Abstract
CaCO3 whiskers, as a micron-level inorganic fiber material, can enhance and toughen composite materials. In order to study the technical feasibility of CaCO3 whisker-modified asphalt, two types of silane coupling agent (SCA), KH-550 and KH-570, were applied to treat the surface of CaCO3 whiskers, and the treatment effects of the original and treated whiskers were characterized by scanning electron microscopy (SEM), energy-dispersive spectrometer (EDS) and contact angle test. Meanwhile, models of CaCO3 whiskers, SCA, and asphalt molecules were established by Material Studio (MS, 2020 version) software, and the adhesion mechanism between the CaCO3 whiskers-and-asphalt interface was predicted. The results of microscopic characterization experiments indicate that the surface of the whiskers treated with SCA became rougher. Compared with the original whiskers, the contact angle between the treated whisker surface and water increased from 50° to 92.2° and 103.4°, and the surface of whiskers changed from hydrophilic to hydrophobic. The results of molecular dynamics simulation analysis show that the adhesion performance between the CaCO3 whisker surface and asphalt increased from 100.1 mJ/m2 to 112.5 mJ/m2 and 126.6 mJ/m2 after modification with SCA, and the increase in adhesion energy of KH550 is greater than that of KH570. The above research results indicate that the micro-characterization results were consistent with the molecular dynamics simulation results; that is, after treatment with SCA, the adhesion energy between the whiskers and asphalt was increased to varying degrees. The research method in this article combines micro-characterization with molecular dynamics simulation, which has a certain degree of innovation.
1. Introduction
Whiskers are high-purity fibrous single crystals grown under controlled conditions. Their crystal structure is almost complete and does not contain crystal defects such as grain boundaries, dislocations, and voids. Therefore, they have excellent mechanical and physical properties. CaCO3 whiskers are made from limestone, with a wide range of sources and low prices, and can be produced in a low-temperature (<100 °C) aqueous system. The process is simple, easy to industrialize, and the production and use processes are clean and pollution-free [1].
Many scholars have conducted research on whiskers as building materials. Chen et al. found that CaCO3 whiskers had a reinforcing and toughening effect on polyethylene fiber-reinforced cement-based composites. As the content of whiskers has been observed to increase, the strengthening and toughening effect has shown a trend of first increasing and then decreasing. An appropriate amount of CaCO3 whiskers can fill the matrix and improve the pore structure [2]. Zhang et al. added CaCO3 whiskers to engineered cementitious composite (ECC) and found that CaCO3 whiskers can significantly improve the tensile strain capacity of ECC. The needle shape of whiskers can improve the roughness of the interface and enhance the frictional bonding force [3]. Chen et al. found that the introduction of CaCO3 whiskers can improve the bending strength and toughness of cement-based materials, but the bending performance of the material decreased with the increase in whisker content [4]. Song et al. found that micrometer-scale CaCO3 whiskers can bridge microcracks in hardened cement-based composites. When the whiskers were pulled out, energy was consumed, and the mechanical properties of composite material were improved, which has the effect of micro-scale crack resistance [5].
The research of the above scholars mainly focuses on improving the toughness and mechanical properties of cement composite materials with whiskers. Whiskers are fibrous inorganic materials with a certain length–diameter ratio. Some scholars have explored whether whiskers can be applied to the preparation of modified asphalt and exert a reinforcing and toughening effect similar to fibers [6,7,8].
In composite materials, the interface area between the reinforcement and the matrix is very important. Good interface bonding can effectively enhance the effect of the reinforcement, while poor bonding can weaken the interface strength and potentially lower the performance of the composite. If there are defects or poor chemical compatibility between the reinforcement and the matrix, the weak links in the interface area can become the source of stress failure or impact failure in the composite material. The poor compatibility between the reinforcement and matrix is an obstacle to the optimization of material combination. To solve this problem, the surface of the reinforcement is generally treated to strengthen the interface [9].
CaCO3 whiskers, as a micron-scale inorganic fibrous material, are inherently inorganic materials. Their surface is smooth and chemically inert, and there are significant differences in material structure and properties with an organic polymer asphalt matrix. The mutual affinity is poor, and the interface bonding is not satisfactory. In order to achieve the goal of using CaCO3 whiskers in modified asphalt, by using SCA to treat the surface of CaCO3 whiskers, chemical molecular bonds were established between inorganic whiskers and organic asphalt. Through an SCA, the whisker and asphalt combine more closely, and the preparation of high-performance modified asphalt is realized.
Two types of SCA (KH-550 and KH-570) were used to treat the surface of CaCO3 whiskers in this article. The surface-treatment effect of the whiskers was studied using SEM and contact angle tests. Using the multi-scale research method of composite materials, the interface effect between the whiskers and asphalt was studied. The molecular behavior of the whisker surface and asphalt before and after modification was simulated by molecular dynamics, and the mechanism of whisker surface treatment and its adhesion to asphalt were analyzed. The research results have a reference value for improving the performance of whisker-modified asphalt. The research framework of this study is shown in Figure 1.
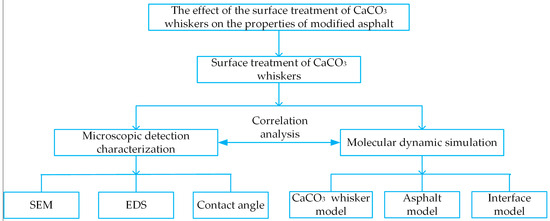
Figure 1.
Research framework.
2. Microscopic Characterization of Surface Modification of CaCO3 Whiskers
2.1. Test Materials
2.1.1. CaCO3 Whiskers
The appearance of CaCO3 whiskers is as of a white powder, which shows needle-like structure in an SEM test. Figure 2a and Figure 2b, respectively, show the appearance and microstructure of CaCO3 whiskers. The CaCO3 whiskers used in this article were purchased from Shanghai Fengzhu Co., Ltd. (Shanghai, China).
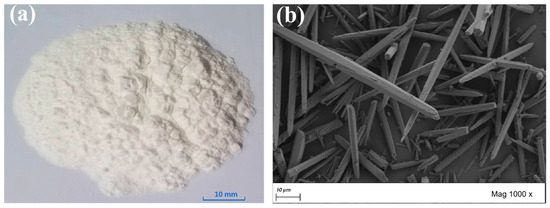
Figure 2.
CaCO3 whiskers. (a) Appearance; (b) microstructure by SEM (1000×).
The main component of CaCO3 whiskers is CaCO3, with a content of over 97%. Other components include MgO and some trace elements.
