Abstract
It has previously been shown that epilithic bacterial biopolymers used as coatings influenced the physical properties (surface hardness and color change) at different levels and decreased the surface disaggregation of experimental limestone when evaluated at the laboratory level. A short-term study (30 days) was conducted to evaluate the performance under natural conditions of limestone blocks exposed to tropical conditions of a selected bacterial biopolymer (TM1B-488, after the producing bacterium) and a previously unreported Mayan plant biopolymer known as “Escobilla”, Sida rhombifolia (Malvaceae) used in conservation procedures. Surface hardness (Leeb units) and color (L*a*b* coordinates) were measured and statistically tested for two types of limestone blocks (sound and deteriorated limestone). Both biopolymers increased surface hardness, decreased surface disaggregation, and did not alter color. Escobilla polymer is a carbohydrate-rich biopolymer characterized by tangential filtration, global chemical composition, and monosaccharide composition of hydrolyzed polymer. These results indicate that biopolymers of a heteropolysaccharide nature are constituted by some anionic charge residues that could contribute to surface stabilization and consolidation, but compatibility with traditional building materials (mortars) and longer time of exposure (a year) are necessary to fully assess their applicability in the restoration of architectural heritage.
1. Introduction
Biopolymers, which consist of a conglomerate of biomolecules, such as polysaccharides, proteins, lipids, and nucleic acids, have attracted the interest of the scientific community due to their characteristics, including biodegradability, biocompatibility, renewability, and physical properties. Biopolymer-based composites have recently emerged for building purposes [1,2]. Biopolymers can be recovered from various sources, such as microorganisms, plants, and animals. Animal-based materials, such as blood, urine, and eggs, and also plant biopolymers like straw, bark, cactus juice, and flour have been used historically in the building industry as admixtures to improve the properties of mortars and plasters [3]. With the renewed interest in green materials, the use of biopolymers in the conservation of cultural heritage has gained importance in recent years as a sustainable and environmentally friendly alternative. Aztecs used fermented juice of the nopal cactus Opuntia ficusindica to improve the biopolymers’ plasticity and water absorption [4]. Biopolymers extracted from cactus plants, rice (amylopectin), and tree bark are considered “traditional natural materials”, and their use has transcended generations through oral information and local practice, which has allowed for their biotechnological application in the field of conservation and restoration [5,6,7]. Recently, Rodriguez-Navarro and coworkers [8], using a multidisciplinary approach, elegantly demonstrated that polysaccharide-rich bark extracts from Mayan trees contributed to the production of a calcite cement with meso-to-nanostructural features, matching those of calcite biominerals (e.g., shells). These authors proved the hypothesis that organics (biopolymers) could play a similar toughening role as (bio)macromolecules in calcium carbonate biominerals. These studies show that plant-biobased polymers have properties that are compatible with materials of relevance for the modern building industry and the conservation of historic monuments.
The biopolymer extracted from Sida rhombifolia has been reported in consolidation procedures [9,10]. The biopolymer has been used in two different ways: as a building material for structural stabilization by mixing it with clay soil and volcanic soil and as a natural consolidant by spraying or brushing it into an aqueous suspension [9]. This traditional use has gained popularity in the preservation of various building materials, thanks to the high content of sticky mucoid substances, mainly composed of polysaccharides, present in the leaves of the plant. Although the application of Sida rhombifolia biopolymer has been reported in the humid tropical regions of Central America for bioconsolidation [10,11], to our knowledge, a characterization of the polysaccharide component of this biopolymer has not been carried out.
Similarly, microbial biopolymers in the construction industry have been used in concrete and dry-mix mortars; these include welan gum and xanthan gum, among others, which provide viscosity-enhancing admixtures to achieve high resistance to segregation of concrete [3]. In a recent study employing a photoacoustic technique, we studied the influence of xanthan, microbactan (bacterial), and arabic gum (plant-based) biopolymers in the water transport process of limestone rock. All three biopolymer-coated samples slowed down the transport of water at different levels, probably related to their varying chemistries [12]. The implications for reduced water transport in stone conservation under the influence of biopolymers may include both enhanced and lower deterioration rates. In addition, in another preliminary laboratory study by our group, we evaluated the use of bacterial biopolymers on sound limestone surfaces. The producing bacteria were originally isolated from biofilms colonizing the Mayan sites. This study showed that certain biopolymers improved the physical properties of the stone specimens, decreasing the loss of surface material and hardening the surface. Furthermore, they did not cause substantial chromatic changes in the treated surface. The best-performing biopolymer was chemically characterized and was found to have a probable glycoprotein nature, which was attributed to the consolidation effect [13].
In the present research work, we extended previous findings of the consolidating capacity of the bacterial biopolymer (TM1B-488) that was beforehand tested [13] and included a new plant biopolymer Sida rhombifolia (Malvaceae) and compared with the performance of the conventional inorganic treatment based on (CaOH2) commonly used by restorers, in a short-term study exposed to natural (tropical) conditions. Caneva and Nugari [9] have studied the biodegradability of the biopolymer of Sida rhombifolia known as “Escobilla”, but to our knowledge, there is no previous report of the testing for consolidation purposes. Blocks of limestone of a built wall exposed to tropical conditions in Southern Mexico were chosen for experiments (details below) and showed different degrees of deterioration, with marked visual differences between apparently sound blocks and severely deteriorated (recessed surfaces) blocks. In this study, the consolidating effect was assessed using in situ surface hardness testing, while color change was used as a compatibility (surface alteration) indicator. Surface hardness changes are precursors to erosion and may be utilized to describe stone weathering behavior.
2. Materials and Methods
2.1. Study Site
San Francisco de Campeche is a historic fortified city facing the Gulf of Mexico, located in the Yucatan Peninsula, southern Mexico (Figure 1). It is known for its preserved baroque colonial buildings, military architecture, and historic walled enclosures built to defend against pirate attacks. The city was fortified in the 17th century and is recognized as one of the few walled cities in the Americas; it was declared a UNESCO World Heritage Site in 1999 [14].
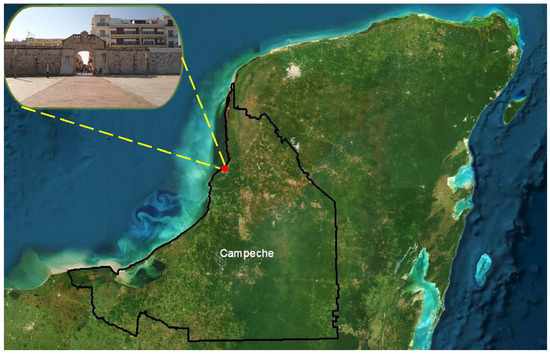
Figure 1.
Geographical location of the study site in the City of San Francisco de Campeche, Southern Gulf of Mexico. Source: Own elaboration using ArcMap 10.5 (ArcGIS 10.5).
