Selection of PEG-Matrix Combinations to Achieve High Performance Form-Stable Phase Change Materials for Building Applications
Abstract
1. Introduction
- Their high heat storage capacity allows them to store and release more thermal energy during phase transitions, enabling effective indoor temperature regulation.
- The high compatibility of organic PCMs with common building materials minimizes unwanted interactions between these materials, ensuring the durability of the integrated system.
- Compared with inorganic PCMs, organic PCMs do not cause corrosion in building materials, thus improving the overall longevity of the construction.
- Flexibility in the formulation and encapsulation methods of organic PCMs allows for tailored designs that meet specific project requirements.
- Finally, organic PCMs can be produced from renewable resources or compounds with low environmental impact, contributing to sustainability in construction.
2. Properties and Characteristics of Polyethylene Glycol
2.1. Features of PEG as Phase Change Material
- ▪
- Since PEG is available in a wide range of molecular weights corresponding to different melting/solidification temperature ranges, it is possible to select the most suitable PEG for the building where the PCM will be integrated, in terms of geographical position and climatic conditions.
- ▪
- Offering high latent heat, PEG-based PCMs can store great amounts of thermal energy during phase transition.
- ▪
- Providing good compatibility with common building materials, PEG can be incorporated into various components, such as insulation materials, wall panels, or other building elements, without significantly altering their integrity.
- ▪
- Its use, finally, greatly contributes to reducing the need for traditional heating and cooling devices, the presence of which may not be permitted due to, for example, the artistic or historical constraints of the building.
3. Application of PEG as PCM in Buildings
- ▪
- Direct Mixing. PCM can be dispersed directly into the binder during the process of production of the building material. This method is applied to materials such as mortars, cement, plaster, or plasterboard, to increase their heat storage capacity. Although this technique appears to be the simplest, fastest, and cheapest one, the PCM can interfere with the hydration process and kinetic reaction, slowing down the reaction rate, and causing a severe reduction of the final mechanical properties of the material. The mentioned phenomena have been observed also when different procedures are used to introduce PEG into building materials; however, these phenomena are more marked with the direct incorporation of PEG [57].
- ▪
- Microencapsulation. The PCM can be encapsulated within tiny capsules. The microcapsules containing the PCM are then dispersed in building materials such as concrete, plaster, or gypsum [5]. This method allows for the controlled absorption/release of thermal energy. However, it is reported that PEG is poorly suited for encapsulation methods [58].
- ▪
- Macroencapsulation. In this case, larger containers enclose the PCM that is dispersed within the building material. Macroencapsulation makes greater quantities of PCM available; the capsules are often integrated into walls, ceilings, or floors. However, (i) macrocapsules can cause structure stiffness when incorporated into building materials; (ii) their size can take up too much volume in the structure; (iii) although the macroencapsulation technique is less expensive than the microencapsulation, it is still more expensive than other methods [59].
- ▪
- Form-stable or impregnation method. PCM can be introduced and stabilized in a porous matrix. In the case of PEG, a physical link between the polymer and the matrix is obtained through the association of soft segments of PEG with the stiff segments of the matrix. Different techniques (i.e., blending, soaking, adsorbing) can be employed to force the soft PEG segments within a rigid polymeric or an inorganic porous matrix [60]. This is the most widely used method, as it offers numerous advantages: (i) its cost-effectiveness and ease of implementation; (ii) the possibility to limit PEG leakage during its phase transition; (iii) the form-stable technique provides stability to the PCM, reducing the risk of degradation or alteration during its handling, transportation, and integration into building materials. In addition, this method allows for tuning the properties of the PCM by appropriately choosing the chemical composition of the matrix. In fact, by selecting different matrices, it is possible to modify the mechanical resistance, thermal conductivity, and other characteristics of the PCMs to adapt them to application needs. All of the mentioned advantages make the form-stable method the most attractive approach to producing a PEG-based PCM.
4. PEG-Based Form-Stable PCMs
4.1. PEG-Based PCMs with a Polymer Matrix
| PEG | Matrix | Tm (°C) | ΔHm (J⋅g−1) | Tc (°C) | ΔHc (J⋅g−1) | Amount of PEG (% wt.) | Ref. |
|---|---|---|---|---|---|---|---|
| 1000 | Epoxy resin | 34.9 | 92.7 | 31.6 | 89.8 | 70.0 | [31,61] |
| 1500 | 45.8 | 108.5 | 38.8 | 103.4 | |||
| 2000 | 52.6 | 116.2 | 37.9 | 107.8 | |||
| 6000 | 58.0 | 115.0 | 44.2 | 113.6 | |||
| 8000 | Polyaryl polymethylene isocyanate and polycarbonatediol | 50.9 | 113.5 | 39.7 | 110.3 | - | [49] |
| 10000 | 52.8 | 112.3 | 39.7 | 109.5 | - | ||
| 600 | Polyurethane foam | 18.1 | 62.3 | - | - | 44.0 | [62] |
| 1000 | 37.8 | 76.7 | - | - | 49.0 | ||
| 1500 | 49.6 | 138.1 | - | - | 53.0 | ||
| 600–1000–1500 | 17.0–31.0–42.4 | 72.4 | - | - | 38.0 | ||
| 4000 | 4,4′-Diphenylmethane di-isocyanate | 51.1 | 144.1 | 33.0 | 117.5 | 90.0 | [63] |
| 4000 | Polyvinyl alcohol | 44.5 | 132.3 | 27.2 | 115.6 | 80.0 | |
| 49.7 | 107.1 | 31.2 | 93.3 | 60.0 | |||
