Synthesis and Properties of a Photocurable Coating Based on Waste Cooking Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
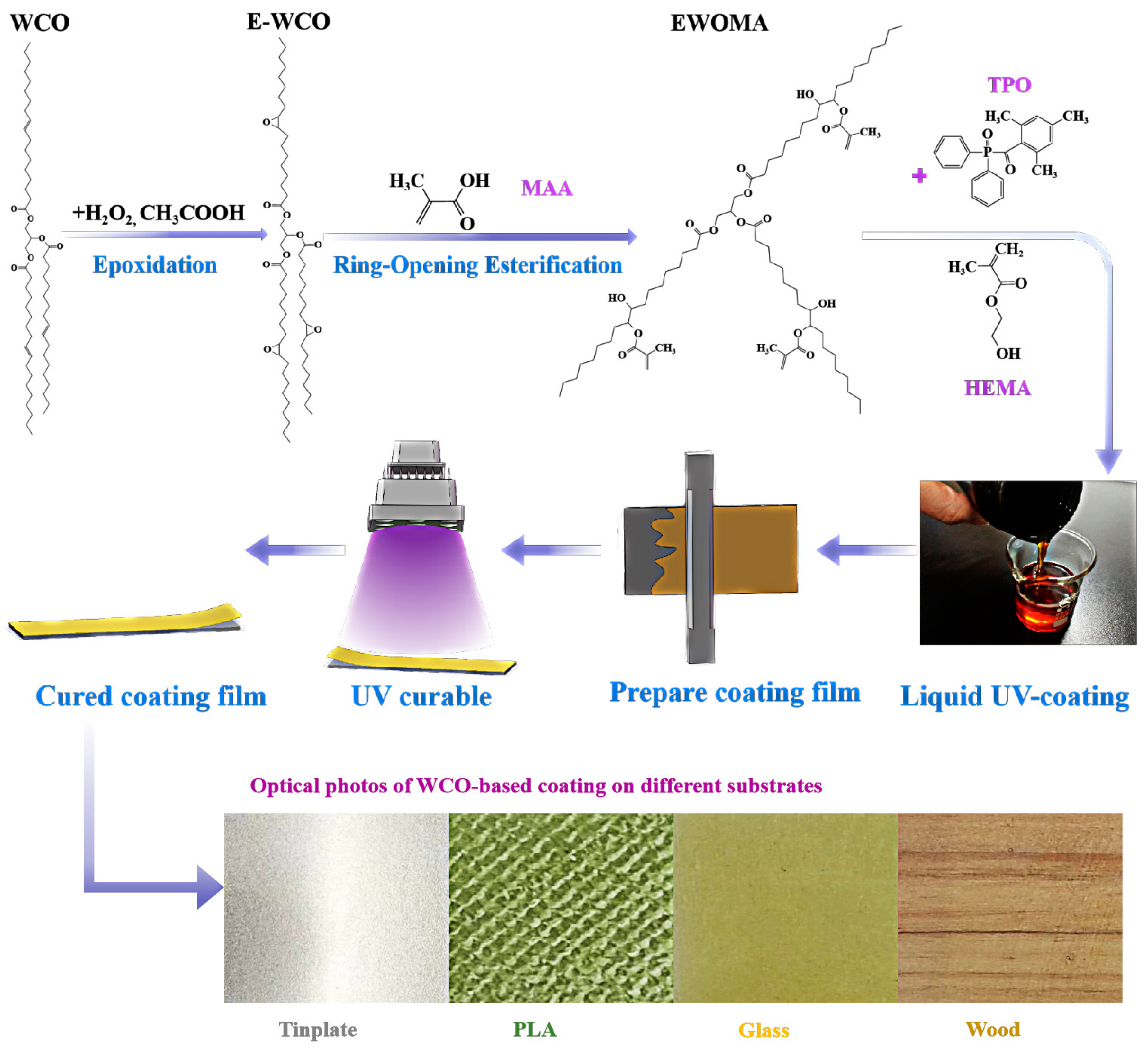
2.2. Synthesis of the WCO-Based Photocurable Coating
2.3. Application and Curing of the WCO-Based Photocurable Coating
2.4. Characterization
3. Results and Discussion
3.1. Photocurable Behavior
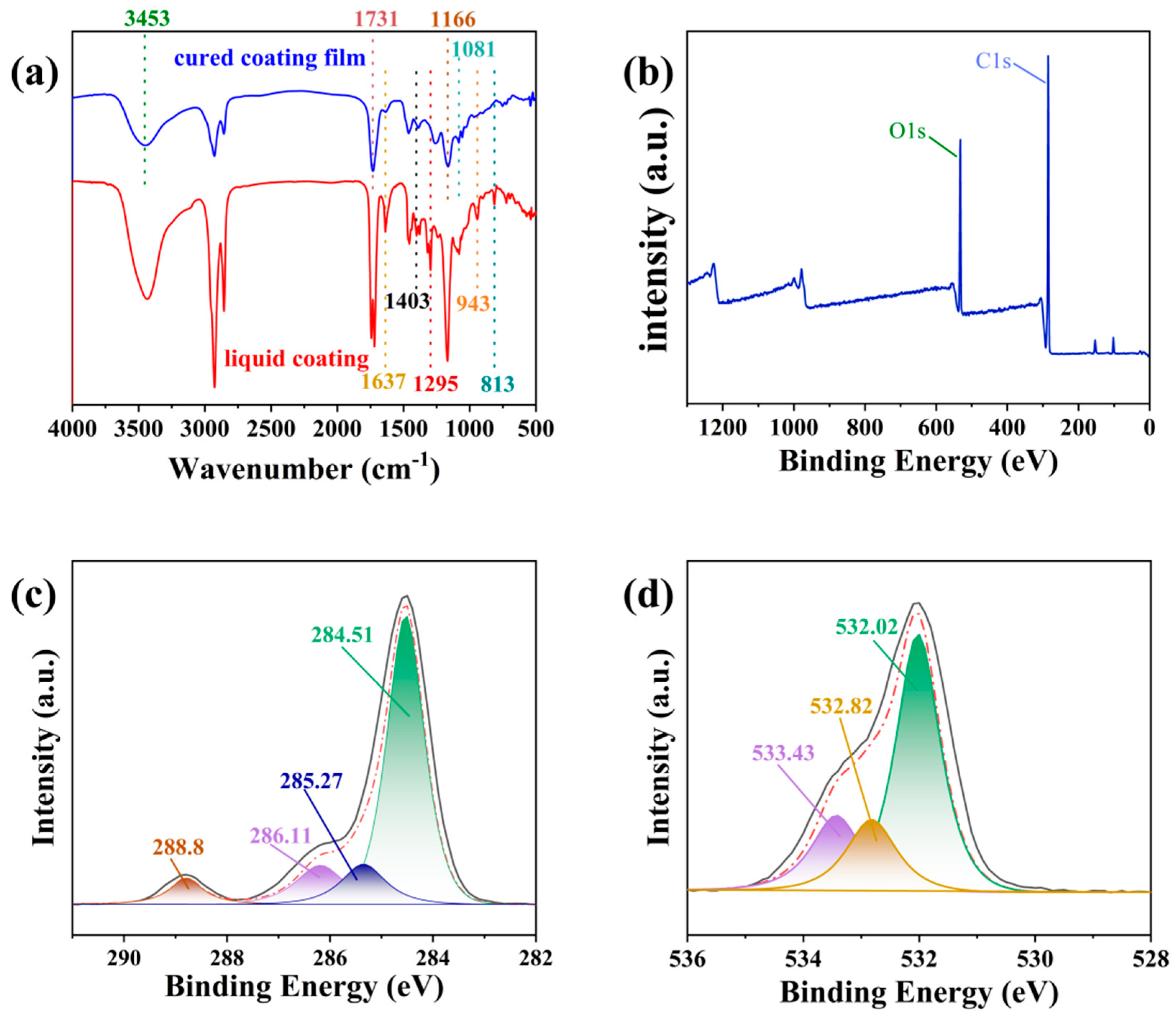
3.2. Structural Characterization
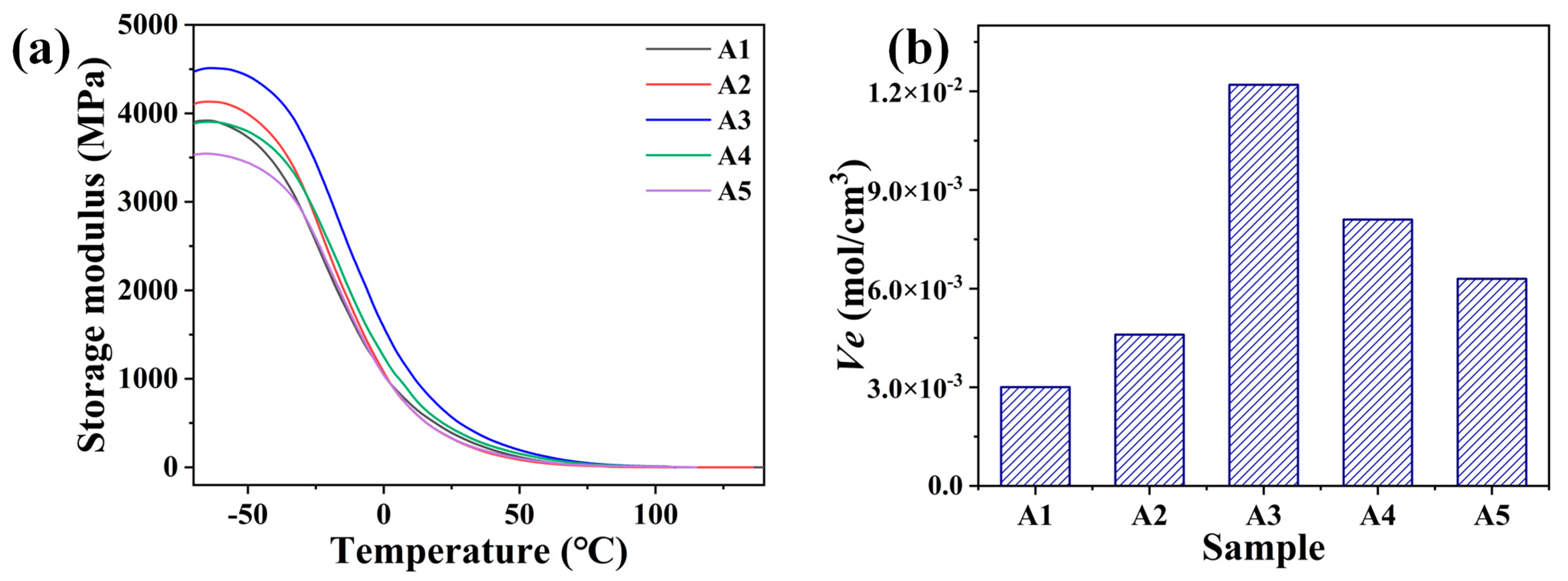
3.3. Thermal Analysis
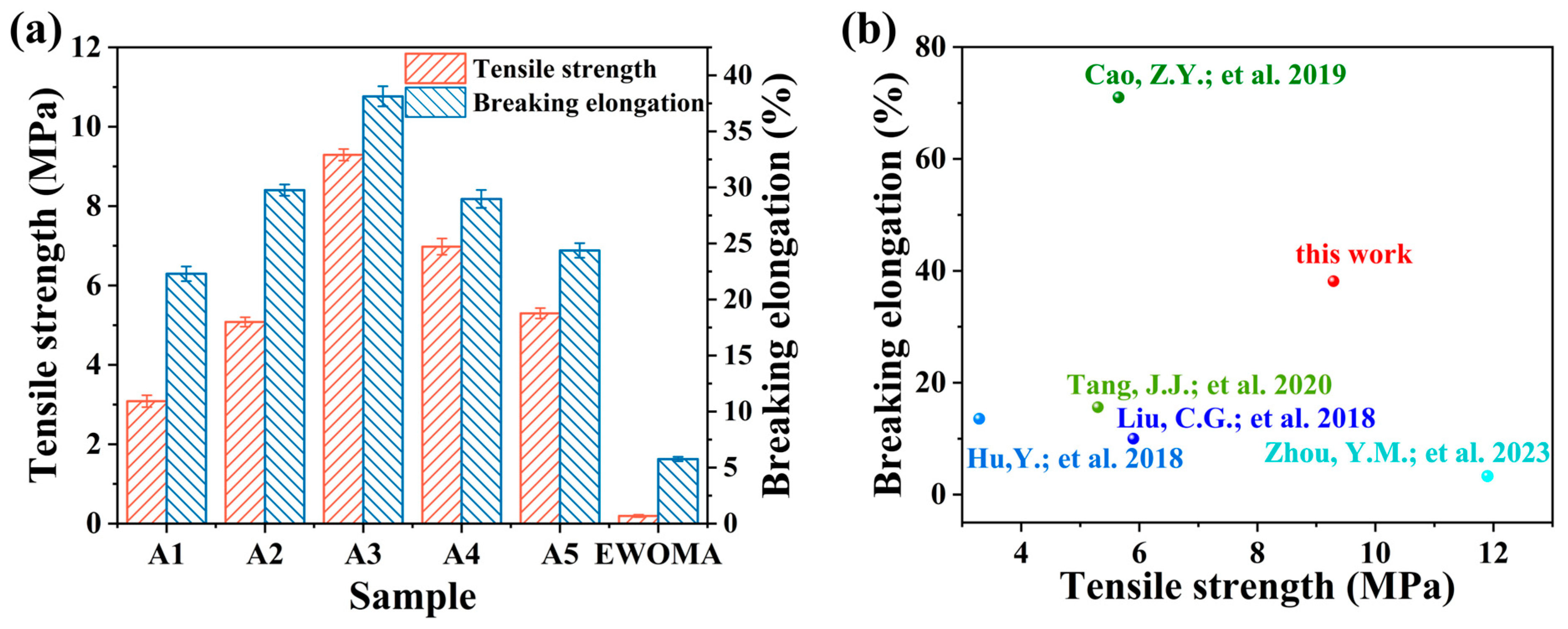
3.4. Mechanical Property
3.5. Coating Properties
3.6. Antismudge Property
3.7. Anticorrosive Property
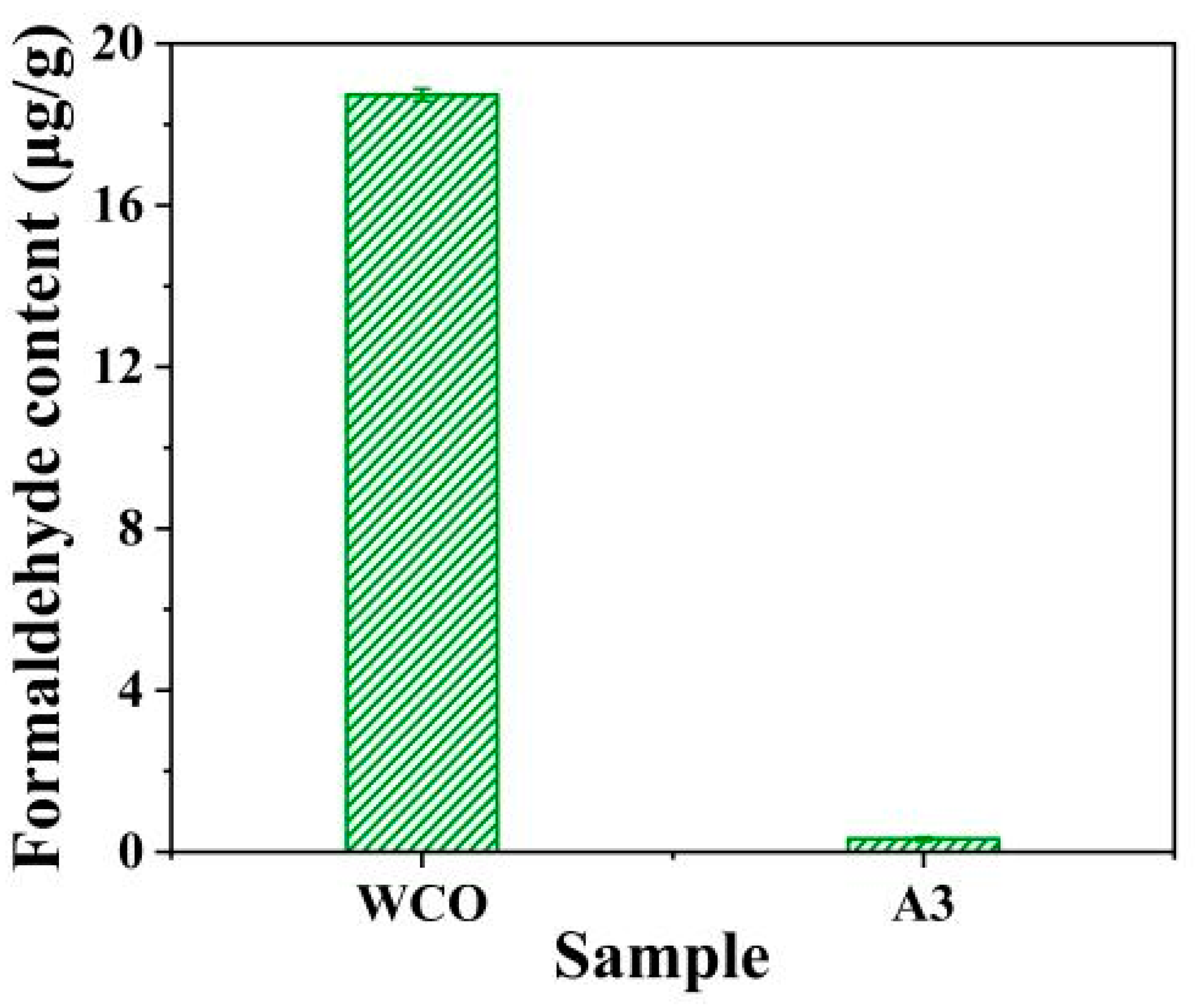
3.8. Aldehyde Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WCO | Waste cooking oil |
| EWOMA | Epoxy waste oil methacrylate |
| HEMA | 2-hydroxyethyl methacrylate |
| MAA | Methacrylic acid |
| TPO | 2,4,6-trimethylbenzoyldiphenyl phosphine oxide |
| PPh3 | Triphenylphosphine |
| HQ | Hydroquinone |
| Symbols | |
| A1–A5 | WCO-based coating samples with different dosages of HEMA |
References
- Landi, F.F.A.; Fabiani, C.; Castellani, B.; Cotana, F.; Pisello, A.L. Environmental assessment of four waste cooking oil valorization pathways. Waste Manag. 2022, 138, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, G.; Kanna, P.R.; Taler, D.; Sobota, T. Review of Waste Cooking Oil (WCO) as a Feedstock for Biofuel-Indian Perspective. Energies 2023, 16, 1739. [Google Scholar] [CrossRef]
- Bandbafha, H.H.; Li, C.; Chen, X.M.; Peng, W.X.; Aghbashlo, M.; Lam, S.S.; Tabatabaei, M. Managing the hazardous waste cooking oil by conversion into bioenergy through the application of waste-derived green catalysts: A review. J. Hazard. Mater. 2022, 424, 127636. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Dalai, A.K. Waste Cooking OilsAn Economical Source for Biodiesel: A Review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Foo, W.H.; Chia, W.Y.; Tang, D.Y.Y.; Koay, S.S.N.; Lim, S.S.; Chew, K.W. The conundrum of waste cooking oil: Transforming hazard into energy. J. Hazard. Mater. 2021, 47, 126129. [Google Scholar] [CrossRef]
- Andrew, N.A.; Nelson, I.E.; Obahiagbon, K. Optimum biodiesel production from waste vegetable oil using functionalized cow horn catalyst: A comparative evaluation of some expert systems. Clean. Eng. Technol. 2021, 4, 100184. [Google Scholar]
- Kamel, D.A.; Farag, H.A.; Amin, N.K.; Zatout, A.A.; Fouad, Y.O. Optimum biodiesel production from waste vegetable oil using functionalized cow horn catalyst: A comparative evaluation of some expert systems. Environ. Sci. Pollut. Res. 2019, 26, 32804–32814. [Google Scholar] [CrossRef]
- Wang, E.P.; Ma, X.; Tang, S.Z.; Yan, R.; Wang, Y.; Riley, W.W.; Reaney, M.J.T. Synthesis and oxidative stability of trimethylolpropane fatty acid triester as a biolubricant base oil from waste cooking oil. Sci. Direct 2014, 175, 371–378. [Google Scholar] [CrossRef]