Authorization to conduct this study was obtained from the National Institute of Anthropology and History (INAH in Spanish), which is the Mexican official institution in charge of the protection and conservation of cultural heritage of historical relevance. A 50 m long reconstructed section (2014, Adriana Velázquez Morlet Pers. Communication) of the original enclosure known as “Puerta de Mar” was chosen for the experiments (Figure 2).

Figure 2.
Reconstructed section of the fortress wall at “Puerta de Mar” used in this study. The wall is about 8 m in height. Note the heterogeneous nature of weathering states of different building blocks. (A) Sound blocks, red arrow. (B) Block with advanced deterioration (recessed blocks), blue arrow.
This site is located across “Calle 8”, one of the main streets in the historic center of the city of San Francisco de Campeche (19°50′44″ N, 90°32′19″ W; 3 m above sea level) and is located at ≈240 m from the seacoast. It is exposed to a subtropical environment (Table 1). Local transit is limited, and protection from vandalism is ensured, so the deterioration features are most likely the result of ongoing natural weathering processes. In addition, INAH determined this site as the intervention wall, given its relevance.

Table 1.
Weather conditions at San Francisco de Campeche, Campeche, Mexico [15].
2.2. Selection of Experimental Limestone Building Blocks
Limestone from local quarries was used to build the reconstructed wall section. The wall was assessed to determine the observable surface deterioration features, following the classification system of rock deterioration forms proposed by [16]. According to this assessment, blocks were divided into two categories (Figure 3).

Figure 3.
Comparative visual analysis of building block types. (A) Sound limestone block that does not display observable deterioration features. (B) Block with advanced deterioration and surfaces with deep, homogenous material loss. Recession with loss of mass occurs toward the interior of the block in the form of a uniform cavity.
2.3. Preparation of the Test Areas over Experimental Blocks
The building blocks to be used for treatments were divided using a grid system (8 rectangular areas) to delimit the areas. The grid covered a total area of 700 cm2 for each selected block; each rectangular area was 87.5 cm2. Two rectangular areas comprising an area of 175 cm2 were used for the application of each treatment; this area was considered for color measurements, while the upper row was reserved for the evaluation of surface hardness (Figure 4).
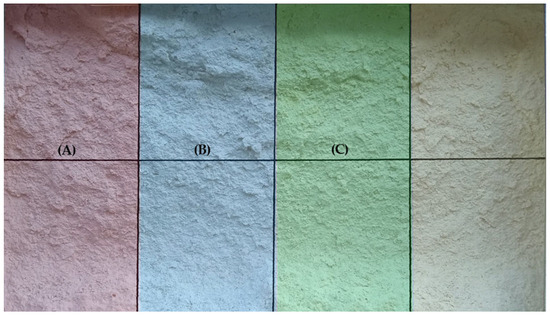
Figure 4.
Surfaces intended for consolidation. Three types of treatments were used: (A) Biopolymer obtained from leaves of the Mayan plant Sida rhombifolia (Escobilla); (B) TM1B-488 Bacterial biopolymer [13]; (C) Conventional treatment: Calcium hydroxide (CaOH) solution.
Briefly, the application of calcium hydroxide (CaOH) solution is traditionally used to consolidate calcareous stones. The formulation was prepared using 1.7 g/L of CaOH in distilled water, according to [17]. Biopolymers were obtained from Sida rhombifolia (Malvaceae), a perennial herb that has been reported in consolidation procedures [9,10]. The extraction of the biopolymer was performed according to a modified protocol described previously [18]. The bacterial biopolymer TM1B-488 was produced and extracted after fermentation, according to our previous study [13]. All treatments were applied using a 6 cm wide brush. A total of 40 applications were performed for 4 consecutive days, in a sequence of 10 applications per day, waiting 2 min between each application or until saturation of the substrate surface with the treatment was observed [10].
2.4. Evaluation of the Effect of Biopolymer-Based Coatings on Surface Hardness of Exposed Limestone Blocks
The physical properties of the hardness of the surface of the blocks were evaluated as indicators of the effectiveness (consolidation) of the coating. The color change was measured to determine compatibility. A consolidation effect is reflected in a change in these properties, an increase in surface hardness on the surfaces of the building blocks concomitant with increased surface cohesion. Compatibility was assessed based on a total color change threshold value of less than 5 for all treatments. The application of the coatings was carried out on 21 April 2021. The measurement campaigns were subsequently carried out after 15 and 30 days of the application of the different treatments (7 May and 21 May 2021), respectively. These measurements correspond to the dry season and were carried out on dry and sunny days. This was performed to avoid high levels of humidity in the rainy season that could alter surface hardness readings [19].
2.4.1. Surface Hardness
Surface hardness was measured using a portable hardness tester (Equotip 3) by means of the impact device “D”, with an impact energy of 11.5 (Newton millimeter). The Single Impact Method (SIM) was used, which consisted of taking several individual measurements at different points (randomly) within the sample area. Thus, a series of 8 readings were taken randomly for each treatment area (87.5 cm2) [19]. Measurements were made before application (Day 0) of the coatings and then carried out 15 and 30 days later.
2.4.2. Analysis of Surface Color Change
The color change induced by the treatments and the total color change (ΔE × ab) were determined by reading the color parameter before (day 0), immediately after, and then 15 and 30 days after the application of the biopolymers.
The colorimetric measurements were carried out with a portable spectrophotometer, Konica Minolta cm-700D, to obtain the CIELAB coordinates (International Commission of Echairage 1976-L*a*b*). The parametric conditions used were illuminant D65, observer 2°, and 10-mm diameter viewing [20]. The CIEL*a*b* coordinates were transformed into RGB channels to obtain a visual representation of the color [21].
The ΔL*, Δa*, and Δb* values needed to calculate the total color change were calculated as described by [20,22]. Finally, using the ΔE × ab formula proposed by La Comision Internacional de l’Echairage 1976-L*a*b* (CIE1976), the total color change was calculated for each surface treated.
where represents the total color difference in each surface after treatment application.
A total of 72 color measurements were carried out randomly per treatment area, that is, in an area of 175 cm2, according to the protocol described by [23].
2.4.3. Statistical Analysis
The size of the effect of the different treatments on the variable surface hardness (Leeb units) (no-normal data) was analyzed by non-parametric confidence intervals using the adjusted bootstrap percentile (BCa) method [24]. We used BCa confidence intervals (1 − α = 0.95) for no-normal data [25]. BCa is a second-order interval that uses an accelerated bias-corrected method to correct bias and skewness in the distribution of bootstrap estimates (10,000 times) [26], resulting in an improvement in the coverage of these intervals. We considered a significant difference between time (days) when bootstrap Bca confidence intervals overlapping of upper or lower limits was approximately 50% or less between treatments [27]. The statistical analyses were calculated using the Groupwise mean function of the R companion package [28], and results were plotted using ggplot 2 [29] in R software (version 4.2.3).