| 47.0 | 105.5 | 31.1 | 65.3 | 40.0 | |||
| 1000 | Triethylene glycol methyl ether methacrylate | 35.2 | 108.0 | - | - | - | [64] |
| 33.5 | 101.0 | - | - | - | |||
| 30.6 | 95.0 | - | - | - | |||
| 25.3 | 75.0 | - | - | - | |||
| 6000 | Polybutylene terephthalate and adipic acid | 45.6 | 136.8 | 33.4 | 110.1 | 91.0 | [65] |
| 6000 | Polyvinyl alcohol | 59.0 | 144.6 | 46.2 | 132.7 | 94.5 | [48] |
| 6000 | Polyvinyl alcohol and graphene oxide | 59.1 | 153.9 | 47.4 | 150.6 | 97.2 | |
| 6000 | Polyvinyl alcohol and reduced graphene oxide | 59.6 | 155.5 | 49.0 | 149.9 | 96.9 | |
| 20000 | Carboxylic acid | 60.2 | 188.8 | 46.3 | 167.8 | 90.0 | [45] |
4.2. PEG-Based PCMs with a Natural Organic Matrix
| PEG | Matrix | Tm (°C) | ΔHm (J⋅g−1) | Tc (°C) | ΔHc (J⋅g−1) | Amount of PEG (% wt.) | Ref. |
|---|---|---|---|---|---|---|---|
| 5000 | Cellulose-graft with expanded graphite | 58.9 | 166.6 | 37.6 | 165.3 | 84.8 | [43] |
| 8000 | Cellulose acetate | 60.6 | 155.35 | - | - | 82.0 | [67] |
| 2000 | Cellulose | 56.2 | 177.7 | 29.1 | 168.9 | 98.1 | [68] |
| 2000 | Cellulose with 0.05% Fe3O4 | 55.8 | 176.6 | 28.8 | 167.7 | 97.5 | |
| Cellulose with 0.1% Fe3O4 | 56.1 | 175.5 | 28.4 | 166.4 | 96.9 | ||
| Cellulose with 0.15% Fe3O4 | 57.4 | 173.9 | 28.5 | 164.3 | 96.0 | ||
| Cellulose with 0.2% Fe3O4 | 56.7 | 172.6 | 28.8 | 163.7 | 95.3 | ||
| 800 | Delignified wood with an epoxy resin | 32.2 | 28.2 | 21.7 | 21.5 | 50.0 | [70] |
| 32.7 | 71.7 | 21.7 | 46.5 | 60.0 | |||
| 31.3 | 106.7 | 22.3 | 79.7 | 70.0 | |||
| 30.7 | 134.1 | 22.2 | 122.9 | 80.0 | |||
| 800–1000 | Balsa wood and 0% of carbon black | 29.4 | 119.3 | 23.6 | 118.5 | - | [71] |
| Balsa wood and 1% of carbon black | 28.7 | 140.3 | 18.4 | 133.9 | - | ||
| Balsa wood and 2% of carbon black | 27.7 | 112.8 | 16.2 | 89.49 | - | ||
| Balsa wood and 4% of carbon black | 23.7 | 100.3 | 18.7 | 91.1 | - | ||
| Balsa wood and 6% of carbon black | 40.1 | 137.00 | 19.6 | 135.3 | - | ||
| 800–1000 | Delignified bamboo | 28.8–41.6 | 84.3 | 7.0 | 72.2 | 81.9 | [72] |
| 2000 | Rough rice husk ash | 55.2 | 98.7 | 38.5 | 88.6 | 53.1 | [73] |
| Modified rice husk ash | 55.7 | 119.3 | 40.4 | 107.9 | 64.6 | ||
| 1000 | Waste apricot kernel shell-derived activated carbon | 26.6 * | 110.6 | 28.8 * | 112.5 | 70 | [74] |
| 2000 | Corn straw | 54.0 | 143.2 | 38.1 | 140.2 | 90 | [75] |
| Corn straw coated by polypyrrole | 52.1 | 40.8 | 29.1 | 35.6 | 60 | ||
| 52.6 | 69.5 | 33.1 | 65.7 | 70 | |||
| 53.0 | 105.8 | 37.0 | 102.6 | 85 | |||
| 52.7 | 145.8 | 37.2 | 141.5 | 90 | |||
| 1000 | Coral sand | 28.6 | 43.6 | 20.5 | 48.9 | - | [76] |
4.3. PEG-Based PCMs with a Carbon Matrix
4.4. PEG-Based PCMs with an Inorganic Porous Matrix
4.4.1. PEG-Based PCMs with an LWA-Based Porous Matrix
4.4.2. PEG-Based PCMs with a Silica-Based Porous Matrix
| PEG | Matrix | Tm (°C) | ΔHm (J⋅g−1) | Tc (°C) | ΔHc (J⋅g−1) | Amount of PEG (% wt.) | Ref. |
|---|---|---|---|---|---|---|---|
| 1000 | Mesoporous calcium silicate | 58.0 | 28.6 | 41.1 | 21.1 | 40.0 | [82] |
| 2000 | 58.4 | 65.8 | 41.5 | 52.9 | 50.0 | ||
| 4000 | 58.3 | 104.6 | 43.3 | 89.8 | 60.0 | ||
| 6000 | 57.0 | 122.1 | 44.1 | 106.8 | 70.0 | ||
| 4000 | Radial-like mesoporous silica | 57.8 | 121.7 | 46.4 | 104.4 | 70.0 | [83] |
| 2000 | Graphene-based mesoporous silica sheets | 49.2 | 136.3 | - | - | 80.0 | [84] |
| 6000 | Silica nanosheets | 57.8 | 114.8 | 43.5 | 104.6 | - | [85] |
| Silica nanosheets with Sn2+ | 57.9 | 114.7 | 44.0 | 104.1 | - | ||
| Silica nanosheets with Sn2+ and Ag nanoparticles | 58.0 | 113.9 | 44.3 | 103.8 | 66.1 | ||
| 4000 | Silica fume | 56.9 | 169.7 | 24.1 | 159.6 | 75.0 | [40] |
| 56.0 | 120.5 | 17.6 | 107.3 | 50.0 | |||
| 55.7 | 101.0 | 15.3 | 79.6 | 25.0 | |||
| 600 | Silica fume | 19.6 | 71.6 | 13.7 | 74.1 | - | [33] |
| 600 | Raw silica fume | 14.2 | 56.1 | 9.0 | 53.3 | 40.0 | [87] |
| Raw silica fume with carbon nanofiber | 15.2 | 56.1 | 8.1 | 54.2 | |||
| 15.1 | 55.3 | 8.2 | 54.1 | ||||
| Raw silica fume with carbon nanotube | 14.0 | 55.2 | 9.2 | 53.5 | |||
| 13.8 | 55.7 | 8.9 | 53.5 | ||||
| Raw silica fume with graphene nanoplatelet | 15.0 | 55.0 | 10.0 | 54.1 | |||
| 14.9 | 55.1 | 10.0 | 53.1 | ||||
| 2000 | SiO2 | 57.4 | 128.4 | - | - | 80.0 | [88] |
| 800 | SiO2 | 25.6 | 14.1 | 16.0 | 11.6 | 70.0 | [89] |
| 25.8 | 25.4 | 17.3 | 21.9 | 80.0 | |||
| 25.3 | 39.4 | 17.7 | 33.4 | 90.0 | |||
| 6000 | SiO2 | 65.6 | 133.4 | - | - | 80.0 | [90] |
| 58.9 | 79.4 | - | - | 60.0 | |||
| 4000 | SiO2 | 58.09 | 151.8 | 42.3 | 141.0 | 80.0 | [39] |
| 1000 | SiO2 | 36.8 | 113.6 | 21.5 | 115.4 | 80.0 | [28] |
| 1000 | SiO2 | 29.7 | 143.0 | 40.0 | 117.2 | 86.7 | [92] |
4.4.3. PEG-Based PCMs with Other Mineral Porous Matrices
| PEG | Matrix | Tm (°C) | ΔHm (J⋅g−1) | Tc (°C) | ΔHc (J⋅g−1) | Amount of PEG (%wt.) | Ref. |
|---|---|---|---|---|---|---|---|
| 1000 | Diatomite | 27.7 | 87.1 | 32.2 | 82.2 | 50.0 | [93] |
| 600 | Diatomite and 1% CNTs | 7.9 | 51.4 | 6.9 | 53.2 | 41.0 | [95] |
| Diatomite and 2% CNTs | 7.3 | 53.8 | 8.2 | 57.9 | 42.8 | ||
| Diatomite and 3% CNTs | 6.9 | 55.9 | 8.1 | 59.8 | 44.5 | ||
| Diatomite and 4% CNTs | 7.2 | 62.9 | 8.8 | 69.5 | 50.7 | ||
| 600 | Bentonite | 4.0 | 56.7 | 10.3 | 55.1 | 43.0 | [96] |
| 600 | Pumice | 8.8 * | 98.4 | 13.9 * | 96.1 | 56.0 | [23] |
| 2000 | Modified attapulgite | 45.2 | 38.7 | 35.7 | 35.2 | 25.0 | [97] |
| 45.2 | 54.8 | 36.2 | 51.1 | 35.0 | |||
| 45.8 | 75.6 | 36.2 | 70.8 | 45.0 | |||
| 2000 | Expanded vermiculite with 1.59 CNTs | 41.9 | 19.4 | 11.8 | 46.2 | 77.8 | [98] |
| Expanded vermiculite with 3.30 CNTs | 42.8 | 25.6 | 16.2 | 58.1 | 79.3 | ||
| Expanded vermiculite with 5.20 CNTs | 45.1 | 45.1 | 21.1 | 77.5 | 81.5 | ||
| Expanded vermiculite with 7.09 CNTs | 48.0 | 83.9 | 27.3 | 104.2 | 81.5 | ||
| - | Mica | 57.7 | 91.8 | 42.4 | 91.9 | 53.6 | [99] |
