Influence of the Annealing Environment on the Structure and Ferroelectric Properties of Lead Titanate Thin Films
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Wang, Y.; Yang, M.; Miao, J.; Lin, K.; Li, Q.; Chen, X.; Deng, J.; Xing, X. Ferroelectric Thin Films: Performance Modulation and Application. Mater. Adv. 2022, 3, 5735–5752. [Google Scholar] [CrossRef]
- Martin, L.W.; Rappe, A.M. Thin-Film Ferroelectric Materials and Their Applications. Nat. Rev. Mater. 2016, 2, 16087. [Google Scholar] [CrossRef]
- Singh, R.; Tripathi, A.; Gautam, M. 23—Ferroelectric Perovskite Thin Films as Nonvolatile Computer Memories. In Perovskite Metal Oxides; Moharana, S., Badapanda, T., Satpathy, S.K., Mahaling, R.N., Kumar, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 595–616. [Google Scholar] [CrossRef]
- Müller, M.; Efe, I.; Sarott, M.F.; Gradauskaite, E.; Trassin, M. Ferroelectric Thin Films for Oxide Electronics. ACS Appl. Electron. Mater. 2023, 5, 1314–1334. [Google Scholar] [CrossRef]
- Sun, L.L.; Tan, O.K.; Liu, W.G.; Zhu, W.G.; Yao, X. Poling of Multilayer Pb(Zr0.3Ti0.7)O3/PbTiO3 Thin Film for Pyroelectric Infrared Sensor Application. Infrared Phys. Technol. 2003, 44, 177–182. [Google Scholar] [CrossRef]
- Fang, B.; Qian, K.; Yuan, N.; Ding, J.; Zhao, X.; Luo, H. Large Pyroelectric Response of 0.8Pb(Mg1/3Nb2/3)O3–0.2PbTiO3 Ceramics Prepared by Reaction-Sintering Method. Mater. Lett. 2012, 84, 91–93. [Google Scholar] [CrossRef]
- Szwagierczak, D.; Kulawik, J. Thick Film Capacitors with Relaxor Dielectrics. J. Eur. Ceram. Soc. 2004, 24, 1979–1985. [Google Scholar] [CrossRef]
- Cochran, S. 1—Piezoelectricity and Basic Configurations for Piezoelectric Ultrasonic Transducers. In Ultrasonic Transducers; Nakamura, K., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 3–35. [Google Scholar] [CrossRef]
- Muralt, P. Ferroelectric Thin Films for Micro-Sensors and Actuators: A Review. J. Micromech. Microeng. 2000, 10, 136. [Google Scholar] [CrossRef]
