Study on the Effect of Lignin Removal Rate on the Dielectric Properties of Delignified Materials
Abstract
1. Introduction
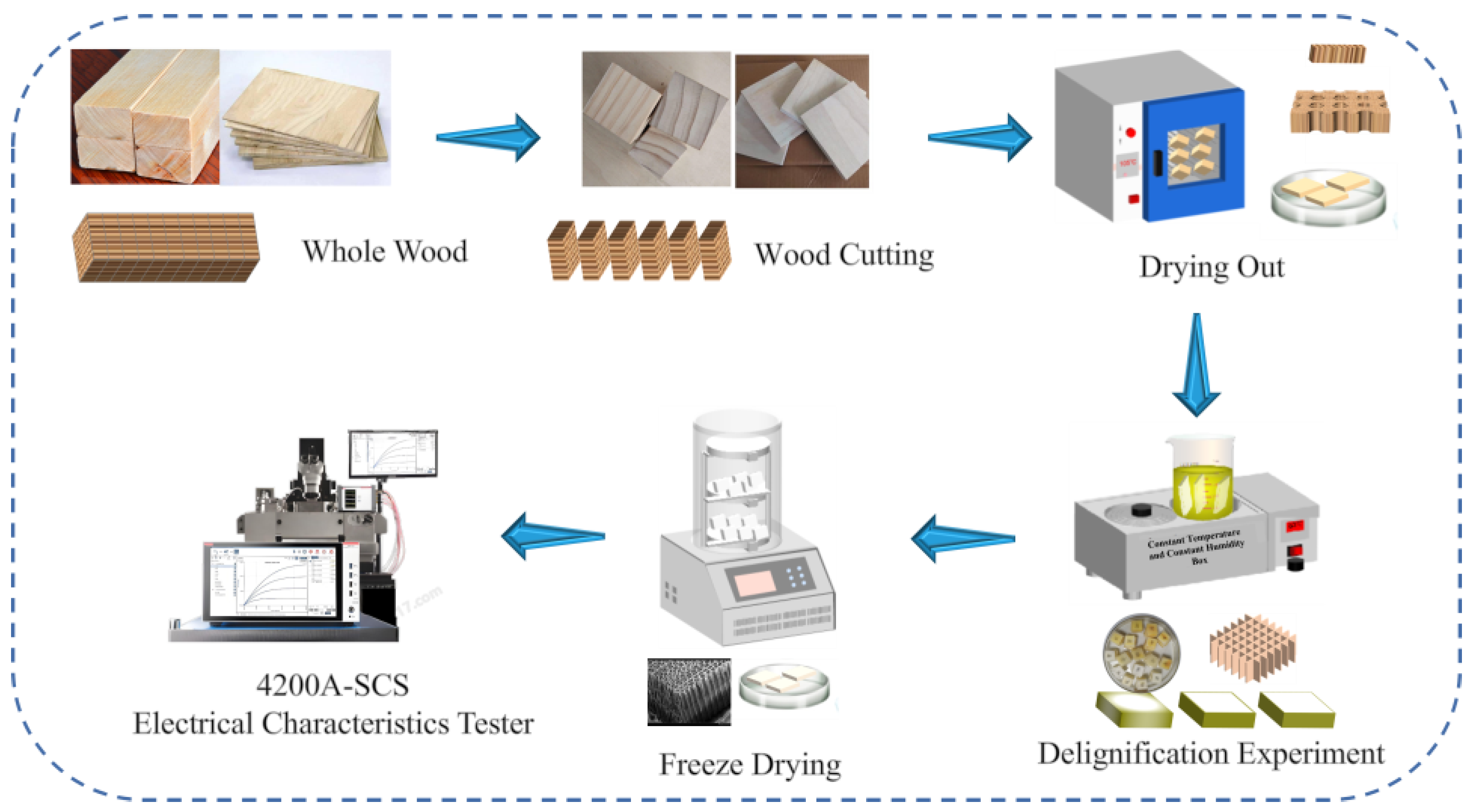
2. Experimental Procedure
2.1. Materials and Reagents
2.2. Instruments and Equipment
2.3. Test Method
Lignin Removal Experiment
2.4. Testing and Characterization
2.4.1. Determination of Lignin Content and Removal Rate
2.4.2. Determination of Dielectric Properties
3. Results and Analysis
3.1. Influence of Different Reaction Times on the Morphology Analysis of DW
3.2. Effects of Different Reaction Times on Changes in Lignin Content
3.3. Effect of Different Reaction Times on the Molecular Structure of Wood
3.4. Effect of Different Reaction Times on the Dielectric Properties of Wood
| Measuring Materials | Test Condition | Dielectric Constant (Ε′) | Dielectric Loss (Ε″) | Dielectric Loss Angle Tangent (TanΔ) | Source |
|---|---|---|---|---|---|
| Natural wood Keranji NaOH-treated Keranji | Frequency (Hz) | Natural wood low frequency: 2.56 NaOH-treated LF: 4.63. | Natural wood low frequency: low frequency; 0.57 High frequency: 0.08 NaOH treated Low frequency: 0.74 High frequency: 0.092 | - | [32] |
| Natural wood Bubinga wood | Different test directions 8.2–12.4 GHz | X:2.691 Y:2.767 Z:3.270 | X:0.2958 Y:0.2173 Z:0.4941 | - | [33] |
| Norway spruce, Juniper, Aspen | 10−2–107 Hz | N:1.425–1.375 J:1.6–1.41 A:1.36–1.30 | - | N:0.06–0.002 J:0.01–0.007 A:0.1–0.0001 | [34] |
| cellulose | Frequency (Hz) | 5.9–7.5 | tanδ less than 0.1 | [35] | |
| Cellulose crystals Iα and Iβ | Different XC generalized computational fits | Iα: 0.6~0.75 Iβ: 0.7~0.95 | - | - | [5] |
| lignin | 10−1~107 Hz | 2.2–2.9 | 1.5–0.6 | - | [36] |
| CMC composite film CMC-lignin composite film | 103–106 Hz | CMC:5.1–4.2 CMC-lingin:5.8–4.6 | - | - | [37] |
| chemically modified kraft lignin | 105 Hz 80–240 °C | 0.31–0.12 4.2–2.1 | Tanδ ML:0.4–0.15 | [38] |
4. Conclusions and Prospects
- (1)
- As the removal time is increased, the lignin content in the wood decreases, the lignin removal rate rises, reaching approximately 82.5%, and the intensity of the lignin benzene ring skeleton vibration characteristic peaks decreases, while the intensity of the phenol hydroxyl, hydroxyl, carbonyl, and carboxyl characteristic peaks is generally elevated.
- (2)
- As the rate of lignin removal increases, the value of ε′ varies in a roughly linear fashion with the lignin removal rate, with tanδ exhibiting an initial upward trend followed by a downward trend.
- (3)
- Further discussion is required regarding the relationship between the dielectric properties of electrical parameters and sample internal lignin content, as well as changes in the latter. It is yet unclear which ε′ and tanδ are affected by changes in chemical composition. While it has been proposed that the main polarization group number and degrees of freedom are restricted, the dielectric chirp mechanism of different polarity groups at different frequencies has not been sufficiently analyzed. To gain insight into the differing polarity groups in the AC electric field with a specific orientation, it is essential to consider the changes in ε′ and tanδ as a consequence of the combined influence of multiple factors. Further studies are required to elucidate the changing law of dielectric properties in Mongolian Scotch Pine and Paulownia.