- Cai, Z.Z.; Zhuang, X.C.; Yang, X.H.; Huang, F.R.; Wang, Y.; Li, Y. Litsea cubeba kernel oil as a promising new medium-chain saturated fatty acid feedstock for biolubricant base oil synthesis. Ind. Crops Prod. 2021, 167, 113564. [Google Scholar] [CrossRef]
- Hussein, R.Z.K.; Attia, N.K.; Fouad, M.K.; ElSheltawy, S.T. Experimental investigation and process simulation of biolubricant production from waste cooking oil. Biomass Bioenergy 2021, 144, 105850. [Google Scholar] [CrossRef]
- Chen, C.Y.; Li, D.S.; Suna, N.; Ma, X.F.; Xiao, G.Q.; Zhou, J. Oil recovery from drilling cuttings by biosurfactant from kitchen waste oil. Energy Sources 2021, 43, 314–325. [Google Scholar] [CrossRef]
- Kurt, I.; Acar, I.; Güclü, G. Preparation and characterization of water reducible alkyd resin/colloidal silica nanocomposite coatings. Prog. Org. Coat. 2014, 77, 949–956. [Google Scholar] [CrossRef]
- Ma’nczyk, K.; Szewczyk, P. Highly branched high solids alkyd resins. Prog. Org. Coat. 2002, 44, 99–109. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Odi, H.D.; Maliki, M.; Omorogbe, S.O.; Aigbodion, A.I.; Ikhuoria, E.U. Correlative studies on the properties of rubber seed and soybean oil-based alkyd resins and their blends. J. Coat. Technol. Res. 2021, 18, 459–467. [Google Scholar] [CrossRef]
- Milhem, S.A.; Verriele, M.; Nicolas, M.; Thevenet, F. Indoor use of essential oil-based cleaning products: Emission rate and indoor air quality impact assessment based on a realistic application methodology. Atmos. Environ. 2021, 246, 118060. [Google Scholar] [CrossRef]
- Patil, P.D.; Buradkar, A.M.; Mulla, M.Z.; Prabhu, P.A.; Nagla, J.R. Taguchi Optimization for Waste Cooking Oil Based Biodiesel Preparation. Int. J. Eng. Adv. Technol. 2020, 9, 1088–1097. [Google Scholar] [CrossRef]
- Xiao, M.S.; Lin, D.R.; Li, Z.H.; Zhao, J.J.; Long, X.M.; Wu, Z.J. Synthesis of Biodiesel from Waste Cooking Oil by One-step Esterification and Its Structural Characterization. Waste Biomass Valorization 2020, 11, 2087–2100. [Google Scholar] [CrossRef]
- Mohadesi, M.; Aghel, B.; Maleki, M.; Ansari, A. Study of the transesterification of waste cooking oil for the production of biodiesel in a microreactor pilot: The effect of acetone as the co-solvent. Fuel 2020, 273, 117736. [Google Scholar] [CrossRef]
- Das, S.; Das, B.; Misra, R.D. Emergy-based assessment of biodiesel production in India using edible and non-edible oil. Int. J. Environ. Sci. Technol. 2022, 19, 11117–11144. [Google Scholar] [CrossRef]
- Alkhafaje, Z.A.; Mohammed, A.K.; Rashid, I.M. Development of two-step noncatalytic esterification of waste cooking oil for biodiesel preparation. Mech. Catal. 2020, 131, 645–659. [Google Scholar] [CrossRef]
