Borgioli, F.; Galvanetto, E.; Bacci, T. Corrosion resistance properties of glow-discharge nitrided AISI 316L austenitic stainless steel in NaCl solutions. Corros. Sci. 2006, 48, 1513–1527. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Z.B.; Long, J.; Wang, J.; Zheng, K.H.; Ke, Z.M.; Luo, Z.C.; Pokrovsky, A.I.; Khina, B.B. Recent advances in wear-resistant steel matrix composites: A review of reinforcement particle selection and preparation processes. J. Mater. Res. Technol. 2024, 29, 1779–1797. [Google Scholar] [CrossRef]
- Maznichevsky, A.N.; Sprikut, R.V.; Goikhenber, Y.N. Investigation of nitrogen containing austenitic stainless steel. Mater. Sci. Forum. 2020, 989, 152–159. [Google Scholar] [CrossRef]
- Gao, F.Y.; Qiao, Y.X.; Chen, J.; Yang, L.L.; Zhou, H.L.; Zheng, Z.B.; Zhang, L.M. Effect of nitrogen content on corrosion behavior of high-nitrogen austenitic stainless steel. NPJ Mater. Degrad. 2023, 7, 75. [Google Scholar] [CrossRef]
- Satpati, A.K.; Phadnis, S.V.; Sundaresan, R.I. Electrochemical and XPS studies and the potential scan rate dependent pitting corrosion behavior of Zircaloy-2 in 5% NaCl solution. Corros. Sci. 2005, 47, 1445–1458. [Google Scholar] [CrossRef]
- Ghosh, S.; Kain, V. Effect of surface machining and cold working on the ambient temperature chloride stress corrosion cracking susceptibility of AISI 304L stainless steel. Mater. Sci. Eng. A 2010, 527, 679–683. [Google Scholar] [CrossRef]
- Wang, X.L.; Hu, Q.X.; Liu, W.K.; Yuan, W.; Shen, X.W.; Gao, F.Y.; Tang, D.X.; Hu, Z.C. Microstructure and corrosion properties of wire arc additively manufactured multi-trace and multilayer stainless steel 321. Metals 2022, 12, 1039. [Google Scholar] [CrossRef]
- Mu, J.; Li, Y.Z.; Wang, X. Crevice corrosion behavior of X70 steel in NaCl solution with different pH. Corros. Sci. 2021, 182, 109310. [Google Scholar] [CrossRef]
- Wang, Q.C.; Zhang, B.C.; Ren, Y.B.; Yang, K. Eliminating detrimental effect of cold working on pitting corrosion resistance in high nitrogen austenitic stainless steels. Corros. Sci. 2017, 123, 351–355. [Google Scholar] [CrossRef]
- Abdelfatah, A. Corrosion Characteristics of 304 stainless steel in sodium chloride and sulfuric acid solutions. Int. J. Electrochem. Sci. 2022, 17, 220417. [Google Scholar] [CrossRef]
- Li, S.H.; Li, J.; Zhang, J.; Shi, C.B. Effect of nitrogen on microstructure and microsegregation of martensitic stainless steel 4Cr13 produced by electroslag remelting. J. Iron Steel Res. Int. 2022, 30, 1854–1861. [Google Scholar] [CrossRef]
- Li, H.B.; Jiang, Z.H.; Yang, Y.; Cao, Y.; Zhang, Z.R. Pitting corrosion and crevice corrosion behaviors of high nitrogen austenitic stainless steels. Int. J. Miner. Metall. Mater. 2009, 16, 517–524. [Google Scholar] [CrossRef]
- Deyab, M.A.; Mohamed, N.H.; Moustafa, Y.M. Corrosion protection of petroleum pipelines in NaCl solution by microcrystalline waxes from waste materials: Electrochemical studies. Corros. Sci. 2017, 122, 74–79. [Google Scholar] [CrossRef]
- Dai, N.W.; Zhang, L.C.; Zhang, J.X.; Chen, Q.M.; Wu, M.L. Corrosion behavior of selective laser melted Ti-6Al-4V alloy in NaCl solution. Corros. Sci. 2016, 102, 484–489. [Google Scholar] [CrossRef]
- Li, J.Q.; Han, Q.L.; Zou, Y.B.; Yu, Z.X.; Zhu, G.X.; Xu, W.; Shi, S.H. Electrochemical behavior of additive manufactured TC4 alloy in different concentrated NaCl solutions. J. Appl. Electrochem. 1022, 52, 1419–1431. [Google Scholar] [CrossRef]
