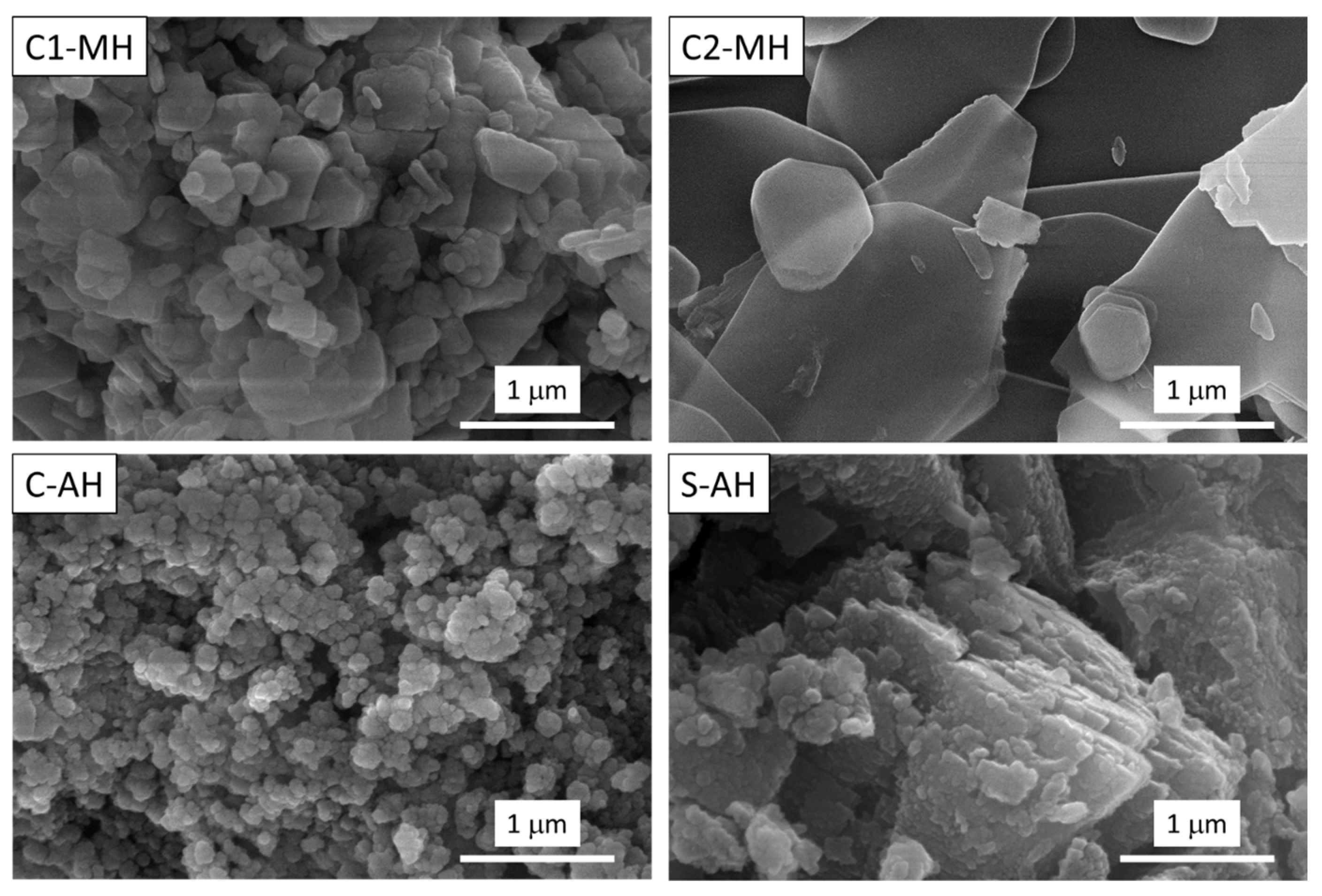
3.1. Fire Retardant Performances
Preliminarily, in order to evaluate how the reaction to fire of the coated steel samples evolves during the flame impingement test,
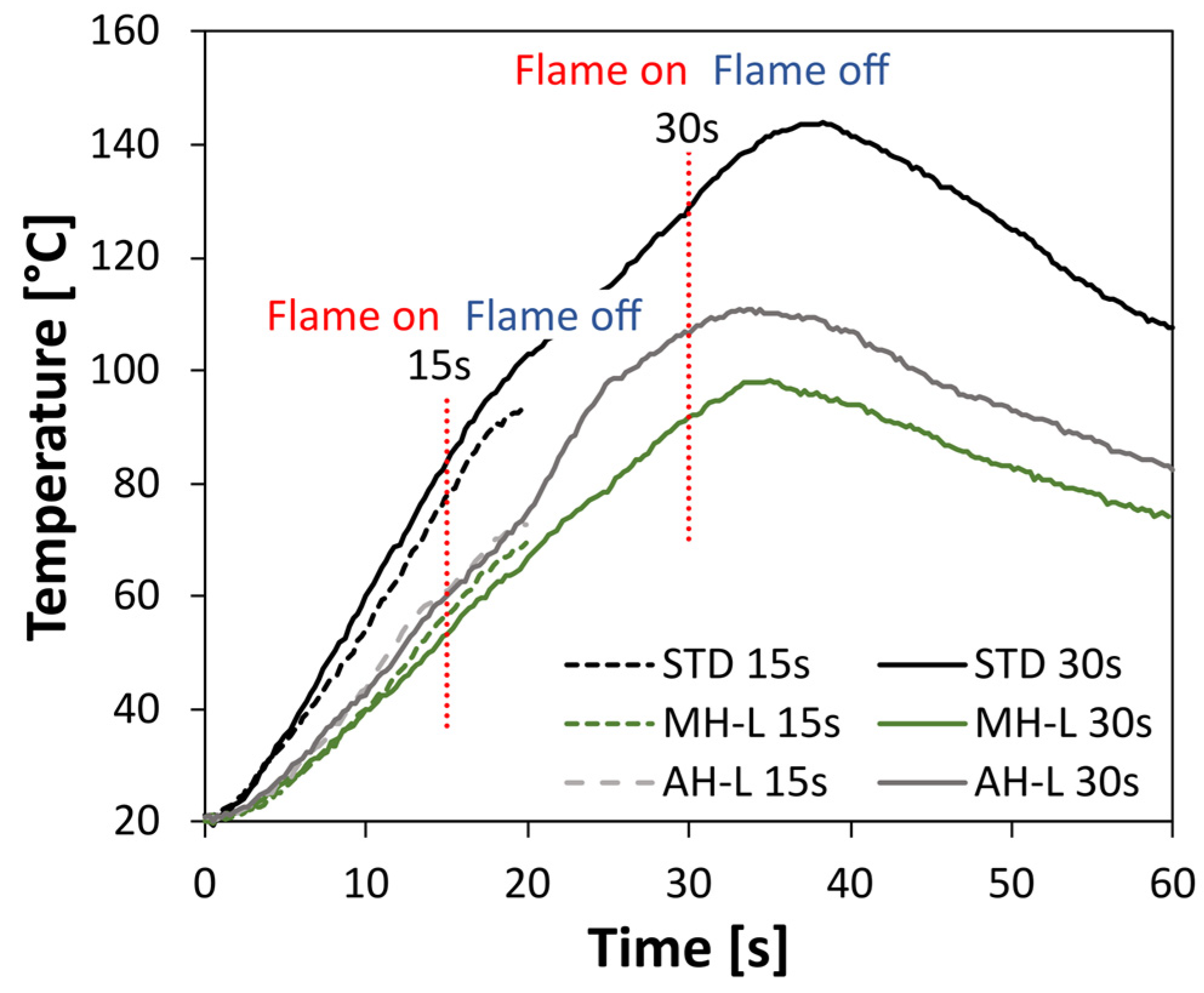
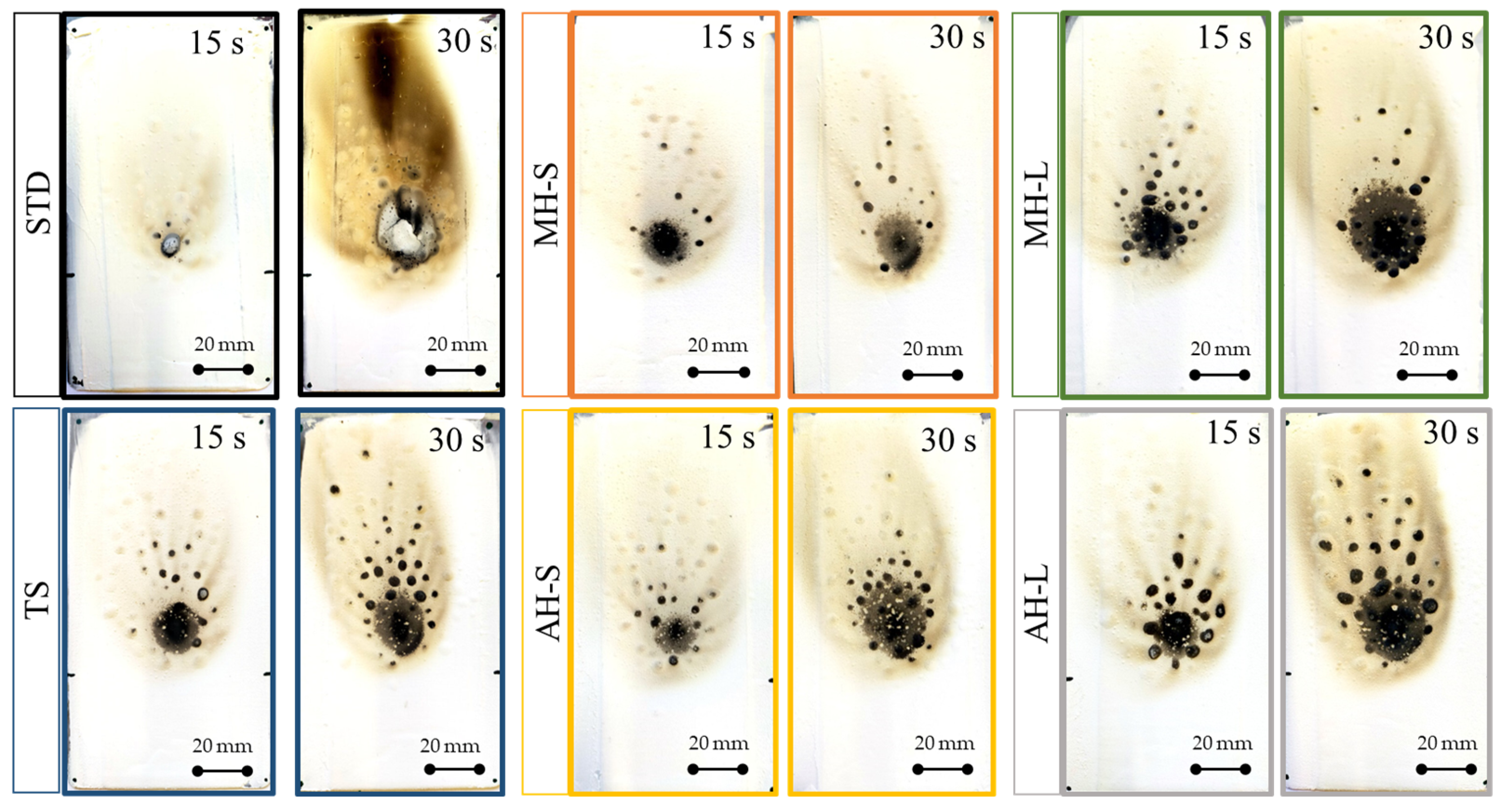
Figure 3 shows the evolution of the backside temperature of three types of coatings, a standard coating (STD), which is not flame retardant, and two heat insulating coatings. For this comparison, the magnesium hydroxide (MH-L) and aluminum hydroxide (AH-L) based coatings were considered. Results related to a fire exposure of 15 s and 30 s are, furthermore, compared.
The STD samples exhibited a relevant sensitivity to the direct flame impingement. At 30 s, the STD coated surface is largely degraded, and the backside temperature (measured by the thermocouple probe) shows a relevant and progressive increase in temperature. In particular, after 15 s and 30 s of flame exposition, the temperature is 83.8 °C and 129.5 °C, respectively.
Further information can be obtained by considering the trend of the curves relating to 15 s and 30 s. The trend is very similar during the flame on phase indicating that the specimens underwent a similar heating condition. A discordant behavior is found at the end of the heating phase (beginning of the flame off phase). After the flame is put out within 15 s, the temperature continues to escalate due to thermal inertia, but doesn’t attain a stable state by the end of the 20 s measurement. This indicates that flame retardants might require longer periods of exposure to provide comprehensive protection. The findings imply that brief exposure durations might not suffice to ensure complete defense against fire, and it might be essential to utilize stronger flame-retardant substances for the best possible fire safety. The STD 30 s sample has a maximum temperature equal to 143.7 °C at 37 s. Subsequently, the specimen is cooled with a progressive reduction in the temperature. However, after 60 s (30 s after the flame goes out), the backside temperature of the