Fabrication and Characterization of Micro-Arc Oxidation Films on β-Titanium Alloy in Silicate and Silicate/Glycerin Electrolyte
Abstract
1. Introduction
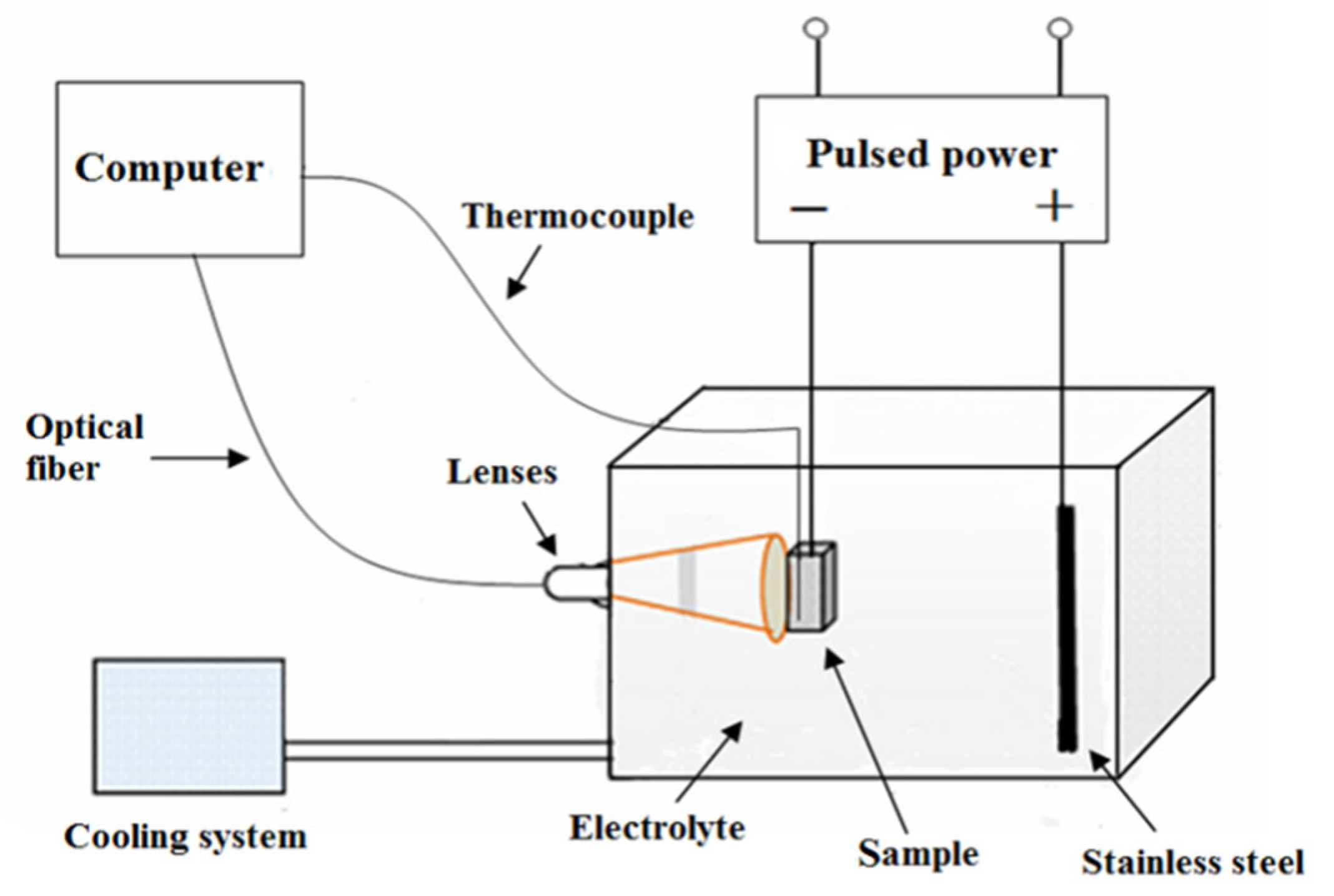
2. Materials and Methods
3. Results
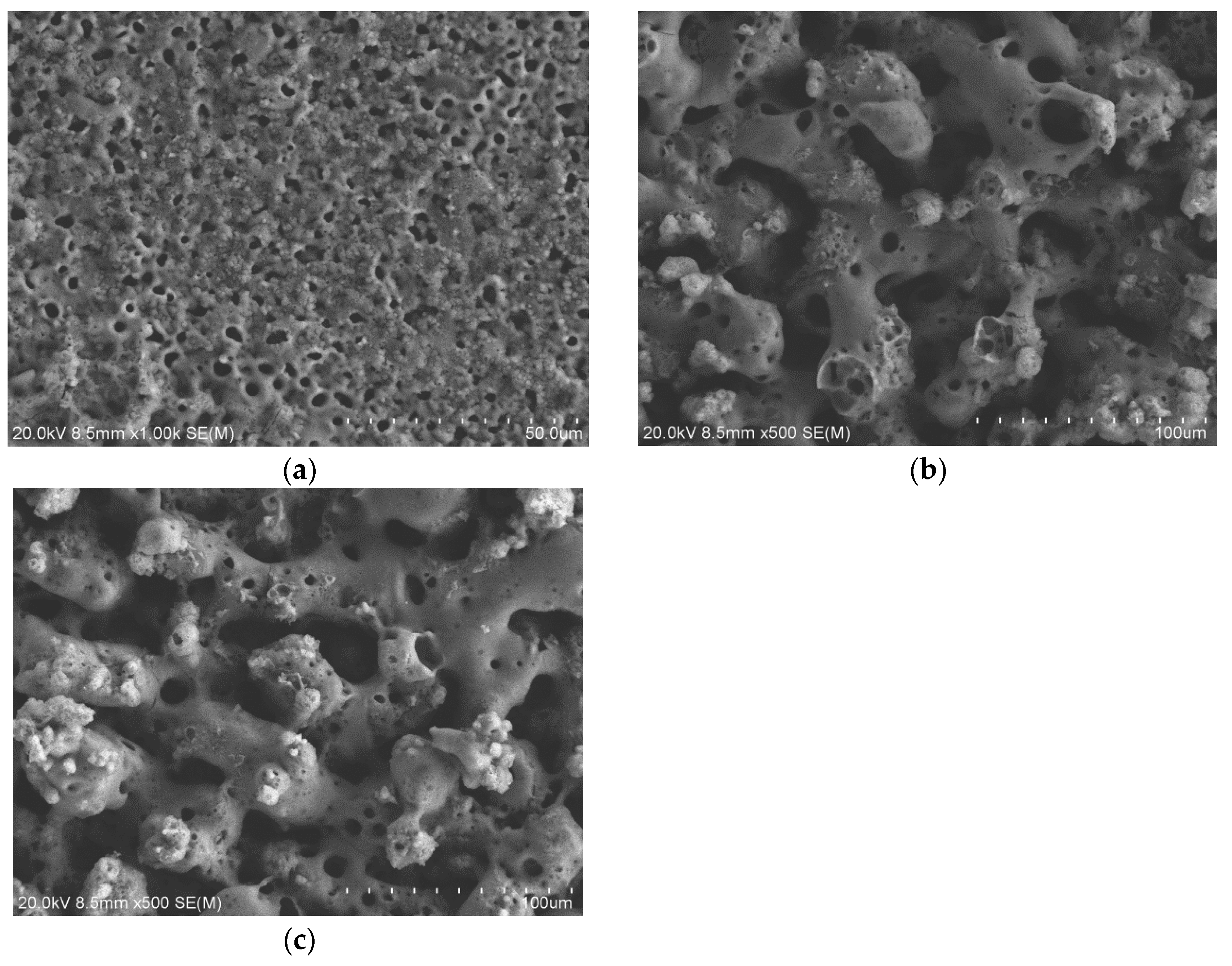
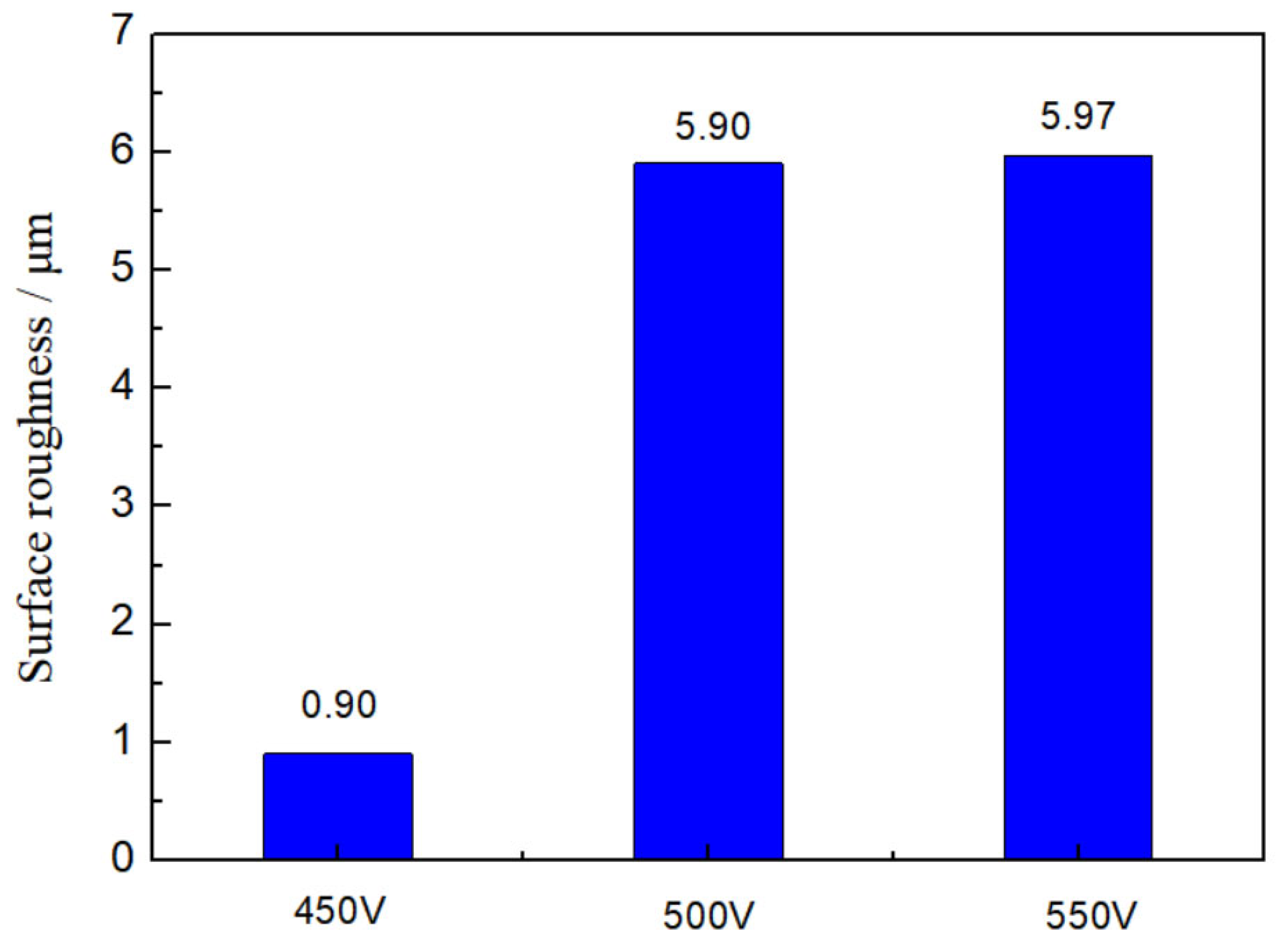