2.5. Biopolymer Extraction
2.5.1. Sida rhombifolia Biopolymer
Biopolymer was obtained from Sida rhombifolia (Malvaceae), a perennial herb also known as “Escobilla”. It is distributed throughout most of Mexico [30] (Figure 5).

Figure 5.
Sida rhombifolia located in an urbanized area of the city of Campeche, Mexico. Own source.
Escobilla is distinguished by having yellow flowers and a calyx with ten ribs at the base (Figure 6). The species Sida rhombifolia is recognized by its leaves that are serrated only on the upper part, arranged in a spiral, not lobed, elongated, often rhombic, but also lanceolate or elliptical; the main ones have blades of more than 2 cm long and thin stipules [31].
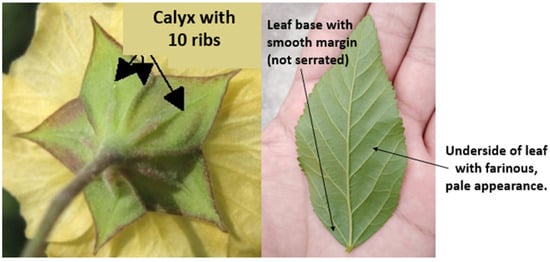
Figure 6.
Characteristics of the calyx of the flower and the underside of the leaf of Sida rhombifolia [31].
The extraction of the biopolymer was performed according to a modified protocol described previously [18]. Dry leaves were powdered (10 g) and depigmented with absolute alcohol in a 1:25 w/v ratio by stirring. Once the sample was depigmented, it was dried for 48 h to eliminate the solvent. Subsequently, a maceration process was carried out in deionized water for 5 h. Finally, the supernatant was precipitated with absolute alcohol, and the crude biopolymer was recovered.
2.5.2. Bacterial Biopolymer TM1B-488
The bacterial biopolymer used in this research work derives from the bacterium TM1B-488, which has a genetic identity of Xanthomonas citri. The bacterial biopolymer TM1B-488 was produced and extracted after fermentation, according to our previous study [13].
2.6. Sida rhombifolia Biopolymer Characterization
2.6.1. Semi-Purification by Ultrafiltration
The crude extract of the biopolymer was semi-purified by enrichment of polysaccharides using a tangential flow filtration (TFF) system using a General Electric Mode-lo-ÄKTAFlux S/2128851. This technique involves the application of membranes with different molecular sizes. The smaller components that pass through the membrane are known as permeate, and the fraction with the larger components that do not pass through the membrane is called retained. With the application of this technique, an increase in the percentage concentration of carbohydrates that make up the biopolymer was obtained.
The solution was then filtered through an ultrafiltration membrane of 500,000 NMWC (Nominal molecular weight cutoff) in order to concentrate the high molecular weight polysaccharides in the retentate. In this way, the final retained product was recovered and stored in the freezer for 24 h, then lyophilized.
2.6.2. Chemical Characterization
Different colorimetric techniques were used. For the total carbohydrate content, the method of [32] was followed by recording the absorbances at a wavelength of 488 nm. The uronic acid content was determined by the method of [33,34], recording absorbances at a wavelength of 525 nm. The determination of total proteins was carried out according to the method of [35], recording the absorbances at a wavelength of 750 nm. In all cases, measurements were performed in triplicate, and the absorbances were recorded using a UV-Visible spectrophotometer (Thermo-Scientific model Evolution 201, Waltham, MA, USA).
2.6.3. High-Performance Liquid Chromatography (HPLC) Analysis
The analysis of the monosaccharides present in Sida rhombifolia biopolymer was performed following a modification of the protocol described by [36]. This method consisted of dissolving 250 μL of the biopolymer samples in 1 mL of 4 M trifluoroacetic acid. The solution was then heated in an oven for 2 h at 120 °C so that the polysaccharides were completely hydrolyzed to monosaccharide fractions. The solution was then filtered through a pore size of 0.22 μm. The filtrate was dried in a nitrogen atmosphere and dissolved in HPLC-grade deionized water injected into the column. The monosaccharide composition of the biopolymers was analyzed by high-performance liquid chromatography (Agilent Infinity 1260, Agilent Technologies, Santa Clara, CA, USA) using a refractive index detector (RID) at a temperature of 45 °C equipped with a ligand exchange column (Agilent Hi-Plex H, 300 × 7.7 mm, Agilent Technologies, CA, USA). The mobile phase was 0.05 M sulphuric acid (H2SO4) at a flow rate of 0.6 mL/min. A sample of 20 μL was injected with a separation time of 26 min. The column temperature was 35 °C. For the identification of monosaccharides, the standards D-(+)-galactose, D-(+)-galacturonic acid, D-glucuronic acid, D-(+)-xylose, L-(+)-arabinose, D-(+)-glucose and L-rhamnose (Sigma® standards, Marlborough, MA, USA) were used.
3. Results
The hardness of a material is quantified as the resistance of the material to an impacting device. Equotip surface hardness data are useful discriminators of the degree of weathering and the impact of consolidant treatments. Equotip hardness values are related to physical properties, such as porosity and density. Stones are intrinsically heterogeneous in regard to these properties. To minimize heterogeneity between different experimental blocks, it was decided to select single-block samples of two levels of weathering.
3.1. Evaluation of Consolidation in Limestone Blocks as Determined by Surface Hardness
Figure 7 illustrates the behavior of surface hardness of sound stone blocks as a function of the type of consolidation and time of testing (15 and 30 days). Measurements over limestone surface coated with the bacterial biopolymer TM1B-488 recorded an initial mean hardness value of 271 Leeb units, with ranges of Bca-CI = 238–304 Leeb units. After 15 days, the hardness decreased to 248 Leeb units (Bca-CI = 222–285). However, after 30 days, the hardness increased to 287 Leeb units (Bca-CI = 253–326). From day 15 to day 30, the average increase was significant (overlap of intervals of 46%). At day 30, the larger mean effect would be expected with 326 Leeb upper limit of the 95% confidence interval.
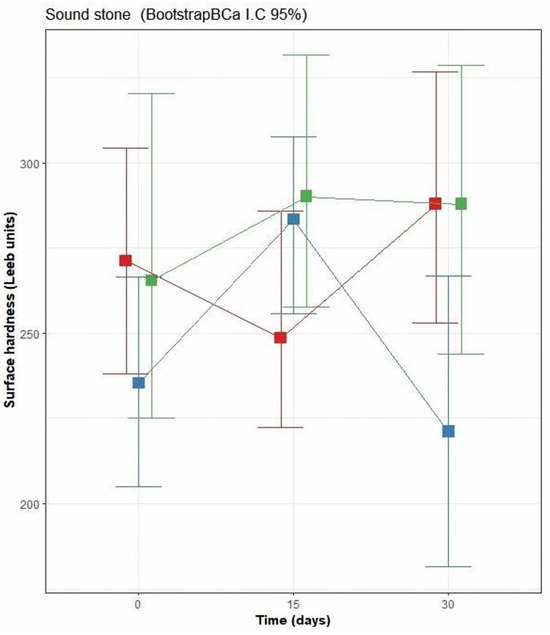
Figure 7.