sample is still 107.5 °C. This settles that the fire barrier capability guaranteed by the STD coating is quite limited. The coating does not supply a protection against the thermal flux imposed during the direct flame impingement. Consequently, the choice of adding thermally active particles represents a potentially effective approach to increase the flame stability and protection of the composite coating under direct exposure to fire service conditions.
The MH-L sample showed a more effective reaction to fire and flame stability than the STD one. Evaluating the evolution of the temperature over time, it can be seen that the coating treated with FR filler has a better heat barrier action, which can be seen from the lower temperature reached throughout the test time. In particular, after 15 s and 30 s of direct flame exposure, the temperature on the rear side of the sample is 54.1 °C and 91.8 °C, respectively. These values are approximately 30–40 °C lower than the STD sample. These findings suggest that the heat transfer, facilitated by the heat dispersion caused by magnesium hydroxide, is significantly restricted, leading to positive impacts on the coating’s resistance to flames. The AH-L batch exhibits an intermediate trend of the temperature evolution compared to MH-L and STD samples, suggesting a higher tendency for heat diffusion from the flame source towards the back side of the sample, compared to the MH-L flame retardant coating. However, a more accurate evaluation of the comparison between the various batches and of the extent of the flame-induced damage is believed to be productive to effectively evaluate the performance of the compared coatings.
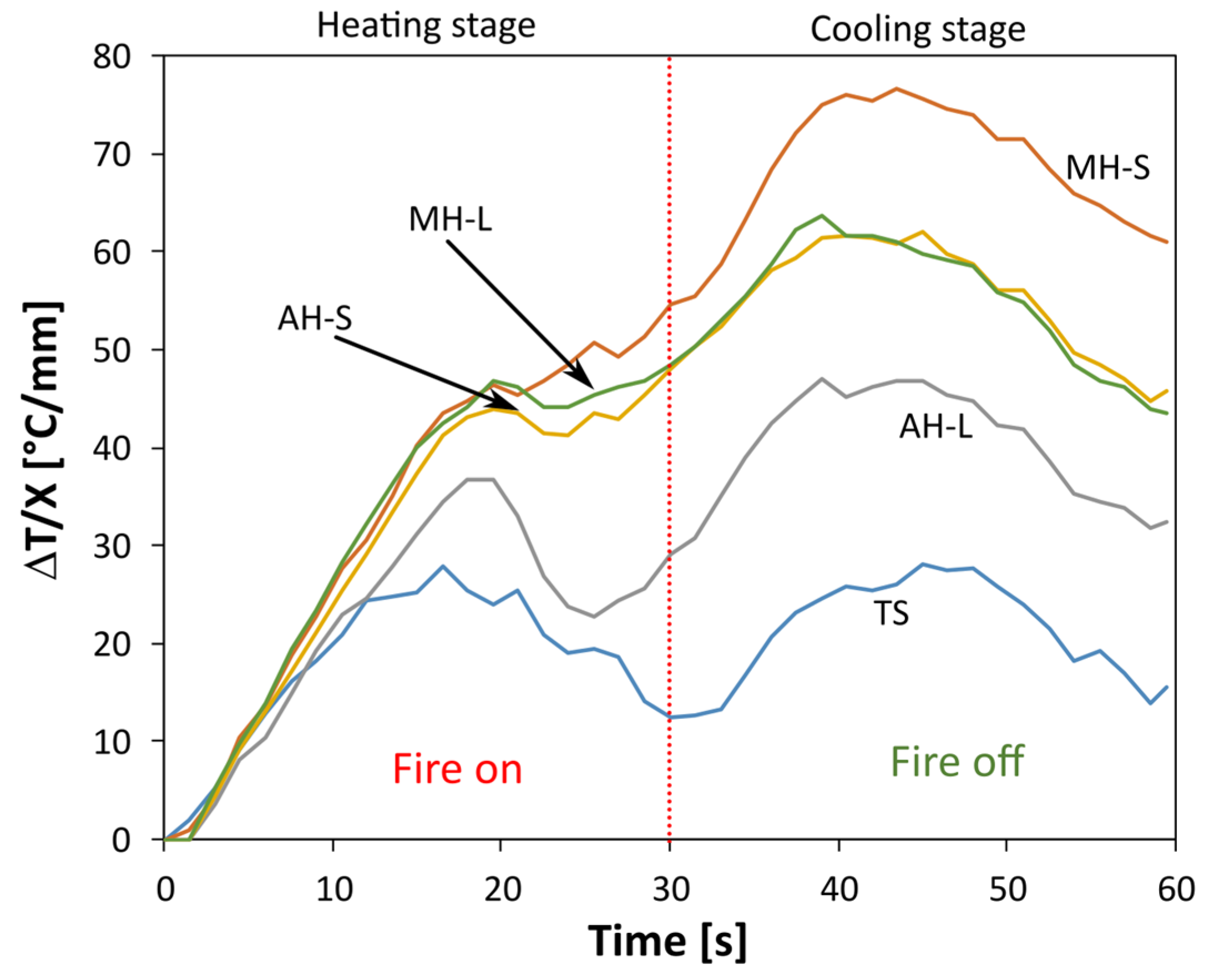
In order to assess the effect of magnesium hydroxide and aluminum hydroxide on the flame-retardant behavior of acrylic based coating, the backside temperature reduction of the FR filled coatings, normalized to the coating thickness (X), compared to the STD one, and at increasing time during the fire retardant behavior tests performed at 30 s, are reported in
Figure 4.
