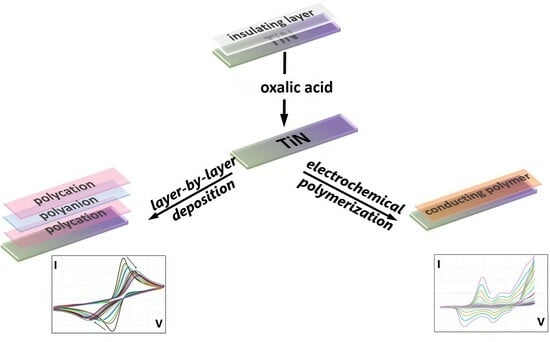
Deposition of Polymers on Titanium Nitride Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Procedures
3. Results and Discussion
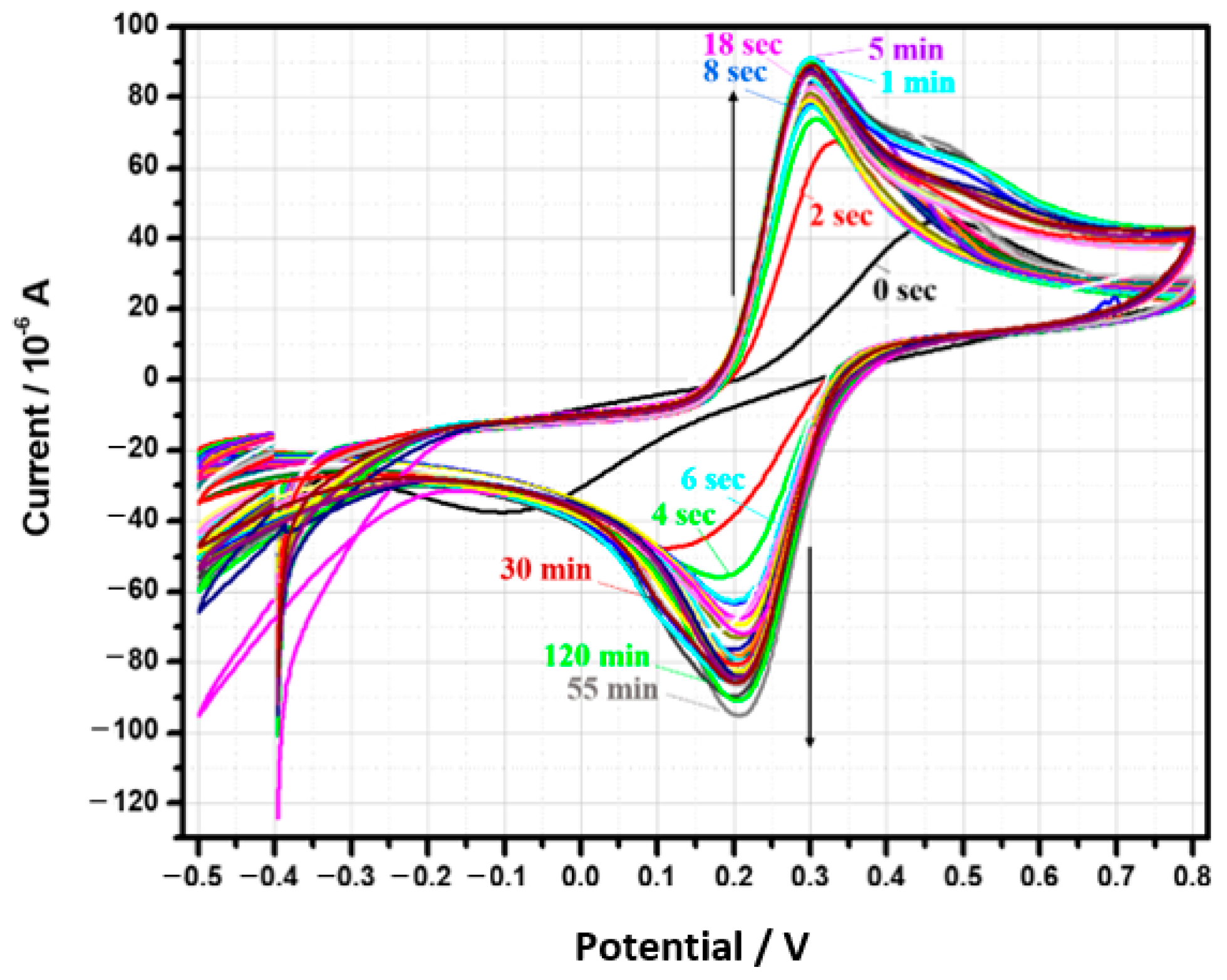
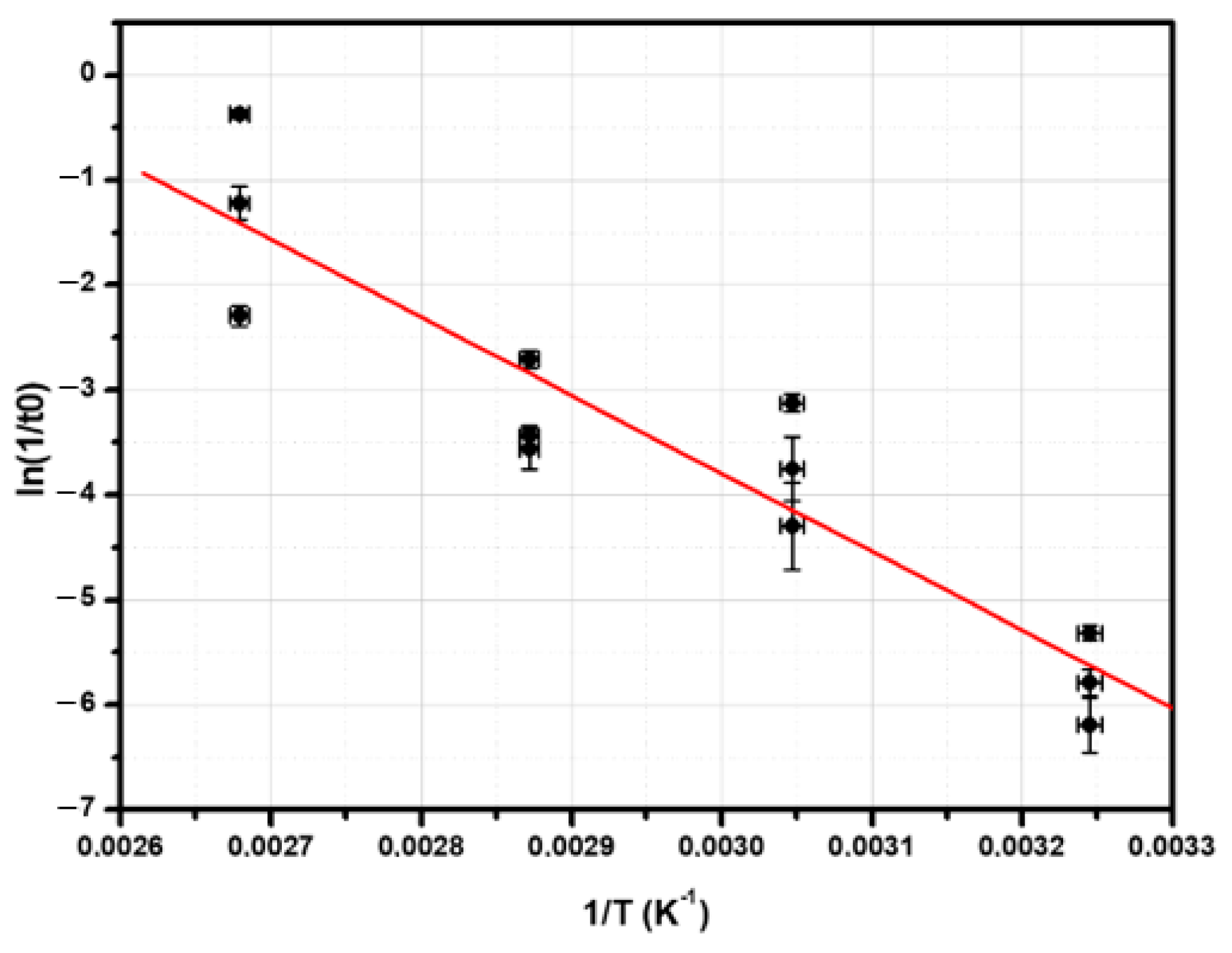
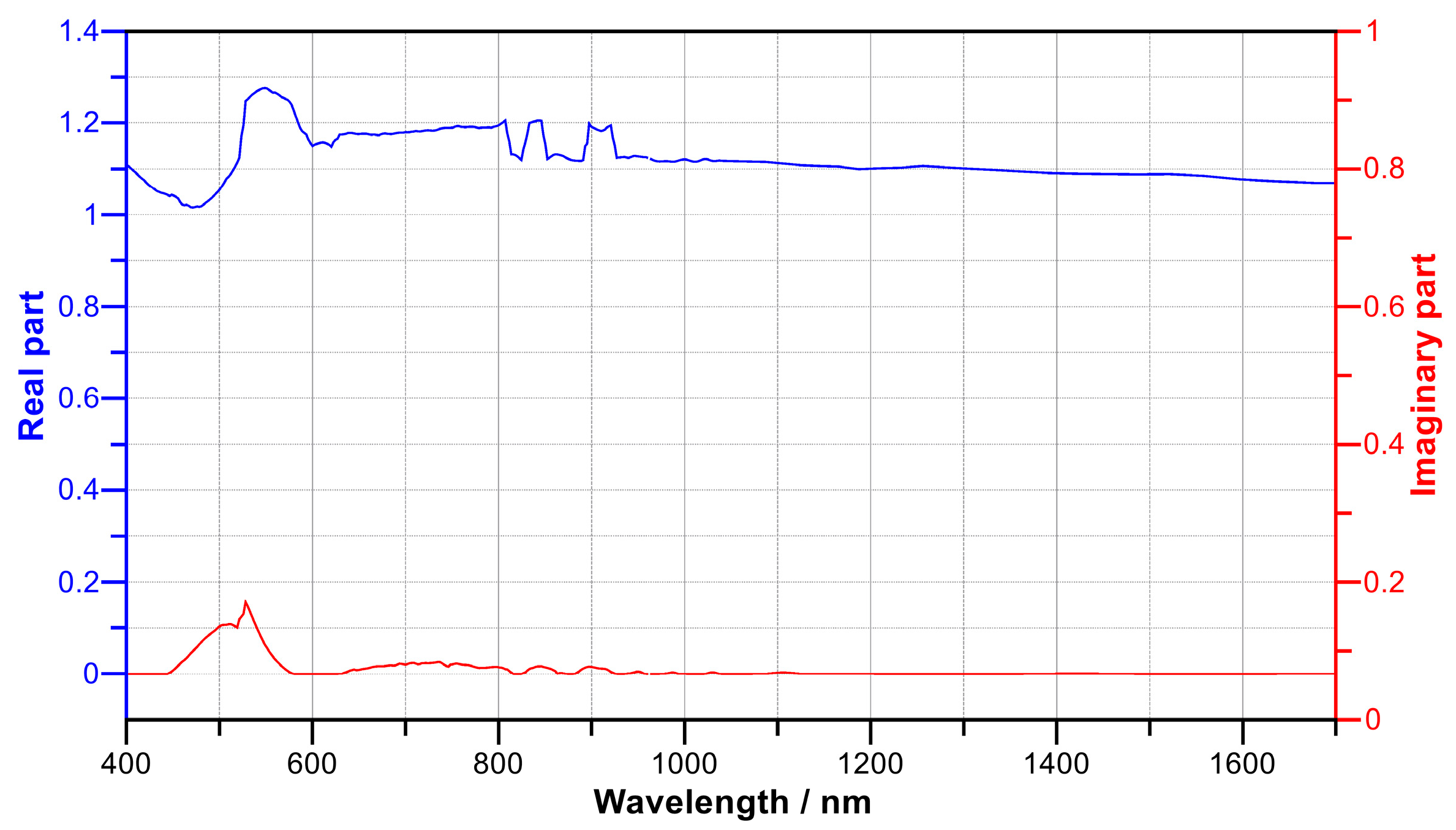
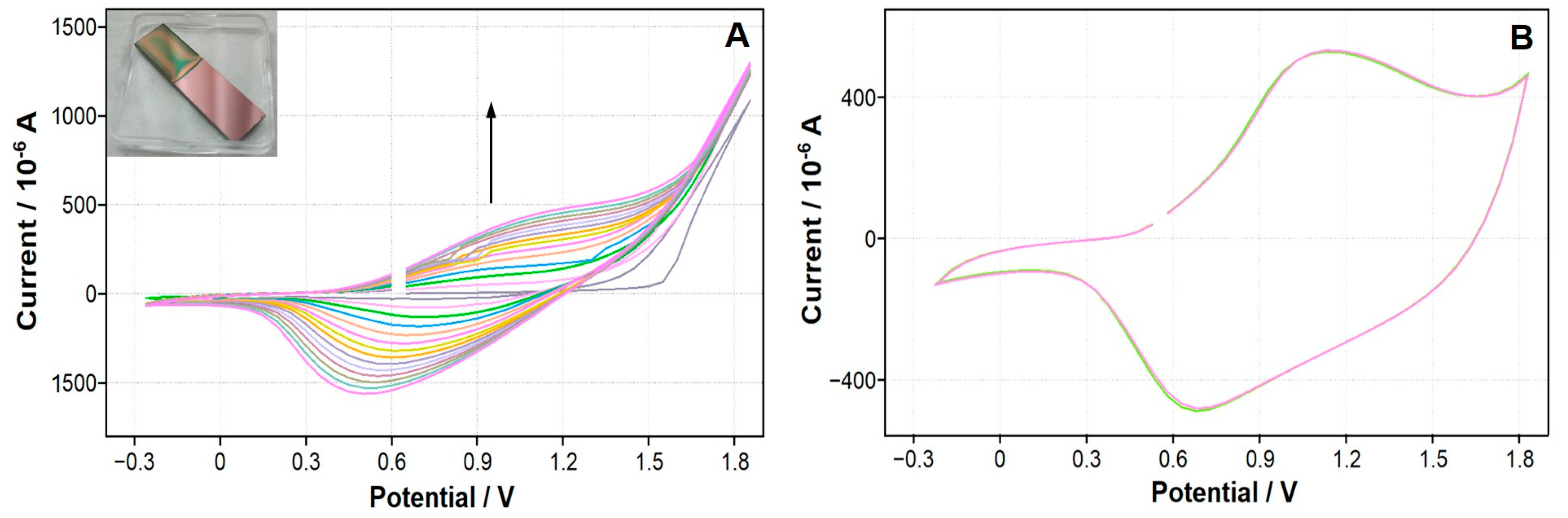
3.1. The Removal of the Insulating Layer from the TiN Surface
3.2. Layer-by-Layer Deposition of Polyelectrolytes
3.3. In Situ Electrochemical Synthesis of Conducting Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toth, L.E. Transition Metal Carbides and Nitrides, 1st ed.; Academic Press: Cambridge, MA, USA, 1971; p. 296. [Google Scholar]
- Sproul, W.D.; Rudnik, P.J.; Gogol, C.A. The effect of target power on the nitrogen partial pressure level and hardness of reactively sputtered titanium nitride coatings. Thin Solid Films 1989, 171, 171–181. [Google Scholar] [CrossRef]
- Perry, A.J. A contribution to the study of poisons’ ratios and elastic constants of TiN, ZrN, and HfN. Thin Solid Films 1990, 193–194, 463–471. [Google Scholar] [CrossRef]
- Kužel, R.; Černý, R., Jr.; Valvoda, V.; Blomberg, M.; Merisalo, M. Complex XRD microstructural studies of hard coatings applied to PVD-deposited TiN films. Part I. Problems and methods. Thin Solid Films 1994, 247, 64–78. [Google Scholar] [CrossRef]
- Rao, A.U.; Tiwari, S.K.; Goyat, M.S.; Chawla, A.K. Recent developments in magnetron-sputtered silicon nitride coatings of improved mechanical and tribological properties for extreme situations. J. Mater. Sci. 2023, 58, 9755–9804. [Google Scholar] [CrossRef]
- Avasarala, B.; Haldar, P. Electrochemical oxidation behavior of titanium nitride based electrocatalysts under PEM fuel cell conditions. Elechtrochimica Acta 2010, 55, 9024–9034. [Google Scholar] [CrossRef]
- Wei, B.; Ming, F.; Liang, H.; Qi, Z.; Hu, W.; Wang, Z. All nitride asymmetric supercapacitors of niobium titanium nitride-vanadium nitride. J. Power Sources 2021, 481, 228842. [Google Scholar] [CrossRef]
- Patsalas, P.; Kalfagiannis, N.; Kassavetis, S. Optical properties and plasmonic performance of titanium nitride. Materials 2015, 8, 3128–3154. [Google Scholar] [CrossRef]
- Ghanashyam Krishna, M.; Padmanabhan, K.A. Titanium nitride based multi-functional thin films. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2022. [Google Scholar] [CrossRef]
