The Corrosion Performance of Hybrid Polyurea Coatings Modified with TiO2 Nanoparticles in a CO2 Environment
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
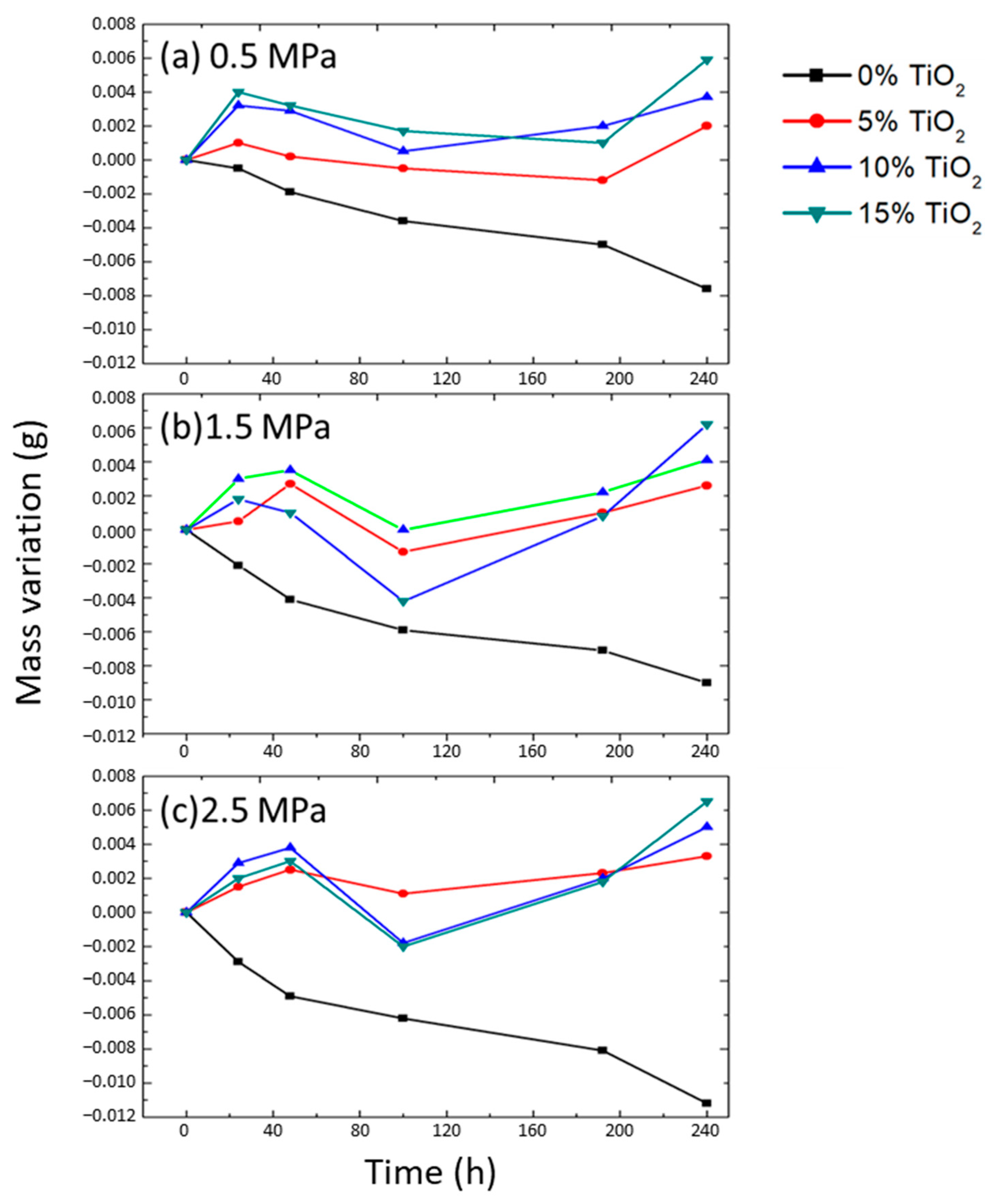
3.1. Mass Variation
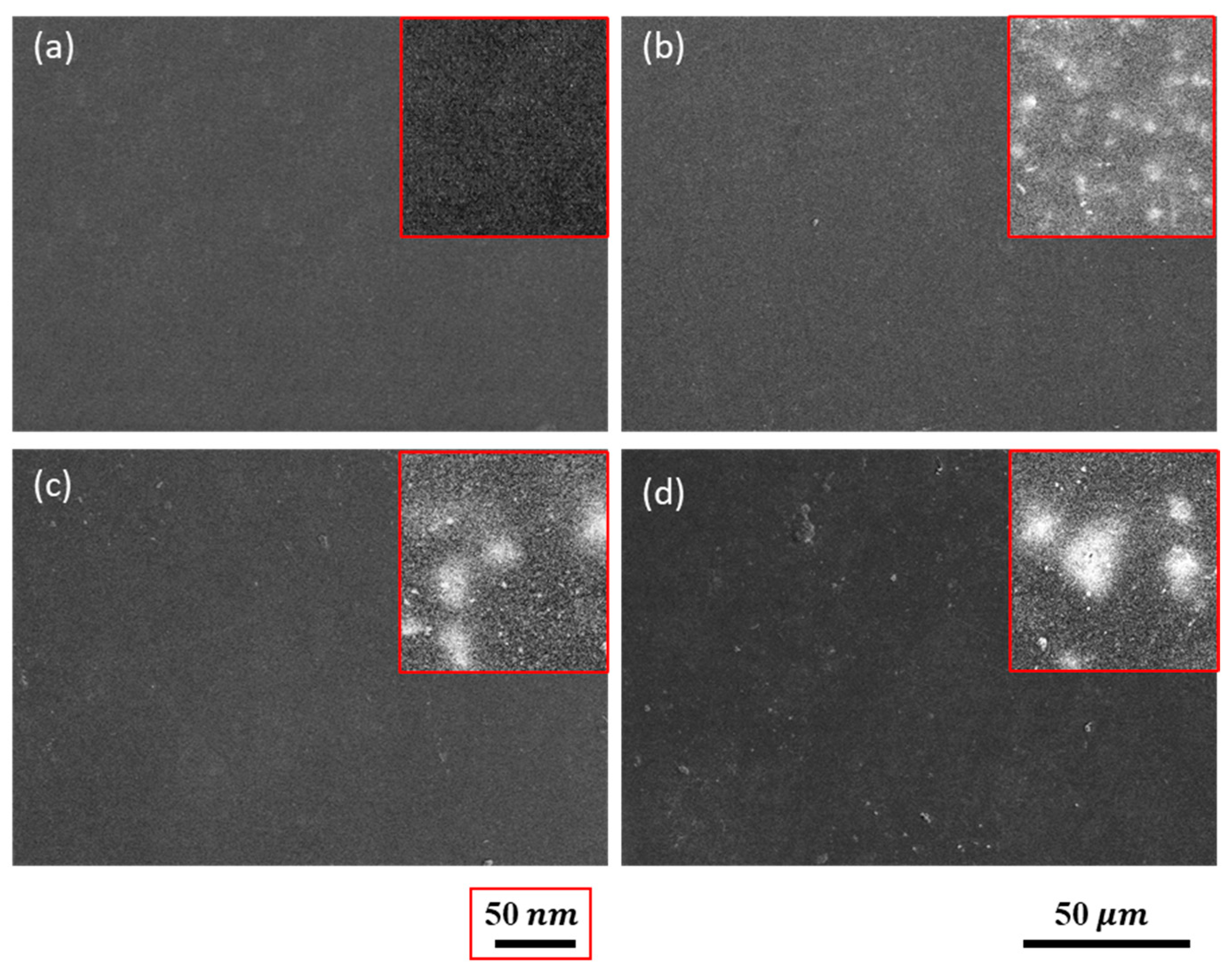
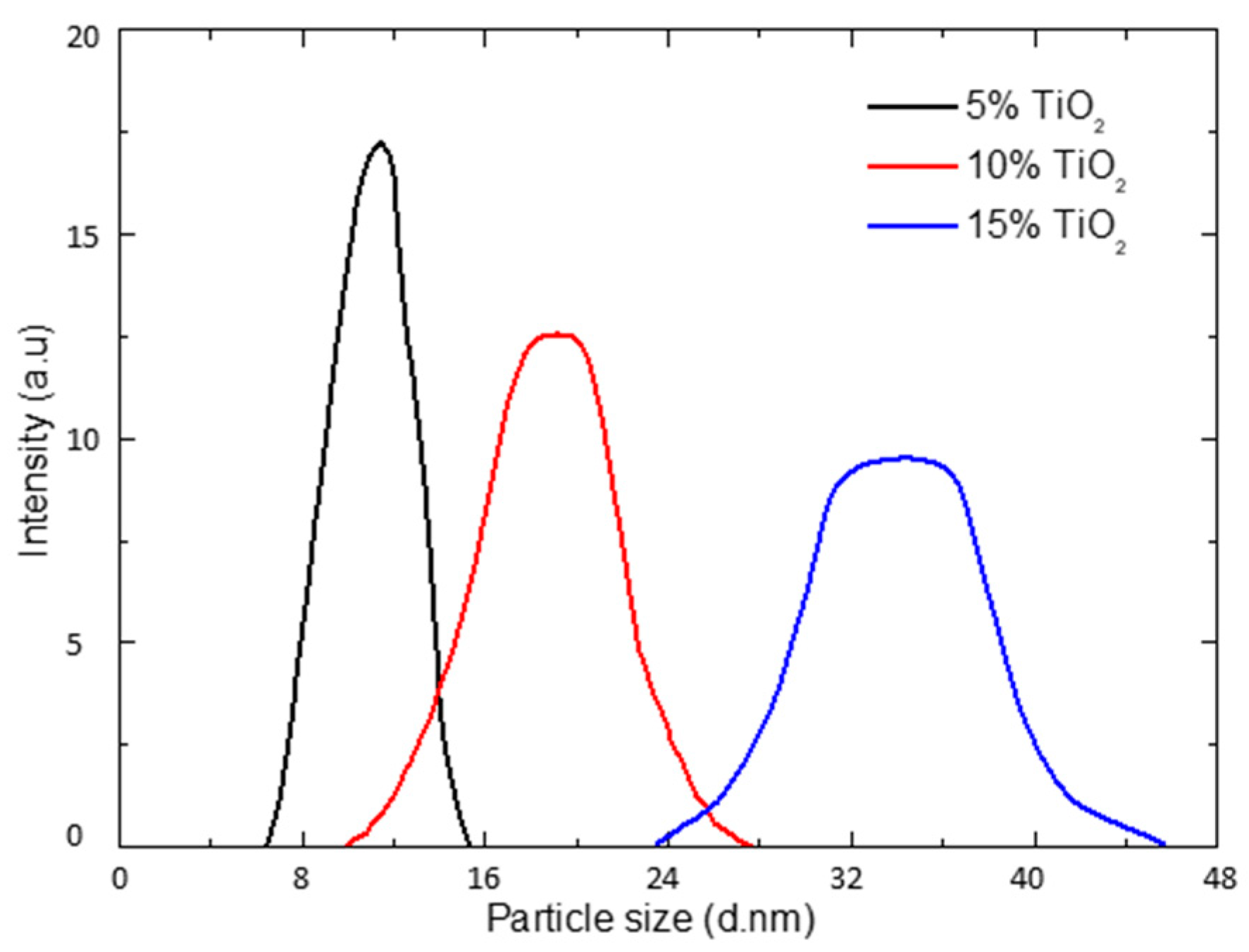
3.2. Microstructures
3.3. Open-Circuit Potential
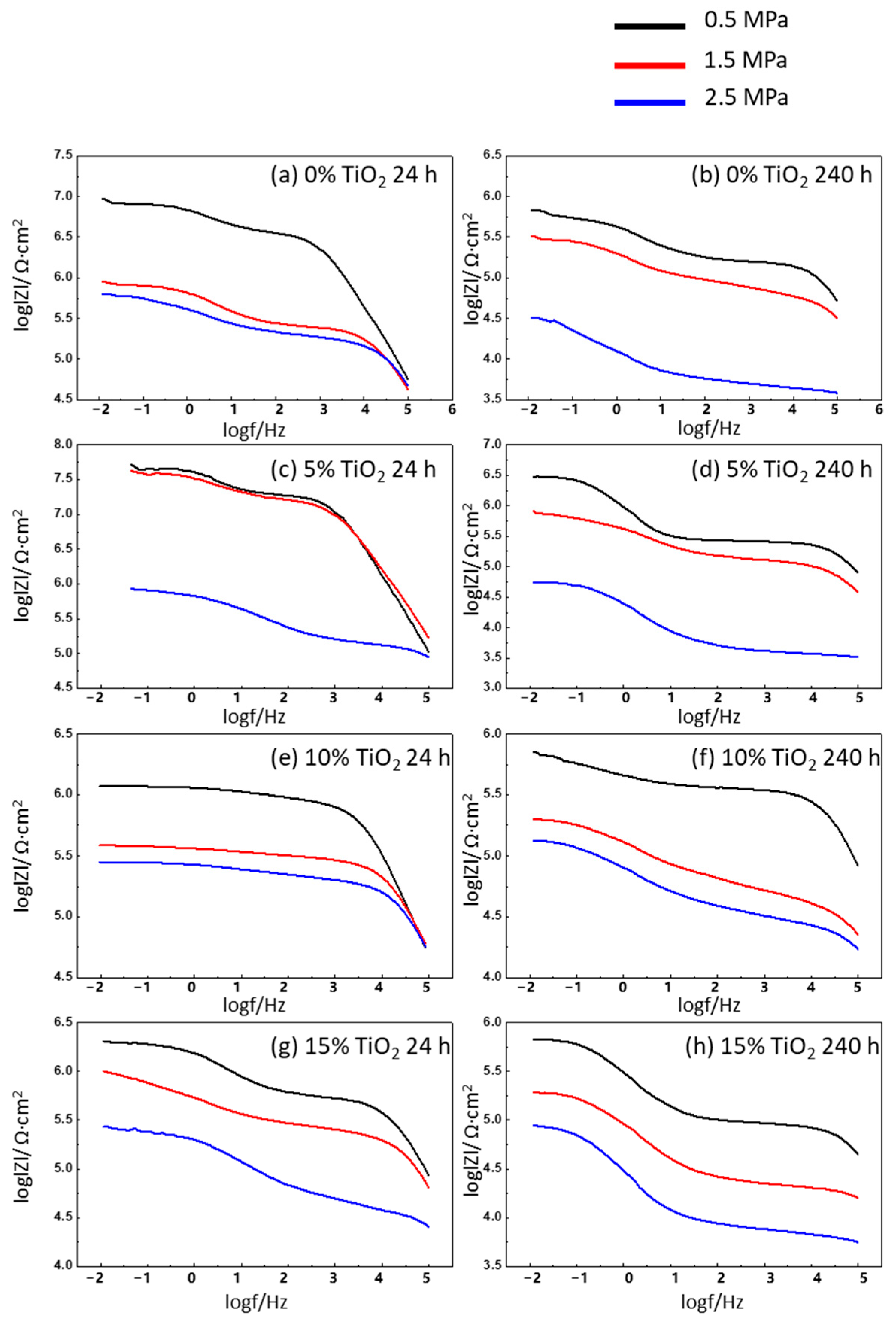
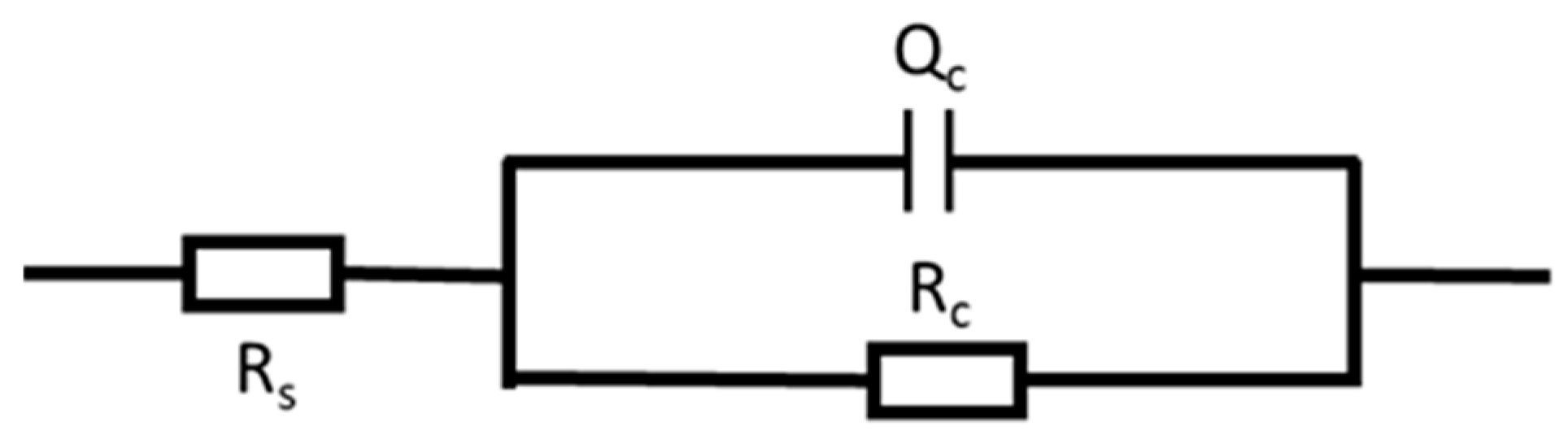
3.4. EIS Analysis
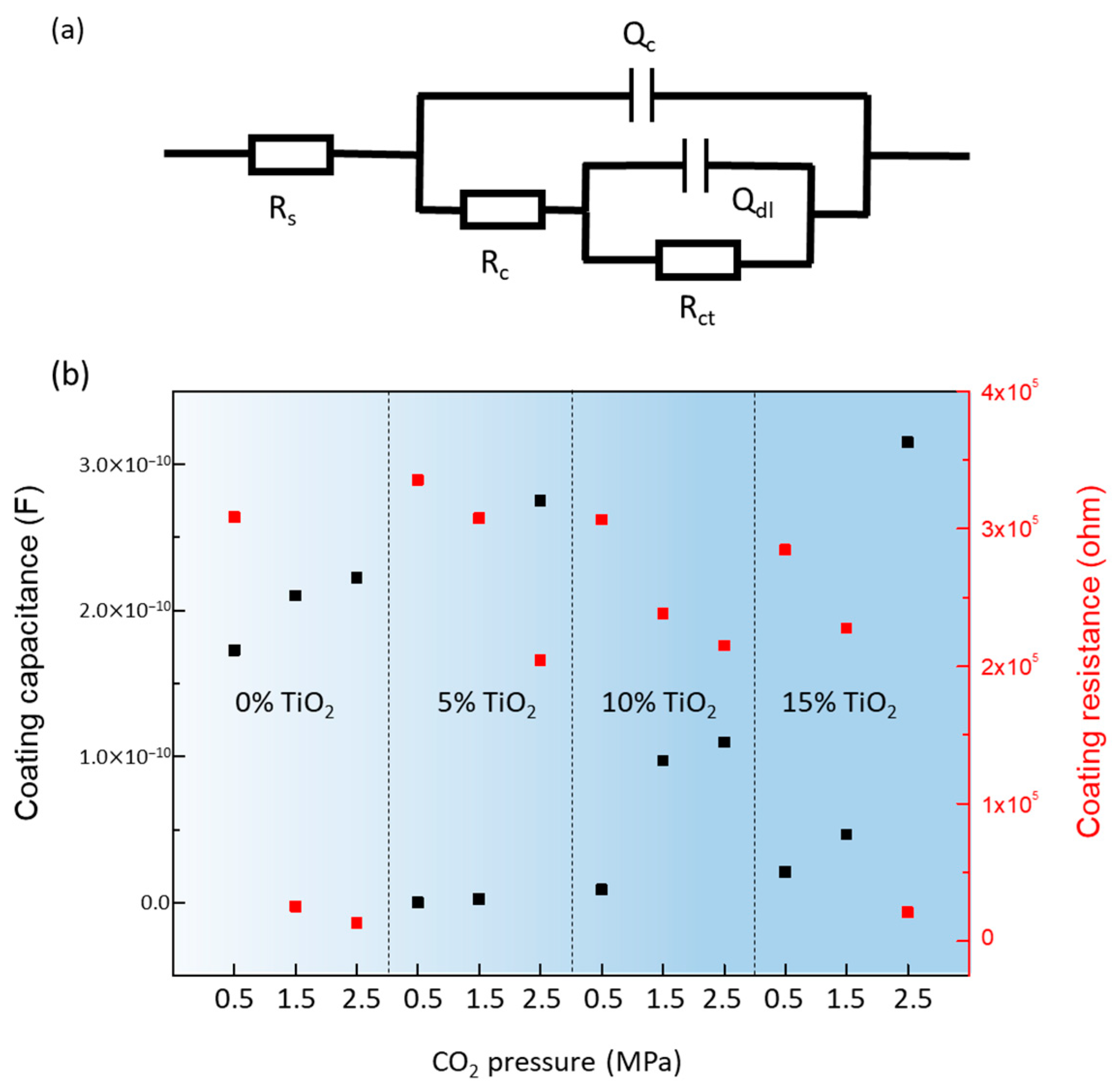
3.4.1. Effect of CO2 Pressure
3.4.2. Effect of Content of TiO2 Nanoparticles
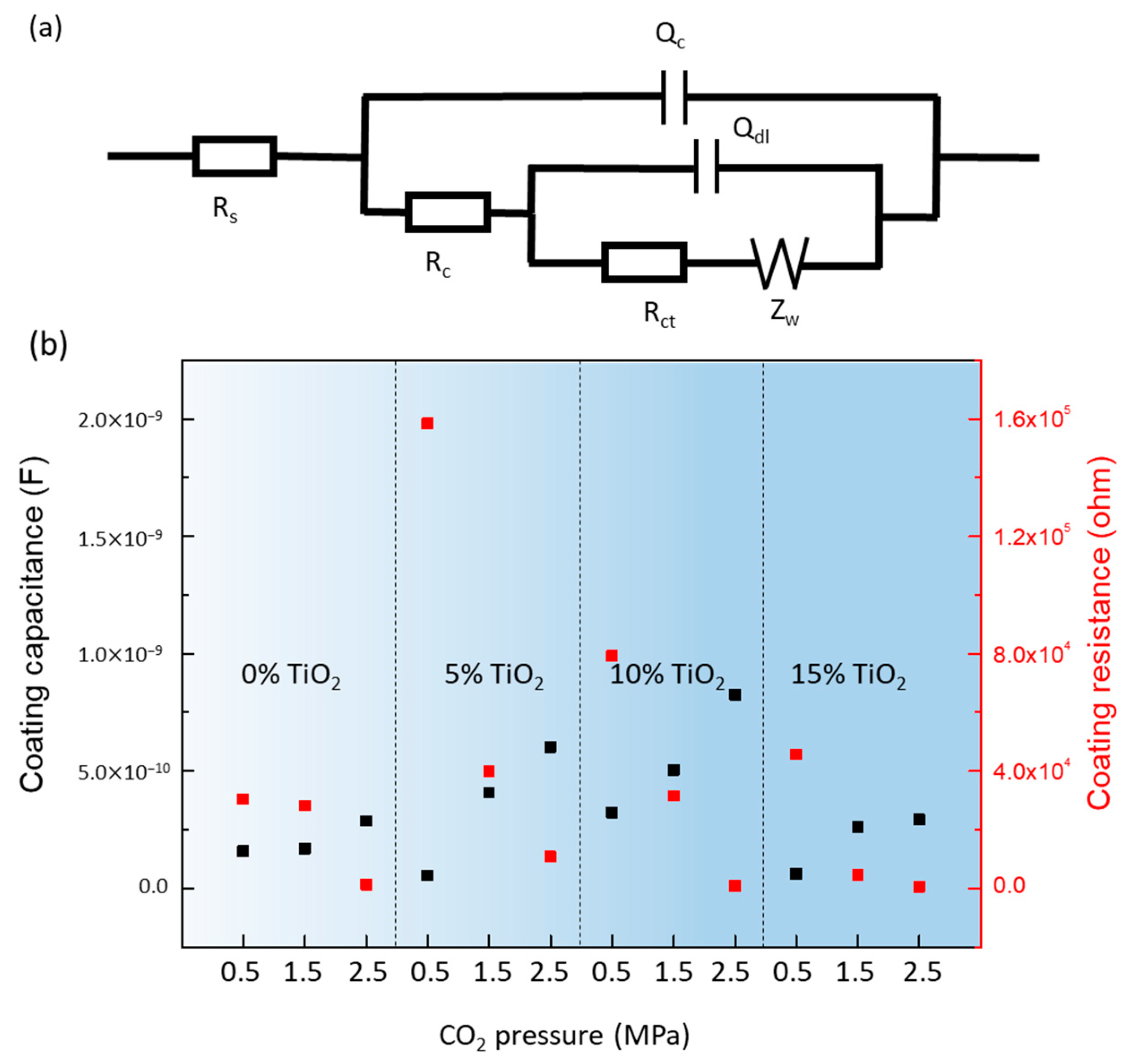
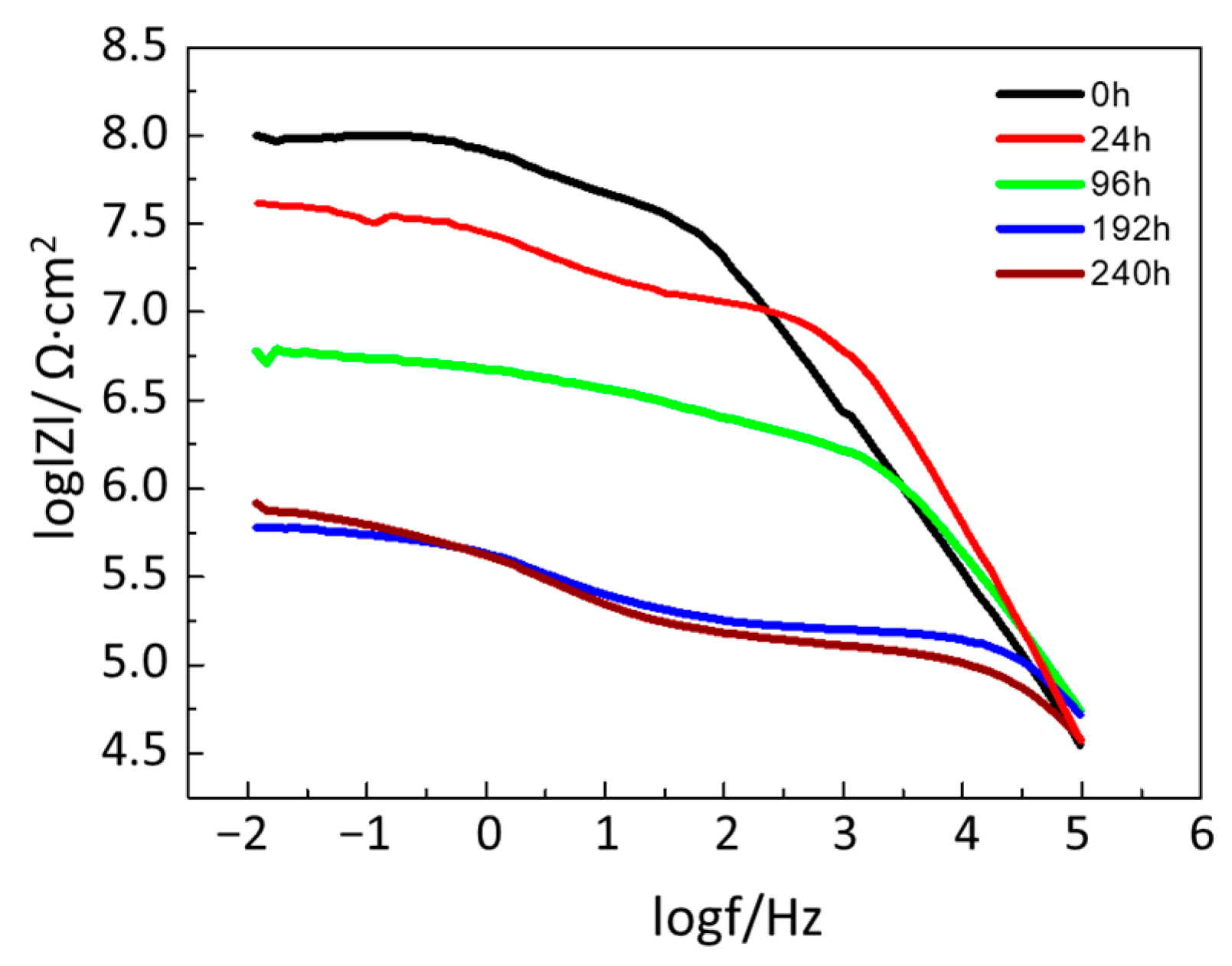
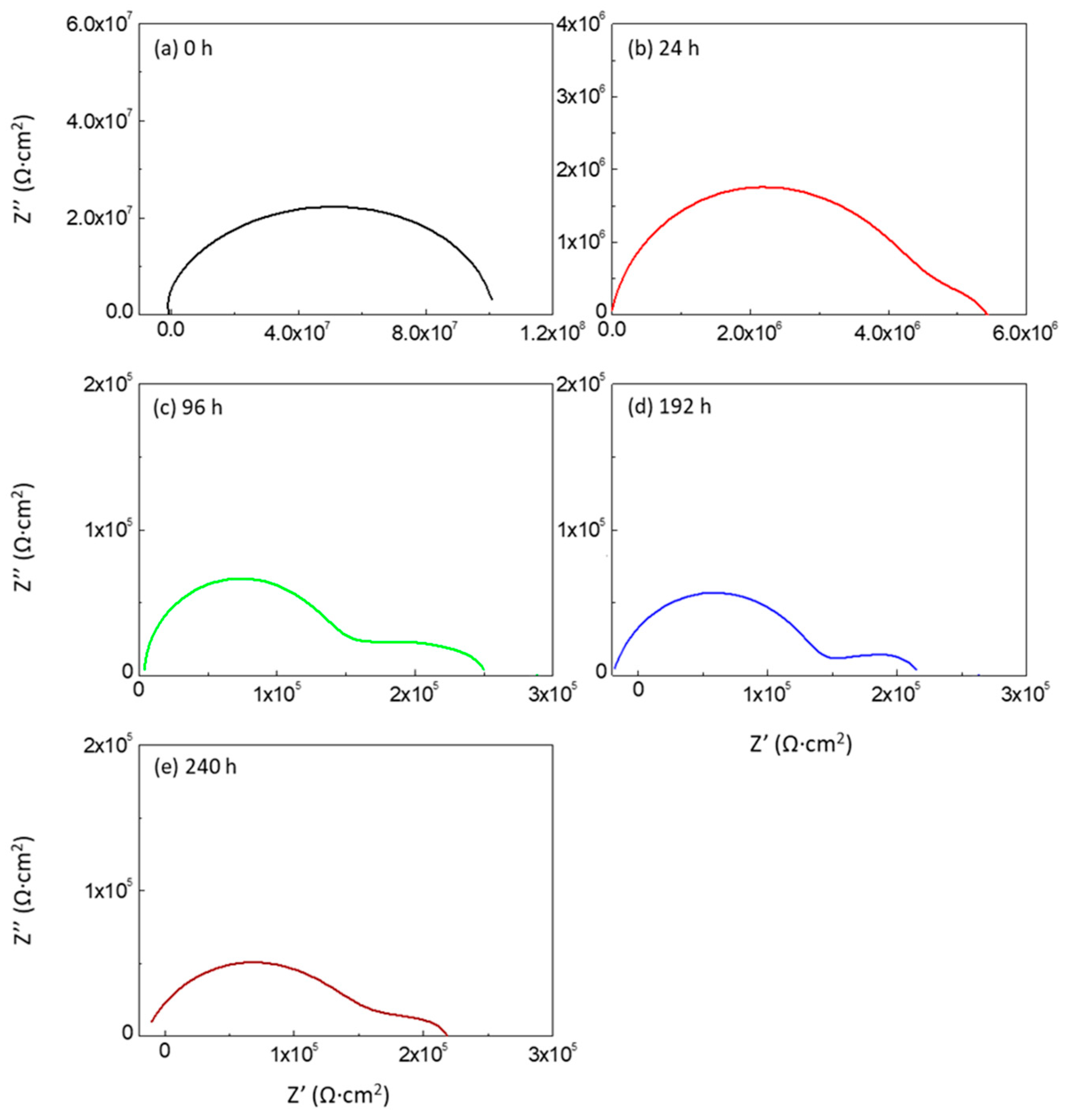
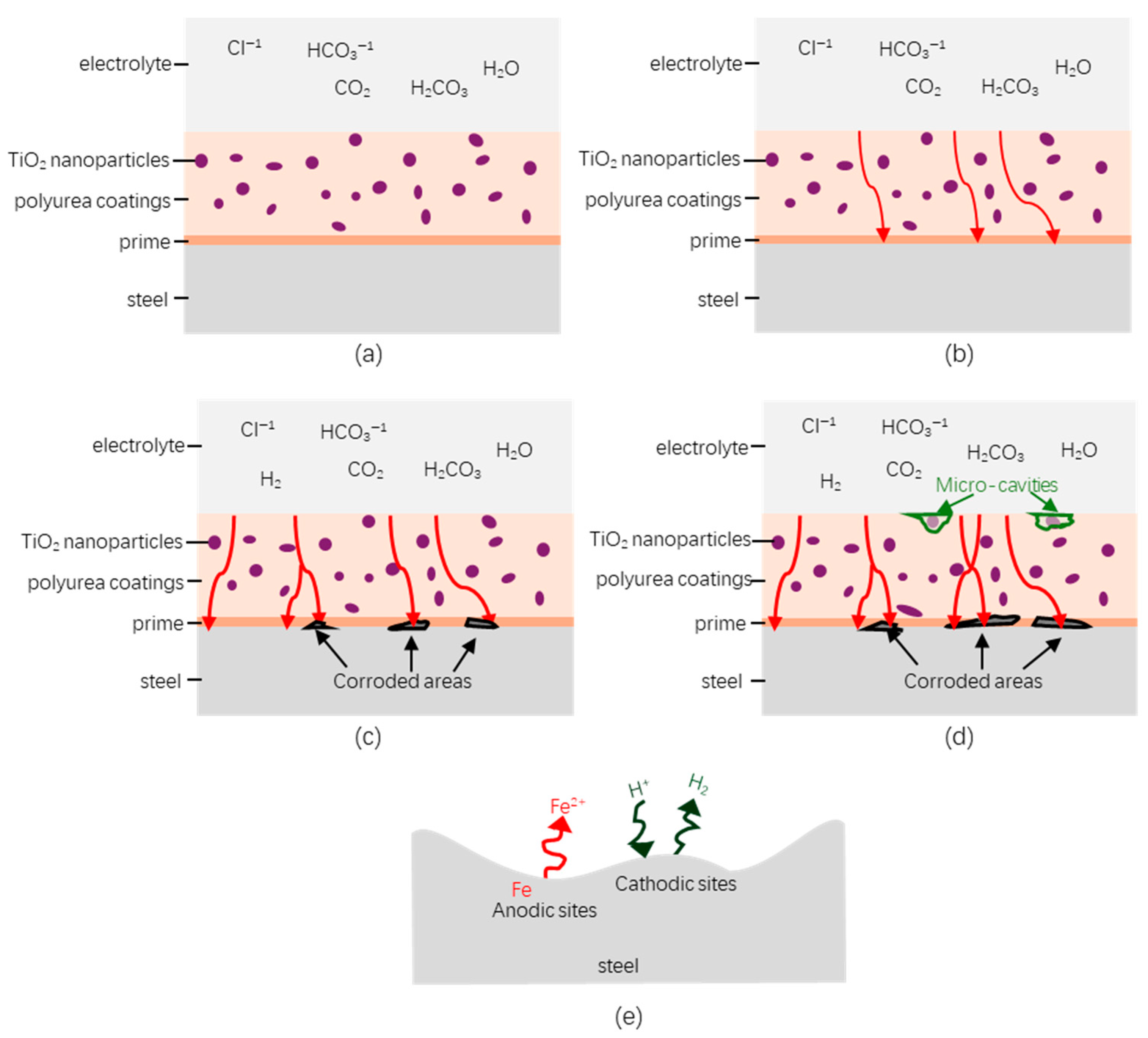
3.4.3. Degradation of Hybrid Polyurea Coating (Effect of Immersion Time)
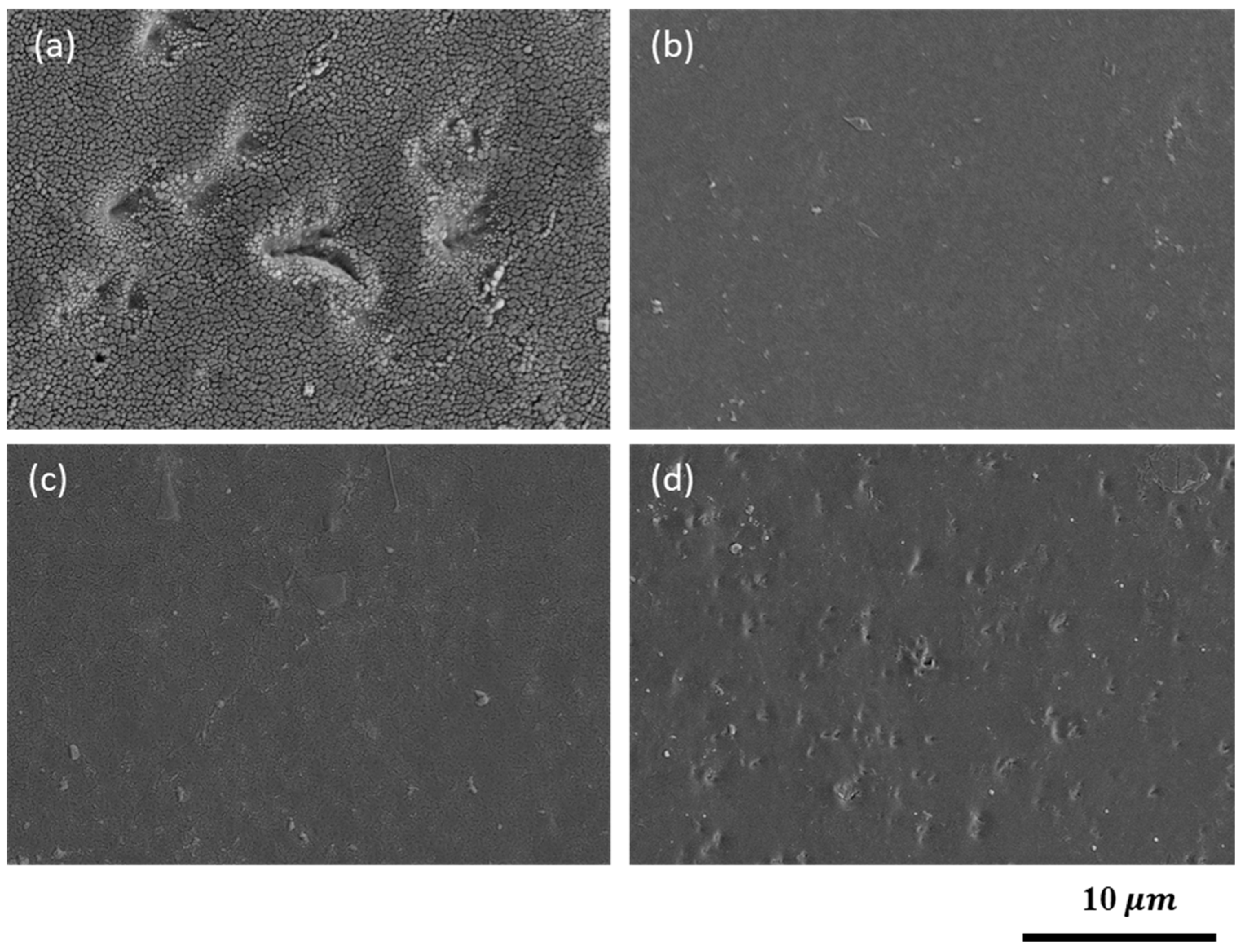
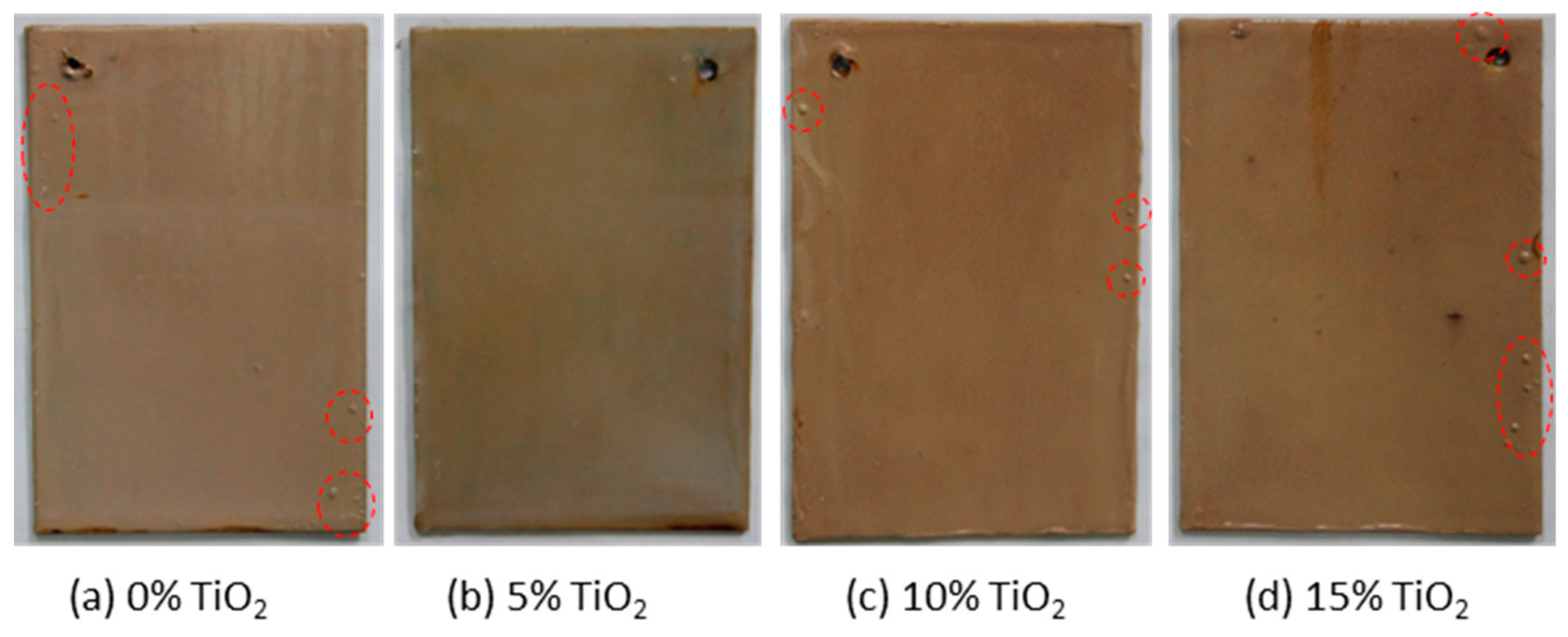
3.5. NSS Testing Results
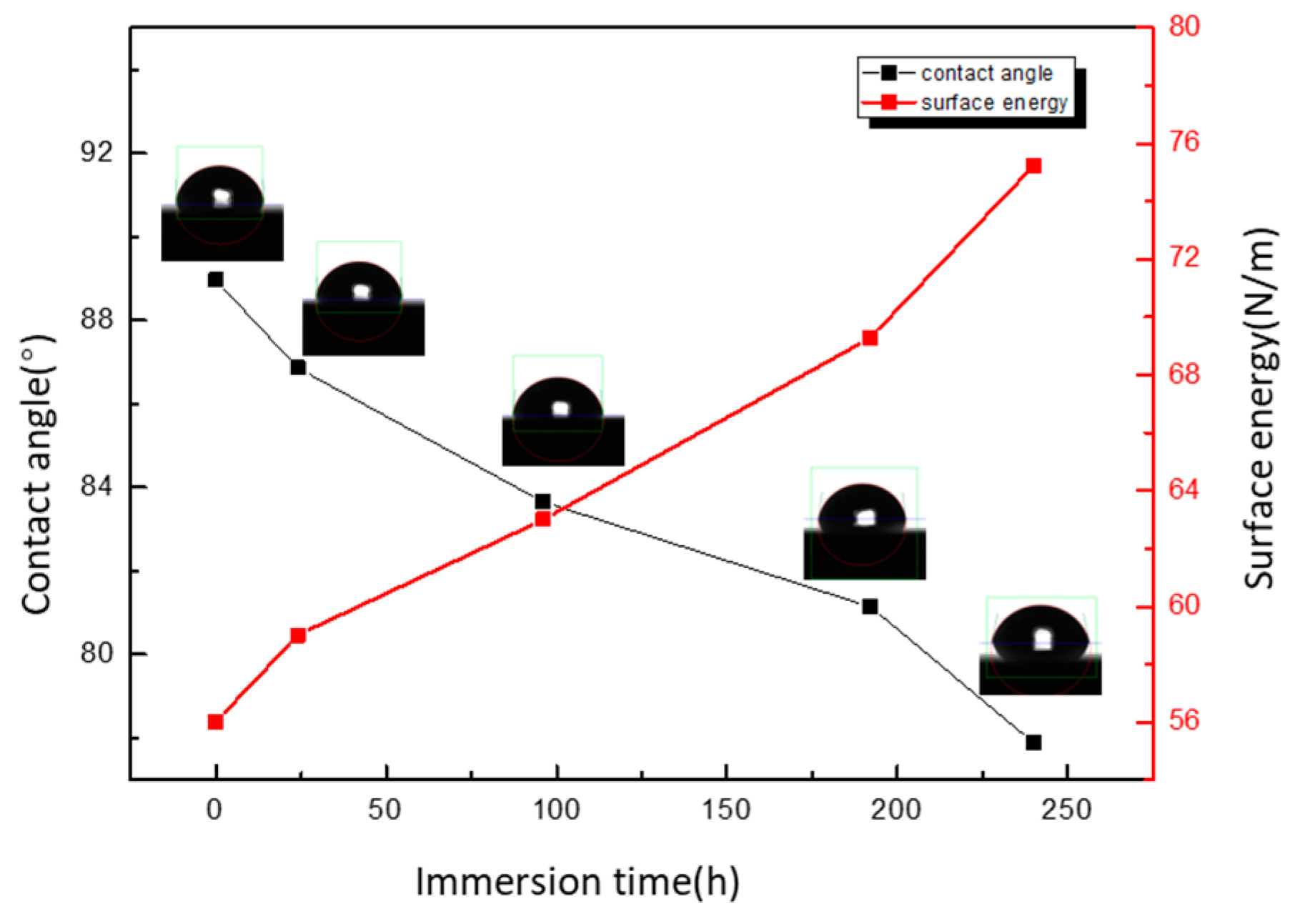
3.6. Contact Angle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| TiO2 Content | CO2 (MPa) | Rs (ohm) | Qc (F) | Rc (ohm) | Qdl (F) | Rct (ohm) |
|---|---|---|---|---|---|---|
| 0% | 0.5 | 2376 | 1.728 × 10−10 | 3.083 × 105 | 1.399 × 10−8 | 3.084 × 106 |
| 1.5 | 803.2 | 2.100 × 10−10 | 2.555 × 104 | 1.274 × 10−7 | 2.545 × 105 | |
| 2.5 | 691 | 2.224 × 10−10 | 1.325 × 104 | 4.1924 × 10−8 | 1.325 × 105 | |
| 5% | 0.5 | 1.105 × 104 | 9.061 × 10−15 | 3.353 × 105 | 5.599 × 10−9 | 7.035 × 106 |