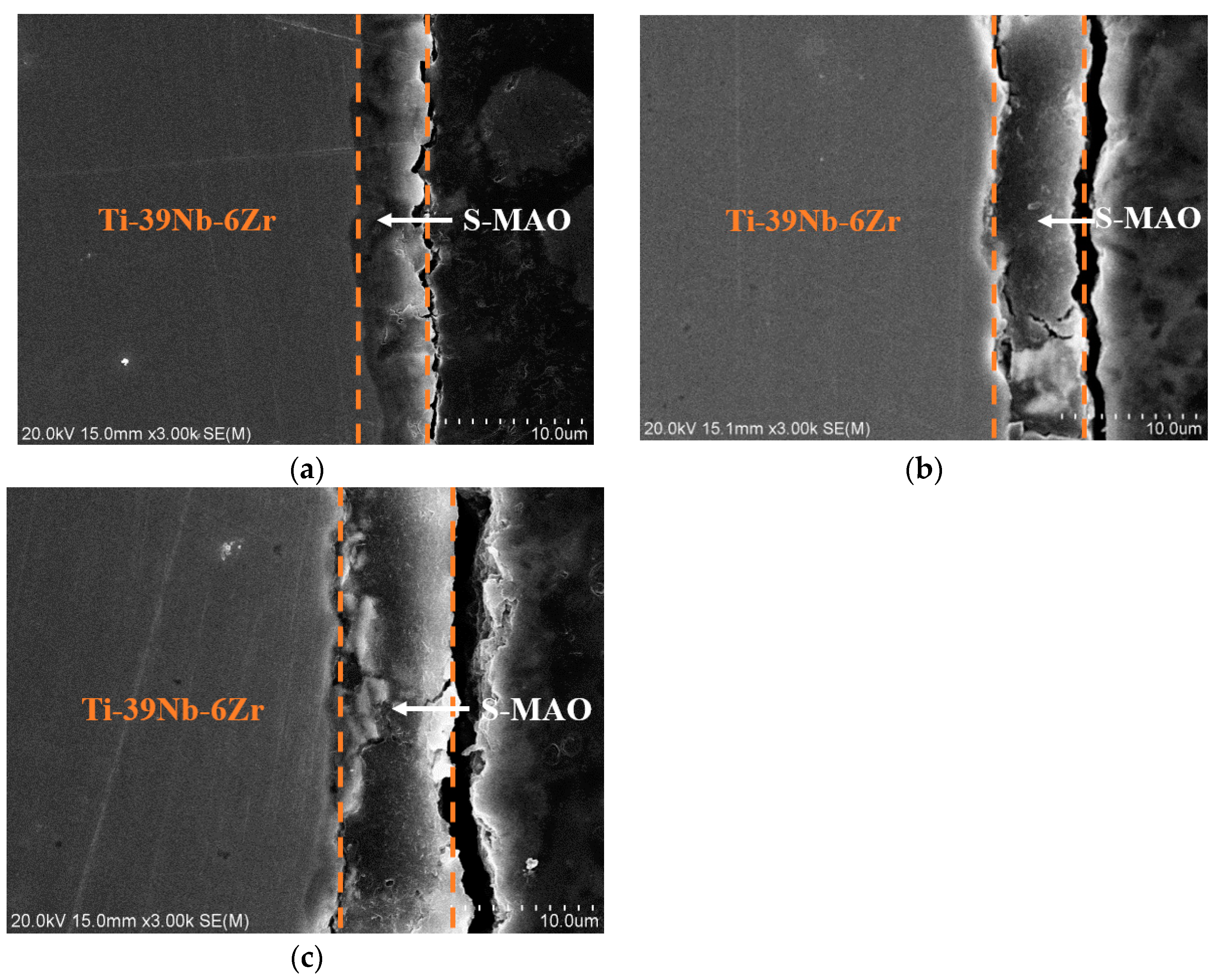
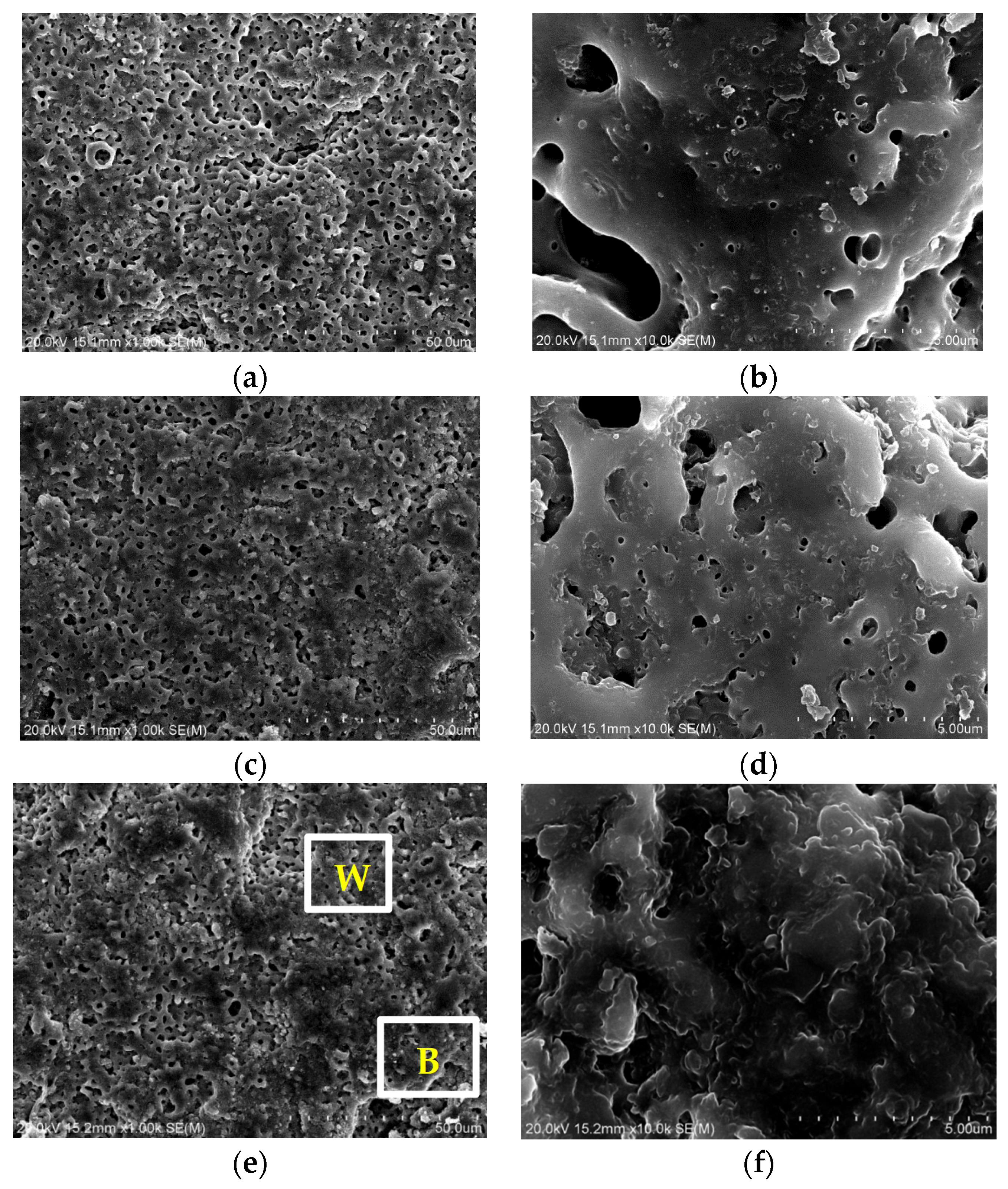
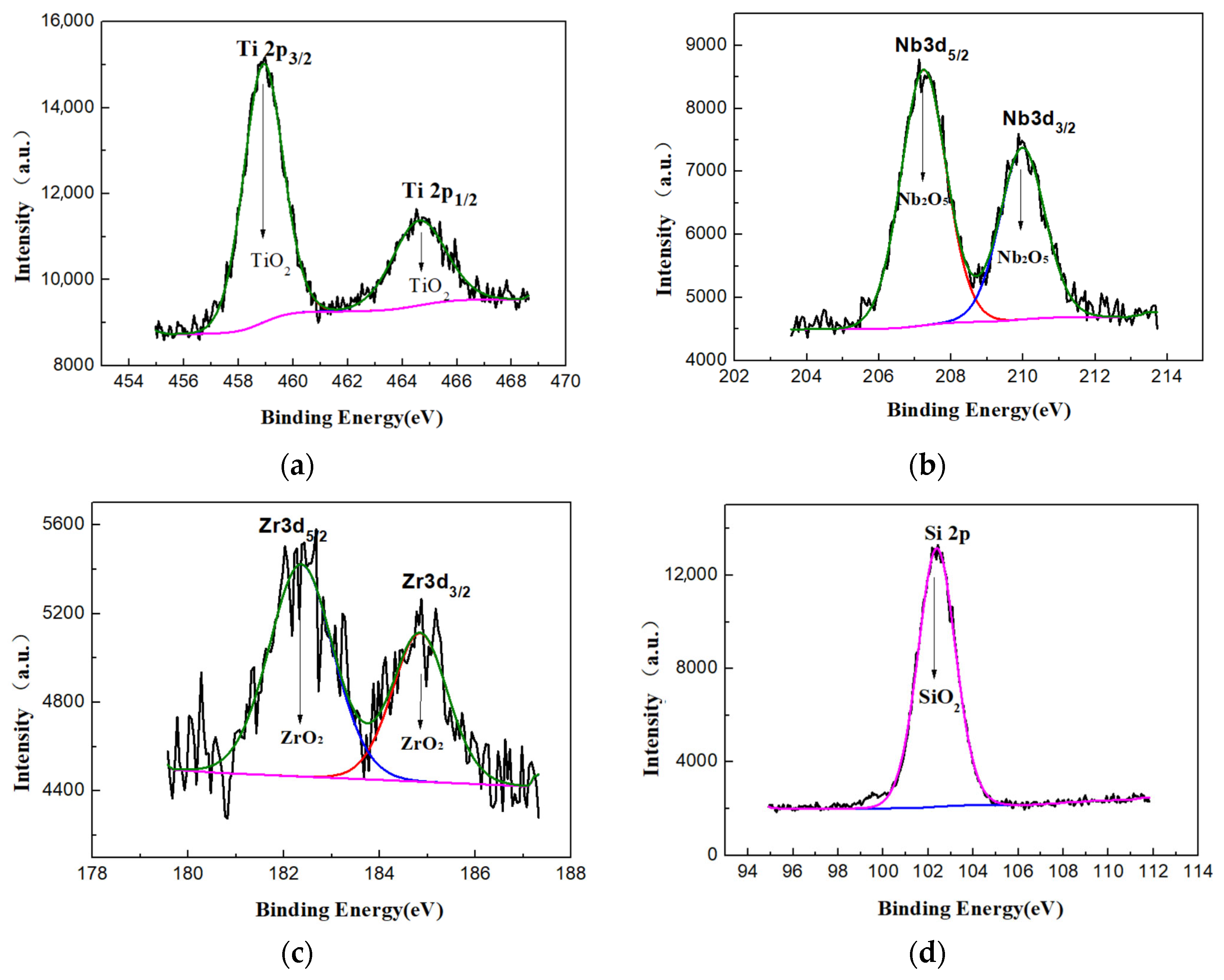
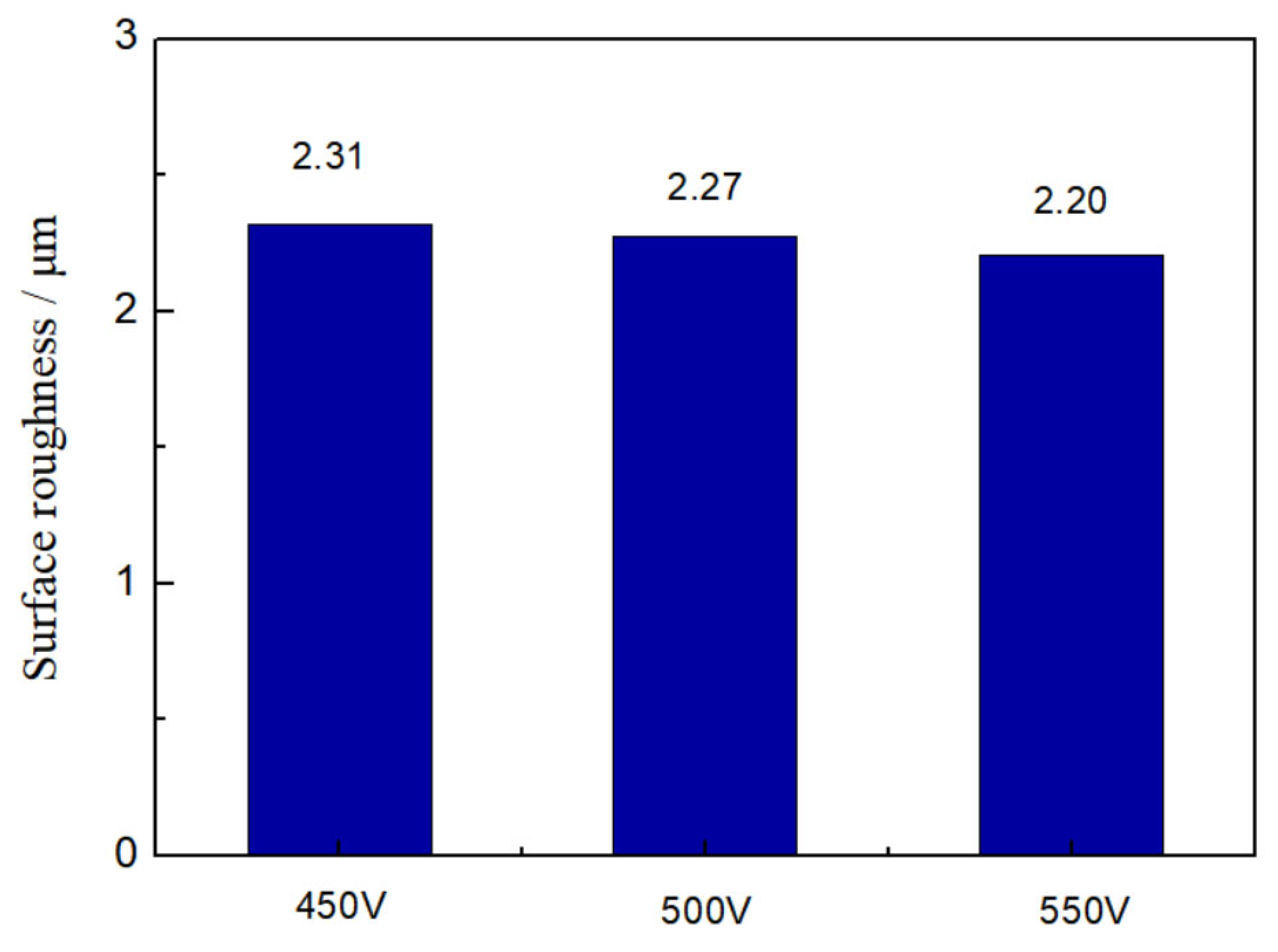
3.1. Morphologies, Chemical Compositions and Roughness Analysis of Micro-Arc Oxidized Films
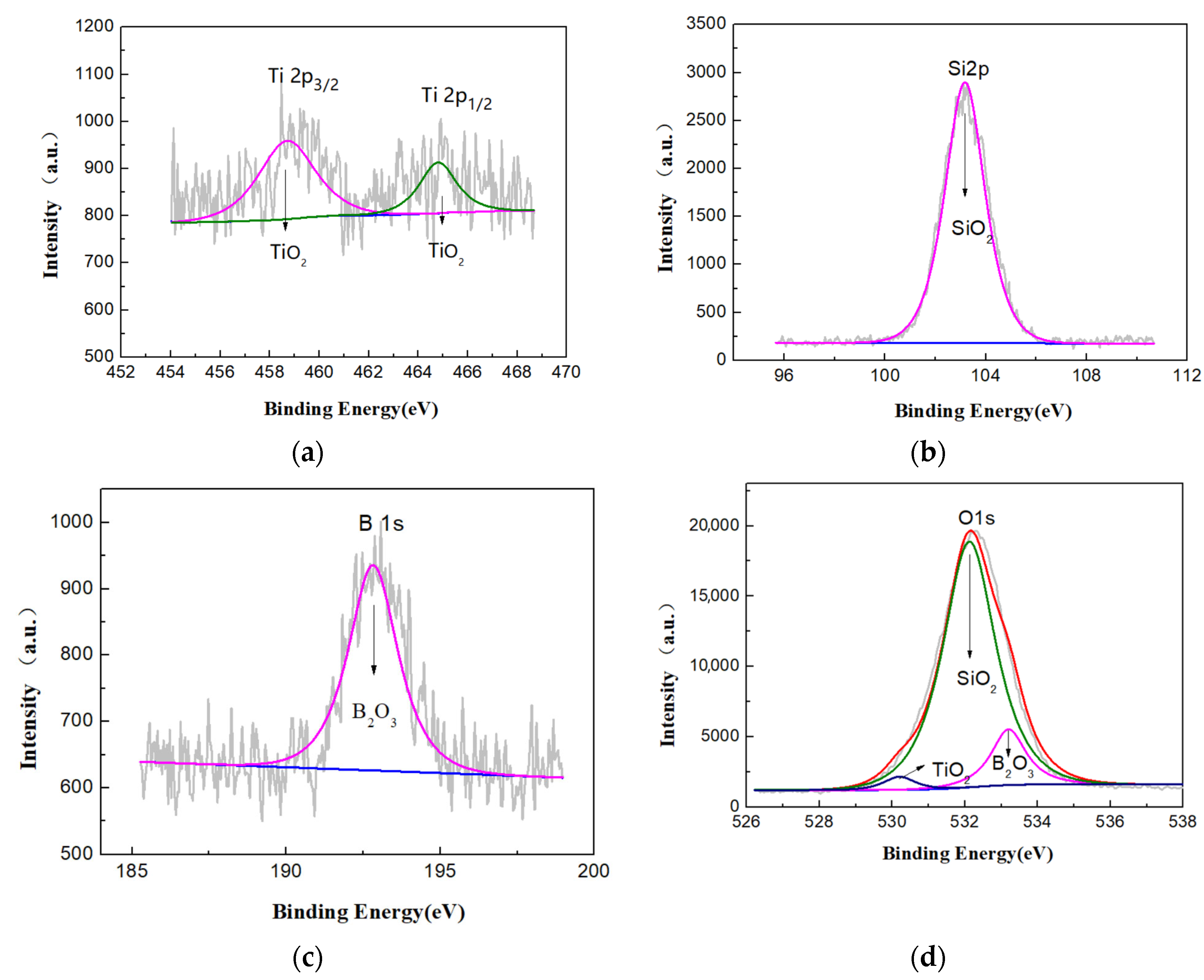