- Zhou, X.; Shang, C.; Gu, L.; Dong, S.; Chen, X.; Han, P.; Li, L.; Yao, J.; Liu, Z.; Xu, H.; et al. Mesoporous coaxial titanium nitride fibers of core-shell structures for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2011, 3, 3058–3063. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Li, M.; Zhang, J.; Zhang, L.; Song, Y.; Xiao, W.; Cruz, A.; Tong, Y.; Li, Y. TiN paper for ultrafast-charging supercapacitors. Nano-Micro Lett. 2020, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Jiang, B.; Liu, Z.; Li, C.; Yan, F.; Liu, X.; Li, H.; Yang, C.; Dong, D.; Hao, J. Sputtered titanium nitride films on nanowires Si substrate as pseudocapacitive electrode for supercapacitors. Ceram. Int. 2021, 47, 26758–26767. [Google Scholar] [CrossRef]
- Yoo, B.; Kim, K.-J.; Kim, Y.H.; Ko, M.J.; Kim, W.M.; Park, N.G. Titanium nitride thin film as a novel charge collector in TCO-less dye-sensitized solar cell. J. Mater. Chem. 2011, 21, 3077–3084. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Yus, J.; Moratalla, R.; Ferrari, B. Electrophoretic deposition of binder-free TiN nanoparticles to design 3D microstructures. The role of sintering in the microstructural robustness of supercapacitor electrodes. Elechtrochim. Acta 2021, 369, 137654. [Google Scholar] [CrossRef]
- Wang, R.; Xia, C.; Wei, N.; Alshareef, H.N. NiCo2O4@TiN core-shell electrodes trought conformal atomatic layer deposition for all-solid-state supercapacitors. Electrochim. Acta 2016, 196, 611–621. [Google Scholar] [CrossRef]
- Hasegawa, G.; Kitada, A.; Kawasaki, S.; Kanamori, K.; Nakanishi, K.; Kobayashi, Y.; Kageyama, H.; Abe, T. Impact of electrolyte on pseudocapacitance and stability of porous titanium nitride (TiN) monolithic electrode. J. Electrochem. Soc. 2015, 162, A77–A85. [Google Scholar] [CrossRef]
- Östling, M.; Nygren, S.; Petersson, C.S.; Norström, H.; Buchta, R.; Blom, H.O.; Berg, S. A comparative study of the diffusion barrier properties of TiN and ZrN. Thin Solid Films 1986, 145, 81–88. [Google Scholar] [CrossRef]
- Zeng, Y.; Russell, S.W.; Mckerrow, A.J.; Chen, L.; Alford, T.L. Effectiveness of TiN, Ta, TaN, and W2N as barriers for the integration of low-k dielectric hydrogen silsesquioxane. J. Vac. Sci. Technol. 2000, 18, 221–230. [Google Scholar] [CrossRef]
- Randhawa, H. Hard coatings for decorative applications. Surf. Coat. Technol. 1988, 36, 829–836. [Google Scholar] [CrossRef]
- Niyomsoan, S.; Grant, W.; Olson, D.L.; Mishra, B. Variation of color in titanium and zirconium nitride decorative thin films. Thin Solid Films 2002, 415, 187–194. [Google Scholar] [CrossRef]
- Ruterana, P.; Nouet, C.; Kehagias, T.; Komninou, P.; Karakostas, T.; di Forte Poisson, M.A.; Huet, F.; Morkoc, H. The microstructure of Ti/Al and TiN ohmic contacts to gallium nitride. Phys. Stat. Sol. 1999, 176, 767–771. [Google Scholar] [CrossRef]
- Matenoglou, G.M.; Koutsokeras, L.E.; Patsalas, P. Plasma energy and work function of conducting transition metal nitrides for electronic applications. Appl. Phys. Lett. 2009, 94, 152108. [Google Scholar] [CrossRef]