| 1.5 | 1.335 × 104 | 2.371 × 10−12 | 3.074 × 105 | 8.342 × 10−9 | 7.754 × 105 | |
| 2.5 | 1.126 × 104 | 2.752 × 10−10 | 2.046 × 105 | 3.245 × 10−5 | 625 | |
| 10% | 0.5 | 1.181 × 104 | 9.039 × 10−12 | 3.066 × 105 | 1.435 × 10−9 | 1046 |
| 1.5 | 1.205 × 104 | 9.715 × 10−11 | 2.386 × 105 | 1.35 × 10−8 | 786 | |
| 2.5 | 1.102 × 104 | 1.098 × 10−10 | 2.150 × 105 | 1.662 × 10−7 | 1053 | |
| 15% | 0.5 | 1.011 × 104 | 2.095 × 10−11 | 2.846 × 105 | 4.004 × 10−9 | 4.166 × 105 |
| 1.5 | 1.015 × 104 | 4.656 × 10−11 | 2.277 × 105 | 1.898 × 10−7 | 1.88 × 105 | |
| 2.5 | 1.131 × 104 | 3.148 × 10−10 | 2.109 × 104 | 2.104 × 10−8 | 4.886 × 104 |
| TiO2 Content | CO2 (MPa) | Rs (ohm) | Qc (F) | Rc (ohm) | Qdl (F) | Rct (ohm) | Warburg |
|---|---|---|---|---|---|---|---|
| 0% | 0.5 | 1195 | 1.586 × 10−10 | 3.047 × 105 | 5.468 × 10−11 | 3.243 × 105 | 9.342 × 105 |
| 1.5 | 2012 | 1.630 × 10−10 | 2.820 × 105 | 1.260 × 10−10 | 6494 | 4.152 × 105 | |
| 2.5 | 1953 | 2.871 × 10−10 | 1292 | 3.921 × 10−9 | 2181 | 4.470 × 105 | |
| 5% | 0.5 | 5420 | 5.439 × 10−11 | 1.583 × 105 | 1.587 × 10−7 | 6.966 × 105 | 1.142 × 104 |
| 1.5 | 8266 | 4.087 × 10−10 | 3.982 × 104 | 1.094 × 10−6 | 1.15 × 105 | 3.002 × 105 | |
| 2.5 | 2746 | 6.014 × 10−10 | 1.089 × 104 | 2.655 × 10−6 | 5.663 × 104 | 1.733 × 105 | |
| 10% | 0.5 | 9005 | 3.236 × 10−11 | 7.922 × 104 | 0.004749 | 17.9 | 1.97 × 107 |
| 1.5 | 8695 | 5.054 × 10−11 | 3.145 × 104 | 1.58 × 10−8 | 2.11 × 105 | 5.998 × 106 | |