| - | Decorated mica (Md) | 57.9 | 99.6 | 45.9 | 99.4 | 57.2 | |
| - | Md with expanded graphite | 57.7 | 136.3 | 46.4 | 132.6 | 76.7 | |
| 6000 | Expanded perlite | 58.4 | 145.1 | 46.3 | 137.3 | 70.1 | [44] |
| Expanded perlite with carbon layer | 55.2 | 134.9 | 46.7 | 129.3 | 66.4 | ||
| 4000 | Expanded perlite | 56.8 | 139.9 | 41.3 | 132.1 | 73.4 | [100] |
| Carbon-modified expanded perlite | 57.5 | 127.8 | 41.3 | 122.0 | 73.9 | ||
| 6000 | Expanded perlite | 59.5 | 150.7 | 41.4 | 134.6 | 79.5 | [47] |
| 6000 | Expanded perlite with 0.5% CNTs | 61.6 | 132.1 | 41.2 | 118.5 | 69.7 | |
| Expanded perlite with 1% CNTs | 61.8 | 121.9 | 39.2 | 109.2 | 64.2 | ||
| 800 | Untreated expanded perlite | 23.8 | 101.2 | 12.8 | 97.2 | 65.0 | [101] |
| Treated expanded perlite | 28.2 | 136.4 | 21.6 | 123.2 | 85.0 | ||
| 600 | Gypsum | 10.6 | 24.2 | 15.8 | 21.6 | 18.0 | [102] |
| Natural clay | 10.9 | 28.8 | 16.3 | 26.0 | 22.0 | ||
| 600 | Kaolin | 5.2 | 32.8 | −15.4 | 29.8 | 20.0 | [104] |
| 1000 | Acid-modified halloysite and graphene | 38.4 | 83.0 | 27.2 | 82.2 | 55.0 | [105] |
| 6000 | 5% of SrBaCO3 | 60.2 | 148.8 | 39.1 | 145.0 | 78.5 | [106] |
| 10% of SrBaCO3 | 60.3 | 141.8 | 39.9 | 139.2 | 74.8 | ||
| 15% of SrBaCO3 | 60.1 | 124.1 | 37.4 | 121.5 | 65.5 | ||
| 1000 | Lecce stone | 39.3 | 27.7 | 19.4 | 26.2 | 23.0 | [30] |
| 800 | 12.7 | 28.3 | 9.3 | 28.1 | 23.0 | [108,109] |
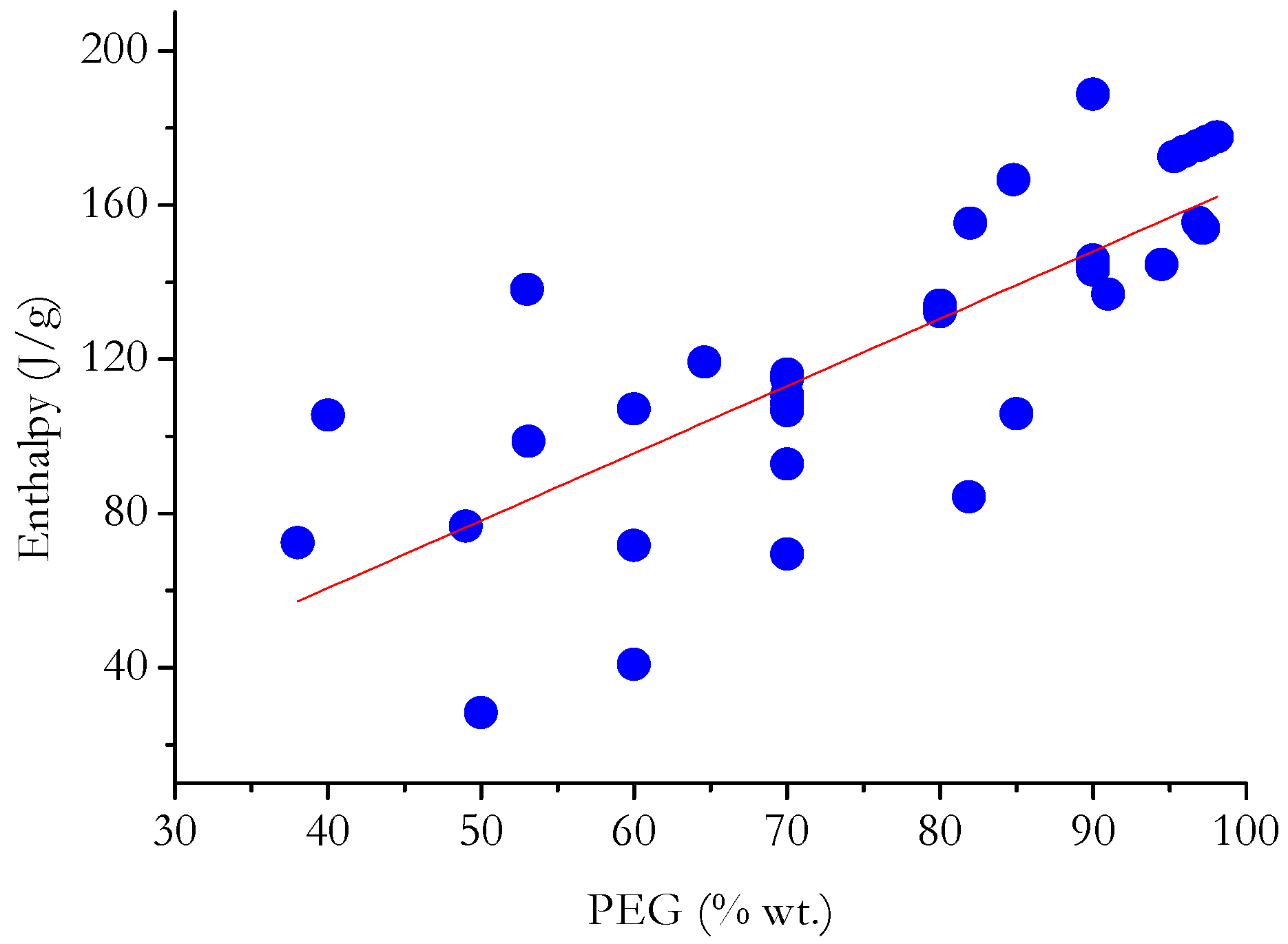
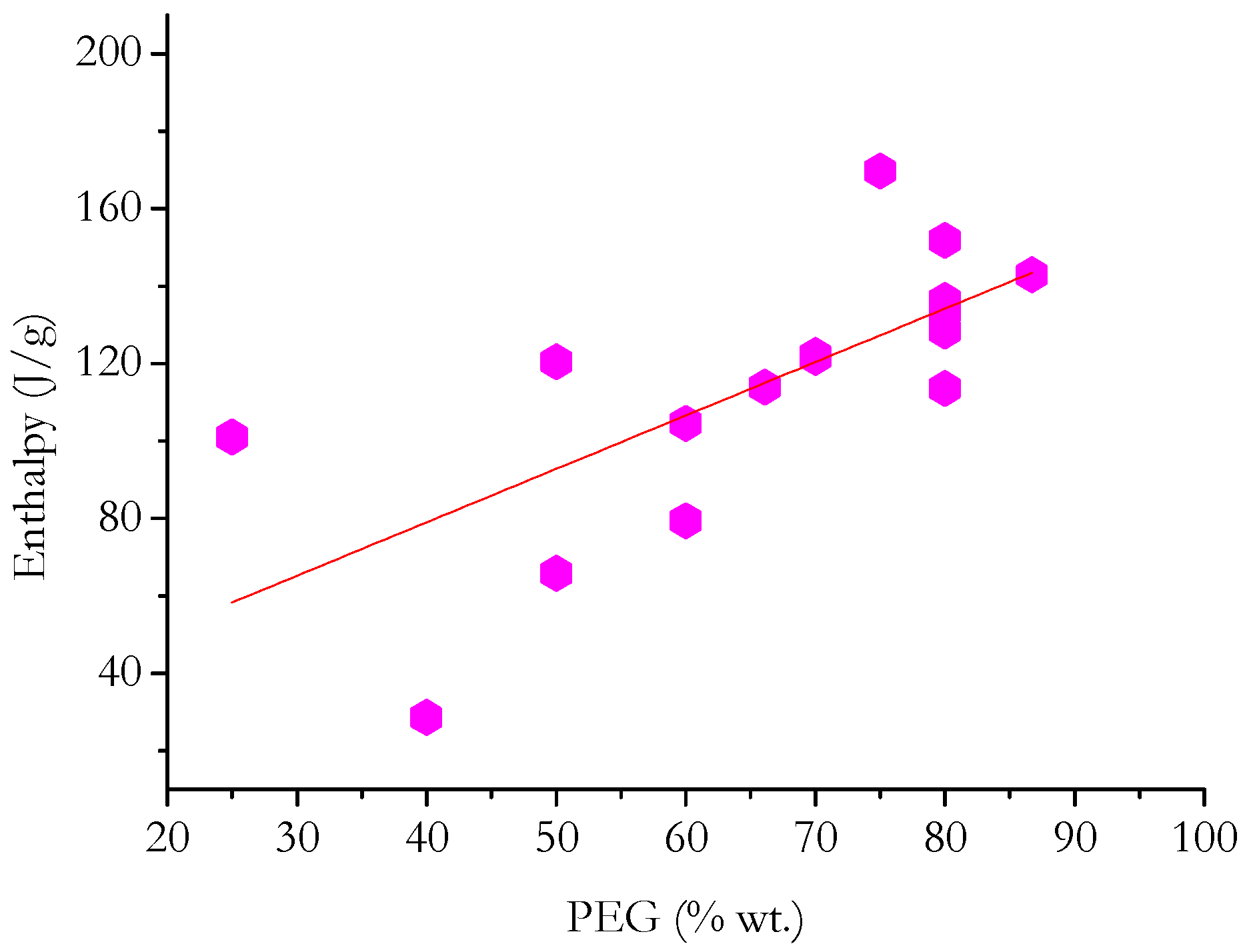
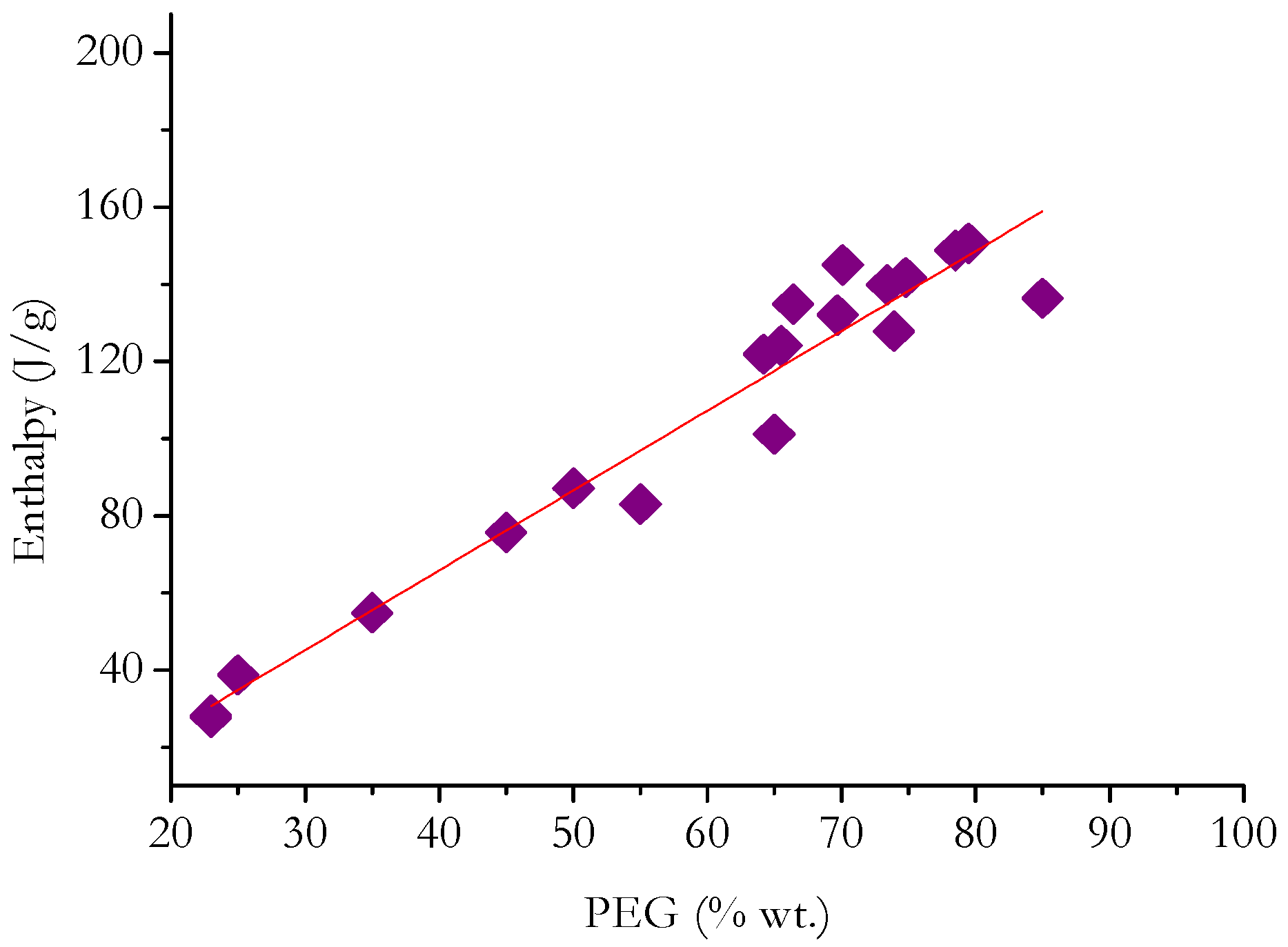
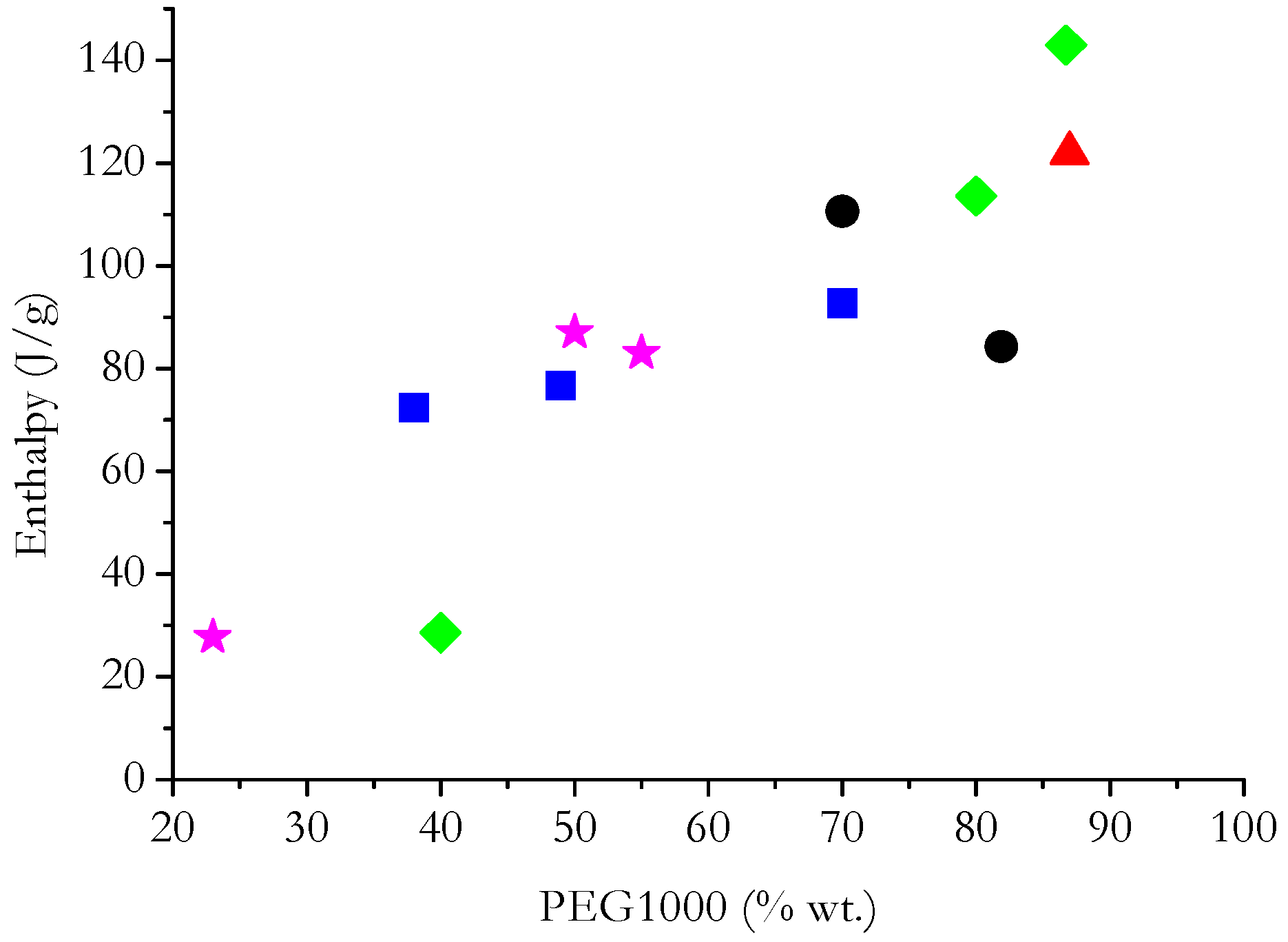
5. Statistical Analysis and Discussion
6. Conclusions
- ▪
- In general, ΔH increases with PEG content (in terms of weight percentage) for PEG grades with comparable thermal characteristics.
- ▪
- By separately analyzing PCMs having matrices of a specific nature, it is possible to deduce that the enthalpy developed by PCMs with carbon-based or mineral non-siliceous porous matrices depends greatly on the PEG content compared with PCMs with polymer-based or siliceous matrices.
- ▪
- Even sorting the matrices based on their chemical nature, it is not possible to find linear relationships that precisely fit the heat developed during the phase change and the PEG content in each PCM. This suggests that, in most cases, the nature of the matrix has a diverse effect on the ΔH measured during the phase change. This may depend on physical interactions that are established between PEG and the matrix; difficulty for PEG to crystallize in the pores of the matrix; and limits in the crystallization of PEG due to the presence of cross-links/crystalline macromolecules of the polymer matrix.
- ▪
- While the selection of the PEG grade is made based on the thermal fluctuations that must be reduced, the choice of the matrix must also be made based on the final expected characteristics of the PCM. For instance, to increase the low thermal conductivity of PEG, carbon-based matrices can be used. To ensure optimal PCM performance that is maintained over time, it is necessary to use a matrix that allows the stabilization of the PEG inside the support. The PCM must not significantly compromise the mechanical properties or weight of the element in which it is inserted, for example, if it is added to mortars.
- ▪
- Keeping sustainability in mind, the choice of the matrix must also be made based on its availability onsite, limiting fuel resources and costs for its transportation and favoring the exploitation of byproducts from other industries, according to the principles of circular economy.
- ▪
- Finally, the choice of the optimal PEG/matrix couple must also be made based on the quantity of PCM that can be included in the building component; for example, the percentage that can be added to a mortar.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rousselot, M.; Pinto Da Rocha, F. Energy Efficiency Trends in Buildings in the EU; European Union: Brussels, Belgium, 2021; pp. 1–4. [Google Scholar]
- Da Cunha, S.R.L.; de Aguiar, J.L.B. Phase Change Materials and Energy Efficiency of Buildings: A Review of Knowledge. J. Energy Storage 2020, 27, 101083. [Google Scholar] [CrossRef]
- Ben Romdhane, S.; Amamou, A.; Ben Khalifa, R.; Saïd, N.M.; Younsi, Z.; Jemni, A. A Review on Thermal Energy Storage Using Phase Change Materials in Passive Building Applications. J. Build. Eng. 2020, 32, 101563. [Google Scholar] [CrossRef]
- Frigione, M.; Lettieri, M.; Sarcinella, A. Phase Change Materials for Energy Efficiency in Buildings and Their Use in Mortars. Materials 2019, 12, 1260. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.; Sarcinella, A.; Aguiar, J.; Frigione, M. Perspective on the Development of Energy Storage Technology Using Phase Change Materials in the Construction Industry: A Review. Energies 2023, 16, 4806. [Google Scholar] [CrossRef]
- Junaid, M.F.; Rehman, Z.; Čekon, M.; Čurpek, J.; Farooq, R.; Cui, H.; Khan, I. Inorganic Phase Change Materials in Thermal Energy Storage: A Review on Perspectives and Technological Advances in Building Applications. Energy Build. 2021, 252, 111443. [Google Scholar] [CrossRef]
- Junaid, M.F.; Rehman, Z.; Ijaz, N.; Čekon, M.; Čurpek, J.; Babeker Elhag, A. Biobased Phase Change Materials from a Perspective of Recycling, Resources Conservation and Green Buildings. Energy Build. 2022, 270, 112280. [Google Scholar] [CrossRef]
- Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Versatility of Polyethylene Glycol (PEG) in Designing Solid–Solid Phase Change Materials (PCMs) for Thermal Management and Their Application to Innovative Technologies. J. Mater. Chem. A 2017, 5, 18379–18396. [Google Scholar] [CrossRef]
- Kim, A.; Wert, N.A.; Gowd, E.B.; Patel, R. Recent Progress in PEG-Based Composite Phase Change Materials. Polym. Rev. 2023, 63, 1078–1129. [Google Scholar] [CrossRef]