2.1.2. Silane Coupling Agent (SCA)
SCA contains two active groups with different properties on the same silicon atom, where the silicon functional group is chemically bonded to the inorganic material, and the carbon functional group is bound to the organic polymer [10]. The molecular structure formula of SCA is generally as follows:
where, X is an organic functional group and Y is a hydrolysable inorganic functional group.
X − (CH2)n SiY3
The mechanism of action of SCA is based on two different reactive groups in their molecular structure: the organic functional group (X) and the hydrolysable inorganic functional group (Y). The Y group hydrolyzes to generate silicon alcohol (Si(OH)3), producing Si-OH, which reacts with the -OH generated by the hydrolysis of CO32− ions on the surface of CaCO3 whiskers to dehydrate and form covalent bonds, thereby achieving the connection of one end of the SCA to the surface of CaCO3 whiskers. Meanwhile, organic functional groups (X) can react or be compatible with asphalt, forming a strong bonding layer between inorganic and organic materials. After surface treatment, the surface of CaCO3 whiskers has changed from a hydrophilic surface containing more -OH to an oleophilic surface containing organic functional groups. The surface organic coating improves the compatibility between CaCO3 whiskers and asphalt molecules, thereby enhancing the adhesion performance between CaCO3 whiskers and asphalt molecules.
In this article, two types of SCA KH550 and KH570 were used. The difference was in the X group. The former was an amino functional group, while the latter was a methacryloxy functional group. Both groups can dissolve in asphalt and undergo coupling reactions. KH550 is a colorless liquid, alkaline, and highly versatile. KH570 appears as a colorless or slightly yellow liquid with a slightly oily appearance. The chemical structural formulas of the two SCAs are shown in Figure 3.
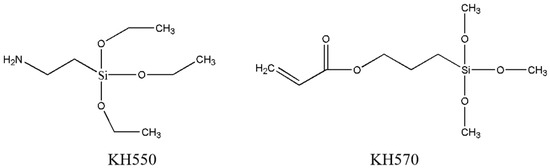
Figure 3.
Chemical structure of SCA (KH550 and KH570) [11].
2.2. Surface Treatment Process of CaCO3 Whiskers
In order to improve the utilization efficiency of SCAs in industry, it is generally necessary to first prepare an SCA treatment solution, and then treat the surface of the reinforcement in different ways. Usually, loose materials are treated with a impregnation method, while those with fixed shapes are treated with a brush coating method. In this paper, the prepared SCA solution was used to impregnate the whiskers. The experimental steps are as follows:
- (a)
- The SCA solution was prepared. At room temperature, the solution with a mass ratio of anhydrous ethanol to deionized water of 3:7 was prepared. Then, SCA was added to the ethanol solution at a mass ratio of 2.5%. The mixture was stirred and allowed to stand for half an hour in order to fully alcoholize the hydrolyzed groups of SCA.
- (b)
- CaCO3 whiskers without impurities were added to the solution in step (a). The mixture was stirred with a glass rod for 15 min, and then allowed to stand at room temperature for 30 min.
- (c)
- The solution is filtered to obtain a whisker filter cake. To avoid the residual unreacted SCA on the surface of CaCO3 whiskers, distilled water was used to rinse and wash the filter cake at room temperature.
- (d)
- CaCO3 whiskers were recycled and dried in an 80 °C vacuum drying oven for 10 h.
2.3. Microscopic Characterization Results and Discussion
2.3.1. SEM
In this study, the original and surface-modified whiskers were treated with gold spray. The surface morphology of the whiskers was observed using a ZEISS EVO 10 scanning electron microscope (SEM) (Carl Zeiss AG, Oberkochen, Germany) to analyze the surface modification effects of two types of SCA, KH-550 and KH-570.
The morphology of CaCO3 whiskers are important indicators for evaluating their performance. From Figure 4, it can be observed that CaCO3 whiskers grow in single-crystal form, with a slender needle-like appearance. The whiskers selected in the test are hexagonal prisms. If the cross-section is approximately circular, the diameter is between 1 μm and 3 μm. At the same time, it can be observed that the length of the whiskers varies, with longer ones reaching over 100 μm and shorter ones only a few micrometers. After comparing the original whiskers with the surface modified whiskers, it can be found that the surface of the original whiskers is relatively flat and smooth. The surface of the whiskers treated with the SCA KH550 show an increase in small protrusions, while the surface of the whiskers treated with KH570 is covered with an oil-like coating layer, indicating an increase in the specific surface area of the treated whiskers and more contact surfaces with asphalt. After the surface of the whiskers was coated with a layer of reagent, its surface becomes rough, which is conducive to improving the friction between the whiskers and asphalt, enhancing the bonding strength, effectively overcoming the shortcomings of the low absorption rate and poor stability of whisker asphalt. On the other hand, it also makes it difficult for the whiskers to stick together, improving the dispersion of the whisker particles.