The greater the ΔT/X, the more effective the thermal barrier provided by the functional coating. The TS system (reference thermal protective coating) exhibits a bimodal trend, characterized by two very distinct peaks, occurring at approximately half of the heating and cooling cycle, respectively. The coating has a good protective action with a maximum ΔT/X equal to ~20 °C/mm. The addition of FR based on Al(OH)3 implies an improvement in thermal performance, with an evident increase in fire protection of the metal substrate. This effect is evident throughout the measurement range of the test. The maximum deviation with respect to the STD specimen is achieved during the cooling phase at approximately 45 s for the AH-S sample equal to 47.5 °C/mm.
The replacement of Al(OH)3 with Mg(OH)2 induces a further increase in performance. In particular, the MH-S specimen shows a monotonous and saddle-free trend at 30 s (usually induced by a burn-on/burn-off transition of the flame). This curve trend confirms the high thermal stability of the functional coating. The limited degradation, even after long periods of exposure to direct flame, enhances the differences with respect to the other specimens.
It should be noted, in particular, that the specimens added with FR, characterized by a smaller particle size (MH-S and AH-S), have better fire protection properties. When the flame is removed (30 s), a further increase in temperature is recorded, due to thermal inertia, for up to 40 s. The other investigated batches do not show significantly different behavior from the TS one, in particular the AH-L specimen. A wider difference is found at longer exposure times. Large-sized particle fillers appear to require longer activation times to provide effective fire protection.
Analyzing the samples images after flame exposure (
Figure 5), the STD batch displays the least effective fire-retardant performance, as demonstrated by the extensive carbonized area resulting, in particular, from 30 s of direct flame exposure. It is observable that the unfilled TS batch, used as a reference, displays a minor affected area during a brief exposure time. However, with longer exposure, there is no significant distinction between the TS batch and the AH-L 30 s batch.
