Effect of Coating Damage on the Micro Area Corrosion Performance of HDR Duplex Stainless Steel
Abstract
1. Introduction
1.1. Sample Preparation
1.2. Electrochemical Tests and Structure Observation
2. Results and Analysis of Experiment
2.1. HDR Metallographic Morphology
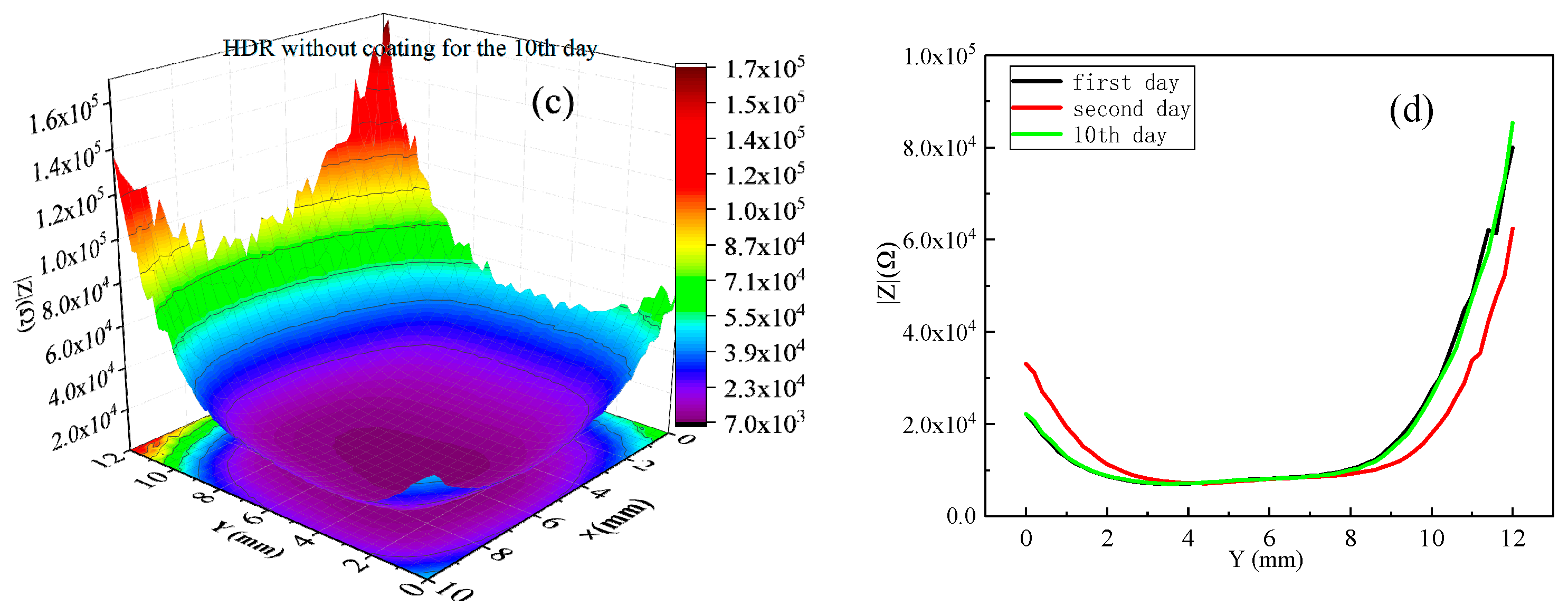
2.2. LEIS Test of HDR Uncoated
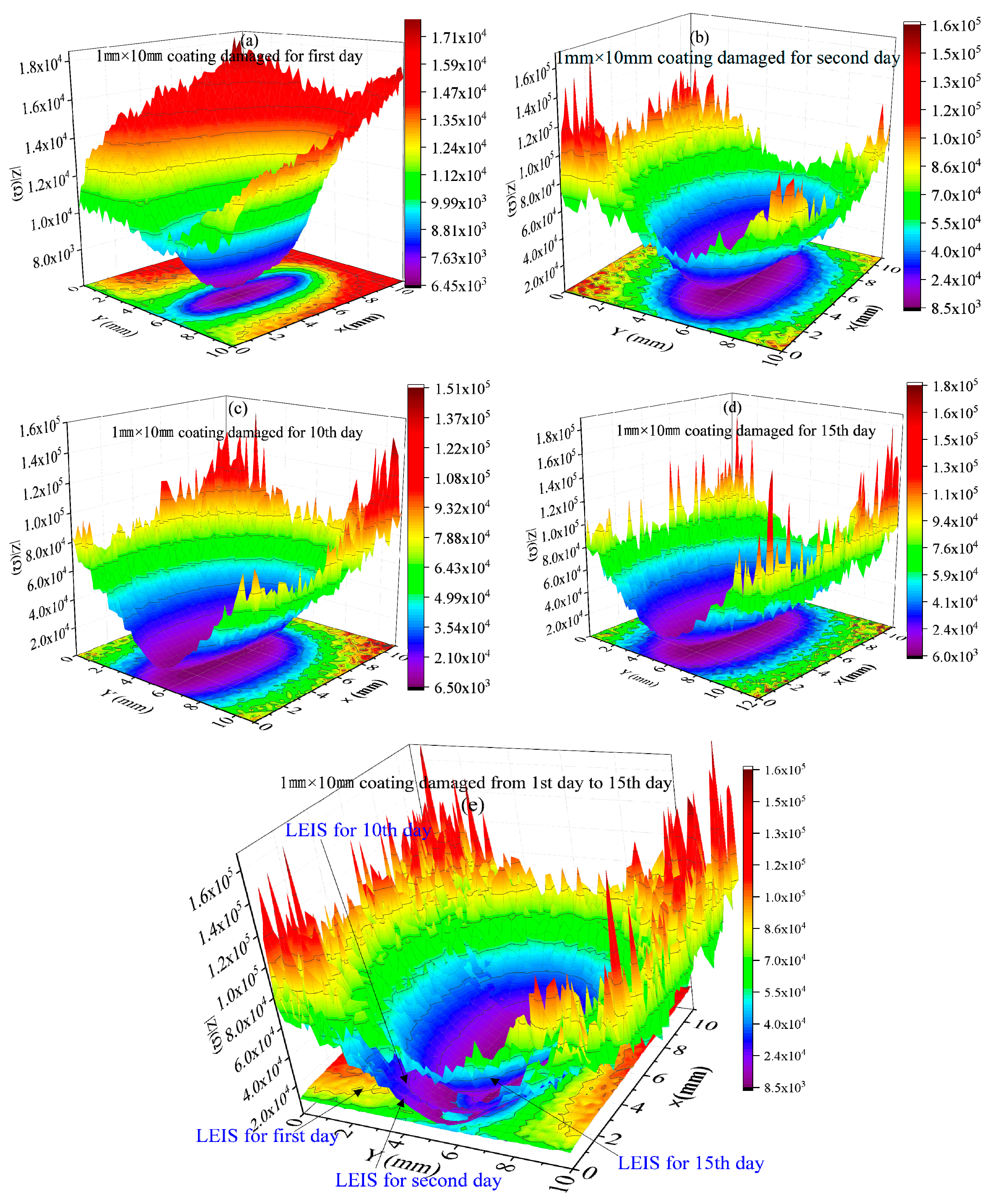
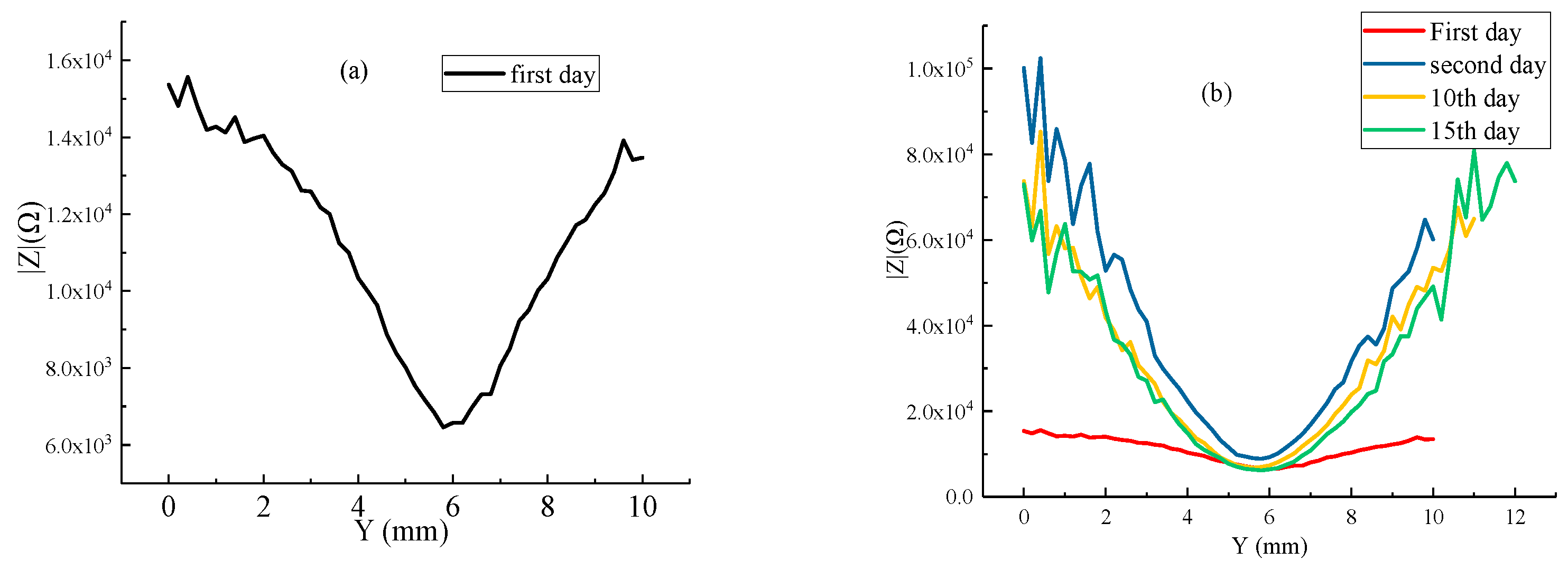
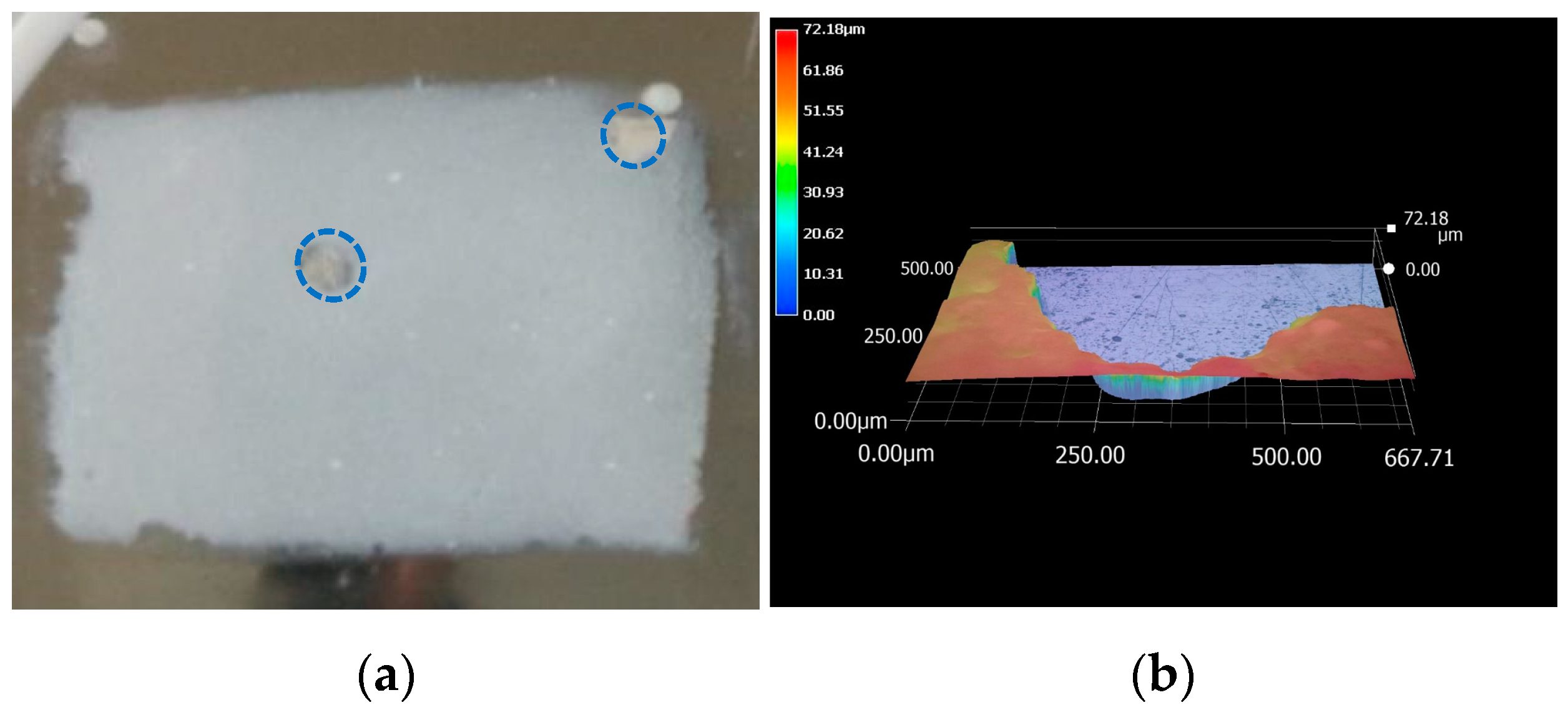
2.3. LEIS Test of HDR with Coating Damaged
3. Discussion
4. Conclusions
- (1)
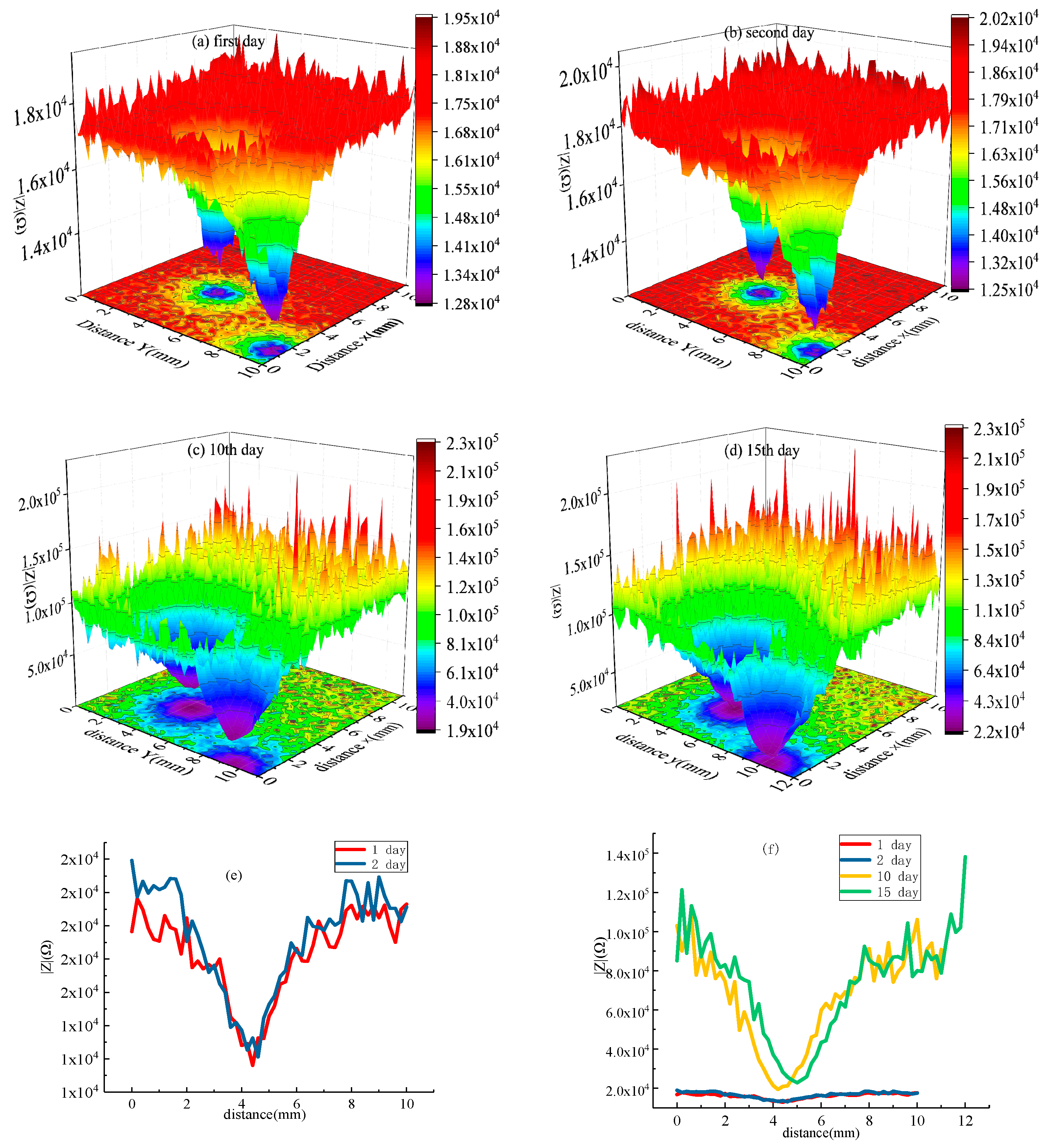
- HDR was not protected by a coating or 1 mm × 10 mm strip coating damage. It was regarded as an open system. The LEIS impedance of the exposed metal matrix was stable, and the corrosion resistance was similar. However, the area of coating damaged was reduced to φ1 mm; with the extension of soaking time, the LEIS impedance of the exposed HDR matrix was 4 times than that of the bare HDR matrix without coating protection after 15 days, and the cumulative effect of corrosion products was obvious, which effectively promoted HDR corrosion resistance in the exposed areas after coating damage.
- (2)
- The coating had a better protective effect on HDR duplex stainless steel. For the thinner coating with 1 mm × 10 mm strip coating damage, compared to that of φ1 mm round coating damage, the LEIS impedance reached a stable state faster. However, the thicker the coating, the greater the LEIS impedance and the better the corrosion protection of the coating.