Behavior of surface hardness in sound stone blocks before application of the coating (0 days) and after 15 and 30 days. Green box corresponds to Escobilla biopolymer; red box corresponds to bacterial biopolymer TM1B-488, and blue box is the CaOH solution.
This irregular behavior suggests that there is a negligible consolidation effect by the bacterial biopolymer but is also probably attributed to the variability of surface hardness of the surface tested under field conditions. In the case of Escobilla biopolymer-coated limestone surfaces, the surface initially recorded a hardness of 265 Leeb units, with ranges from 225 to 320 Leeb units, and subsequent increase at 15 days, reaching 290 Leeb units (Bca-CI = 257–331), and then followed by a slight decrease at 30 days, with a hardness of 287 Leeb units (Bca-CI = 243–328). From day 15 to day 30, the average increase in hardness was not significant (overlap of intervals of 88%). At day 15, the largest mean effect would be expected with 331 Leeb upper limit of the 95% confidence interval.
In this sense, the Escobilla biopolymer appeared to have a short-term but low bioconsolidating effect on sound stone. The limestone surface coated with the reference consolidant, CaOH, showed a clear trend; at the beginning, it had a hardness of 235 Leeb units with ranges (Bca-CI) from 204 to 266 Leeb units, increasing significantly after 15 days to 284 Leeb units (Bca-CI). CI = 255–307) (overlap of intervals of 12%), but then decreasing significantly at 30 days to 221 Leeb units (Bca-CI = 181–266) (overlap of intervals of 11%).
Figure 8 illustrates the behavior of the surface hardness of coated limestones that present a high degree of weathering. Comparatively, the Leeb hardness values of the weathered limestone were slightly lower than those observed in healthy stone blocks. The decrease in hardness properties is evident as the deterioration becomes more advanced and deeper. Measurements on the limestone surface coated with the bacterial biopolymer TM1B-488 recorded an initial average hardness value of 231 Leeb units, with a range of Bca-CI = 200–265 Leeb units. After application of the coating, an increase in this property was observed after 15 days, reaching 244 Leeb units (Bca-CI = 219–272). However, after 30 days of exposure, the hardness effect decreased, recording a hardness of 236 Leeb units (Bca-CI = 215–257). Regarding the limestone surface coated with Escobilla biopolymer, an initial hardness of 246 Leeb units (Bca-CI = 206–287) was recorded. In addition, an increase in hardness was observed 15 days after the application of the coating, reaching 256 Leeb units (Bca-CI = 227–302). However, this effect decreased after 30 days, reaching a hardness of 247 Leeb units (Bca-CI = 225–267). Both biopolymers showed a similar pattern and an increase in hardness at 15 days, followed by a decrease at 30 days. For both polymers, the overlap of intervals was larger than 50%; thus, the effects were not significant. The surfaces coated with CaOH had an initial hardness of 181 Leeb units, with intervals (Bca-CI) of 159–204 Leeb units. From day 0 to 15, the hardness increased significantly to 221 Leeb units (Bca-CI = 159–244) (overlap of intervals of 11%). After 15 days, the hardness continued progressively until 30 days, reaching a hardness of 224 Leeb units (Bca-CI = 205–247), not significantly showing the lowest average expected values for all time points.
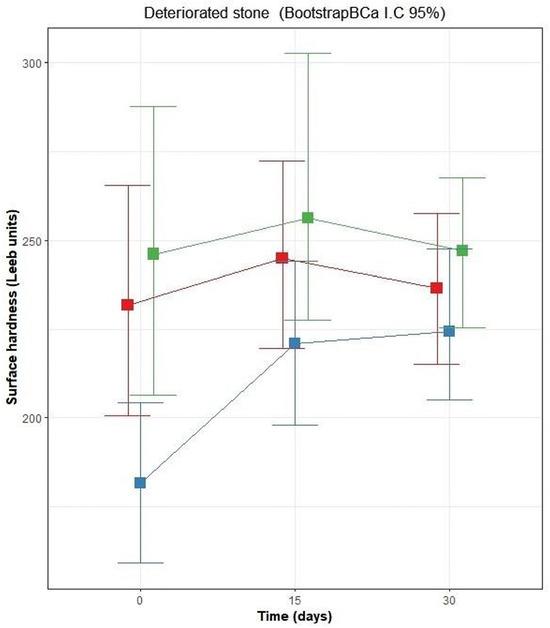
Figure 8.
Behavior of surface hardness in deteriorated stone blocks before application of the coating (0 days) and after 15 and 30 days. Green box corresponds to Escobilla biopolymer; red box corresponds to bacterial biopolymer TM1B-488, and blue box is the CaOH solution.
3.2. Color Changes
Table 2 shows the color variation in the sound stone blocks. The surface treated with CaOH initially presented the following values in the CIELab* coordinates: 86.07 in L*; 1.43 in a*; and 4.26 in b*. After application of the coating, a slight decrease was observed in the L* values, which represented luminosity, and in b* values, referring to the yellow-blue color. The color change was more notable at 30 days, and a more pronounced decrease in L* and an increase in b* were noted, highlighting the color difference. Regarding the surfaces treated with the Escobilla biopolymer, the initial values were 85.95 in L*, 1.53 in a*, and 10.05 in b*. The most notable changes occurred immediately after coating application (84.46 in L*, 1.59 in a* and 12.75 in b*) and after 30 days (81.77 in L*, 1.95 in a* and 12.72 in b*), observing a reduction in the luminosity parameter (L*) and an increase in the b* parameter, associated with the yellow–blue color, with a prevalence in the yellow spectrum. In the case of the surfaces treated with the bacterial biopolymer, the initial values were 85.97 in L*, 1.50 in a*, and 9.91 in b*. The greatest variation was observed after application at 30 days (81.26 in L*, 2.05 in a*, and 13.23 in b*), with the L* and b* coordinates presenting the greatest changes.

Table 2.
Comparison of the color of sound stone blocks before, after, and 15 and 30 days after applying coatings.