- Leng, C.; Yang, H.; Yi, G.; Lo, H.; Li, J.; Li, Y. The effect of carbon addition on the mechanical properties and fracture behaviors of cobalt-chromium-iron-manganese-nickel high entropy alloy. Materialwiss. Werkst. 2024, 55, 7–12. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhao, X.Y.; Gao, S.; Zhu, Y.S.; Zheng, Y.; Huang, Y.; Xu, Y.Z. Visualizing and understanding corrosion evolution beneath a condensed droplet using the multi-electrode array. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133252. [Google Scholar] [CrossRef]
- Cai, X.; Yang, M.M.; Rui, Y.P.; Wang, Z.; Zhou, J.; Xue, F. Investigation of mechanical and corrosion behavior of multi-pass nickel-aluminum bronze fabricated through wire-arc directed energy deposition. Addit. Manuf. 2024, 80, 103967. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, Z.H.; Yang, L.L.; Tang, Y.B.; Qiao, Y.X.; Lu, D.H. Corrosion behavior of different building planes of selective laser melting 316L stainless steel in 0.1 M HCl solution. J. Mater. Res. Technol. 2024, 28, 4738–4753. [Google Scholar] [CrossRef]
- Nie, S.J.; Yi, X.N.; Zhou, H.L.; Zhu, H.J.; Yang, L.L.; Fu, F.L.; Li, J.Y.; Yang, H.K.; Xu, G.X.; Lu, S.; et al. Corrosion behavior of as-cast Al0.75CoFeCr1.25Ni high entropy alloy in 0.5 mol/L NaOH solution. J. Iron Steel Res. Int. 2024. [Google Scholar]
- Liao, X.Y.; Zheng, Z.B.; Liu, T.L.; Long, J.; Wang, S.; Zhang, H.Y.; Zheng, K.H. Achieving high impact–abrasion–corrosion resistance of high–chromium wear–resistant steel via vanadium additions. J. Mater. Res. Technol. 2024, 29, 2425–2436. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Yang, X.L.; Tang, A. Corrosion behavior of nickel-based 718 alloy determined by in situ electrochemical methods at different partial pressures of H2S in 25 wt% NaCl solution at 150 °C. Rare Met. 2019, 38, 855–863. [Google Scholar] [CrossRef]
- Kong, W.C.; Li, M.K.; Hu, J. Immersion corrosion behavior, electrochemical performance and corrosion mechanism of subsonic flame sprayed FeCoCrMoSi amorphous coating in 3.5% NaCl solution. Int. J. Hydrogen Energy 2022, 47, 6911–6923. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zheng, Z.B.; Xu, L.L.; Xu, Z.B.; Yin, F.X.; Zheng, K.H. Unraveling the interfacial structure of TA2 titanium-A36 steel composite plate and its corrosion behavior in marine environment. Corros. Sci. 2024, 230, 111923. [Google Scholar] [CrossRef]
- Zhao, Q.C.; Wang, X.F.; Pan, Z.M.; Wei, Y.; Cheng, H.X.; Ma, Y.C.; Luo, H.; Li, X.G. Effects of rare earth elements addition on mechanical properties and corrosion behavior of GCr15 bearing steel under different heat treatment conditions. Corros. Commun. 2023, 9, 65–76. [Google Scholar] [CrossRef]
- Grubac, Z.; Hukovic, M.M. EIS study of solid-state transformations in the passivation process of bismuth in sulfide solution. J. Electroanal. Chem. 2004, 565, 85–94. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Wang, X.Y.; Yang, L.L.; Wang, X.J.; Chen, J.; Wang, Z.B.; Zhou, H.L.; Zou, J.S.; Wang, F.H. Effect of aging treatment on microstructure and corrosion behavior of a Fe-18Cr-15Mn-0.66N stainless steel. J. Mater. Sci. Technol. 2022, 107, 197–206. [Google Scholar] [CrossRef]
- Moayed, M.H.; Newman, R.C. Evolution of current transients and morphology of metastable and stable pitting on stainless steel near the critical pitting temperature. Corros. Sci. 2006, 48, 1004–1018. [Google Scholar] [CrossRef]
- Galvele, J.R.; Torresi, R.M.; Carranza, R.M. Passivity breakdown its relation to pitting and stress corrosion cracking processes. Corros. Sci. 1990, 31, 563–571. [Google Scholar] [CrossRef]