| 2.5 | 3393 | 1.258 × 10−10 | 875.4 | 8.103 × 10−6 | 0.5669 | 1.872 × 106 | |
| 15% | 0.5 | 7635 | 6.189 × 10−11 | 4.57 × 104 | 4.243 × 10−7 | 3.275 × 105 | 6.354 × 106 |
| 1.5 | 7632 | 2.623 × 10−10 | 4590 | 1.941 × 10−6 | 8.314 × 104 | 1.072 × 106 | |
| 2.5 | 2651 | 2.921 × 10−10 | 655.2 | 4.86 × 10−6 | 4.137 × 104 | 1.208 × 107 |
References
- Blanco, H.; Faaij, A. A review at the role of storage in energy systems with a focus on Power to Gas and long-term storage. Renew. Sustain. Energy Rev. 2018, 81, 1049–1086. [Google Scholar] [CrossRef]
- Bachu, S.; Dusseault, M. Underground Injection of Carbon Dioxide in Salt Beds. Dev. Water Sci. 2005, 52, 637–648. [Google Scholar]
- Li, W.; Nan, X.; Chen, J.; Yang, C. Investigation of thermal-mechanical effects on salt cavern during cycling loading. Energy 2021, 232, 120969. [Google Scholar] [CrossRef]
- Li, L.; Gong, W.; Li, J. Prediction on service life of concrete pipeline buried in chlorinated environment under nonuniformly distributed earth pressure. Constr. Build. Mater. 2020, 243, 118162. [Google Scholar] [CrossRef]
- Hua, Y.; Shamsa, A.; Barker, R.; Neville, A. Protectiveness, morphology and composition of corrosion products formed on carbon steel in the presence of Cl−, Ca2+ and Mg2+ in high pressure CO2 environments. Appl. Surf. Sci. 2018, 455, 667–682. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, K.; Dong, C.; Wu, J.; Li, X.; Huang, Y. In situ Raman spectroscopy study of corrosion products on the surface of carbon steel in solution containing Cl− and SO42−. Eng. Fail. Anal. 2011, 18, 1981–1989. [Google Scholar] [CrossRef]