- Czekaj, D.; Jaszczyszyn, E.; Orkisz, T.; Kozielski, L.; Lisinska-Czekaj, A.; Federowicz, E.; Modelski, J. Synthesis and Basic Properties of Ferroelectric Thin Films. Methods Materials and Novel Applications. In Proceedings of the 2006 International Conference on Microwaves, Radar & Wireless Communications, Krakow, Poland, 22–24 May 2006; pp. 692–699. [Google Scholar]
- Lima, E.C.; Guerra, J.D.S.; Rodrigues, A.D.; Bernardi, M.I.B.; M’Peko, J.C.; Hernandes, A.C. Investigation of the Structural Properties of PbTiO3 thin films. In Proceedings of the 2021 IEEE International Symposium on Applications of Ferroelectrics (ISAF), Virtual, 16–21 May 2021; pp. 1–4. [Google Scholar]
- Luo, C.; Li, D.; Liu, J.; Ma, Z. Investigation of Dielectric Constant for PbTiO3 Film in THz Range. Ferroelectrics 2023, 613, 22–30. [Google Scholar] [CrossRef]
- Palkar, V.R.; Purandare, S.C.; Pinto, R. Ferroelectric Thin Films of PbTiO3 on Silicon. J. Phys. D Appl. Phys. 1999, 32, R1. [Google Scholar] [CrossRef]
- Ali, H.E.H.; Ricote, J.; Calzada, M.L.; Bretos, I.; Jiménez, R. The Influence of the Crystallization Temperature on the Reliability of PbTiO3 Thin Films Prepared by Chemical Solution Deposition. J. Eur. Ceram. Soc. 2017, 37, 1449–1458. [Google Scholar] [CrossRef]
- Nishino, R.; Fujita, T.C.; Kagawa, F.; Kawasaki, M. Evolution of Ferroelectricity in Ultrathin PbTiO3 Films as Revealed by Electric Double Layer Gating. Sci. Rep. 2020, 10, 10864. [Google Scholar] [CrossRef] [PubMed]
- Sakai, J.; Roque, J.M.; Vales-Castro, P.; Padilla-Pantoja, J.; Sauthier, G.; Santiso, J. Pulsed Laser Deposition of Epitaxial Non-Doped PbTiO3 Thin Films from PbO–TiO2 Mosaic Targets. Coatings 2021, 11, 662. [Google Scholar] [CrossRef]
- Basit, N.A.; Kim, H.K.; Blachere, J. Temperature Dependence of Lead Loss in R.F. Magnetron Sputtering of a Stoichiometric Pb(Zr,Ti)O3 Target. Thin Solid Film. 1997, 302, 155–161. [Google Scholar] [CrossRef]
