Microstructural and Mechanical Properties Analysis of Phosphate Layers Deposited on Steel Rebars for Civil Constructions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
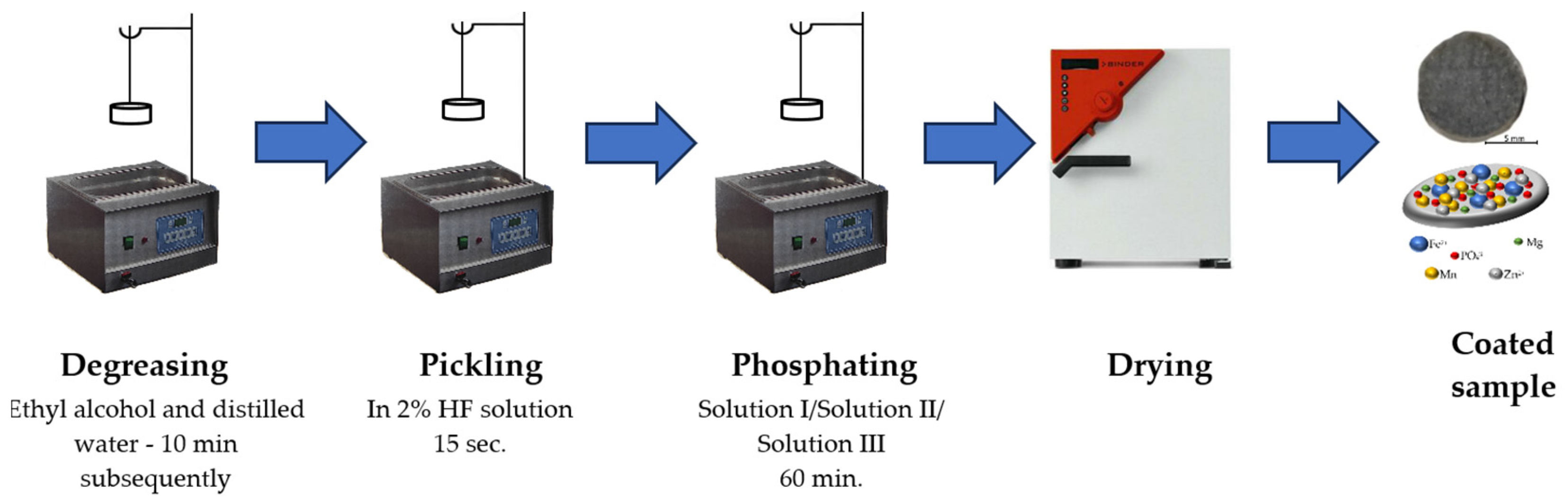
2.2. Sample Preparation
2.3. Methods
3. Results and Discussion
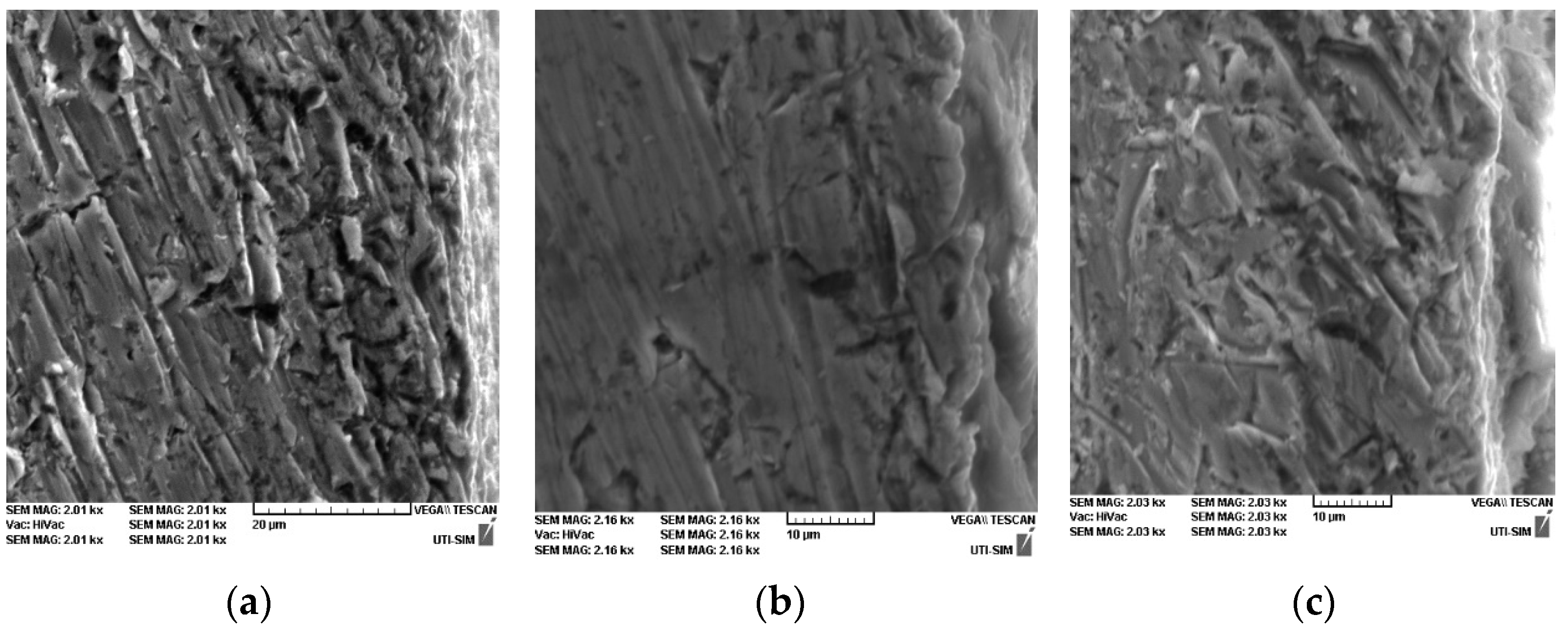
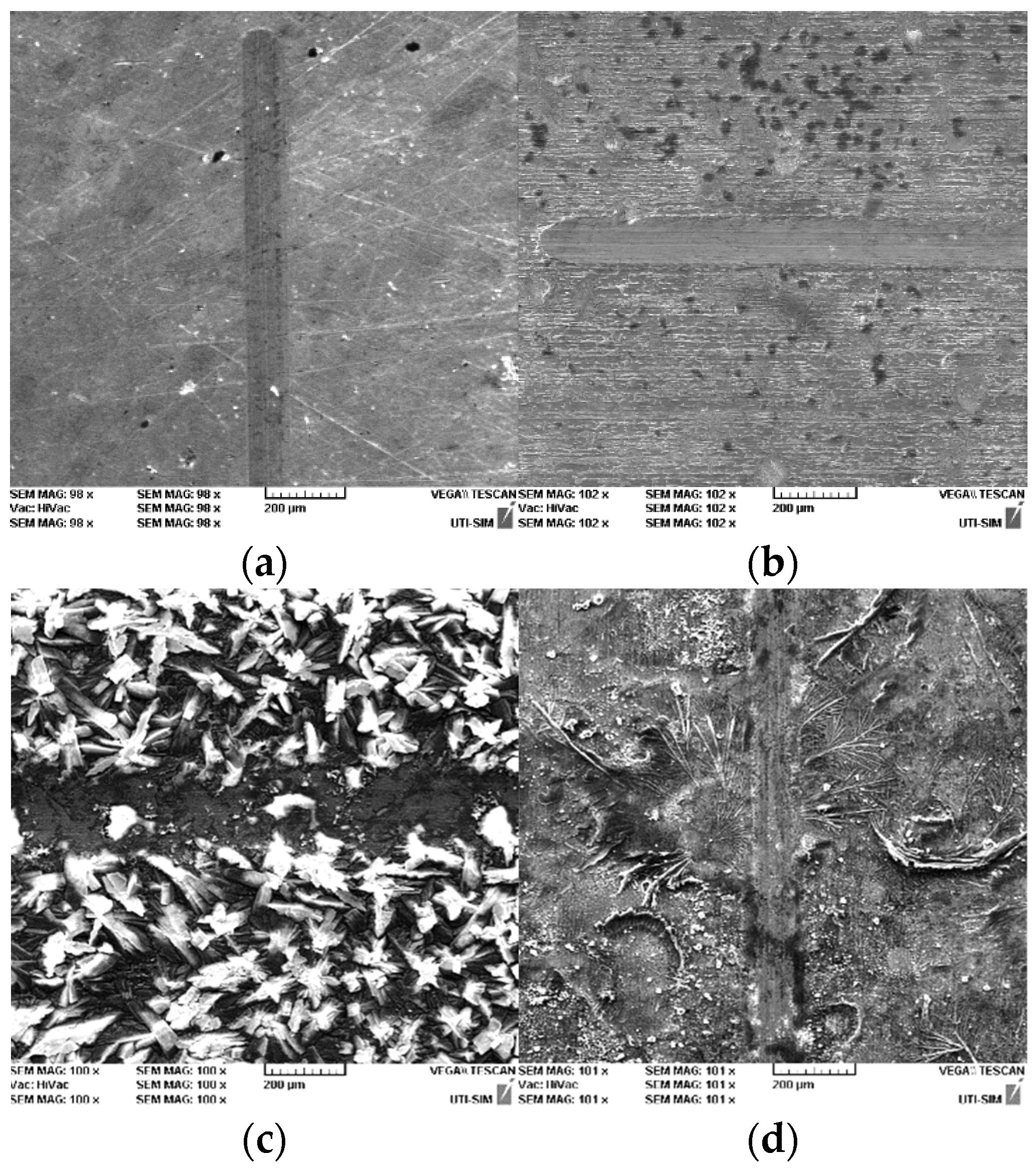
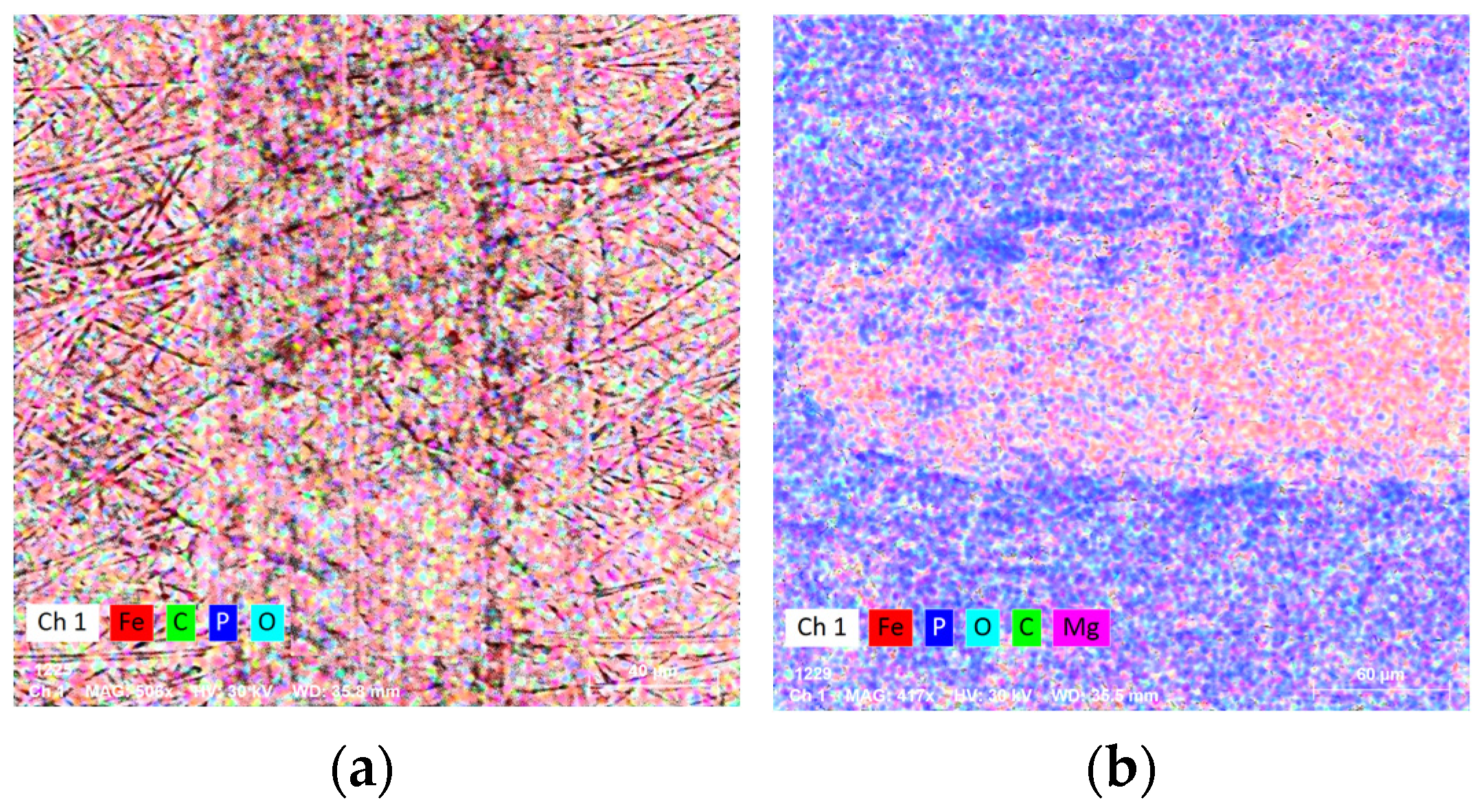
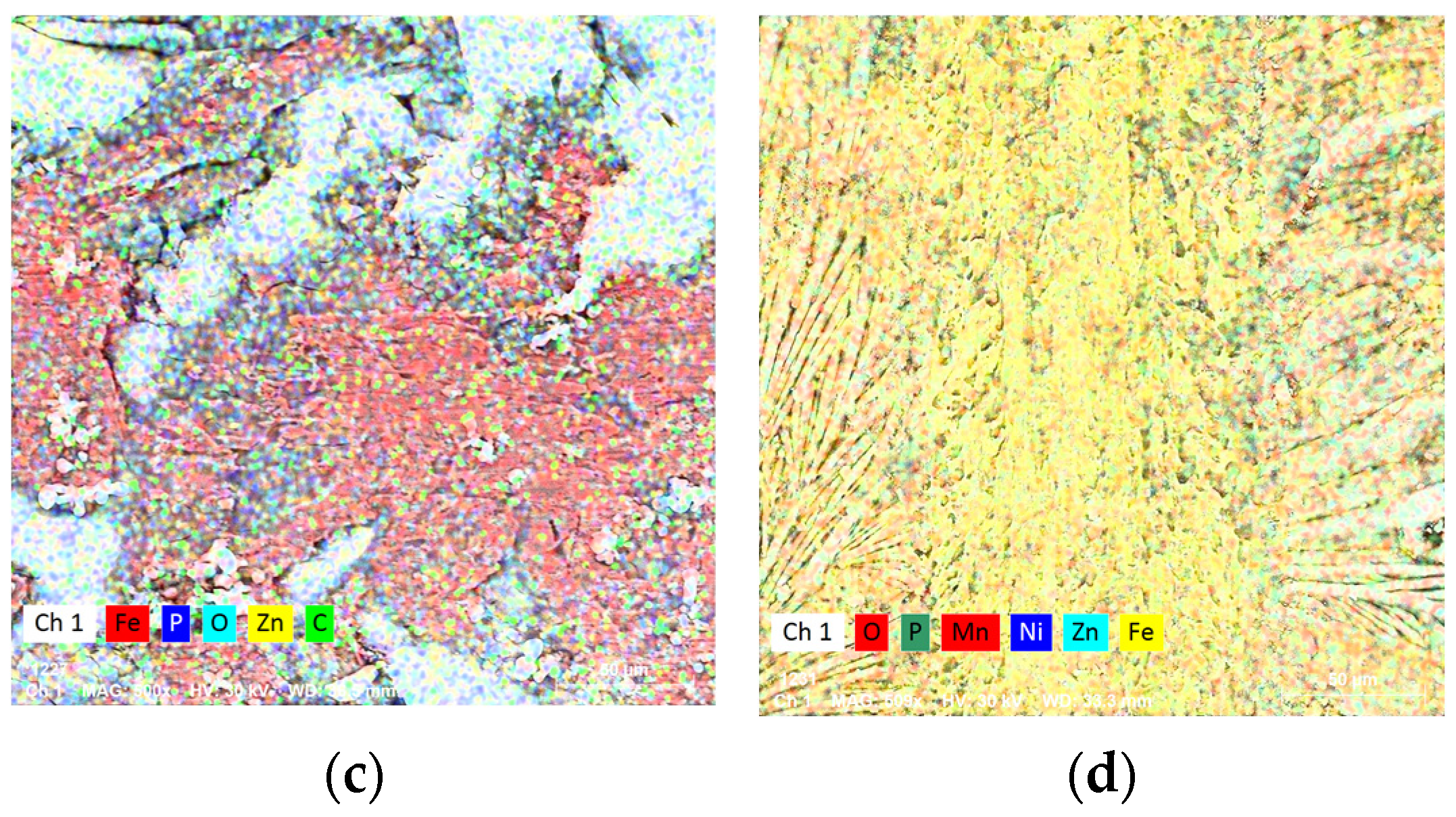
3.1. Structural and Chemical Analysis
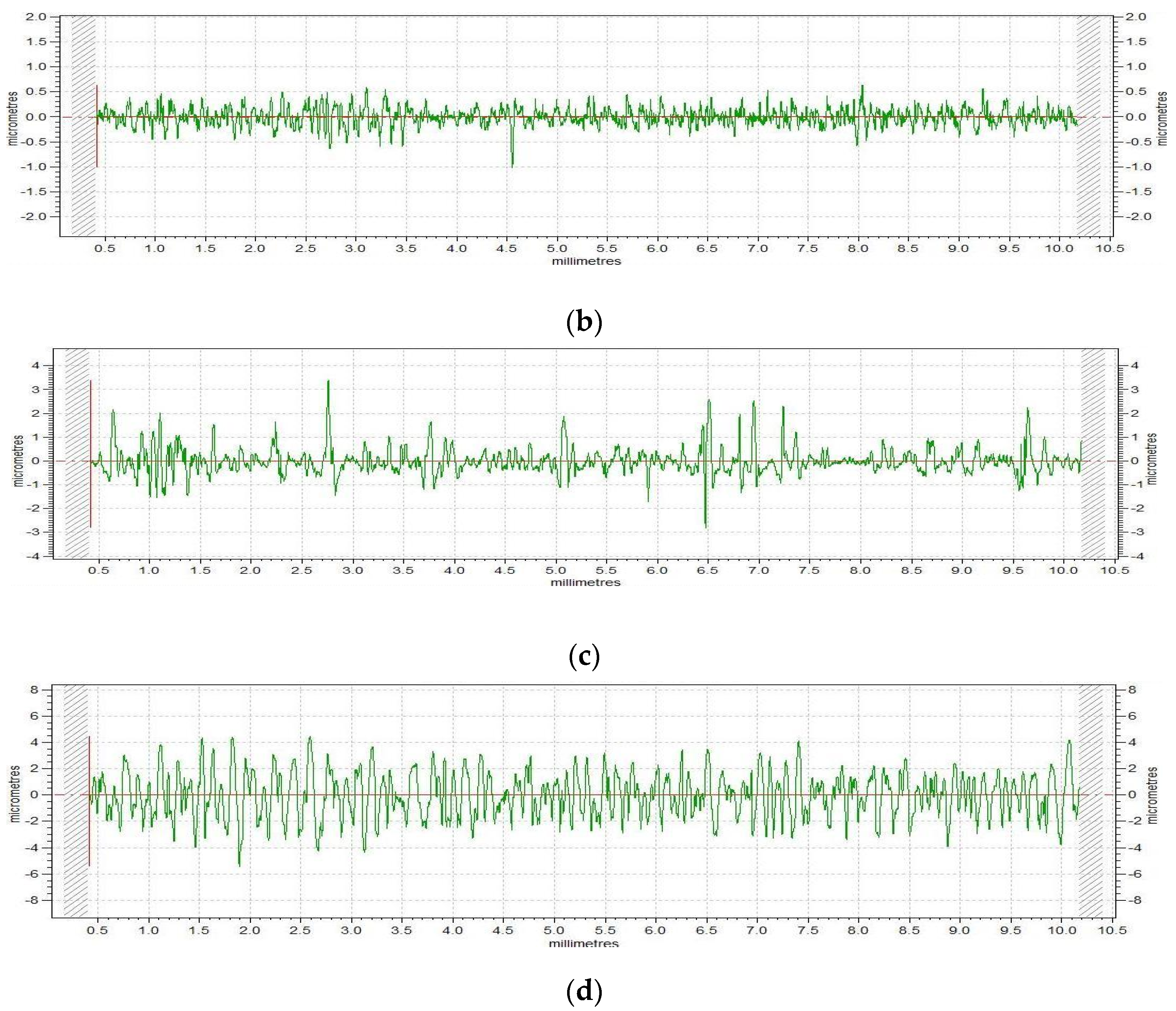
3.2. Surface Roughness
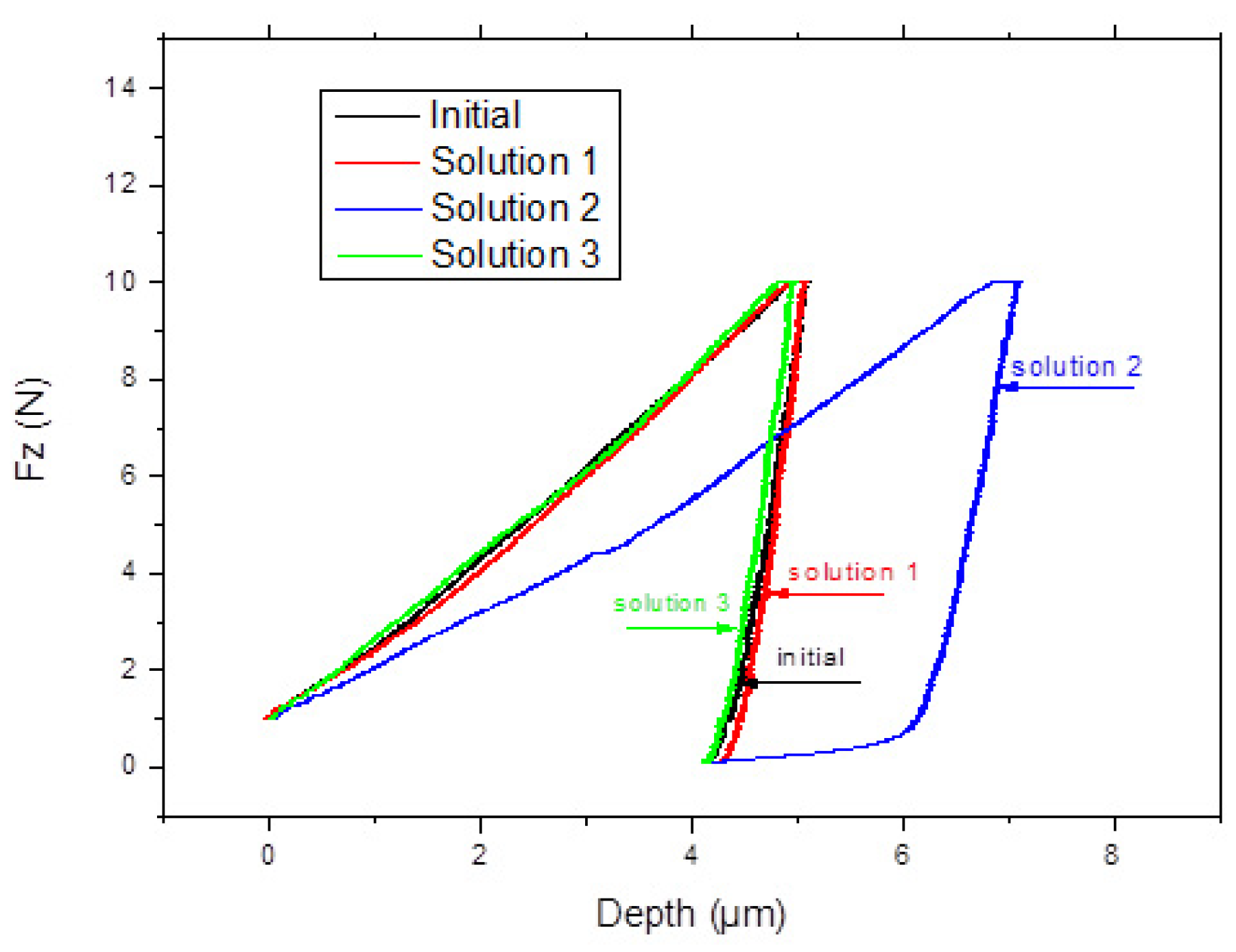
3.3. Microindentation
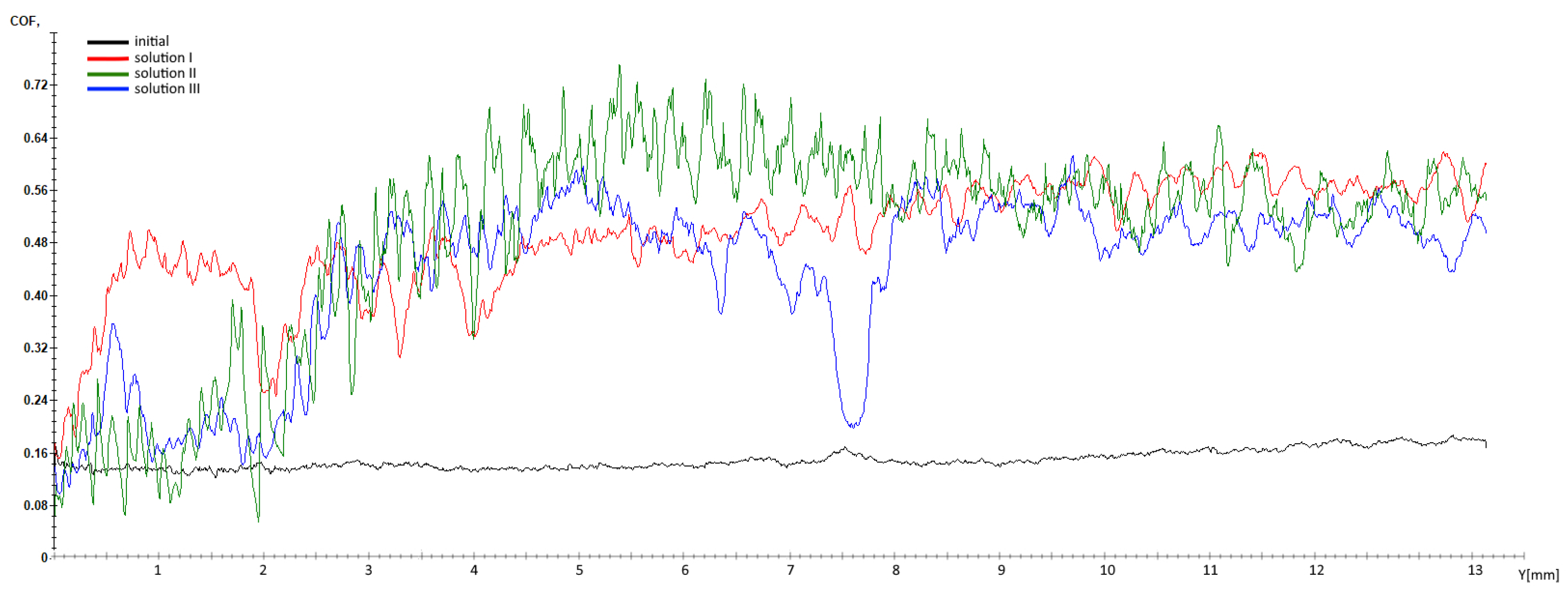
3.4. Scratch Resistance Analysis
4. Conclusions
- SEM micrographs highlight the appearance of crystals in various forms (the smaller sizes of these crystals (5–25 µm) can be seen on the samples phosphated with solution I and II, having a cylindrical shape, and the largest are obtained with solution III, the crystals of which are larger (30–50 µm) and have an acicular shape);
- Samples phosphated with solution I and solution III show negative values for the Rsk factor, and the metal substrate and the surface phosphated with solution II show a positive one. For the sample phosphated with solution III, the profile is very balanced, and the value of the asymmetry parameter is very close to zero;
- The initial sample shows the lowest maximum deformation, and the sample phosphated with solution III shows the highest value. The initial sample has an average hardness of 1.595 GPa with an elasticity modulus of 148.46 GPa, and the phosphated samples with solution II have a hardness of 1.165 GPa with a Young modulus of 117.98 GPa, which are lower than the solution I and III phosphated samples;
- The friction coefficient obtained for the phosphated samples presents three times higher values (0.49 for the phosphated samples with solutions I and III, respectively, 0.44 for the phosphated sample with solution II) compared with the average value obtained on the metal substrate (0.15);