- Patsalas, P.; Charitidis, C.; Logothetidis, S.; Dimitriadis, C.A.; Valassiades, O. Combined electrical and mechanical properties of titanium nitride thin films as metallization materials. J. Appl. Phys. 1999, 86, 5296–5298. [Google Scholar] [CrossRef]
- Hultman, L.; Ljungcrantz, H.; Hallin, C.; Janzén, E.; Sundgren, J.E.; Pécz, B.; Wallenberg, L.R. Growth and electronic properties of epitaxial TiN thin films on 3C-SiC(001) and 6H-SiC(0001) substrates by reactive magnetron sputtering. J. Mater. Res. 1996, 11, 2458–2462. [Google Scholar] [CrossRef]
- Dimitriadis, C.A.; Lee, J.I.; Patsalas, P.; Logothetidis, S.; Tassis, D.H.; Brini, J.; Kamarinos, G. Characterization of TiNx/n-Si Schottky diodes by reactive magnetron sputtering. J. Appl. Phys. 1999, 85, 4238–4242. [Google Scholar] [CrossRef]
- Shiao, M.-H.; Lin, C.-T.; Huang, H.J.; Chen, P.-H.; Liao, B.-H.; Tseng, F.-G.; Lin, Y.-S. Novel gold dendritic nanoflowers deposited on titanium nitride for photoelectrochemical cells. J. Solid State Electrochem. 2018, 22, 3077–3084. [Google Scholar] [CrossRef]
- Kuk, S.K.; Ham, Y.; Gopinath, K.; Boonmongkolras, P.; Lee, Y.; Lee, Y.W.; Kondaveeti, S.; Ahn, C.; Shin, B.; Lee, J.-K.; et al. Continuous 3D titanium nitride nanoshell structure for solar-driven unbiased biocatalytic CO2 reduction. Adv. Energy Mater. 2019, 9, 1900029. [Google Scholar] [CrossRef]
- Meng, Z.; Zheng, S.; Luo, R.; Tang, H.; Wang, R.; Zhang, R.; Tian, T.; Tang, H. Transition metal nitrides for electrocatalytic application: Progress and rational design. Nanomaterials 2022, 12, 2660. [Google Scholar] [CrossRef]
- Khan, R.K.; Farghaly, A.A.; Silva, T.A.; Ye, D.; Collinson, M.M. Gold nanoparticle-decorated titanium nitride electrodes prepared by glancing angle deposition for sensing applications. ACS Appl. Nano Mater. 2019, 2, 1562–1569. [Google Scholar] [CrossRef]
- Chin, Y.-L.; Chou, J.-C.; Lei, Z.-C.; Sun, T.-P.; Chung, W.-Y.; Hsiung, S.-K. Titanium nitride membrane application to extended gate field effect transistor pH sensor using VLSI technology. J. Appl. Phys. 2001, 40, 6311–6315. [Google Scholar] [CrossRef]
- Stanković, D.M.; Ognjanović, M.; Martin, F.; Švorc, Ľ.; Mariano, J.F.M.L.; Antić, B. Design of titanium nitride- and wolfram carbide-doped RGO/GC electrodes for determination of gallic acid. Anal. Biochem. 2017, 539, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Chen, X.; Gu, L.; Zhang, L.; Zhou, X.; Liu, Z.; Han, P.; Xu, H.; Yao, J.; Zhang, X.; et al. A biocompatible titanium nitride nanorods derived nanostructured electrode for biosensing and bioelectrochemical energy conversion. Biosens. Bioelectron. 2011, 26, 4088–4094. [Google Scholar] [CrossRef] [PubMed]