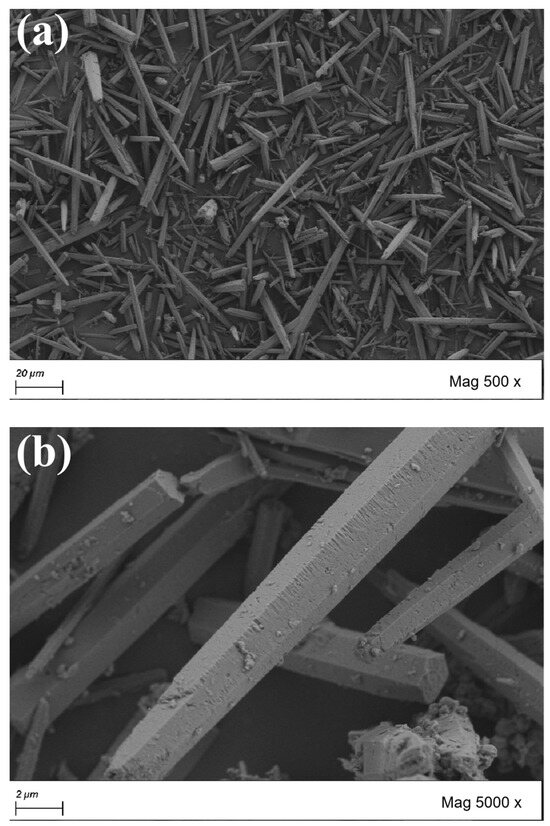
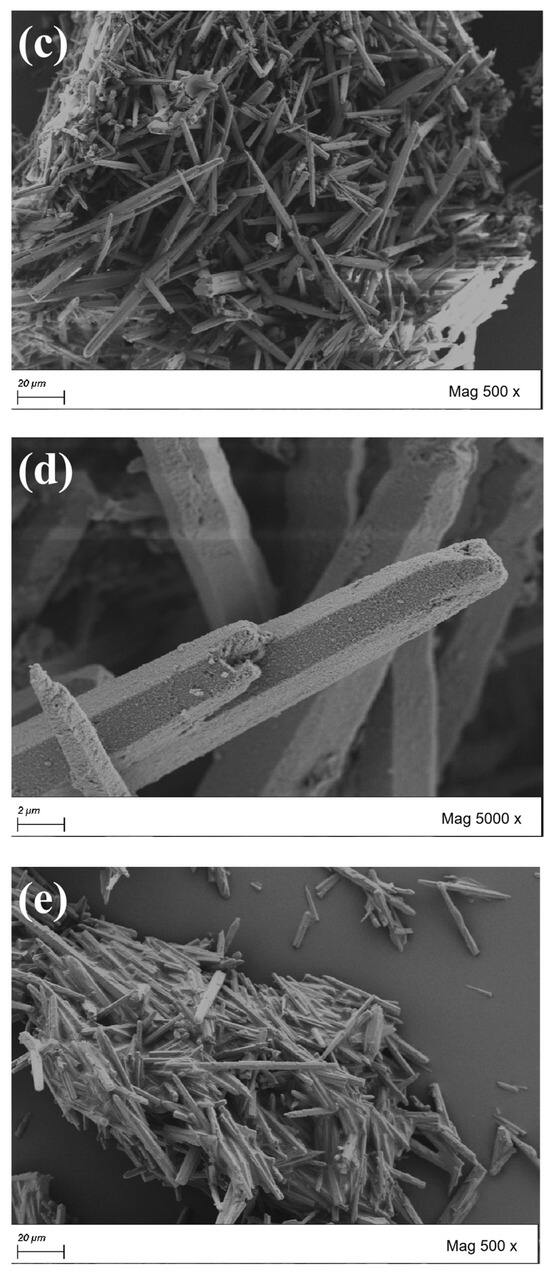
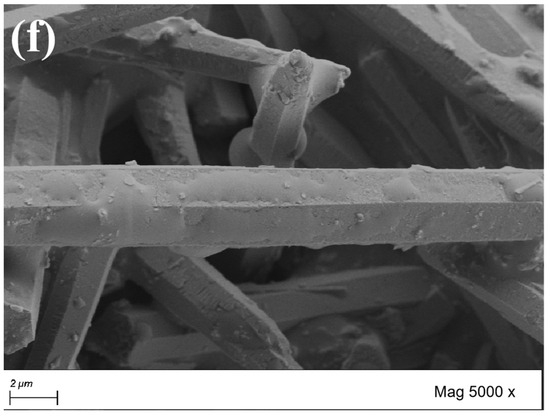
Figure 4.
Scanning electron micrographs of CaCO3 whiskers before and after surface treatment. (a) Original whisker (500×); (b) original whisker (5000×); (c) whiskers (KH550 treatment, 500×); (d) whiskers (KH550 treatment, 5000×); (e) whiskers (KH570 treatment, 500×); (f) whiskers (KH570 treatment, 5000×).
2.3.2. EDS
An Energy-Dispersive Spectrometer (EDS) is generally used in conjunction with SEM to analyze and calculate the types, distribution, and content of elements in selected material micro-areas during SEM scanning. It is a device for detecting the surface elements of materials.
An EDS was used to detect the micro-areas of the original sample and the whisker material surface treated with silane coupling agent. The experimental results are shown in Figure 5. It can be seen that spectral peaks of various elements were detected on the surface of the whiskers. Among them, Ca and O elements have the highest energy values and intensities, followed by the C element, indicating that Ca, O, and C elements are the most abundant elements in whiskers. In Figure 5a, C, O, and Ca elements can be detected in the micro-regions of the original whisker material. Figure 5b shows the whiskers treated with KH550. Compared with the original whiskers, the most significant changes were observed in the addition of Si and N. KH550 contains a large amount of Si element and amino groups, which can form stronger hydrogen bonds with polar hydrogen atoms in asphalt molecules, thereby enhancing the adhesion between whiskers and asphalt. Figure 5c shows the whiskers treated with KH570, which can be seen to be mainly composed of C, O, Si, and Ca elements.
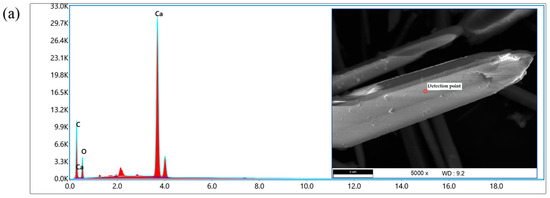
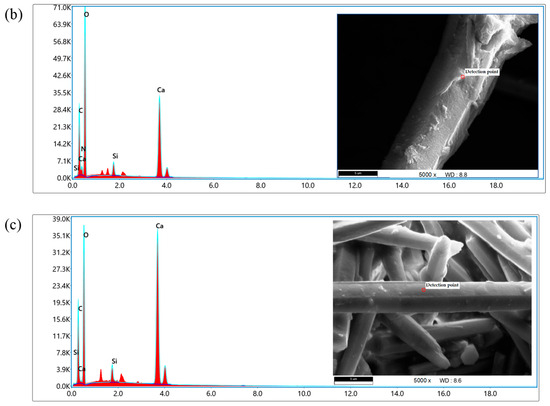
Figure 5.
EDS test results of CaCO3 whiskers. (a) CaCO3 whiskers; (b) CaCO3 whiskers (KH-550 treatment); (c) CaCO3 whiskers (KH-570 treatment).
2.3.3. Contact Angle
The equipment used was the CA500S fully automatic contact-angle measuring device from Guangdong Beidou Precision Instrument Co., Ltd. (Dongguan, China). Deionized water was used as a detection liquid to measure the contact angle. During the test, a certain amount of CaCO3 whiskers were made into a sample with a diameter of 12 mm using the compression method. Then, the sample was automatically measured using the equipment.
The contact angle is the main criterion for the wettability of solid materials. The equilibrium contact angle is usually defined as the angle from the solid–liquid interface to the gas–liquid interface through the interior of the droplet at the junction of three phases, represented by the symbol θ. The stop-drop method was applied to measure the contact angle between the surface of the whisker sample and the water. This method is simple, convenient, and has a high accuracy in measurement. Figure 6 shows the experimental results.