- The sample phosphated with solution II shows areas where the phosphate layer resisted the external scratching stress and was not completely removed by the indenter.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gani, M.S.J. Fiber Reinforced Cement and Concrete Composites; Chapman & Hall: Sarasota, FL, USA, 1997; pp. 128–145. [Google Scholar]
- Mehta, K.; Monteiro, P. Concrete: Microstructure, Properties and Materials; McGraw-Hill Education: Chicago, IL, USA, 2014; ISBN 9780071797870. [Google Scholar]
- Duffó, G.; Reinoso, M.; Ramos, C.; Farina, S. Characterization of steel rebars embedded in a 70-year old concrete structure. Cem. Concr. Res. 2012, 42, 111–117. [Google Scholar] [CrossRef]
- Shin, C.B.; Kim, E.K. Modeling of chloride ion ingress in coastal concrete. Cem. Concr. Res. 2002, 32, 757–762. [Google Scholar] [CrossRef]
- Yohai, L.; Valcarce, M.; Vázquez, M. Testing phosphate ions as corrosion inhibitors for construction steel in mortars. Electrochim. Acta 2016, 202, 316–324. [Google Scholar] [CrossRef]
- Simescu, F.; Idrissi, H. Corrosion behaviour in alkaline medium of zinc phosphate coated steel obtained by cathodic electrochemical treatment. Corros. Sci. 2009, 51, 833–840. [Google Scholar] [CrossRef]
- Samardžija, M.; Alar, V.; Aljinović, F.; Kapor, F. Influence of phosphate layer on adhesion properties between steel surface and organic coating. Rud. Geološko Naft. Zb. 2022, 37, 11–17. [Google Scholar] [CrossRef]
- Bajat, J.; Mišković-Stanković, V.; Popić, J.; Dražić, D. Adhesion characteristics and corrosion stability of epoxy coatings electrodeposited on phosphated hot-dip galvanized steel. Prog. Org. Coat. 2008, 63, 201–208. [Google Scholar] [CrossRef]
- Marcos-Meson, V.; Michel, A.; Solgaard, A.; Fischer, G.; Edvardsen, C.; Skovhus, T.L. Corrosion resistance of steel fibre reinforced concrete—A literature review. Cem. Concr. Res. 2018, 103, 1–20. [Google Scholar] [CrossRef]