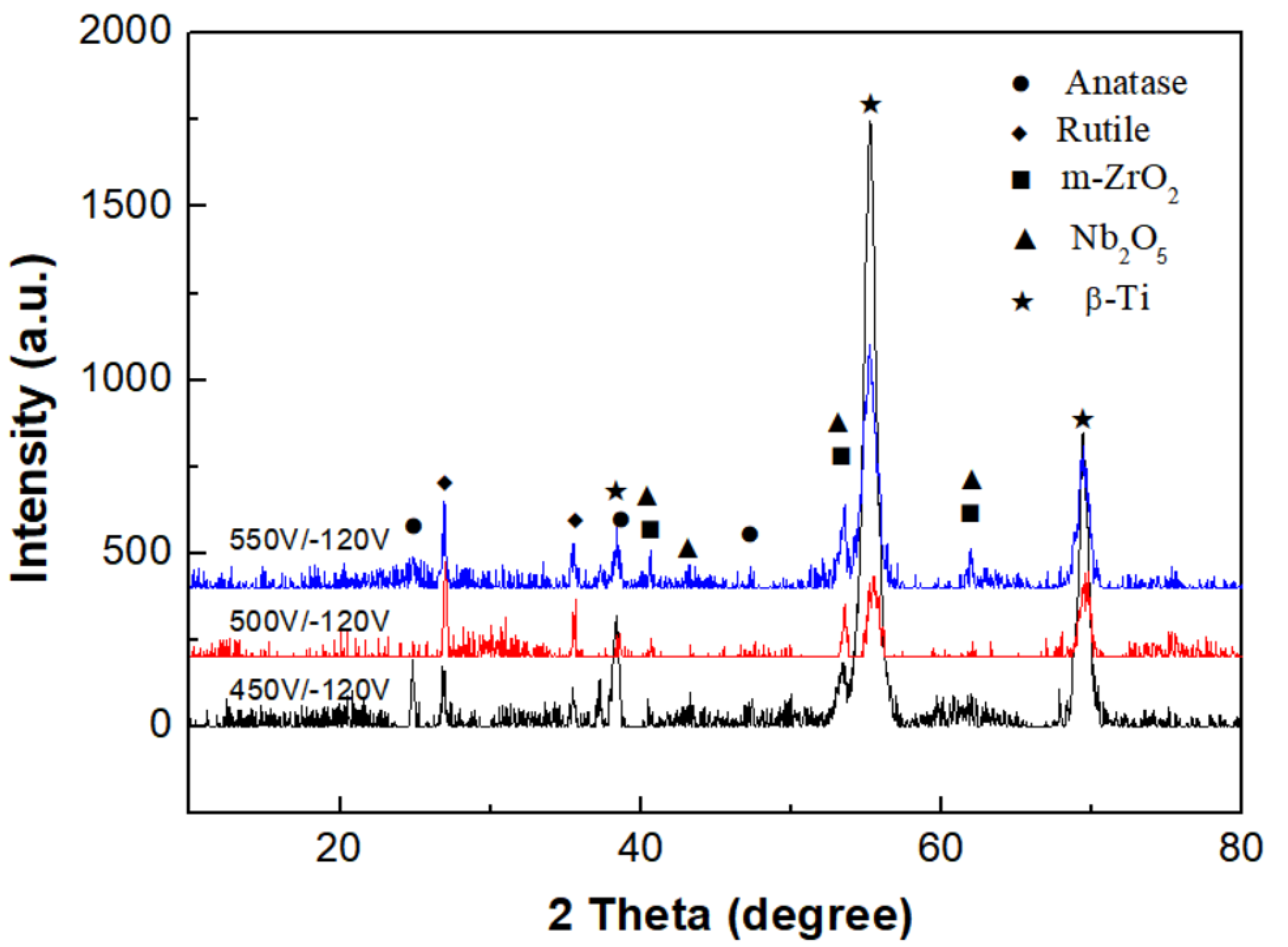
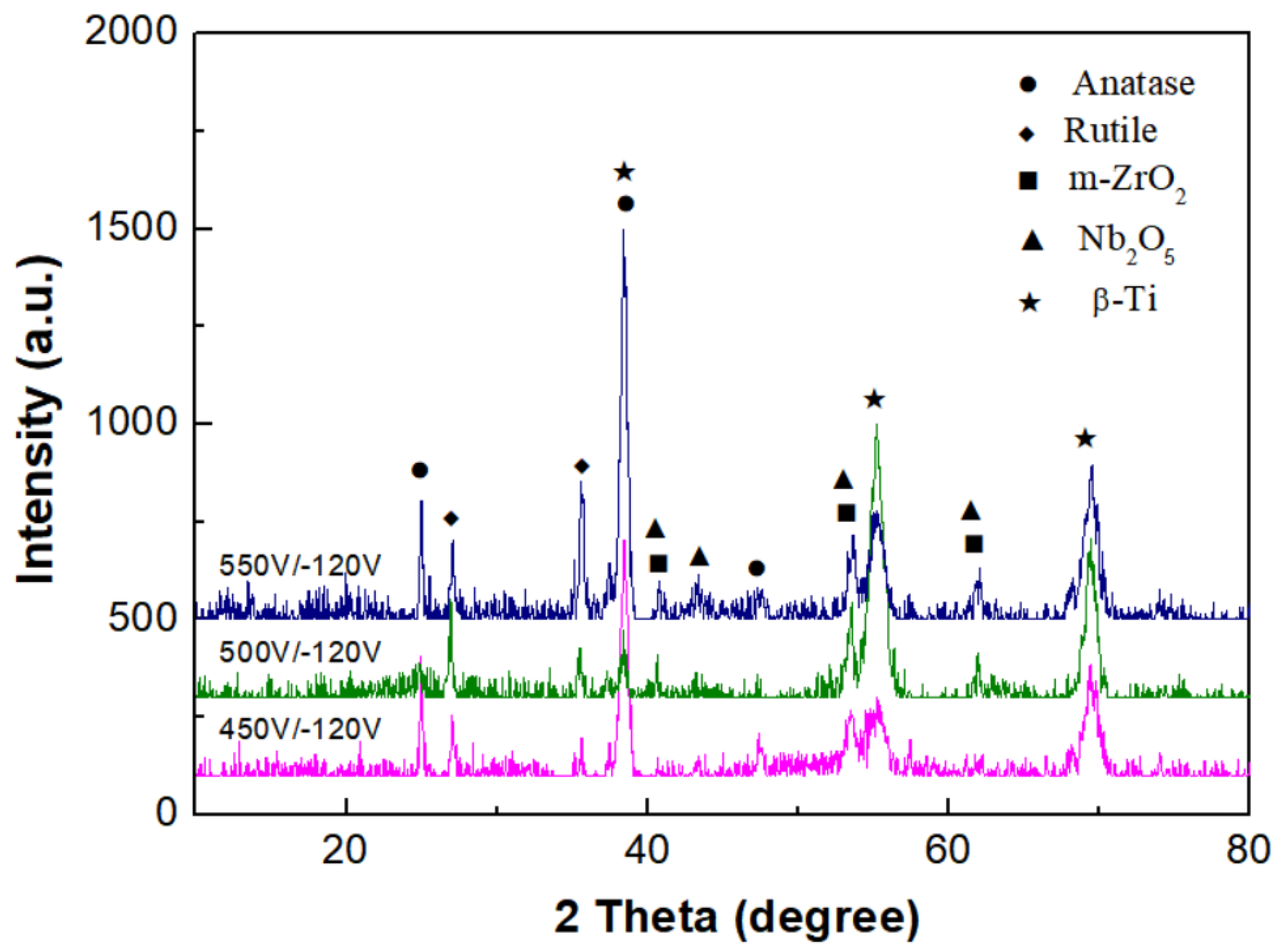
3.2. Phase Analysis of Micro-Arc Oxidized Films
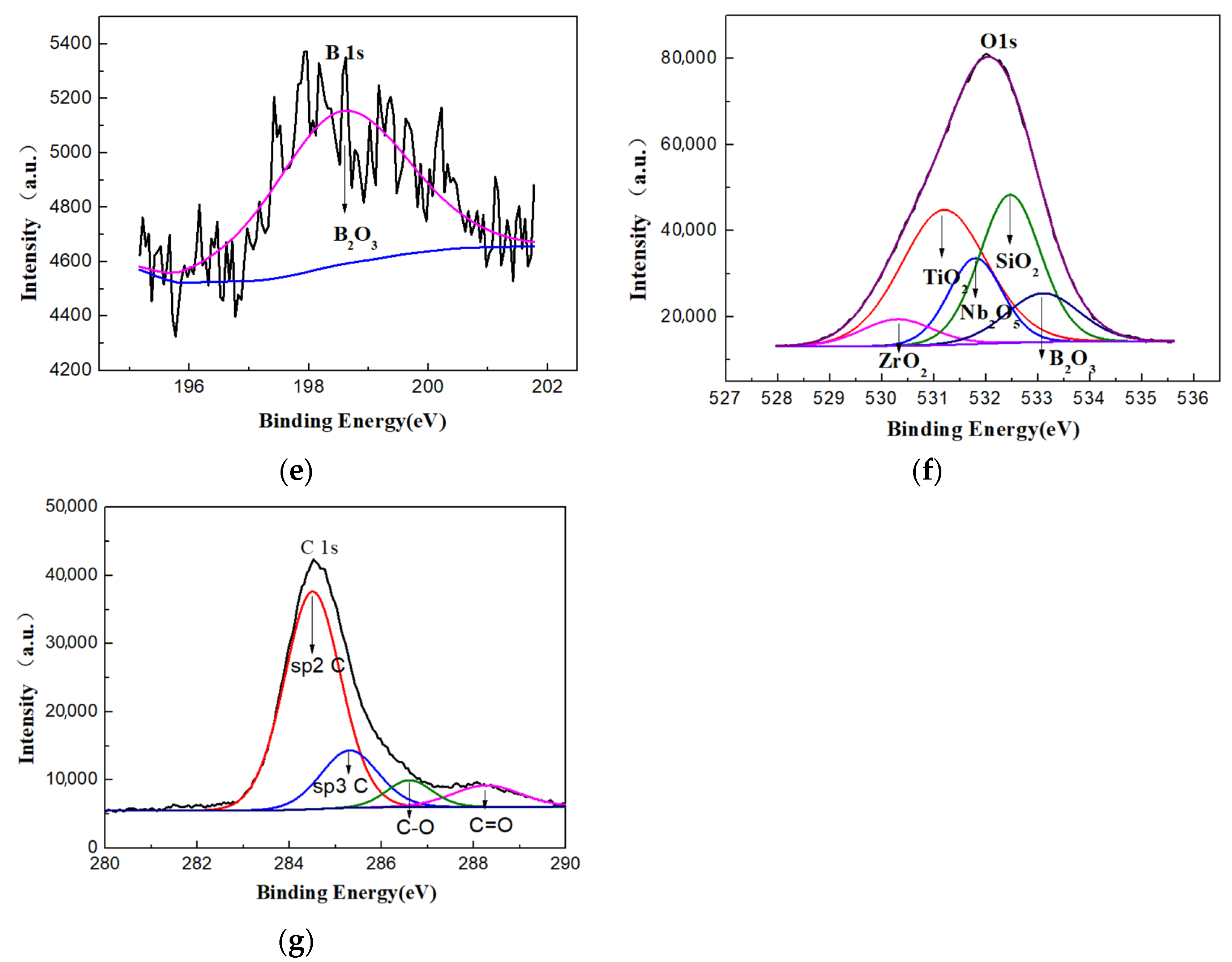
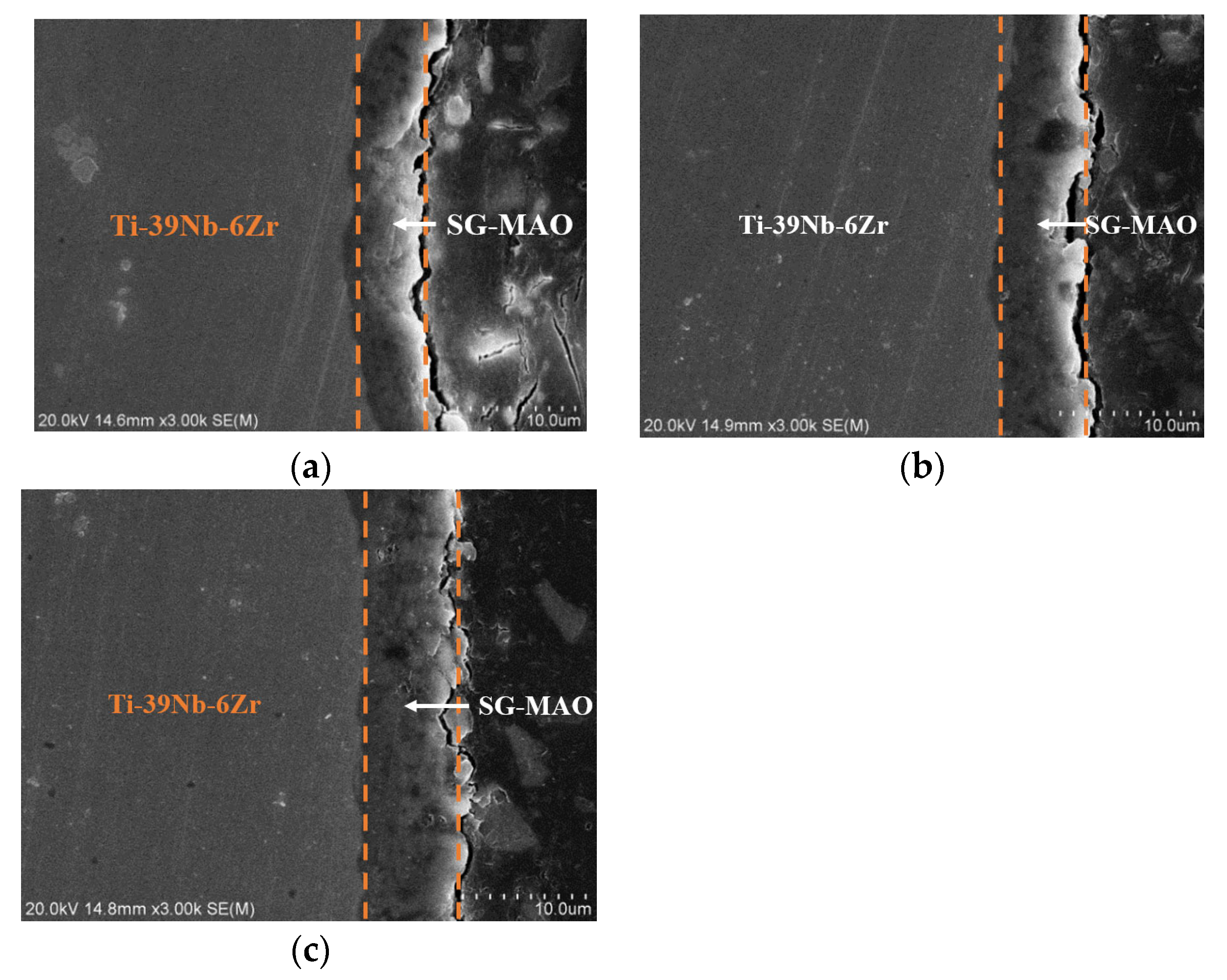
3.3. Preparation and Characterizations of Micro-Arc Oxidized Films of