- Demirbas, A.; Bafail, A.; Ahmad, W.; Sheikh, M. Biodiesel production from non-edible plant oils. Energy Explor. Exploit. 2016, 34, 290–318. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.Y.; Fan, X.G.; Wang, L.; Liang, J.Y.; Jin, X.Y.; Che, R.J.; Ying, W.Y.; Chen, S.P. Four-Dimensional Printing of Multifunctional Photocurable Resin Based on Waste Cooking Oil. ACS Sustain. Chem. Eng. 2022, 10, 16344–16358. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.Y.; Qi, Y.X.; Jin, X.Y.; Xu, H.R.; Chen, Y.X.; Chen, S.P.; Su, H.P. Synthesis and properties of wax based on waste cooking oil. RSC Adv. 2022, 12, 3365–3371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, X.G.; Liu, M.Y.; Wang, L.; Wang, P.Y.; Xu, H.R.; Chen, Y.X.; Chen, S.P. Fatty acid wax from epoxidation and hydrolysis treatments of waste cooking oil: Synthesis and properties. RSC Adv. 2022, 12, 36018–36027. [Google Scholar] [CrossRef]
- Xiong, Y.; Miao, W.F.; Wang, N.N.; Chen, H.M.; Wang, X.R.; Wang, J.Y.; Tan, Q.L.; Chen, S.P. Solid alcohol based on waste cooking oil: Synthesis, properties, micromorphology and simultaneous synthesis of biodiesel. Waste Manag. 2019, 85, 295–303. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Sheng, Y.M.; Wang, M.H.; Lu, X. UV-curable self-healing, high hardness and transparent polyurethane acrylate coating based on dynamic bonds and modified nano-silica. Prog. Org. Coat. 2022, 172, 107051. [Google Scholar] [CrossRef]
- Jovičić, M.; Radičević, R.; Pavličević, J.; Bera, O.; Govedarica, D. Synthesis and characterization of ricinoleic acid based hyperbranched alkyds for coating application. Prog. Org. Coat. 2020, 148, 105832. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, J.S.; Zhu, G.Q.; Yu, X.X.; Hu, Y.; Shang, Q.Q.; Cheng, J.Q.; Hu, L.H.; Zhou, Y.H.; Liu, C.G. Self-healing, high-performance, and high-biobased-content UV-curable coatings derived from rubber seed oil and itaconic acid. Prog. Org. Coat. 2021, 159, 106391. [Google Scholar] [CrossRef]
- Ang, D.T.C.; Gan, S.N. Development of palm oil-based alkyds as UV curable coatings. Pigment Resin Technol. 2012, 41, 302–310. [Google Scholar]
- Mishra, V.; Mohanty, I.; Patel, M.R.; Patel, K.I. Development of Green Waterborne UV-Curable Castor Oil-Based Urethane Acrylate Coatings: Preparation and Property Analysis. Int. J. Polym. Anal. Charact. 2015, 20, 504–513. [Google Scholar] [CrossRef]
- Yu, X.X.; Hu, Y.; Lei, W.; Liu, C.G.; Zhou, Y.H. Development of Catalyst-Free Self-Healing Biobased UV-Curable Coatings via Maleate Monoester Transesterification. Coatings 2023, 13, 110. [Google Scholar] [CrossRef]
- Bashkirova, K.A.; Gazeev, M.V.; Sviridov, A.V. Features of planning an experiment to develop a new paint and varnish composition for the formation of protective and decorative coatings on wood products. IOP Conf. Ser. Earth Environ. Sci. 2022, 949, 012065. [Google Scholar] [CrossRef]
- Jeon, M.; Cho, B.R.; Han, M.; Lee, C.; Yoon, S.C. Synthesis of Photocurable Fluorenylidene Diacrylate for Optical Application. Mol. Cryst. Liq. Cryst. 2008, 492, 303–311. [Google Scholar] [CrossRef]
- Ortiz, R.A.; Padilla, E.E.G.; Valdez, A.E.G.; Flores, R.A.; Muñoz, J.F.E. Development of a photocurable glass-fiber reinforced epoxy-amine/thiol-ene composite. J. Polym. Res. 2016, 23, 30. [Google Scholar] [CrossRef]
