Abstract
For the synthesis of ionogels containing microcrystalline cellulose (MCC) and Na-bentonite (Na-Bent), ionic liquid (IL) 1-butyl-3-methylimidazolium acetate was used as an MCC solvent. Characterization and research of the physicochemical properties of the synthesized materials were carried out using methods such as SEM, WAXS, thermal analysis, FTIR, conductometry, and viscometry. WAXS analysis showed an increase in the interlayer distance of Na-bentonite in composites due to the intercalation of IL molecules. Based on the data on the characteristic temperatures of thermal degradation, enhanced thermal stability of triple IL/Na-Bent/MCC ionogels was revealed compared to that for cellulose-free systems. It was found that the electrical conductivity of both triple IL/Na-Bent/MCC and binary IL/MCC ionogels was non-monotonous. The data obtained can be used in the formation of multifunctional coatings with enhanced thermal stability.
1. Introduction
Currently, obtaining multifunctional materials from natural polysaccharides has attracted great interest from researchers due to the development of environmentally harmless technologies and the reduction in natural resources [1]. Cellulose is the most abundant polysaccharide in nature and has many advantages such as biodegradability, renewability, non-toxicity, and low cost. From a structural point of view, cellulose is a linear polymer whose macromolecules are built from monomeric units of repeating fragments of D-glucose. Numerous hydroxyl groups are periodically located along the cellulose chain which can form a strong network of both intramolecular and intermolecular hydrogen bonds [2].
Over the past few decades, special attention has been paid to one of the cellulose derivatives: microcrystalline cellulose (MCC). Compared to other known cellulosic materials, MCC has the highest degree of crystallinity and density, a large specific surface area, and the ability to form thixotropic gels. MCC has a high sorption capacity which is determined by the dispersion of its particles and microporosity [3]. Due to its physical and chemical properties such as its high chemical resistance, physiological inertness, biocompatibility, significant mechanical strength, insolubility in water, and no taste and smell, MCC is widely used as a filler, stabilizer, and emulsifier in medicine and pharmaceuticals as well as in the cosmetic and food industries [4]. MCC is also used as a multifunctional auxiliary substance in the manufacture of soft and solid dosage forms and as a starting material for the creation of non-toxic bioresorbable materials for medical purposes [5,6].
However, one of the main problems encountered in the processing of cellulose is its low solubility in water or in common organic and inorganic solvents such as 1,3-dimethyl-2-imidazolidinone, N-oxide monohydrate, N-methylmorpholine-N-oxide, etc., which is due in part to the crystalline structure and close packing of polymer chains due to inter- and intramolecular hydrogen bonds. It should be noted that the disadvantages of the above solvents for cellulose include their high cost, toxicity, and the impossibility of regeneration [7,8,9].
In this regard, the development of new approaches to the dissolution cellulose based on the principles of “green chemistry” is of great interest. Recently, new direct cellulose solvents such as ionic liquids (ILs) have emerged as alternatives to traditional solvents. ILs are organic salts with a relatively low melting point (<373 K) consisting of small inorganic anions and large asymmetric organic cations. Physico-chemical properties of ionic liquids are determined by the structure, chemical nature, and interaction of anions and cations. The typical representatives of ILs are the salts of tetraalkylammonium as well the salts with cations formed by cyclic amines and saturated amines [10,11,12]. Due to their properties, such as non-volatility, non-combustibility, low vapor pressure, environmental friendliness, good chemical, and thermal stability, ILs are widely used to create catalytic systems, supercapacitors as coolants, electrolytes in chemical current sources, and biosensors; moreover, they are used for the purification of petroleum products from aromatic compounds, etc. [13].
However, along with their advantages, ILs have significant disadvantages. For example, according to [14], ILs are generally 5–20 times more expensive than molecular solvents. In many cases, this is due to expensive their multi-stage synthesis. In addition, because their ionic nature, ILs are often corrosive. Therefore, special containers are needed in potential industrial processes. In addition, many ionic liquids are more toxic than common organic solvents such as methanol and dichloromethane [15].
