Abstract
Three pretreatment methods including calcination, carbonization, and a carbonization-calcination combined pretreatment were studied to understand the pretreatment mechanisms for cement-based recycled powder (CRP). The mineral and microstructure of the CRP sample were investigated through X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), thermal gravity (TG) analysis, and scanning electron microscopy (SEM) after exposure to different thermal temperatures (400 °C, 600 °C, and 800 °C), carbonization times (6 h, 1 d, and 3 d), and pre-carbonization for 1 d followed by heating at 800 °C. The results showed that the optimal thermal pretreatment temperature was approximately 720–800 °C. Through the process of calcination, the C-S-H, Ca(OH)2, and CaCO3 minerals in the CRP sample underwent decomposition to produce CaO or C2S. During carbonation, the pretreatment not only results in the increased production of CaCO3 owing to the reaction of the C-S-H gel and Ca(OH)2 with CO2, but also enhances its properties and the strength of chemical bond between CaCO3 and the post-hydration products. Both CaCO3 and CaO were present after the combined pretreatment, which indicates that the CaCO3 mineral formed superior stability after it had been pre-carbonated. Due to fewer impurities in CRP, the positive effect of the pretreatment on CRP was significantly better than that on recycled powder derived from construction and demolition waste.
1. Introduction
The carbon peak and carbon neutrality (dual-carbon) strategies necessitate the green and low-carbon development of the building materials industry and have become common sustainability goals worldwide [1,2,3]. With accelerated urbanization and industrialization, large amounts of construction and demolition waste (CDW) are generated [4,5,6,7], which results in significant adverse effects on the environment and prevents the achievement of the dual-carbon target [8,9,10]. Therefore, reusing CDWs to mitigate environment pollution is necessary [11,12,13].
Currently, recycling CDW to produce coarse or fine aggregates for concrete is a completely effective way to preserve natural resources and reduce the amount of land occupied [14,15,16]. In addition, the by-product of recycled powder (RP) accounts for more than 20%–30% of CDW by weight during reclamation. Thus, to increase the use of CDW, previous studies have investigated the RP [17,18,19,20]. Zhang [21] found that the RP can help promote pozzolanic activity, similar to that of fly ash, owning to its rich content of SiO2 and Al2O3. However, the RP still cannot be used as a supplementary cementitious material in concrete production and is frequently used as an inert filler in constructional engineering due to its relatively low reactivity level [18,22,23]. Xiao [24] used the RP as a partial replacement for OPC to produce recycled powder concrete (RPC) and found that RP has a negative effect on the mechanical properties of RPC. Thus, directly utilizing RP as a mineral admixture is not the most sustainable approach, and new pretreatment methods are urgently needed to improve the activity level and utilization rate of RP [25].
In recent decades, many researchers have tried to improve the properties of RP using different pretreatment methods [26,27,28], such as grinding it down (to a particle size below 75 μm) [29], a calcination thermal pretreatments [30], and a carbonization pretreatments [14]. Currently, calcination and carbonization pretreatments are the two most frequently employed modification methods [31,32,33]. Wu [34] found that after a calcination pretreatment, the C-S-H gel, Ca(OH)2, and CaCO3 minerals in RP were decomposed into the CaO mineral and C2S and C3S crystals that can take part in the post-hydration reaction in preparing recycled concrete, which improves the activity of RP. Additionally, a carbonation treatment can effectively improve the performance of RP due to the reactions of CO2 with Ca(OH)2 and the C-S-H gel of cement paste. Wang [32] reported that Ca(OH)2 and the C-S-H gel in RP were fully consumed and formed into a stronger mineral, CaCO3 and amorphous silica gel, during the carbonation process, which improves the bonding interface between RP and the hydration products. However, the RP derived from CDW is insensitive to this pretreatment because of the effect of stable mineral SiO2 in the RP [30]. This means that pretreatment mechanisms remain unclear, particularly due to the influence of impurities. In addition, the optimal thermal temperature and carbonation time have been insufficiently investigated, and there is limited research on the combined pre-carbonization at high temperatures, indicating the potential for future investigations.
Therefore, in this study, the CRP was obtained from cement paste to ensure the purity of the final product. Three pretreatment methods, namely a calcination pretreatment carried out at different temperatures (400 °C, 600 °C, and 800 °C), a carbonization pretreatment carried out for different durations (6 h, 1 d, and 3 d), and a carbonization–calcination pretreatment, were investigated. The mineral and microstructure of CRP were studied in their entirety via X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), thermal gravity (TG), and scanning electron microscope (SEM) analyses. This study provides a valuable understanding of the effect of different pretreatment methods on the properties of CRP.
2. Experimental Program
2.1. Recycled Powder Preparation
The cement-based recycled powder (CRP) was derived from cement pastes with a water–cement ratio of 0.55. The cement paste was prepared using 42.5R OPC, complying with the Chinese standard GB 175-2007 [35], and the 100 d compressive strength was 43.4 MPa. The production process of CRP is depicted in Figure 1. Firstly, the cement specimens were broken into coarse aggregates. Then, the coarse aggregates were further crushed into fine aggregates with a jaw crusher. Subsequently, the cement-based fine aggregates were ground into powders, and the powders were filtered with a vibrating sieve into a particle size below 0.075 mm as the CRP. Table 1 shows chemical compositions of the precursors determined via X-ray fluorescence (XRF).

Figure 1.
The production process of CRP.

Table 1.
The chemical composition of CRP (%).
2.2. Pretreatment Method
Table 2 lists detailed information about the different treatments applied to each sample. In this study, three pretreatment methods for CRP were conducted, namely a calcination thermal treatment, a carbonization pretreatment, and a carbonization–calcination combination pretreatment. The calcination and carbonization pretreatments of CRP were carried out in a muffle furnace (SX2-28-13) and carbonizing box (CCB-70A), respectively. The heating rate was 4 °C/min from room temperature to the target temperature (400 °C, 600 °C, and 800 °C), and the target temperature was kept constant for 300 min before cooling down naturally in the oven to room temperature, based on a previous study [31,32]. Additionally, the carbonation treatment involved keeping the CRP under (20 ± 3)% CO2, (70 ± 5)% RH, and (20 ± 2) °C for the duration (6 h, 1 d, and 3 d) according to the Chinese standard GB/T50082-2009 [36]. Furthermore, the carbonization–calcination combination treatment involved pre-carbonation for 1 d, and then calcination at 800 °C.

Table 2.
Detailed information about different treatments on each sample group.
2.3. X-ray Diffraction (XRD)
The CRP samples were dried in an electrothermal blast drying oven (101-1) at 40 °C for one day. The samples were measured using a D8 Advance X-ray diffractometer under 50 kV and 60 mA. The scanning angle range and the scanning time were 5–80° (2θ) and 18 min, respectively.
2.4. Thermal Gravity (TG)
Same as the XRD test, the CRP samples were subjected to a thermal gravity (TG) test after drying in the oven at 40 °C for one day. A thermal gravimetric analyzer (TGA 4000) was used for the TG test. In a N2 atmosphere, the sample was heated from room temperature to 900 °C at a heating rate of 10 °C/min to obtain the TG results.
2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
The dried samples were subjected to the Fourier-transform infrared spectroscopy (FTIR) test. The FTIR spectra (form 4000 to 400 cm−1) of the sample were collected using a spectrometer (Nicolet IS50). The FTIR test was performed using a 1 mg CRP and 150 mg KBr.