- Sethayospongsa, R.; Chuayprakong, S.; Srisawadi, S.; Nuansing, W.; Chokevivat, W.; Methachan, B.; Srimongkol, S.; Suksanong, P. Multi-material additive manufacturing of MWCNT-based conductive photocurable resin and its antimicrobial property. J. Mater. Res. 2022, 38, 708–719. [Google Scholar] [CrossRef]
- Wu, J.; Qian, Y.; Sutton, A.C.; Scala, J.J.L.; Webter, D.C.; Sibi, M.P. Bio-Based Furanic Di(meth)acrylates as Reactive Diluents for UV Curable Coatings: Synthesis and Coating Evaluation. ACS Sustain. Chem. Eng. 2021, 9, 15537–15544. [Google Scholar] [CrossRef]
- Choi, W.C.; Lee, W.K.; Ha, C.S. Low-viscosity UV-curable polyurethane acrylates containing dendritic acrylates for coating metal sheets. J. Coat. Technol. Res. 2019, 16, 377–385. [Google Scholar] [CrossRef]
- Chan, D.; Maikawa, C.L.; Aquino, A.I.; Raghavan, S.S.; Troxell, M.L.; Appel, E.A. Polyacrylamide-based hydrogel coatings improve biocompatibility of implanted pump device. J. Biomed. Mater. Res. 2023, 111, 910–920. [Google Scholar] [CrossRef]
- Yeh, S.L.; Deval, P.; Tsai, W.B. Fabrication of Transparent PEGylated Antifouling Coatings via One-Step Pyrogallol Deposition. Polymers 2023, 15, 2731. [Google Scholar] [CrossRef]
- Liu, M.; Tong, S.; Tong, Z.; Guan, Y.; Sun, Y. A strong, biodegradable and transparent cellulose-based bioplastic stemmed from waste paper. J. Appl. Polym. Sci. 2023, 140, e53671. [Google Scholar] [CrossRef]
- Quan, Z.; Zhang, Q.; Li, H.; Sun, S.; Xu, Y. Fluorescent cellulose-based materials for information encryption and anti-counterfeiting. Coord. Chem. Rev. 2023, 493, 215287. [Google Scholar]
- Yang, X.; Zhang, Y.; Ye, M.; Tang, Y.; Wen, Z.; Liu, X.; Li, C.C. Renewable lignin and its macromolecule derivatives: An emerging platform toward sustainable electrochemical energy storage. Green Chem. 2023, 25, 4154–4179. [Google Scholar]
- Vasile, C.; Baican, M. Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials. Polymers 2023, 15, 3177. [Google Scholar]
- Wang, Q.; Thomas, J.; Soucek, M.D. Investigation of UV-curable alkyd coating properties. J. Coat. Technol. Res. 2023, 20, 545–557. [Google Scholar] [CrossRef]
- Lei, H.; Yao, N.; Wang, S.; Fang, X.; Wu, J.; Yang, G.; Hua, Z. Plant oil-based branched polymer coatings enhanced with nucleobases for achieving strong adhesion and outstanding environmental tolerance. Chem. Eng. J. 2023, 471, 144602. [Google Scholar] [CrossRef]
- Shafranska, O.; Dahlgren, J.; Jones, A.; Tardiff, J.; Webster, D.C. Differing unsaturation levels of soybean oils impact the properties of peroxide-vulcanized carbon black-filled ethylene-propylene-diene monomer rubber. J. Appl. Polym. Sci. 2023, 140, e53872. [Google Scholar] [CrossRef]
- Ma, Y.; Bei, Y.; Zhang, M.; Song, F.; Hu, F.; Kou, Z.; Hu, L.; Zhou, Y.; Jia, P. Synthesis of woody oil-based plasticizer via solvent-free Diels-Alder reaction and its biodegradability. Ind. Crops Prod. 2022, 188, 115646. [Google Scholar] [CrossRef]