Table 3 shows the color variation for the deteriorated blocks. The surface coated with CaOH recorded the following initial values: 87.47 in L*; 0.66 in a*; and 7.75 in b*. After its application, the values of L* and b* began to decrease and increase, respectively. The most notable change occurred after 30 days (82.82 in L*, 1.37 in a*, and 10.22 in b*), in which the three parameters, L*, a*, and b*, were altered, making the color change more noticeable. Regarding the surfaces treated with the Escobilla biopolymer, the initial values were 86.71 in L*, 0.69 in a*, and 7.83 in b*. The changes were very variable throughout the exposure time, but the color changes at 15 (86.21 in L*, 0.74 in a* and 8.62 in b*) and 30 days (82.59 in L*, 1.43 in a* and 10.56 in b*) were more notable. In this last exposure, the luminosity parameter (L*) decreased by four units, and a* and b* increased by one and three units, respectively, compared to the initial records. In the case of limestone surfaces coated with the bacterial biopolymer TM1B-488, the initial values were 87.32 in L*, 0.65 in a*, and 7.87 in b*. The greatest variation in these parameters was observed at 30 days (83.60 in L*, 1.29 in a*, and 9.93 in b*), with a decrease in four units in L* and an increase in one and two units in a* and b*, respectively, in relation to the initial records.

Table 3.
Comparison of the color of deteriorated stone blocks before, after, and 15 and 30 days after applying coatings.
Table 4 details the total color change values (ΔE) recorded after 30 days for the stone building blocks. The surfaces coated with CaOH presented values of ∆E = 4.76 and 8.19 in sound and deteriorated blocks, respectively. The values of ∆E = 6.09 and 2.26 were obtained for the surfaces on which the Escobilla biopolymer was applied for the sound and deteriorated blocks, respectively. For the surfaces coated with the bacterial biopolymer, the total color change was ∆E 6.32 and 7.33 for the sound and deteriorated blocks, respectively. The greatest heat changes occurred for the deteriorated stone blocks, except for the surfaces coated with the Escobilla biopolymer.

Table 4.
Interpretation of ΔE values obtained to determine the color change in surfaces after application of treatments [37].
The variability of the total color change can be interpreted as follows [37]. Compared to sound stone blocks, for surfaces coated with CaOH, the color difference is perceptible to an inexperienced observer. For surfaces treated with biopolymers, the color difference is clearly perceptible. In relation to the deteriorated stone blocks, for the surfaces coated with CaOH and the bacterial biopolymer, the color difference is clearly perceptible. On the other hand, for the surface coated with the S. rhombifolia biopolymer, the color difference is only perceptible to an expert observer. In general terms, most treatments exceeded the acceptability threshold (∆E = 5) after 30 days of exposure. However, it is important to note that factors such as humidity, porosity, and surface heterogeneity can influence measurements. With the results obtained, it can be determined that the color change caused by the application of the coatings is evident; however, the magnitude of the change will be determined by the composition of the coating used, as well as the characteristics of the surface of the material where it is applied.
3.3. Biomolecular Characterisation of Escobilla (Sida rhombifolia) Biopolymer
Spectrophotometric determinations revealed the dominance of polysaccharides of the Sida rhombifolia biopolymer. The results of the chemical characterization (Table 5) showed that the biopolymer recovered from the crude biopolymer had a total carbohydrate concentration of 34%. It was observed that they are mostly made up of charged sugars (27%) and a small portion of protein (approximately 14%). However, the carbohydrate concentration was not comparable with that reported by [18], who reported a carbohydrate composition of 64%–75% for this plant species. This discrepancy could be related to the differences in methods employed.

Table 5.
Percentage chemical characterization of Sida rhombifolia biopolymer.
Therefore, in order to obtain a biopolymer of higher purity, which would allow us to approach the values reported in the literature, the biopolymer was semi-purified by an ultrafiltration system, increasing the percentage concentration of carbohydrates and then chemically characterized. Table 5 compares the chemical composition concentrations obtained from the crude biopolymer and the semi-purified biopolymer. It is important to mention that the ultrafiltration system generates two fractions: the permeate, where the smaller molecules are concentrated, and the retentate (according to the membrane size), where a conglomerate of larger molecules is collected. The highest concentration of total carbohydrates was quantified in the retained biopolymer (63%), while in the permeate (36%), the latter is close to the concentration obtained from the organic extraction (34.1%). Similarly, it was determined that the sugars with charge (uronic acids) are those that predominate, 46% for the retained biopolymer and 26% for the permeate. Finally, the protein concentration increased in the retained biopolymer by 24%, with a lower concentration than that obtained in the permeate (9.23%).
The proportion (percentage content) of the chemical fraction not detected by the colorimetric tests may be associated with the cell debris or be present as other molecules, such as lipids, extracellular nucleic acids, or low molecular weight molecules that could be present.
On the other hand, monosaccharide content analysis of Sida rhombifolia biopolymer was determined by HPLC after hydrolysis with trifluoroacetic acid. The standards for glucose, arabinose, rhamnose, galacturonic acid, and glucuronic acid appeared individually at retention times of 8.46, 11.85, 10.85, 9.59, and 9.23, respectively (Figure 9). In the case of xylose, galactose, and mannose, the peaks appear overlapping and are observed at a retention time of 10.39.
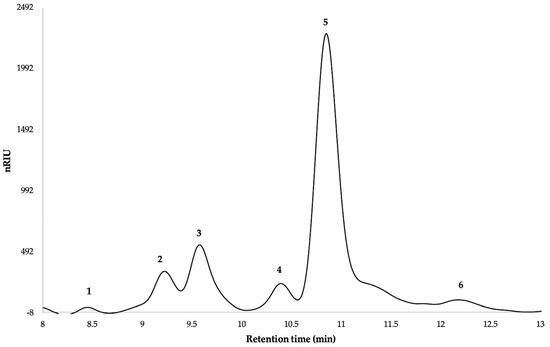
Figure 9.
Chromatographic profile of monosaccharides standards. Components are as follows: 1. Glucose; 2. Glucuronic acid; 3. Galacturonic acid; 4. Overlapping in Xylose, Galactose, or Mannose; 5. Rhamnose; 6. Arabinose.
It was observed that the biopolymer of Sida rhombifolia is mainly composed of glucose (1.08%), glucuronic acid (8.26%), galacturonic acid (15.47%), and rhamnose (69.54%), the latter being the major component (Figure 10). The presence of one component was also observed at retention time 10.392, which could correspond to monosaccharides of xylose, galactose, or mannose (5.6%), whose signals, in general, tend to overlap with each other as they have a similar retention time. The presence of arabinose was not detected.
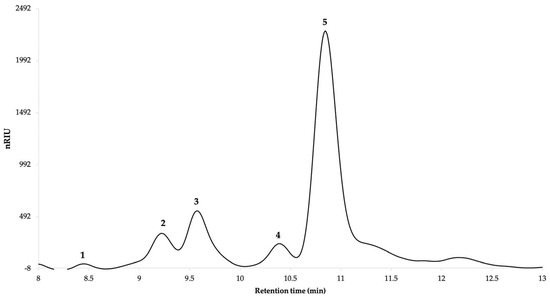
Figure 10.