In recent years, ionic liquids for the dissolution and regeneration of cellulose-containing materials have been actively studied in connection with the possibility of their reuse and ability to close technological cycles. According to the published literature, ILs containing imidazolium, phosphonium, and ammonium cations contribute to the dissolution of cellulose while a growth in the size of the alkyl radical in the cation reduces the solubility of cellulose [9,16,17,18,19]. In the review [20], the authors reported that imidazolium-based ionic liquids can be used to dissolve biopolymers. Moreover, they concluded that intramolecular and intermolecular hydrogen bonds existing in the structure of cellulose and chitosan can be broken by 1-Butyl-3-methylimidazolium acetate (BMImAc) which leads to the effective dissolution of polysaccharides.
To improve the physical-mechanical properties and ionic conductivity and expand the possibilities of using cellulose ionogels, inorganic fillers are introduced into the polymer matrix [21,22,23]. Of particular interest among inorganic fillers are bentonite (Bent) and montmorillonite (MMT) which is the main component of bentonite. MMT is a readily available, inexpensive, and biocompatible layered clay mineral. The crystal structure of it consists of three-layer packages that contain silicon–oxygen and aluminum–hydroxyl layers with exchange cations between them as well as reactive –OH groups on the surface. The thickness of each clay sheet is approximately 1 nm [24]. In the current study, Na-bentonite was used as the inorganic component of the ionogels since exchangeable Na cations can be replaced by IL cations.
In the literature, there are publications on the physico-mechanical properties of cellulose ionogels filled with various clay minerals. For example, M. Soheilmoghaddam et al. [22] reported an enhancement in the strength and thermal stability of films cast from solutions of regenerated cellulose in an ionic liquid with the addition of zeolite. Guo et al. [25] showed that the introduction of halloysite into cellulose-based ionogels also improves the ionic conductivity and the mechanical properties of the ionogels. Therefore, it can be assumed that ionogels containing IL, clay, and cellulose (or its derivatives) are promising materials for studying their physicochemical properties. Obviously, such studies can contribute to the search for conditions for the targeted synthesis of ionogels with controlled properties and, as a result, with important practical applications.
The aim of the current work was the synthesis of IL/Na-Bent/MCC ionogels and the study of the influence of polymer content on the morphology, structure, thermal behavior, and ionic conductivity of the obtained biomaterials. XRD analysis showed an increase in the interlayer distance of Na-bentonite in composites due to the intercalation of IL molecules. The prepared triple IL/Na-Bent/MCC ionogels were characterized by a greater thermal stability than those without cellulose. The electrical conductivity of both triple IL/Na-Bent/MCC and binary IL/MCC ionogels was non-monotonous.
The present paper is a continuation of our studies of triple ionogels with halloysite as a clay filler [26]. Therefore, the information on the properties of triple ionogels (but with bentonite as a clay filler) obtained in the current study is new and can be used in describing the effect of the clay filler nature in such systems.
2. Materials and Methods
2.1. Chemicals
The following reagents were used to prepare triple ionogels: ionic liquid of 1-butyl-3-methylimidazolium acetate (purity ≥ 95%, water content ≤ 2.0%) purchased from Sigma-Aldrich (Darmstadt, Germany), Na-bentonite and microcrystalline cellulose (Avicel type, with an average size of 20 µm) purchased from Sigma-Aldrich (St. Louis, MO, USA). All reagents were used without further cleansing. Moreover, to reduce uncertainty, all reagents were dried prior to synthesis.
2.2. Preparation of the BMImAc/Na-Bent/MCC Composite Materials
The IL/Bent/MCC composite materials were prepared according to the following procedure. First, the dried cellulose was dissolved in the BMImAc (3–4 g) to obtain the IL/MCC ionogels with a polymer concentration of 1, 2, 3, 4, 6, 8, and 10 wt. %. Then, sodium bentonite was gradually added under continuous vibratory mixing for 1 h until a supersaturated solution was reached. Then, the mixture was sonicated in a CT-431D2 ultrasonic bath (CTbrand Wahluen Electronic Tool Co., Ltd., Shantou, China) for 2 h and then dried in a LT-VO/20 vacuum drying oven (Labtex, Moscow, Russia) at 80 °C for 24 h.