- Han, J.M.; Shin, H.Y.; Min, B.-M.; Han, K.-H.; Cho, A. Measurement and correlation of high pressure phase behavior of carbon dioxide+water system. J. Ind. Eng. Chem. 2009, 15, 212–216. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Nazdracheva, T. Anti-corrosion coatings for protection of steel railway structures exposed to atmospheric environments: A review. Constr. Build. Mater. 2011, 288, 123115. [Google Scholar] [CrossRef]
- Wang, X.; Melchers, R.E. Long-term under-deposit pitting corrosion of carbon steel pipes. Ocean Eng. 2017, 133, 231–243. [Google Scholar] [CrossRef]
- Iqbal, N.; Sharma, P.K.; Kumar, D.; Roy, P.K. Protective polyurea coatings for enhanced blast survivability of concrete. Constr. Build. Mater. 2018, 175, 682–690. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Radeva, T. Composite coatings with polymeric modified ZnO nanoparticles and nanocontainers with inhibitor for corrosion protection of low carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125741. [Google Scholar] [CrossRef]
- Fadl, A.M.; Abdou, M.I.; Hamza, M.A.; Sadeek, S.A. Corrosion-inhibiting, self-healing, mechanical-resistant, chemically and UV stable PDMAS/TiO2 epoxy hybrid nanocomposite coating for steel petroleum tanker trucks. Prog. Org. Coat. 2020, 146, 105715. [Google Scholar] [CrossRef]