- Lazar, P.; Bejinariu, C.; Cazac, A.M.; Sandu, A.V.; A Bernevig, M.; Burduhos-Nergis, D.P. Phosphate coatings for the protection of steels reinforcement for concrete. J. Phys. 1960, 1960, 012013. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, Z.; Pan, H.; Cheng, X. The initiation and formation of a double-layer phosphate conversion coating on steel. Electrochem. Commun. 2020, 114, 106676. [Google Scholar] [CrossRef]
- Ezekiel, S.; Ayoola, A.; Durodola, B.; Odunlami, O.; Olawepo, A. Data on zinc phosphating of mild steel and its behaviour. CDC 2022, 38, 100838. [Google Scholar] [CrossRef]
- Fernandez, I.; Bairán, J.M.; Marí, A.R. Corrosion effects on the mechanical properties of reinforcing steel bars. Fatigue and σ-ε behavior. Constr. Build Mater. 2015, 101, 772–783. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.-P.; Vizureanu, P.; Sandu, A.V.; Bejinariu, C. Phosphate surface treatment for improving the corrosion resistance of the c45 carbon steel used in carabiners manufacturing. Materials 2020, 13, 3410. [Google Scholar] [CrossRef]
- American Standards Association; American Society of Mechanical Engineers; Society of Automotive Engineers. Surface Texture: Surface Roughness, Waviness, and Lay; OCLC 1197629204; American Society of Mechanical Engineers: New York, NY, USA, 2020; ISBN 978-0-7918-7325-0. [Google Scholar]
- Zhang, W.; Song, X.; Gu, X.; Li, S. Tensile and fatigue behavior of corroded rebars. Constr. Build. Mater. 2012, 34, 409–417. [Google Scholar] [CrossRef]
- Apostolopoulos, C.A. Mechanical behavior of corroded reinforcing steel bars S500s tempcore under low cycle fatigue. Constr. Build. Mater. 2007, 21, 1447–1456. [Google Scholar] [CrossRef]
- Apostolopoulos, C.; Papadopoulos, M.; Pantelakis, S. Tensile behaviour of corroded reinforcing steel bars BSt 500s. Constr. Build. Mater. 2006, 20, 782–789. [Google Scholar] [CrossRef]
