3D Porous Sponge/Carbon Nanotube/Polyaniline/Chitosan Capacitive Bioanode Material for Improving the Power Generation and Energy Storage Performance of Microbial Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Sponge Pretreatment
2.2. Preparation of Capacitive Anode Materials
2.3. MFC Construction
2.4. Characterizations and Measurements
3. Results and Discussion
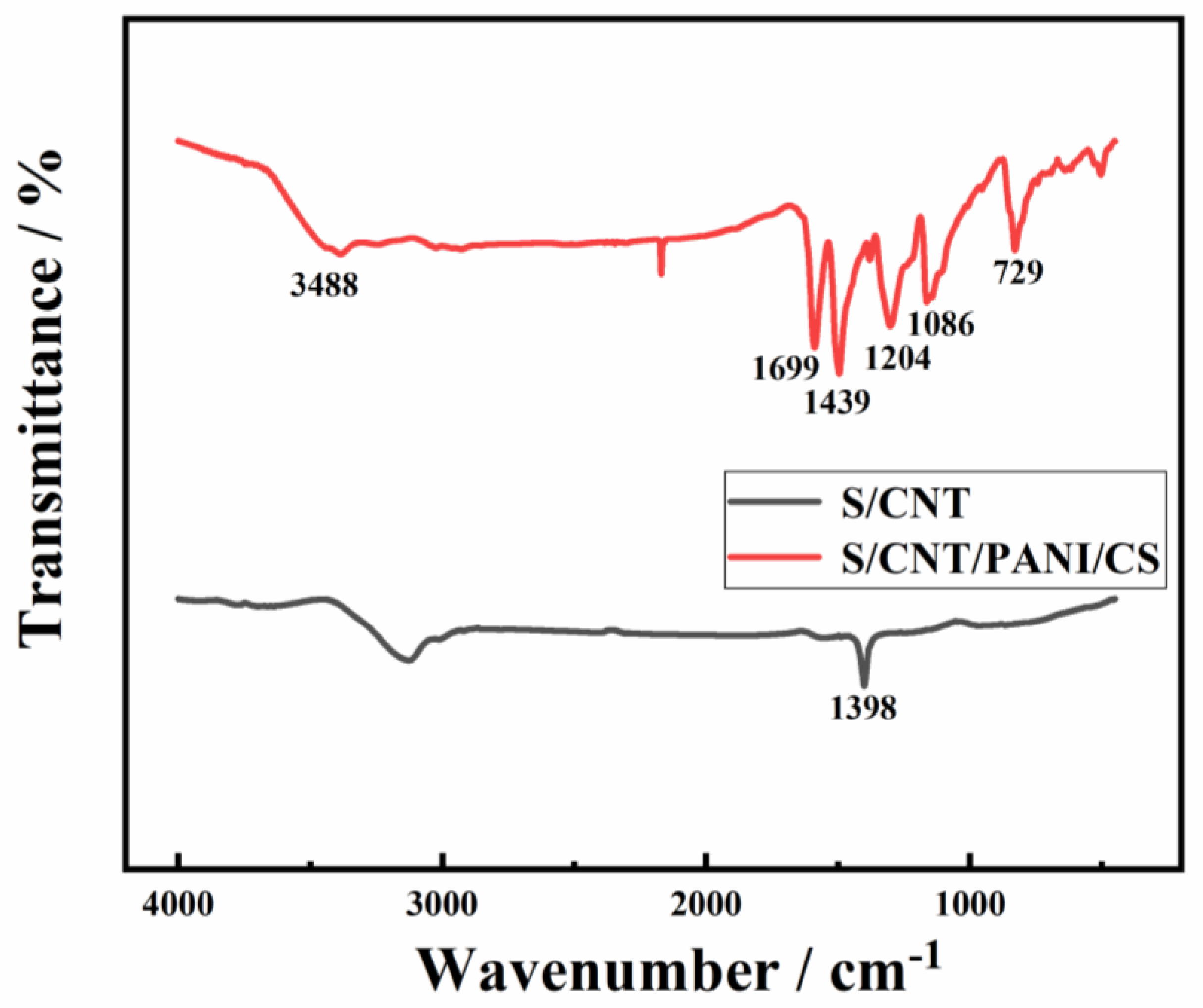
3.1. Physicochemical Characterization of the S/CNT/PANI/CS Electrode
3.2. Performance of MFCs Equipped with S/CNT and S/CNT/PANI/CS Anodes
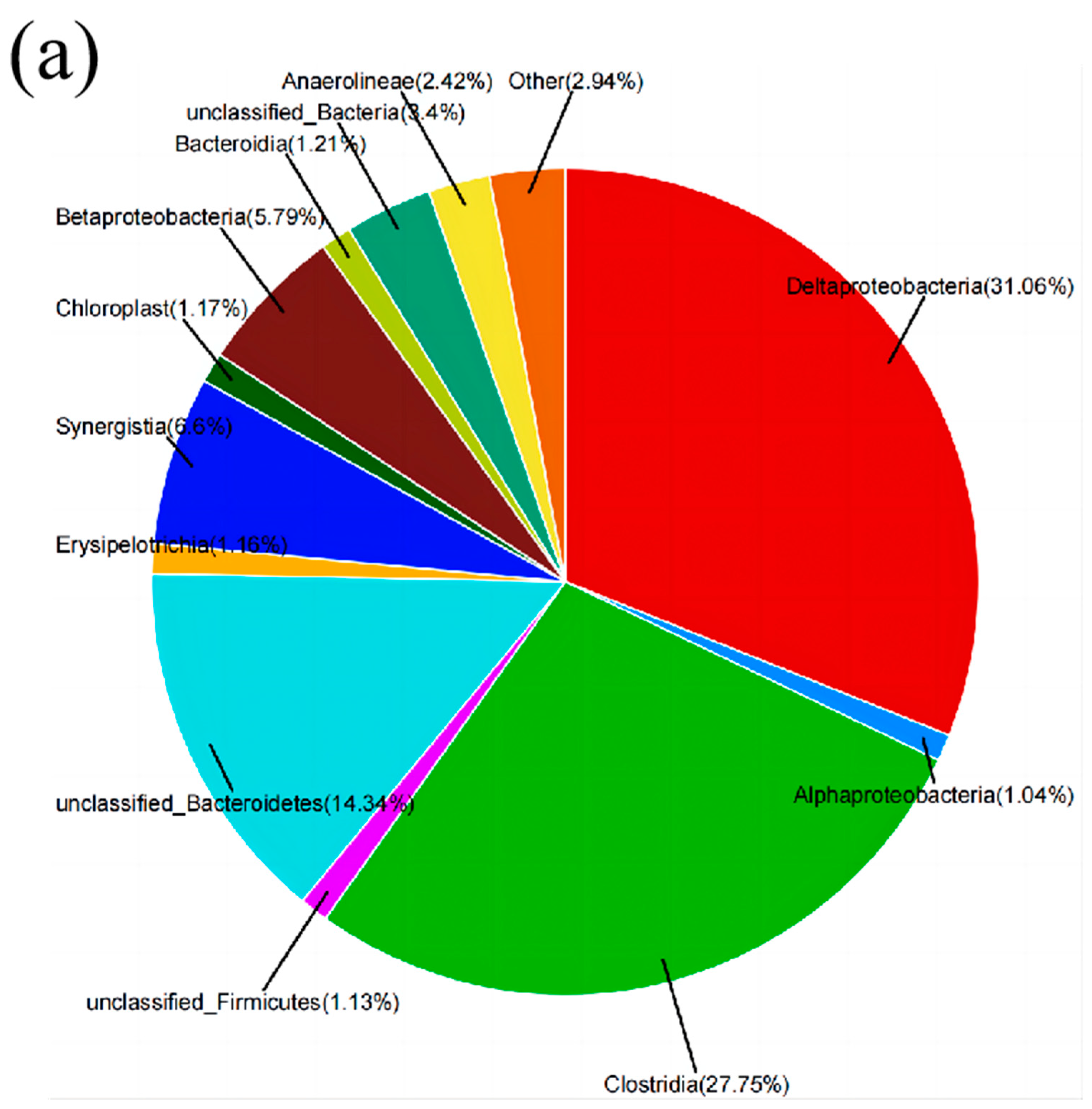
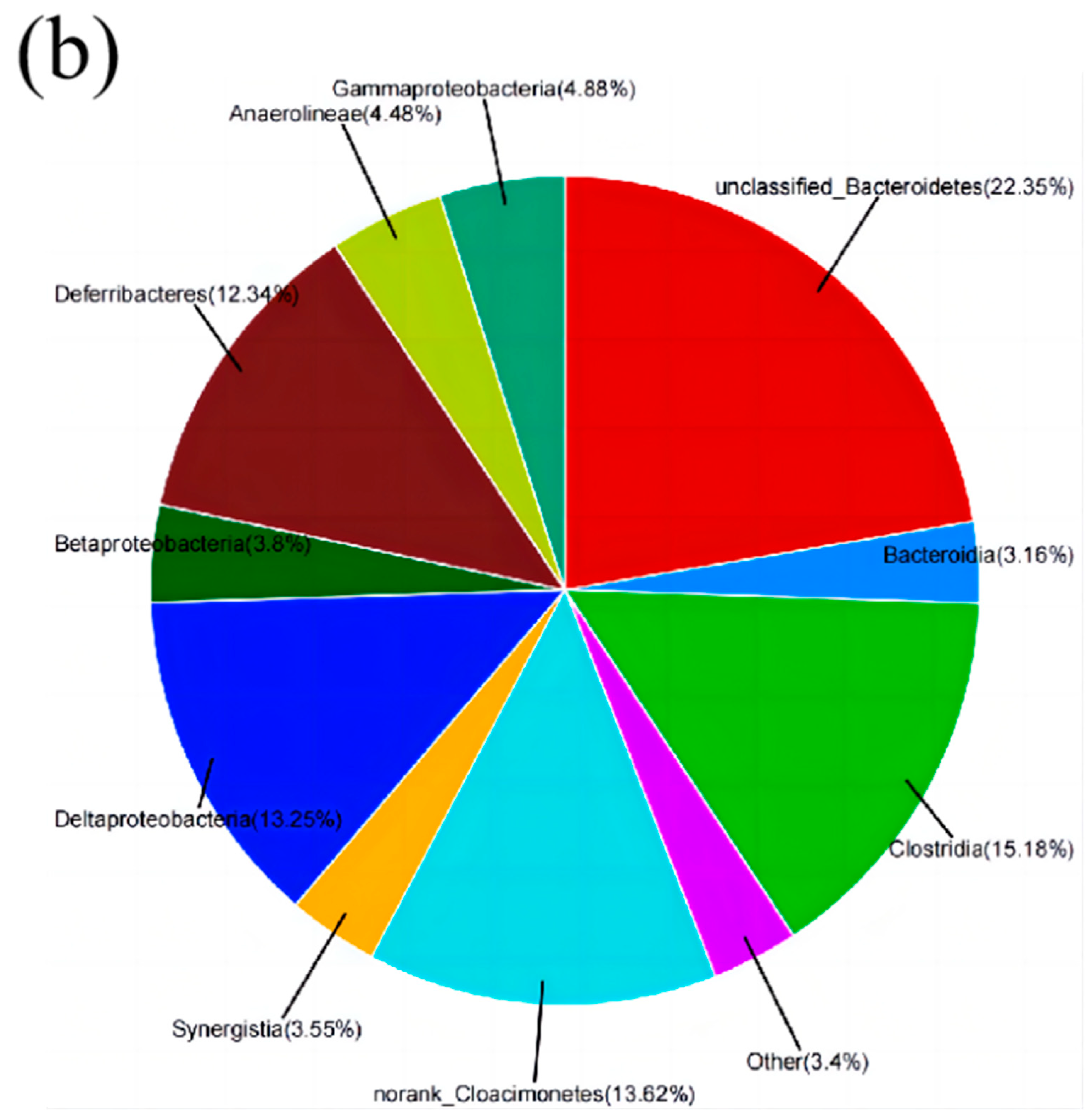
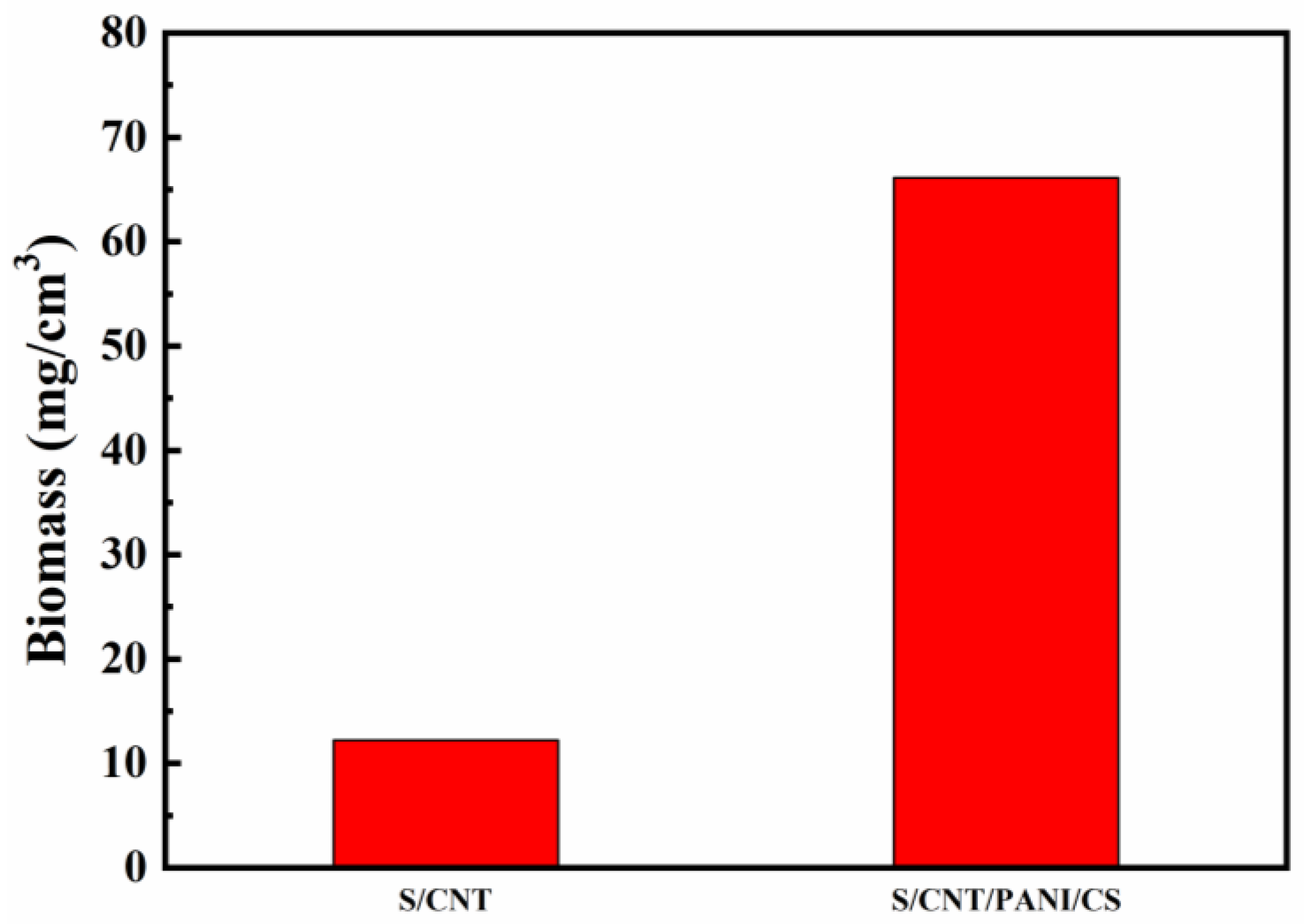
3.3. Biocompatibility Test
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewan, A.; Beyenal, H.; Lewandowski, Z. Intermittent Energy Harvesting Improves the Performance of Microbial Fuel Cells. Environ. Sci. Technol. 2009, 43, 4600–4605. [Google Scholar] [CrossRef] [PubMed]
- Ci, S.; Wen, Z.; Chen, J.; He, Z. Decorating anode with bamboo-like nitrogen-doped carbon nanotubes for microbial fuel cells. Electrochem. Commun. 2012, 14, 71–74. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, P.; Jiang, Y.; Huang, X. Enhanced power generation of microbial fuel cell using manganese dioxide-coated anode in flow-through mode. J. Power Sources 2015, 273, 580–583. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schroder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.; Tang, X.; Li, H.; Du, Z.; Liu, X.; Zhang, Q. Power production enhancement with a polyaniline modified anode in microbial fuel cells. Biosens. Bioelectron. 2011, 28, 373–377. [Google Scholar] [CrossRef]
- Kang, Y.L.; Ibrahim, S.; Pichiah, S. Synergetic effect of conductive polymer poly (3,4-ethylenedioxythiophene) with different structural configuration of anode for microbial fuel cell application. Bioresour. Technol. 2015, 189, 364–369. [Google Scholar] [CrossRef]
- Feng, C.; Ma, L.; Li, F.; Mai, H.; Lang, X.; Fan, S. A polypyrrole/anthraquinone-2,6-disulphonic disodium salt (PPy/AQDS)-modified anode to improve performance of microbial fuel cells. Biosens. Bioelectron. 2010, 25, 1516–1520. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, C.; Zhang, J.; Wu, X. Appropriate mechanical strength of carbon black-decorated loofah sponge as anode material in microbial fuel cells. Int. J. Hydrog. Energy 2016, 41, 23156–23163. [Google Scholar] [CrossRef]
- Tang, J.; Yuan, Y.; Liu, T.; Zhou, S. High-capacity carbon-coated titanium dioxide core–shell nanoparticles modified three dimensional anodes for improved energy output in microbial fuel cells. J. Power Sources 2015, 274, 170–176. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Q.; Chen, Y.; Yin, J.; Duan, T. Enhanced Performance of a Microbial Fuel Cell with a Capacitive Bioanode and Removal of Cr (VI) Using the Intermittent Operation. Appl. Biochem. Biotech. 2016, 180, 1372–1385. [Google Scholar] [CrossRef]