- Haldorai, Y.; Hwang, S.-K.; Gopalan, A.-I.; Huh, Y.S.; Han, Y.-K.; Voit, W. Direct electrochemistry of cytochrome c immobilized on titanium nitride/multi-walled nanotube composite for amperometric nitrite biosensor. Biosens. Bioelectron. 2016, 79, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Reiter, S.; Schlipf, J.; Mai, C.; Spirito, D.; Jose, J.; Wenger, C.; Fischer, I.A. Strongly enhanced sensitivities of CMOS compatible plasmonic titanium nitride nanohole arrays for refractive index sensing under oblique incidence. Opt. Express 2023, 31, 17389–17407. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface modification of gold nanoparticles with small molecules for biochemical analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.D.; Troughton, E.B.; Tao, Y.-T.; Evall, J.; Whitesides, G.M.; Nuzzo, R.G. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J. Am. Chem. Soc. 1989, 111, 321–335. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Onclin, S.; Ravoo, B.J.; Reinhoudt, D.N. Engineering silicon oxide surfaces using self-assembled monolayers. Angew. Chem. Int. Ed. 2005, 44, 6282–6304. [Google Scholar] [CrossRef] [PubMed]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T.M.; Zuilhof, H. Covalent surface modification of oxide surfaces. Angew. Chem. Int. Ed. 2014, 53, 6322–6356. [Google Scholar] [CrossRef] [PubMed]
- Lange, U.; Roznyatovskaya, N.V.; Mirsky, V.M. Conducting polymers in chemical sensors and arrays. Anal. Chim. Acta 2008, 614, 1–26. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Komsthöft, T.; Bovone, G.; Bernhard, S.; Tibbitt, M.W. Polymer functionalization of inorganic nanoparticles for biomedical applications. Curr. Opin. Chem. Eng. 2022, 37, 100849. [Google Scholar] [CrossRef]
- Krutty, J.D.; Schmitt, S.K.; Gopalan, P.; Murphy, W.L. Surface functionalization and dynamics of polymeric cell culture substrates. Curr. Opin. Biotech. 2016, 40, 164–169. [Google Scholar] [CrossRef]
- Achour, A.; Chaker, M.; Achour, H.; Arman, A.; Islam, M.; Mardani, M.; Boujtita, M.; Brizoual, L.L.; Djouadi, M.A.; Brousse, T. Role of nitrogen doping at the surface of titanium nitride thin films towards capacitive charge storage enhancement. J. Power Sources 2017, 359, 349–354. [Google Scholar] [CrossRef]
- Iniesta, J.; González-García, J.; Fernández, J.; Montiel, V.; Aldaz, A. On the voltammetric of a platinized titanium surface with respect to the specific hydrogen and anion adsorption and charge transfer processes. J. Mater. Chem. 1999, 9, 3141–3145. [Google Scholar] [CrossRef]