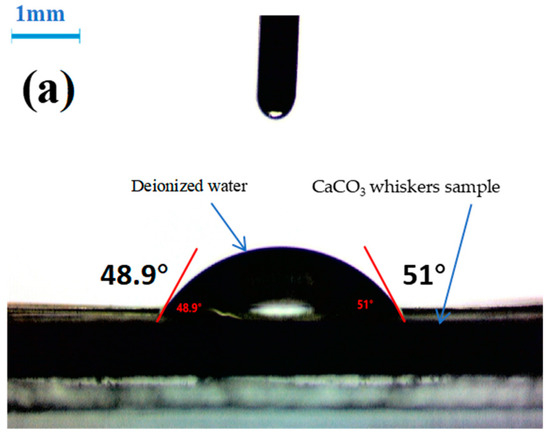
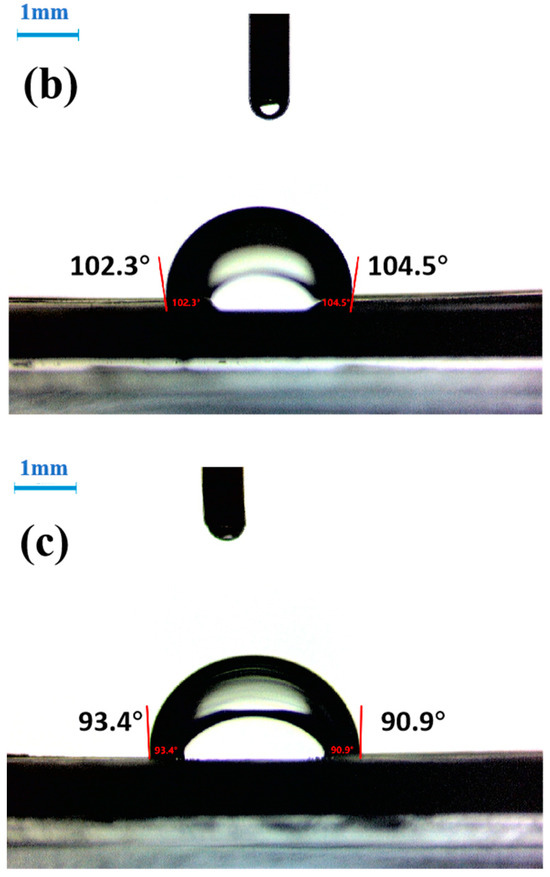
Figure 6.
Contact angle of whisker with water: (a) original whisker; (b) whisker (KH550 treatment); (c) whisker (KH570 treatment).
The contact angle can be used to evaluate the wettability of the material surface. The small contact angle θ indicates a strong wettability on the surface of the sample to be tested. If θ is large, it indicates a poor wettability. If θ is 0°, it indicates complete wetting. If θ is 180°, the detection liquid condenses into small balls on the surface of the sample. From Figure 6, it can be observed that the contact angle is 50° between the original CaCO3 whiskers and water, and the water partially wets the surface of the whiskers. After treatment with KH550 on the surface of CaCO3 whiskers, the measured contact angle with water is 92.2°. After treatment with KH570, the measured contact angle with water is 103.4°. This indicates that after the surface of the whiskers is treated with SCA, the degree of surface organization increases. As θ increases, the wettability decreases, and the surface energy of the whiskers decreases. Sajid et al. have found that surface treatment with ionic liquids reduces the surface free energy of steel, making it easier to wet [12,13]. In this study, the surface of CaCO3 whiskers was treated with SCA, which increased the contact angle with polar detection liquid (water), reduced the surface free energy of the whiskers, and made them hydrophobic and oleophilic, making it easier for them to bond with the asphalt.
3. Molecular Dynamics Mechanism of Surface Modification of CaCO3 Whiskers
Figure 7 is a schematic diagram of the contact interface between fibers and the matrix. The adhesion performance of the fiber–matrix interface is extremely important for transmitting stress and enhancing the mechanical properties of composite materials. Although CaCO3 whiskers have excellent mechanical and physical properties, their main components are inorganic materials. The surface of the finished whiskers is smooth and chemically inert, which makes it difficult to combine with organic asphalt. To further reveal the interface interaction between whiskers and asphalt, a molecular dynamics (MD) simulation method was adopted in this study. Material Studio software was used to construct models of CaCO3 whiskers, SCA, and asphalt molecules, and their rationality was verified. On this basis, the adhesion energy between CaCO3 whiskers and the asphalt interface was used as an indicator to evaluate the adhesion between CaCO3 whiskers and asphalt interface before and after surface modification.
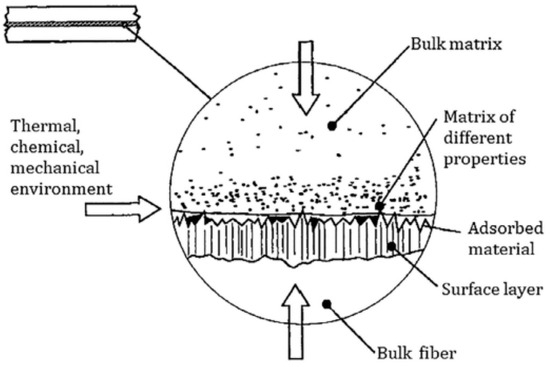
Figure 7.
A schematic diagram of the contact interface between the fiber and the matrix [14].
MD simulation can characterize the relationship between the atomic and molecular structures of materials and their engineering properties. So far, various atomic models have been developed for asphalt and aggregates through MD simulation. Some rheological and thermodynamic properties of asphalt can be calculated, such as the viscosity, diffusion rate, and adhesion performance between asphalt and aggregates [15,16,17,18]. The use of SCA to modify a mineral surface can effectively enhance their dispersibility and interfacial adhesion [19,20,21]. Compared to experiments, molecular dynamics simulation can not only save time and cost, but also explore the essential interaction mechanism from a molecular perspective. In this section, KH550 and KH570 were used as grafting materials to modify the surface of CaCO3 whiskers, in order to explore their influence on the adhesion effect of asphalt.
3.1. Establishment of CaCO3 Whiskers and Asphalt Model Structure
3.1.1. Molecular Dynamics (MD)
A flow chart can reveal the procedure of MD simulation more intuitively in Figure 8.
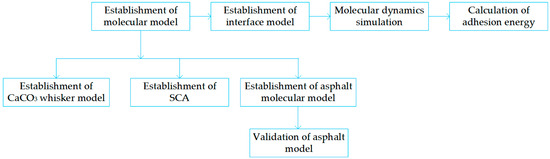
Figure 8.
Flow chart of MD simulation.
The force field is an important component of MD simulation, and the selected force field varies for different research objects. The commonly used force fields in MD include COMPASS, CVFF, and PCFF. Among them, COMPASS is more suitable for organic and inorganic molecular systems and is widely used in asphalt research [22]. In this study, the COMPASS force field was used in MD simulation.