Ti-39Nb-6Zr Alloy in Silicate Electrolyte with Glycerin Additive
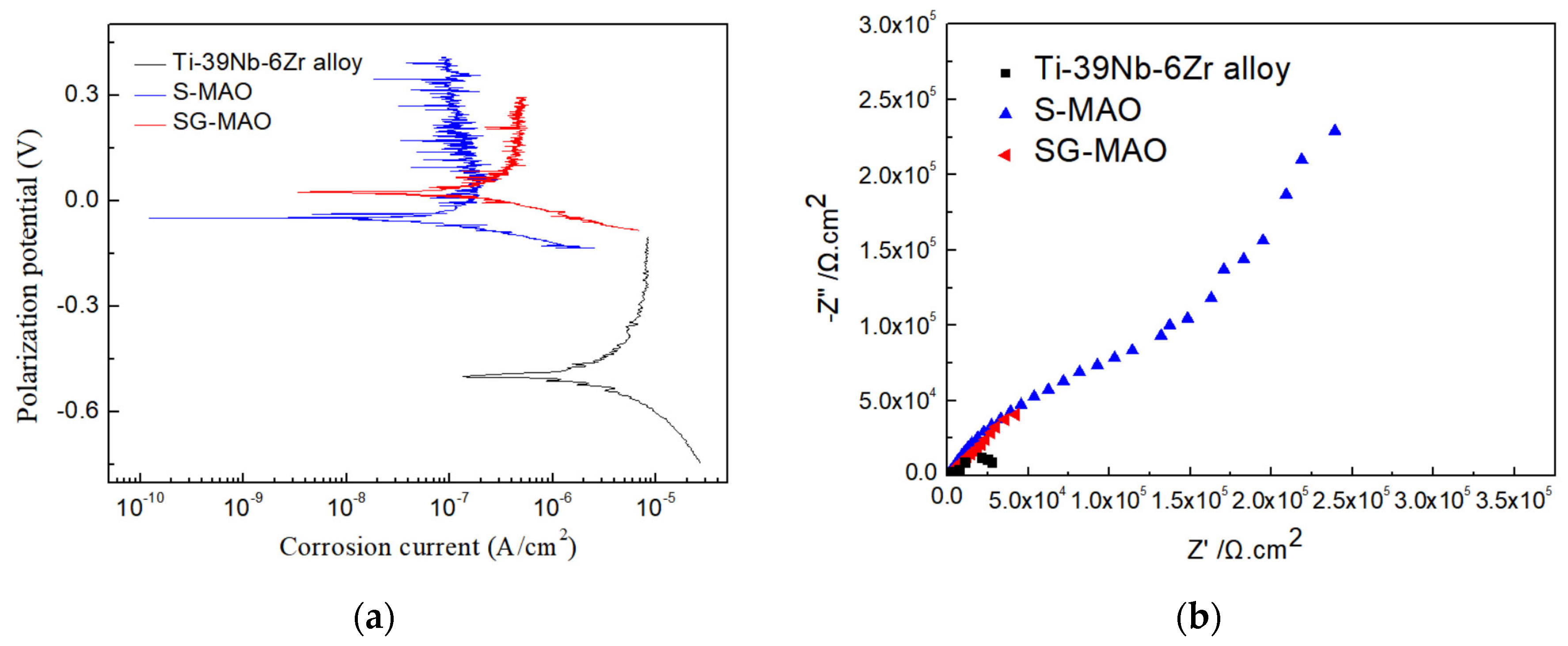
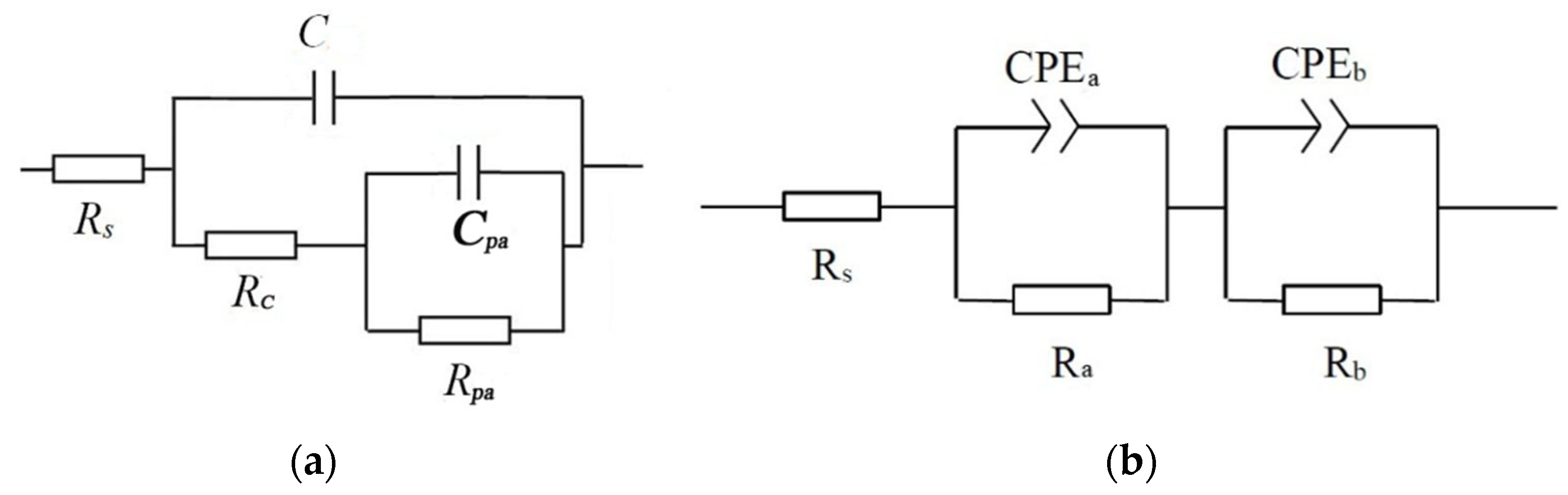
3.4. Electrochemical Corrosion Properties of Micro-Arc Oxidized Films at Room Temperature
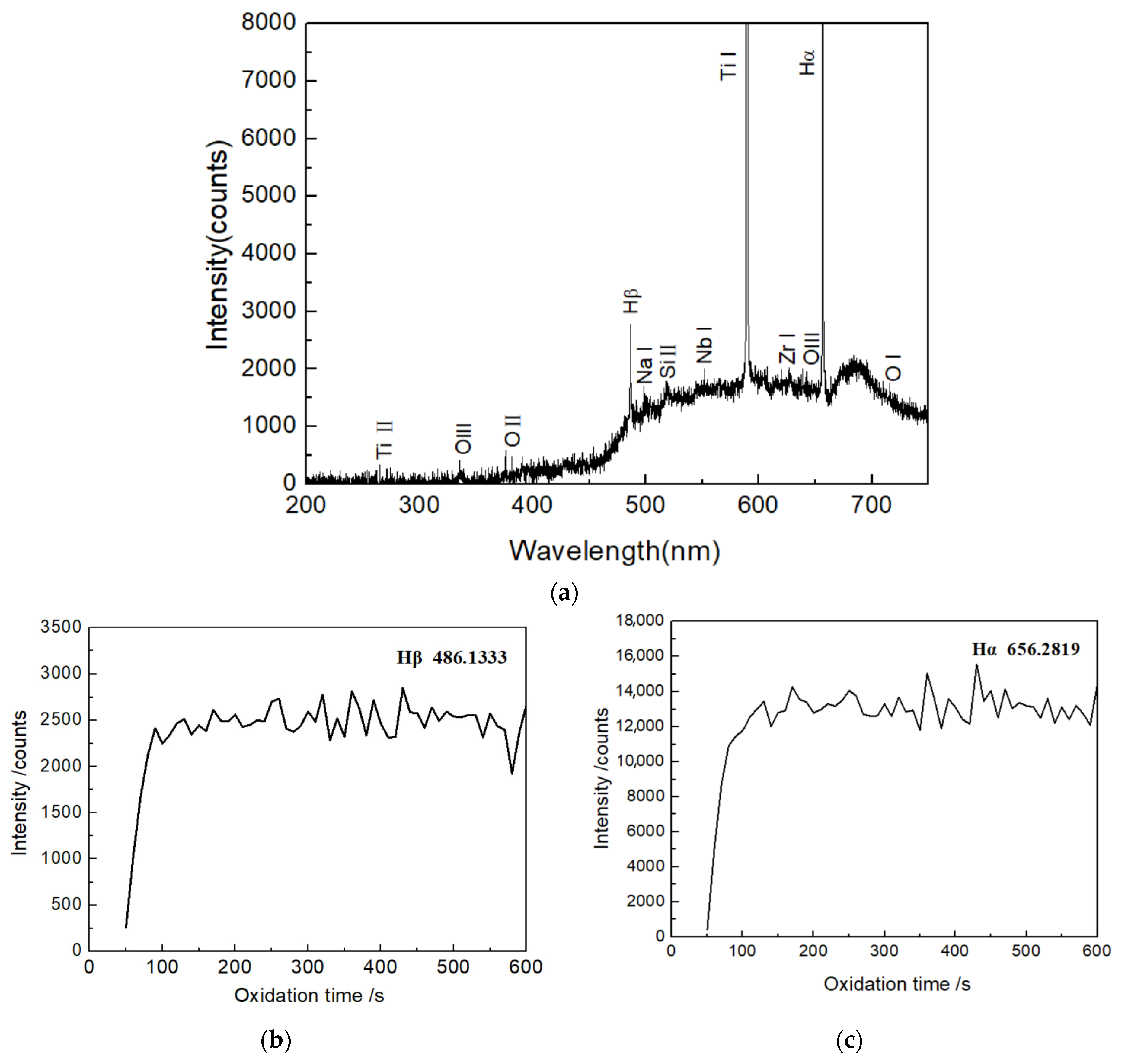