- Zhang, L.N.; Ojo, O.A. Corrosion behavior of wire arc additive manufactured Inconel 718 superalloy. J. Alloys Compd. 2020, 829, 154455. [Google Scholar] [CrossRef]
- Genevieve, B.; Christine, B.; Nadine, P. AC impedance spectroscopy in characterizing time-dependent corrosion of AZ91 and AM50 magnesium alloys. J. Electrochem. Soc. 2001, 148, 489–496. [Google Scholar]
- Fattah-alhosseini, A.; Golozar, M.A.; Saatchi, A.; Raeissi, K. Effect of solution concentration on semiconducting properties of passive films formed on austenitic stainless steels. Corros. Sci. 2010, 52, 205–209. [Google Scholar] [CrossRef]
- Sikora, J.; Sikora, E.; Macdonald, D.D. The electronic structure of the passive film on tungsten. Electrochim. Acta 2000, 45, 1875–1883. [Google Scholar] [CrossRef]
- Sunseri, C.; Piazza, S.; Quarto, F.D. Photocurrent spectroscopic investigations of passive films on chromium. J. Electrochem. Soc. 1990, 137, 2411–2417. [Google Scholar] [CrossRef]
- Hamadou, L.; Kadri, A.; Benbrahim, N. Impedance investigation of thermally formed oxide films on AISI 304L stainless steel. Corros. Sci. 2010, 52, 859–864. [Google Scholar] [CrossRef]
- Jun, L.; Macdonald, D.D. The passivity of iron in the presence of ethylenediaminetetraacetic acid. II. The defect and electronic structures of the barrier layer. J. Electrochem. Soc. 2001, 148, 425–430. [Google Scholar]
- Zhou, Y.T.; Xu, A.N.; Mao, F.X.; Yu, J.K.; Kong, D.C.; Dong, C.F.; Macdonald, D.D. Passivity breakdown on copper: Influence of borate anion. Electrochim. Acta 2019, 320, 134545. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Ismail, K.M.; Sikora, E. Characterization of the passive state on zinc. J. Electrochem. Soc. 1998, 145, 3141–3149. [Google Scholar] [CrossRef]
- Cheng, H.X.; Luo, H.; Wang, X.F.; Pan, Z.M.; Jiang, Y.; Li, X.G. Electrochemical corrosion and passive behavior of a new high-nitrogen austenitic stainless steel in chloride environment. Mater. Chem. Phys. 2022, 292, 126837. [Google Scholar] [CrossRef]
- Xie, H.Y.; Chen, J.L.; Zhang, P.; Gao, L.K.; Liu, D.W.; Chen, L.Z. Separation of galena and chalcopyrite using the difference in their surface acid corrosion characteristics. Int. J. Miner. Metall. Mater. 2023, 30, 2157–2168. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Qin, Y.; Zhou, H.L.; Yang, L.L.; Wang, X.J.; Wang, Z.B.; Liu, Z.G.; Zou, J.S. Electrochemical hydrogen charging on corrosion behavior of Ti-6Al-4V alloy in artificial seawater. Chin. J. Mech. Eng. 2024, 37, 2. [Google Scholar] [CrossRef]
- Wang, D.P.; Chen, G.; Wang, A.D.; Wang, Y.X.; Qiao, Y.X.; Liu, Z.G.; Qi, Z.X.; Liu, C.T. Corrosion behavior of single- and poly-crystalline dual-phase TiAl-Ti3Al alloy in NaCl solution. Int. J. Miner. Metall. Mater. 2023, 30, 689–696. [Google Scholar] [CrossRef]
- Sun, S.C.; Wei, S.F.; Wang, G.Y.; Jiang, Z.H.; Lian, J.S.; Ji, C.T. The synthesis and electrochemical behavior of high-nitrogen nickel-free austenitic stainless steel. J. Mater. Eng. Perform. 2014, 23, 3957–3962. [Google Scholar] [CrossRef]
- Leia, M.K.; Zhu, X.M. Role of Nitrogen in pitting corrosion resistance of a high-nitrogen face-centered-cubic phase formed on austenitic stainless steel. J. Electrochem. Soc. 2005, 152, 291–295. [Google Scholar] [CrossRef]
- Luo, H.; Su, H.Z.; Li, B.S.; Ying, G.B. Electrochemical and passive behaviour of tin alloyed ferritic stainless steel in concrete environment. Appl. Surf. Sci. 2018, 439, 232–239. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).