Stainless Steel Deposits on an Aluminum Support Used in the Construction of Packaging and Food Transport Containers
Abstract
1. Introduction
- –
- To have a high degree of physicochemical stability, which does not allow the release of foreign substances into the extraction media, which reproduce the exploitation conditions;
- –
- Not to confer toxicity to the food product it comes into contact with;
- –
- To ensure efficient protection for the food product against other accidental contamination during the entire period of processing, storage, and transport of the respective product.
2. Materials and Methods
2.1. Materials Used
2.2. Obtaining Coating
2.3. Coating Characterization
2.4. Organoleptic Analysis of Food Liquids
3. Results and Discussion
4. Conclusions
- –
- The deposits of 80T of food-grade food-grade stainless steel, which have low porosity (below 3.8%), can be used for the purpose of isolating the aluminum alloy and stopping the phenomenon of migration of aluminum ions inside food liquids;
- –
- The adhesion and high microhardness of the deposits make them resistant to shock and variable tests that occur during the transport of liquid food products;
- –
- Increasing the acidity of the milk causes the multiplication of lactic bacteria. The aluminum alloy has a higher specific density than the alloy of aluminum alloy combined with food-grade stainless steel. That is why the new material in contact with the milk will not influence the development of lactic bacteria;
- –
- The innovative solution of the new material composed of aluminum alloy combined with food-grade stainless steel offers advantages for the advanced preservation of acidophilic milk, drinking milk, wines, juices, and oils because the low porosity of food-grade stainless steel deposits prevents the migration of chemical elements from the packaging material into the liquid food;
- –
- The coating solution for aluminum alloy containers with food-grade stainless steel on the inside prevents the oxidation and alteration of the wines, reducing the loss of alcohol and bouquet through evaporation and implicitly reducing the costs as well;
- –
- Also, the innovative material significantly improves the sensory characteristics of the juices and the oils, like the appearance, color, and clarity of the oils that have been stored in aluminum alloy containers covered with food-grade stainless steel through the arc spraying process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Partha, P.D.; Ragesh, P.; Ng, K.W. Advances in biomaterials based food packaging systems: Current status and the way forward. Biomater. Adv. 2024, 164, 213988. [Google Scholar]
- Boboescu, R.; Sporea, I.; Tokar, A.; Bujor, V.; Grincenau, G. Reliability—Durability, Reliability & Durability; House Publishing Academica Brâncusi: Târgu Jiu, Romania, 2018; Volume 1. [Google Scholar]
- Swarup, R.; Ruchir, P.; Łopusiewicz, Ł.; Biswas, D.; Chandel, V.; Rhim, J.W. Recent progress in pectin extraction, characterization, and pectin-based films for active food packaging applications: A review. Int. J. Biol. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef]
- Hadidi, M.; Jafarzadeh, S.; Forough, M.; Garavand, F.; Alizadeh, S.; Salehabadi, A.; Khaneghah, A.M.; Jafari, S.M. Plant protein-based food packaging films; recent advances in fabrication, characterization, and applications. Trends Food Sci. Technol. 2022, 120, 154–173. [Google Scholar] [CrossRef]
- Wang, H.; Cao, Z.; Yao, L.; Feng, T.; Song, S.; Sun, M. Insights into the Edible and Biodegradable Ulvan-Based Films and Coatings for Food Packaging. Foods 2023, 12, 1622. [Google Scholar] [CrossRef] [PubMed]
- Thulasisingh, A.; Kumar, K.; Yamunadevi, B.; Poojitha, N.; SuhailMadharHanif, S.; Kannaiyan, S. Biodegradable packaging materials. Polym. Bull. 2021, 79, 4467–4496. [Google Scholar] [CrossRef]
- Plastic Can Be Found Everywhere in the Ocean. Available online: https://plasticsoep.sites.uu.nl/en/plastic-found-everywhere-ocean/ (accessed on 18 August 2024).
- Erginkaya, Z.; Kalkan, S.; Ünal, E. Use of Antimicrobial Edible Films and Coatings as Packaging Materials for Food Safety. In Food Processing: Strategies for Quality Assessment; Springer: Berlin/Heidelberg, Germany, 2014; pp. 261–295. [Google Scholar]
- Amin, U.; Khan, M.U.; Majeed, Y.; Rebezov, M.; Khayrullin, M.; Bobkova, E.; Shariati, M.A.; Chung, I.M.; Thiruvengadam, M. Potentials of polysaccharides, lipids and proteins in biodegradable food packaging applications. Int. J. Biol. Macromol. 2021, 183, 2184–2198. [Google Scholar] [CrossRef]
- Morris, S.A. Food and Package Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Yaashikaa, P.R.; Kamalesh, R.; Senthil Kumar, P.; Saravanan, A.; Vijayasri, K.; Rangasamy, G. Recent advances in edible coatings and their application in food packaging. Food Res. Int. 2023, 173, 113366. [Google Scholar] [CrossRef]
- Huang, C.C.; Ma, H.W. A multidimensional environmental evaluation of packaging materials. Sci. Total Environ. 2004, 324, 161–172. [Google Scholar] [CrossRef]
- Santos, J.S.; Rodrigues, A.; Simon, A.P.; Ferreira, C.H.; Santos, V.A.Q.; Sikora, M.S.; Cruz, N.C.; Mambrini, G.P.; Trivinho-Strixino, F. One-Step Synthesis of Antibacterial Coatings by Plasma Electrolytic Oxidation of Aluminum. Adv. Eng. Mater. 2019, 21, 1900119. [Google Scholar] [CrossRef]
- Yin, Z.; Tao, S.; Zhou, X.; Ding, C. Microstructure and Mechanical Properties of Al2O3-Al Composite Coatings Deposited by Plasma Spraying Appl. Appl. Surf. Sci. 2008, 254, 1636–1643. [Google Scholar] [CrossRef]
- Huanhuan, S.; Shuqin, M. Microstructural characteristics and protection performances of plasma-sprayed Al2O3–Al composite coatings on AZ91D magnesium alloy. Sci. Eng. Compos. Mater. 2016, 23, 467–474. [Google Scholar] [CrossRef]
- Maragou, N.C.; Tzachristas, A.; Tsochatzis, E.D.; Thomaidis, N.S. Chemical Migration from Wine Contact Materials. Appl. Sci. 2024, 14, 6507. [Google Scholar] [CrossRef]
- Fatimah, S.; Kamil, M.; Kwon, J.; Kaseem, M.; Ko, Y. Dual incorporation of SiO2 and ZrO2 nanoparticles into the oxide layer on 6061 Al alloy via plasma electrolytic oxidation: Coating structure and corrosion properties. J. Alloys. Compd. 2017, 707, 358–364. [Google Scholar] [CrossRef]
- Burrell, S.A.M.; Exley, C. There is (still) too much aluminium in infant formulas. BMC Pediatr. 2010, 10, 63. [Google Scholar] [CrossRef]
- Redgrove, J.; Rodriguez, I.; Mahadevan-Bava, S.; Exley, C. Prescription Infant Formulas Are Contaminated with Aluminium. Int. J. Environ. Res. Public Health 2019, 16, 899. [Google Scholar] [CrossRef]
- Zheng, J.; Tian, L.; Bayen, S. Chemical contaminants in canned food and can-packaged food: A review. Crit. Rev. Food Sci. Nutr. 2021, 63, 2687–2718. [Google Scholar] [CrossRef]
- Packaging and Machinery—Norm. Available online: https://lege5.ro/Gratuit/ge4dcnjx/ambalaje-si-utilaje-norma?dp=geydkmrzg42te (accessed on 22 August 2024).