- (3)
- When the HDR duplex stainless steel surface coating was damaged, the LEIS impedance gradually increased along with the coating damage to the coating, and the further away from the coating damaged, the greater the increase in impedance, and the coating protection effect was obvious. HDR corrosion occurs preferentially on the ferrite structure after the coating is damaged, which is related to the weak phase of the structure and Cl− adsorption.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AI, J.; Hu, Y.; Wang, H.; Liu, X. Research Progress on Corrosion of Duplex Stainless Steel and Its Welded Joint. Surf. Technol. 2022, 51, 77–91. [Google Scholar]
- Yao, J. The Microeletrochemical Behavior and the Stability of Passive Film on 2205 Duplex Stainless Steel; University of Science and Technology Beijing: Beijing, China, 2019; pp. 53–80. [Google Scholar]
- Wang, S.; Hu, Y.; Fang, K.; Zhang, W.; Wang, X. Effect of surface machining on the corrosion behaviour of 316 austenitic stainless steel in simulated PWR water. Corros. Sci. 2017, 126, 104–120. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, D.; Li, Y.; Yang, K.; Gu, T. Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry 2015, 101, 14–21. [Google Scholar] [CrossRef]
- Gao, Y.; Ren, S.; Liu, C.; Wu, X. Application of Micro Zone Electrochemical Technology in Corrosion and Assessment on the Coating Steel Structural. J. Ningbo Univ. Technol. 2021, 33, 13–20. [Google Scholar]
- Zhang, H.; Ye, W.; Sun, B.; Huang, L. Corrosion and prevention of Duplex stainless steel in seawater pipline. Corros. Prot. 2013, 34, 45–48. [Google Scholar]
- Lin, Y.; Wang, X.; Li, Z.; Zhang, X.; Wang, W. Effect of axial and radial micro-structure difference on corrosion resistance of HDR duplex stainless steel tubes. J. Nav. Univ. Eng. 2023, 35, 94–100. [Google Scholar]
- Wang, J.; Qiu, X. Failure behavior of iron red epoxy primer in seawater by LEIS and EIS. Mater. Prot. 2016, 49, 23–25. [Google Scholar]
- Huang, B.; Tang, B.; Feng, Y.; Wu, T.; Zeng, X.; Zhong, J.; Fu, X.; Huang, X.; Zhong, S. Analysis of pipeline stray current interference and corrosion protection coating damage. NDT 2023, 45, 1–6. [Google Scholar]
- Zhang, R.; Xu, A.; Zhang, Z. Corrosion Behavior of Grey Organic Composite Coating for Vehicles at Different Damage Degree. J. Mil. Transp. Univ. 2017, 19, 92–95. [Google Scholar]
- Wang, H.; Lian, B.; Wang, W.; Ma, S. Study on Synergic Protection Effect of Coating and Cathodic Protection for Three-Phase Separator. Coat. Ang Prot. 2023, 44, 54–61. [Google Scholar]
- Balusamy, T.; Nishimura, T. In-Situ Monitoring of Local Corrosion Process of Scratched Epoxy Coated Carbon Steel in Simulated Pore Solution Containing Varying percentage of Chloride ions by Localized Electrochemical Impedance Spectroscopy. Electrochim. Acta 2016, 305–313. [Google Scholar] [CrossRef]