- Peng, X.; Yu, H.; Wang, X.; Zhou, Q.; Zhang, S.; Geng, L.; Sun, J.; Cai, Z. Enhanced performance and capacitance behavior of anode by rolling Fe3O4 into activated carbon in microbial fuel cells. Bioresour. Technol. 2012, 121, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; He, H.; Wang, H.; Zhou, M.; Gu, T. Graphite Felt Anode Modified by Electropolymerization of NanoPolypyrrole to Improve Microbial Fuel Cell (MFC) Production of Bioelectricity. J. Microb. Biochem. Technol. 2013, S12, 004. [Google Scholar]
- Wang, Y.; Zhu, L.; An, L. Electricity generation and storage in microbial fuel cells with porous polypyrrole-base composite modifified carbon brush anodes. Renew. Energy 2020, 162, 2220–2226. [Google Scholar] [CrossRef]
- Zhao, G.; Yin, Z.; Zhang, L.; Wei, X. Direct electrochemistry of cytochrome c on a multi-walled carbon nanotubes modified electrode and its electrocatalytic activity for the reduction of H2O2. Electrochem. Commun. 2005, 7, 256–260. [Google Scholar] [CrossRef]
- Tsai, H.; Wu, C.; Lee, C.; Shih, E.P. Microbial fuel cell performance of multiwall carbon nanotubes on carbon cloth as electrodes. J. Power Sources 2009, 194, 199–205. [Google Scholar] [CrossRef]