2.6. Scanning Electron Microscopy (SEM)
Similarly, the SEM samples were also dried at 40 °C for a day before the SEM test. Subsequently, the samples were sputter-coated with a layer of gold by using a sputter-coating instrument (Quorum SC7620), and microstructure pictures of the CRP were obtained using a scanning electron microscope (Tescan Mira Lms) at an accelerating voltage of 3 kV. The observations were carried out under high-level vacuum conditions.
3. Results and Discussions
3.1. X-ray Diffraction (XRD) Analysis
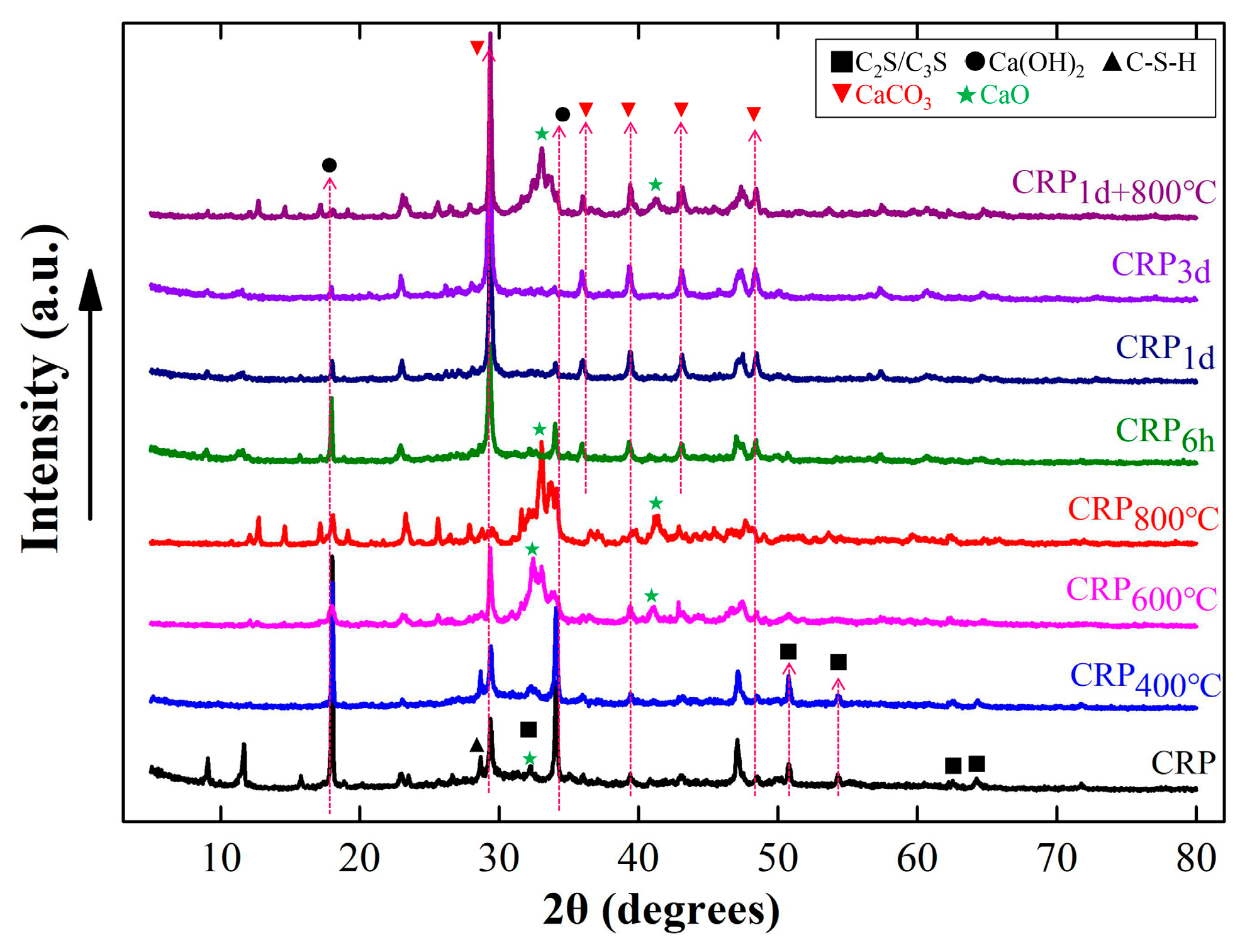
Figure 2 shows the XRD results of the CRP with or without the pretreatment. The XRD patterns reveal that the CRP primarily consisted of incompletely hydrated CaO, Ca(OH)2, and C2S/C3S, as well as hydration products such as C-S-H and CaCO3. Furthermore, the Ca(OH)2 peak (at approximately 18° (2θ) and 35° (2θ)) is evident in the CRP sample, and upon increasing the thermal treatment to 400 °C, the intensity of this peak in CRP400°C decreased compared to that of the CRP sample. However, the mineral compositions of CRP400°C did not exhibit significant differences from those of the CRP sample, suggesting that the calcination temperature of 400 °C has a minimal impact on the CRP. This result is consistent with a previous report [30]. By increasing the calcination temperature to 600 °C and 800 °C, the peak intensities of Ca(OH)2 and CaCO3 were noticeably reduced. Additionally, the C-S-H intensity peaks in both the CRP600°C and CRP800°C samples disappeared, while those of CRP and CRP400°C did not. Furthermore, new peak diffractions of CaO in CRP600°C and CRP800°C samples can be clearly observed at 32° and 41° (2θ). These finding indicated that the mineral composition of CRP began to decompose and form new minerals at a calcination temperature exceeding 600 °C [37,38]. On the other hand, in the same way as the calcination pretreatment proceeded, the peak intensities of Ca(OH)2 and C-S-H also decreased and disappeared as carbonization continued. Differently, the peak intensity of CaCO3 increased after carbonizing for 6 h, 1 d, and 3 d, and a new peak of CaCO3 occurred at 36° (2θ). This XRD result reveals that some combination reactions occurred during the CRP carbonization pretreatment. Compared with the CRP sample, the peaks of Ca(OH)2, C-S-H, and C2S/C3S in the CRP1d+800°C sample were lower, while the peak intensity of CaCO3 became higher, and a new peak of CaO appeared, which indicates that CRP1d+800°C both has the characteristics of carbonization for 1 d and calcination at 800 °C. Similar conclusions have been reported in a previous study [32]. Therefore, according to the above analysis, the calcination and combination pretreatment may decompose the minerals of the CRP samples, while the carbonization pretreatment may synthesize some new minerals from the CRP samples [21].
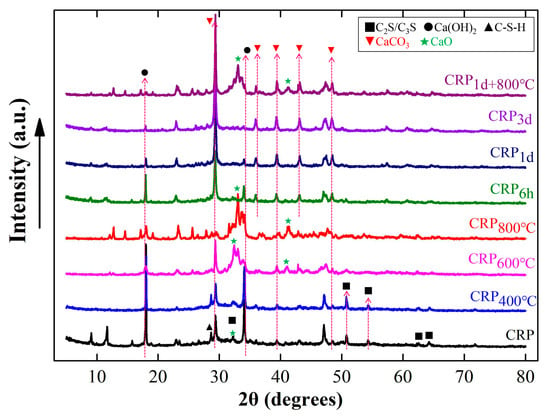
Figure 2.