- Toma, S.L.; Bejinariu, C.; Gheorghiu, D.A.; Baciu, C. The improvement of the physical and mechanical properties of steel, deposits obtained by thermal spraying in electric arc. Struct. Integr. Welded Struct. 2013, 814, 173–179. [Google Scholar] [CrossRef]
- Toma, S.L.; Chicet, D.-L.; Cazac, A.-M. Numerical Calculation of the Arc-Sprayed Particles’ Temperature in Transient Thermal Field. Coatings 2022, 12, 877. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Saviuc, A.; Tudose-Sandu-Ville, F.; Toma, S.; Nedeff, F.; Rusu, C.M.; Agop, M. Non-Linear Behaviors of Transient Periodic Plasma Dynamics in a Multifractal Paradigm. Symmetry 2020, 12, 1356. [Google Scholar] [CrossRef]
- Calin, M.A.; Curteza, A.; Toma, S.; Agop, M. Morphological properties of polyamide 6-cnt nanofibers obtained by electrospinning method. Metal. Int. 2013, 18, 19–22. [Google Scholar]
- Stescu, C.; Chicet, D.; Tufescu, A.; Istrate, B.; Munteanu, C.; Strugaru-Iacob, S. Contact stress simulation problem in case of thermal spray coatings. IOP Conf. Ser. Mater. Sci. Eng. 2020, 916, 012114. [Google Scholar] [CrossRef]
- Bejinariu, C.; Burduhos-Nergis, D.P.; Cimpoesu, N.; Bernevig-Sava, A.; Toma, S.L. Study on the anticorrosive phosphated steel carabiners used at personal protective equipment. Qual. Access Success 2019, 20, 71–76. [Google Scholar]
- Cazac, A.M.; Bejinariu, C.; Baciu, C.; Toma, S.L.; Florea, C.D. Experimental Determination of Force and Deformation Stress in Nanostructuring Aluminum by Multiaxial Forging Method. Appl. Mech. Mater. 2014, 657, 137–141. [Google Scholar] [CrossRef]
- Zare, M.A.; Taghiabadi, R.; Ghoncheh, M.H. Effect of Cooling Rate on Microstructure and Mechanical Properties of AA5056 Al-Mg Alloy. Int. J. Met. 2021, 16, 1533–1543. [Google Scholar] [CrossRef]
- Toma, B.F.; Baciu, R.E.; Bejinariu, C.; Cimpoieșu, N.; Ciuntu, B.M.; Toma, S.L.; Burduhos-Nergis, D.P.; Timofte, D. Researches on the improvement of the bioactivity of TiO2 deposits, obtained by magnetron sputtering–DC. IOP Conf. Ser. Mat. Sci. Eng. 2018, 374, 012017. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Frankowski, M.; Simeonov, V.; Polkowska, Ż.; Namieśnik, J. Determination of metals content in wine samples by inductively coupled plasma-mass spectrometry. Molecules 2018, 23, 2886. [Google Scholar] [CrossRef]
- Pasvanka, K.; Kostakis, M.; Tarapoulouzi, M.; Nisianakis, P.; Thomaidis, N.S.; Proestos, C. ICP–MS Analysis of Multi-Elemental Profile of Greek Wines and Their Classification According to Variety, Area and Year of Production. Separations 2021, 8, 119. [Google Scholar] [CrossRef]
- Soares, F.; Anzanello, M.J.; Fogliatto, F.S.; Marcelo, M.C.; Ferrão, M.F.; Manfroi, V.; Pozebon, D. Element selection and concentration analysis for classifying South America wine samples according to the country of origin. Comput. Electron. Agric. 2018, 150, 33–40. [Google Scholar] [CrossRef]
- Determination of the Total Acidity of the Wine. Available online: https://isjtulcea.ro/wp-content/uploads/2018/06/Determinarea-aciditatii-totale-a-vinului.pdf (accessed on 16 August 2024).
- Radu, S.; Voinea, O. Biochemical Expertise of Juices Obtained from Local and Exotic Fruits; PIM Publishing House: Iasi, Romania, 2016; pp. 63–64. [Google Scholar]
- Kumar, K.A.; Viswanathan, K. Study of UV transmission through a few edible oils and chicken oil. J. Spectrosc. 2013, 2013, 540417. [Google Scholar]
- Orozco, F.D.A.; Sousa, A.C.; Araujo, M.C.U.; Domini, C.E. A new flow UV-Vis kinetics spectrophotometric method based on photodegradative reaction for determining the oxidative stability of biodiesel. Fuel 2020, 26, 116–197. [Google Scholar]
- Statsoft Inc. STATISTICA for Windows; Statsoft Inc.: Tulsa, OK, USA, 2013. [Google Scholar]
- General Technologies in the Food Industry—Laboratory Work. Available online: https://chimie-biologie.ubm.ro/Cursuri%20on-line/MIHALI%20CRISTINA/Lucrari-laborator_tehnologii_generale-IA.pdf (accessed on 23 August 2024).