- Mazor, A.; Srolovitz, D.J.; Hagan, P.S.; Bukiet, B.G. Columnar growth in thin films. Phys. Rev. Lett. 1988, 60, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.H. Emergent Properties of Plasmonic Systems in the Weak to Strong Coupling Regimes. Doctoral Dissertation, Boston College, Boston, MA, USA, 2019. [Google Scholar]
- Reiter, S.; Han, W.; Mai, C.; Spirito, D.; Jose, J.; Zöllner, M.; Fursenko, O.; Schubert, M.A.; Stemmler, I.; Wenger, C.; et al. Titanium nitride plasmonic nanohole arrays for CMOS—Compatible integrated refractive index sensing: Influence of layer thickness on optical properties. Plasmonics 2023, 18, 831–843. [Google Scholar] [CrossRef]
- Molina, J.; del Río, A.I.; Cases, F. Influence of the scan rate on the morphology of polyaniline grown on conducting fabrics. Centipede-like morphology. Synth. Met. 2010, 160, 99–107. [Google Scholar] [CrossRef]
- Farndon, E.E.; Pletcher, D.; Saraby-Reintjes, A. The electrodeposition of platinum onto a conducting ceramic, Ebonex. Electrochimica Acta 1997, 42, 1269–1279. [Google Scholar] [CrossRef]
- González-García, J.; Sánchez-Cano, G.; Aldaz, A.; Montiel, V.; Fierro, J.L.G. Surface analysis of titanium electrodes. Appl. Surf. Sci. 1994, 78, 457–466. [Google Scholar] [CrossRef][Green Version]
- Keil, R.G. Resistive electrode effects on cyclic voltammetry. J. Electrochem. Soc. 1986, 133, 1375. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Habbache, N.; Alane, N.; Djerad, S.; Tifouti, L. Leaching of copper oxide with different acid solutions. J. Chem. Eng. 2009, 152, 503–508. [Google Scholar] [CrossRef]
- Yakusheva, E.A.; Gorichev, I.G.; Atanasyan, T.K.; Lainer, Y.A. Kinetics if dissolution of cobalt oxides in acidic media. Russ. Metallurgy 2010, 1, 18–23. [Google Scholar] [CrossRef]
- Horlait, D.; Clavier, N.; Szenknect, S.; Dacheux, N.; Dubois, V. Dissolution of Cerium(IV)–Lanthanide(III) Oxides: Comparative Effect of Chemical Composition, Temperature, and Acidity. Inorg. Chem. 2012, 51, 3868–3878. [Google Scholar] [CrossRef]
- McPherson, J.; Kim, J.-Y.; Shanware, A.; Mogul, H. Thermochemical description of dielectric breakdown in high dielectric constant materials. Appl. Phys. Lett. 2003, 82, 2121–2123. [Google Scholar] [CrossRef]
- Ulrich, R.K.; Brown, W.D.; Ang, S.S.; Barlow, F.D.; Elshabini, A.; Lenihan, T.G.; Naseem, H.A.; Nelms, D.M.; Parkerson, J.; Schaper, L.W.; et al. Getting aggressive with passive devices. IEEE Circuits Devices Mag. 2000, 16, 16–25. [Google Scholar] [CrossRef]
- Wu, M.; Alivov, Y.I.; Morkoç, H. High-k dielectrics and advanced channel concepts for Si MOSFET. J. Mater. Sci. Mater. Electron. 2008, 19, 915–951. [Google Scholar] [CrossRef]