The prepared mixture was centrifuged for 10 min at 5000 rpm and the separation was monitored. During centrifugation, the ionogel is separated into the main quasi-solid mass and the IL phase. The liquid is taken with a volumetric pipette and then, based on the ratio of the main mass in the ionogel (IL:MCC:clay), the required amount of clay is mixed with the selected IL, introduced into the main ionogel, and all the previous steps (mixing, ultrasonic treatment, and drying) are repeated to obtain the ionogel (Figure 1).
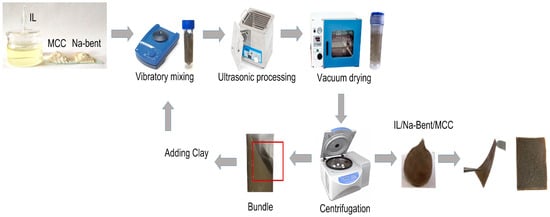
Figure 1.
Scheme of the synthesis procedure.
Table 1 shows the final compositions of the synthesized ionogels. In this table, we used the abbreviations, such as IL/Bent/MCC-1, IL/Bent/MCC-2, IL/Bent/MCC-3, IL/Bent/MCC-4, IL/Bent/MCC, IL/Bent/MCC-6, IL/Bent/MCC-8, and IL/Bent/MCC-10 to denote ionogels which were prepared using the IL/MCC systems with 1, 2, 3, 4, 6, 8, and 10 wt. % of MCC, respectively, as precursors.

Table 1.
Final compositions of the synthesized ionogels.
2.3. Techniques
The microstructure of the BMImAc/Na-Bent/MCC ionogels was studied by scanning electron microscopy (SEM, Tescan Vega 3 SBH, Brno, the Czech Republic). Before SEM experiments, the samples were dried to obtain more reliable information. Wide-angle X-ray scattering (WAXS, Bruker D8 Advance, Karlsruhe, Germany) was used to investigate the crystal structure of the Na-bentonite, BMImAc, MCC, and resulting ionogels. In WAXS experiments, the cuvettes were completely filled with the test substance. For infrared (IR, Thermo Nicolet, Waltham, MA, USA) spectroscopy, the samples were prepared by dispersing into KBr and pressing to pellets. To study the thermal behavior of ionogels and initial components, differential scanning calorimetry (DSC, Netzsch, Selb, Germany) and thermogravimetry (TG, Netzsch, Selb, Germany) were used. In DSC tests, to minimize the role of water, the data obtained in the second heating were used. Impedance spectroscopy was used to determine the electrical conductivity (κ) of the ionic liquid and synthesized ionogels. Dynamic viscosity was determined at constant temperature and an angular rotation rate of 3 to 200 rpm. In all measurements, the errors of the results corresponded to the errors of the instruments.
3. Results and Discussion
3.1. Surface Morphology of the Studied Materials
The surface morphology of the original Na-bentonite powder and IL/Na-Bent/MCC ionogels with various cellulose concentrations was studied by SEM (Figure 2a–d). As can be seen from Figure 2a, the original clay has a typical layered structure. Particles are aggregated with various morphologies and sizes of <50 μm.

Figure 2.
SEM-images of surface: Na-Bent (a), IL/Na-Bent/MCC-2 (b), IL/Na-Bent/MCC-8 (c), and IL/Na-Bent/MCC-10 (d).
Figure 2b–d show that the ionogel is an ionic liquid densely filled with Na-bentonite nanoparticles. An increase in the MCC concentration in the ionogel leads to the formation of regions that differ in density from the main solution (Figure 2d). In addition, the thickness of the IL/MCC solution layer on the surface of clay nanoparticles increases and the covering ability improves which may be due to the strengthening of bonds at the solid surface/liquid interface. It should be noted that the Na-bentonite particles in an ionogel with an initial MCC concentration above 6% are completely covered with a liquid phase layer in contrast to ionogels with a lower cellulose content.