X-ray diffraction (XRD) results of the un-pretreated and pretreated CRP samples.
3.2. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
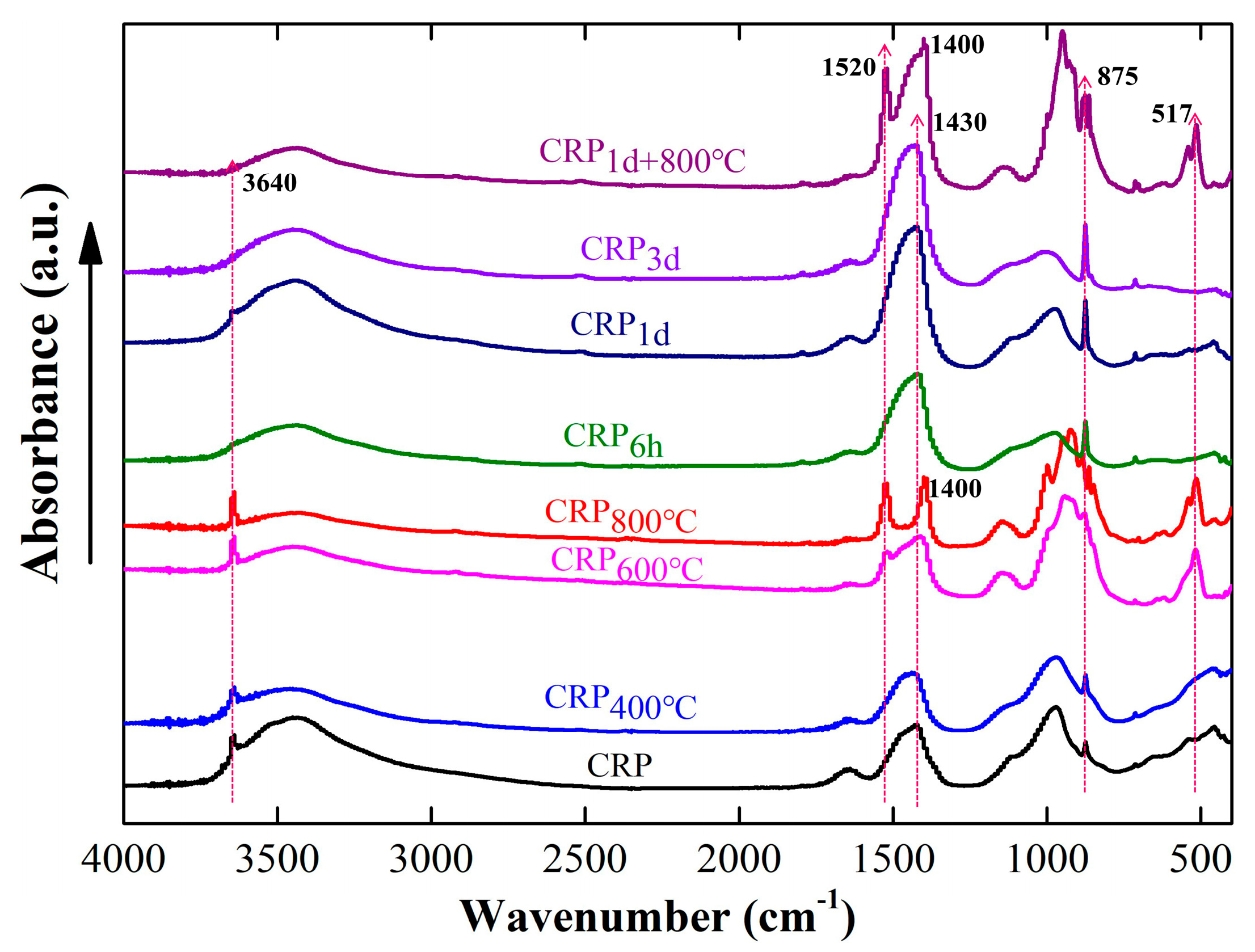
Figure 3 shows the Fourier-transform infrared spectroscopy (FTIR) results of all the samples with or without the pretreatment. Three absorption peaks of CRP at 3640 cm−1, 1430 cm−1, and 875 cm−1 were obvious, suggesting that some minerals such as Ca(OH)2 (3640 cm−1) [39,40] and CaCO3 (1430 cm−1 and 875 cm−1) [30,41] were present in the CRP sample, which is consistent with the XRD result. After undergoing calcination at 400 °C, the FTIR spectra of CRP400°C exhibited a resemblance to that of the CRP sample, suggesting that the impact of calcination at 400 °C on the CRP was negligible. As the calcination temperature increased to 600 °C and 800 °C, the absorption peak of CRP600°C and CRP800°C was broken into two peaks with slightly lower intensities compared to those in the FTIR spectra of CRP and CRP400°C samples within the range of 1430 cm−1–1600 cm−1. Furthermore, the absorption peaks of CRP600°C and CRP800°C at 875 cm−1 were non-existent in the spectra. This change indicates that the CaCO3 mineral might have been destroyed and began to decompose [30,32]. In addition, it can be seen that new spectra peaks of CRP600°C and CRP800°C sample appeared at 517 cm−1, suggesting that new minerals were formed after calcination at 600 °C and 800 °C. On the other hand, the spectra absorption peaks of CRP6h, CRP1d, and CRP3d cannot be observed at 3640 cm−1, suggesting that the Ca(OH)2 mineral has reacted with CO2. Furthermore, the absorption peaks at 1430 cm−1 and 875 cm−1 became sharper as carbonation proceeded, proving further evidence for the formation of higher amounts of CaCO3. Similar to the XRD results, the FTIR spectra of CRP1d+800°C has both the characteristics of the CRP1d and CRP800°C samples. The absorption peak at 3640 cm−1 in CRP1d+800°C also cannot be observed, and the higher absorption peak intensity at 875 cm−1 is more obvious than that of CRP. Additionally, compared to the CRP, the absorption peak at 1400 cm−1 shifted to a lower position, and a new peak at 1520 cm−1 presented in the CRP1d+800°C sample.
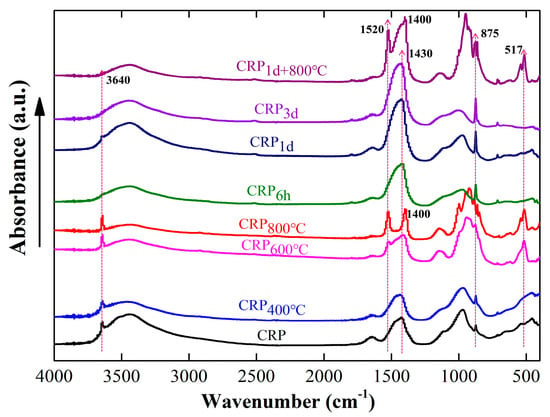
Figure 3.
FTIR results of the un-pretreated and pretreated CRP samples.