- Pontes, F.M.; Rangel, J.H.G.; Leite, E.R.; Longo, E.; Varela, J.A.; Araújo, E.B.; Eiras, J.A. Low Temperature Synthesis and Electrical Properties of PbTiO3 Thin Films Prepared by the Polymeric Precursor Method. Thin Solid Film. 2000, 366, 232–236. [Google Scholar] [CrossRef]
- Harjuoja, J.; Kosola, A.; Putkonen, M.; Niinistö, L. Atomic Layer Deposition and Post-Deposition Annealing of PbTiO3 Thin Films. Thin Solid Film. 2006, 496, 346–352. [Google Scholar] [CrossRef]
- Iljinas, A.; Marcinauskas, L.; Stankus, V. In Situ Deposition of PbTiO3 Thin Films by Direct Current Reactive Magnetron Sputtering. Appl. Surf. Sci. 2016, 381, 6–11. [Google Scholar] [CrossRef]
- Iljinas, A.; Stankus, V.; Kaliasas, R. Synthesis of PbTiO3 Thin Films by Annealing Multilayer Oxide Structures in Vacuum. Acta Phys. Pol. A 2016, 129, 121–124. [Google Scholar] [CrossRef]
- Iljinas, A.; Stankus, V.; Čyvienė, J.; Abakevičienė, B. Formation of PbTiO3 Thin Films on Seed Layers Using DC Magnetron Layer-by-Layer Deposition. Vacuum 2015, 122, 310–313. [Google Scholar] [CrossRef]
- Wu, J.-G.; Zhu, J.-L.; Xiao, D.-Q.; Zhu, J.-G.; Tan, J.-Z.; Zhang, Q.-L. Preparation and Properties of PbTiO3/(Pb1−xLax)TiO3/PbTiO3 Multi-Layer Films by Rf Magnetron Sputtering. Ferroelectrics 2007, 357, 271–275. [Google Scholar] [CrossRef]
- Weymann, C.; Lichtensteiger, C.; Fernandez-Peña, S.; Cordero-Edwards, K.; Paruch, P. Improved Thin Film Growth Using Slow Kinetics Intermittent Sputtering. Appl. Surf. Sci. 2020, 516, 146077. [Google Scholar] [CrossRef]
- Fu, Y.; Singh, D.J. Thermal Conductivity of Perovskite KTaO3 and PbTiO3 from First Principles. Phys. Rev. Mater. 2018, 2, 094408. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.; Jing, H.; Li, Z.; Wang, D.; Jia, C.-L. Correlations between Polarization and Structural Information of Supertetragonal PbTiO3. Phys. Rev. B 2022, 105, 024106. [Google Scholar] [CrossRef]