- Oktay, B.; Türkcan, J.H.; Özdemir, O.K.; Apohan, N.K. Vegetable oil-based epoxy coating materials for self-healing and anticorrosive applications. Macromol. Res. 2023, 14. [Google Scholar] [CrossRef]
- Rosu, L.; Varganici, C.D.; Mustata, F.; Rosu, D.; Rosca, I.; Rusu, T. Epoxy Coatings Based on Modified Vegetable Oils for Wood Surface Protection against Fungal Degradation. ACS Appl. Mater. Interfaces 2020, 12, 14443–14458. [Google Scholar] [CrossRef]
- Nepomuceno, N.C.; Fook, M.V.L.; Rives, A.; Mija, A.; Wellen, R.M.R. Bio-Based Epoxy Resins of Epoxidized Soybean oil Cured with Salicylic acid Loaded with Chitosan: Evaluation of Physical-Chemical Properties. J. Polym. Environ. 2023, 31, 2566–2575. [Google Scholar] [CrossRef]
- Dinu, R.; Bejenari, I.; Volf, I.; Mija, A. Vegetable Oil-Based Resins Reinforced with Spruce Bark Powder and with Its Hydrochar Lignocellulosic Biomass. Appl. Sci. 2021, 11, 10649. [Google Scholar]
- Mauro, C.D.; Genua, A.; Rymarczyk, M.; Dobbels, C.; Malburet, S.; Graillot, A.; Mija, A. Chemical and mechanical reprocessed resins and bio-composites based on five epoxidized vegetable oils thermosets reinforced with flax fibers or PLA woven. Compos. Sci. Technol. 2021, 205, 108678. [Google Scholar]
- Thomas, J.; Nwosu, J.; Soucek, M.D. Sustainable biobased composites from norbornylized linseed oil and biomass sorghum fillers. Compos. Commun. 2023, 42, 101695. [Google Scholar] [CrossRef]
- Miao, S.D.; Zhu, W.N.; Castro, J.; Nowicki, M.; Zhou, X.; Cui, H.T.; Fisher, J.P.; Zhang, L.J.G. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci. Rep. 2016, 6, 27226. [Google Scholar]
- Zhang, P.; Zhang, J. One-step acrylation of soybean oil (SO) for the preparation of SO-based macromonomers. Green Chem. 2013, 15, 641–645. [Google Scholar] [CrossRef]
- Zhang, P.; Xin, J.; Zhang, J. Effects of Catalyst Type and Reaction Parameters on One-Step Acrylation of Soybean Oil. ACS Sustain. Chem. Eng. 2014, 2, 181–187. [Google Scholar]
- Zou, K.; Soucek, M.D. UV-Curable Cycloaliphatic Epoxide Based on Modified Linseed Oil: Synthesis, Characterization and Kinetics. Macromol. Chem. Phys. Homepage 2005, 206, 967–975. [Google Scholar]
- Kalita, D.; Tarnavchyk, I.; Webster, D.C.; Chisholm, B.J. Synthesis and evaluation of novel plant oil-based polymers as binders for artist paints: Controllable drying behavior and low yellowness. Prog. Org. Coat. 2022, 163, 106607. [Google Scholar]
- Kalita, D.J.; Tarnavchyk, I.; Sibi, M.; Moser, B.R.; Webster, D.C.; Chisholm, B.J. Biobased poly(vinyl ether)s derived from soybean oil, linseed oil, and camelina oil: Synthesis, characterization, and properties of crosslinked networks and surface coatings. Prog. Org. Coat. 2018, 125, 453–462. [Google Scholar]
- Thomas, J.; Patil, R. Enabling Green Manufacture of Polymer Products via Vegetable Oil Epoxides. Ind. Eng. Chem. Res. 2023, 62, 1725–1735. [Google Scholar] [CrossRef]