- Gao, J.; Qian, H.; Sun, X.; Guo, W.; Li, X. Effect of seawater pressure on protection properties of epoxy coating used in deep sea. CIESC J. 2015, 66, 4572–4577. [Google Scholar]
- Kovačina, J.; Jegdić, B.; Radojković, B.; Marunkić, D.; Stevanović, S.; Simović, A. Influence of microstructure and roughness level on corrosion resistance of the austenitic stainless steel welded joint. Mater. Corros. 2021, 72, 1–17. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L.; Zhang, J.; Lu, X.; Xu, Y.; Zhang, X.; Shi, R.; Chen, D. Effect of Aluminum Tri-polyphosphate on Corrosion Behavior of Reinforcing Steel in Seawater Prepared Coral Concrete. J. Wuhan Univ. Technol.-Mater. 2019, 34, 906–913. [Google Scholar] [CrossRef]
- Wang, S.; Gu, Y.; Geng, Y.; Liang, J.; Zhao, J.; Kang, J. Investigating local corrosion behavior and mechanism of MAO coated 7075 aluminum alloy. J. Alloys Compd. 2020, 826, 1–13. [Google Scholar] [CrossRef]
- Nardeli, J.V.; Fugivara, C.S.; Taryba, M.; Montemor, M.F.; Benedetti, A.V. Biobased self-healing polyurethane coating with Zn micro-flakes for corrosion protection of AA7475. Chem. Eng. J. 2020, 404, 126478–126492. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, Y.; Zeng, M.; Li, M. Effect of dissolved oxygen on electrochemical corrosion behavior of 2205 duplex stainless steel in hot concentrated seawater. J. Mater. Sci. Technol. 2021, 66, 177–185. [Google Scholar] [CrossRef]