3.1.2. Establishment of CaCO3 Whisker Molecular Model
In Figure 9, a CaCO3 whisker model was constructed, and the (104) surface of the CaCO3 whisker was selected as the reference plane. In previous studies, researchers observed through an atomic force microscope (AFM) and other methods that the surface of CaCO3 (104) was the most stable cleavage surface of CaCO3 whiskers in nature, with the highest probability of exposure in nature [23,24,25]. Two types of SCA were used to modify the surface of the constructed CaCO3 (104) to obtain a modified CaCO3 surface model.
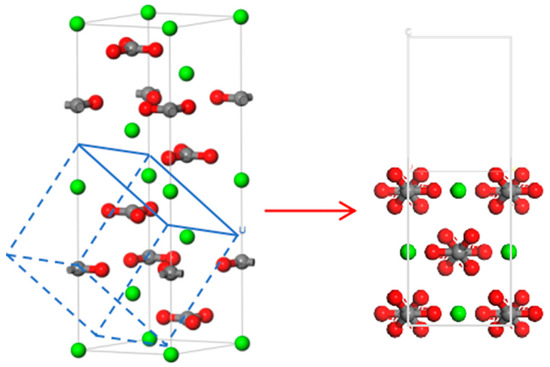
Figure 9.
CaCO3 crystal model.
3.1.3. Establishment of Molecular Model for SCA
According to the chemical structure formula in Figure 3, the molecular models of KH550 and KH570 were established in Figure 10.
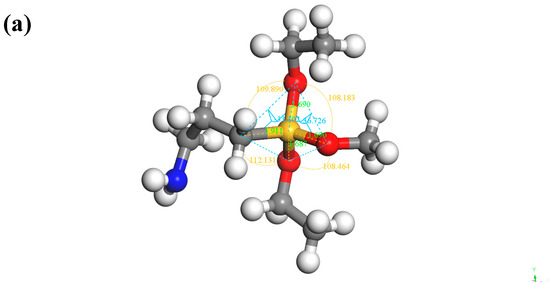
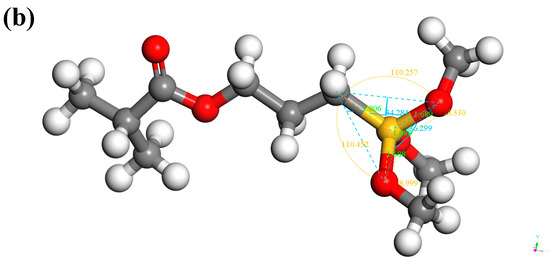
Figure 10.
SCA model: (a) KH550; (b) KH570.
3.1.4. Establishment of Asphalt Molecular Model
In this paper, a 12-component asphalt molecular model was adopted, which has been widely used in academia and has a relatively high accuracy. The Amorphous Cell tool in Materials Studio 2020 was adopted to construct the asphalt model [26]. The 12-component asphalt molecular model was proposed by Li and Greenfield in 2014 [16]. In the model, asphaltene is represented by three molecules (asphaltene phenol, asphaltene pyridine, and asphaltene thiophene). Colloids are represented by five molecules (quinolinohopane, thioisoreneiratane, benzobisbenzothiophene, pyridinohopane, and trimethylbenzeneneoxane). Aromatic phenols are represented by two molecules (PHPN and DOCHN). Saturated phenols are also represented by two molecules (squalane and hopane). The specific molecular model and composition are shown in Figure 11 and Table 1.
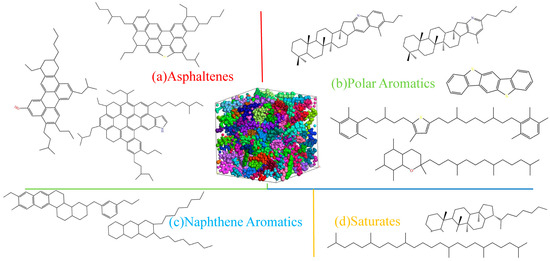
Figure 11.
The 12-component asphalt molecular model.

Table 1.
Composition of 12-component asphalt molecules.
A molecular model of asphalt was constructed using the Materials Studio software, and a 12-component asphalt model was formed using the Amorphous Cell module. Then, the dynamic equilibrium of the model was achieved through the Forcite dynamic calculation module. Under NPT (isothermal and isobaric) conditions, the model gradually equilibrates from an initial loose state to a tightly contacted state. Figure 12 shows the states of asphalt molecules after initial loosening and final equilibrium, respectively.
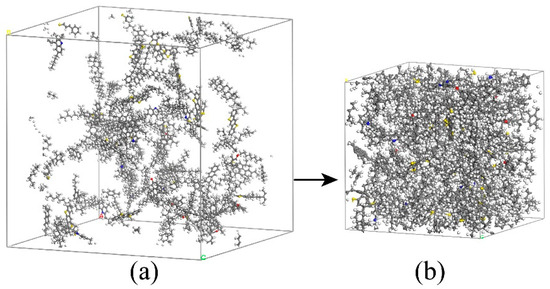
Figure 12.
Aggregation state of asphalt molecules before and after NPT dynamic equilibrium. (a) Initial loosening state; (b) final equilibrium state.
3.1.5. Asphalt Model Validation
In this paper, density and cohesive energy density (CED) were used to verify the rationality of the asphalt model. Figure 13 and Figure 14, respectively, represent the changes in the grain cell edge length and density of the asphalt model from initial construction to NPT equilibrium.
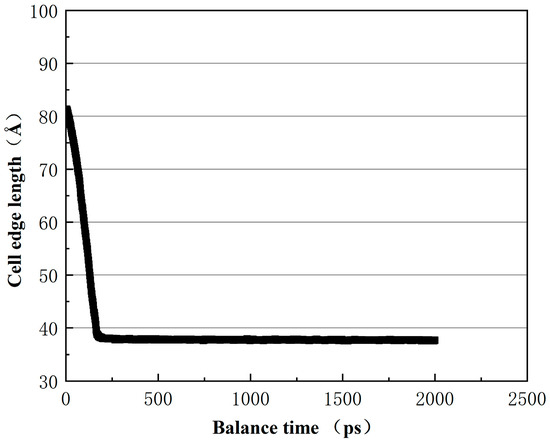
Figure 13.
The change in asphalt unit cell side length with equilibrium time.