3.3. Scanning Electron Microscope (SEM) Analysis
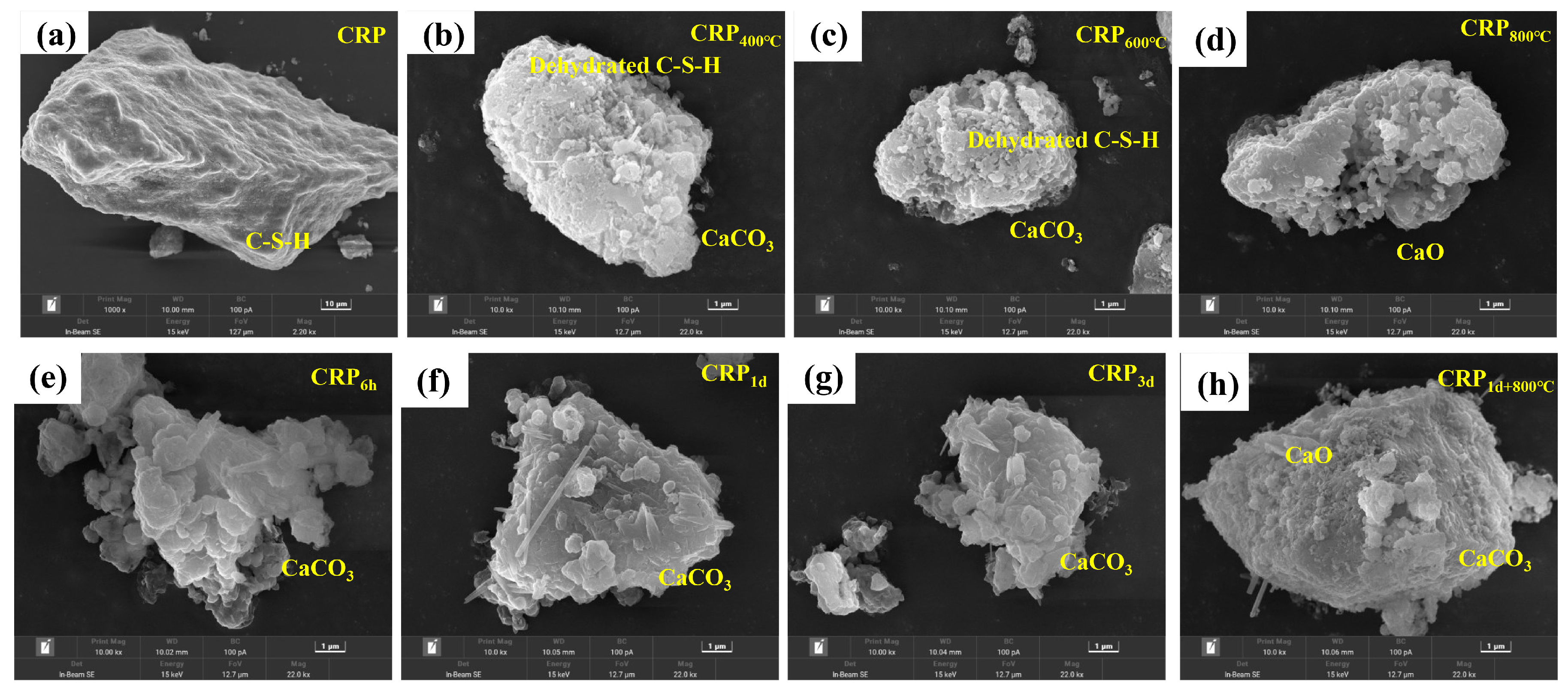
Figure 4 shows the SEM image of all the CRP samples. It can be obviously seen that the microstructure surface of the un-pretreatment CRP is smooth and is attached to the hydration gel (C-S-H), as shown in Figure 4a. With an increasing temperature, as shown in Figure 4b–d, the dehydration degree of C-S-H increased and the adhesion between the C-S-H gels deteriorated significantly, which resulted in more pore structure appearing in the CRP400°C, CRP600°C, and CRP800°C samples. When the calcination temperature reached 800 °C, CaO existed in the CRP800°C sample, suggesting that CaCO3 was broken down into CaO. On the other hand, Figure 4e–g display that more CaCO3 was found in the microstructure surfaces of CRP6h, CRP1d, and CRP3d, indicating that the C-S-H gel changed to CaCO3 after the carbonation pretreatment. In addition, compared to the CRP1d sample, the microstructure of CRP3d minimally changed, revealing that some minerals of CRP were completely reacted after the 1 day carbonation treatment. Figure 4h shows several minerals (CaCO3 and CaO) and the pore structure both observed on the morphology surface of CRP1d+800°C, suggesting that CRP1d+800°C had the structure of both CRP1d and CRP800°C. This finding is consistent with the XRD and FTIR results.
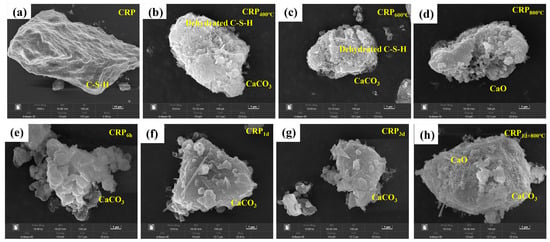
Figure 4.
Microstructure image of the un-pretreated and pretreated CRP samples. (a) CRP. (b) CRP400°C. (c) CRP600°C. (d) CRP800°C. (e) CRP6h. (f) CRP1d. (g) CRP3d. (h) CRP1d+800°C.