- Feng, Y.; Tang, Y.; Ma, D.; Zhu, Y.; Zou, M.; Han, M.; Ma, J.; Ma, X. Thickness-Dependent Evolution of Piezoresponses and Stripe 90° Domains in (101)-Oriented Ferroelectric PbTiO3 Thin Films. ACS Appl. Mater. Interfaces 2018, 10, 24627–24637. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.W.; Son, J.Y. Tetragonally Strained Crystal Structure and Ferroelectric Properties of Epitaxial PbTiO3 Thin Films Grown on Single-Crystal Rh Substrates. Mater. Chem. Phys. 2021, 264, 124477. [Google Scholar] [CrossRef]
- Azaroual, O.; El Hasnaoui, M.; Akharchach, B.; Narjis, A. Ferroelectric Properties and Structural Characterisation of Lead Titanate Thin Films Prepared by Polymeric Precursor Method. Adv. Mater. Process. Technol. 2022, 8, 3674–3684. [Google Scholar] [CrossRef]
- Beklešovas, B.; Iljinas, A.; Stankus, V.; Čyvienė, J.; Andrulevičius, M.; Ivanov, M.; Banys, J. Structural, Morphologic, and Ferroelectric Properties of PZT Films Deposited through Layer-by-Layer Reactive DC Magnetron Sputtering. Coatings 2022, 12, 717. [Google Scholar] [CrossRef]
- Banerjee, R.; Purandare, S.C.; Palkar, V.R.; Pinto, R. Effect of Silicon on the Microstructure of Pulsed Laser Ablated Ferroelectric PbTiO3 Thin Films. J. Phys. D Appl. Phys. 2001, 34, 1037. [Google Scholar] [CrossRef]
- Martín-Arbella, N.; Bretos, Í.; Jiménez, R.; Calzada, M.L.; Sirera, R. Photoactivation of Sol–Gel Precursors for the Low-Temperature Preparation of PbTiO3 Ferroelectric Thin Films. J. Am. Ceram. Soc. 2011, 94, 396–403. [Google Scholar] [CrossRef]
- Schulze, G.F.; Jona, F.; Shirane, G. Ferroelectric Crystals. 402 S. Oxford/London/New York/Paris 1962. Pergamon Press. Preis Geb. 84 S. Net. Z. Angew. Math. Mech. 1963, 43, 512. [Google Scholar] [CrossRef]
- Yang, J.I.; Welsh, A.; Sbrockey, N.M.; Tompa, G.S.; Polcawich, R.G.; Potrepka, D.M.; Trolier-McKinstry, S. Annealing Behavior and Electrical Properties of Atomic Layer Deposited PbTiO3 and PZT Films. J. Cryst. Growth 2018, 493, 45–50. [Google Scholar] [CrossRef]