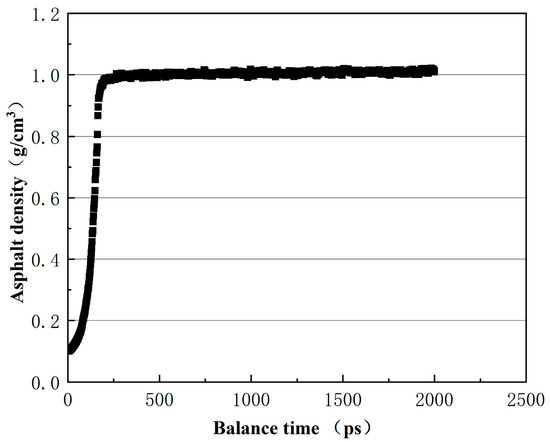
Figure 14.
Density change during equilibrium process of asphalt cell.
From Figure 13, it can be seen that after 300 ps, the asphalt crystal cells tend to stabilize, indicating a gradual equilibrium of the system. In Figure 14, the density of the 12–component asphalt model stabilizes at 1.01 g/cm3, which is consistent with most experimental values (1.0–1.04 g/cm3) [27,28].
Cohesive energy density (CED) represents the energy released by the aggregation of asphalt molecules. The larger the CED value, the greater the viscosity of asphalt molecules. In MD simulation, the interaction between asphalt molecules was evaluated by Van der Waals force δvdw and electrostatic force δele. The definition formula of CED is as follows in Equations (2) and (3) [29,30].
where CED is the cohesive energy density of the asphalt molecule, δ is the solubility parameter, δvdw is the van der Waals force component of solubility parameter, and δele is the electrostatic force component of the solubility parameter.
Figure 15 shows the CED values of the 12–component asphalt molecules at different temperatures. It can be seen that as the temperature increases, the CED of asphalt molecules gradually decreases. This is because as the temperature increases, the activity intensity of asphalt molecules intensifies, leading to an increase in the intermolecular spacing and a decrease in CED. The CED value is close to or within the reference range [31,32], indicating that the established asphalt molecular model and parameter settings are reasonable and effective.
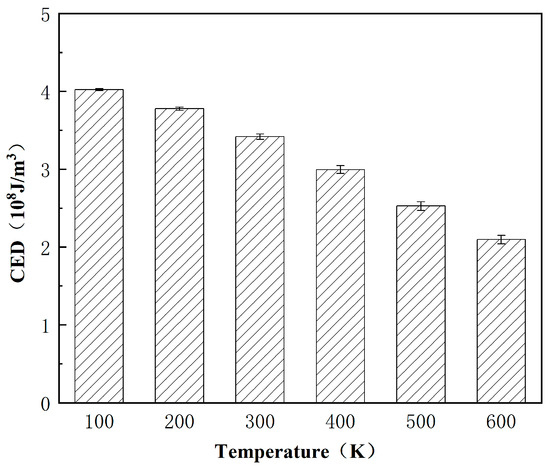
Figure 15.
The relationship between CED and temperature.
3.2. Research on Interface Adhesion Between CaCO3 Whiskers and Asphalt
3.2.1. Interface Model Establishment
The surface model supercell is treated to combine with the asphalt model to form an asphalt–whisker interface model. On the asphalt–whisker interface model, a 30-ohm vacuum layer is set to eliminate the influence of periodic boundary conditions on the model. Finally, the interface model shown in Figure 16e was obtained.
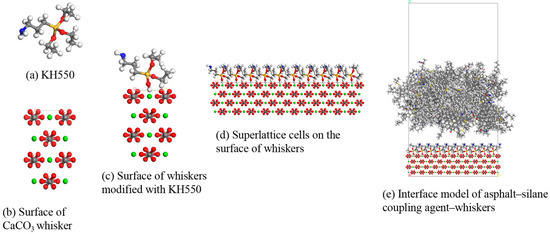
Figure 16.
Construction of interface model between asphalt and CaCO3 whiskers after surface modification (KH550 as example).
3.2.2. Molecular Dynamics Simulation
In the process of ensemble optimization, annealing, and the dynamic calculation of the constructed interface model, the COMPASS force field was used. The molecular dynamics simulation process was conducted in the isothermal–isocratic NVT ensemble for 300 ps, and the final equilibrium kinetic temperature was 298 K. The time step of all dynamic equilibrium based on MD simulation was 1 fs, and the cut-off distance was 12.5 Å. The temperature control was carried out using the Nose method and the pressure-control method was the Berendsen method.
3.2.3. Adhesion Energy Calculation
As shown in Figure 17, after a 300 ps NVT dynamic calculation, the energy indicators of the interface model tend to stabilize, indicating that asphalt gradually adheres to the surface of CaCO3 whiskers to form a stable interface bonding model. After obtaining a stable interface model, the last 30 frames of the trajectory file were selected for adhesion energy calculation. By calculating the potential energy, kinetic energy, non-bond energy, and total energy during the optimization process of the NVT dynamic structure, it was found that the energy at the interface was initially large, but remained in a certain stable state as the dynamic optimization process progressed.
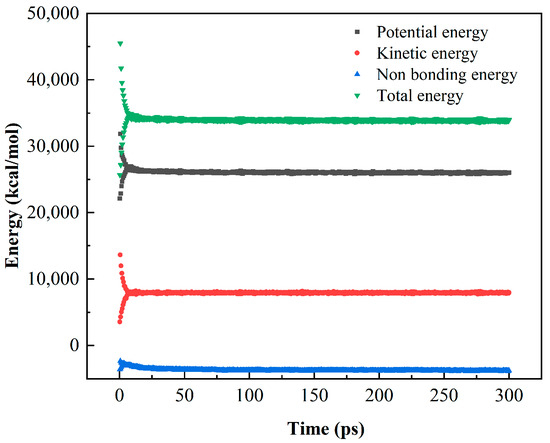
Figure 17.
Energy variation during NVT dynamic calculation process.
When calculating interface adhesion energy, the adhesion energy calculation formula [33] is used, as shown in Equation (4).
where is the final adhesion energy between asphalt and whiskers (mJ/m2). is the energy of the asphalt portion in the interface model (kcal/mol). is the energy of the whisker portion in the interface model (kcal/mol). is the total potential energy of the interface model after NVT equilibrium (kcal/mol). A is the interface contact area (Å2), and 695 is the unit convention factor. Figure 16 shows the model used for interface energy calculation, and Figure 18 shows the results of adhesion energy calculation.
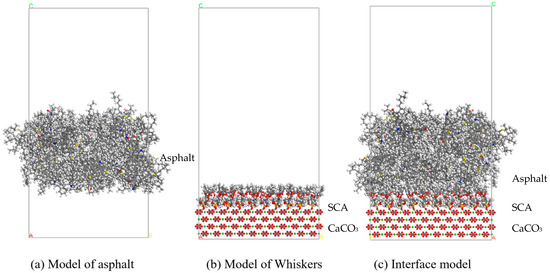
Figure 18.