- Minea, A.A. State of the Art in PEG-Based Heat Transfer Fluids and Their Suspensions with Nanoparticles. Nanomaterials 2021, 11, 86. [Google Scholar] [CrossRef]
- Junaid, M.F.; Rehman, Z.; Ijaz, N.; Farooq, R.; Khalid, U.; Ijaz, Z. Performance Evaluation of Cement-Based Composites Containing Phase Change Materials from Energy Management and Construction Standpoints. Constr. Build. Mater. 2024, 416, 135108. [Google Scholar] [CrossRef]
- Yao, X.; Qi, C.; Sun, C.; Huo, F.; Jiang, X. Poly(Ethylene Glycol) Alternatives in Biomedical Applications. Nano Today 2023, 48, 101738. [Google Scholar] [CrossRef]
- Soni, J.; Sahiba, N.; Sethiya, A.; Agarwal, S. Polyethylene Glycol: A Promising Approach for Sustainable Organic Synthesis. J. Mol. Liq. 2020, 315, 113766. [Google Scholar] [CrossRef]
- Sarkar, S.; Mestry, S.; Mhaske, S.T. Developments in Phase Change Material (PCM) Doped Energy Efficient Polyurethane (PU) Foam for Perishable Food Cold-Storage Applications: A Review. J. Energy Storage 2022, 50, 104620. [Google Scholar] [CrossRef]
- Leungtongkum, T.; Flick, D.; Hoang, H.M.; Steven, D.; Delahaye, A.; Laguerre, O. Insulated Box and Refrigerated Equipment with PCM for Food Preservation: State of the Art. J. Food Eng. 2022, 317, 110874. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic Phase Change Materials and Their Textile Applications: An Overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Zhou, X.; Liu, B.; Wang, X.; Lin, X. Research and Exploration of Phase Change Materials on Solar Pavement and Asphalt Pavement: A Review. J. Energy Storage 2021, 35, 102246. [Google Scholar] [CrossRef]
- Hu, H. Recent Advances of Polymeric Phase Change Composites for Flexible Electronics and Thermal Energy Storage System. Compos. Part B Eng. 2020, 195, 108094. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, X. Progress of Research on Phase Change Energy Storage Materials in Their Thermal Conductivity. J. Energy Storage 2023, 61, 106772. [Google Scholar] [CrossRef]
- Tao, J.; Luan, J.; Liu, Y.; Qu, D.; Yan, Z.; Ke, X. Technology Development and Application Prospects of Organic-Based Phase Change Materials: An Overview. Renew. Sustain. Energy Rev. 2022, 159, 112175. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, M.; Liu, Q.; Wan, J.; Hu, J. Preparation and Thermal Properties of Molecular-Bridged Expanded Graphite/Polyethylene Glycol Composite Phase Change Materials for Building Energy Conservation. Materials 2018, 11, 818. [Google Scholar] [CrossRef]
- Ru, C.; Li, G.; Guo, F.; Sun, X.; Yu, D.; Chen, Z. Experimental Evaluation of the Properties of Recycled Aggregate Pavement Brick with a Composite Shaped Phase Change Material. Materials 2022, 15, 5565. [Google Scholar] [CrossRef] [PubMed]
- Sarı, A.; Hekimoğlu, G.; Tyagi, V.V.; Sharma, R.K. Evaluation of Pumice for Development of Low-Cost and Energy-Efficient Composite Phase Change Materials and Lab-Scale Thermoregulation Performances of Its Cementitious Plasters. Energy 2020, 207, 118242. [Google Scholar] [CrossRef]
- Cui, S.; Kishore, R.A.; Kolari, P.; Zheng, Q.; Kaur, S.; Vidal, J.; Jackson, R. Model-Driven Development of Durable and Scalable Thermal Energy Storage Materials for Buildings. Energy 2023, 265, 126339. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, Y.; Liu, Y.; Guo, H. Preparation and Thermal Properties of Polyethylene Glycol/Expanded Graphite as Novel Form-Stable Phase Change Material for Indoor Energy Saving. Mater. Lett. 2018, 216, 220–223. [Google Scholar] [CrossRef]
- Xu, J.; Sun, J.; Zhao, J.; Zhang, W.; Zhou, J.; Xu, L.; Guo, H.; Liu, Y.; Zhang, D. Eco-Friendly Wood Plastic Composites with Biomass-Activated Carbon-Based Form-Stable Phase Change Material for Building Energy Conversion. Ind. Crops Prod. 2023, 197, 116573. [Google Scholar] [CrossRef]
- Alkan, C.; Günther, E.; Hiebler, S.; Ensari, Ö.F.; Kahraman, D. Polyurethanes as Solid–Solid Phase Change Materials for Thermal Energy Storage. Sol. Energy 2012, 86, 1761–1769. [Google Scholar] [CrossRef]
- Xu, T.; Chen, Q.; Zhang, Z.; Gao, X.; Huang, G. Investigation on the Properties of a New Type of Concrete Blocks Incorporated with PEG/SiO2 Composite Phase Change Material. Build. Environ. 2016, 104, 172–177. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S.; Luo, J.; Sun, K.; Zhang, J.; Tan, Z.; Shi, Q. Thermal Analysis and Heat Capacity Study of Polyethylene Glycol (PEG) Phase Change Materials for Thermal Energy Storage Applications. J. Chem. Thermodyn. 2019, 128, 259–274. [Google Scholar] [CrossRef]
- Frigione, M.; Lettieri, M.; Sarcinella, A.; Barroso de Aguiar, J. Sustainable Polymer-Based Phase Change Materials for Energy Efficiency in Buildings and Their Application in Aerial Lime Mortars. Constr. Build. Mater. 2020, 231, 117149. [Google Scholar] [CrossRef]
- Constantinescu, M.; Dumitrache, L.; Constantinescu, D.; Anghel, E.M.; Popa, V.T.; Stoica, A.; Olteanu, M. Latent Heat Nano Composite Building Materials. Eur. Polym. J. 2010, 46, 2247–2254. [Google Scholar] [CrossRef]
- Bi, L.; Long, G.; Xiao, R.; Zeng, X.; Xie, Y. Properties and Characterization of High-Performance Steam-Cured Cement-Based Materials Modified by Phase Change Materials. J. Clean. Prod. 2021, 328, 129669. [Google Scholar] [CrossRef]
- Abbasi Hattan, H.; Madhkhan, M.; Marani, A. Thermal and Mechanical Properties of Building External Walls Plastered with Cement Mortar Incorporating Shape-Stabilized Phase Change Materials (SSPCMs). Constr. Build. Mater. 2021, 270, 121385. [Google Scholar] [CrossRef]
- Wang, C.; Dong, W.; Li, A.; Atinafu, D.G.; Wang, G.; Lu, Y. The Reinforced Photothermal Effect of Conjugated Dye/Graphene Oxide-Based Phase Change Materials: Fluorescence Resonance Energy Transfer and Applications in Solar-Thermal Energy Storage. Chem. Eng. J. 2022, 428, 130605. [Google Scholar] [CrossRef]