- Erbay, C.; Yang, G.; Figueiredo, P.; Sadr, R.; Yu, C.; Han, A. Three-dimensional porous carbon nanotube sponges for high-performance anodes of microbial fuel cells. J. Power Sources 2015, 298, 177–183. [Google Scholar] [CrossRef]
- Wen, Z.; Ci, S.; Mao, S.; Cui, S.; Lu, G.; Yu, K.; Luo, S.; He, Z.; Chen, J. TiO2 nanoparticles-decorated carbon nanotubes for significantly improved bioelectricity generation in microbial fuel cells. J. Power Sources 2013, 234, 100–106. [Google Scholar] [CrossRef]
- Mehdinia, A.; Ziaei, E.; Jabbari, A. Multi-walled carbon nanotube/SnO2 nanocomposite: A novel anode material for microbial fuel cells. Electrochim. Acta 2014, 130, 512–518. [Google Scholar] [CrossRef]
- Zou, Y.; Pisciotta, J.; Baskakov, I.V. Nanostructured polypyrrole-coated anode for sun-powered microbial fuel cells. Bioelectrochemistry 2010, 79, 50–56. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Q.; Chen, Y.; Zheng, H.; Wang, S. Enhanced performance of microbial fuel cell with polyaniline/sodium alginate/carbon brush hydrogel bioanode and removal of COD. Energy 2020, 202, 117780. [Google Scholar] [CrossRef]
- Higgins, S.R.; Foerster, D.; Cheung, A.; Lau, C.; Bretschger, O.; Minteer, S.D.; Nealson, K.; Atanassov, P.; Cooney, M.J. Fabrication of macroporous chitosan scaffolds doped with carbon nanotubes and their characterization in microbial fuel cell operation. Enzym. Microb. Technol. 