3.4. Thermal Gravity (TG) Analysis
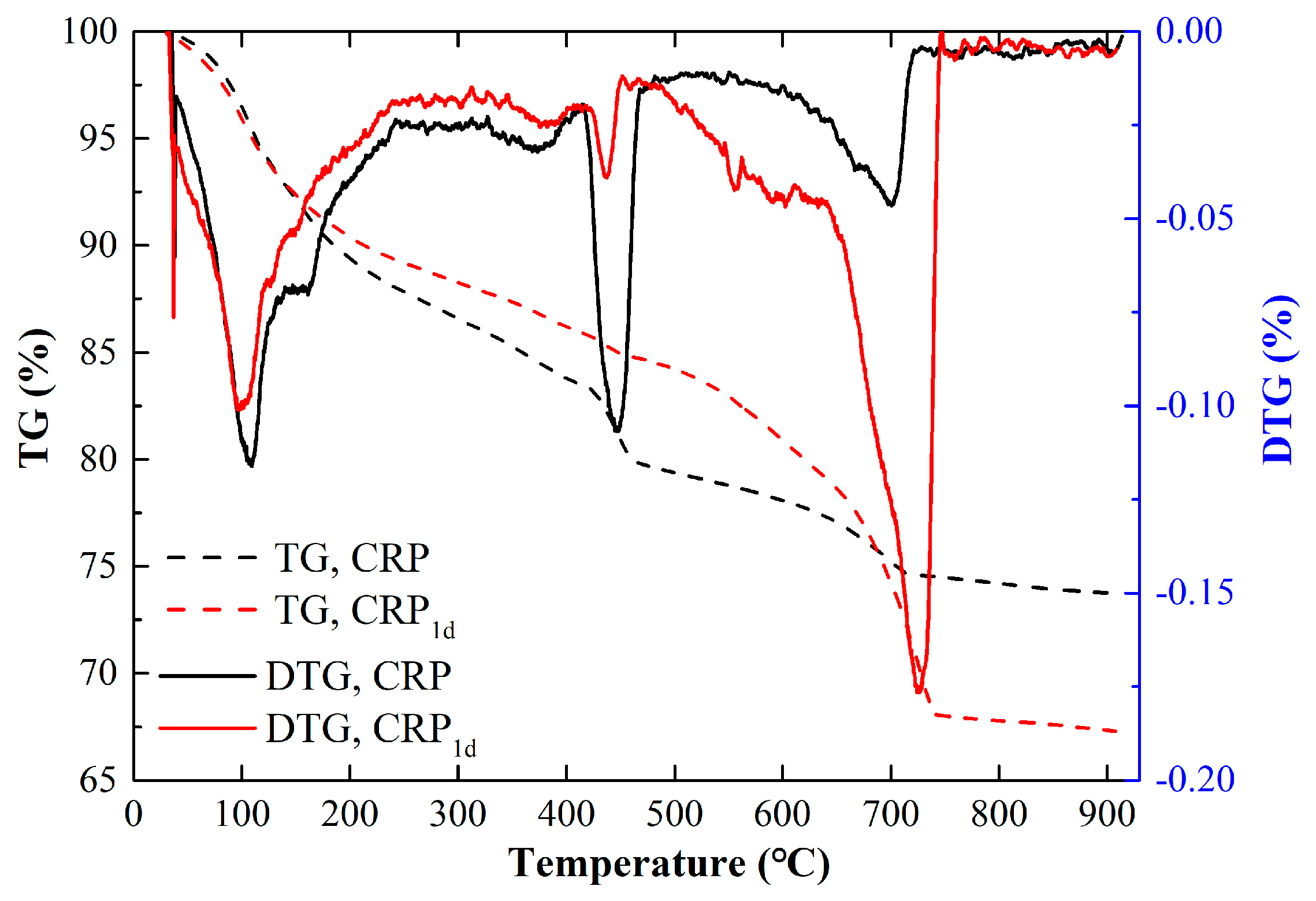
Based on the analyses in Section 3.1, Section 3.2 and Section 3.3, it can be seen that the thresholds for carbonization duration and calcination temperature were 1 day and 800 °C, respectively. However, the thermal gravity (TG) test result barely changed after the calcination pretreatment [32]. Thus, in this study, the changes in the TG and DTG curves of the CRP and CRP1d samples were studied, as depicted in Figure 5. It can be seen from Figure 5 that both samples exhibited three stages of weight loss with a continuously increasing temperature. The first stage happened at about 120 °C, which represents the loss of free water in the sample and the partial physical or chemical bonding of water in C-S-H [42]. The second stage occurred at around 450 °C, which represents the decomposition of Ca(OH)2 [43,44]. The third stage happened at about 720 °C, which reflects the decomposition of dehydrated C-S-H and CaCO3 [45]. In addition, Figure 5 also demonstrated that the peak value of the DTG curve in CRP1d at 450 °C was significantly lower than that of CRP due to the reaction between the C-S-H gel or Ca(OH)2 and CO2, forming CaCO3. Therefore, the CRP1d sample exhibited a more pronounced loss of weight at 720 °C due to of the excessive decomposition of CaCO3 compared to that of the CRP. The TG and DTG results are in agreement with the XRD, FTIR, and SEM data.
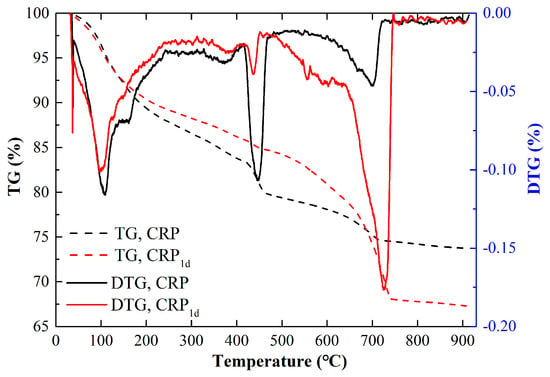
Figure 5.
TG and DTG curves of CRP and CRP1d.
3.5. Discussions
From the above results and analyses, different mineral products and microstructures in CRP were induced using different pretreatment methods. The calcination pretreatment can improve the activity level of CRP when the pretreatment temperature reaches the effective threshold, which is better for their application in recycled concrete. The primary minerals within the CRP, comprising C-S-H gel, Ca(OH)2, and CaCO3, were decomposed during calcination at different temperatures. The free and bound water in C-S-H decomposed and formed into dehydrated C-S-H (CS) after heating at 120 °C and beyond, as shown in Equation (1). As the temperature increased to 450 °C, the Ca(OH)2 mineral began to undergo a dihydroxylation reaction and formed the active mineral CaO (Equation (2)). Moreover, at temperatures exceeding 720 °C, it is demonstrated in Equations (3) and (4) that the minerals of CS and CaCO3 decomposed into β-C2S and CaO. These compounds can then participate in the process of cement hydration [46]. Previous studies have demonstrated similar results [30,31,32].
Equations (5)–(7) demonstrate that the carbonation pretreatment consumed some of the CaO, C-S-H gel, and Ca(OH)2 to form a significant amount of CaCO3 [47]. Although the resulting products of carbonation do not take part in hydration, and thereby, do not enhance the CRP activity level; the newly formed CaCO3 is a highly strong mineral that can improve the properties of CRP [33]. Additionally, during carbonation, the surface of CRP was covered by a layer of amorphous silica gel, which can significantly improve the interfacial properties between CaCO3 and C-S-H [48].
Both the CaCO3 and CaO minerals were present in CRP after the carbonation–calcination combined pretreatment. CaCO3 was formed in the pre-carbonation stage (Equations (5)–(7)), followed by the emergence of the newly formed CaO mineral in the post-calcination stage (Equations (1)–(4)). This result indicates that the pre-carbonated products exhibited greater stability when exposed to high temperatures, which improved the physical properties of CRP. Additionally, the CaO mineral in CRP helped to promote the hydration reactions, which makes the CRP more useful. Therefore, the combined pretreatment may result in both carbonization and calcination modification effects on the CRP, which is ultimately better than how the singular carbonization and calcination pretreatments perform. However, it is imperative that this conclusion is thoroughly examined and substantiated.