- Liu, Z.; He, F.; Li, Y.; Jiang, Z.; He, G.; Lin, C.; Zhang, Q.; Zhou, Y.; Yang, W. Enhanced Solar/Electric-to-Thermal Energy Conversion Capability of Double Skeleton Based Shape-Stabilized Phase Change Materials. Sol. Energy Mater. Sol. Cells 2023, 252, 112171. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, J. A Poly(Ethylene Glycol)-Based Smart Phase Change Material. Sol. Energy Mater. Sol. Cells 2008, 92, 1260–1268. [Google Scholar] [CrossRef]
- Pielichowska, K.; Nowak, M.; Szatkowski, P.; Macherzyńska, B. The Influence of Chain Extender on Properties of Polyurethane-Based Phase Change Materials Modified with Graphene. Appl. Energy 2016, 162, 1024–1033. [Google Scholar] [CrossRef]
- Tang, B.; Cui, J.; Wang, Y.; Jia, C.; Zhang, S. Facile Synthesis and Performances of PEG/SiO2 Composite Form-Stable Phase Change Materials. Sol. Energy 2013, 97, 484–492. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Ma, H.; Yang, J. The Preparation of a Green Shape-Stabilized Composite Phase Change Material of Polyethylene Glycol/SiO2 with Enhanced Thermal Performance Based on Oil Shale Ash via Temperature-Assisted Sol–Gel Method. Sol. Energy Mater. Sol. Cells 2015, 132, 29–39. [Google Scholar] [CrossRef]
- Wang, C.L.; Yeh, K.L.; Chen, C.W.; Lee, Y.; Lee, H.L.; Lee, T. A Quick-Fix Design of Phase Change Material by Particle Blending and Spherical Agglomeration. Appl. Energy 2017, 191, 239–250. [Google Scholar] [CrossRef]
- Zhao, Y.; Min, X.; Huang, Z.; Liu, Y.; Wu, X.; Fang, M. Honeycomb-like Structured Biological Porous Carbon Encapsulating PEG: A Shape-Stable Phase Change Material with Enhanced Thermal Conductivity for Thermal Energy Storage. Energy Build. 2018, 158, 1049–1062. [Google Scholar] [CrossRef]
- Wu, B.; Lyu, S.; Han, H.; Li, T.; Sun, H.; Wang, J.-K.; Li, D.; Lei, F.; Huang, J.; Sun, D. Biomass-Based Shape-Stabilized Phase Change Materials from Artificially Cultured Ship-Shaped Diatom Frustules with High Enthalpy for Thermal Energy Storage. Compos. Part B Eng. 2021, 205, 108500. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Liu, R.; Huang, Y. Cellulose-Based Solid–Solid Phase Change Materials Synthesized in Ionic Liquid. Sol. Energy Mater. Sol. Cells 2009, 93, 1321–1328. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, R.; Tang, C.; Wu, B.; Huang, Z.; Min, X.; Huang, Y.; Liu, Y.; Fang, M.; Wu, X. Thermal Conductivity Enhancement of Polyethylene Glycol/Expanded Perlite with Carbon Layer for Heat Storage Application. Energy Build. 2016, 130, 113–121. [Google Scholar] [CrossRef]
- Tan, Y.; Xiao, Y.; Chen, R.; Zhou, C.; Wang, L.; Liu, Y.; Li, D. High Latent Heat and Recyclable Form-Stable Phase Change Materials Prepared via a Facile Self-Template Method. Chem. Eng. J. 2020, 396, 125265. [Google Scholar] [CrossRef]
- Zahir, M.H.; Rahman, M.M.; Basamad, S.K.S.; Mohaisen, K.O.; Irshad, K.; Rahman, M.M.; Aziz, M.A.; Ali, A.; Hossain, M.M. Preparation of a Sustainable Shape-Stabilized Phase Change Material for Thermal Energy Storage Based on Mg2+-Doped CaCO3/PEG Composites. Nanomaterials 2021, 11, 1639. [Google Scholar] [CrossRef] [PubMed]
- Mohaisen, K.O.; Zahir, M.H.; Maslehuddin, M.; Al-Dulaijan, S.U. Development of a Shape-Stabilized Phase Change Material Utilizing Natural and Industrial Byproducts for Thermal Energy Storage in Buildings. J. Energy Storage 2022, 50, 104205. [Google Scholar] [CrossRef]
- Luo, L.; Luo, W.; Chen, W.; Hu, X.; Ma, Y.; Xiao, S.; Li, Q.; Jiang, X. Form-Stable Phase Change Materials Based on Graphene-Doped PVA Aerogel Achieving Effective Solar Energy Photothermal Conversion and Storage. Sol. Energy 2023, 255, 146–156. [Google Scholar] [CrossRef]
- Kong, W.; Yang, Y.; Zhou, C.; Lei, J. Novel Thermosetting Phase Change Materials with Polycarbonatediol Based Curing Agent as Supporting Skeleton for Thermal Energy Storage. Energy Build. 2017, 146, 12–18. [Google Scholar] [CrossRef]
- Chen, C.; Chen, R.; Zhao, T.; Wang, L. A Comparative Study of Linear Polyurea and Crosslinked Polyurea as Supports to Stabilize Polyethylene Glycol for Thermal Energy Storage. Renew. Energy 2022, 183, 535–547. [Google Scholar] [CrossRef]
- Zhao, S.; Yuan, A.; Zhao, Y.; Liu, T.; Fu, X.; Jiang, L.; Lei, J. Preparation of Mechanically Robust and Thermochromic Phase Change Materials for Thermal Energy Storage and Temperature Indicator. Energy Build. 2022, 261, 111993. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Tu, J.; Shi, R.; Luo, Z.; Xiong, C.; Jiang, M. Shape Stability of Polyethylene Glycol/Acetylene Black Phase Change Composites for Latent Heat Storage. Adv. Mater. Sci. Eng. 2018, 2018, 3954163. [Google Scholar] [CrossRef]
- Liang, B.; Lu, X.; Li, R.; Tu, W.; Yang, Z.; Yuan, T. Solvent-Free Preparation of Bio-Based Polyethylene Glycol/Wood Flour Composites as Novel Shape-Stabilized Phase Change Materials for Solar Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2019, 200, 110037. [Google Scholar] [CrossRef]
- Liao, H.; Chen, W.; Liu, Y.; Wang, Q. A Phase Change Material Encapsulated in a Mechanically Strong Graphene Aerogel with High Thermal Conductivity and Excellent Shape Stability. Compos. Sci. Technol. 2020, 189, 108010. [Google Scholar] [CrossRef]
- Feng, L.; Ding, J.; Hu, H.; Lv, Z.; Zhang, Y.; Xu, B.; Quan, J.; Hao, S.; Fan, H.; Hang, Z. Preparation and Characterization of Bio-Based PLA/PEG/g-C3N4 Low-Temperature Composite Phase Change Energy Storage Materials. Polymers 2023, 15, 2872. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hammami, N.; Trovalet, L.; Bigot, D.; Habas, J.-P.; Malet-Damour, B. Description of Phase Change Materials (PCMs) Used in Buildings under Various Climates: A Review. J. Energy Storage 2022, 56, 105760. [Google Scholar] [CrossRef]