- Gu, S.; Shi, H.; Zhang, C.; Wang, W.; Liu, F.; Han, E.-H. Mesoporous CeO2 containers in water-borne epoxy coatings for dual active corrosion protection of mild steel. Prog. Org. Coat. 2021, 158, 106376. [Google Scholar] [CrossRef]
- Bierwagen, G.P. Reflections on corrosion control by organic coatings. Prog. Org. Coat. 1996, 28, 43–48. [Google Scholar] [CrossRef]
- Corrales, T.; Peinado, C.; Allen, N.S.; Edgeb, M.; Sandoval, G.; Catalina, F. A chemiluminescence study of micron and nanoparticle titanium dioxide: Effect on the thermal stability of metallocene polyethylene. J. Photochem. Photobiol. A Chem. 2003, 156, 151–160. [Google Scholar] [CrossRef]
- Liu, J.-M.H.L.; Leng, W.-H.; Zhang, J.-Q.; Cao, C.-N. Novel bis-silane/TiO2 bifunctional hybrid films for metal corrosion protection both under ultraviolet irradiation and in the dark. Scr. Mater. 2007, 57, 549–552. [Google Scholar] [CrossRef]
- Shen, Y.C.C.G.X.; Lin, C.J. Corrosion protection of 316 L stainless steel by a TiO2 nanoparticle coating prepared by sol–gel method. Thin Solid Film. 2005, 489, 130–136. [Google Scholar] [CrossRef]
- Jalili, M.M.; Moradian, S.; Dastmalchian, H.; Karbasi, A. Investigating the variations in properties of 2-pack polyurethane clear coat through separate incorporation of hydrophilic and hydrophobic nano-silica. Prog. Org. Coat. 2007, 59, 81–87. [Google Scholar] [CrossRef]