- Zanchin, V.R.; Cavallari, M.R.; Fonseca, F.J.; Albertin, K.F.; Pereyra, I.; Andrade, A.M. Low voltage organic devices with high-k TiOxNy and PMMA dielectrics for future application on flexible electronics. ECS Trans. 2011, 39, 455–460. [Google Scholar] [CrossRef]
- Albertin, K.F.; Pereyra, I. Study of metal-oxide-semiconductor capacitors with r.f. magnetron sputtering TiOxNy films dielectric layer. Phys. Status Solidi 2010, 3–4, 937–940. [Google Scholar] [CrossRef]
- Laarz, E.; Carlsson, M.; Vivien, B.; Johnsson, M.; Nygren, M.; Bergström, L.J. Colloidal processing of Al2O3-based composites reinforced with TiN and TiC particulates, whiskers and nanoparticles. Eur. Ceram. Soc. 2001, 21, 1027–1035. [Google Scholar] [CrossRef]
- Mortalò, C.; Cannio, M.; Zin, V.; Miorin, E.; Montagner, F.; Pasquali, L.; Montecchi, M.; Boccaccini, D.N.; Fabrizio, M.; Deambrosis, S.M. Surface optimization of commercial porous Ti substrates of titanium nitride. Membranes 2022, 12, 531. [Google Scholar] [CrossRef]
- Hiller, J.; Mendelsohn, J.D.; Rubner, M.F. Reversibly erasable nanoporous anti-reflection coatings from polyelectrolyte multilayers. Nature Mater. 2002, 1, 59–63. [Google Scholar] [CrossRef]
- Suryajaya, S.; Nabok, A.; Davis, F.; Hassan, A.; Higson, S.P.J.; Evans-Freeman, J. Optical and AFM study of electrostatically assembles films of CdS and ZnS colloid nanoparticles. Appl. Surf. Sci. 2008, 254, 4891–4898. [Google Scholar] [CrossRef][Green Version]
- Kyung, K.-H.; Fujimoto, K.; Shiratori, S. Control of structure and film thickness using spray layer-by-layer method: Application to double anti-reflection film. Jpn. J. Appl. Phys. 2011, 50, 035803. [Google Scholar] [CrossRef]
- Hao, Q.; Rahm, M.; Weiss, D.; Mirsky, V.M. Morphology of Electropolymerized poly(N-methylaniline) films. Microchim. Acta 2003, 143, 147–153. [Google Scholar] [CrossRef]
- Tsakova, V.; Milchev, A. Electrochemical formation and stability of polyaniline films. Electrochim. Acta 1991, 36, 1579–1583. [Google Scholar] [CrossRef]
- Watanabe, A.; Mori, K.; Iwasaki, Y.; Nakamura, Y.; Niizuma, S. Electrochemism of polyaniline film prepared by electrochemical polymerization. Macromolecules 1987, 20, 1739–1796. [Google Scholar] [CrossRef]
- Baba, A.; Tian, S.; Stefani, F.; Xia, C.; Wang, Z.; Advincula, R.C.; Johannsmann, D.; Knoll, W. Electropolymerization and doping/dedoping properties of polyaniline thin films as studied by electrochemical-surface plasmon spectroscopy and by the quartz crystal microbalance. J. Electroanal. Chem. 2004, 562, 95–103. [Google Scholar] [CrossRef]
- Lindfors, T.; Ivaska, A. pH sensitivity of polyaniline and its substituted derivatives. J. Electroanal. Chem. 2002, 531, 43–52. [Google Scholar] [CrossRef]