- Du, Y.; Liu, P.; Quan, X.; Ma, C. Characterization and Cooling Effect of a Novel Cement-Based Composite Phase Change Material. Sol. Energy 2020, 208, 573–582. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Zhang, G.H. Review on Microencapsulated Phase Change Materials (MEPCMs): Fabrication, Characterization and Applications. Renew. Sustain. Energy Rev. 2011, 15, 3813–3832. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Shukla, S.K. Potential of Macroencapsulated PCM for Thermal Energy Storage in Buildings: A Comprehensive Review. Constr. Build. Mater. 2019, 225, 723–744. [Google Scholar] [CrossRef]
- Li, M.; Shi, J. Review on Micropore Grade Inorganic Porous Medium Based Form Stable Composite Phase Change Materials: Preparation, Performance Improvement and Effects on the Properties of Cement Mortar. Constr. Build. Mater. 2019, 194, 287–310. [Google Scholar] [CrossRef]
- Anghel, E.M.; Pavel, P.M.; Constantinescu, M.; Petrescu, S.; Atkinson, I.; Buixaderas, E. Thermal Transfer Performance of a Spherical Encapsulated PEG 6000-Based Composite for Thermal Energy Storage. Appl. Energy 2017, 208, 1222–1231. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Thermal Insulation Capability of PEG-Containing Polyurethane Foams. Thermochim. Acta 2008, 475, 15–21. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Fang, G. Synthesis and Characterization of Chain-Extended and Branched Polyurethane Copolymers as Form Stable Phase Change Materials for Solar Thermal Conversion Storage. Sol. Energy Mater. Sol. Cells 2018, 186, 14–28. [Google Scholar] [CrossRef]
- Khomein, P.; Nallapaneni, A.; Lau, J.; Lilley, D.; Zhu, C.; Kaur, S.; Prasher, R.; Liu, G. Random Copolymer of Poly(Polyethylene Glycol Methyl Ether)Methacrylate as Tunable Transition Temperature Solid-Solid Phase Change Material for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2021, 225, 111030. [Google Scholar] [CrossRef]
- Yan, D.; Zhao, S.; Ge, C.; Gao, J.; Gu, C.; Fan, Y. PBT/Adipic Acid Modified PEG Solid-Solid Phase Change Composites. J. Energy Storage 2022, 52, 104753. [Google Scholar] [CrossRef]
- Gowthami, D.; Sharma, R.K. Influence of Hydrophilic and Hydrophobic Modification of the Porous Matrix on the Thermal Performance of Form Stable Phase Change Materials: A Review. Renew. Sustain. Energy Rev. 2023, 185, 113642. [Google Scholar] [CrossRef]
- Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Shape-Stabilized Poly(Ethylene Glycol) (PEG)-Cellulose Acetate Blend Preparation with Superior PEG Loading via Microwave-Assisted Blending. Sol. Energy 2017, 144, 32–39. [Google Scholar] [CrossRef]
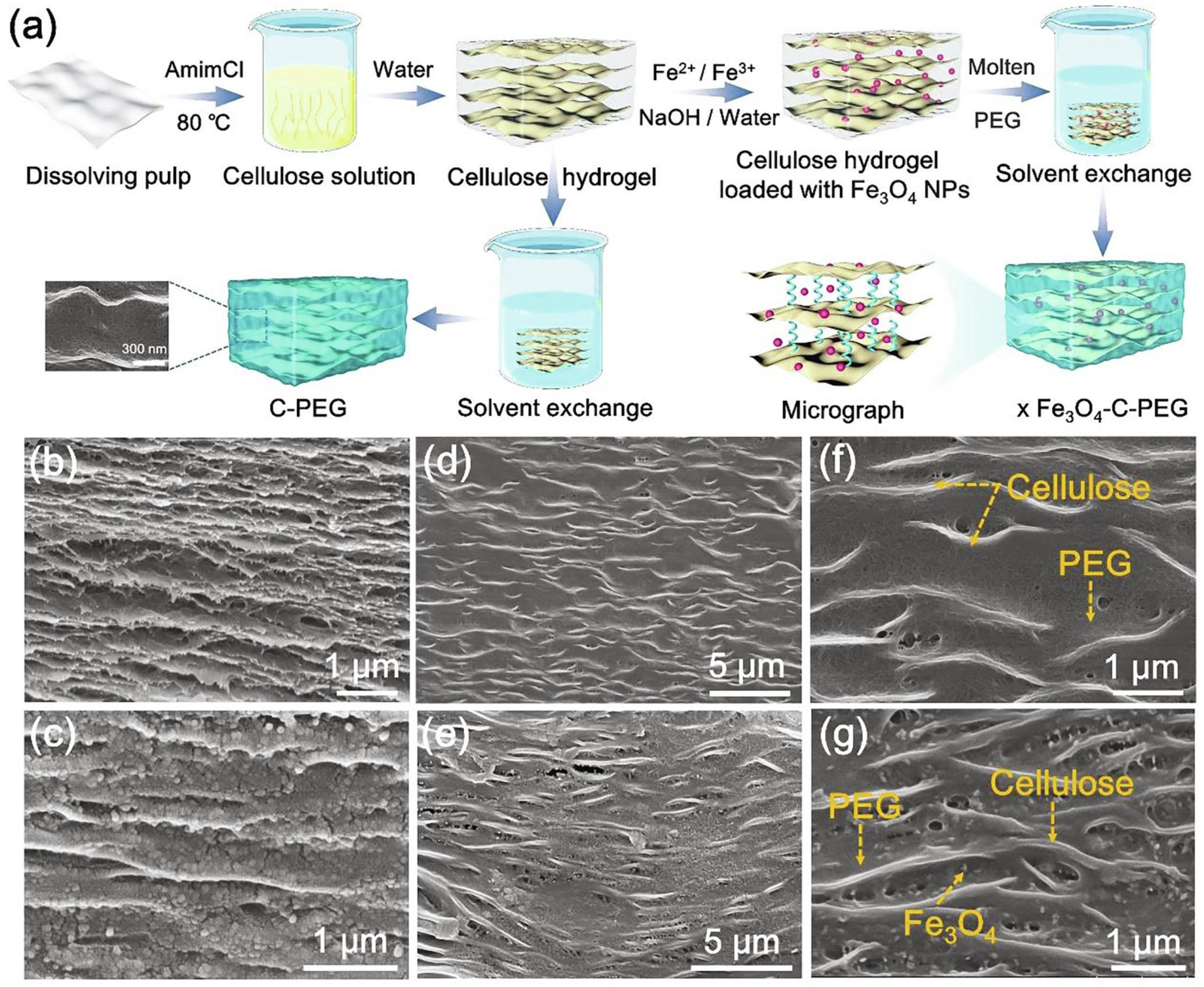
- Zhang, J.; Xu, Y.; Li, X.; Li, H.; Yao, C.; Chen, S.; Xu, F. Leak-Free, High Latent Heat and Self-Cleaning Phase Change Materials Supported by Layered Cellulose/Fe3O4 Skeleton for Light-to-Thermal Energy Conversion. Energy Convers. Manag. 2022, 256, 115357. [Google Scholar] [CrossRef]
- Qi, C.; Zhang, F.; Mu, J.; Zhang, Y.; Yu, Z. Enhanced Mechanical and Thermal Properties of Hollow Wood Composites Filled with Phase-Change Material. J. Clean. Prod. 2020, 256, 120373. [Google Scholar] [CrossRef]
- Xia, R.; Zhang, W.; Yang, Y.; Zhao, J.; Liu, Y.; Guo, H. Transparent Wood with Phase Change Heat Storage as Novel Green Energy Storage Composites for Building Energy Conservation. J. Clean. Prod. 2021, 296, 126598. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Zhang, W.; Zhao, J.; Wang, B.; Fang, X.; Guo, H.; Liu, Y. A Photo-to-Thermal Energy Conversion and Heat Regulation Wood Supported by Alkylated Carbon Black for Thermal Conductive Filler. Sol. Energy Mater. Sol. Cells 2023, 251, 112165. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, X.; Lang, S.; Liu, G.; Wang, H.; Zhou, X.; Du, G. Dimensionally Stable Delignified Bamboo Matrix Phase-Change Composite under Ambient Temperature for Indoor Thermal Regulation. Polymers 2023, 15, 1727. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Liu, Y.; Yang, Y. Enhanced Thermal Properties of Polyethylene Glycol/Modified Rice Husk Ash Eco-Friendly Form-Stable Phase Change Material via Optimizing Support Pore Structure. J. Energy Storage 2021, 43, 103172. [Google Scholar] [CrossRef]
- Hekimoğlu, G.; Sarı, A.; Önal, Y.; Gencel, O.; Tyagi, V.V.; Aslan, E. Utilization of Waste Apricot Kernel Shell Derived-Activated Carbon as Carrier Framework for Effective Shape-Stabilization and Thermal Conductivity Enhancement of Organic Phase Change Materials Used for Thermal Energy Storage. Powder Technol. 2022, 401, 117291. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, J.; Ren, J.; Fan, P.; Yang, K.; Cai, H. Corn Straw Supported High-Performance Phase Change Composites: Strategy to Turn Agricultural Residues into High Efficient and Stable Thermal Energy Storage Materials. Mater. Today Sustain. 2023, 24, 100571. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Jin, C.; Lu, L.; Bai, M. Experimental Study on the Preparation and Physical Thermal Performance of Coral Sand-Based Phase Change Material Concrete Composites. Constr. Build. Mater. 2023, 389, 131631. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Feng, W.; Nian, H. Single-Walled Carbon Nanotube for Shape Stabilization and Enhanced Phase Change Heat Transfer of Polyethylene Glycol Phase Change Material. Energy Convers. Manag. 2017, 143, 96–108. [Google Scholar] [CrossRef]
- Cui, H.; Wang, P.; Yang, H.; Shi, Y. Large-Scale Fabrication of Expanded Graphite Aerogel-Based Phase Change Material Composite for Efficient Solar Harvesting. J. Energy Storage 2022, 56, 105890. [Google Scholar] [CrossRef]
- Bentz, D.P.; Turpin, R. Potential Applications of Phase Change Materials in Concrete Technology. Cem. Concr. Compos. 2007, 29, 527–532. [Google Scholar] [CrossRef]
- Sukontasukkul, P.; Uthaichotirat, P.; Sangpet, T.; Sisomphon, K.; Newlands, M.; Siripanichgorn, A.; Chindaprasirt, P. Thermal Properties of Lightweight Concrete Incorporating High Contents of Phase Change Materials. Constr. Build. Mater. 2019, 207, 431–439. [Google Scholar] [CrossRef]