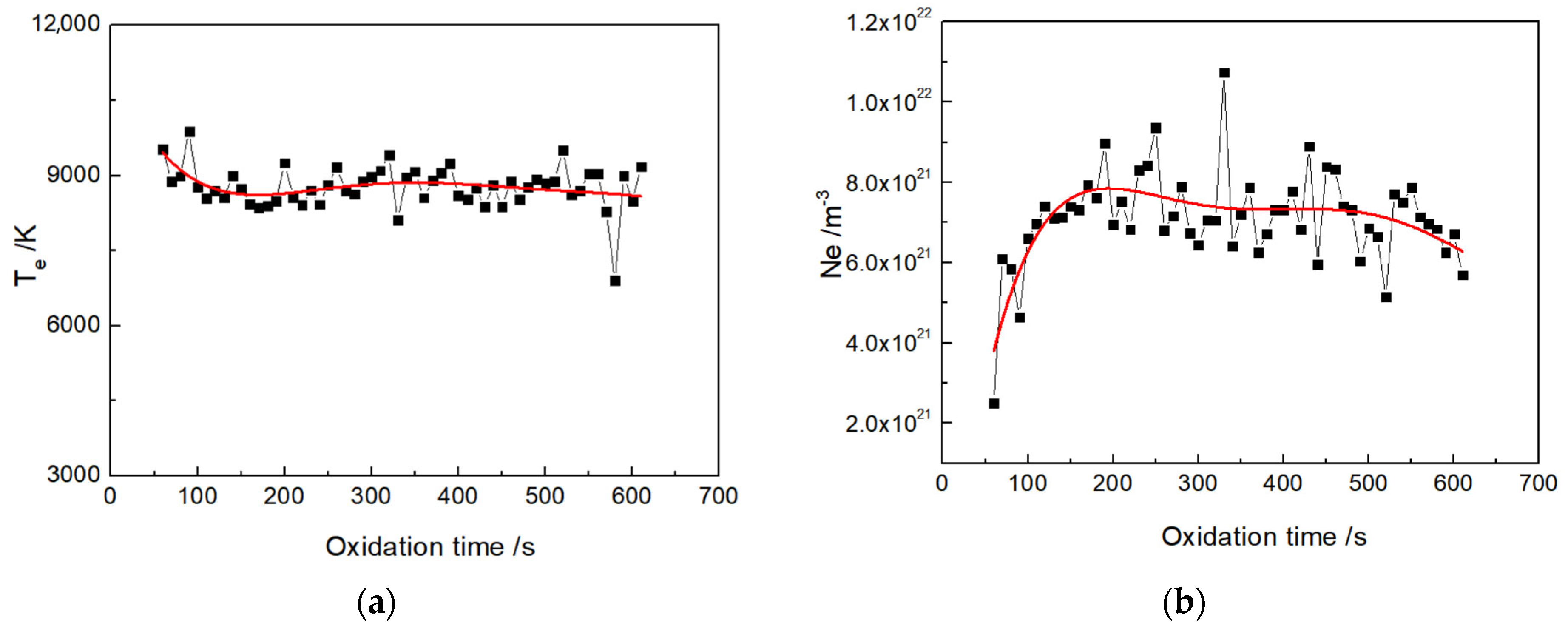
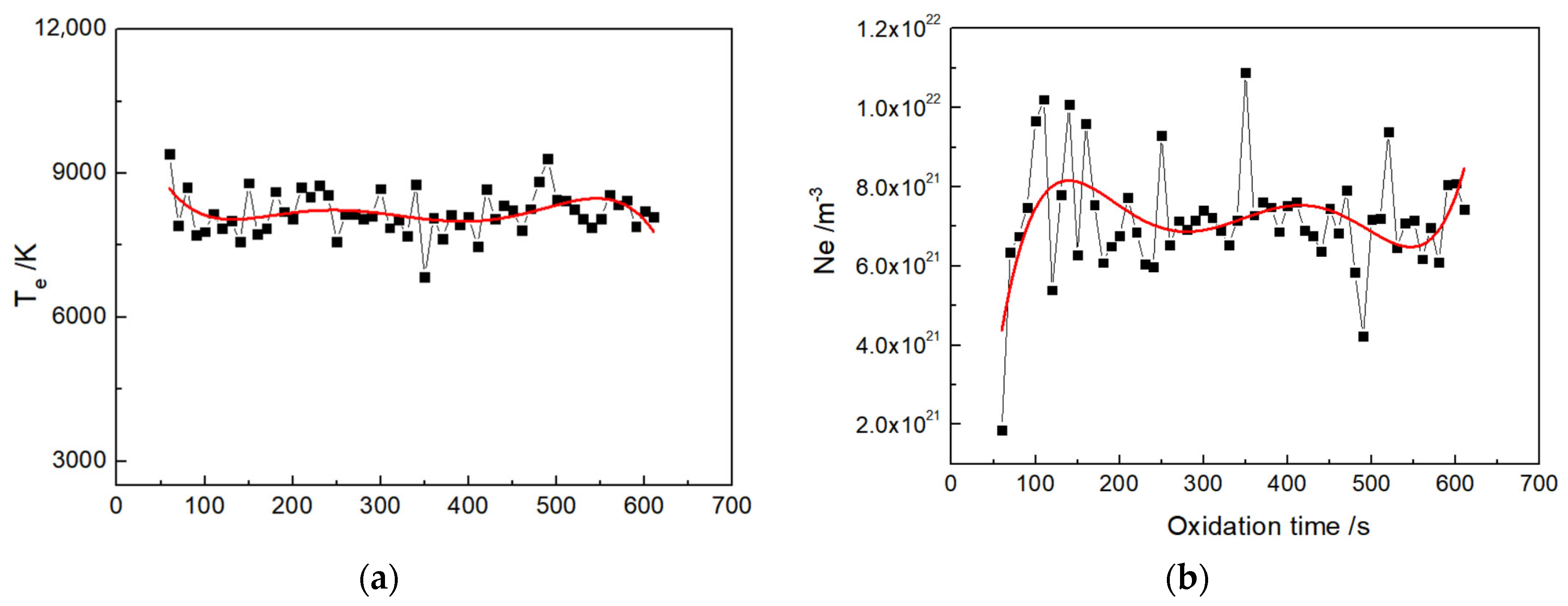
3.5. Optical Emission Spectroscopy of Micro-Arc Discharge
4. Discussion
5. Conclusions
- For the micro-arc oxidized films of the Ti-39Nb-6Zr alloy prepared in silicate electrolyte with or without glycerin additive, their phase structures are mainly composed of the anatase phase (A-TiO2), rutile phase (R-TiO2), Nb2O5 phase, and monoclinic m-ZrO2 phase. Moreover, the content of the anatase phase is significantly increased after the addition of glycerin.
- The addition of glycerin into silicate electrolyte not only brings about the involvement of the C element into the reaction, but also inhibits the deposition of SiO2. Accordingly, the thickness of the loose layer is reduced, and the surface roughness of the micro-arc oxidized film is improved, leading to the enhanced compactness and adhesion force with the substrate.