Model diagram of asphalt, whiskers, and asphalt–whisker interfaces.
From Figure 19, it can be seen that the adhesion energy between CaCO3 whiskers and asphalt has been improved to varying degrees after modification with SCA. After treatment with KH570 or KH550, the van der Waals force increased from 81.8 mJ/m2 to 88.5 mJ/m2 and 93.3 mJ/m2, the Coulomb electrostatic force increased from 18.3 mJ/m2 to 24 mJ/m2 and 33.3 mJ/m2, and the total interfacial adhesion energy increased from 100.1 mJ/m2 to 112.5 mJ/m2 and 126.6 mJ/m2. The increase in adhesion energy for modification with KH550 is greater than that of KH570. The adhesion energy between asphalt and CaCO3 whiskers is composed of van der Waals forces and Coulomb electrostatic forces. After modification with SCA, both Coulomb electrostatic forces and van der Waals forces increase. The simulation results indicate that the treatment of the CaCO3 whisker surface with SCA can improve the bonding strength with asphalt, thereby enhancing the ability to resist water damage. Zhao et al. found that silane coupling agents with a certain degree of crosslinking can enhance interfacial adhesion, which also confirms the same conclusion [34].
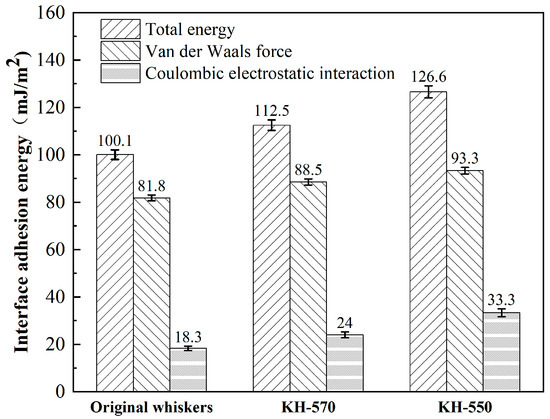
Figure 19.
Calculation results of adhesion energy.
3.3. Correlation Analysis Between Molecular Dynamics Results and Microscopic Characterization
The microscopic characterization results of CaCO3 whiskers mentioned above showed that after treatment with KH550 and KH570, the surfaces of the whiskers observed by SEM become rougher. In terms of contact angle, after treatment, the contact angle θ increased and the wettability with water decreased, which made it difficult for external moisture to invade the interface and reduced the occurrence of moisture damage. This also indicated an increase in the degree of organification on the surface of CaCO3 whiskers. The interface changed from hydrophilic to hydrophobic, and the bonding between the whiskers and asphalt was tighter, thereby improving the adhesion energy with asphalt. This is consistent with the MD simulation results; that is, after being treated with SCA, the adhesion energy between the whiskers and asphalt increases to varying degrees.
In addition, two types of SCA (KH550 and KH570) contain different functional groups, resulting in different strength effects on adhesion energy. KH-550 contains amino groups, while KH570 contains epoxy groups. Amino groups may form stronger hydrogen bonds (O-H… N and N-H… O) with polar hydrogen atoms in asphalt molecules, resulting in stronger adhesion energy on the treated CaCO3 surface. The content of this study is limited to physical adsorption, and in the future, more in-depth research will be conducted on topics related to graft bonding and chemical cross-linking.
4. Conclusions
This study has a certain reference value for improving the application of CaCO3 whiskers in modified asphalt. However, further analysis is still needed in the study of the consistency between microscopic experimental results and molecular dynamics simulation results. The following are the main conclusions of this article.
- (1)
- The SEM results indicate that the surface of CaCO3 whiskers becomes rough after treatment with SCA. The EDS results showed that the amino group contained in KH550 can form stronger hydrogen bonds with polar hydrogen atoms in asphalt molecules, enhancing the adhesion between whiskers and asphalt.
- (2)
- The contact angle test showed that treating the surface of CaCO3 whiskers with SCA increased the contact angle with polarity detection liquid (water) and reduced the surface free energy of the whiskers. This makes the whiskers surface hydrophobic and oleophilic, making it easier to bond with asphalt.
- (3)
- MD simulation results show that the adhesion energy between whiskers and asphalt increased to varying degrees after treatment with SCA; KH550 had a much stronger effect than KH570, which is consistent with the results of microscopic characterization experiments.
Author Contributions
Writing—original draft, X.X.; data curation, J.W.; visualization, Q.Z. and G.S.; writing—review and editing, X.X. and J.W.; supervision, J.Z., Y.W., and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R&D Program of China (no. 2021YFB2600600 and 2021YFB2600603), China Postdoctoral Science Foundation (no. 2019M653521 and 2023M731427), Fundamental Research Program of Shanxi Province (no. 202303021211243), Scientific and Technologial Innovation Programs of Higher Education Institutions in Shanxi (no. 2022L579), Taiyuan University Talent Introduction and Research Start up Fund Project (no. 24TYKY021), Research Results of Education Science Planning Project in Shanxi Province (No. GH-220783). The authors gratefully acknowledge this financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Authors Xiangyang Xing and Qingyue Zhou were employed by the company Jiangsu Zengguang New Materials Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Liu, J. CaCO3 Whiskers Prepared by Sol-Gel Method and Modification. Ph.D. Dissertation, Dalian University of Technology, Dalian, China, 2012. [Google Scholar]
- Chen, Y.; Tang, C. Mechanical properties of calcium carbonate whisker hybrid polyethylene fiber reinforced cement-based composite. Bull. Chin. Ceram. Soc. 2024, 43, 16–26. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, H.; Hu, J. Mechanical properties of high cost performance hybrid fiber engineered cementitious composites. Bull. Chin. Ceram. Cociety 2023, 42, 3816–3826. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, Z.; Yu, Z. Flexural properties of calcium carbonate whisker reinforced cementitious composites after exposed to elevated temperature. J. Funct. Mater. 2023, 54, 9100–9106. [Google Scholar] [CrossRef]
- Song, M.; Wang, J.; Luan, C. Effect of calcium carbonate whisker on SHCC properties. Concrete 2022, 3, 14–19. [Google Scholar] [CrossRef]
- Xing, X.; Pei, J.; Li, R.; Tan, X. Effect and mechanism of calcium carbonate whisker on asphalt binder. Mater. Res. Express 2019, 6, 055306. [Google Scholar] [CrossRef]