4. Effect of Source on Recycled Powder
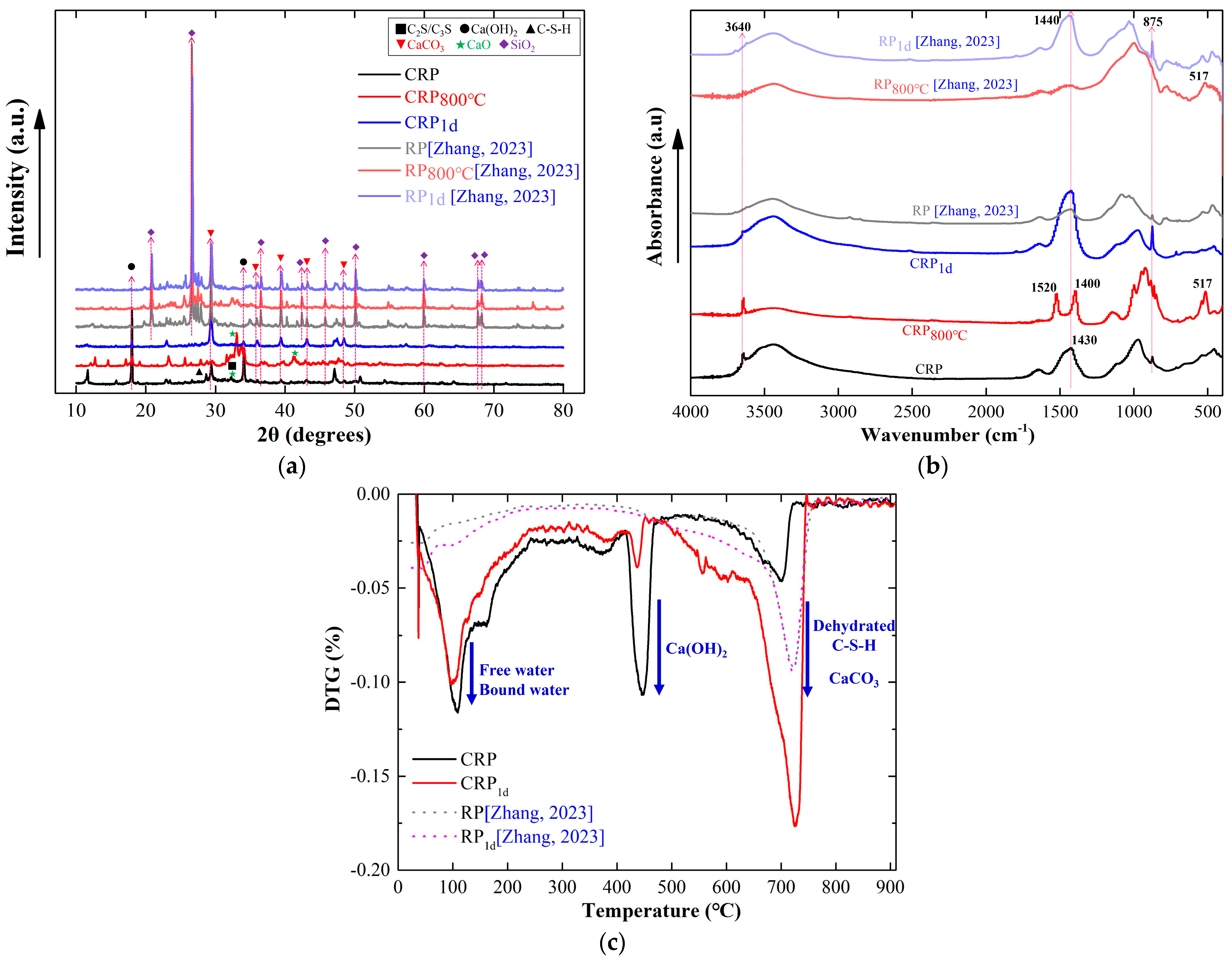
A number of studies have found that the by-product RP derived from CDW is mainly composed of SiO2 and CaO, which together account for 79.2% of its composition, as CDW mainly consists of concrete and bricks [18,30,37]. Owing to the stability of SiO2, the pretreatment may have a small positive effect on this RP. In this study, the CRP was derived from pure cement paste, which is more susceptible to the pretreatment. For a more comprehensive exploration of the influence of pretreatment on different RPs, two types of RP obtained from cement paste in this study and CDW in a previous study [30] were compared, as shown in Figure 6.
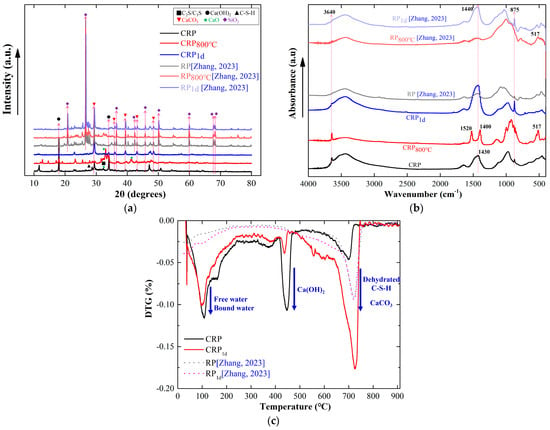
Figure 6.
The mineral comparison between CRP and RP [30]. (a) XRD pattern. (b) FTIR spectrum. (c) DTG curve.
Figure 6a displays the XRD results of the CRP and RP with or without the pretreatment. It can be clearly seen from the XRD spectrum that the SiO2 peaks are more evident in the RP sample than those in the CRP sample. In addition, the Ca(OH)2 peak is observable in the CRP, but cannot be found in the RP sample, which indicates that the hydration degree of CRP is weaker than that of RP. After undergoing the calcination pretreatment at 800 °C, the CaCO3 peak in RP800°C (28° and 48°) vanished, as indicated by the results of the CRP samples. However, the CRP exhibited noticeable peaks for CaO (32° and 41°), whereas RP did not display any such peak. These results suggest that the calcination pretreatment has a more favorable effect on the CRP compared with that on the RP. On the other hand, there was no considerable difference between the XRD patterns of the raw RP and the RPs subjected to carbonation, which contrasts the carbonization results of the CRP. This finding also demonstrates that the carbonization pretreatment has a smaller effect on the RP samples than that on the CRP. Figure 6b shows that the absorption peak at 3640 cm−1 was not apparent in the RP sample, suggesting the absence of Ca(OH)2. This finding is in agreement with the XRD results shown in Figure 6a, which demonstrates that the activity level of the RP was lower than that of the CRP. Similar to the CRP800°C, a new peak in RP800°C emerged at the 3640 cm−1 after undergoing the calcination treatment at 800 °C. However, in comparison to the RP, the peak in RP800°C at 1430 cm−1 remained relatively stable, which differs from that of CRP800°C. This result demonstrates that the mineral CaCO3 in the RP800°C sample slightly decomposed. Therefore, it can be found that the calcination pretreatment has the potential to improve the activity of the RP, but the impact was less significant than that of the CRP. Furthermore, although the absorbance peaks in RP1d at 1430 cm−1 and 875 cm−1 became stronger than those of the RP, it is still lower than that of CRP1d, due to there being fewer active minerals in the RP. Additionally, it can be observed from Figure 6c that there is only one stage of weight loss in the RP and RP1d samples, which occurred at approximately 720 °C due to CaCO3 decomposition. This result is obviously in contrast to the CRP and CRP1d data. At a temperature of 720 °C, the peak value of CRP was smaller than that of the RP and RP1d, suggesting that the carbonation reaction of RP in CDW fully occurred before the pretreatment. Furthermore, Figure 6c reveals that two DTG curves of RP and RP1d almost overlap with each other, further demonstrating that the impact of the carbonation pretreatment on the RP is weaker than that of the CRP because the minimal SiO2 impurity component in the CRP.
5. Conclusions
In this study, the effects of a calcination pretreatment, carbonation pretreatment, and carbonation-calcination combined pretreatment on the properties of the cement-based recycled powder (CRP) were investigated. Based on the results obtained in this study, some conclusions can be drawn as follows:
- (1)
- CRP activity can be significantly enhanced through the pretreatment of calcination and the combined carbonation-calcination pretreatments. Therefore, these two pretreatment methods can be employed to augment the utilization rate of CRP.