- Dinu, R.; Briand, N.; Mija, A. Influence of Keratin on Epoxidized Linseed Oil Curing and Thermoset Performances. ACS Sustain. Chem. Eng. 2021, 9, 15641–15652. [Google Scholar] [CrossRef]
- Mauro, C.D.; Malburet, S.; Graillot, A.; Mija, A. Recyclable, Repairable, and Reshapable (3R) Thermoset Materials with Shape Memory Properties from Bio-Based Epoxidized Vegetable Oils. ACS Appl. Bio Mater. 2020, 3, 8094–8104. [Google Scholar] [CrossRef]
- Mauro, C.D.; Malburet, S.; Genua, A.; Graillot, A.; Mija, A. Sustainable Series of New Epoxidized Vegetable Oil-Based Thermosets with Chemical Recycling Properties. Biomacromolecules 2020, 21, 3923–3935. [Google Scholar] [CrossRef]
- Thomas, J.; Nwosu, J.; Soucek, M.D. Acid-Cured Norbornylized Seed Oil Epoxides for Sustainable, Recyclable, and Reprocessable Thermosets and Composite Application. ACS Appl. Polym. Mater. 2023, 5, 2230–2242. [Google Scholar] [CrossRef]
- Chen, Z.; Chisholm, B.J.; Patani, R.; Wu, J.F.; Fernando, S.; Jogodzinski, K.; Webster, D.C. Soy-based UV-curable thiol-ene coatings. J. Coat. Technol. Res. 2010, 7, 603–613. [Google Scholar]
- Wu, Y.; Fei, M.; Chen, T.; Li, C.; Fu, T.; Qiu, R.; Liu, W. H-bonds and metal-ligand coordination-enabled manufacture of palm oil-based thermoplastic elastomers by photocuring 3D printing. Addit. Manuf. 2021, 47, 102268. [Google Scholar]
- Dai, J.Y.; Liu, X.Q.; Ma, S.Q.; Wang, J.G.; Shen, X.B.; You, S.S.; Zhu, J. Soybean oil-based UV-curable coatings strengthened by crosslink agent derived from itaconic acid together with 2-hydroxyethyl methacrylate phosphate. Prog. Org. Coat. 2016, 97, 210–215. [Google Scholar]
- Yang, X.F.; Cheng, F.; Fan, Y.X.; Song, Y.; He, N.; Lai, G.Q.; Gong, Z.S.; Shen, J.B. Highly transparent acrylate epoxidized soybean oil based UV-curable silicone-modified coatings with good thermal stability and flame retardancy. Prog. Org. Coat. 2022, 165, 106769. [Google Scholar]
- Bas¸türk, E.; Intan, T.; Güngör, A. Flame retardant UV-curable acrylated epoxidized soybean oil based organic-inorganic hybrid coating. Prog. Org. Coat. 2013, 76, 985–992. [Google Scholar]
- Liu, H.J.; Liu, W.C.; Liu, S.J. Development of acrylated soybean oil-based UV-curable coatings with high impact strength from low viscosity oligomer. J. Appl. Polym. Sci. 2018, 135, 45698. [Google Scholar] [CrossRef]
- Ge, X.Y.; Yu, L.; Liu, Z.S.; Liu, H.S.; Chen, Y.; Chen, L. Developing acrylated epoxidized soybean oil coating for improving moisture sensitivity and permeability of starch-based film. Int. J. Biol. Macromol. 2019, 125, 370–375. [Google Scholar] [CrossRef]
- Wu, B.; Sulfi, A.; Biswas, R.G.; Hisatsune, A.; Paquette, V.M.; Ning, P.; Soong, R.; Dicks, A.P.; Simpson, A.J. Direct Conversion of McDonald’s Waste Cooking Oil into a Biodegradable High-Resolution 3D-Printing Resin. ACS Sustain. Chem. Eng. 2020, 8, 1171–1177. [Google Scholar] [CrossRef]
- China Standard GB/T 1727-1992; General Preparation of Paint Film. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 1992.