- Apostolopoulos, C.; Papadakis, V. Consequences of steel corrosion on the ductility properties of reinforcement bar. Constr. Build. Mater. 2008, 22, 2316–2324. [Google Scholar] [CrossRef]
- Capozucca, R. Damage to reinforcement concrete due to reinforcement corrosion. Constr. Build. Mater. 1995, 9, 295–303. [Google Scholar] [CrossRef]
- Apostolopoulos, C.A.; Demis, S.; Papadakis, V.G. Chloride-induced corrosion of steel reinforcement—Mechanical performance and pit depth analysis. Constr. Build. Mater. 2013, 38, 139–146. [Google Scholar] [CrossRef]
- Demis, S.; Pilakoutas, K.; Apostolopoulos, C.A. Effect of corrosion on bond strength of steel and non-metallic reinforcement. Mater. Corros. 2010, 61, 328–331. [Google Scholar] [CrossRef]
- Batis, G.; Rakanta, E. Corrosion of steel reinforcement due to atmospheric pollution. Cem. Concr. Compos. 2005, 27, 269–275. [Google Scholar] [CrossRef]
- Moreno, E.; Cobo, A.; Cánovas, M.F. Mechanical properties variation of B500SD high ductility reinforcement regarding its corrosion degree. Mater. Constr. 2011, 61, 517–532. [Google Scholar] [CrossRef]
- Fang, C.; Lundgren, K.; Chen, L.; Zhu, C. Corrosion influence on bond in reinforced concrete. Cem. Concr. Res. 2004, 34, 2159–2167. [Google Scholar] [CrossRef]
- Apostolopoulos, C.A. The influence of corrosion and cross-section diameter on the mechanical properties of B500c steel. J. Mater. Eng. Perform. 2009, 18, 190–195. [Google Scholar] [CrossRef]
- Cairns, J.; Plizzari, G.A.; Du, Y.; Law, D.W.; Frnazoni, C. Mechanical properties of corrosion-damaged reinforcement. ACI Mater. J. 2005, 102, 256–264. [Google Scholar]
- Almusallam, A.A. Effect of degree of corrosion on the properties of reinforcing steel bars. Constr. Build Mater. 2001, 15, 361–368. [Google Scholar] [CrossRef]
- Du, Y.G.; Clark, L.A.; Chan, A.H.C. Residual capacity of corroded reinforcing bars. Mag. Concr. Res. 2005, 57, 135–147. [Google Scholar] [CrossRef]