- Leng, A.; Streckel, H.; Stratmann, M. The delamination of polymeric coatings from steel. Part 2: First stage of delamination, effect of type and concentration of cations on delamination, chemical analysis of the interface. Corros. Sci. 1998, 41, 579–597. [Google Scholar] [CrossRef]
- Duan, T.; Xu, L.; Ding, K.; Peng, W.; Hou, J.; Guo, W.; Cheng, W. Corrosion behaviour investigation of 460 low alloy steels exposed in the natural deep-sea environment. Corros. Eng. Sci. Technol. 2019, 54, 485–492. [Google Scholar] [CrossRef]
- Duan, T.; Peng, W.; Ding, K.; Guo, W.; Hou, J.; Cheng, W.; Liu, S.; Xu, L. Long-term field exposure corrosion behavior investigation of 316L stainless steel in the deep sea environment. Ocean. Eng. 2019, 189, 106405. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, S.; Zhang, H.; Zhu, M.; Lei, C.; Li, B. Corrosion behaviors of 316 stainless steel and Inconel 625 alloy in chloride molten salts for solar energy storage. High Temp. Mater. Process. 2020, 39, 340–350. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Q.; Li, X.; Hu, J.; Cao, F. Insight into the triggering effect of (Al, Mg, Ca, Mn)-oxy-sulfide inclusions on localized corrosion of weathering steel. J. Mater. Sci. Technol. 2021, 64, 99–113. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, P.; Zhu, F.X.; Shi, M.H. Effect of Nickel Content on Mechanical Property and Microstructure for Sulfide Stress Corrosion High-Strength Steel. Mater. Sci. Forum 2018, 939, 16–22. [Google Scholar] [CrossRef]