- China Standard GB/T 5532-2008; Animal and Vegetable Fats and Oils—Determination of Iodine Value. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 2008.
- China Standard GB/T 1677-2008; Determinating the Epoxy Value of Plasticizers. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 2008.
- China Standard GB 5009.168-2016; National Standards for Food Safety—Determination of Fatty Acids in Food. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 2016.
- China Standard GB/T 14571.3-2008; Ethylene Glycol for Industrial Use—Determination of Content of Total Aldehydes Present—Spectrophotometric Method. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 2008.
- China Standard GB/T 1040.2-2006; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 2006.
- China Standard GB/T 6739-2006; Paints and Varnishes—Determination of Film Hardness by Pencil Test. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 2006.
- China Standard GB/T 9286-1998; Paints and Varnishes—Cross Cut Test for Films. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 1998.
- China Standard GB/T 1732-2020; Determination of Impact Resistance of Film. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 2020.
- China Standard GB/T 5209-1985; Paints and Varnishes—Determination of Resistance to Water—Water Immersion Method. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 1985.
- China Standard GB/T 1763-1979; Methods of Test for Chemical Resistance of Paint Films. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 1979.
- China Standard GB/T 9754-2007; Paints and Varnishes—Determination of Specular Gloss of Non-Metallic Paint Films at 20, 60 and 85. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 2007.
- China Standard GB/T 23983-2009; Test Method for Yellowing Resistance of Wood Coatings. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 2009.
- Ha, Z.M.; Lei, L.; Zhou, M.Y.; Xia, Y.Z.; Chen, X.N.; Mao, P.; Fan, B.F.; Shi, S.X. Bio-Based Waterborne Polyurethane Coatings with High Transparency, Antismudge and Anticorrosive Properties. ACS Appl. Mater. Interfaces 2023, 15, 7427–7441. [Google Scholar] [CrossRef]
- Zhou, M.Y.; Ha, Z.M.; Lei, L.; Xia, Y.Z.; Mao, P.; Chen, X.N.; Fan, B.F.; Shi, S.X. Castor oil-based transparent and omniphobic polyurethane coatings with high hardness, anti-smudge and anti-corrosive properties. Prog. Org. Coat. 2022, 172, 107120. [Google Scholar] [CrossRef]
- Rabnawaz, M.; Liu, G.J.; Hu, H. Fluorine-Free Anti-Smudge Polyurethane Coatings. Angew. Chem. Int. Ed. 2015, 54, 12722–12727. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.H.; Wei, H.; Wang, L.; Gao, Q.; Chen, Y.; Xiang, J.; Fan, H.J. Anti-smudge and self-cleaning characteristics of waterborne polyurethane coating and its construction. J. Colloid Interface Sci. 2022, 628, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, C.G.; Shang, Q.Q.; Zhou, Y.H. Synthesis and characterization of novel renewable castor oil-based UV-curable polyfunctional polyurethane acrylate. J. Coat. Technol. Res. 2018, 15, 77–85. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Feng, L.X.; Qu, J.Q. Preparation of high-performance epoxy soybean oil-based UV-curable oligomers and coatings. J. Coat. Technol. Res. 2023, 1–11. [Google Scholar] [CrossRef]
- Tang, J.J.; Zhang, J.S.; Lu, J.Y.; Huang, J.; Zhang, F.; Hu, Y.; Liu, C.G.; An, R.R.; Miao, H.C.; Chen, Y.Y.; et al. Preparation and Properties of Plant-Oil-Based Epoxy Acrylate-Like Resins for UV-Curable Coatings. Polymers 2020, 16, 2165. [Google Scholar] [CrossRef]
- Liu, C.G.; Wang, C.; Hua, Y.; Zhang, F.; Shang, Q.Q.; Lei, W.; Zhou, Y.H.; Cai, Z.C. Castor oil-based polyfunctional acrylate monomers: Synthesis and utilization in UV-curable materials. Prog. Org. Coat. 2018, 121, 236–246. [Google Scholar] [CrossRef]
- Cao, Z.Y.; Gao, F.; Zhao, J.Z.; Wei, X.; Cheng, Q.; Zhong, J.; Lin, C.; Shu, J.B.; Fu, C.Q.; Shen, L. Bio-Based Coating Materials Derived from Acetoacetylated Soybean Oil and Aromatic Dicarboxaldehydes. Polymers 2019, 11, 1089. [Google Scholar] [CrossRef]
- Noè, C.; Lannucci, L.; Malburet, S.; Graillot, A.; Sangermano, M.; Grassini, S. New UV-Curable Anticorrosion Coatings from Vegetable Oils. Macromol Mater. Eng. 2021, 306, 2100029. [Google Scholar] [CrossRef]
- Wu, Q.; Tang, J.J.; Zhang, J.; Wang, C.N.; Shang, Q.Q.; Feng, G.D.; Liu, C.G.; Zhou, Y.H.; Lei, W. High-Performance Soybean-Oil-Based Epoxy Acrylate Resins: “Green” Synthesis and Application in UV-Curable Coatings. ACS Sustain. Chem. Eng. 2018, 6, 8340–8349. [Google Scholar] [CrossRef]