- Kobertz, D.; Müller, M.; Molak, A. Vaporization and Caloric Studies on Lead Titanate. Calphad 2014, 46, 62–79. [Google Scholar] [CrossRef]
- Li, H.-L.; Zhang, Y.; Zhou, J.-J.; Zhang, X.-W.; Liu, H.; Fang, J.-Z. Phase Structure and Electrical Properties of xPZN–(1−x)PZT Piezoceramics near the Tetragonal/Rhombohedral Phase Boundary. Ceram. Int. 2015, 41, 4822–4828. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Longo, E.; Varela, J.A.; Zaghete, M.A. Synthesis and Characterization of Lead Zirconate Titanate (PZT) Obtained by Two Chemical Methods. Ceram. Int. 2014, 40, 1717–1722. [Google Scholar] [CrossRef]
- Tipakontitikul, R.; Ananta, S.; Yimnirun, R. Phase Formation and Transitions in the Lead Magnesium Niobate–Lead Zirconate Titanate System. Curr. Appl. Phys. 2006, 6, 307–311. [Google Scholar] [CrossRef]
- Wongsaenmai, S.; Tan, X.; Ananta, S.; Yimnirun, R. Dielectric and Ferroelectric Properties of Fine Grains Pb(In1/2Nb1/2)O3–PbTiO3 Ceramics. J. Alloys Compd. 2008, 454, 331–339. [Google Scholar] [CrossRef]
- He, C.; Zhang, Y.; Sun, L.; Wang, J.; Wu, T.; Xu, F.; Du, C.; Zhu, K.; Liu, Y. Electrical and Optical Properties of Nd3+-Doped Na0.5Bi0.5TiO3 Ferroelectric Single Crystal. J. Phys. D Appl. Phys. 2013, 46, 245104. [Google Scholar] [CrossRef]
- Rujiwatra, A.; Jongphiphan, J.; Ananta, S. Stoichiometric Synthesis of Tetragonal Phase Pure Lead Titanate under Mild Chemical Conditions Employing NaOH and KOH. Mater. Lett. 2005, 59, 1871–1875. [Google Scholar] [CrossRef]
- Idrissi, H.; Aboujalil, A.; Deloume, J.P.; Fantozzi, G.; Durand, B. Molten Salt Prepared Lead Titanate: Powder Characterization, Sintering and Physical Properties. J. Eur. Ceram. Soc. 1999, 19, 1997–2004. [Google Scholar] [CrossRef]