- Xing, X.; Pei, J.; Shen, C.; Li, R.; Zhang, J.; Huang, J.; Hu, D. Performance and Reinforcement Mechanism of Modified Asphalt Binders with Nano-Particles, Whiskers, and Fibers. Appl. Sci. 2019, 9, 2995. [Google Scholar] [CrossRef]
- Yutong, L.; Zeliang, Y.; Hui, L.; Lei, X. Preparation, characterization, and properties of asphalt modified by surface-treated anhydrous calcium sulfate whiskers. Constr. Build. Mater. 2023, 384, 131370. [Google Scholar] [CrossRef]
- Hu, F.; Chen, G.; Du, Y. Material List Interface, 2nd ed.; East China University of Science and Technology Press: Shanghai, China, 2007; pp. 116–133. [Google Scholar]
- Xiang, Y.; Xie, Y.; Long, G. Effect of basalt fiber surface silane coupling agent coating on fiber-reinforced asphalt: From macro-mechanical performance to micro-interfacial mechanism. Constr. Build. Mater. 2018, 179, 107–116. [Google Scholar] [CrossRef]
- Peng, C.; Chen, P.; You, Z.; Lv, S.; Zhang, R.; Xu, F.; Zhang, H.; Chen, H. Effect of silane coupling agent on improving the adhesive properties between asphalt binder and aggregates. Constr. Build. Mater. 2018, 169, 591–600. [Google Scholar] [CrossRef]
- Sajid, H.U.; Kiran, R. Influence of corrosion and surface roughness on wettability of ASTM A36 steels. J. Constr. Steel Res. 2018, 144, 310–326. [Google Scholar] [CrossRef]
- Sajid, H.U.; Kiran, R. Improving the wettability of structural steels by employing ionic liquids. J. Mol. Liq. 2021, 324, 115137. [Google Scholar] [CrossRef]
- Teklal, F.; Djebbar, A.; Allaoui, S.; Hivet, G.; Joliff, Y.; Kacimi, B. A review of analytical models to describe pull-out behavior—Fiber/matrix adhesion. Compos. Struct. 2018, 201, 791–815. [Google Scholar] [CrossRef]
- Hansen, J.S.; Lemarchand, C.; Nielsen, E.; Dyre, J.C.; Schrøder, T. Four-component united-atom model of bitumen. J. Chem. Phys. 2013, 138, 94508. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Greenfield, M.L. Chemical compositions of improved model asphalt systems for molecular simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Q.; Yang, J.; Yang, R.; Zhu, J. Study of adhesion between crack sealant and pavement combining surface free energy measurement with molecular dynamics simulation. Constr. Build. Mater. 2020, 240, 117900. [Google Scholar] [CrossRef]
- Zhang, L.; Greenfield, M.L. Relaxation time, diffusion, and viscosity analysis of model asphalt systems using molecular simulation. J. Chem. Phys. 2007, 127, 194502. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Xiao, S. Molecular dynamics simulation of thermodynamic properties of modified SiO2 enhanced epoxy resin. High Volt. Technol. 2018, 44, 740–749. [Google Scholar] [CrossRef]
- Li, Y.; Meng, F.; Zhang, X.; Zhou, X.; Mei, Y. The effect of SiO2 surface modification on the properties of meta aramid insulating paper. Insul. Mater. 2019, 52, 22–28. [Google Scholar] [CrossRef]
- Wang, X. Microscopic Mechanism Study of Silane Coupling Agent Modified Nano SiO2 on Its Modified Cellulose Insulation Paper Properties. Ph.D. Dissertation, Southwest University, Chengdu, China, 2019. [Google Scholar]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Hillner, P.E.; Manne, S.; Hansma, P.K.; Gratz, A.J. Atomic force microscope: A new tool for imaging crystal growth processes. Faraday Discuss. 1993, 95, 191–197. [Google Scholar] [CrossRef]
- Stipp, S.L.S.; Eggleston, C.M.; Nielsen, B.S. Calcite surface structure observed at microtopographic and molecular scales with atomic force microscopy (AFM). Geochim. Cosmochim. Acta 1994, 58, 3023–3033. [Google Scholar] [CrossRef]
- Herman, A.; Addadi, L.; Weiner, S. Interactions of sea-urchin skeleton macromolecules with growing calcite crystals|[mdash]|a study of intracrystalline proteins. Nature 1988, 331, 546–548. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, Z.; Liu, J.; Ma, F.; Peng, C.; Li, C.; Chang, X.; Zhao, P. Molecular dynamics simulation and experimental analysis on fluidity improvement of liquid rubber modified asphalt binder. Constr. Build. Mater. 2023, 402, 133027. [Google Scholar] [CrossRef]
- Jin, J.; Chen, H.; Liu, S.; Xiao, M.; Liu, L. Study on preparation and properties of phase change modified asphalt for the functional pavement. Constr. Build. Mater. 2024, 439, 137248. [Google Scholar] [CrossRef]
- Wei, H.; Liu, Y.; Li, J.; Wang, F.; Zheng, J.; Yuan, Z. Characterizing fatigue damage evolution in asphalt mixtures using acoustic emission and Gaussian mixture model analysis. Constr. Build. Mater. 2023, 409, 133973. [Google Scholar] [CrossRef]
- Guoqing, S.; Zhenxing, N.; Jiupeng, Z.; Xiaoyong, T.; Yufei, J.; Zixuan, C. Impacts of asphalt and mineral types on interfacial behaviors: A molecular dynamics study. Case Stud. Constr. Mater. 2022, 17, e01581. [Google Scholar]
- Xu, G.; Wang, H. Study of cohesion and adhesion properties of asphalt concrete with molecular dynamics simulation. Comput. Mater. Sci. 2016, 112, 161–169. [Google Scholar] [CrossRef]
- Zhi, Z.; Naisheng, G.; Zhanping, Y.; Jiawei, W. Research on compatibility mechanisms between waste wood oil and petroleum asphalt through molecular dynamics. CIESC J. 2023, 74, 4037–4050. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Yang, J.; Luo, S.; Liu, Y.; Huang, W.; Hu, J.; Xu, G.; Min, Z. Force field benchmark of asphalt materials: Density, viscosity, glass transition temperature, diffusion coefficient, cohesive energy density and molecular structures. J. Mol. Liq. 2024, 398, 124166. [Google Scholar] [CrossRef]
- Chu, L.; Luo, L.; Fwa, T.F. Effects of aggregate mineral surface anisotropy on asphalt-aggregate interfacial bonding using molecular dynamics (MD) simulation. Constr. Build. Mater. 2019, 225, 1–12. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Xu, Q.; Wang, K.; Yu, Y.; Zhao, Q.; Jiang, M.; Liu, P. Molecular dynamics simulation: The roles of silane coupling agent structural configurations on quartz fiber-epoxy interface. Comput. Mater. Sci. 2024, 235, 112833. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).