2011, 48, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, X.; Huang, Y.; Sheng, G.; Wang, S.; Yu, H. Carbon nanotube/chitosan nanocomposite as a biocompatible biocathode material to enhance the electricity generation of a microbial fuel cell. Energy Environ. Sci. 2011, 4, 1422–1427. [Google Scholar] [CrossRef]
- Du, Q.; An, J.; Li, J.; Zhou, L.; Li, N.; Wang, X. Polydopamine as a new modification material to accelerate startup and promote anode performance in microbial fuel cells. J. Power Sources 2017, 343, 477–482. [Google Scholar] [CrossRef]
- Hou, J.; Liu, Z.; Zhang, P. A new method for fabrication of graphene/polyaniline nanocomplex modified microbial fuel cell anodes. J. Power Sources 2013, 224, 139–144. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, S.; Liu, Y.; Tang, J. Nanostructured Macroporous Bioanode Based on Polyaniline-Modified Natural Loofah Sponge for High-Performance Microbial Fuel Cells. Environ. Sci. Technol. 2013, 47, 14525–14532. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xu, Y.; Yao, Z.; Liu, A.; Shi, G. Supercapacitors Based on Flexible Graphene/Polyaniline Nanofiber Composite Films. ACS Nano 2010, 4, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Cabuk, M.; Yavuz, M.; Unal, H.I.; Alan, Y. Synthesis, characterization, and enhanced antibacterial activity of chitosan-based biodegradable conducting graft copolymers. Polym. Compos. 2014, 36, 497–509. [Google Scholar] [CrossRef]