- Toma, S.L. The influence of jet gas temperature on the characteristics of steel coating obtained by wire arc spraying. Surf. Coat. Technol. 2012, 220, 261–265. [Google Scholar] [CrossRef]
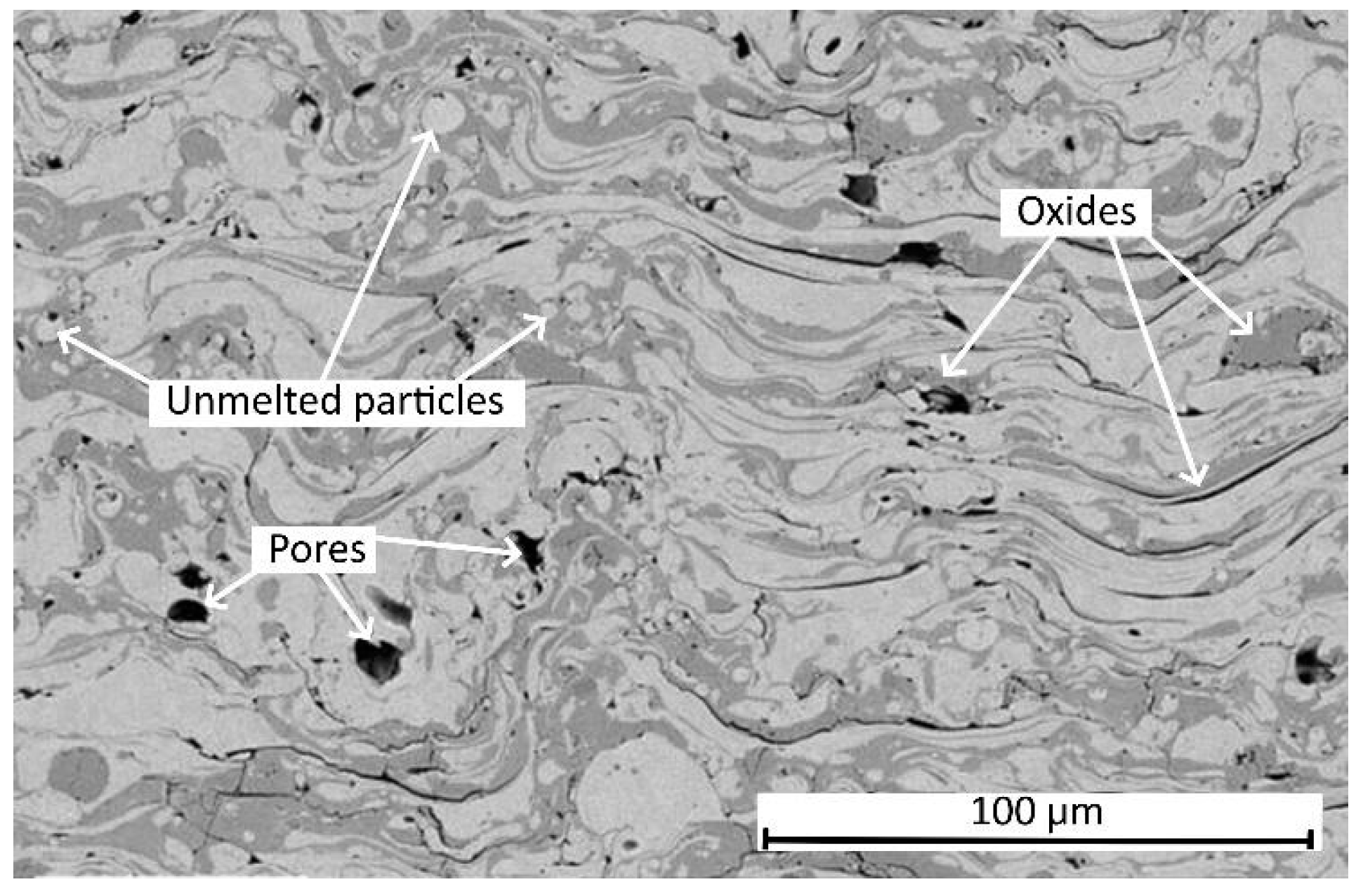
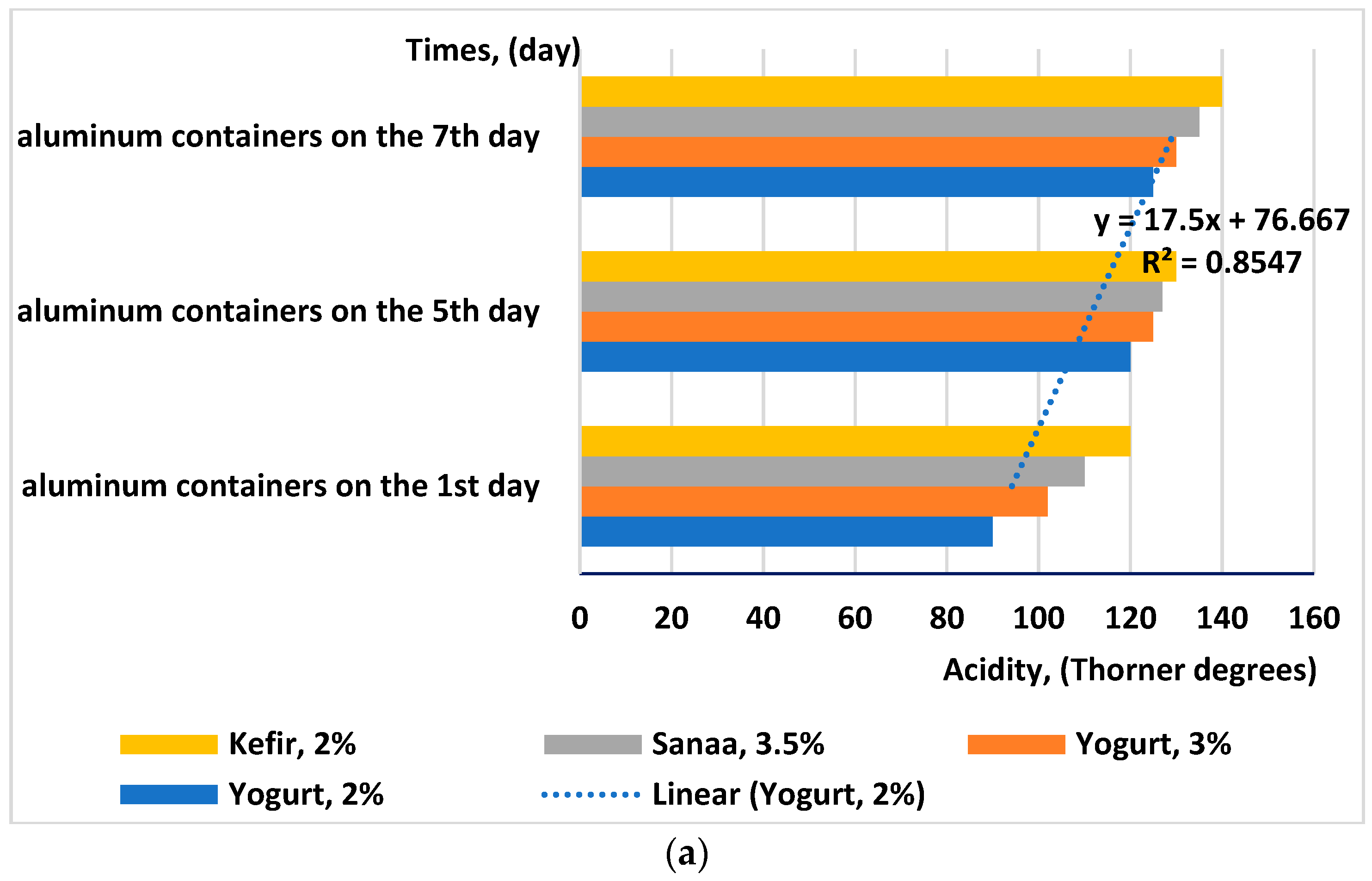

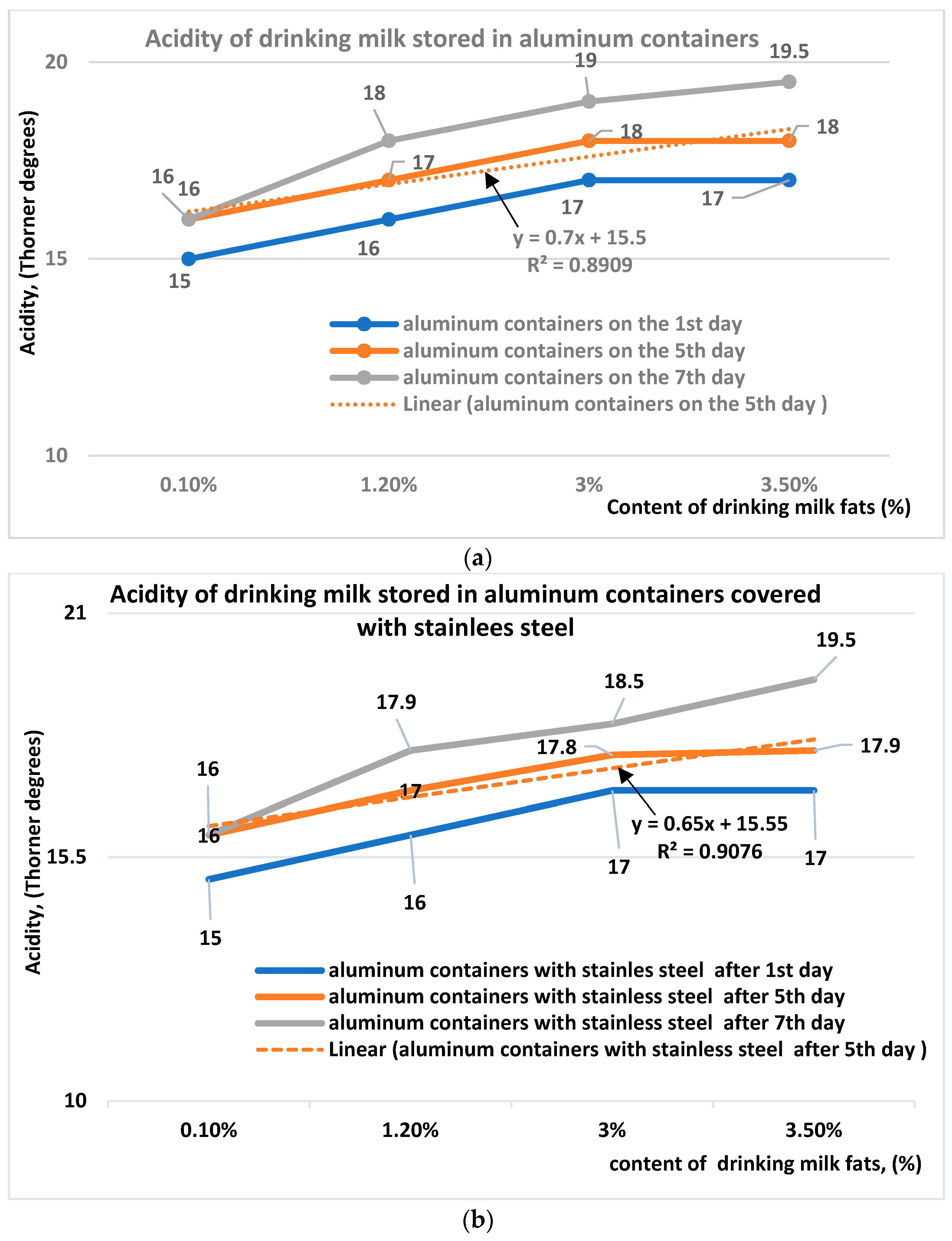
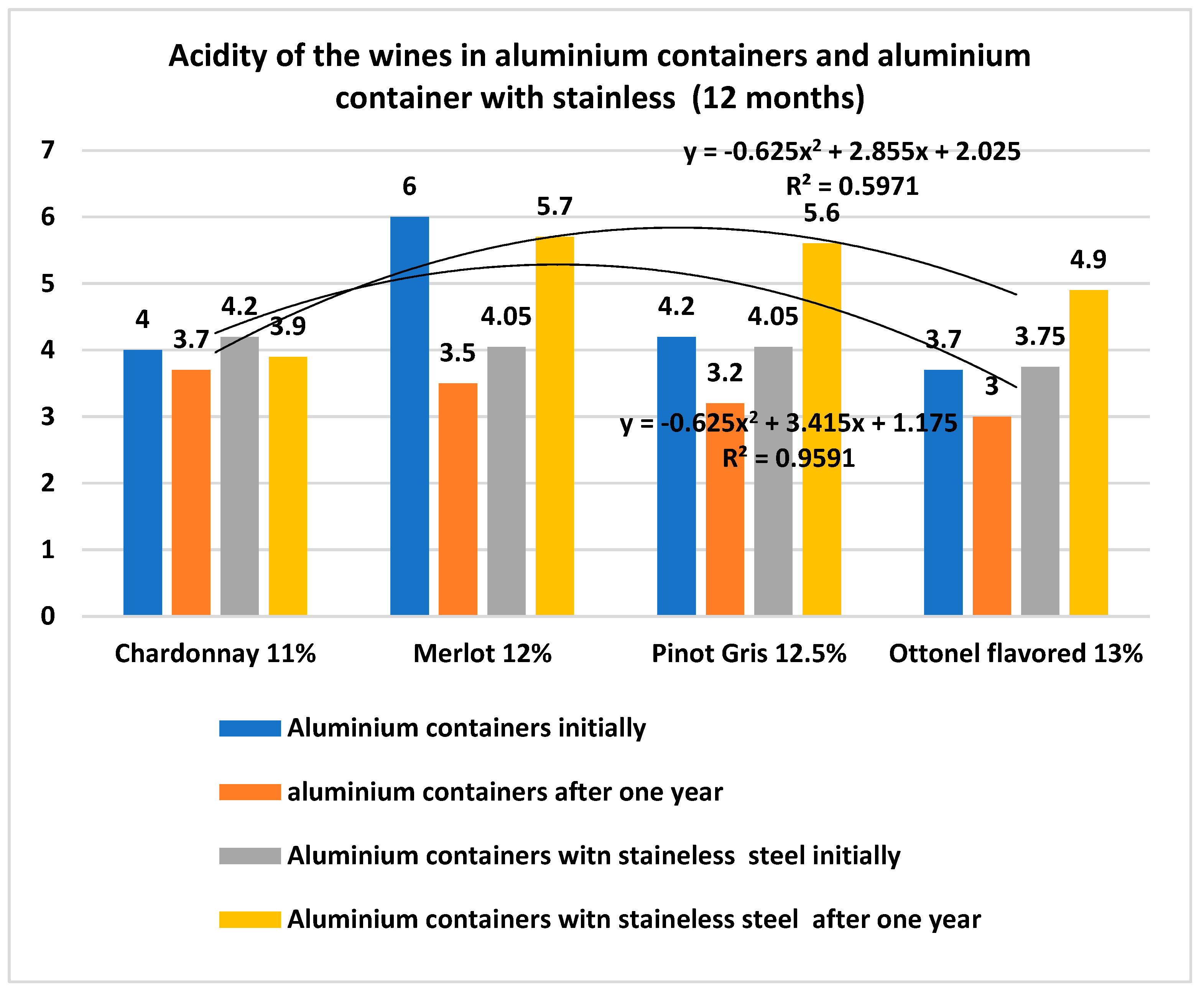
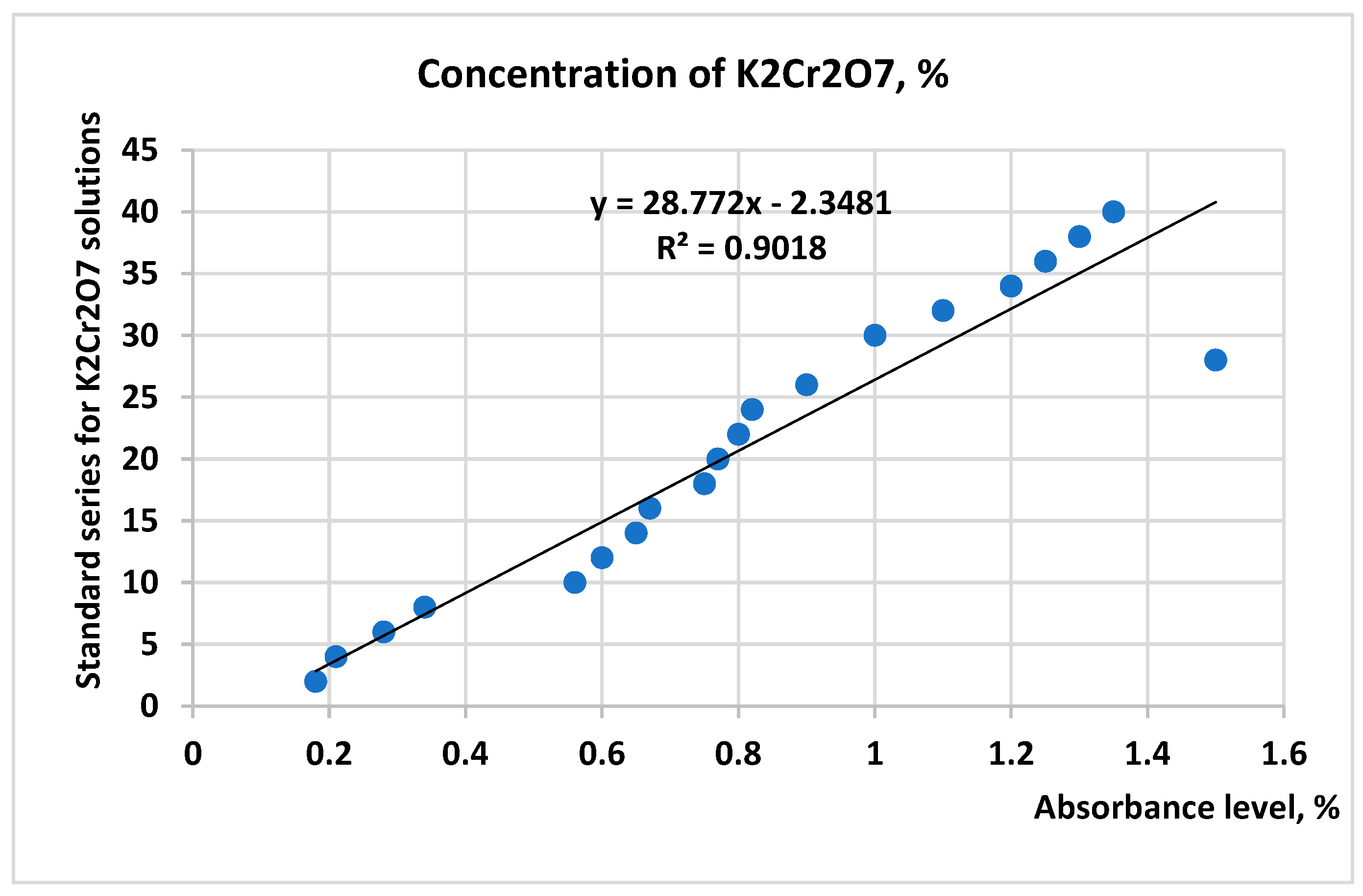
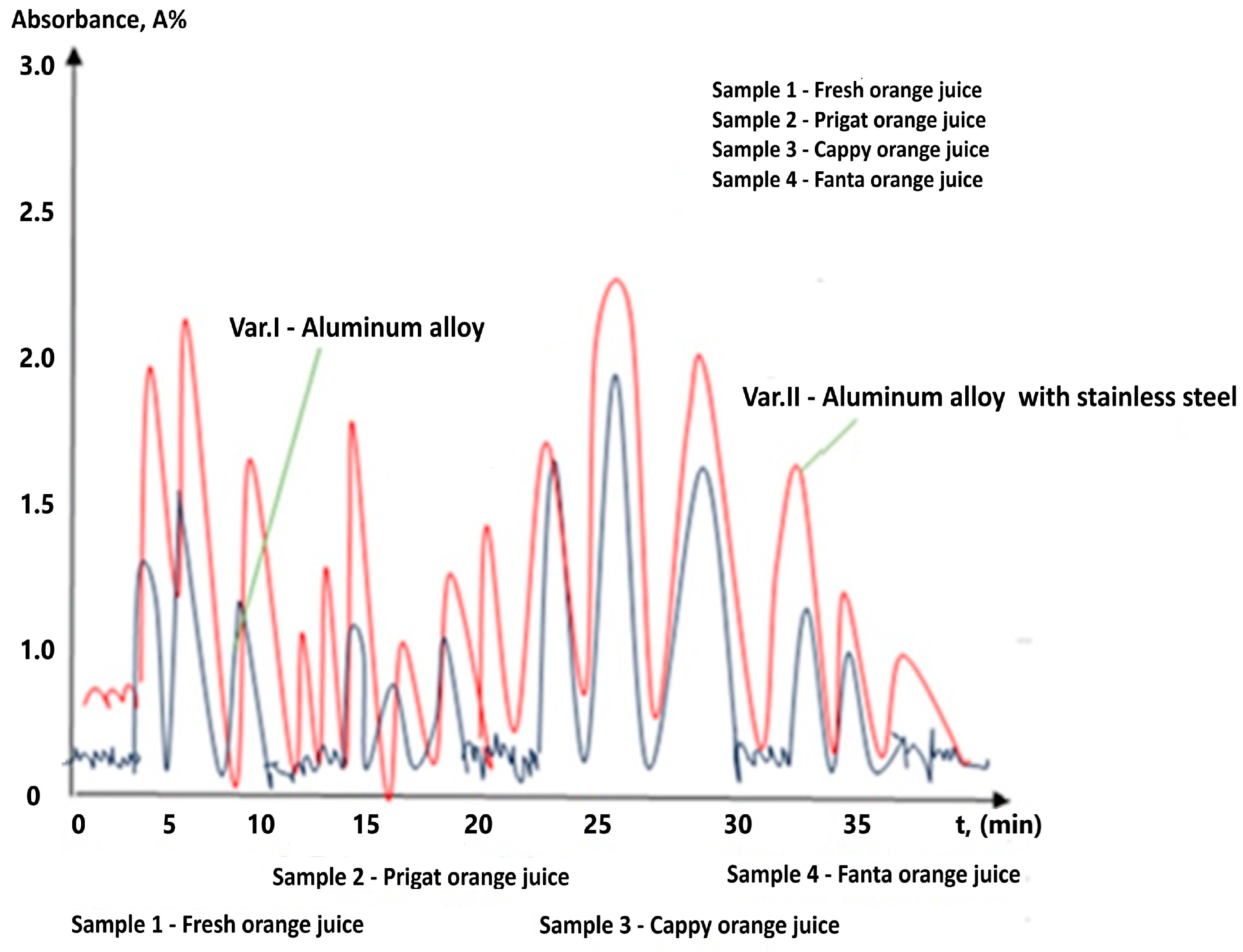
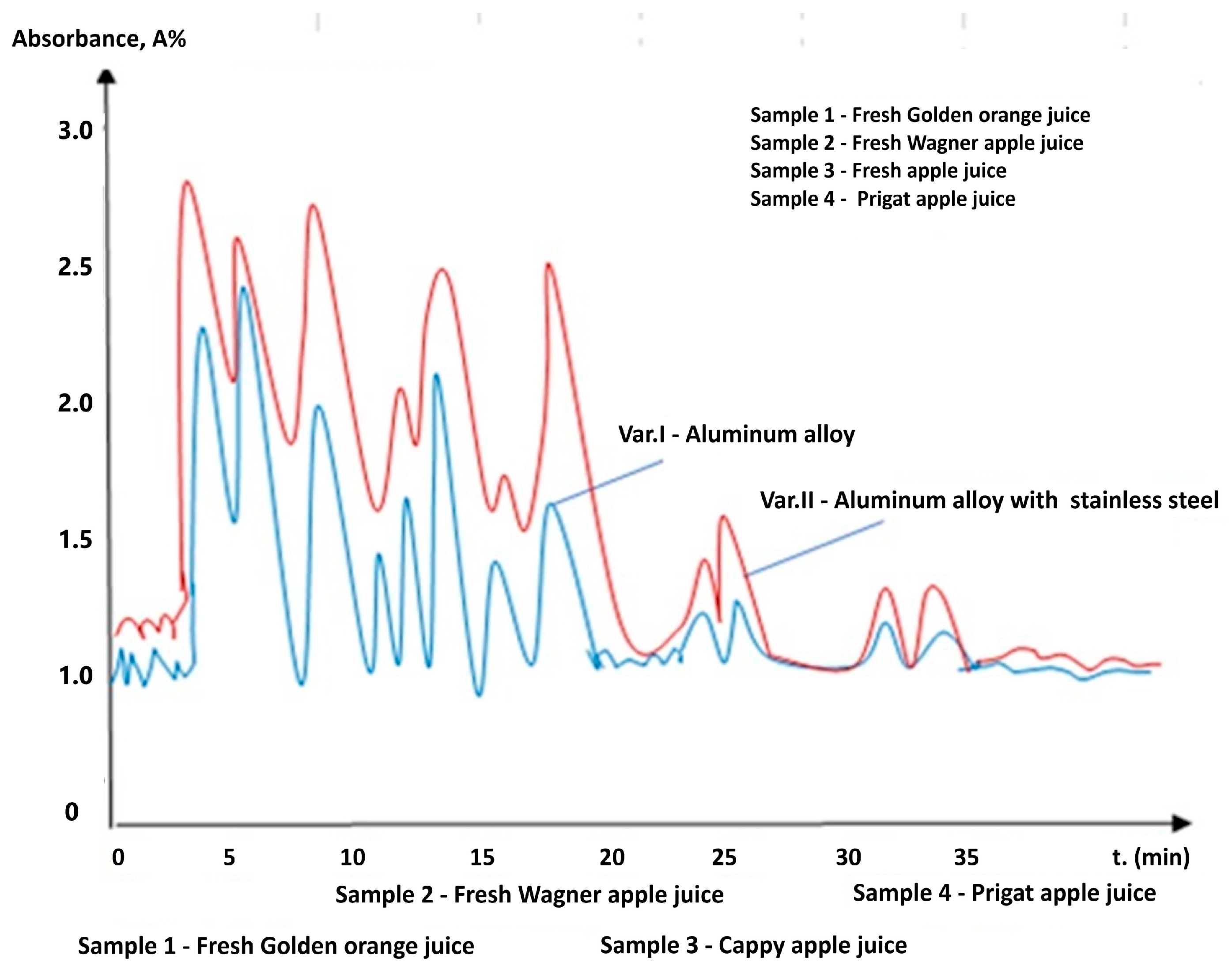
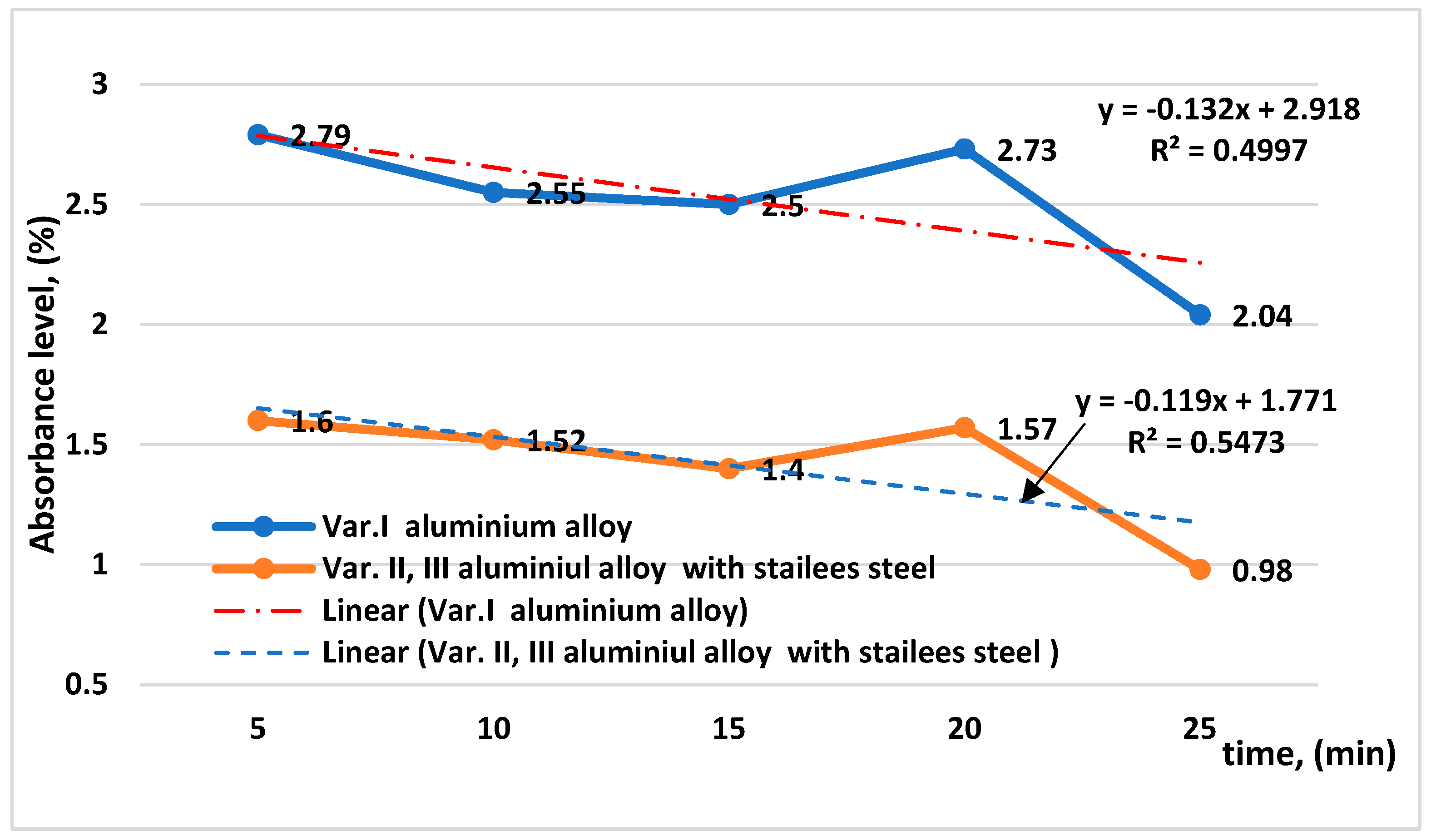
| Materials | Chemical Compositions (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Cr | Mn | Ni | Si | Ti | Cu | Mg | Al | Zn | Fe | |
| 80T | 0.12 | 18 | 1.5 | 8 | 0.5 | - | - | - | - | - | Balance |
| EN AW-2007 | - | 0.1 | 0.8 | 0.1 | 0.8 | 0.2 | 3.7 | 0.8 | Balance | 0.8 | 0.8 |