3.2. Crystal Structure of the BMImAc/Na-Bent/MCC Ionogels
To obtain data on structural changes occurring during the formation of the ionogel, WAXS analysis can be used. Figure 3 and Figure 4 show the corresponding patterns for a number of BMImAc/Na-Bent/MCC ionogels and their components.
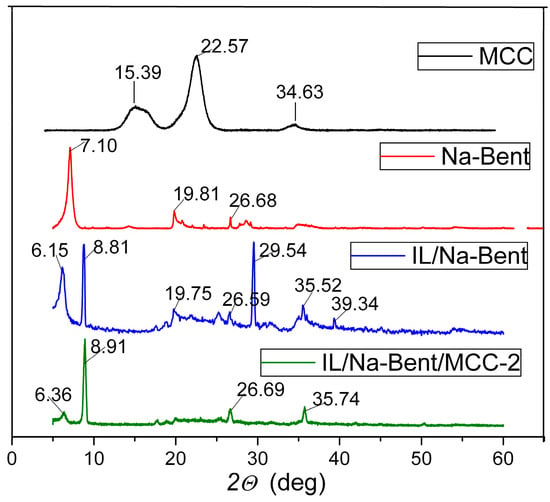
Figure 3.
WAXS patterns for the BMImAc/Na-Bent/MCC-2 ionogel and its components.

Figure 4.
WAXS patterns for BMImAc/Na-Bent/MCC ionogels with various MCC concentrations.
The diffraction pattern of the MCC sample is shown in Figure 3. Reflections characteristic of the polymer are observed at 2θ = 15.39°, 22.57°, and 34.63°. This is consistent with the previously reported [27,28,29].
The diffractogram of Na-bentonite shows a peak at 2θ = 7.10°. This reflection corresponds to the basal spacing (d001) of 1.26 nm and is consistent with data previously obtained for montmorillonite (JCPDS chart N 13-0135) which is the main component of bentonite. Other peak positions at diffraction angles of 2θ = 19.81° and 26.68° correspond to crystallographic planes (110) and (112).
Diffraction peaks corresponding to bentonite also appear in the spectra of the studied ionogels (Figure 4). In addition, for all synthesized ionogels, the characteristic reflection assigned to the (001) plane is shifted towards smaller angles compared to that for the initial Na-bentonite. The positions (2θ°) of this peak for ionogels with different compositions are given in Table 2. The presented information indicates the intercalation of IL molecules into the interlayer space of Na-bentonite since the basal distance (d001) for ionogels is greater than that for the initial clay.

Table 2.
XRD data for the BMImAc/Na-Bent/MCC ionogel samples.
Apparently, the BMImAc molecules intercalated into Na-bentonite are more difficult to thermally decompose than those that do not interact with clay. As can be seen from the comparison of the TG data (Section 3.5), for the BMImAc/Na-Bent composite, the characteristic temperatures of the second stage are higher than for pure IL.
It should be noted that, in contrast to the initial Na-bentonite, reflections are observed in the WAXS patterns of ionogels at angles of approximately 8.8 degrees. According to [30,31], these peaks are caused by the formation of structurally ordered IL crystallites between clay particles. The sizes of these crystallites (L), calculated according to the Scherrer equation, are shown in Table 2.
3.3. FTIR Analysis of Ionogels
The IR transmission spectra of the BMImAc ionic liquid, MCC, initial Na-Bent, IL/Na-Bent, and IL/Na-Bent/MCC ionogels in the range of 4000–400 cm–1 are shown in Figure 5.
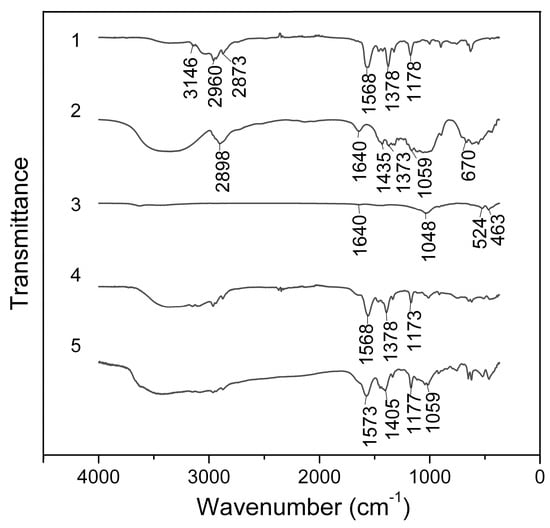
Figure 5.