- Thames, S.F.; Yu, H. Cationic UV-cured coatings of epoxide-containing vegetable oils. Surf. Coat. Technol. 1999, 115, 208–214. [Google Scholar] [CrossRef]
- Su, Y.; Ma, S.; Wang, B.; Xu, X.; Feng, H.; Hu, K.; Zhang, W.; Zhou, S.; Weng, G.; Zhu, J. High-performance castor oil-based polyurethane thermosets: Facile synthesis and properties. React. Funct. Polym. 2023, 183, 105496. [Google Scholar] [CrossRef]
- Li, J.; Shen, H.; Zhao, Z.; Cao, D.; Zeng, M.; Cai, H.; Wei, J.; Fan, Q.; Deng, J.; Ming, F.; et al. Protective effects of Clostridium butyricum against oxidative stress induced by food processing and lipid-derived aldehydes in Caco-2 cells. Appl. Microbiol. Biotechnol. 2020, 104, 9343–9361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Le, T.; Larsen, L.B.; Li, L.; Qin, D.; Su, G.; Li, B. Effect of glycation derived from α-dicarbonyl compounds on the in vitro digestibility of β-casein and β-lactoglobulin: A model study with glyoxal, methylglyoxal and butanedione. Food Res. Int. 2017, 102, 313–322. [Google Scholar] [CrossRef]
- Lynch, J.; Jin, L.; Richardson, A.; Conklin, D. Tobacco Smoke and Endothelial Dysfunction: Role of Aldehydes? Curr. Hypertens. Rep. 2020, 22, 73. [Google Scholar] [CrossRef]
- Suh, K.S.; Choi, E.M.; Rhee, S.Y.; Kim, Y.S. Methylglyoxal induces oxidative stress and mitochondrial dysfunction in osteoblastic MC3T3-E1 cells. Free Radic. Res. 2014, 48, 206–217. [Google Scholar] [CrossRef]
- Sena, C.M.; Matafome, P.; Crisóstomo, J.; Rodrigues, L.; Fernandes, R.; Pereira, P.; Seiça, R.M. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol. Res. 2012, 65, 497–506. [Google Scholar] [CrossRef]



| Sample | EWOMA Dosage (g) | HEMA Dosage (g) | Mass Ratio of EWOMA to HEMA | TPO (g) |
|---|---|---|---|---|
| A1 | 100 | 50 | 2:1 | 3 |
| A2 | 150 | 50 | 3:1 | 4 |
| A3 | 200 | 50 | 4:1 | 5 |
| A4 | 250 | 50 | 5:1 | 6 |
| A5 | 300 | 50 | 6:1 | 7 |
| EWOMA (control sample) | 100 | - | - | 2 |
| Sample | Viscosity (MPa·s) | Pencil Hardness | Adhesion (Grade) | Impact Strength (kg/cm) | Water Resistance (24 °C, 96 h) | Gloss °60 (GU) | Yellowing Index (ΔE, 96 h) |
|---|---|---|---|---|---|---|---|
| A1 | 207 | 2B | 4 | 33 | Small amount of tiny bubbles | 101.6 | 0.27 |
| A2 | 433 | B | 3 | 38 | Small amount of tiny bubbles | 103.7 | 0.29 |
| A3 | 681 | 2H | 2 | 62 | No significant change | 110.7 | 0.24 |
| A4 | 1039 | B | 3 | 23 | No significant change | 104.4 | 0.43 |
| A5 | 1628 | B | 4 | 18 | No significant change | 102.3 | 0.46 |
| EWOMA | 4321 | 6B | 4 | 1 | Small amount of tiny bubbles | 94 | 4.36 |
| Serial Number | Major Compositions | Pencil Hardness | Adhesion (Grade) | References |
|---|---|---|---|---|
| 1 | Epoxidized soybean oil (ESO) Hydroxyethyl methacrylated maleate (HEMAMA) | HB | 3 | [96] |
| 2 | Acrylated epoxidized soybean oil (AESO) Hydroxyethyl methacrylate (HEMA) | 2H | 2 | [92] |
| 3 | Epoxidized rose hip seed oil (ERHO) | B | 2 | [95] |
| 4 | Epoxidized grape-seed oil (EGRP) | B | 3 | |
| 5 | Dimethylolpropionic acid (DMPA) Methacrylic anhydride (MAAH) 4-Dimethylaminopyridine (DMAP) Trimethylol-propane triacrylate (TMPTA) | H | 2 | [91] |
| 6 | Epoxidized soybean oil (AESO) Castor oil (CO) Pentaerythritol (PER) Acryloyl chloride (AC) | 4B | 2 | [93] |
| 7 | Epoxidized soybean oil (ESO) 3,4-epoxycyclohexylmethyl-3,4-epoxycyclohexanecarboxylate (UVR 6110) 4-Thiophenyl phenyl diphenyl sulfonium hexafluoroantimonate (UVI 6974) Trifunctioncal primary e-caprolactone triol (Tone Polyol 0305) | H | 0 | [97] |
| 8 | thioglycerol modified castor oil (TCO) Isophorone diisocyanate (IPDI) | 4H | 2 | [98] |
| 9 | Epoxy waste oil methacrylate (EWOMA) Hydroxyethyl methacrylate (HEMA) | 2H | 2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Liu, Y.; Wang, P.; Ying, W.; Liu, Q.; Ding, G.; Chen, S. Synthesis and Properties of a Photocurable Coating Based on Waste Cooking Oil. Coatings 2023, 13, 1553. https://doi.org/10.3390/coatings13091553
Liu M, Liu Y, Wang P, Ying W, Liu Q, Ding G, Chen S. Synthesis and Properties of a Photocurable Coating Based on Waste Cooking Oil. Coatings. 2023; 13(9):1553. https://doi.org/10.3390/coatings13091553
Chicago/Turabian StyleLiu, Mengyu, Yan Liu, Pengyu Wang, Wanying Ying, Qing Liu, Guanzhi Ding, and Shuoping Chen. 2023. "Synthesis and Properties of a Photocurable Coating Based on Waste Cooking Oil" Coatings 13, no. 9: 1553. https://doi.org/10.3390/coatings13091553
APA StyleLiu, M., Liu, Y., Wang, P., Ying, W., Liu, Q., Ding, G., & Chen, S. (2023). Synthesis and Properties of a Photocurable Coating Based on Waste Cooking Oil. Coatings, 13(9), 1553. https://doi.org/10.3390/coatings13091553