- Yang, L.H.; Liu, F.C.; Han, E.H. Effects of P/B on the properties of anticorrosive coatings with different particle size. Prog. Org. Coat. 2005, 53, 91–98. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M. An evaluation of the corrosion resistance and adhesion properties of an epoxy-nanocomposite on a hot-dip galvanized steel (HDG) treated by different kinds of conversion coatings. Surf. Coat. Technol. 2011, 205, 4649–4657. [Google Scholar] [CrossRef]
- Behzadnasab, S.M.M.M.; Kabiri, K.; Jamali, S. Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. Corros. Sci. 2011, 53, 89–98. [Google Scholar] [CrossRef]
- Bordbar, S.; Rezaeizadeh, M.; Kavian, A. Improving thermal conductivity and corrosion resistance of polyurea coating on internal tubes of gas heater by nano silver. Prog. Org. Coat. 2020, 146, 105722. [Google Scholar] [CrossRef]
- Beiki, H.; Mosavi, S.J. Silver Nanoparticles-Polyurea Composite Coatings on ASTM A194 Steel: A Study of Corrosion Behavior in Chloride Medium. J. Bio-Tribo-Corros. 2020, 6, 66. [Google Scholar] [CrossRef]
- Dhoke, S.K.; Khanna, A.S. Effect of nano-Fe2O3 particles on the corrosion behavior of alkyd based waterborne coatings. Corros. Sci. 2009, 51, 6–20. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, X.; Yang, X.; Lu, L. Nanometre-sized TiO2 as applied to the modification of unsaturated polyester resin. Mater. Chem. Phys. 2022, 77, 609–611. [Google Scholar]