IR transmission spectra of IL (1), MCC (2), Na-Bent (3), IL/Na-Bent (4), and IL/Na-Bent/MCC-10 (5).
Figure 5 (trace 1) shows that the IR spectrum of the ionic liquid contains absorption bands characteristic of BMImAc functional groups. The small peak at 3146 cm−1 is due to C–H stretching in imidazolium rings. The peaks at 2960 cm−1 and 2873 cm−1 can be attributed to the asymmetric and symmetric stretching vibrations (C–H) [32]. The bands at 1568 cm−1 and 1378 cm−1 refer to vibrations of the COO– groups. The peak at 1178 cm−1 probably belongs to the stretching vibrations of the imidazolium rings.
In the spectrum of microcrystalline cellulose (Figure 5, trace 2), the broad band at 3600–3000 cm−1 can be attributed to the H-bonded –OH groups. The peak at 2898 cm−1 corresponds to the stretching vibrations of C–H bonds in polymers. The small peak at 1640 cm−1 belongs to the bending mode of absorbed water in MCC [33]. The bands at 1435 cm−1 and 1373 cm−1 can be attributed to CH2 and C–H bending vibrations, respectively. The band at 1059 cm−1 corresponds to C–O stretching vibration groups in polysaccharides. The small peak at 670 cm−1 can be attributed to the C–OH bending mode of cellulose [34].
In the IR spectrum of Na-bentonite (Figure 5, trace 3) in the range of 3700–3300 cm−1, there are absorption bands of stretching vibrations of –OH groups associated with octahedral cations. A peak at 1640 cm−1 refers to vibrations of water molecules. The intense band at 1035 cm−1 corresponds to the Si–O–Si stretching vibrations of the clay mineral. Peaks at 524 cm−1 and 463 cm−1 refer to vibrations of Al–O and Si–O groups, respectively.
The IL/Na-Bent ionogel spectrum (Figure 5, trace 4) shows that the bands belong to the functional groups of the organic and inorganic components. However, in this spectrum, compared to the IL spectrum, there is a shift in the absorption bands at 1568 and 1378 cm−1 which can be attributed to the stretching vibrations of the COO anion of the IL by 5 and 16 cm−1, respectively [31].
In the spectrum of the IL/Na-Bent/MCC ionogel (Figure 5, trace 5), there are changes in contour and an expansion of the absorption band in the range of 3700–3000 cm−1 which can be associated with the stretching of H-bonded –OH groups as well as with a change in the number and strength of hydrogen bonds in the ionogel [35]. Shifts in the stretching vibrations of the COO– group of the BMImAc from 1568 to 1573 cm−1 by 5 cm−1, as well a change in the band contour and a shift of this band from 1378 to 1405 cm−1 by 27 cm−1, are found. A shift of the band of vibrations of the imidazolium ring at 1178 cm−1 to the long-wave region by 5 cm−1 is also observed.
It should be noted that the band of the C–O group of cellulose (1059 cm−1) is overlapped by the band related to vibrations of the Si–O–Si groups in clay of 1035 cm−1; it is shifted to the long wavelength region by 4 cm−1.