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Items |
| DW | Delignified wood |
| FSP | Cellulose saturation point |
| MC | Moisture content |
| M | Pinus sylvestris Linn. var. mongolica Litv |
| P | Paulownia fortunei (Seem.) Hemsl |
| ε′ | Dielectric constant |
| tanδ | Dielectric loss angle tangent |
| σ | Electrical conductivity |
References
- O’Dea, R.M.; Willie, J.A.; Epps, T.H. 100th Anniversary of Macromolecular Science Viewpoint: Polymers from Lignocellulosic Biomass. Current Challenges and Future Opportunities. ACS Macro Lett. 2020, 9, 476–493. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Ansari, F.; Gowda, V.K.; Brouzet, C.; Chen, P.; Larsson, P.T.; Roth, S.V.; Lundell, F.; Wågberg, L.; Kotov, N.A.; et al. Multiscale Control of Nanocellulose Assembly: Transferring Remarkable Nanoscale Fibril Mechanics to Macroscale Fibers. ACS Nano 2018, 12, 6378–6388. [Google Scholar] [CrossRef]
- Kumar, A.; Jyske, T.; Petrič, M. Delignified Wood from Understanding the Hierarchically Aligned Cellulosic Structures to Creating Novel Functional Materials: A Review. Adv. Sustain. Syst. 2021, 5, 2000251. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Sun, P.-P.; Singhania, R.R.; Patel, A.K.; Dong, C.-D. Journey of Lignin from a Roadblock to Bridge for Lignocellulose Biorefineries: A Comprehensive Review. Sci. Total Environ. 2023, 861, 160560. [Google Scholar] [CrossRef] [PubMed]
- Yaadav, A.; Boström, M.; Malyi, O. Understanding of Dielectric Properties of Cellulose. Cellulose 2024, 31, 2783–2794. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, D.; Gao, Y.; Yue, Y.; Yang, W.; Mei, C.; Xu, X.; Xu, Y.; Xiao, H.; Han, J. Solar-Powered High-Performance Lignin-Wood Evaporator for Solar Steam Generation. Adv. Funct. Mater. 2023, 33, 2306947. [Google Scholar] [CrossRef]
- Lunev, I.; Greenbaum (Gutina), A.; Feldman, Y.; Petrov, V.; Kuznetsova, N.; Averianova, N.; Makshakova, O.; Zuev, Y. Dielectric Response of Hydrated Water as a Structural Component of Nanofibrillated Cellulose (NFC) from Different Plant Sources. Carbohydr. Polym. 2019, 225, 115217. [Google Scholar] [CrossRef]
- Zhu, S.; Biswas, S.K.; Qiu, Z.; Yue, Y.; Fu, Q.; Jiang, F.; Han, J. Transparent Wood-Based Functional Materials via a Top-down Approach. Prog. Mater. Sci. 2023, 132, 101025. [Google Scholar] [CrossRef]
- Qin, J.; Li, X.; Shao, Y.; Shi, K.; Zhao, X.; Feng, T.; Hu, Y. Optimization of Delignification Process for Efficient Preparation of Transparent Wood with High Strength and High Transmittance. Vacuum 2018, 158, 158–165. [Google Scholar] [CrossRef]