| Parameters | Value |
|---|---|
| Current intensity (A) | 220 |
| Voltage (U) | 30/32/34 |
| Primary air pressure (bar) | 6 |
| Movement speed of the gun (m/s) | 0.14 |
| Stand-off distance—SOD (mm) | 100 |
| Number of passes | 3 |
| Coating | Sample | Voltage, [V] | Adhesion, [MPa] | Porosity, [%] | HV300 | Thickness, [mm] |
|---|---|---|---|---|---|---|
| 80T | 01CS30 | 30 | 28.3 ± 7.3 | 6.7 ± 2.1 | 308 ± 25 | 1.2 ± 0.3 |
| 02CS32 | 32 | 32.7 ± 3.6 | 3.8 ± 0.7 | 1.3 ± 0.2 | ||
| 03CS34 | 34 | 33.1 ± 2.8 | 3.1 ± 1.2 | 1.2 ± 0.4 |
| Chemical Element | Control Sample | 01CS30 | 02CS32 | 03CS34 |
|---|---|---|---|---|
| Cr, [wt.%] | 0.0006 | 0.00061 | 0.000606 | 0.000604 |
| Mn, [wt.%] | 0.00014 | 0.00021 | 0.000145 | 0.000146 |
| Ni, [wt.%] | 0.00037 | 0.00038 | 0.000371 | 0.00037 |
| Si, [wt.%] | 0.000012 | 0.00003 | 0.000012 | 0.000012 |
| Ti, [wt.%] | 0.0001 | 0.0052 | 0.0001 | 0.0001 |
| Cu, [wt.%] | 0.04 | 0.0586 | 0.04 | 0.04 |
| Mg, [wt.%] | 56.124 | 56.237 | 56.125 | 56.124 |
| Al, [wt.%] | 0.0006 | 0.42263 | 0.0006 | 0.0006 |
| Zn, [wt.%] | 0.0016 | 0.0036 | 0.0016 | 0.0016 |
| Fe, [wt.%] | 0.0032 | 0.0033 | 0.0032 | 0.0032 |
| Na, [wt.%] | 14.14505 | 14.1451 | 14.14505 | 14.14505 |
| Se, [wt.%] | 0.003 | 0.0031 | 0.0031 | 0.0032 |
| Be, [wt.%] | 0.0005 | 0.0005 | 0.0005 | 0.0005 |
| Ca, [wt.%] | 0.0012 | 0.0012 | 0.0012 | 0.0012 |
| Nb, [wt.%] | 0.00002 | 0.00002 | 0.00002 | 0.00002 |
| Te, [wt.%] | 0.00003 | 0.00003 | 0.00003 | 0.00003 |
| Pd, [wt.%] | 0.0005 | 0.0005 | 0.0005 | 0.0005 |