Chromatographic profile of monosaccharides of Sida rhombifolia biopolymer. Components are as follows: 1. Glucose; 2. Glucuronic acid; 3. Galacturonic acid; 4. Overlapping in Xylose, Galactose, or Mannose; 5. Rhamnose.
In this sense, the analysis of the hydrolyzed monosaccharides demonstrated that the biopolymer is a heteropolysaccharide with some residues with an anionic charge.
4. Discussion
Limestone is particularly prone to surface exfoliation and subsequent recession [38]; this was the type of weathering observed in the exposed testing wall of Campeche, Mexico. This deterioration form was prevalent in this wall, despite the fact that it was rebuilt less than 10 years before this study was carried out, and is likely related to the combined action of thermal stress (high light levels of solar irradiation) and salt spray, typical of a coastal environment [39] and is consistent with the expected weathering due to the prevailing microenvironmental conditions (See Table 1). In our previous study, a preliminary assessment was conducted to assess the influence of bacterial biopolymers on the physical properties of experimental limestone blocks, determining the surface consolidation effect based on the peeling test (disaggregation) and hardness measurements. Leeb hardness (HL) was 406.7 ± 27.2 [13]. Comparatively, the evaluation performed with samples of sound stone under laboratory conditions, showed toward those of this study, increased surface hardness after bioconsolidation, ranging from 34% (Biopolymer TM1B-488) to 32% (Biopolymer TM1B-489), to 19% (Biopolymer TM1B-464), and to 10% (Biopolymer TM1B-349) as a bioconsolidating effect. It is important to emphasize that the Leeb hardness values recorded in the previous study with sound limestone were higher than the values found in the field in the present study, both for unaltered (230–270 Leeb units) and deteriorated blocks (180–250 Leeb units). These values of hardness are consistent with the varying levels reported for limestone exposed to the environment [40] and are in agreement with the understanding that weathering stress history can be reflected in surface hardness evolution. After consolidation, the weathered areas (Figure 8) responded to the different treatments, in particular, the surfaces treated with Excobilla biopolymer in a more pronounced manner than the sound limestone surfaces. The effect of time was not evident, however, as the influence of time was not durable after 30 days. Better performance over weathered surfaces than on sound limestone blocks is expected as the consolidants provide cohesion of flakes, spalled areas, and granular disintegration and, thus, contribute to mechanically reinforced stone. At this point, it is not possible to determine if this is a temporal evolution of the protecting effect or the result of the quantitative variability of treated substrata.
It is important that any substance applied to the surface of a historic property of cultural importance does not alter its appearance; this is usually determined by a change in color. Environmental (rainfall), anthropogenic (pollution), and/or biological (microbial colonization) factors influence the aesthetics of a surface, for example, limestone. The key coordinates in this study corresponded to b* (related to the color yellow to blue) and L* (luminosity). The decreasing b* coordinate caused a slight yellowish color on the surface that might be related to the loss of integrity of the polymer, while the values of the L* coordinate remained constant during the evaluation period. Melo et al. [41] demonstrated that photodegradation by ultraviolet irradiation of resins used in the conservation of stones slightly alters their coloration. On the other hand, Kaplan et al. [42] demonstrated, like Melo and colleagues, that the photodegradation of commercial polymers tends to modify the coloration of limestone, making it whiter. This is mainly due to the modification of the L* coordinate, resulting from the cleavage of polymer chains, the breaking of polymer bonds, and the formation of volatile products. The values of these coordinates can be interpreted in the total color change.
Previously, we showed that bacterial polymers at the laboratory scale level did not influence the color regime. In this case, our treatments did not cause a significant color change to the limestone samples; the total color change was below the threshold value of ∆E < 5, indicating a non-significant color alteration on the limestone surfaces over time. This finding is interesting as it shows that bacterial biopolymer-based coatings are compatible with limestone from the user’s point of view, an important aspect to consider in the color stability of polymer-treated surfaces. Kaplan et al. [42] showed that synthetic polymeric coatings of Paraloid B72 modified the surface color of rocks, with values of ∆E being above the permissible threshold. Our biopolymer-coated limestone did not induce a significant chromatic alteration, at least after 30 days, but the results of [42] suggest that this time should probably be extended.
Given the partial chemical characterization of the Escobilla biopolymer, it suggested a probable mucilage caused by hydrocolloids that are normally made up of neutral monosaccharides such as arabinose, galactose, glucose, mannose, xylose, and various uronic acids [43]. The monosaccharide composition of polysaccharides is essential to elucidate the chemical properties and to better understand the structure–activity relationship. HPLC separation is one of the best techniques for the analysis of these sugars since it is capable of simultaneously determining neutral, acidic, and basic sugars [44]. The composition of the polysaccharide was important because the presence of two uronic acids was confirmed, which are known to be able to interact with Ca+ ions through their hydroxyl groups, forming egg-box type structures and, in some cases, forming gels [45].
This mucilaginous (sticky) feature likely contributed to higher adherence to the limestone surfaces, contributing to surface stabilization and hardening. Moreover, it has been reported that mucilage, such as that of the cactus, which also has galacturonic acid in its structure, can interact with Ca(OH)2 crystals, having positive effects on the microstructure of lime mortars [46]. Something similar could be happening with the coating used in this work on limestone rocks.
Restorers, as a very relevant, experienced working group, are generally reluctant to use synthetic consolidants because of past experience showing that synthetic polymeric consolidants are not readily reversible and may also pose health risks to humans. This is one of the main reasons supporting the increasing interest in using bio-based materials as potential novel consolidants. Biopolymers and concomitant biomineralization can be simultaneously operating, paralleling biofilms that can colonize the rock and induce the formation of CaCO3 within the porous or via the production of extracellular biopolymers with binding properties [47,48,49].
5. Conclusions
Lime mortars (as surfaces) have been historically modified with the addition of organic additives (plant polymers) in Mayan building constructions, aiding in restoring and protecting historical buildings. These admixtures have improved the physical properties and induced hardening of surfaces, making materials less susceptible to weathering under tropical environments. Short-term evaluation of biopolymers, both bacterially sourced and plant-based, showed a protective but short-lived role. The intrinsic variability of limestone (surface hardness) impeded the assessment when the protective role was evident. The plant-based (Escobilla) polymers, such as heteropolymers (as per the biochemical analysis), had a higher consolidating effect. More detailed biochemical composition studies will help to elucidate the role of the different components of the polymer and the effect of longer-time exposure. This paves the way for their widespread uses in restoration as a modifier and/or consolidant. To our knowledge, no previous research has reported such negligible color changes after coating stones with bacterial biopolymers. It is, therefore, important to develop an effective and environmentally acceptable consolidation treatment for historic limestone monuments.