- Dhatarwal, P.; Sengwa, R.J. Polymer Compositional Ratio-Dependent Morphology, Crystallinity, Dielectric Dispersion, Structural Dynamics, and Electrical Conductivity of PVDF/PEO Blend Films. Macromol. Res. 2019, 27, 1009–1023. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Zha, L.; Koskela, S.; Berglund, L.A.; Zhou, Q. Wood Xerogel for Fabrication of High-Performance Transparent Wood. Nat. Commun. 2023, 14, 2827. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, Q.; Yang, X.; Berglund, L. Transparent Wood for Functional and Structural Applications. Phil. Trans. R. Soc. A 2018, 376, 20170182. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Yashima, Y.; Abe, M.; Miki, T.; Nishida, M. Influence of Delignification on Plastic Flow Deformation of Wood. Cellulose 2022, 29, 4153–4165. [Google Scholar] [CrossRef]
- Ou, J.; Hu, S.; Yao, L.; Chen, Y.; Qi, H.; Yue, F. Simultaneous Strengthening and Toughening Lignin/Cellulose Nanofibril Composite Films: Effects from Flexible Hydrogen Bonds. Chem. Eng. J. 2023, 453, 139770. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Oxidation Treatment on Wood Cell Walls Affects Gas Permeability and Sound Absorption Capacity. Carbohydr. Polym. 2022, 276, 118874. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Effective Changes in Cellulose Cell Walls, Gas Permeability and Sound Absorption Capability of Cocos Nucifera (Palmwood) by Steam Explosion. Cellulose 2021, 28, 5707–5717. [Google Scholar] [CrossRef]
- Liu, R.; Smeds, A.; Tirri, T.; Zhang, H.; Willför, S.; Xu, C. Influence of Carbohydrates Covalently Bonded with Lignin on Solvent Fractionation, Thermal Properties, and Nanoparticle Formation of Lignin. ACS Sustain. Chem. Eng. 2022, 10, 14588–14599. [Google Scholar] [CrossRef]
- Qi, Y.; Zhou, Z.; Xu, R.; Dong, Y.; Zhang, Z.; Liu, M. Effect of NaOH Pretreatment on Permeability and Surface Properties of Three Wood Species. ACS Omega 2023, 8, 40362–40374. [Google Scholar] [CrossRef]
- Li, C.; Yue, J.; Wang, B.; Tao, Y.; Hu, J.; Lu, J.; Du, J.; Wang, H. Unveiling the Mechanism of Lignin Nanofiller in Boosting the Performance of Triboelectric Nanogenerators. Nano Energy 2024, 126, 109631. [Google Scholar] [CrossRef]
- Mudedla, S.K.; Vuorte, M.; Veijola, E.; Marjamaa, K.; Koivula, A.; Linder, M.B.; Arola, S.; Sammalkorpi, M. Effect of Oxidation on Cellulose and Water Structure: A Molecular Dynamics Simulation Study. Cellulose 2021, 28, 3917–3933. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B.; Lv, Y.; Ye, X.; Lin, C.; Liu, M. Insight into the Performance of Lignin-Containing Cellulose Nanofibers (LCNFs) via Lignin Content Regulation by p-Toluenesulfonic Acid Delignification. Cellulose 2022, 29, 2273–2287. [Google Scholar] [CrossRef]
- Wang, K.; Dong, Y.; Ling, Z.; Liu, X.; Shi, S.Q.; Li, J. Transparent Wood Developed by Introducing Epoxy Vitrimers into a Delignified Wood Template. Compos. Sci. Technol. 2021, 207, 108690. [Google Scholar] [CrossRef]