- Lu, X.; Zuo, Y.; Zhao, X.; Tang, Y. The influence of aluminum tri-polyphosphate on the protective behavior of Mg-rich epoxy coating on AZ91D magnesium alloy. Electrochim. Acta 2013, 93, 53–64. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, C.; Wu, H.; Zhao, H.; Mao, F.; Wang, L. Mussel-inspired delivery system for enhancing self-healing property of epoxy coatings. J. Mater. Sci. Technol. 2021, 80, 36–49. [Google Scholar] [CrossRef]
- Ha, H.Y.; Lee, T.H.; Lee, C.G.; Yoon, H. Understanding the relation between pitting corrosion resistance and phase fraction of S32101 duplex stainless steel. Corros. Sci. 2019, 149, 226–235. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, Y.; Liu, L.; Li, M. Pitting and crevice corrosion evolution characteristics of 2205 duplex stainless steel in hot concentrated seawater. J. Solid State Electrochem. 2021, 25, 1555–1565. [Google Scholar] [CrossRef]
- Wang, J.; Shi, W.; Xiang, S.; Ballinger, R.G. Study of the corrosion behaviour of sensitized 904L austenitic stainless steel in Cl- solution. Corros. Sci. 2021, 181, 109234–109241. [Google Scholar] [CrossRef]
- Li, J.; Zhong, X.; Wang, T.; Shang, T.; Hu, J.; Zhang, Z.; Zeng, D.; Hou, D.; Shi, T. Synergistic effect of erosion and hydrogen on properties of passive film on 2205 duplex stainless steel. J. Mater. Sci. Technol. 2021, 67, 1–10. [Google Scholar] [CrossRef]