- Choi, C.; Nam, J.P.; Nah, J.W. Application of chitosan and chitosan derivatives as biomaterials. J. Ind. Eng. Chem. 2016, 33, 1–10. [Google Scholar] [CrossRef]
- Deeke, A.; Sleutels, T.H.J.A.; Heijne, A.T.; Hamelers, H.V.M.; Buisman, C.J.N. Influence of the thickness of the capacitive layer on the performance of bioanodes in Microbial Fuel Cells. J. Power Sources 2013, 243, 611–616. [Google Scholar] [CrossRef]
- Deeke, A.; Sleutels, T.H.J.A.; Hamelers, H.V.M.; Buisman, C.J.N. Capacitive Bioanodes Enable Renewable Energy Storage in Microbial Fuel Cells. Environ. Sci. Technol. 2012, 46, 3554–3560. [Google Scholar] [CrossRef]
- Lv, Z.; Xie, D.; Li, F.; Hu, Y.; Wei, C.; Feng, C. Microbial fuel cell as a biocapacitor by using pseudo-capacitive anode materials. J. Power Sources 2014, 246, 642–649. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, C.; Xie, W.; Yang, S.; Hsu, C.; Lin, M.; Jian, W. Nano Approach Investigation of the Conduction Mechanism in Polyaniline Nanofibers. ACS Nano 2011, 5, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, L.; Gan, M.; Fu, S.; Dai, W.; Zhou, T.; Sun, X.; Wang, H.; Wang, H. Free-standing 3D graphene/polyaniline composite film electrodes for high-performance supercapacitors. J. Power Sources 2015, 299, 347–355. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, C.; Bao, S.; Bao, Q. Carbon nanotube/polyaniline composite as anode material for microbial fuel cells. J. Power Sources 2007, 170, 79–84. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Liu, Y.; Hao, C.; Tian, C.; Feng, C.; Lei, Z.; Huang, W.; Zhang, Z. Identification of removal principles and involed bacteria in microbial fuel cells for sulfide removal and electricity generation. Int. J. Hydrog. Energy 2013, 38, 14348–14355. [Google Scholar] [CrossRef]
- Dunaj, S.J.; Vallino, J.J.; Hines, M.E.; Gay, M.; Kobyljanec, C.; Rooney-Varga, J.N. Relationships between soil organic matter nutrients bacterial community structure, and the performance of microbial fuel cells. Environ. Sci. Technol. 2012, 46, 1914–1922. [Google Scholar] [CrossRef]
- Li, D.; Deng, L.; Yuan, H.; Dong, G.; Chen, J.; Zhang, X.; Chen, Y.; Yuan, Y. N, P-doped mesoporous carbon from onion as trifunctional metal-free electrode modifier for enhanced power performance and capacitive manner of microbial fuel cells. Electrochim. Acta 2018, 262, 297–305. [Google Scholar] [CrossRef]



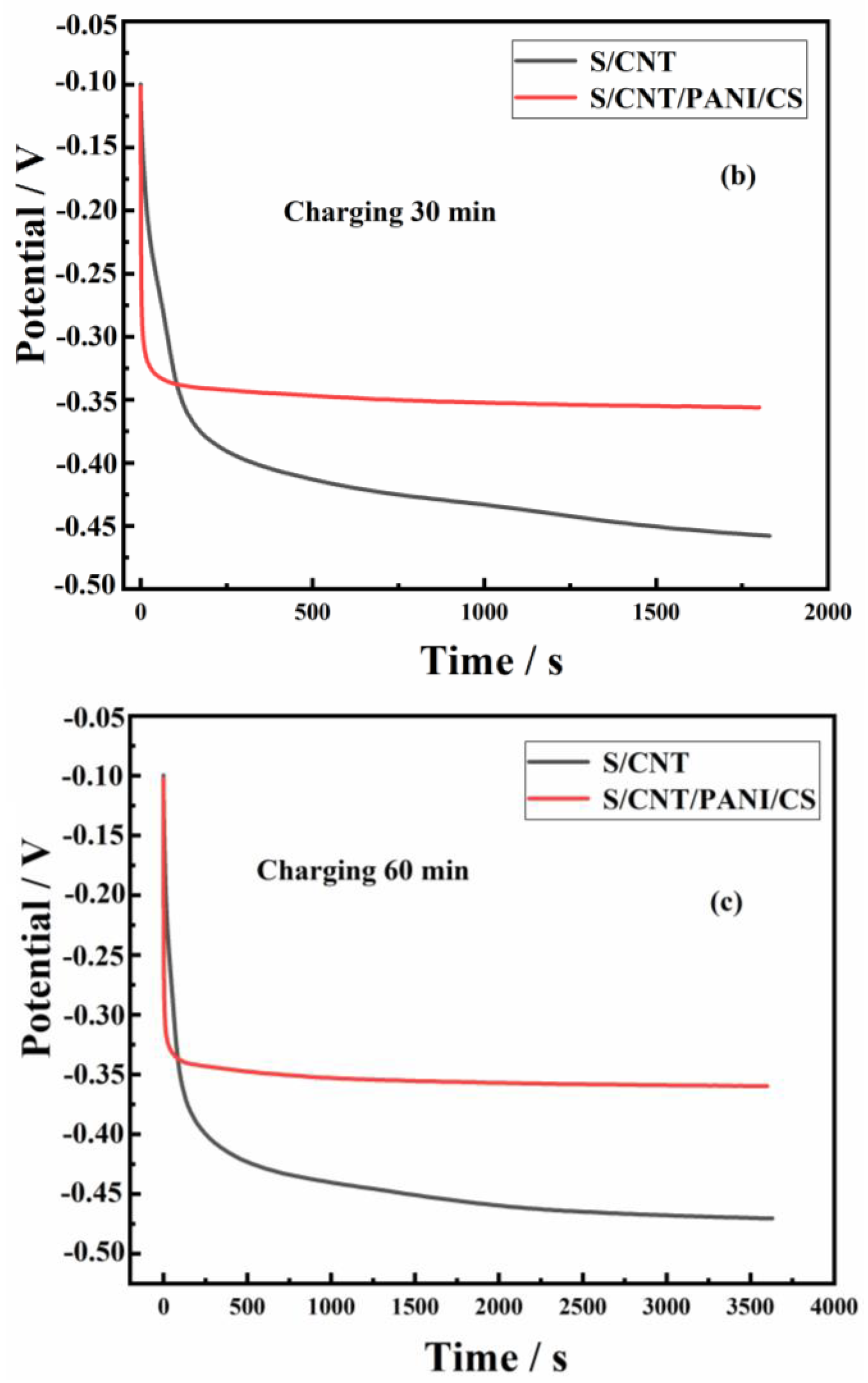
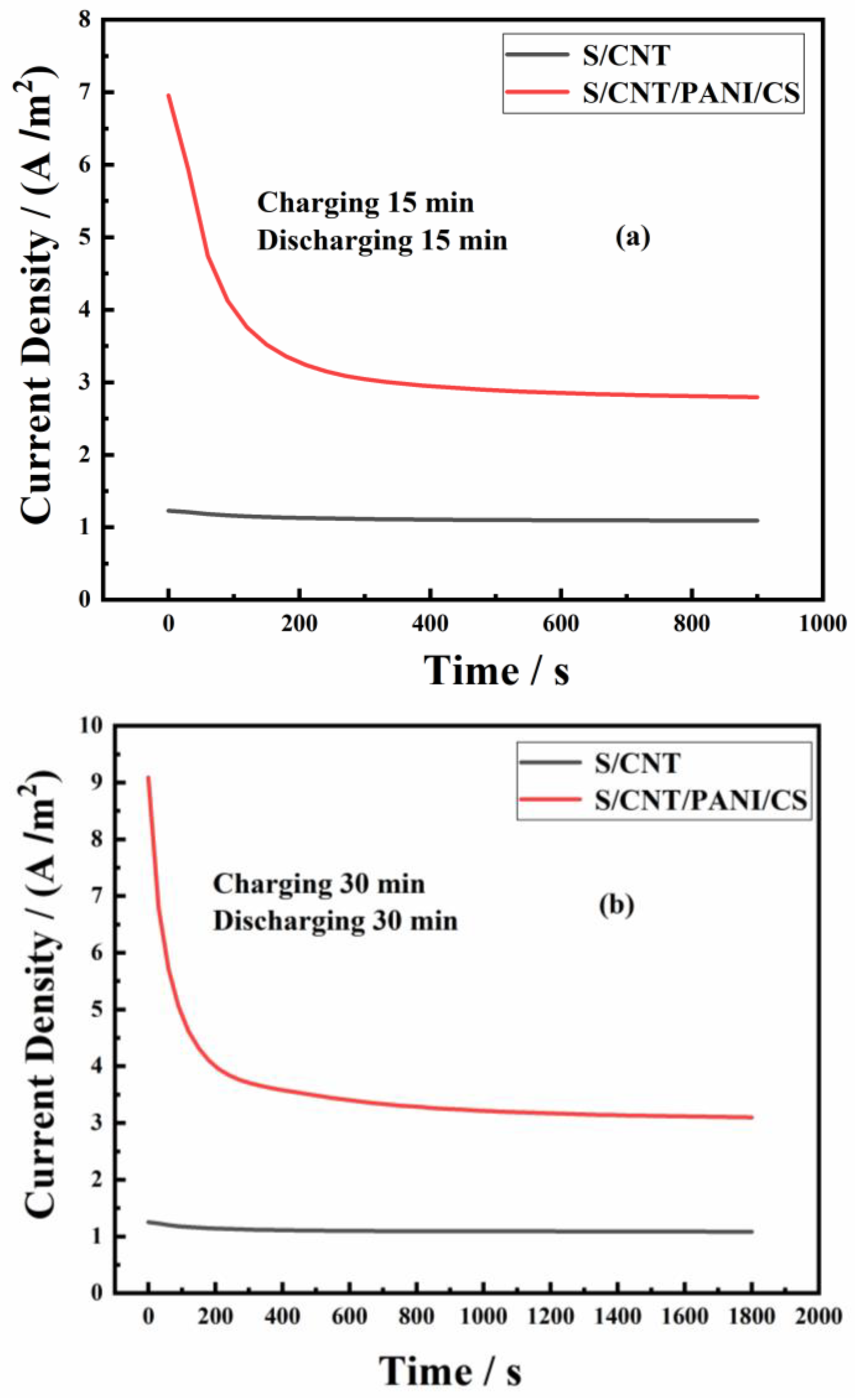
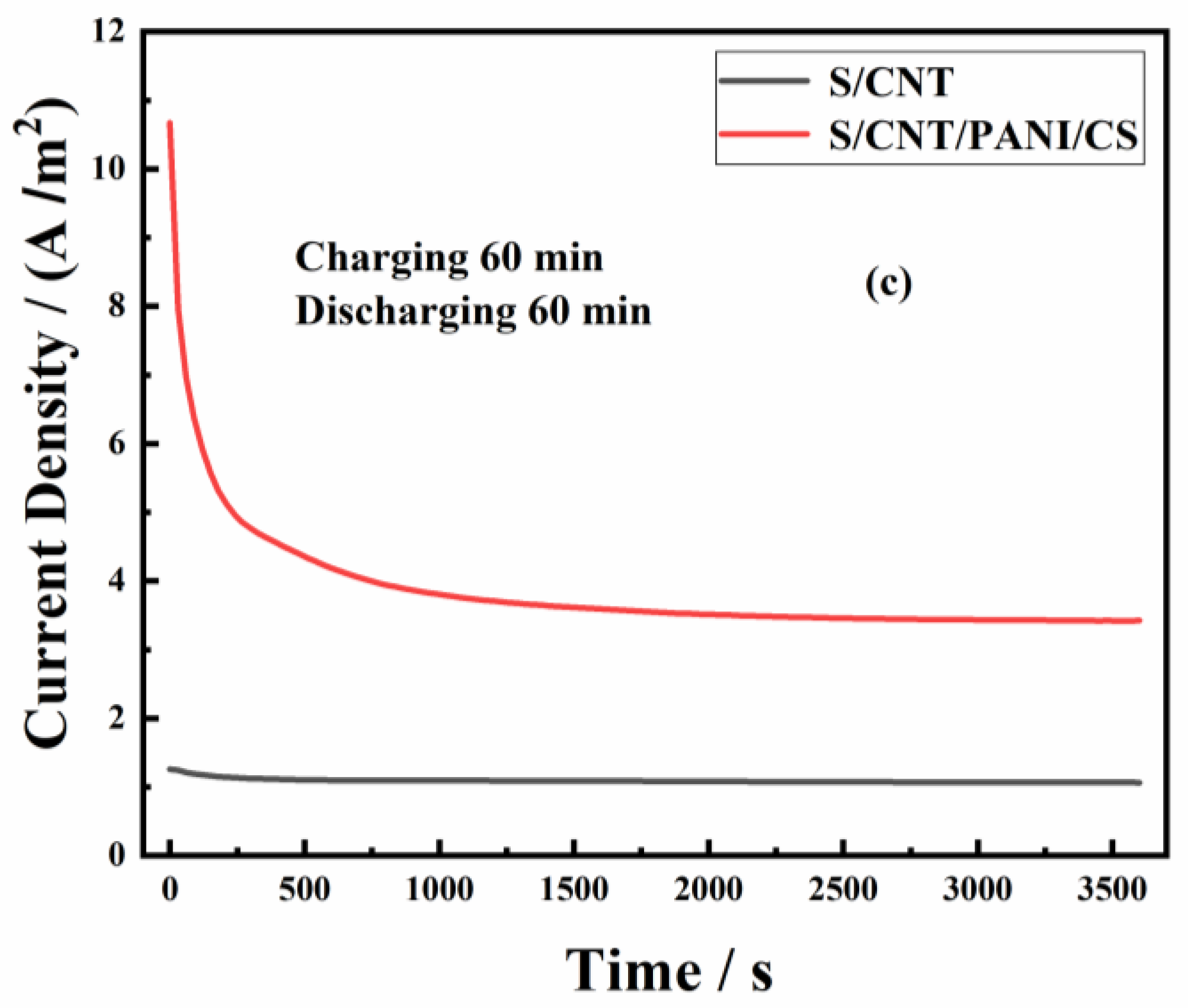
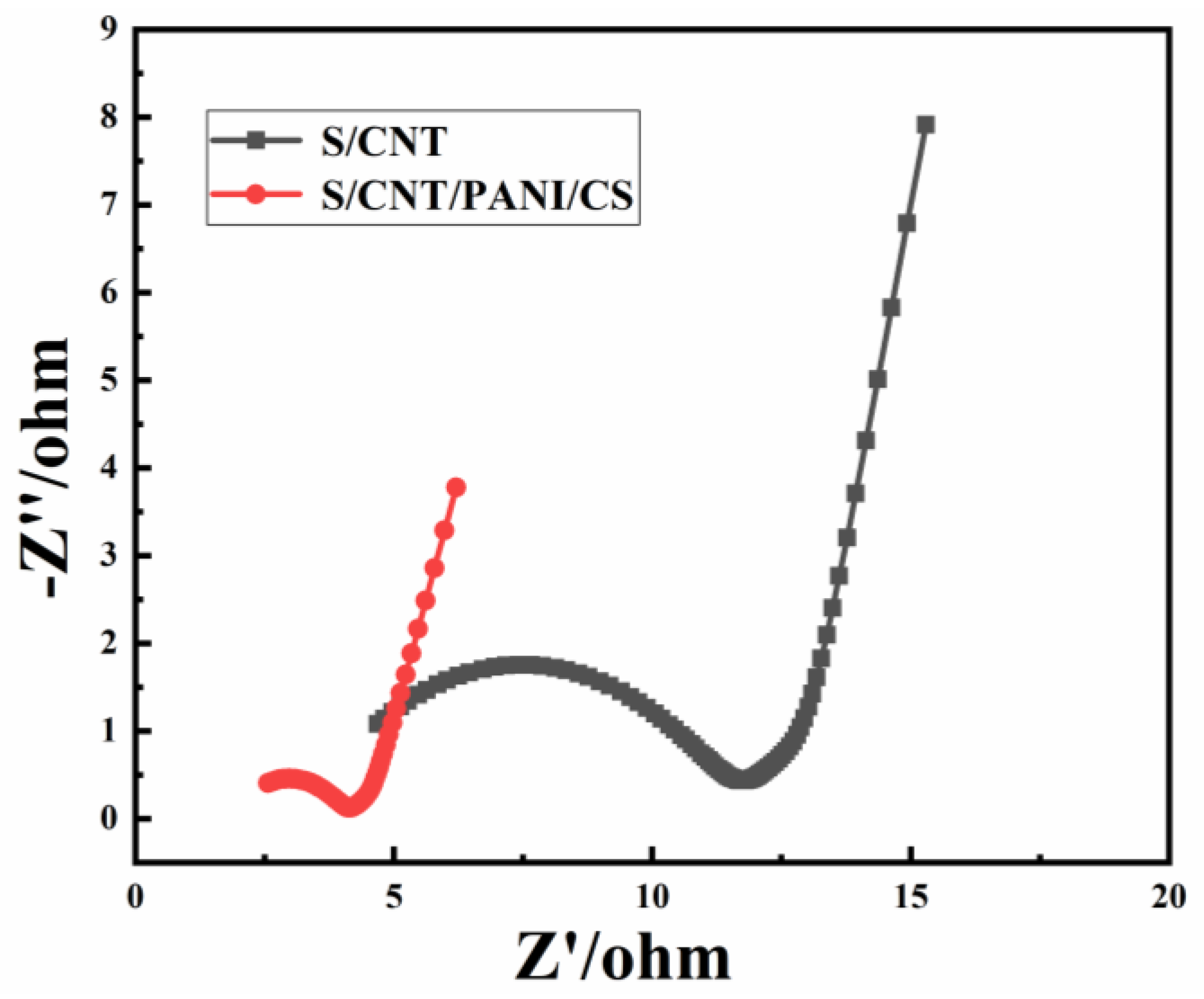
| Anodes | Parameters | C15/D15 | C30/D30 | C60/D60 | Power Density (W/m3) | Rct (Ω) |
|---|---|---|---|---|---|---|
| ip (A/m2) | 1.23 | 1.24 | 1.26 | 2298.8 | 4.4 | |