- Du, Y.G.; Clark, L.A.; Chan, A.H.C. Effect of corrosion on ductility of reinforcing bars. Mag. Concr. Res. 2005, 57, 407–419. [Google Scholar] [CrossRef]
- Valanezhad, A.; Tsuru, K.; Maruta, M.; Kawachi, G.; Matsuya, S.; Ishikawa, K. Novel Ceramic Coating on Titanium with High Mechanical Properties. Bioceram. Dev. Appl. 2011, 1, D110124. [Google Scholar] [CrossRef]
- Seo, Y.H. A study on improving the prediction accuracy of cold forging die life based on quantitative evaluation of phosphate film damage. Sci. Rep. 2023, 13, 16464. [Google Scholar] [CrossRef]
- Duszczyk, J.; Siuzdak, K.; Klimczuk, T.; Strychalska-Nowak, J.; Zaleska-Medynska, A. Modified manganese phosphate conversion coating on low-carbon. Materials 2020, 13, 1416. [Google Scholar] [CrossRef]
- Al-Swaidani, A. Modified zinc phosphate coatings: A promising approach to enhance the anti-corrosion properties of reinforcing steel. MOJ Civil. Eng. 2017, 3, 370–374. [Google Scholar] [CrossRef]
- Jegannathan, S.; Arumugam, T.; Narayanan, T.S.; Ravichandran, K. Formation and characteristics of zinc phosphate coatings obtained by electrochemical treatment: Cathodic vs. anodic. Prog. Org. Coat. 2009, 65, 229–236. [Google Scholar] [CrossRef]
- Sandu, A.V.; Coddet, C.; Bejinariu, C. A Comparative study on surface structure of thin zinc phosphates layers obtained using different deposition procedures on steel. Rev. Chim. 2012, 63, 401–406. [Google Scholar]
- Duszczyk, J.; Siuzdak, K.; Klimczuk, T.; Strychalska-Nowak, J.; Zaleska-Medynska, A. Manganese phosphatizing coatings: The effects of preparation conditions on surface properties. Materials 2018, 11, 2585. [Google Scholar] [CrossRef]
- Herbath, B.; Kovacs, K. The effects of the steel’s surface quality on the properties of anticorrosion coatings. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Balatonkenese, Hungary, 13–15 October 2019. [Google Scholar]
- Nedeff, V.; Bejenariu, C.; Lazar, G.; Agop, M. Generalized lift force for complex fluid. Powder Technol. 2013, 235, 685–695. [Google Scholar] [CrossRef]