- Mohilner, D.M.; Adams, R.N.; Argersinger, W.J. Investigation of the kinetics and mechanism of the anodic oxidation of aniline in aqueous sulfuric acid solution at a platinum electrode. J. Am. Chem. Soc. 1962, 84, 3618–3622. [Google Scholar] [CrossRef]
- Planes, G.A.; Rodriguez, J.L.; Miras, M.C.; Pastor, E.; Barbero, C.A. Spectroelectrochemical studies of poly(N-methyaniline) formation redox behavior and degradation. A comparison with polyaniline. Electrochim. Acta 2014, 122, 39–49. [Google Scholar] [CrossRef]
- Planes, G.A.; Miras, M.C.; Barbero, C. Strong effects of counterions on the electrochemistry of poly(N-methylaniline) thin films. Polym. Int. 2002, 51, 429–433. [Google Scholar] [CrossRef]
- Yağan, A. Electrochemical characterization of poly(N-methylaniline) films. Int. J. Electrochem. Sci. 2019, 14, 2925–2932. [Google Scholar] [CrossRef]
- Blomquist, M.; Lindfors, T.; Vähäsalo, L.; Pivrikas, A.; Ivaska, A. Electropolymerization and characterization of poly(N-methylaniline) and poly(N-butylaniline) in mixtures of aqueous and organic solvents. Synth. Met. 2006, 156, 549–557. [Google Scholar] [CrossRef]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The mechanism of polypyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar] [CrossRef]
- Zhou, M.; Heinze, J. Electropolymerization of pyrrole and electrochemical study of polypyrrole. 3. Nature of “Water effect” in acetonitrile. J. Phys. Chem. 1999, 103, 8451–8457. [Google Scholar] [CrossRef]
- Lange, U.; Mirsky, V.M. Polythiophene films on gold electrodes: A comparison of bulk and contact resistances in aqueous and organic media. J. Solid State Electrochem. 2011, 15, 2377–2382. [Google Scholar] [CrossRef]
- Efremenko, Y.; Mirsky, V.M. Poly-3-thienylboronic acid: A chemosensitive derivative of polythiophene. J. Solid State Electrochem. 2020, 24, 3105–3111. [Google Scholar] [CrossRef]
- Li, X.; Li, Y. Electrochemical preparation of polythiophene in acetonitrile solution with boron fluoride-ethyl ether as the electrolyte. J. Appl. Polym. Sci. 2003, 90, 940–946. [Google Scholar] [CrossRef]
- William, L.; Brooks, A.; Sumerlin, B.S. Synthesis and applications of boronic acid-containing polymers: From materials to medicine. Chem. Rev. 2016, 116, 1375–1397. [Google Scholar] [CrossRef]
- Efremenko, Y.; Mirsky, V.M. 3-Thienylboronic acid as a receptor for diol-containing compounds: A study by isothermal titration calorimetry. Chemosensors 2022, 10, 251. [Google Scholar] [CrossRef]
- Abrantes, L.M.; Correia, J.P.; Savic, M.; Jin, G. Structural modifications during conducting polymer formation—An ellipsometric study. Electrochim. Acta 2001, 46, 3181–3187. [Google Scholar] [CrossRef]