- (2)
- The mineral components of the CRP decomposed gradually at different target temperatures during calcination. The primary minerals of the C-S-H gel, Ca(OH)2, and CaCO3 in the CRP decomposed into CaO after the calcination pretreatment. Based on the mineral composition analysis and microstructure characterization, the optimal thermal activation temperature was around 720–800 °C, generating an extensive quantity of the efficient reactive substance CaO that encourages the hydration reaction, which increased the reactivity of CRP.
- (3)
- The carbonization pretreatment consumed C-S-H gel, CaO, and Ca(OH)2 and produced CaCO3 in the CRP samples. The production of CaCO3 cannot enhance the activity, but it can improve the properties of CRP owing to the superior potency of CaCO3. Furthermore, the interfacial properties between CaCO3 and the C-S-H gel in the rehydration reaction developed a stronger bond due to silica gel, indicating that the carbonized CRP has a much stronger interface than that of un-pretreatment CRP. In addition, it seems that the threshold for carbonation time was 1d.
- (4)
- Both CaCO3 and CaO were found in the combined pretreated CRP, which demonstrated that the pre-carbonated product of CaCO3 displayed superior stability under high temperatures. The combined pretreatment method may have potential advantages over both the carbonation and calcination pretreatments. Nonetheless, future investigations into the impact of combined treatment on the CRP are necessary, with the ultimate goal of increasing the use of CRP.
- (5)
- The impact of the pretreatment on the CRP (obtained from cement past) was markedly superior to that on the RP (derived from CDW) due to the reduced impurity SiO2 content.
Author Contributions
Conceptualization, J.X.; Investigation, J.L.; Methodology, J.L., Y.F., H.Z., B.Z. (Baifa Zhang), J.W., B.Z. (Bin Zhang) and J.X.; Project administration, J.X.; Writing—original draft, J.L.; Writing—review and editing, J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Planning Project of Guangdong Province (Grant No 2022A0505050077), the Guangdong Basic and Applied Basic Research Foundation (Grant Nos 2019B151502004, 2023A1515012180), and the National Natural Science Foundation of China (Grant No 12072078, 12372180).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, S.; Yang, D.; Shi, W.; Deng, C.; Chen, C.; Feng, S. Global evaluation of carbon neutrality and peak carbon dioxide emissions: Current challenges and future outlook. Environ. Sci. Pollut. Res. 2023, 30, 81725–81744. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, K.; Kang, J.; Chen, W.; Wang, X.; Zhang, X. Policy and Management of Carbon Peaking and Carbon Neutrality: A Literature Review. Engineering 2022, 14, 52–63. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, C.; Chen, X.; Jia, L.; Guo, X.; Chen, R.; Zhang, M.; Chen, Z.; Wang, H. Carbon peak and carbon neutrality in China: Goals, implementation path and prospects. China Geol. 2021, 4, 720–746. [Google Scholar] [CrossRef]
- Huang, L.; Tan, J.; Huang, J.; Lu, Z.; Xie, J. Axial-impact resistance of geopolymeric recycled aggregate concrete confined with glass FRP tubes. J. Compos. Constr. 2024, 28, 04023071. [Google Scholar] [CrossRef]
- Bao, Z. Developing circularity of construction waste for a sustainable built environment in emerging economies: New insights from China. Dev. Built Environ. 2023, 13, 100107. [Google Scholar] [CrossRef]
- Yu, K.; Zhu, W.; Ding, Y.; Lu, Z.; Yu, J.; Xiao, J. Micro-structural and mechanical properties of ultra-high performance engineered cementitious composites (UHP-ECC) incorporation of recycled fine powder (RFP). Cem. Concr. Res. 2019, 124, 105813. [Google Scholar] [CrossRef]
- Soto-Paz, J.; Arroyo, O.; Torres-Guevara, L.E.; Brayan, A. Parra-Orobio, Miguel Casallas-Ojeda. The circular economy in the construction and demolition waste management: A comparative analysis in emerging and developed countries. J. Build. Eng. 2023, 78, 107724. [Google Scholar] [CrossRef]
- Chen, H.; Du, Q.; Huo, T.; Liu, P.; Cai, W.; Liu, B. Spatiotemporal patterns and driving mechanism of carbon emissions in China’s urban residential building sector. Energy 2023, 263, 126102. [Google Scholar] [CrossRef]
- Helena, D.; John, B.; Katja, L.; Timo, J.; Pirke, S.; Tuomas, M.; Susanna, S.; Tuuli, M.; Kaarina, S. Construction and demolition waste management—A holistic evaluation of environmental performance. J. Clean. Prod. 2015, 107, 333–341. [Google Scholar] [CrossRef]
- Gu, F.; Xie, J.; Vuye, C.; Wu, Y.; Zhang, J. Synthesis of geopolymer using alkaline activation of building-related construction and demolition wastes. J. Clean. Prod. 2023, 420, 138335. [Google Scholar] [CrossRef]
- Xie, J.; Li, J.; Zhang, B.; Chen, W.; Zhong, H.; Yang, J.; Feng, Y. Effects of pretreated recycled fine aggregates on the mechanical properties and microstructure of alkali-activated mortar. Case Stud. Constr. Mater. 2023, 20, e02819. [Google Scholar] [CrossRef]
- Ilcan, H.; Sahin, O.; Kul, A.; Ozcelikci, E.; Sahmaran, M. Rheological property and extrudability performance assessment of construction and demolition waste-based geopolymer mortars with varied testing protocols. Cem. Concr. Compos. 2023, 136, 104891. [Google Scholar] [CrossRef]
- Corbu, O.; Toma, I.O. Progress in sustainability and durability of concrete and mortar composites. Coatings 2022, 12, 1024. [Google Scholar] [CrossRef]
- Zhang, L.; Sojobi, A.O.; Kodur VK, R.; Liew, K.M. Effective utilization and recycling of mixed recycled aggregates for a greener environment. J. Clean. Prod. 2019, 236, 117600. [Google Scholar] [CrossRef]
- Malazdrewicz, S.; Ostrowski, K.A.; Sadowski, Ł. Self-compacting concrete with recycled coarse aggregates from concrete construction and demolition waste–Current state-of-the art and perspectives. Constr. Build. Mater. 2023, 370, 130702. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Valencia-Saavedra, W.; Mejía de Gutiérrez, R. Construction and demolition waste (CDW) recycling—As both binder and aggregates—In alkali-activated materials: A novel re-use concept. Sustainability 2020, 12, 5775. [Google Scholar] [CrossRef]
- Tang, Q.; Ma, Z.; Wu, H.; Wang, W. The utilization of eco-friendly recycled powder from concrete and brick waste in new concrete: A critical review. Cem. Concr. Compos. 2020, 114, 103807. [Google Scholar] [CrossRef]
- Xiao, J.; Tang, Y.; Chen, H.; Zhang, H.; Xia, B. Effects of recycled aggregate combinations and recycled powder contents on fracture behavior of fully recycled aggregate concrete. J. Clean. Prod. 2022, 366, 132895. [Google Scholar] [CrossRef]