Author Contributions
Conceptualization, J.C.C.-C. and B.O.O.-M.; methodology, P.A.C.-C.; J.E.P.-S. and A.I.A.-C.; formal analysis, J.L.M.-M.; investigation, M.M.R.-E.; funding acquisition, B.O.O.-M.; project administration, J.C.C.-C. and B.O.O.-M.; writing—original draft preparation, L.A.D.-L., J.C.C.-C. and P.A.C.-C.; writing—review and editing, B.O.O.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a CONAHCYT grant CB-2015-01 257449 “Influencia de tratamientos con nano y biomaterials en la colonización microbiana de roca monumental” [“Influence of treatment with nano- and biomaterials on the microbial colonization of monumental stone”].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article.
Acknowledgments
The authors wish to acknowledge funding from projects Influencia de tratamientos con nano y biomateriales en la colonización microbiana de roca monumental 257449 Fondo SEP—CONAHCYT. We also thank Arglga. Adriana Velázquez Morlet (Instituto Nacional de Antropología e Historia, Campeche) for the authorization to conduct this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Venkataravanappa, R.Y.; Lakshmikanthan, A.; Kapilan, N.; Chandrashekarappa, M.P.G.; Der, O.; Ercetin, A. Physico-Mechanical Property Evaluation and Morphology Study of Moisture-Treated Hemp–Banana Natural-Fiber-Reinforced Green Composites. J. Compos. Sci. 2023, 7, 266. [Google Scholar] [CrossRef]
- Dayo, A.Q.; Babar, A.A.; Qin, Q.-R.; Kiran, S.; Wang, J.; Shah, A.H.; Zegaoui, A.; Ghouti, H.A.; Liu, W.B. Effects of accelerated weathering on the mechanical properties of hemp fibre/polybenzoxazine based green composites. Compos. Part A Appl. Sci. Manuf. 2020, 128, 105653. [Google Scholar] [CrossRef]
- Ivanov, V.; Stabnikov, V. Construction biotechnological plastics. In Construction Biotechnology; Springer: Singapore, 2017; pp. 51–75. [Google Scholar] [CrossRef]
- León-Martínez, F.M.; Cano-Barrita, P.F.d.J.; Lagunez-Rivera, L.; Medina-Torres, L. Study of nopal mucilage and marine brown algae extract as viscosity-enhancing admixtures for cement based materials. Constr. Build. Mater. 2014, 53, 190–202. [Google Scholar] [CrossRef]
- Jáider-Benavides, Y. Los Extractos Vegetales Usados como Aditivos en los Morteros de Cal con Fines de Conservación. Bachelor’s Thesis, Escuela Nacional de Conservación, Restauración y Museografía “Manuel del Castillo Negrete” del Instituto Nacional de Antropología e Historia, Mexico City, Mexico, 2006. [Google Scholar]
- Pérez, N.A.; Charua, D.; Fernández, S. Extracción y purificación del mucílago y goma de nopal para su uso en conservación. Estud. Sobre Conserv. Restaur. Museol. 2015, 2, 156–166. [Google Scholar]
- Otero, J.; Charola, A.E.; Starinieri, V. Sticky rice–nanolime as a consolidation treatment for lime mortars. J. Mater. Sci. 2019, 54, 10217–10234. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Monasterio-Guillot, L.; Burgos-Ruiz, M.; Ruiz-Agudo, E.; Elert, K. Unveiling the secret of ancient Maya masons: Biomimetic lime plasters with plant extracts. Sci. Adv. 2023, 9, eadf6138. [Google Scholar] [CrossRef] [PubMed]
- Caneva, G.; Nugari, M.P. Evaluation of escobilla’s mucilage treatments in the archeological site of Joya de Ceren (El Salvador). In Biodeterioration and Biodegradation in Latin America, 1st ed.; Morales, B.O.O., Gaylarde, C.C., Zapata, J.A.N., Gaylarde, P.M., Eds.; Universidad Autónoma de Campeche: Campeche, Mexico, 2005; Volume 1, pp. 59–64. [Google Scholar]
- Kita, Y. The functions of vegetable mucilage in lime and earth mortars. A Review. In Proceedings of the 3rd Historic Mortars Conference, Glasgow, UK, 9–14 September 2013. [Google Scholar]
- Castellanos, C.; Descamps, F.; Isaura Arauz, M. Joya de Cerén, El Salvador, Plan de Manejo; Consejo Nacional para la Cultura y el Arte (CONCULTURA): Los Angeles, CA, USA; El Salvador and The Getty Conservation Institute: Los Angeles, CA, USA, 2002. [Google Scholar]
- May-Crespo, J.; Ortega-Morales, B.O.; Camacho-Chab, J.C.; Quintana, P.; Alvarado-Gil, J.J.; Gonzalez-García, G.; Chan-Bacab, M.J. Photoacoustic monitoring of water transport process in calcareous stone coated with biopolymers. Appl. Phys. A Mater. Sci. Process. 2016, 122, 1060. [Google Scholar] [CrossRef]
- Camacho-Chab, J.C.; Pereañez-Sacarías, J.E.; Camacho-Chab, P.A.; Gaylarde, C.; Arena-Ortiz, M.L.; Ortiz-Alcántara, J.M.; Chan-Bacab, M.J.; Quintana-Owen, P.; Ortega-Morales, B.O. Influence of bacterial biopolymers on physical properties of experimental limestone blocks. World J. Microbiol. Biotechnol. 2022, 38, 254. [Google Scholar] [CrossRef]
- UNESCO. Available online: https://whc.unesco.org/en/list/895/ (accessed on 11 December 2023).