- Li, L.; Yan, J.; Xiao, J.; Sun, L.; Fan, H.; Wang, J. A comparative study of corrosion behavior of S-phase with AISI 304 austenitic stainless steel in H2S/CO2/Cl− media. Corros. Sci. 2021, 187, 109472–109482. [Google Scholar] [CrossRef]
- Nishimoto, M.; Muto, I.; Sugawara, Y.; Hara, N. Cerium addition to CaS inclusions in stainless steel: Insolubilizing water-soluble inclusions and improving pitting corrosion resistance. Corros. Sci. 2021, 180, 109222–109230. [Google Scholar] [CrossRef]
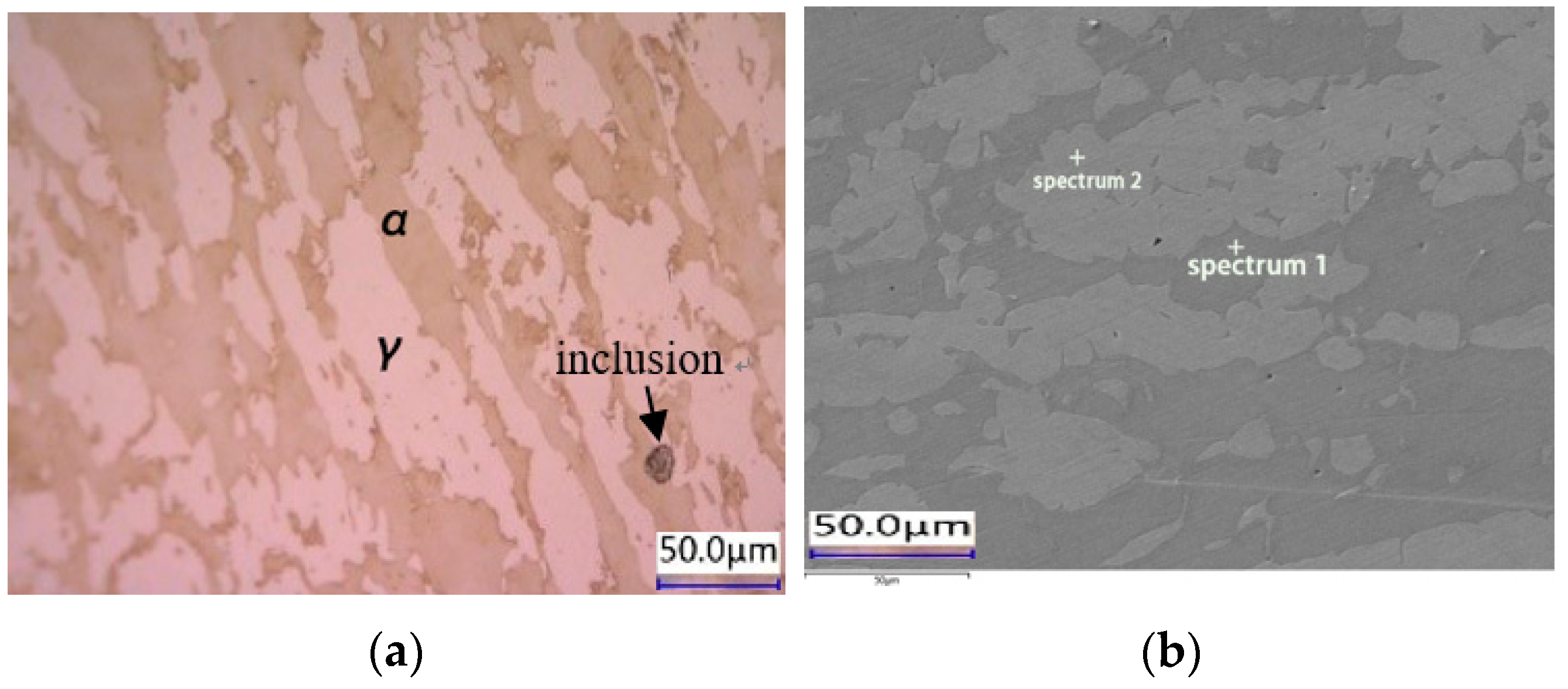


| Chemical Element | C | Mn | Si | S | P | Ni | Cr | Mo | N | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Mass (%) | 0.018 | 1.28 | 0.44 | 0.001 | 0.024 | 6.42 | 24.59 | 2.16 | 0.16 | 64.907 |
| Sample | Structures | Marked | Fe | Cr | Ni | Mn | Mo | Si |
|---|---|---|---|---|---|---|---|---|
| HDR (%) | Austenite (γ) | Spectrum 1 | 64.0 | 27.6 | 4.4 | 1.3 | 2.7 | |
| Ferrite (α) | Spectrum 2 | 66.6 | 23.2 | 6.9 | 1.5 | 1.5 | 0.3 |
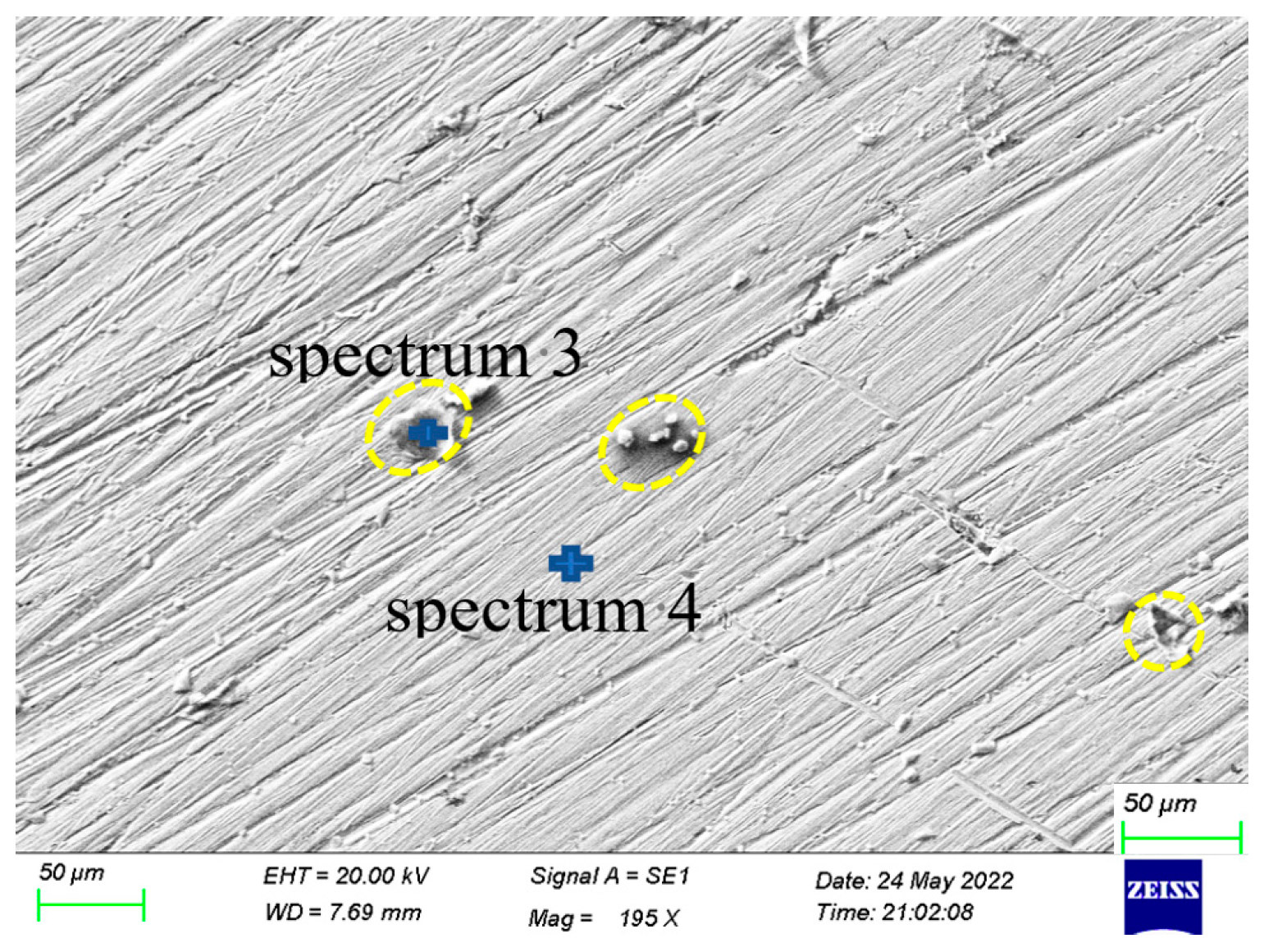
| Marked | Fe | Cr | Ni | Mn | Mo | Si | S | Cl | Na |
|---|---|---|---|---|---|---|---|---|---|
| Spectrum 3 | 62.1 | 27.3 | 3.6 | 1.6 | 0.5 | 0.8 | 0.9 | 3.2 | |
| Spectrum 4 | 65.1 | 23.4 | 7.6 | 1.5 | 1.8 | 0.5 |
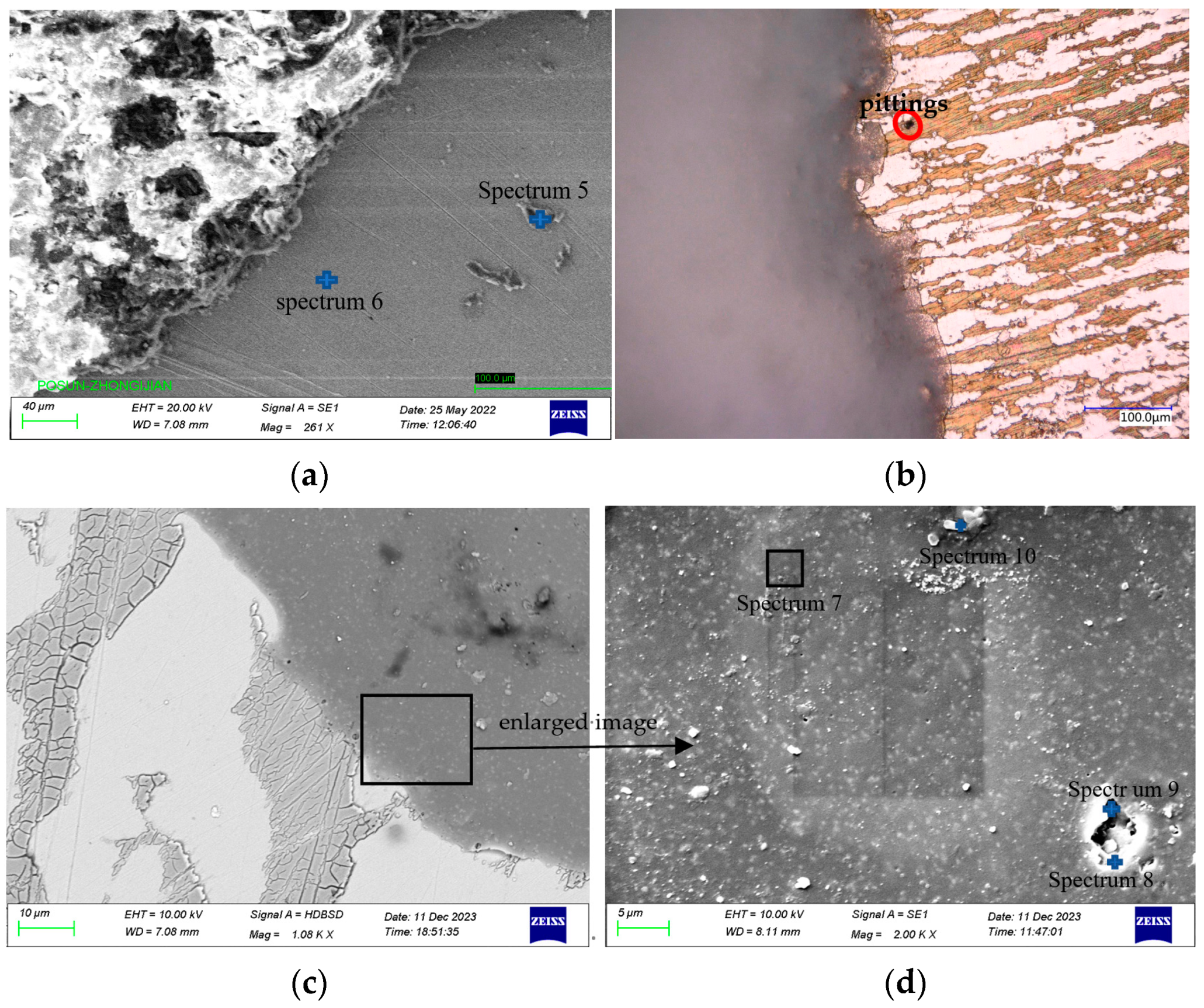
| Marked | Fe | Cr | Ni | Mn | Mo | Si | S | Cl | Na |
|---|---|---|---|---|---|---|---|---|---|