- Sukontasukkul, P.; Sangpet, T.; Newlands, M.; Yoo, D.-Y.; Tangchirapat, W.; Limkatanyu, S.; Chindaprasirt, P. Thermal Storage Properties of Lightweight Concrete Incorporating Phase Change Materials with Different Fusion Points in Hybrid Form for High Temperature Applications. Heliyon 2020, 6, 04863. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Deng, Y.; Guan, W.; Ma, H. Polyethylene Glycol/Mesoporous Calcium Silicate Shape-Stabilized Composite Phase Change Material: Preparation, Characterization, and Adjustable Thermal Property. Energy 2015, 82, 333–340. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Deng, Y.; Guan, W.; Ning, L. Radial-like Mesoporous Silica Sphere: A Promising New Candidate of Supporting Material for Storage of Low-, Middle-, and High-Temperature Heat. Energy 2016, 112, 1074–1083. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, P.; Wang, F.; Kang, M.; Li, R.; Mou, Y.; Huang, Y. Phase Change Materials Based on Polyethylene Glycol Supported by Graphene-Based Mesoporous Silica Sheets. Appl. Therm. Eng. 2016, 101, 217–223. [Google Scholar] [CrossRef]
- Liu, S.; Yan, Z.; Fu, L.; Yang, H. Hierarchical Nano-Activated Silica Nanosheets for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2017, 167, 140–149. [Google Scholar] [CrossRef]
- Pongsopha, P.; Sukontasukkul, P.; Maho, B.; Intarabut, D.; Phoo-ngernkham, T.; Hanjitsuwan, S.; Choi, D.; Limkatanyu, S. Sustainable Rubberized Concrete Mixed with Surface Treated PCM Lightweight Aggregates Subjected to High Temperature Cycle. Constr. Build. Mater. 2021, 303, 124535. [Google Scholar] [CrossRef]
- Hekimoğlu, G.; Sarı, A.; Gencel, O.; Tyagi, V.V. Thermal Conductivity Enhancement of Silica Fume Based Composite Thermal Energy Storage Material Using Different Carbon Nanomaterials. Energy Build. 2022, 257, 111789. [Google Scholar] [CrossRef]
- Li, J.; He, L.; Liu, T.; Cao, X.; Zhu, H. Preparation and Characterization of PEG/SiO2 Composites as Shape-Stabilized Phase Change Materials for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2013, 118, 48–53. [Google Scholar] [CrossRef]
- Xu, J.; Yang, T.; Xu, X.; Guo, X.; Cao, J. Processing Solid Wood into a Composite Phase Change Material for Thermal Energy Storage by Introducing Silica-Stabilized Polyethylene Glycol. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106098. [Google Scholar] [CrossRef]
- Wang, P.; Li, N.; Zhao, C.; Wu, L.; Han, G. A Phase Change Storage Material That May Be Used in the Fire Resistance of Building Structure. Procedia Eng. 2014, 71, 261–264. [Google Scholar] [CrossRef]
- Serrano, A.; Borreguero, A.M.; Iglesias, I.; Acosta, A.; Rodríguez, J.F.; Carmona, M. Diffusion of Shape Stabilized PEG-SiO2 as a Driver for Producing Thermoregulating Facing Bricks. Materials 2021, 14, 1395. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, W.; Sun, W.; Tan, Y.; Zhou, C.; Xiao, X. Preparation of Hydrophobic Shaped Core–Shell Phase Change Pellets and Thermoregulation Effect in Buildings. Appl. Therm. Eng. 2023, 222, 119923. [Google Scholar] [CrossRef]
- Karaman, S.; Karaipekli, A.; Sarı, A.; Biçer, A. Polyethylene Glycol (PEG)/Diatomite Composite as a Novel Form-Stable Phase Change Material for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1647–1653. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Guan, W.; Deng, Y.; Ning, L. Enhanced Thermal Conductivity of PEG/Diatomite Shape-Stabilized Phase Change Materials with Ag Nanoparticles for Thermal Energy Storage. J. Mater. Chem. A 2015, 3, 8526–8536. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Al-Sulaiman, F.A.; Karaipekli, A.; Tyagi, V.V. Diatomite/CNTs/PEG Composite PCMs with Shape-Stabilized and Improved Thermal Conductivity: Preparation and Thermal Energy Storage Properties. Energy Build. 2018, 164, 166–175. [Google Scholar] [CrossRef]
- Sarı, A. Thermal Energy Storage Characteristics of Bentonite-Based Composite PCMs with Enhanced Thermal Conductivity as Novel Thermal Storage Building Materials. Energy Convers. Manag. 2016, 117, 132–141. [Google Scholar] [CrossRef]
- Shi, J.; Li, M. Synthesis and Characterization of Polyethylene Glycol/Modified Attapulgite Form-Stable Composite Phase Change Material for Thermal Energy Storage. Sol. Energy 2020, 205, 62–73. [Google Scholar] [CrossRef]
- Deng, Y.; He, M.; Li, J.; Yang, Z. Polyethylene Glycol-Carbon Nanotubes/Expanded Vermiculite Form-Stable Composite Phase Change Materials: Simultaneously Enhanced Latent Heat and Heat Transfer. Polymers 2018, 10, 889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, C.; Lin, N.; Xie, B.; Chen, J. Enhanced Properties of Mica-Based Composite Phase Change Materials for Thermal Energy Storage. J. Energy Storage 2021, 42, 103106. [Google Scholar] [CrossRef]
- Li, R.; Zhao, Y.; Xia, B.; Dong, Z.; Xue, S.; Huo, X.; Wu, X.; Liu, Y.; Huang, Z.; Fang, M.; et al. Enhanced Thermal Conductivity of Composite Phase Change Materials Based on Carbon Modified Expanded Perlite. Mater. Chem. Phys. 2021, 261, 124226. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, J.; Zhang, W.; Xu, J.; Wang, B.; Wang, X.; Zhou, J.; Guo, H.; Liu, Y. Composites with a Novel Core–Shell Structural Expanded Perlite/Polyethylene Glycol Composite PCM as Novel Green Energy Storage Composites for Building Energy Conservation. Appl. Energy 2023, 330, 120363. [Google Scholar] [CrossRef]
- Sarı, A. Composites of Polyethylene Glycol (PEG600) with Gypsum and Natural Clay as New Kinds of Building PCMs for Low Temperature-Thermal Energy Storage. Energy Build. 2014, 69, 184–192. [Google Scholar] [CrossRef]
- Kulkarni, P.; Muthadhi, A. Polyethylene Glycol-600/Expanded Clay Aggregate with Alccofine1203 in Concrete. Mater. Today Proc. 2021, 43, 1081–1088. [Google Scholar] [CrossRef]