Examining the specimens with the addition of hydroxides, it is noted that only the specimen which uses the fine-grained magnesium hydroxide has excellent fire stability. The area affected by the combustion appears more contained and relatively homogeneous. While aluminum hydroxide-based specimens exposed to fire for a longer time show worse performance, similar to the TS reference sample. This can be explained by the fact that during the dehydration process aluminum hydroxide absorbs less energy (1050 J/g) [
1] than magnesium hydroxide (1389 J/g) [
2]. The dehydration process is an endothermic process, which removes heat from the support, slowing down the thermal degradation of the coating and, in this case, releasing nontoxic and noncorrosive reaction products. However, according to Beyer et al. [
1], Al(OH)
3 triggers its protective function before Mg(OH)
2, affecting the degradation at lower temperatures. The dehydration process of Mg(OH)
2 occurs at higher temperatures, as well as its protective action. Therefore, the fire-retardant behavior becomes more relevant as the hydroxide dehydration temperature is approached.
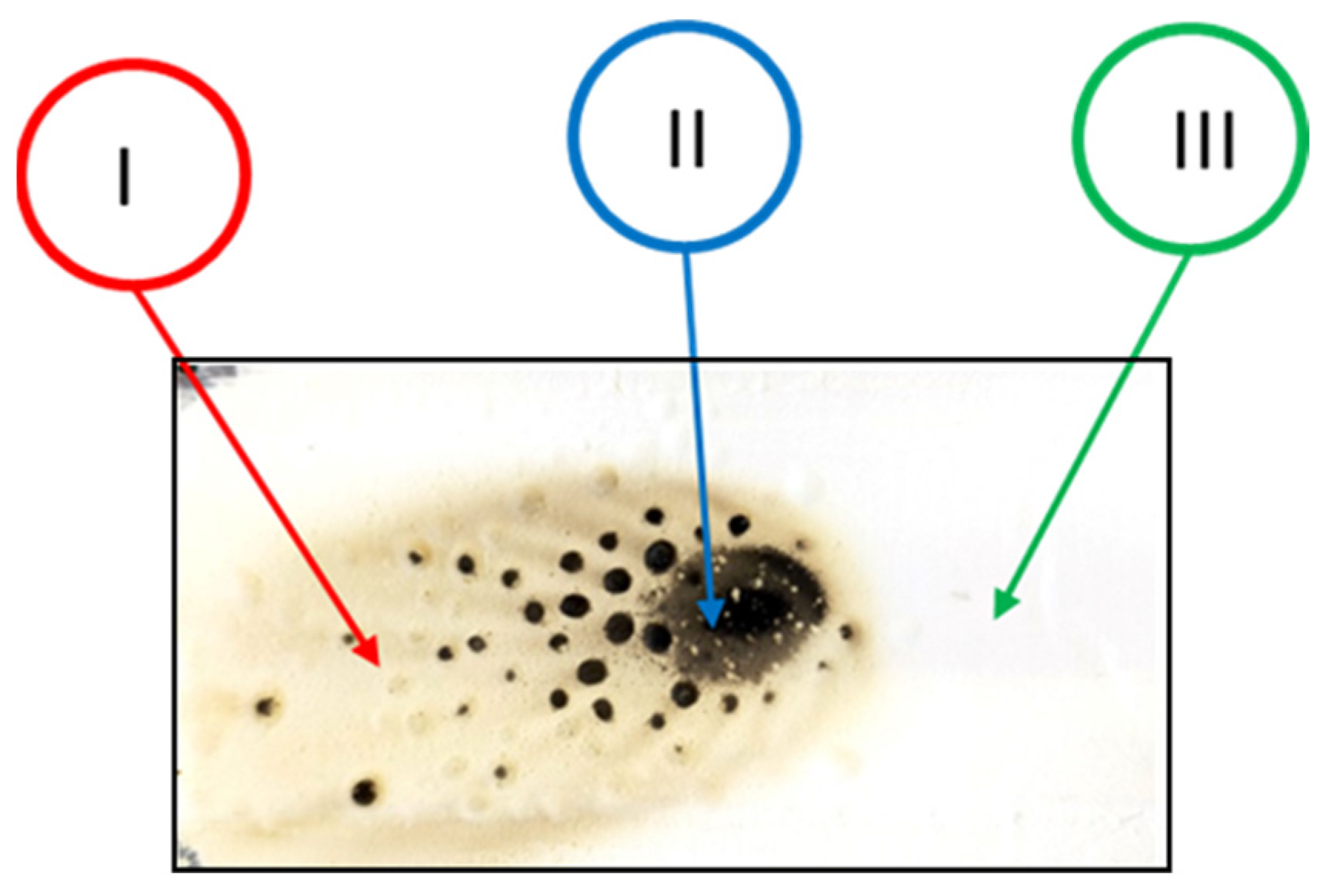
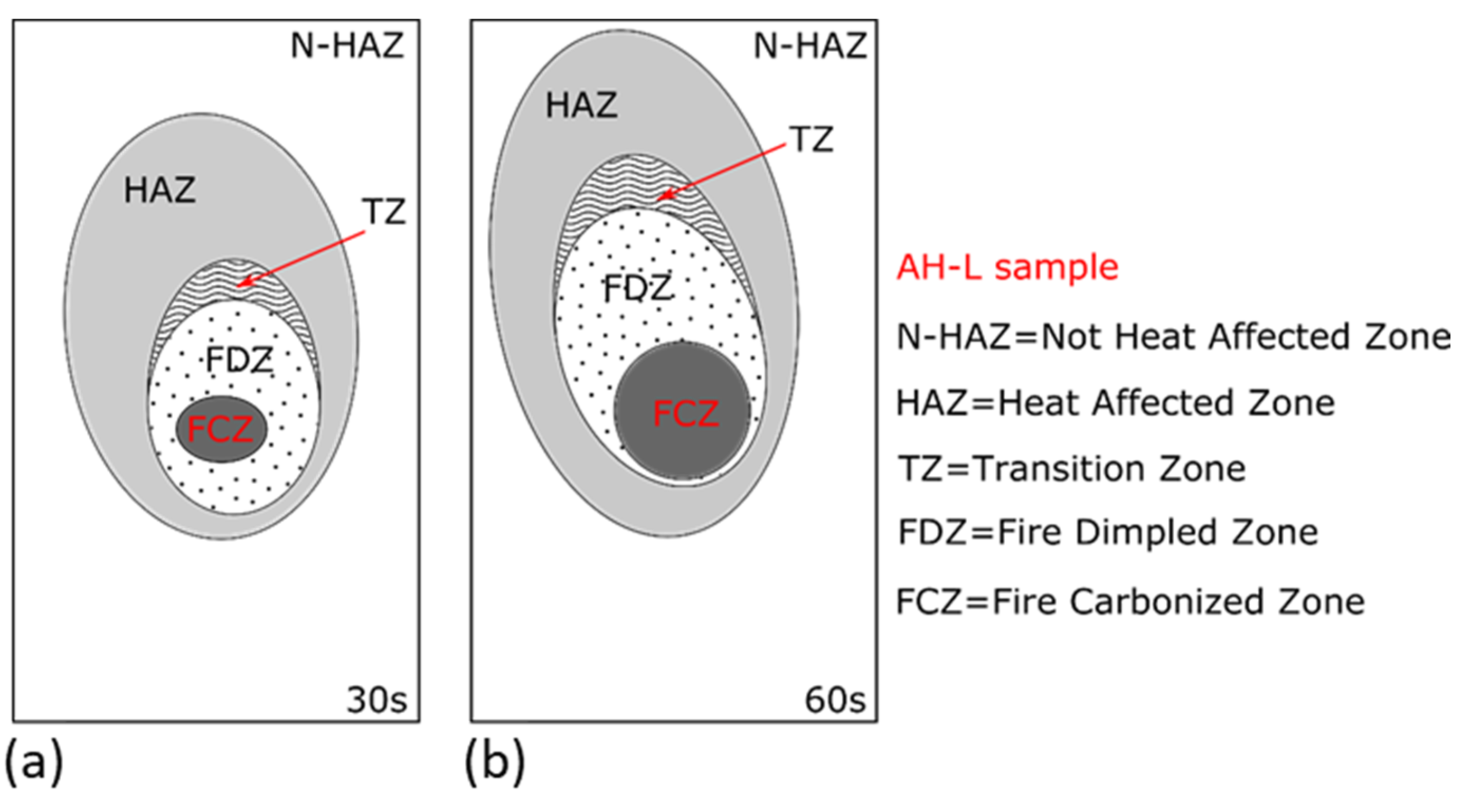
After flame testing, the coatings reveal an area affected by combustion, consisting of three coded zones labeled as I, II, and III. Zone I represents the flame propagation region. Zone II indicates the area of contact between the flame and the paint and, as a result, it is the most darkened portion of the sample. Zone III is the white region where the flame did not propagate, and it closely resembles the fresh sample, i.e., the sample before conducting the tests.
Figure 6 illustrates the three zones just described.
Each zone was structurally characterized by X-Ray diffraction. A similar behavior was observed for all investigated coatings. For brevity,
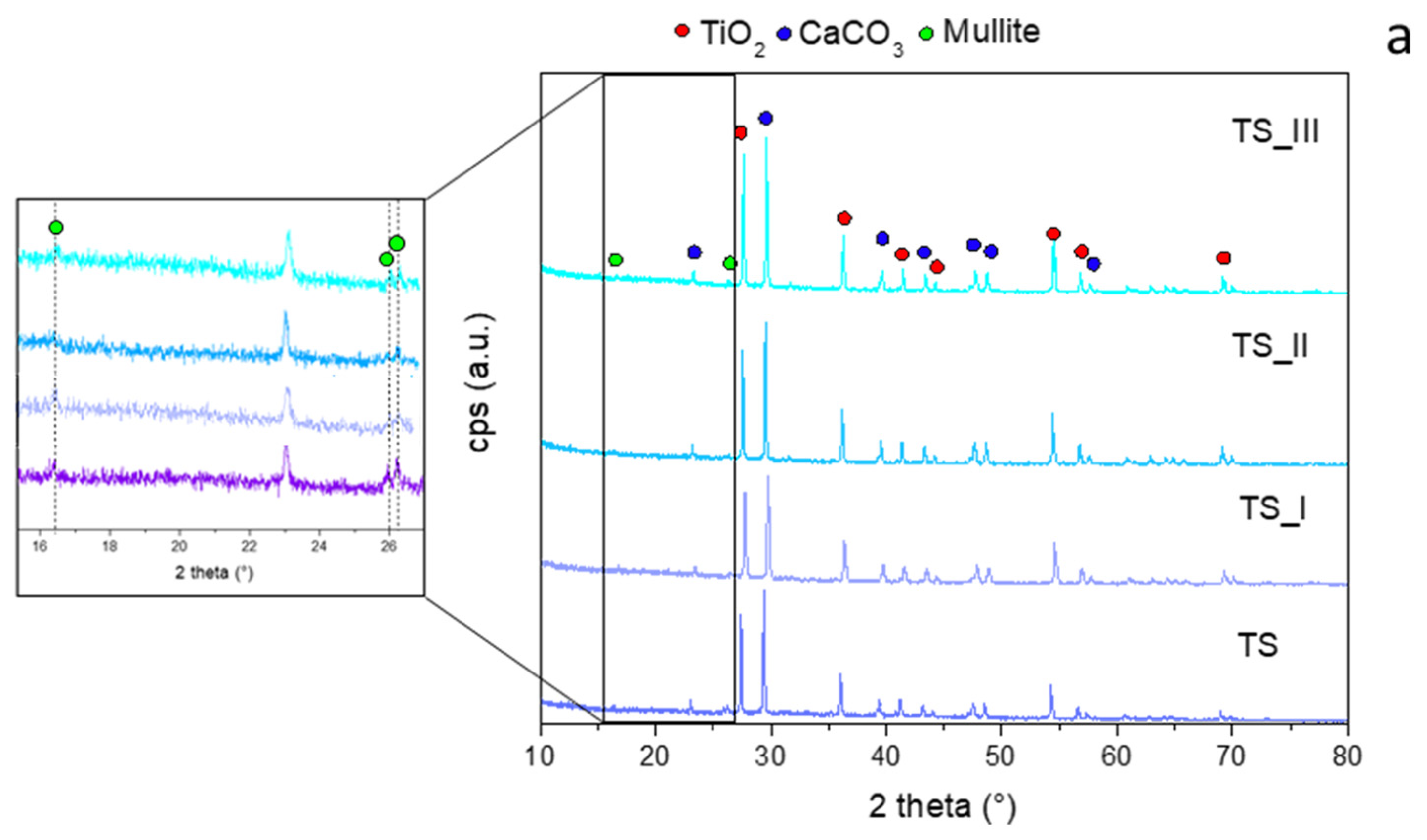
Figure 7 shows the diffractograms of TS and MH-S coatings, after exposure to the flame for 30 s. The diffractograms are compared to the fresh samples, not subjected to the flame test.
In
Figure 7a, it is possible to observe that there are no structural differences between the sample before the fire test and the ones conducted on the three investigated zones after the flame experiment of the TS sample. All diffractograms show a clearly crystalline structure, mainly composed of two phases: TiO
2 (JCPDS #89-552) and CaCO
3 (JCPDS #1-83-1762). The less intense peaks of mullite (JCPDS #4-9-3667) are also noted.