- Bulbuc, V.; Paleu, V.; Pricop, B.; Popa, M.; Cârlescu, V.; Cimpoesu, N.; Bujoreanu, L.G. Variation of wear resistance of T105Mn120 castings, used for railway safety systems, as an effect of dynamic loading under extreme conditions. JMEP 2021, 30, 7128–7137. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.-P.; Sandu, A.; Vizureanu, P.; Bejinariu, C. Phosphate conversion coating—A short review. Arch. Metall. Mater. 2023, 68, 1029–1034. [Google Scholar] [CrossRef]
- Sivakumarana, I.; Alankaram, V. The wear characteristics of heat treated manganese phosphate coating applied to AlSi D2 steel with oil lubricant. Tribol. Ind. 2012, 34, 247–254. [Google Scholar]
- Ernens, D.; de Rooij, M.; Pasaribu, H.; van Riet, E.; van Haaften, W.; Schipper, D. Mechanical characterization and single asperity scratch behaviour of dry zinc and manganese phosphate coatings. Tribol. Int. 2018, 118, 474–483. [Google Scholar] [CrossRef]
- Herbáth, B.; Kovács, K.; Jakab, M.; Makó, É. Crystal structure and properties of zinc phosphate layers on aluminum and steel alloy surfaces. Crystals 2023, 13, 369. [Google Scholar] [CrossRef]
- Konovalova, V.S.; Rumyantseva, V.E. Obtaining red phosphate coatings on steel at room temperature. Eng. Proc. 2023, 52, 54. [Google Scholar]
- Rumyantseva, V.; Konovalova, V.; Narmaniya, B. Modified phosphate coatings applied to steel by cold method. J. Phys. Conf. Ser. 2021, 2131, 042027. [Google Scholar] [CrossRef]
- Rumyantsev, E.; Rumyantseva, V.; Konovalova, V. White phosphate coatings obtained on steel from modified cold phosphating solutions. Coatings 2022, 12, 70. [Google Scholar] [CrossRef]


| Element | C | Si | Mn | S | P | Fe |
|---|---|---|---|---|---|---|
| wt.% | 0.23 | 0.07 | 0.75 | 0.045 | 0.045 | rest |
| Solution I | Solution II | Solution III |
|---|---|---|
| NaOH (7 g) | NaOH (0.9 g) | NaOH (0.75 g) |
| MgCO3 (8.5 g) | NaNO2 (0.6 g) | NaNO2 (0.45 g) |