| Other elements, [wt.%] | Balance | Balance | Balance | Balance |
| Chemical Element | Control Sample | 01CS30 | 02CS32 | 03CS34 |
|---|---|---|---|---|
| Cr, [wt.%] | 0.0000239 | 0.0000241 | 0.0000239 | 0.0000238 |
| Mn, [wt.%] | 0.0000107 | 0.0000183 | 0.0000116 | 0.000109 |
| Ni, [wt.%] | 0.000074 | 0.000081 | 0.000079 | 0.000076 |
| Si, [wt.%] | - | 0.000001 | 0.00000082 | 0.0000086 |
| Ti, [wt.%] | - | - | - | - |
| Cu, [wt.%] | 0.00000015 | 0.00000018 | 0.00000016 | 0.00000015 |
| Mg, [wt.%] | - | 0.000025 | - | - |
| Al, [wt.%] | 0.000395 | 0.008655 | 0.000002 | 0.0000001 |
| Zn, [wt.%] | 0.0032 | 0.0038 | 0.00321 | 0.00319 |
| Fe, [wt.%] | - | - | 0.00000012 | 0.00000012 |
| Na, [wt.%] | 0.248 | 0.24803 | 0.24793 | 0.24801 |
| K, [wt.%] | 1.1468 | 1.1445 | 1.1472 | 1.1462 |