- Istakova, O.I.; Konev, D.V.; Medvedeva, T.O.; Zolotukhina, E.V.; Vorotyntsev, M.A. Efficiency of polypyrrole electropolymerization undr various conditions. Russ. J. Electrochem. 2018, 54, 1243–1251. [Google Scholar] [CrossRef]
- Wood, G.A.; Iroh, J.O. Efficiency of electropolymerization of pyrrole onto carbon fibers. Synth. Met. 1996, 80, 73–82. [Google Scholar] [CrossRef]
- Syed, A.; Dinesan, M.K. Review: Polyaniline—A novel polymeric material. Talanta 1991, 38, 815–837. [Google Scholar] [CrossRef]
- Ivanov, S.; Kurniawan, F.; Tsakova, V.; Mirsky, V.M. Automated layer-by-layer deposition of polyelectrolytes in flow mode. Macromol. Mater. Eng. 2009, 294, 441–444. [Google Scholar] [CrossRef]




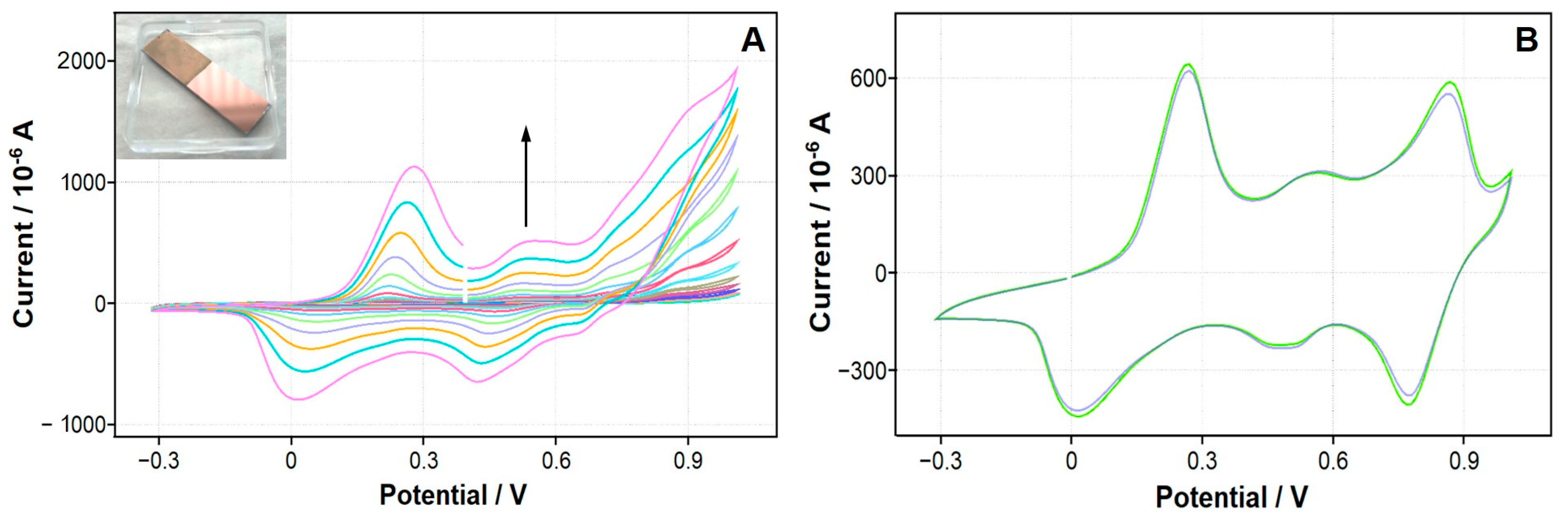
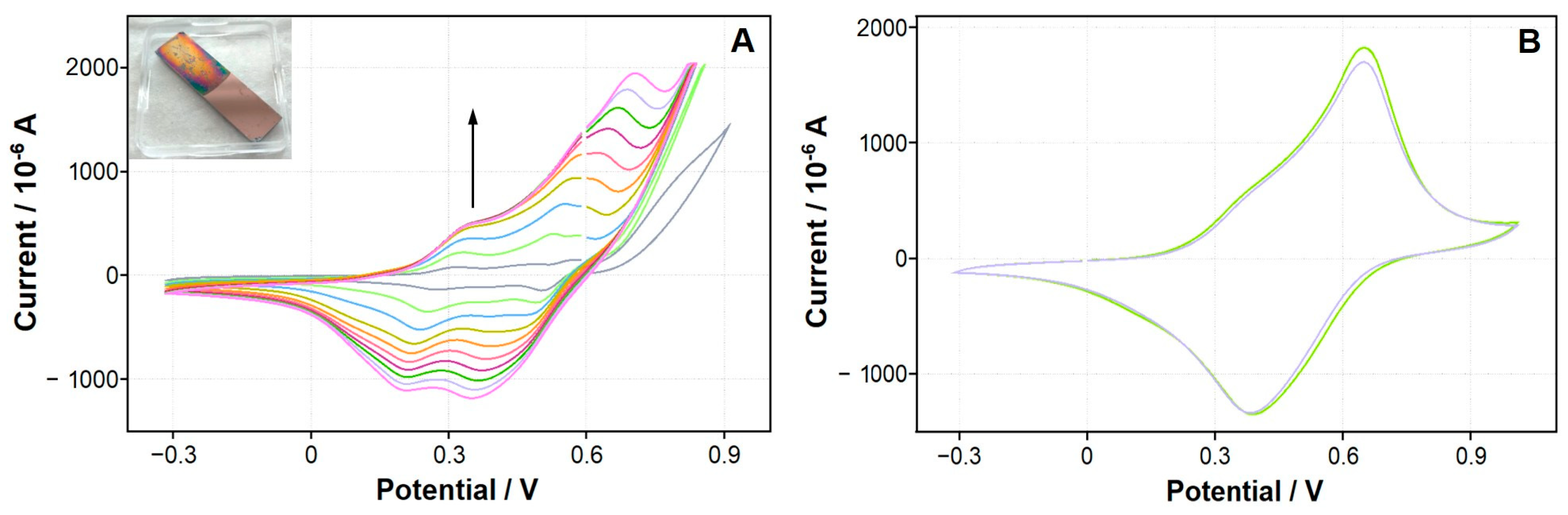
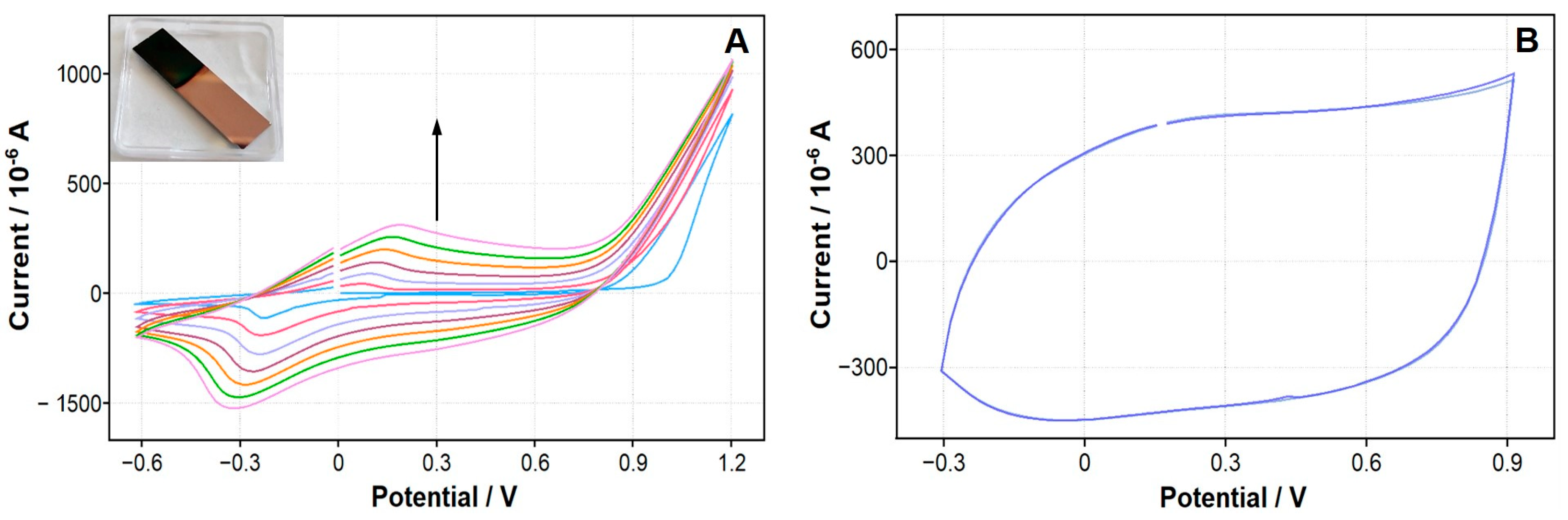
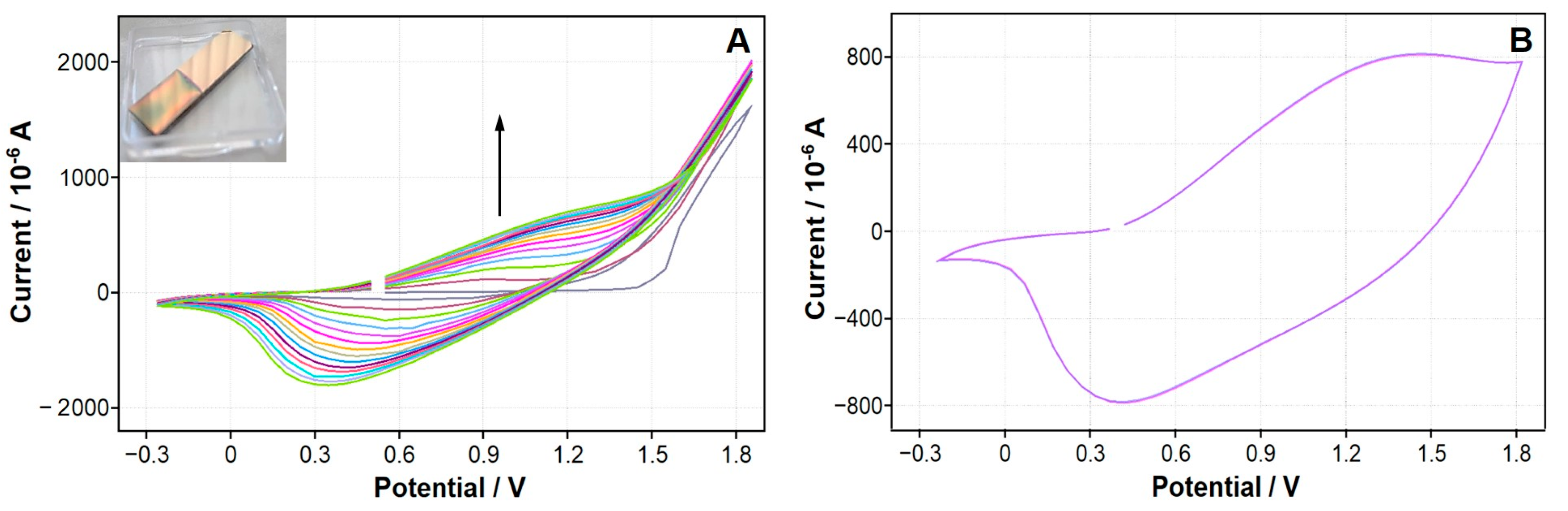
| Polymer | Monomer Concentration | Electrolyte | Potential Range/Number of Cycles |
|---|---|---|---|
| Polyaniline | 50 mM aniline | 0.5 M H2SO4 | −0.3~+1.0 V/15 cycles |
| Poly-N-methylaniline | 100 mM N-methylaniline | 2 M HClO4 | −0.3~+0.85 V/10 cycles |
| Poly-pyrrole | 100 mM pyrrole | ACN, 100 mM NBu4PF6 | −0.2~+1.1 V/7 cycles |
| Polythiophene | 50 mM thiophene | 90% BFEE/10% ACN (v/v), 50 mM DTBP | −0.2~+1.8 V/14 cycles |
| Poly-3-thienylboronic acid | 50 mM 3-thienylboronic acid | 90% BFEE/10% ACN (v/v), 50 mM, DTBP | −0.2~+1.8 V/14 cycles |
| Polymer | Qpolym, mC | Qox, mC | Deposition Efficiency, %, Calculated for the Doping Level 0.5 | Deposition Efficiency, %, Calculated for the Doping Level 0.25 |
|---|---|---|---|---|
| PANI | 78.5 | 2.5 | 16 | 29 |
| PNMA | 41.1 | 3.82 | 47 | 84 |
| PPy | 54.7 | 5.7 | 52 | 94 |
| PTh | 43.9 | 3.41 | 39 | 70 |
| PTBA | 51.3 | 4.3 | 42 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efremenko, Y.; Laroussi, A.; Sengül, A.; Corley-Wiciak, A.A.; Fischer, I.A.; Mirsky, V.M. Deposition of Polymers on Titanium Nitride Electrodes. Coatings 2024, 14, 215. https://doi.org/10.3390/coatings14020215
Efremenko Y, Laroussi A, Sengül A, Corley-Wiciak AA, Fischer IA, Mirsky VM. Deposition of Polymers on Titanium Nitride Electrodes. Coatings. 2024; 14(2):215. https://doi.org/10.3390/coatings14020215
Chicago/Turabian StyleEfremenko, Yulia, Arwa Laroussi, Akant Sengül, Agnieszka Anna Corley-Wiciak, Inga Anita Fischer, and Vladimir M. Mirsky. 2024. "Deposition of Polymers on Titanium Nitride Electrodes" Coatings 14, no. 2: 215. https://doi.org/10.3390/coatings14020215
APA StyleEfremenko, Y., Laroussi, A., Sengül, A., Corley-Wiciak, A. A., Fischer, I. A., & Mirsky, V. M. (2024). Deposition of Polymers on Titanium Nitride Electrodes. Coatings, 14(2), 215. https://doi.org/10.3390/coatings14020215