- Tang, Z.; Li, W.; Lin, X.; Xiao, H.; Miao, Q.; Huang, L.; Chen, L.; Wu, H. TEMPO-Oxidized Cellulose with High Degree of Oxidation. Polymers 2017, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Goldhahn, C.; Schubert, M.; Lüthi, T.; Keplinger, T.; Burgert, I.; Chanana, M. Wood–Gelatin Bio-Composite Membranes with Tunable Flux. ACS Sustain. Chem. Eng. 2020, 8, 7205–7213. [Google Scholar] [CrossRef]
- Dong, T.; Chen, W.; Cai, C.; Bai, F.; Zhou, Z.; Wang, J.; Li, X. Water-Stable, Strong, Biodegradable Lignocellulose Straws Replacement for Plastic Straws. Chem. Eng. J. 2023, 451, 138970. [Google Scholar] [CrossRef]
- Yang, C.; Magallanes, G.; Maldonado, S.; Stephenson, C.R.J. Electro-Reductive Fragmentation of Oxidized Lignin Models. J. Org. Chem. 2021, 86, 15927–15934. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, H.; Wang, X.; Cai, K.; Chen, J.; Xu, Y.; Yu, F.; Nie, S.; Wang, S.; Liu, X. Anti-Moisture, Anti-Bacterial Cellulosic Triboelectric Materials Enabled by Hydroxyl Coordination Effect. Nano Energy 2024, 124, 109472. [Google Scholar] [CrossRef]
- Bogolitsyn, K.G.; Khviuzov, S.S.; Gusakova, M.A.; Pustynnaya, M.A.; Krasikova, A.A. The Differences between Acid–Base and Redox Properties of Phenolic Structures of Coniferous and Deciduous Native Lignins. Wood Sci. Technol. 2018, 52, 1153–1164. [Google Scholar] [CrossRef]
- Fares, O.; AL-Oqla, F.M.; Hayajneh, M.T. Dielectric Relaxation of Mediterranean Lignocellulosic Fibers for Sustainable Functional Biomaterials. Mater. Chem. Phys. 2019, 229, 174–182. [Google Scholar] [CrossRef]
- Shi, S.-C.; Chen, C.; Zhu, J.-L.; Li, Y.; Meng, X.; Huang, H.-D.; Li, Z.-M. Environmentally Friendly Regenerated Cellulose Films with Improved Dielectric Properties via Manipulating the Hydrogen Bonding Network. Appl. Phys. Lett. 2021, 119, 022903. [Google Scholar] [CrossRef]
- Roig, F.; Ramanantsizehena, G.; Lahatra Razafindramisa, F.; Dantras, E.; Dandurand, J.; Hoyet, H.; Bernés, A.; Lacabanne, C. Dielectric and Mechanical Properties of Various Species of Madagascan Woods. Wood Sci. Technol. 2017, 51, 1389–1404. [Google Scholar] [CrossRef]
- Jayamani, E.; Rahman, M.R.; Hamdan, S.; Kyari, M.I.; Bin Bakri, M.K.; Sanaullah, K.; Khan, A. Dielectric Properties of Natural Borneo Woods: Keranji, Kayu Malam, and Kumpang. BioRes 2020, 15, 7815–7827. [Google Scholar] [CrossRef]
- Bossou, O.V.; Mosig, J.R.; Zurcher, J.-F. Dielectric Measurements of Tropical Wood. Measurement 2010, 43, 400–405. [Google Scholar] [CrossRef]
- Albornoz-Palma, G.; Ortega-Sanhueza, I.; Teruel-Juanes, R.; Henríquez-Gallegos, S.; Ribes-Greus, A.; Pereira, M. Effect of Lignin on the Morphological, Rheological, and Dielectric Characteristics of Lignocellulose Nanofibrils from Pinus Radiata. Ind. Crops Prod. 2023, 204, 117323. [Google Scholar] [CrossRef]
- Luo, Q.; Shen, H.; Zhou, G.; Xu, X. A Mini-Review on the Dielectric Properties of Cellulose and Nanocellulose-Based Materials as Electronic Components. Carbohydr. Polym. 2023, 303, 120449. [Google Scholar] [CrossRef]