- Cook, D.C.; Van Orden, A.C.; Reyes, J.; Oh, S.J.; Balasubramanian, R.; Carpio, J.J.; Townsend, H.E. Atmospheric Corrosion in Marine Environments along the Gulf of México. Marine Corrosion in Tropical Environments, ASTM STP 1399; Dean, S.W., Delgadillo, G.H.-D., Bushman, J.B., Eds.; American Society for Testing and Materials: West Conshohocken, PA, USA, 2000; pp. 75–97. ISBN 0-8031-2873-8. [Google Scholar]
- Fitzner, B.; Heinrichs, K. Damage diagnosis on stone monuments–weathering forms, damage categories and damage indices. Acta-Univ. Carol. Geol. 2001, 1, 12–13. [Google Scholar]
- Straulino, L. El fluoruro de sodio, una alternativa para la conservación de roca caliza disgregada. Intervención 2010, 2, 24–33. [Google Scholar] [CrossRef]
- Odalipo, F.Y.; Nwokocha, L.M. Effect of Sida acuta and Corchorus olitorius mucilages on the physicochemical properties of maize and sorghum starches. Asian J. Appl. Sci. 2011, 4, 514–525. [Google Scholar]
- Wilhelm, K.; Viles, H.; Burke, O.; Mayaud, J. Surface hardness as a proxy for weathering behaviour of limestone heritage: A case study on dated headstones on the Isle of Portland, UK. Environ. Earth Sci. 2016, 75, 935. [Google Scholar] [CrossRef]
- Sanmartín, P.; Vázquez-Nion, D.; Silva, B.; Prieto, B. Spectrophotometric color measurement for early detection and moni-toring of greening on granite buildings. Biofouling 2012, 28, 329–338. [Google Scholar] [CrossRef] [PubMed]
- El Halim, M.; Daoudi, L.; El Alaoui El Fels, A. How elemental composition influences the color of igneous and sedimentary rocks: Case of the High Atlas rocks of Morocco. Color Res. Appl. 2022, 47, 475–485. [Google Scholar] [CrossRef]
- Camacho-Chab, J.C.; Ortega-Morales, B.O.; Gaylarde, C.; Pereañez-Sacarías, J.E.; León-Tejera, H.P.; Tun-Che, R.E.; Álvarez-Zapata, R.J.; Almeyda-Cen, A.I.; Talavera-Pech, W.; Illescas-Salinas, J.F. Influence of silver nanoparticles-based coating on calcareous rock surfaces on microbial biofilm colonization in intertidal environments in Campeche, Mexico. Water Air Soil Pollut. 2021, 232, 172. [Google Scholar] [CrossRef]
- Prieto, B.; Sanmartín, P.; Silva, B.; Martínez-Verdú, F. Measuring the color of granite rocks: A proposed prodedure. Color Res. Appl. 2010, 35, 368–375. [Google Scholar] [CrossRef]
- Davison, A.C.; Hinkley, D.V. Confidence Intervals. In Bootstrap Methods and Their Application; Cambridge Series in Statistical and Probabilistic Mathematics; Cambridge University Press: Cambridge, UK, 1997; pp. 191–255. [Google Scholar] [CrossRef]
- Efron, B. Better bootstrap confidence intervals. J. Am. Stat. Assoc. 1987, 82, 171–185. [Google Scholar] [CrossRef]
- Chernick, M.R.; LaBudde, R.A. An Introduction to Bootstrap Methods with Applications to R; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; p. 216. [Google Scholar]
- Cumming, G.; Finch, S. Inference by Eye: Confidence Intervals and How to Read Pictures of Data. Am. Psychol. 2005, 60, 170–180. [Google Scholar] [CrossRef]
- Mangiafico, S.S. Rcompanion: Functions to Support Extension Education Program Evaluation, Version 2.4.34; Rutgers Cooperative Extension: New Brunswick, NJ, USA, 2023. Available online: https://CRAN.R-project.org/package=rcompanion/ (accessed on 29 December 2023).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; pp. 33–74. [Google Scholar]
- Francis, J.K. (Ed.) Sida rhombifolia L. (Malvaceae). In Wildland Shrubs of the United States and Its Territories: Thamnic Descriptions; International Institute of Tropical Forestry, USDA Forest Service: Río Piedras, PR, USA, 2003. [Google Scholar]
- CONABIO. Available online: http://www.conabio.gob.mx/malezasdemexico/malvaceae/sida-rhombifolia/fichas/ficha.htm (accessed on 13 December 2023).
- Dubois, M.; Gille, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Galambos, J.T. The reaction of carbazole with carbohydrates. I. Effect of borate and sulfamate on the carbazole color of sugars. Anal. Biochem. 1967, 19, 119–132. [Google Scholar] [CrossRef]
- Blumerkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1985, 54, 484–489. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, H.; Farr, A.; Randall, R. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Lutfia, F.N.; Isnansetyo, A.; Susidarti, R.A. Chemical composition diversity of fucoidans isolated from three tropical brown seaweed (Phaeophycear) species. Biodiversitas 2020, 21, 3170–3177. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Colour difference ΔE–A survey. Mach. Graph. Vis. 2012, 20, 383–411. [Google Scholar]
- Bonazza, A.; Vidorni, G.; Natali, I.; Ciantelli, C.; Giosuè, C.; Tittarelli, F. Durability assessment to environmental impact of nano-structured consolidants on Carrara marble by field exposure tests. Sci. Total Environ. 2017, 575, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Mottershead, D.; Gorbushina, A.; Lucas, G.; Wright, J. The influence of marine salts, aspect and microbes in the weathering of sandstone in two historic structures. Build. Environ. 2003, 38, 1193–1204. [Google Scholar] [CrossRef]
- Wedekind, W.; Pötzl, C.; López-Doncel, R.; Siegesmund, S. Surface hardness testing for the evaluation of consolidation of porous low bound stones. In Proceedings of the 13th International Congress on the Deterioration and Conservation of Stone, Glasgow, UK, 6–10 September 2016; University of the West of Scotland: Paisley, UK, 2016. [Google Scholar]
- Melo, M.J.; Bracci, S.; Camaiti, M.; Chiantore, O.; Piacenti, F. Photodegradation of acrylic resins used in the conservation of stone. Polym. Degrad. Stab. 1999, 66, 23–30. [Google Scholar] [CrossRef]
- Kaplan, Z.; Böke, H.; Sofuoglu, A.; İpekoğlu, B. Long term stability of biodegradable polymers on building limestone. Prog. Org. Coat. 2019, 131, 378–388. [Google Scholar] [CrossRef]
- Amin, E.S. The mucilages of Hibiscus esculentus (okra or bamia fellahi) and Corchorus olitorius (mulukhia). J. Chem. Soc. 1956, 828–832. [Google Scholar] [CrossRef]
- Dai, J.; Wu, Y.; Chen, S.W.; Zhu, S.; Yin, H.P.; Wang, M.; Tang, J. Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr. Polym. 2010, 82, 629–635. [Google Scholar] [CrossRef]
- Liu, D.; Tang, W.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Monosaccharide composition analysis of polysaccharides from natural sources: Hydrolysis condition and detection method development. Food Hydrocoll. 2021, 116, 106641. [Google Scholar] [CrossRef]
- Ortega-Morales, B.O.; Gaylarde, C.C. Bioconservation of historic stone buildings—An updated review. Appl. Sci. 2021, 11, 5695. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Rodriguez-Gallego, M.; Ben Chekroun, K.; Gonzalez-Muñoz, M.T. Conservation of ornamental stone by Myxococcus xanthus-induced carbonate biomineralization. Appl. Environ. Microbiol. 2003, 69, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Decho, A.W. Overview of biopolymer-induced mineralization: What goes on in biofilms? Ecol. Eng. 2010, 36, 137–144. [Google Scholar] [CrossRef]
- Ivanov, V.; Chu, J.; Stabnikov, V. Basics of Construction Microbial Biotechnology. In Biotechnologies and Biomimetics for Civil Engineering; Pacheco Torgal, F., Labrincha, J., Diamanti, M., Yu, C.P., Lee, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 1, pp. 21–56. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).