- Chen, J.; Shi, H.; Liu, G.; Cheng, J.; Dong, S. Temperature Dependence of Dielectric, Piezoelectric and Elastic Properties of BiScO3–PbTiO3 High Temperature Ceramics with Morphotropic Phase Boundary (Mpb) Composition. J. Alloys Compd. 2012, 537, 280–285. [Google Scholar] [CrossRef]
- de Lazaro, S.; Longo, E.; Sambrano, J.R.; Beltrán, A. Structural and Electronic Properties of PbTiO3 slabs: A Dft Periodic Study. Surf. Sci. 2004, 552, 149–159. [Google Scholar] [CrossRef]
- Kamel, T.M.; de With, G. Poling of Hard Ferroelectric Pzt Ceramics. J. Eur. Ceram. Soc. 2008, 28, 1827–1838. [Google Scholar] [CrossRef]
- He, C.; Wang, Z.; Li, X.; Yang, X.; Long, X.; Ye, Z.-G. Self-Polarized High Piezoelectricity and Its Memory Effect in Ferroelectric Single Crystals. Acta Mater. 2017, 125, 498–505. [Google Scholar] [CrossRef]
- Stankus, V.; Dudonis, J.; Pranevicius, L.; Pranevičius, L.L.; Milcius, D.; Templier, C.; Riviere, J.P. On the Mechanism of Synthesis of PbTiO3 Thin Films by Thermal Annealing of Pb/Ti Layers in Air at Atmospheric Pressure. Thin Solid Film. 2003, 426, 78–84. [Google Scholar] [CrossRef]
- Haimin, L.; Chunli, Q.; Jianguo, Z.; Mingzhe, H.; Qingsong, Y. Effect of Different Annealing Atmosphere on Ferroelectric Properties of 0.7BiFeO3-0.3PbTiO3 Thin Films. Rare Met. Mater. Eng. 2016, 45, 1449–1454. [Google Scholar] [CrossRef]
- Hsu, M.-C.; Sun, Y.-M.; Leu, I.-C.; Hon, M.-H. Structural and Electrical Characterizations of PbTiO3 Thin Films Grown on LaNiO3-Buffered Pt/Ti/SiO2/Si Substrates by Liquid Phase Deposition. J. Electrochem. Soc. 2006, 153, F260. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Castro, A.; Ferreira, P.; Rodriguez, B.J.; Vilarinho, P.M. The Role of Nanoporosity on the Local Piezo and Ferroelectric Properties of Lead Titanate Thin Films. J. Mater. Chem. C 2015, 3, 1035–1043. [Google Scholar] [CrossRef]
- Stancu, V.; Lisca, M.; Boerasu, I.; Pintilie, L.; Kosec, M. Effects of Porosity on Ferroelectric Properties of Pb(Zr0.2Ti0.8)O3 Films. Thin Solid Film. 2007, 515, 6557–6561. [Google Scholar] [CrossRef]