- During the micro-arc oxidation process, the electron concentration in the plasma-discharge zone of silicate electrolyte without glycerin is about 7.5 × 1021 m−3, and the electron temperature is determined as about 8500 K. In contrast, for the discharge zone of silicate electrolyte with glycerin additive, the electron concentration is about 7 × 1021 m−3, while the electron temperature is about 8100 K.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdel-Hady, M.; Hinoshita, K.; Morinaga, M. General approach to phase stability and elastic properties of β-type Ti-alloys using electronic parameters. Scripta Mater. 2006, 55, 477–480. [Google Scholar]
- Martins, G.V.; Silva, C.R.M.; Nunes, C.A.; Trava-Airoldi, V.J.; Borges, L.A.; Machado, J.P.B. Beta Ti-45Nb and Ti-50Nb alloys produced by Powder Metallurgy for aerospace application. Mater. Sci. Forum. 2010, 660, 405–409. [Google Scholar]
- Geetha, M.; Singh, A.K.; Gogia, A.K.; Asokamani, R. Effect of thermomechanical processing on evolution of various phases in Ti–Nb–Zr alloys. J. Alloys Compd. 2004, 384, 131–144. [Google Scholar]
- Dong, H.; Bell, T. Enhanced wear resistance of titanium surfaces by a new thermal oxidation treatment. Wear 2000, 238, 131–137. [Google Scholar] [CrossRef]
- Selvan, J.S.; Subramanian, K.; Nath, A.K.; Kumarb, H.; Ramachandrac, C.; Ravindranathanc, S.P. Laser boronising of Ti–6Al–4V as a result of laser alloying with pre-placed BN. Mater. Sci. Eng. A 1999, 260, 178–187. [Google Scholar] [CrossRef]
- Blau, P.J.; Jolly, B.C.; Qu, J.; Peter, W.H.; Blue, C.A. Tribological investigation of titanium-based materials for brakes. Wear 2007, 263, 1202–1211. [Google Scholar] [CrossRef]
- Farrakhov, R.; Melnichuk, O.; Parfenov, E.; Mukaeva, V.; Raab, A.; Sheremetyev, V.; Zhukova, Y.; Prokoshkin, S. Comparison of Biocompatible Coatings Produced by Plasma Electrolytic Oxidation on cp-Ti and Ti-Zr-Nb Superelastic Alloy. Coatings 2021, 11, 401. [Google Scholar] [CrossRef]
- Xue, W.B.; Deng, Z.W.; Chen, R.Y.; Zhang, T.H. Microstructure of Ceramic Coating Formed by Microarc Oxidation in Silicate Solution on Ti-6Al-4V Alloy. Heat Treat. Met. 2000, 2, 5–7. [Google Scholar]
- Wang, Y.M.; Jiang, B.L.; Lei, T.Q.; Guo, L.X. Microstructure and Tribological Properties of Microarc Oxidation Coatings Formed on Ti6Al4V Alloy in Na2SiO3 System Aqueous Solution. Tribology 2003, 23, 371–375. [Google Scholar]
- Duarte, L.T.; Biaggio, S.R.; Rocha-Filho, R.C.; Bocchi, N. Preparation and characterization of biomimetically and electrochemically deposited hydroxyapatite coatings on micro-arc oxidized Ti–13Nb–13Zr. J. Mater. Sci-Mater. 2011, 22, 1663–1670. [Google Scholar]
- Tsutsumi, Y.; Niinomi, M.; Nakai, M.; Tsutsumi, H.; Doi, H.; Nomura, N.; Hanawa, T. Micro-arc oxidation treatment to improve the hard-tissue compatibility of Ti–29Nb–13Ta–4.6Zr alloy. Appl. Surf. Sci. 2012, 262, 34–38. [Google Scholar]
- Lederer, S.; Lutz, P.; Furbeth, W. Surface Modification of Ti 13Nb 13Zr by Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2018, 335, 62–71. [Google Scholar]
- Liu, R.; Wu, J.; Xue, W.B.; Qu, Y.; Yang, C.L.; Wang, B.; Wu, X.Y. Discharge behaviors during plasma electrolytic oxidation on aluminum alloy. Mater. Chem. Phys. 2014, 148, 284–292. [Google Scholar] [CrossRef]
- Chen, L.; Jin, X.Y.; Qu, Y.; We, K.J.; Zhang, Y.F.; Liao, B.; Xue, W.B. High Temperature Tribological Behaviors of Microarc Oxidation Film on Ti-39Nb-6Zr Alloy. Surf. Coat. Tech. 2018, 347, 29–37. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Vasilic, R.; Petkovic, M.; Nedic, Z.; Kasalica, B.; Belca, I.; Zekovic, L.J. Luminescence properties of oxide films formed by anodization of aluminum in 12-tungstophosphoric acid. Electrochim. Acta 2010, 55, 3857–3863. [Google Scholar] [CrossRef]
- Griem, H.R. Plasma Diagnostics; McGraw Hill: New York, NY, USA, 1964. [Google Scholar]
- Hussein, R.O.; Zhang, P.; Nie, X.; Xia, Y.; Northwood, D.O. The effect of current mode and discharge type on the corrosion resistance of plasma electrolytic oxidation (PEO) coated magnesium alloy AJ62. Surf. Coat. Tech. 2011, 206, 1990–1997. [Google Scholar] [CrossRef]
- Krysmann, W.; Kruze, P.; Dittrich, K.H.; Schneider, H.G. Process characteristics and Parameters of Anodic Oxidation by Spark Discharge (ANOF). Cryst. Res. Technol. 1984, 19, 973–979. [Google Scholar] [CrossRef]
- Jovovic, J.; Stojadinovic, S.; Sisovic, N.M.; Konjevic, N. Spectroscopic characterization of plasma during electrolytic oxidation (PEO) of aluminium. Surf. Coat. Tech. 2011, 206, 24–28. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Influence of process parameters on electrolytic plasma discharging behaviour and aluminum oxide coating microstructure. Surf. Coat. Tech. 2010, 205, 1659–1667. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]

| Voltage/Element | B (wt.%) | O (wt.%) | Si (wt.%) | Ti (wt.%) | Zr (wt.%) | Nb (wt.%) |
|---|---|---|---|---|---|---|
| +400 V/−120 V | 40.18 | 29.92 | 8.16 | 12.53 | 1.91 | 7.09 |
| +450 V/−120 V | 42.09 | 38.64 | 12.68 | 1.83 | 0.95 | 0.68 |
| +500 V/−120 V | 47.97 | 31.96 | 15.13 | 0.31 | 1.00 | 0.03 |
| Voltage/Element | C (wt.%) | O (wt.%) | Si (wt.%) | Ti (wt.%) | Zr (wt.%) | Nb (wt.%) |
|---|---|---|---|---|---|---|
| +400 V/−120 V | 40.18 | 29.92 | 8.16 | 12.53 | 1.91 | 7.09 |
| +450 V/−120 V | 42.09 | 38.64 | 12.68 | 1.83 | 0.95 | 0.68 |
| +500 V/−120 V | 47.97 | 31.96 | 15.13 | 0.31 | 1.00 | 0.03 |
| B region | 13.32 | 39.62 | 7.22 | 19.55 | 6.64 | 12.99 |
| W region | 0.17 | 34.82 | 6.23 | 39.78 | 6.39 | 11.78 |
| Samples | icorr (A·cm−2) | Ecorr (V) | Rp (Ω·cm2) |
|---|---|---|---|
| Ti-39Nb-6Zr alloy | 2.11 × 10−6 | −0.49 | 1.99 × 104 |
| S-MAO | 1.25 × 10−7 | −0.049 | 1.79 × 105 |
| SG-MAO | 3.36 × 10−7 | −0.023 | 76,311 |
| Samples | Rs Ω·cm2 | C F/cm2 | Rc Ω·cm2 | Cpa F/cm2 | Rpa Ω·cm2 |
|---|---|---|---|---|---|
| Ti-39Nb-6Zr alloy | 11.26 | 2.25 × 10−4 | 654.30 | 4.13 × 10−4 | 5790 |
| Samples | Rs Ω·cm2 | CPEa,Y0 Ω−1·cm−2·s−n | na | Ra Ω·cm2 | CPEb,Y0 Ω−1·cm−2·s−n | nb | Rb Ω·cm2 |
|---|---|---|---|---|---|---|---|
| S-MAO | 10.52 | 3.91 × 10−6 | 0.65 | 1.90 × 105 | 1.76 × 10−5 | 0.69 | 4.87 × 106 |
| SG-MAO | 11.28 | 6.09 × 10−6 | 0.65 | 1278 | 4.65 × 10−5 | 0.51 | 8.07 × 105 |
| Line | Λ (nm) | Transition | gk | Energy (eV) | Aki (106 s−1) |
|---|---|---|---|---|---|
| Hβ | 486.05 | 4d 2D→2p 2P | 32 | 2.55 | 8.42 |
| Hα | 656.32 | 3d 2D→2p 2P | 18 | 1.89 | 44.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Jin, X.; Xue, W.; Wu, J. Fabrication and Characterization of Micro-Arc Oxidation Films on β-Titanium Alloy in Silicate and Silicate/Glycerin Electrolyte. Coatings 2024, 14, 1408. https://doi.org/10.3390/coatings14111408
Chen L, Jin X, Xue W, Wu J. Fabrication and Characterization of Micro-Arc Oxidation Films on β-Titanium Alloy in Silicate and Silicate/Glycerin Electrolyte. Coatings. 2024; 14(11):1408. https://doi.org/10.3390/coatings14111408
Chicago/Turabian StyleChen, Lin, Xiaoyue Jin, Wenbin Xue, and Jie Wu. 2024. "Fabrication and Characterization of Micro-Arc Oxidation Films on β-Titanium Alloy in Silicate and Silicate/Glycerin Electrolyte" Coatings 14, no. 11: 1408. https://doi.org/10.3390/coatings14111408
APA StyleChen, L., Jin, X., Xue, W., & Wu, J. (2024). Fabrication and Characterization of Micro-Arc Oxidation Films on β-Titanium Alloy in Silicate and Silicate/Glycerin Electrolyte. Coatings, 14(11), 1408. https://doi.org/10.3390/coatings14111408