| S/CNT | is (A/m2) | 1.09 | 1.08 | 1.07 | ||
| Qs (C/m2) | 24.3 | 46.3 | 70.6 | |||
| Qt (C/m2) | 1005.3 | 1990.3 | 3922.6 | |||
| S/CNT/PANI/CS | ip (A/m2) | 6.95 | 9.09 | 10.67 | 7902.4 | 1.6 |
| is (A/m2) | 2.79 | 3.09 | 3.42 | |||
| Qs (C/m2) | 410.6 | 758.7 | 1578.2 | |||
| Qt (C/m2) | 2921.6 | 6320.7 | 13,890.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y. 3D Porous Sponge/Carbon Nanotube/Polyaniline/Chitosan Capacitive Bioanode Material for Improving the Power Generation and Energy Storage Performance of Microbial Fuel Cells. Coatings 2024, 14, 152. https://doi.org/10.3390/coatings14020152
Wang Y. 3D Porous Sponge/Carbon Nanotube/Polyaniline/Chitosan Capacitive Bioanode Material for Improving the Power Generation and Energy Storage Performance of Microbial Fuel Cells. Coatings. 2024; 14(2):152. https://doi.org/10.3390/coatings14020152
Chicago/Turabian StyleWang, Yuyang. 2024. "3D Porous Sponge/Carbon Nanotube/Polyaniline/Chitosan Capacitive Bioanode Material for Improving the Power Generation and Energy Storage Performance of Microbial Fuel Cells" Coatings 14, no. 2: 152. https://doi.org/10.3390/coatings14020152
APA StyleWang, Y. (2024). 3D Porous Sponge/Carbon Nanotube/Polyaniline/Chitosan Capacitive Bioanode Material for Improving the Power Generation and Energy Storage Performance of Microbial Fuel Cells. Coatings, 14(2), 152. https://doi.org/10.3390/coatings14020152