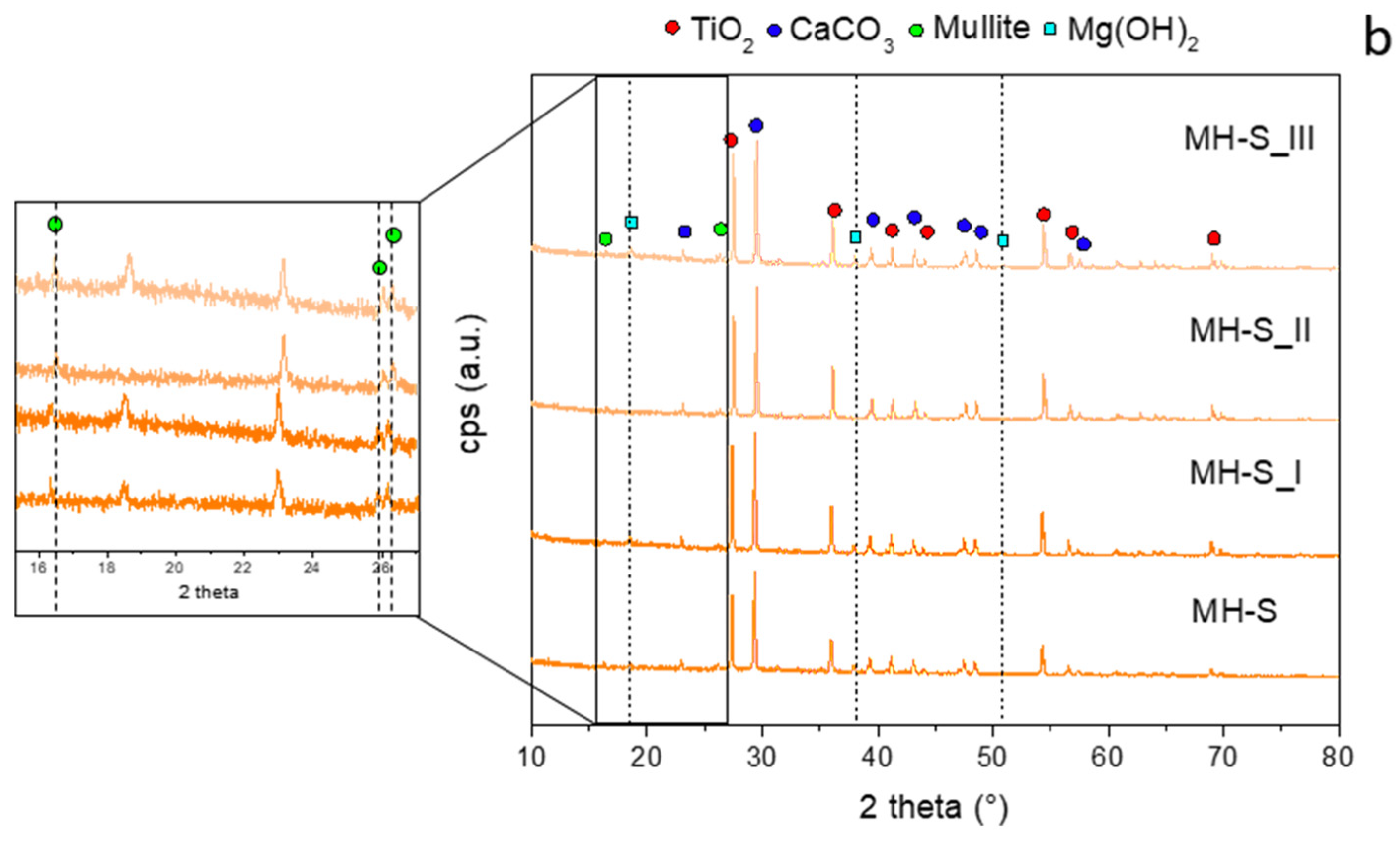
However, in the case of the MH-S sample,
Figure 7b shows a clear difference between the diffractogram of MH-S_II and the other ones. In fact, in this case, the brucite peaks (JCPDS #7-0239, 2theta: 18.6°, 38°, and 51°) disappear, resulting from its dehydration during the combustion of the coating.
In the diffractogram of Zone I, the aforementioned peaks are still clearly visible, probably because in this region the temperature did not exceed 300 °C, which is necessary for the dehydration of Mg(OH)2.
Furthermore, it is interesting to note that the peaks related to periclase MgO (JCPDS #96-100-0054, 2theta: 36.86°, 42.8°, 62.19°, 74.43°, and 78.56°), formed during the dehydration reaction, are not clearly visible because they overlap with the more intense peaks of the standard vehicle. For both formulations, there are no evident differences concerning the mullite phase peaks (magnifications in
Figure 7) in the three investigated zones in comparison with the samples before the flame exposure.
3.2. Digital Image Analysis
In order to better discriminate the evolution of the damage induced by the exposure of the coating to direct flame, the distribution of the degraded area was investigated by means of digital images analysis at varying exposure time and flame retardant in the coating. For the sake of brevity,
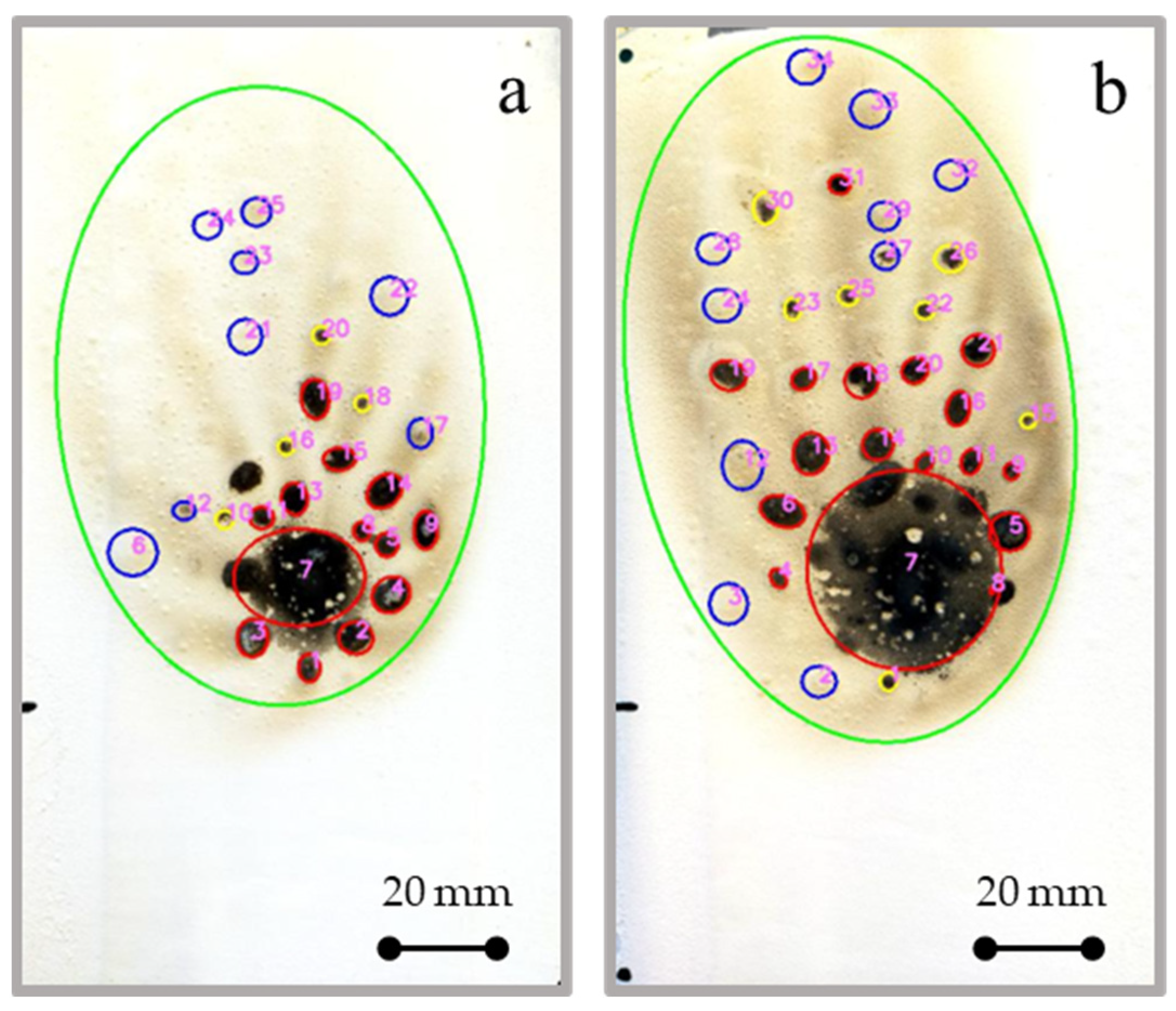
Figure 8 shows the discretization results, obtained by digital image analysis, for a reference AH-L coating at varying exposure time to direct flame. The same discretization approach was applied for all investigated batches.
The algorithm has been tailored to discriminate the area that has undergone the direct exposure to fire (central area highlighted with a large red circle in
Figure 8). In addition, a wide and very large area (green circle in
Figure 8) was distinguished. It identifies the zone of the coating that was affected by the severe local heat treatment. Similarly, various degradation phenomena have been distinguished within this large area.
At first, it was possible to identify with good approximation the local carbonization events which occurred randomly in the neighboring area of the flame contact zone (small red circles in
Figure 8). This area, having been strongly affected by the thermal source, underwent a rise in temperature, which led to the degradation of the low melting materials. The consequence is the formation of small carbonaceous and randomly dispersed dimples that follow the heat flow induced by the flame. These can be due to the charring of local regions characterized by a high concentration of acrylic matrix [
25].