- Shi, H.W.; Liu, F.C.; Yang, L.H.; Han, E.H. Characterization of protective performance of epoxy reinforced with nanometer-sized TiO2 and SiO2. Prog. Org. Coat. 2008, 62, 359–368. [Google Scholar] [CrossRef]
- Radhakrishnana, S.; Siju, C.R.; Mahanta, D.; Patil, S.; Madras, G. Conducting polyaniline–nano-TiO2 composites for smart corrosion resistant coatings. Electrochim. Acta 2009, 54, 1249–1254. [Google Scholar] [CrossRef]
- Mansfeld, F.; Kendig, M.W. Impedance spectroscopy as quality control and corrosion test for anodized Al alloys. Corros. Sci. 1985, 41, 490–492. [Google Scholar] [CrossRef]
- Harringtona, D.A.; Driessche, P.v.D. Mechanism and equivalent circuits in electrochemical impedance spectroscopy. Electrochim. Acta 2011, 56, 8005–8013. [Google Scholar] [CrossRef]
- Wolstenholme, J. Electrochemical methods of assessing the corrosion of painted metals—A review. Corros. Sci. 1973, 17, 521–530. [Google Scholar] [CrossRef]
- Nazari, M.H.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106753. [Google Scholar]
- Hao, R.; Miao, W.; Xu, W.; Lin, Y.; Xiao, Q.; Wang, Z.; Li, Q.; Wang, P.; Wang, T.; Nie, A.; et al. Unraveling the atomic structure evolution of titanium nitride upon oxidation. Corros. Sci. 2024, 240, 112465. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Wakeel, A.; Nasir, M.A.; Zubair, M.M. Ammarmproving anti-corrosion and mechanical properties of mild steel pipelines by using polyurea with nanoparticles. Int. J. Thermofluids 2024, 23, 100729. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Han, J.; Yao, B.H. A numerical solution to the efects of surface roughness on water–coal contact angle. Sci. Rep. 2011, 11, 459. [Google Scholar]