| NaNO2 (0.4 g) | Na5P3O10 (0.1 g) | Na5P3O10 (0.05 g) |
| H3PO4 (85%, 23 mL) | H3PO4 (22 mL) | H3PO4 (7 mL) |
| HNO3 (11 mL) | HNO3 (0.4 mL) | |
| Zn (9 g) | Ni (0.03 g) | |
| Fe (0.03) | ||
| Mn (1.5 g) |
| Surface | Solution I | Solution II | Solution III | EDS Error % | |
|---|---|---|---|---|---|
| O% | wt | 19.96 | 38.06 | 33.12 | 2.9 |
| at | 33.89 | 66.55 | 62.01 | ||
| Fe% | wt | 61.78 | 15.1 | 47.66 | 1.5 |
| at | 30.05 | 7.56 | 25.56 | ||
| C% | wt | 14.33 | - | - | 1.9 |
| at | 32.4 | - | - | ||
| P% | wt | 3.05 | 12.31 | 6.4 | 0.25 |
| at | 2.68 | 11.12 | 6.2 | ||
| Mg% | wt | 0.88 | - | - | 0.12 |
| at | 0.98 | - | - | ||
| Mn% | wt | - | - | 3.6 | 0.11 |
| at | - | - | 1.96 | ||
| Ni% | wt | - | - | 0.58 | 0.07 |
| at | - | - | 0.3 | ||
| Zn% | wt | - | 34.52 | - | 0.2 |
| at | - | 14.76 | - | ||
| Specimen | Ra [µm] | Rsk | Rq [µm] | Rku |
|---|---|---|---|---|
| Substrate | 0.05 | 0.54 | 0.07 | 4.71 |
| MgCo3 | 0.14 | −0.14 | 0.17 | 0.17 |
| MnZnNi | 0.37 | 1.22 | 0.55 | 7.99 |
| Zn | 1.37 | −0.01 | 1.67 | 0.57 |
| Crt. no. | Young’s Indentation Module [GPa] | Hardness [GPa] | Maximum Load [N] | Maximum Displacement [µm] | Contact Stiffness [N/µm] | Contact Area [µm2] | |
|---|---|---|---|---|---|---|---|
| Initial | Point 1 | 92.55 | 1.63 | 8.99 | 5.31 | 7.76 | 5517.57 |
| Point 2 | 212.19 | 1.54 | 9.00 | 5.12 | 16.56 | 5856.25 | |
| Point 3 | 140.64 | 1.62 | 8.99 | 5.07 | 11.33 | 5559.55 | |
| Average | 148.46 | 1.59 | 8.99 | 5.17 | 11.88 | 5644.45 | |
| Solution I | Point 1 | 150.64 | 1.54 | 9.02 | 5.27 | 12.36 | 5866.21 |
| Point 2 | 126.15 | 1.63 | 9.02 | 5.12 | 10.27 | 5539.07 | |
| Point 3 | 187.87 | 1.46 | 9.03 | 5.41 | 15.34 | 6166.61 | |
| Average | 154.89 | 1.54 | 9.02 | 5.27 | 12.65 | 5857.29 | |
| Solution II | Point 1 | 86.29 | 0.98 | 9.02 | 8.17 | 9.33 | 9181.95 |
| Point 2 | 100.39 | 1.13 | 9.00 | 7.12 | 10.02 | 7974.12 | |
| Point 3 | 167.28 | 1.38 | 9.00 | 5.72 | 14.26 | 6507.06 | |