| Spectrum 5 | 51.9 | 19.6 | 4.9 | 1.5 | 0.4 | 0.6 | 9.3 | 11.9 | |
| Spectrum 6 | 66.2 | 23.2 | 8.0 | 1.7 | 0.4 | 0.5 |
| Position | Markered | C | O | Si | Ti | Cl |
|---|---|---|---|---|---|---|
| Well-coated area | Spectrum 7 | 67.3 | 14.8 | 17.8 | ||
| Edges of coating hole edge | Spectrum 8 | 78.5 | 11.7 | 7.9 | 1.9 | |
| Coating holes | Spectrum 9 | 63.1 | 21.3 | 13.8 | 1.8 | |
| White particulate matter | Spectrum 10 | 44.6 | 23.0 | 32.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Li, Z.; Wang, X.; Liu, X.; Chi, J.; Zhang, Z. Effect of Coating Damage on the Micro Area Corrosion Performance of HDR Duplex Stainless Steel. Coatings 2024, 14, 174. https://doi.org/10.3390/coatings14020174
Lin Y, Li Z, Wang X, Liu X, Chi J, Zhang Z. Effect of Coating Damage on the Micro Area Corrosion Performance of HDR Duplex Stainless Steel. Coatings. 2024; 14(2):174. https://doi.org/10.3390/coatings14020174
Chicago/Turabian StyleLin, Yufeng, Zhuying Li, Xiaoqiang Wang, Xin Liu, Junhan Chi, and Zhenhai Zhang. 2024. "Effect of Coating Damage on the Micro Area Corrosion Performance of HDR Duplex Stainless Steel" Coatings 14, no. 2: 174. https://doi.org/10.3390/coatings14020174
APA StyleLin, Y., Li, Z., Wang, X., Liu, X., Chi, J., & Zhang, Z. (2024). Effect of Coating Damage on the Micro Area Corrosion Performance of HDR Duplex Stainless Steel. Coatings, 14(2), 174. https://doi.org/10.3390/coatings14020174