| Se, [wt.%] | 0.000048 | 0.00004802 | 0.00004812 | 0.0000481 |
| Ca, [wt.%] | 0.8882 | 0.882205 | 0.88241 | 0.88224 |
| Rb, [wt.%] | 0.00195 | 0.00191 | 0.0019508 | 0.00192 |
| Se, [wt.%] | 0.000082 | 0.0000821 | 0.00008187 | 0.0000821 |
| Br, [wt.%] | 0.00084 | 0.0008402 | 0.0008411 | 0.0008398 |
| Other elements, [wt.%] | Balance | Balance | Balance | Balance |
| Factors Effect | Parameter | Sum of Squares | Factor Effect | Parameter | p-Value |
|---|---|---|---|---|---|
| Intercept | 2.514 | 25.64 | 25.64 | 467.88 | <0.05 |
| WL | −0.00129 | 10.301 | 10.291 | 470 | <0.05 |
| TP | 0.0058 | 2.578 | 2.578 | 89.0089 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, S.; Toma, S.L. Stainless Steel Deposits on an Aluminum Support Used in the Construction of Packaging and Food Transport Containers. Coatings 2024, 14, 1431. https://doi.org/10.3390/coatings14111431
Radu S, Toma SL. Stainless Steel Deposits on an Aluminum Support Used in the Construction of Packaging and Food Transport Containers. Coatings. 2024; 14(11):1431. https://doi.org/10.3390/coatings14111431
Chicago/Turabian StyleRadu, Steluța, and Stefan Lucian Toma. 2024. "Stainless Steel Deposits on an Aluminum Support Used in the Construction of Packaging and Food Transport Containers" Coatings 14, no. 11: 1431. https://doi.org/10.3390/coatings14111431
APA StyleRadu, S., & Toma, S. L. (2024). Stainless Steel Deposits on an Aluminum Support Used in the Construction of Packaging and Food Transport Containers. Coatings, 14(11), 1431. https://doi.org/10.3390/coatings14111431