| Average | 117.98 | 1.16 | 9.01 | 7.01 | 11.21 | 7887.71 | |
| Solution III | Point 1 | 221.41 | 1.42 | 9.02 | 5.52 | 17.89 | 6377.38 |
| Point 2 | 118.57 | 1.69 | 8.99 | 5.00 | 9.53 | 5335.96 | |
| Point 3 | 103.51 | 1.42 | 9.02 | 5.84 | 9.18 | 6337.26 | |
| Average | 147.83 | 1.51 | 9.01 | 5.45 | 12.20 | 6016.86 |
| Solution | Fx [N] | CoF |
|---|---|---|
| Initial | 0.84 | 0.15 |
| Solution I | 2.86 | 0.49 |
| Solution II | 2.59 | 0.44 |
| Solution III | 2.94 | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazar, P.; Cimpoesu, N.; Istrate, B.; Cazac, A.M.; Burduhos-Nergis, D.-P.; Benchea, M.; Berbecaru, A.-C.; Badarau, G.; Vasilescu, G.D.; Popa, M.; et al. Microstructural and Mechanical Properties Analysis of Phosphate Layers Deposited on Steel Rebars for Civil Constructions. Coatings 2024, 14, 182. https://doi.org/10.3390/coatings14020182
Lazar P, Cimpoesu N, Istrate B, Cazac AM, Burduhos-Nergis D-P, Benchea M, Berbecaru A-C, Badarau G, Vasilescu GD, Popa M, et al. Microstructural and Mechanical Properties Analysis of Phosphate Layers Deposited on Steel Rebars for Civil Constructions. Coatings. 2024; 14(2):182. https://doi.org/10.3390/coatings14020182
Chicago/Turabian StyleLazar, Petru, Nicanor Cimpoesu, Bogdan Istrate, Alin Marian Cazac, Diana-Petronela Burduhos-Nergis, Marcelin Benchea, Andrei-Constantin Berbecaru, Gheorghe Badarau, Gabriel Dragos Vasilescu, Mihai Popa, and et al. 2024. "Microstructural and Mechanical Properties Analysis of Phosphate Layers Deposited on Steel Rebars for Civil Constructions" Coatings 14, no. 2: 182. https://doi.org/10.3390/coatings14020182
APA StyleLazar, P., Cimpoesu, N., Istrate, B., Cazac, A. M., Burduhos-Nergis, D.-P., Benchea, M., Berbecaru, A.-C., Badarau, G., Vasilescu, G. D., Popa, M., & Bejinariu, C. (2024). Microstructural and Mechanical Properties Analysis of Phosphate Layers Deposited on Steel Rebars for Civil Constructions. Coatings, 14(2), 182. https://doi.org/10.3390/coatings14020182