- Khviyuzov, S.S.; Volkov, A.S. Influence of Ionic Impurities on the Dielectric Properties of Lignin. Polym. Adv. Technol. 2024, 35, e6467. [Google Scholar] [CrossRef]
- Chen, W.; Li, C.; Tao, Y.; Lu, J.; Du, J.; Wang, H. Modulating Spatial Charge Distribution of Lignin for Eco-Friendly and Recyclable Triboelectric Nanogenerator. Nano Energy 2023, 116, 108802. [Google Scholar] [CrossRef]
- Culebras, M.; Sanchis, M.J.; Beaucamp, A.; Carsí, M.; Kandola, B.K.; Horrocks, A.R.; Panzetti, G.; Birkinshaw, C.; Collins, M.N. Understanding the Thermal and Dielectric Response of Organosolv and Modified Kraft Lignin as a Carbon Fibre Precursor. Green Chem. 2018, 20, 4461–4472. [Google Scholar] [CrossRef]








| Mongolian Scotch Pine Absorption Signal cm−1 | Paulownia Absorption Signal cm−1 | Functional Group (Chemistry) |
|---|---|---|
| 3323 | 3323 | Mainly -OH stretching vibrations in the free and bound water fractions |
| 2871 | 2907 | -CH3, -CH2 stretching vibrations of cellulose |
| 1729 | 1730 | -C=O stretching vibrational peaks of non-conjugated acetyl or acid groups |
| 1593 | 1598 | Stretching vibrations induced by the conjugate group-C=O of lignin |
| 1508 | 1509 | Lignin benzene ring backbone vibrations and -CH2 deformation vibrations |
| 1450 | 1425 | Stretching vibrations of the -C=O bond of lignin with the carbon skeleton of the benzene ring |
| 1369, 1312 | 1367, 1313 | C-H absorption peaks of aliphatic methyl and ether hydroxyl groups |
| 1266 | 1244 | Characteristic -C-O and -C-C peaks of hemicellulose acetyl, sugar carbonyl ester groups |
| 1203 | 1202 | Mainly includes -C-H in-plane bending vibration and -C-O telescopic vibration |
| 1157 | 1158 | Stretching vibrations of the -C-O bond of cellulose |
| 1104 | 1106 | Intra-lignin benzene ring -CH stretching vibrations |
| 1056, 1029 | 1054, 1027 | Vibrations induced by -C-O-C and -C=O of cellulose and hemicellulose |
| 1418, 877 | 891 | β-glycosidic bond vibrations of cellulose stretching vibrations of glycosidic bonds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Qiu, F.; Han, W.; Xue, Z. Study on the Effect of Lignin Removal Rate on the Dielectric Properties of Delignified Materials. Coatings 2024, 14, 1421. https://doi.org/10.3390/coatings14111421
Xu H, Qiu F, Han W, Xue Z. Study on the Effect of Lignin Removal Rate on the Dielectric Properties of Delignified Materials. Coatings. 2024; 14(11):1421. https://doi.org/10.3390/coatings14111421
Chicago/Turabian StyleXu, Hui, Fengqi Qiu, Weishuai Han, and Zhenhua Xue. 2024. "Study on the Effect of Lignin Removal Rate on the Dielectric Properties of Delignified Materials" Coatings 14, no. 11: 1421. https://doi.org/10.3390/coatings14111421
APA StyleXu, H., Qiu, F., Han, W., & Xue, Z. (2024). Study on the Effect of Lignin Removal Rate on the Dielectric Properties of Delignified Materials. Coatings, 14(11), 1421. https://doi.org/10.3390/coatings14111421