Furthermore, in the area far from the fire exposition, local degradation events have been distinguished, in which there is an evident browning of the surface (small blue circles in
Figure 8). In this area, the reached temperature is considerably lower than in the red zone, thus not inducing the pyrolysis of their constituents. However, a broad and diffuse surface oxidation process of the sample is triggered. Finally, in the transition zone between the soft (blue cycles) and the hard (red circles) heated zone, some very small black degraded dimples (yellow cycles) are observed.
Based on the applied discretization process, an analysis of the frequency distribution of the fire-induced defect areas was conducted.
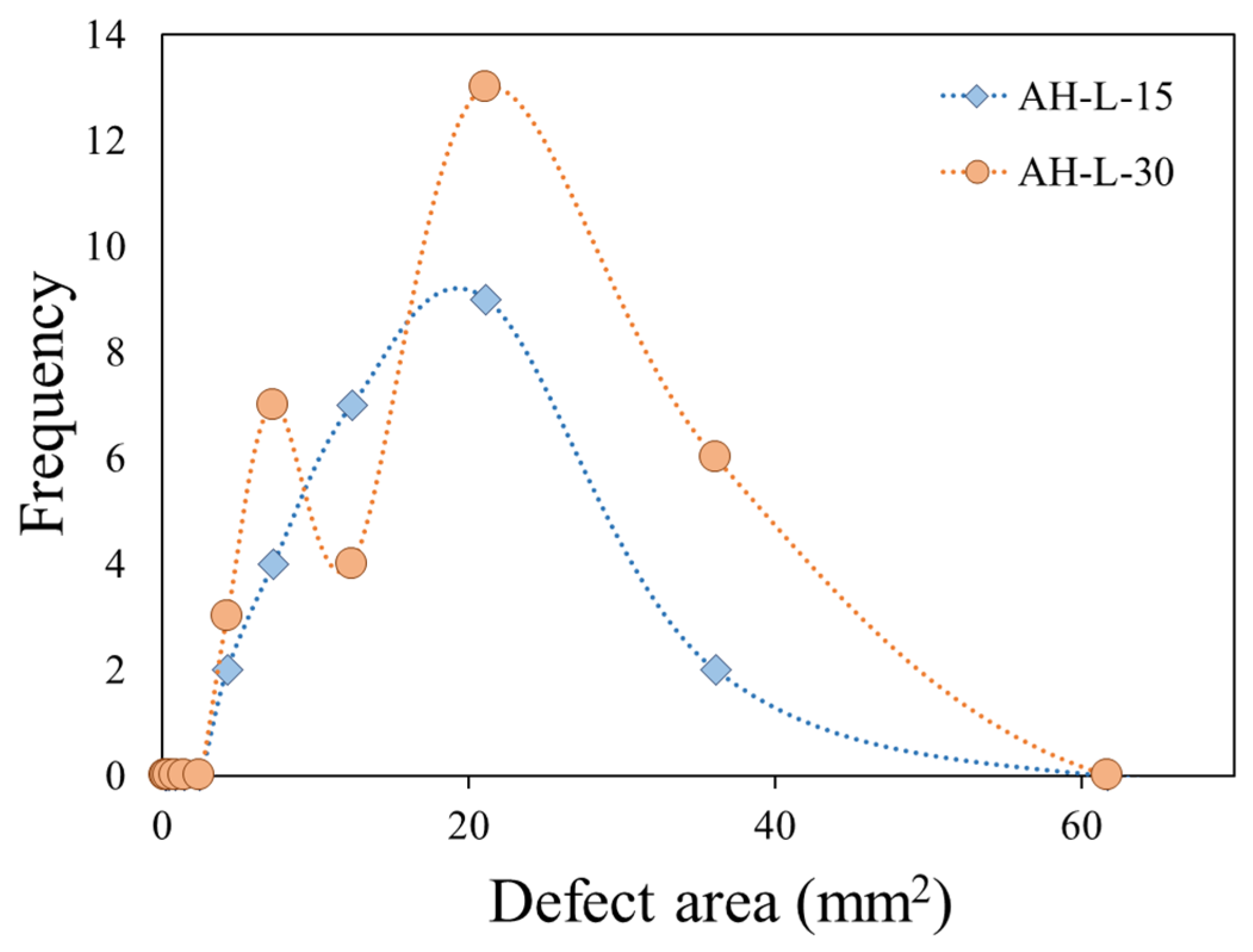
Figure 9 shows the frequency plot in AH-L samples exposed for 15 s and 30 s to direct flame. The AH-L-15 sample exhibits a single cluster of defects with an intermediate area (~12.5–22.5 mm
2), due to a partial activation of fire-induced degradation phenomena. Instead, the AH-L-30 sample, exposed for 30 s to direct flame, is characterized by two well-defined peaks at low and high dimension. The former peak, with a maximum at 7.2 mm
2, can be related to the formation of new degraded nuclei, which extend further away from the flame zone. The second peak correlated to greater area defects (the maximum of the peak at about 21.1 mm
2) is instead attributable to the growth of the defects colony observed at the end of the 15 s exposure test.
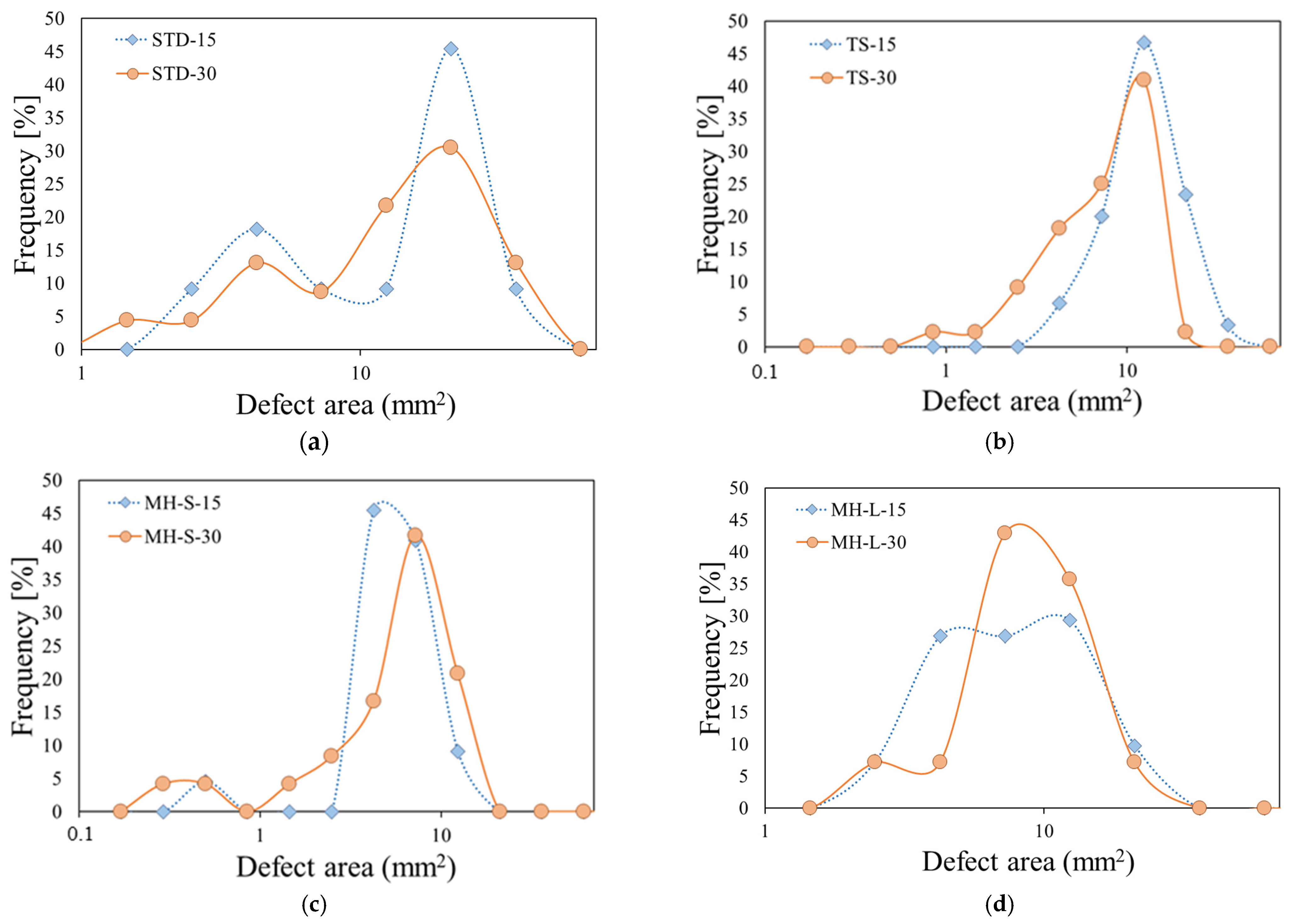
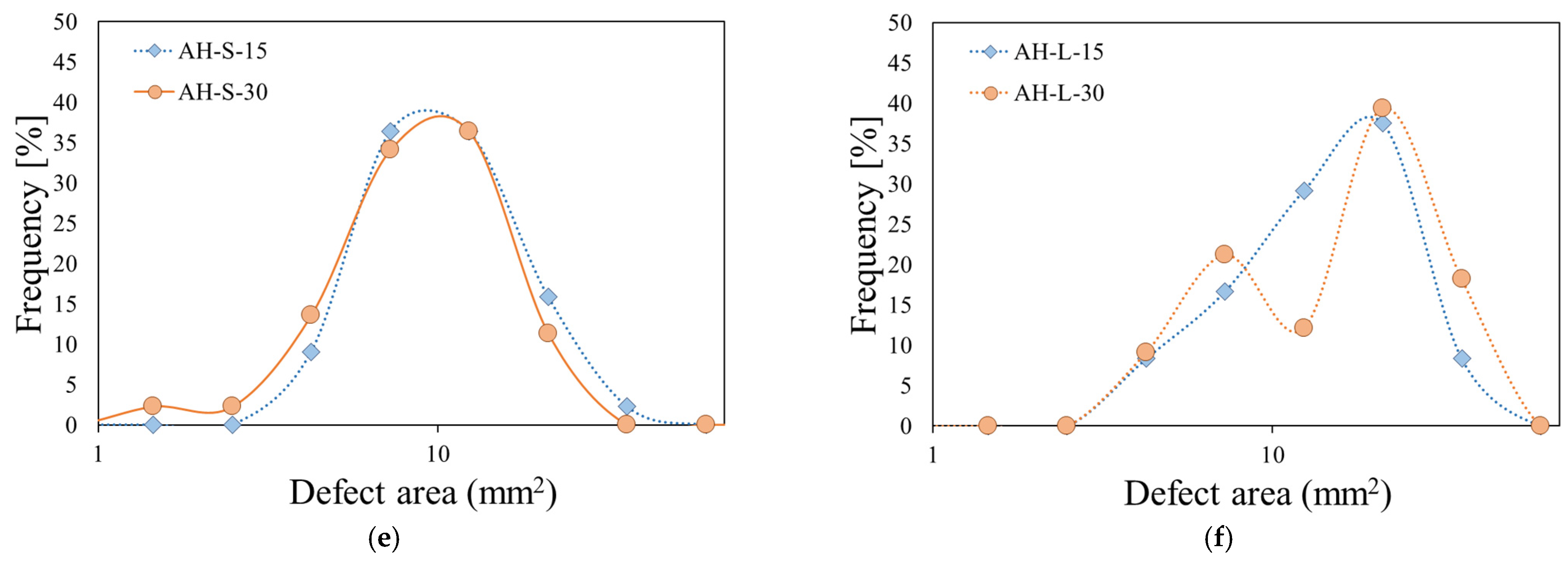
Similar results, with evidence of a bimodal trend in the distribution of the defect areas with a long exposure time to fire, were found for all the investigated batches. Obviously, the size of the defect areas in correspondence with the peak varied according to the greater or lesser tendency to deteriorate, during the direct exposure to fire. In this concern, for clarity,
Figure 10 compares the frequency distribution (expressed in percentage to facilitate the comparison among all batches) of the flame-induced defect area for all samples exposed for 15 s and 30 s to direct flame. In order to increase the plot readability, the defect area axis was defined in log scale.
3.3. Fire Damage Maps
Based on the previous consideration, in all coated samples, the flame-induced damage can be discriminated into four zones:
FCZ. Fire Carbonized Zone. It is the area directly exposed to the flame. The coating reaches high temperatures undergoing pyrolysis. It is easily identified by the black carbonaceous surface obtained at the end of the test.
FDZ. Fire Dimpled Zone. It is the area near the flame exposure area. Fire-induced degradation is relevant, and its morphology is characterized by colonies of dark dimples.
TZ. Transition zone. Progressively increasing the distance from the flame exposition area, the effect is gradually less relevant. In this zone, the dimples do not become carbonaceous. Although, a local, circular shaped browning is detected, indicating that the degradation phenomenon has been activated but sufficient temperatures have not been reached to activate local pyrolysis.
HAZ. Heat affected zone. This region suffers a limited modification induced by the flame test, showing, in particular, a slight superficial browning.
N-HAZ. Not Heat affected zone. This region does not suffer thermal effects induced by the flame test. No surface modifications are observed.
Figure 11 shows, as reference, the fire damage zones occurring by varying the exposure time to direct flame for the AH-L coating batch.
Increasing the time of exposure to fire increases the degradation areas that can be identified. In particular, the heat-affected zone extends for a large part of the surface, confirming the severe thermal treatment imposed by the direct flame exposition (e.g., increase in HAZ and FCZ areas). Interesting considerations can be argued by comparing the extent of these areas as a function of the fire-retardant compound used in the composite coating.
Figure 12 shows the area of FDZ (fire dimpled zone, expressed in cm
2) for all batches exposed for 15 s and 30 s to direct flame.
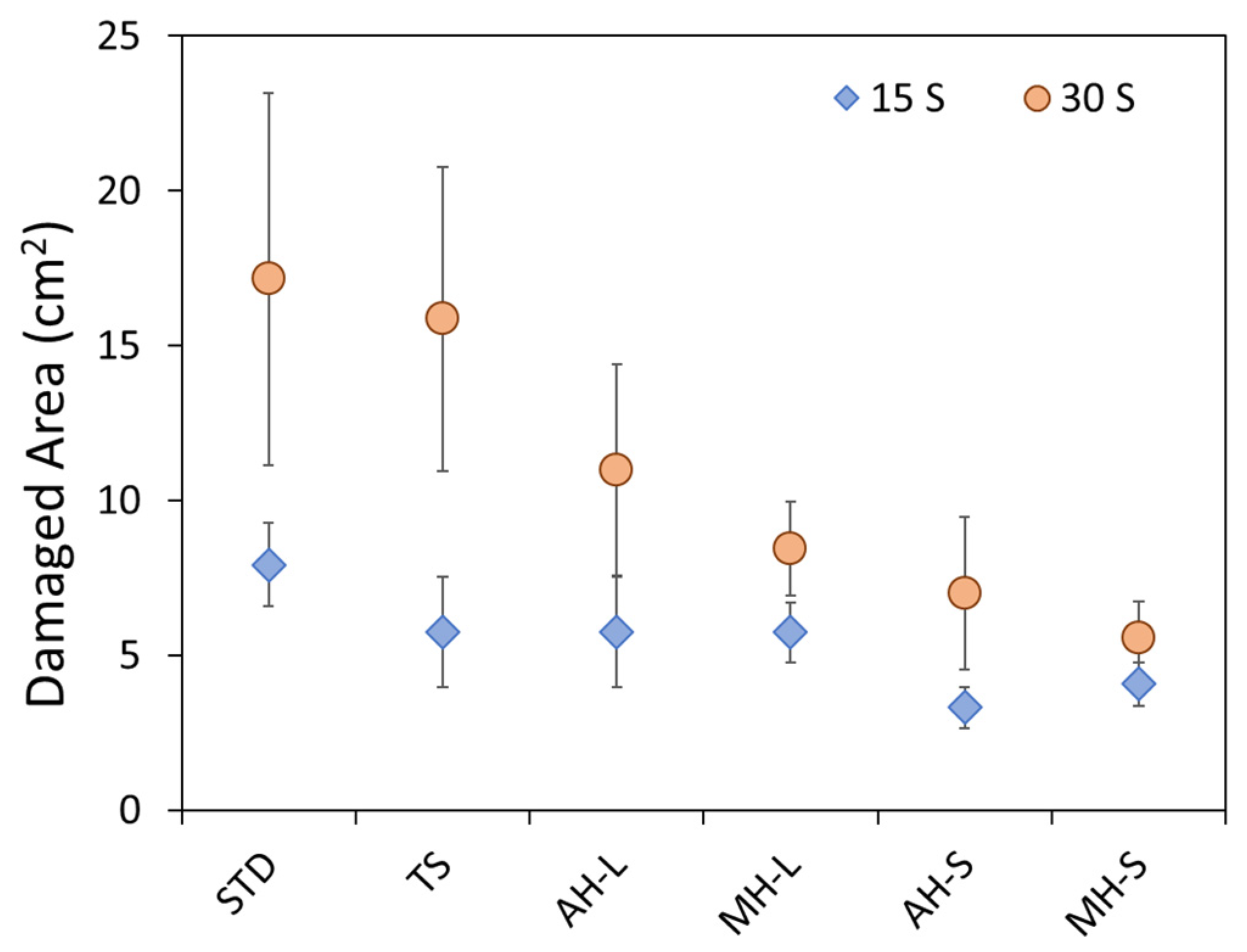
As attended, the STD batch exhibits the worst fire-retardant behavior, as evidenced by the large FDZ area that after 30 s of exposition to direct flame reached a value of ~17.2 cm2. This indicates that a large carbonization area along the flame direction occurred, indicating that the protective capacity supplied by this reference batch is not effective. The addition of thermos-active fillers can offer a clear increase in flame stability and improve the protection of the coating in severe thermal conditions (e.g., direct contact with fire).