- Zhang, Y.; Roscow, J.; Lewis, R.; Khanbareh, H.; Topolov, V.Y.; Xie, M.; Bowen, C.R. Understanding the Effect of Porosity on the Polarisation-Field Response of Ferroelectric Materials. Acta Mater. 2018, 154, 100–112. [Google Scholar] [CrossRef]
- Kormondy, K.J.; Popoff, Y.; Sousa, M.; Eltes, F.; Caimi, D.; Rossell, M.D.; Fiebig, M.; Hoffmann, P.; Marchiori, C.; Reinke, M.; et al. Microstructure and Ferroelectricity of BaTiO3 thin Films on Si for Integrated Photonics. Nanotechnology 2017, 28, 075706. [Google Scholar] [CrossRef]
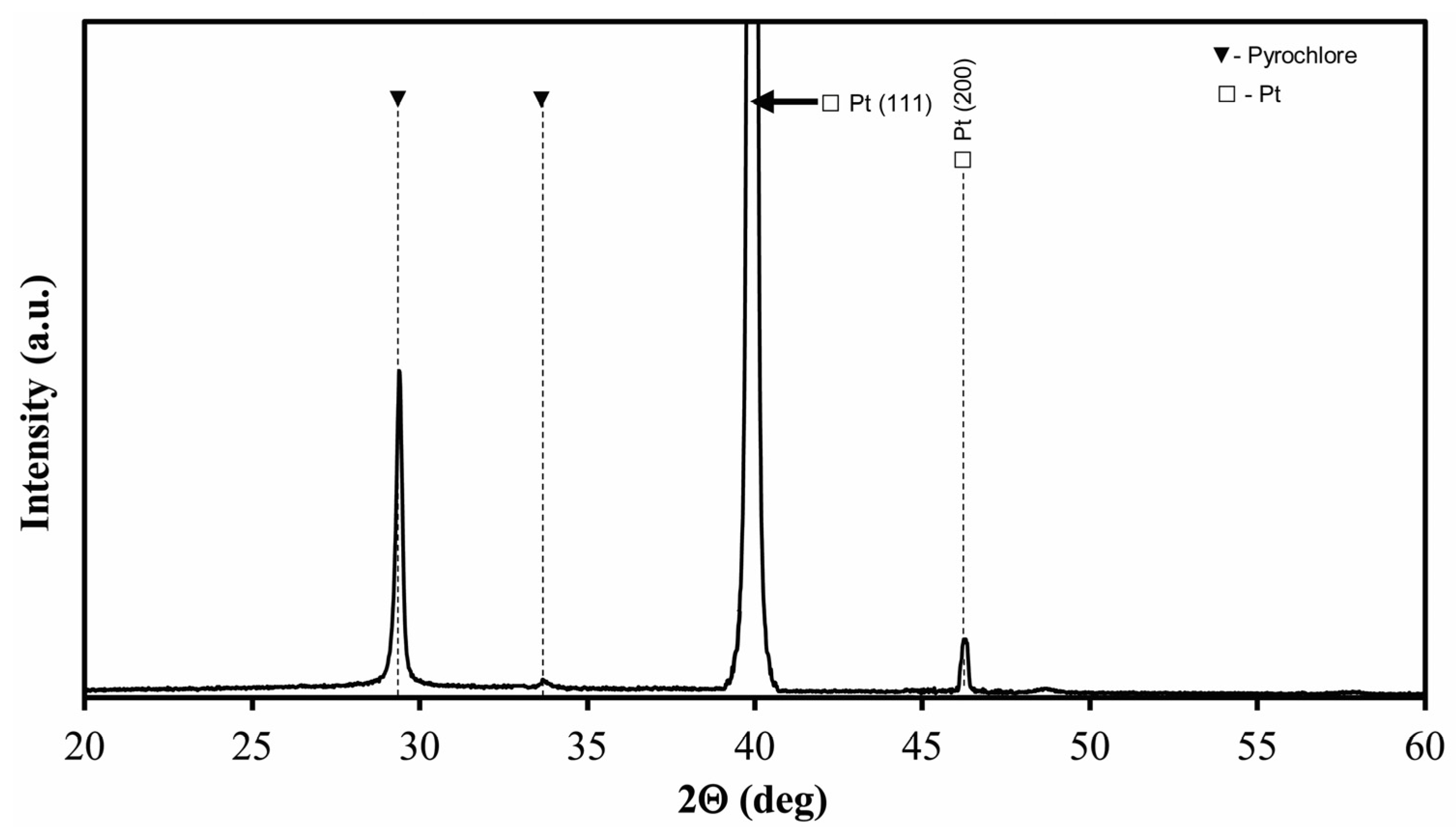
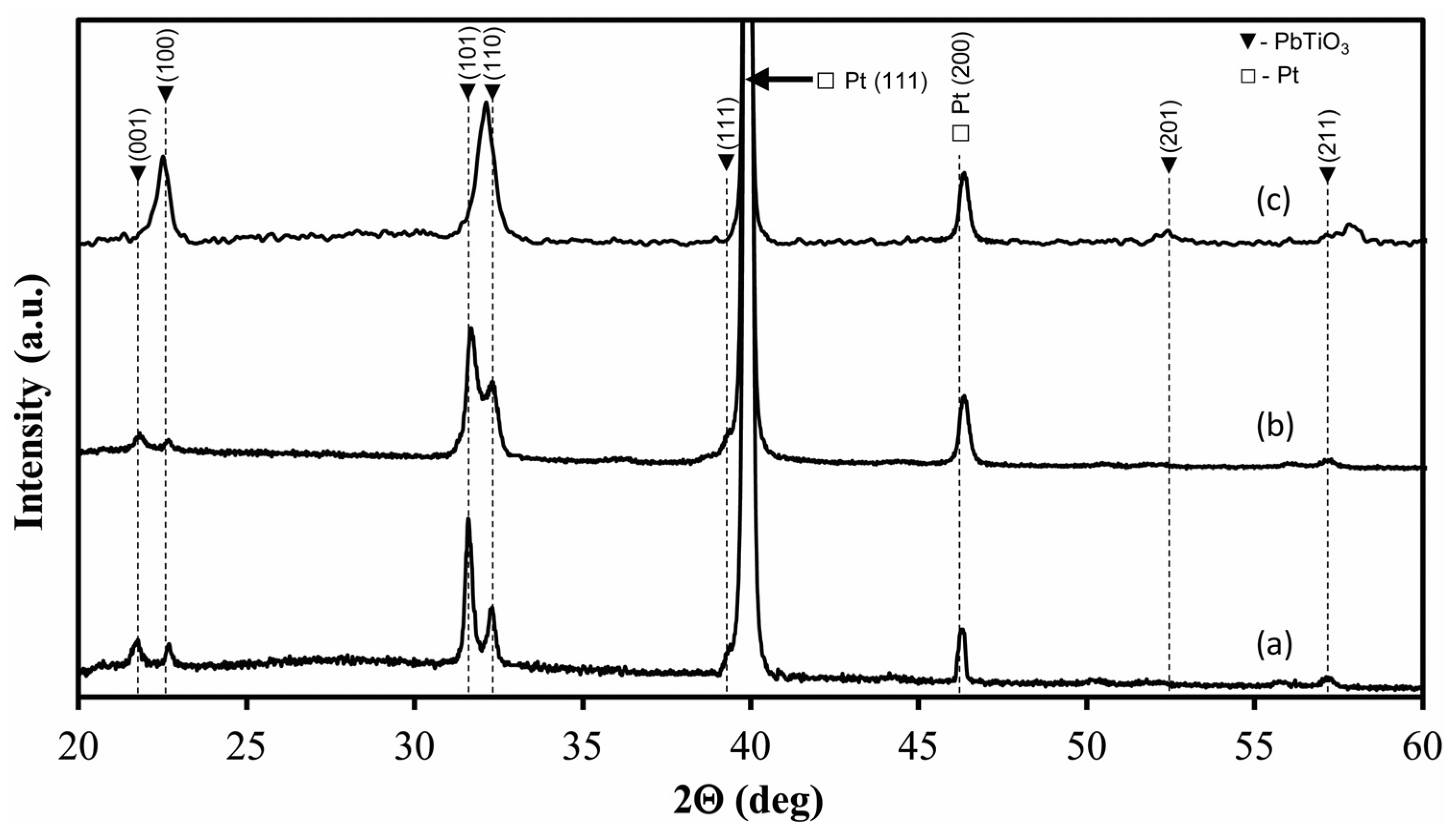


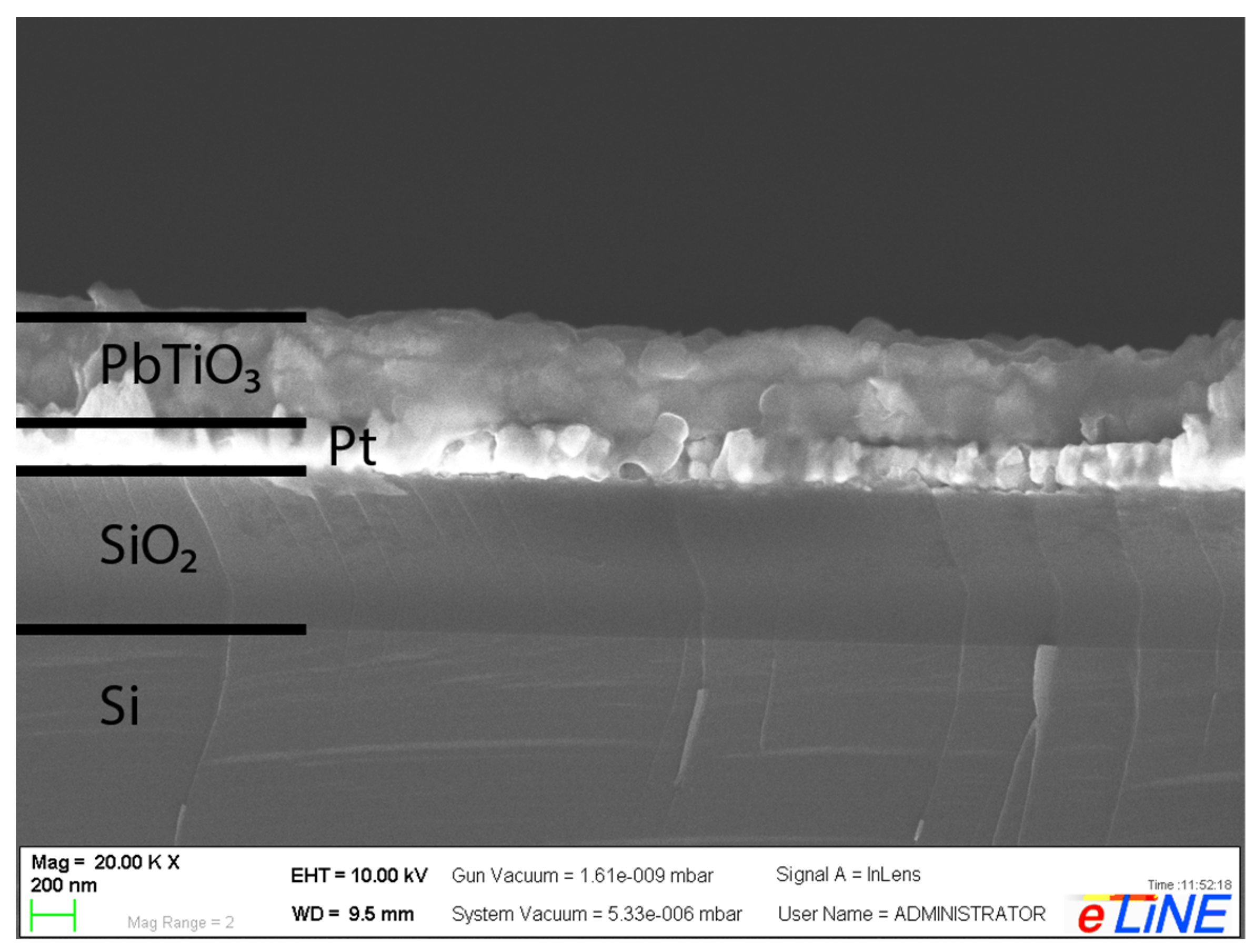
| Film | c/a Ratio | Crystallite Size, nm | Remnant Polarization, μC/cm2 | Coercive Field, kV/cm |
|---|---|---|---|---|
| Annealed in air | 1.042 | 26 | 38 | 130 |
| Annealed in oxygen gas | 1.039 | 23 | 20 | 70 |
| Annealed in vacuum | - | 20 | 7 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iljinas, A.; Stankus, V.; Marcinauskas, L. Influence of the Annealing Environment on the Structure and Ferroelectric Properties of Lead Titanate Thin Films. Coatings 2024, 14, 58. https://doi.org/10.3390/coatings14010058
Iljinas A, Stankus V, Marcinauskas L. Influence of the Annealing Environment on the Structure and Ferroelectric Properties of Lead Titanate Thin Films. Coatings. 2024; 14(1):58. https://doi.org/10.3390/coatings14010058
Chicago/Turabian StyleIljinas, Aleksandras, Vytautas Stankus, and Liutauras Marcinauskas. 2024. "Influence of the Annealing Environment on the Structure and Ferroelectric Properties of Lead Titanate Thin Films" Coatings 14, no. 1: 58. https://doi.org/10.3390/coatings14010058
APA StyleIljinas, A., Stankus, V., & Marcinauskas, L. (2024). Influence of the Annealing Environment on the Structure and Ferroelectric Properties of Lead Titanate Thin Films. Coatings, 14(1), 58. https://doi.org/10.3390/coatings14010058