- Sarı, A. Fabrication and Thermal Characterization of Kaolin-Based Composite Phase Change Materials for Latent Heat Storage in Buildings. Energy Build. 2015, 96, 193–200. [Google Scholar] [CrossRef]
- Gu, X.; Peng, L.; Liu, P.; Bian, L.; Wei, B. Enhanced Thermal Properties and Lab-Scale Thermal Performance of Polyethylene Glycol/Modified Halloysite Nanotube Form-Stable Phase Change Material Cement Panel. Constr. Build. Mater. 2022, 323, 126550. [Google Scholar] [CrossRef]
- Mohaisen, K.O.; Zahir, M.H.; Maslehuddin, M. Shape-Stabilized Phase Change Material for Thermal Energy Storage: Sr+2 Doped BaCO3 Matrix Incorporating Polyethylene Glycol. J. Energy Storage 2023, 58, 106369. [Google Scholar] [CrossRef]
- Frigione, M.; Lettieri, M.; Sarcinella, A.; Barroso de Aguiar, J.L. Applications of Sustainable Polymer-Based Phase Change Materials in Mortars Composed by Different Binders. Materials 2019, 12, 3502. [Google Scholar] [CrossRef] [PubMed]
- Sarcinella, A.; de Aguiar, J.L.B.; Frigione, M. Physical Properties of an Eco-Sustainable, Form-Stable Phase Change Material Included in Aerial-Lime-Based Mortar Intended for Different Climates. Materials 2022, 15, 1192. [Google Scholar] [CrossRef]
- Sarcinella, A.; de Aguiar, J.L.B.; Frigione, M. Physical Properties of Eco-Sustainable Form-Stable Phase Change Materials Included in Mortars Suitable for Buildings Located in Different Continental Regions. Materials 2022, 15, 2497. [Google Scholar] [CrossRef]
- Frigione, M.; Sarcinella, A.; Barroso de Aguiar, J.L. Development and Performance of Eco-Sustainable Form-Stable Phase Change Materials (PCMs) for Mortars to Be Applied in Buildings Located in Different Climatic Areas. Coatings 2023, 13, 258. [Google Scholar] [CrossRef]
- Sarcinella, A.; De Aguiar, J.L.B.; Lettieri, M.; Cunha, S.; Frigione, M. Thermal Performance of Mortars Based on Different Binders and Containing a Novel Sustainable Phase Change Material (PCM). Materials 2020, 13, 2055. [Google Scholar] [CrossRef]
- Sarcinella, A.; de Aguiar, J.L.B.; Frigione, M. Use of Sustainable Phase Change Material (PCM) in Mortars for Building Energy Efficiency. J. Phys. Conf. Ser. 2022, 2385, 012009. [Google Scholar] [CrossRef]
- Sarcinella, A.; Barroso de Aguiar, J.L.; Jesus, C.; Frigione, M. Thermal Properties of PEG-Based Form-Stable Phase Change Materials (PCMs) Incorporated in Mortars for Energy Efficiency of Buildings. J. Energy Storage 2023, 67, 107545. [Google Scholar] [CrossRef]






| PEG Mw (g⋅mol−1) | Heating Process | Cooling Process | Thermal Conductivity (W/m·K) | Ref. | ||
|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J⋅g−1) | Tc (°C) | ΔHc (J⋅g−1) | |||
| PEG400 | 3.2–3.7 | 91.4–98.8 | −23.4–24.0 | 85.0–98.9 | 0.19 | [8,21,22] |
| PEG600 | 9.9–21.3 | 137.0–148.5 | 6.2–6.5 | 129.8–146.3 | 0.19 | [8,21,23,24] |
| PEG800 | 21.8–32.4 | 133.6–187.3 | 17.6–21.7 | 126.6–130.3 | 0.26 | [21,25,26] |
| PEG1000 | 32.4–42.8 | 113.6–153.0 | 21.5–30.7 | 115.4–155.0 | 0.28 | [21,27,28,29,30] |
| PEG1500 | 46.9–49.5 | 170.3–196.6 | 31.6–39.7 | 167.3–188.2 | 0.30 | [21,31,32,33] |
| PEG2000 | 46.5–56.1 | 170.3–193.7 | 19.2–39.7 | 151.1–178.6 | 0.31 | [21,31,34,35] |
| PEG3000 | 50.4–56.6 | 144.9–186.5 | 23.0–43.2 | 143.8–160.9 | 0.33 | [21,36,37] |
| PEG4000 | 54.5–59.6 | 181.4–205.7 | 33.3–40.1 | 171.8–199.0 | 0.33 | [29,38,39,40,41,42] |
| PEG5000 | 60.1–62.2 | 172.4–204.7 | 34.2–40.6 | 168.7–203.2 | 0.33 | [8,29,43] |
| PEG6000 | 53.0–63.0 | 168.6–190.1 | 36.2–46.8 | 163.6–187.1 | 0.34 | [29,44,45,46,47,48] |
| PEG8000 | 50.9–60.4 | 113.5–173.0 | 37.5–47.6 | 110.3–161.8 | 0.33 | [29,49,50,51] |
| PEG10000 | 52.8–66.6 | 112.3–189.2 | 38.1–43.9 | 109.5–178.3 | 0.33 | [29,49,52,53,54] |
| PEG20000 | 57.5–68.0 | 163.1–189.8 | 33.6–44.9 | 114.9–171.5 | 0.32 | [29,45,55] |
| PEG | Matrix | Tm (°C) | ΔHm (J⋅g−1) | Tc (°C) | ΔHc (J⋅g−1) | Amount of PEG (% wt.) | Ref. |
|---|---|---|---|---|---|---|---|
| 6000 | Single-walled carbon nanotubes | 55.3 | 165.4 | 43.6 | 151.6 | 90.0 | [77] |
| 55.8 | 169.4 | 43.3 | 154.6 | 92.0 | |||
| 56.0 | 172.4 | 43.0 | 157.6 | 94.0 | |||
| 56.3 | 176.4 | 42.8 | 160.6 | 96.0 | |||
| 56.7 | 179.4 | 42.7 | 163.6 | 98.0 | |||
| 800 | Expanded graphite | 30.1 | 78.06 | 12.6 | 81.5 | - | [25] |
| 25.9 | 98.6 | 19.1 | 97.6 | - | |||
| 25.8 | 98.3 | 18.7 | 95.0 | - | |||
| 800 | Expanded graphite | 22.6 * | 97.7 | 21.9 * | 92.9 | 87.0 | [21] |
| 1000 | 30.2 * | 121.9 | 33.6 * | 116.8 | |||
| 1500 | 42.7 * | 122.9 | 33.9 * | 114.9 | |||
| 2000 | 51.6 * | 143.8 | 41.7 * | 137.5 | |||
| 3000 | 54.6 * | 141.6 | 41.7 * | 124.1 | |||
| 4000 | Expanded graphite aerogel | 53.8 | 170.1 | 37.9 | 144.7 | 90.8 | [78] |
| 53.8 | 166.4 | 36.0 | 146.5 | 90.2 | |||
| 53.8 | 155.2 | 40.0 | 134.8 | 83.7 | |||
| 53.4 | 148.9 | 36.6 | 128.5 | 80.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarcinella, A.; Frigione, M. Selection of PEG-Matrix Combinations to Achieve High Performance Form-Stable Phase Change Materials for Building Applications. Coatings 2024, 14, 250. https://doi.org/10.3390/coatings14030250
Sarcinella A, Frigione M. Selection of PEG-Matrix Combinations to Achieve High Performance Form-Stable Phase Change Materials for Building Applications. Coatings. 2024; 14(3):250. https://doi.org/10.3390/coatings14030250
Chicago/Turabian StyleSarcinella, Antonella, and Mariaenrica Frigione. 2024. "Selection of PEG-Matrix Combinations to Achieve High Performance Form-Stable Phase Change Materials for Building Applications" Coatings 14, no. 3: 250. https://doi.org/10.3390/coatings14030250
APA StyleSarcinella, A., & Frigione, M. (2024). Selection of PEG-Matrix Combinations to Achieve High Performance Form-Stable Phase Change Materials for Building Applications. Coatings, 14(3), 250. https://doi.org/10.3390/coatings14030250