- Cantero, B.; Bravo, M.; de Brito, J.; Sáez del Bosque, I.F.; Medina, C. Thermal performance of concrete with recycled concrete powder as partial cement replacement and recycled CDW aggregate. Appl. Sci. 2020, 10, 4540. [Google Scholar] [CrossRef]
- Wu, H.; Zuo, J.; Zillante, G.; Wang, J.; Yuan, H. Status quo and future directions of construction and demolition waste research: A critical review. J. Clean. Prod. 2019, 240, 118163. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, Y.; Xie, J.; Chen, W.; Xue, Z.; Zhao, G.; Li, Y.; Li, J.; Yang, J. Compressive behaviour and microstructures of concrete incorporating pretreated recycled powder/aggregates: The coupling effects of calcination and carbonization. J. Build. Eng. 2023, 68, 106158. [Google Scholar] [CrossRef]
- Gao, Y.; Cui, X.; Lu, N.; Hou, S.; He, Z.; Liang, C. Effect of recycled powders on the mechanical properties and durability of fully recycled fiber-reinforced mortar. J. Build. Eng. 2022, 45, 103574. [Google Scholar] [CrossRef]
- Feng, Y.; Li, J.; Zhang, B.; Fu, H.; Chen, W.; Xue, Z.; Lu, Z.; Yang, J.; Xie, J. Concrete improvement incorporating recycled powder and aggregates treated via a combination of calcination and carbonation: The impact behaviors. J. Clean. Prod. 2023, 48, 138069. [Google Scholar] [CrossRef]
- Xiao, J.; Ma, Z.; Sui, T.; Akbarnezhad, A.; Duan, Z. Mechanical properties of concrete mixed with recycled powder produced from construction and demolition waste. J. Clean. Prod. 2018, 188, 720–731. [Google Scholar] [CrossRef]
- Xu, J.; Kang, A.; Wu, Z.; Gong, Y.; Xiao, P. The effect of mechanical-thermal synergistic activation on the mechanical properties and microstructure of recycled powder geopolymer. J. Clean. Prod. 2021, 327, 129477. [Google Scholar] [CrossRef]
- Kazmi, S.M.S.; Munir, M.J.; Wu, Y.-F.; Patnaikuni, I.; Zhou, Y.; Xing, F. Influence of different treatment methods on the mechanical behavior of recycled aggregate concrete: A comparative study. Cem. Concr. Compos. 2019, 104, 103398. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, S.; Huang, B.; Yang, Q.; Li, J. Comparison of mechanical, chemical, and thermal activation methods on the utilisation of recycled concrete powder from construction and demolition waste. J. Build. Eng. 2022, 61, 105295. [Google Scholar] [CrossRef]
- Ouyang, K.; Shi, C.; Chu, H.; Guo, H.; Song, B.; Ding, Y.; Guan, X.; Zhu, J.; Zhang, H.; Wang, Y.; et al. An overview on the efficiency of different pretreatment techniques for recycled concrete aggregate. J. Clean. Prod. 2020, 263, 121264. [Google Scholar] [CrossRef]
- Du, J.; Zhang, T.; Chen, P.; Guo, Y.; Zhan, B.; Wei, J.; Yu, Q. Phase separation of recycled concrete powder during grinding and consequent influences on its hydration behaviors in cement paste. Cem. Concr. Compos. 2023, 142, 105203. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, Y.; Xie, J.; Dai, J.; Chen, W.; Xue, Z.; Li, L.; Li, Y.; Li, J. Effects of pretreated recycled powder substitution on mechanical properties and microstructures of alkali-activated cement. Constr. Build. Mater. 2023, 406, 133360. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Li, K.; Lin, S.; Li, M.; Hao, T.; Ling, Z.; Xiang, D.; Wang, T. A systematic review of factors affecting properties of thermal-activated recycled cement. Resour. Conserv. Recycl. 2022, 185, 106432. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Li, M.; He, H.; Wang, Y.; Xiang, D.; Lin, S.; Zhong, Y.; Zhao, H. Effect of pre-carbonation on the properties of cement paste subjected to high temperatures. J. Build. Eng. 2022, 51, 104337. [Google Scholar] [CrossRef]
- Ouyang, X.; Wang, L.; Xu, S.; Ma, Y.; Ye, G. Surface characterization of carbonated recycled concrete fines and its effect on the rheology, hydration and strength development of cement paste. Cem. Concr. Compos. 2020, 114, 103809. [Google Scholar] [CrossRef]
- Wu, H.; Yang, D.; Ma, Z. Micro-structure, mechanical and transport properties of cementitious materials with high-volume waste concrete powder and thermal modification. Constr. Build. Mater. 2021, 313, 125477. [Google Scholar] [CrossRef]
- GB175-2007; Common Portland Cement. China Architecture and Building Press: Beijing, China, 2008.
- GBT50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Oridinary Concrete. Ministy of Housing and Urban-Rural Developmengt of the People’s Republic of China: Beijing, China, 2010.
- Rocha, J.H.A.; Toledo Filho, R.D. The utilization of recycled concrete powder as supplementary cementitious material in cement-based materials: A systematic literature review. J. Build. Eng. 2023, 76, 107319. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, R.; Yao, P.; Wang, C. Utilizing heat-mechanical synergistic treatment for separating concrete waste into high-quality recycled aggregate, active recycled powder and new concrete. J. Build. Eng. 2023, 68, 106161. [Google Scholar] [CrossRef]
- Khachani, M.; El Hamidi, A.; Halim, M.; Arsalane, S. Non-isothermal kinetic and thermodynamic studies of the dehydroxylation process of synthetic calcium hydroxide Ca(OH)2. J. Mater. Environ. Sci. 2014, 5, 615–624. [Google Scholar]
- Chen, Z.; Lee, Y.; Cho, H.; Lee, H.; Lim, S. Improvement in carbonation resistance of portland cement mortar incorporating γ-dicalcium silicate. Adv. Mater. Sci. Eng. 2019, 2019, 9856734. [Google Scholar] [CrossRef]
- Noor, M.S.M.; Afandi, N.N.A.M.; Noor, A.F.M.; Ismail, Y.M.B. Effect of Carbonate to Phosphate Molar Ratios on the physico-chemical properties of carbonated hydroxyapatite nanopowder. J. Eng. Sci. 2020, 16, 101–110. [Google Scholar] [CrossRef]
- Gallucci, E.; Zhang, X.; Scrivener, K.L. Effect of temperature on the microstructure of calcium silicate hydrate (CSH). Cem. Concr. Res. 2013, 53, 185–195. [Google Scholar] [CrossRef]
- Alonso, C.; Fernandez, L. Dehydration and rehydration processes of cement paste exposed to high temperature environments. J. Mater. Sci. 2004, 39, 3015–3024. [Google Scholar] [CrossRef]
- Vyšvařil, M.; Bayer, P.; Chromá, M.; Rovnaníková, P. Physico-mechanical and microstructural properties of rehydrated blended cement pastes. Constr. Build. Mater. 2014, 54, 413–420. [Google Scholar] [CrossRef]
- Hager, I. Behaviour of cement concrete at high temperature. Bull. Pol. Acad. Sci. Tech. Sci. 2013, 61, 145–154. [Google Scholar] [CrossRef]
- Pesce, C.; Ball, R.J.; Molinari, M.; Reeksting, S.; Pesce, G.L. Effects of carbohydrates and sulfonates during CaO hydration on portlandite microstructure. Cem. Concr. Res. 2024, 175, 107372. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Investigation of the carbonation mechanism of CH and C-S-H in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef]
- Hu, X.; He, P.; Shi, C. Carbonate binders: Historic developments and perspectives. Cem. Concr. Res. 2024, 175, 107352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).