| C | Si | Mn | P | S | Cr | Mo | Ni | V | Ti | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.270 | 0.260 | 1.410 | 0.014 | 0.003 | 0.089 | 0.080 | 0.049 | 0.007 | 0.036 | 0.030 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, S.; Wei, Q.; Li, B.; Jiang, Y.; Zhang, D.; Wang, Y.; Yang, Y.; Wang, B. The Corrosion Performance of Hybrid Polyurea Coatings Modified with TiO2 Nanoparticles in a CO2 Environment. Coatings 2024, 14, 1562. https://doi.org/10.3390/coatings14121562
Si S, Wei Q, Li B, Jiang Y, Zhang D, Wang Y, Yang Y, Wang B. The Corrosion Performance of Hybrid Polyurea Coatings Modified with TiO2 Nanoparticles in a CO2 Environment. Coatings. 2024; 14(12):1562. https://doi.org/10.3390/coatings14121562
Chicago/Turabian StyleSi, Shanshan, Qi Wei, Binzhou Li, Yuanbo Jiang, Dayue Zhang, Yijia Wang, Yu Yang, and Bingying Wang. 2024. "The Corrosion Performance of Hybrid Polyurea Coatings Modified with TiO2 Nanoparticles in a CO2 Environment" Coatings 14, no. 12: 1562. https://doi.org/10.3390/coatings14121562
APA StyleSi, S., Wei, Q., Li, B., Jiang, Y., Zhang, D., Wang, Y., Yang, Y., & Wang, B. (2024). The Corrosion Performance of Hybrid Polyurea Coatings Modified with TiO2 Nanoparticles in a CO2 Environment. Coatings, 14(12), 1562. https://doi.org/10.3390/coatings14121562