It can be noted that the unmodified reference TS batch shows a small FDZ damaged area at low exposition time. At high exposition time, the results are almost compatible with the STD one. This implies that the TS specimen shows adequate fire-retardant behavior only at low exposure times. Its protective efficacy is significantly reduced for longer exposure times. Conversely, there is a significant reduction in the damaged area, even at long exposure times, for the modified coatings. Magnesium hydroxide appears to show better efficacy than aluminum hydroxide. Furthermore, low grain size fire-retardant compounds exhibit the most satisfactory results. Best results were observed for the MH-S batch, where an FDZ damage area equal to 4.1 cm2 and 5.6 cm2 was observed for 15 s and 30 s of exposition to direct flame, respectively.
The aluminum hydroxide modified coatings (AH-L and AH-S batches) evidenced a slightly larger damaged area than corresponding magnesium hydroxide modified coatings. The hydroxides influence the thermal degradation of the modified coating in a temperature range strictly related to the dehydration process of each hydroxide. In particular, the decomposition reaction of Al(OH)
3 takes place in a temperature range of 180–230 °C [
26,
27]. Instead, the dehydration reaction of Mg(OH)
2 occurs at about 200–400 °C [
28]. Considering the temperatures reached by the specimen during the fire test, this latter offers a better flame-retardant capacity providing a suitable thermal degradation barrier.
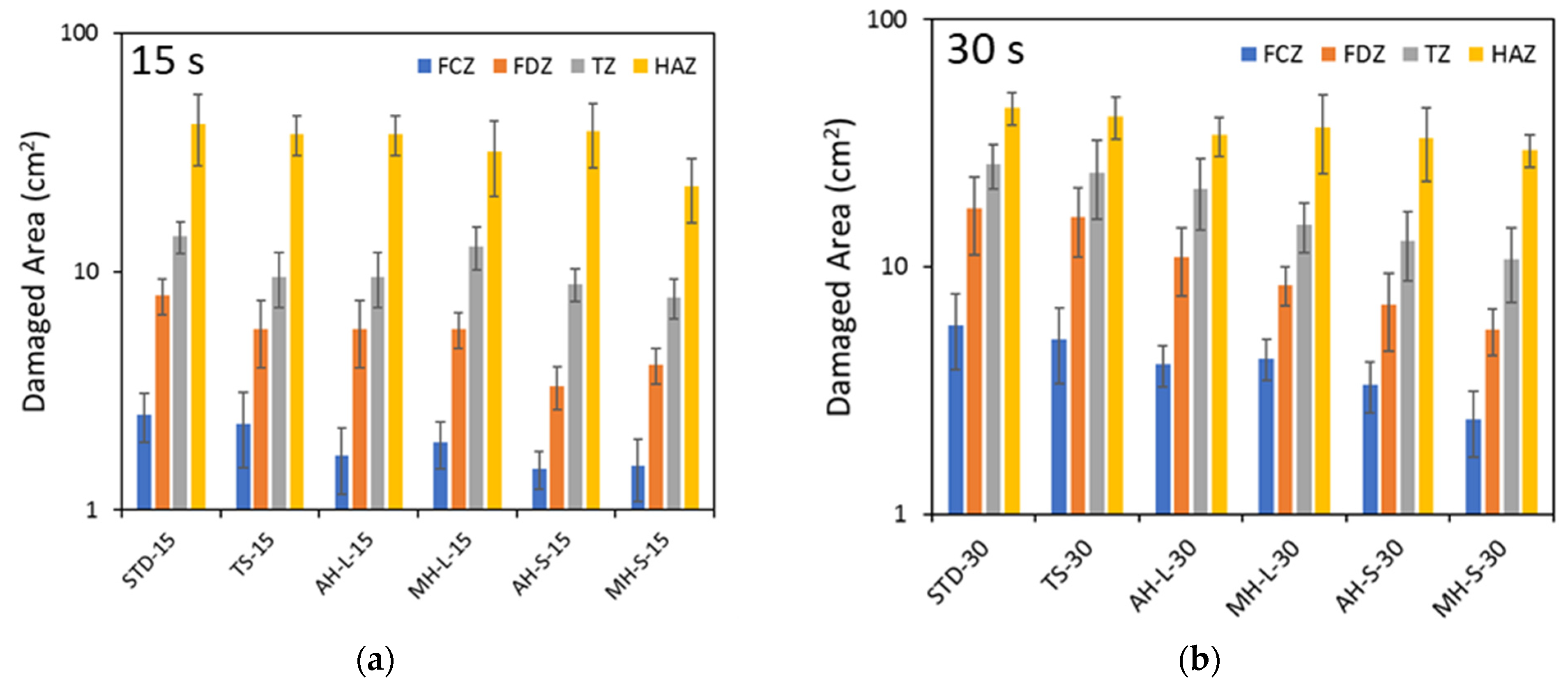
With the aim of having a further assessment of the fire-retardant behavior of the different modified coatings,
Figure 13 compares all areas of damage zones for all batches exposed for 15 s and 30 s to direct flame. The results show that the HAZ area does not have a clear dependence on the modification coating. There is a slight decrease in the average value of HAZ area due to the addition of the fire-resistant compound and choosing a fine particle size of it. However, the dispersion of the results is such that this trend is not statistically significant. Different considerations can be drawn by analyzing the other zones (FCZ, FDZ, TZ), for which there is an evident reduction in the damage area induced by the flame test following the modification of the coating. Especially for the areas near the flame, e.g., FCZ and FDZ, it is evidenced that the fire-retardant protective action has a marked effect. The selection of a proper fire-retardant compound has a key role in the fire protection of the coating. The MH-S-30 specimen shows a reduction in the FCZ and FDZ areas of 53% and 65%, compared to the TS-30 specimen (identified as unmodified reference fire resistant batch). These results suggest the clear shielding action to heat diffusion induced by the fire-resistant compound addition. In particular, a better thermal barrier effect is noted for the system based on Mg(OH)
2 compared to the Al(OH)
3 formulations.
Magnesium and aluminum hydroxides play a relevant role in the thermal energy dissipation during the fire test. According to reaction (1) and reaction (2) both of them, due to the endothermic dehydration reaction, decompose in oxides and water vapor [
7,
26]. However, the incipient decomposition temperature of the dehydration process and the related enthalpy of endothermic reaction is different for the two oxides. Mg(OH)
2 dehydrates at 332 °C, about 150 °C higher than Al(OH)
3 filler (that dehydrates at 180 °C) involving, furthermore, an enthalpy of 1389 J/g [
8], despite the Al(OH)
3 being characterized by 1050 J/g [
9].
In summary, it can be highlighted that the coatings modified with fine-grained compounds (MH-S and AH-S) evidenced a more effective fire protection than the complementary large-grained ones (MH-L and AH-L), as identifiable by the areas of damage observed for these batches. This behavior could be related considering that a small particle size favors a suitable distribution of the thermal active filler thus exalting the thermal exchange and fire-retardant capacity of the coatings. Furthermore, a lower activation time required by the fine-grained additives, compared to the coarse-grained ones, triggers an effective fire protection thus providing a more effective fire-retardant capacity for this class of coatings [
29].
Magnesium (Mg) and Aluminum (Al) hydroxides have proven to be highly effective as fireproof coatings due to their chemical structure and composition. These materials provide a high level of resistance to burns and their thermal decomposition process effectively prevents ignition of the metal substrate. Additionally, their flame-retardant effect is highly homogeneous and efficient when the surface area is large, which is achieved through the use of small particle sizes. Practical engineering applications will depend on addressing challenges related to compatibility with different base vehicles, mechanical properties, and application techniques. With ongoing research and development, these additives could find increasing promotion prospects in the field of fire-resistant coatings. The results of the study have been highly promising, particularly in the case of the MH-S batch. This type of coating could serve as a long-term protection mechanism against accidental fire hazards, making it highly efficient in fire protection.