3.4. Phase Transitions in the BMImAc/Na-Bent/MCC Ionogels
It is known that during the thermal treatment of materials containing ionic liquids, phase transformations can be observed such as the glass transition, crystallization, and melting. In the present paper, thermal behavior of pure BMImAc also synthesized for BMImAc/Na-Bent/MCC ionogels was researched when heated in the region of −110~+100 °C. The DSC diagrams of the second heating cycles for the listed materials are shown in Figure 6. Inflections are observed on these thermograms which may be associated with phase transitions due to glass transition processes. The following characteristic temperatures can be identified in DSC patterns: the glass transition temperature (Tg) and the temperatures of onset (Tonset) and end (Tend) of phase transition. The found values are listed in Table 3
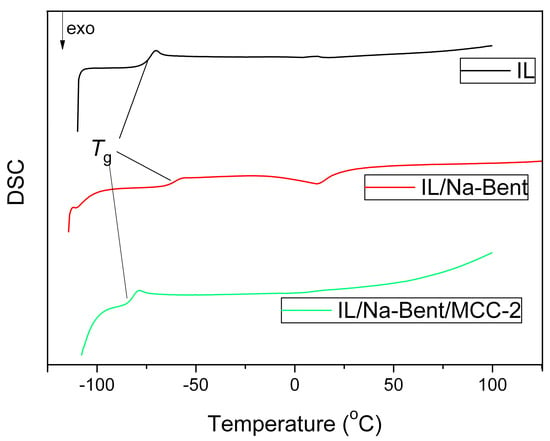
Figure 6.
DSC curves for BMImAc and BMImAc/Na-Bent/MCC ionogels.

Table 3.
Characteristic phase-transition temperatures in the initial BMImAc and synthesized BMImAc/Na-Bent/MCC ionogels.
By analyzing the data presented in Figure 6 and Table 3, we can conclude that when Na-bentonite is introduced into the IL, all the characteristic temperatures are shifted in the positive direction. For example, the Tg value became almost 12 °C higher compared to the ionic liquid. A similar conclusion concerning the influence of the clay mineral was made by us earlier for the BMImAc/Halloysite ionogels [26] as well as for the BMImNTf2/Bent and BMImNTf2/Na-MMT ionogels [36]. The presented data indicate the confinement in the IL/clay systems.
However, subsequent introduction of MCC into the IL/Na-Bent system results in the opposite effect. In particular, all the synthesized BMImAc/Na-Bent/MCC ionogels are characterized by more negative glass transition temperatures than those without MCC.
3.5. Thermal Decomposition of Starting Materials and Ionogels BMImAc/Na-Bent/MCC Based on Them
The TG and DTG curves of the initial components included in the composition of the ionogels are shown in Figure 7a,b. All initial components (namely BMImAc, Na-Bent, and MCC) are characterized by the presence of two stages of thermal decomposition. The first stage is the removal of adsorbed water. Depending on the component, the amount of water removed was from 2 to 5%. The tangential crossing method was used to determine the beginning (T1) and end (T2) of the temperature interval. The IL is characterized by a wider temperature range for the removal of adsorbed water which is shown in Table 4. For IL, the T1 and T2 values are 30~60 degrees higher than those of Na-bent and MCC which indicates a strong chemical interaction between the ionic liquid and water.
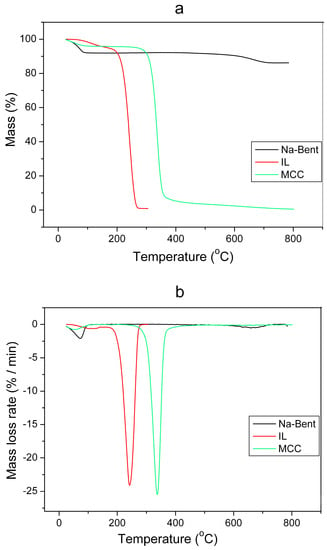
Figure 7.
TG (a) and DTG (b) curves for initial components of ionogels.

Table 4.
Characteristic temperatures and weight loss of initial components of ionogels.
The second stage is characterized by thermal decomposition of the initial component which leads to an intense decrease in the mass of the sample. From Figure 7a,b, it can be seen that the IL and MCC completely decompose when heated to 360 °C while Na-bent shows a weight loss of only 13% at a temperature of 800 °C. Apparently, this is due to the removal of chemically bound water.
Figure 8 shows the TG and DTG data for a three-stage process of changing the state of heated ionogels with a polymer concentration from 1 to 10%. A wider temperature range than that of pure components characterizes the initial stage of ionogel decomposition; namely, the removal of water (58~138 °C). Earlier [37], we identified a similar effect for montmorillonite/IL ionogels.
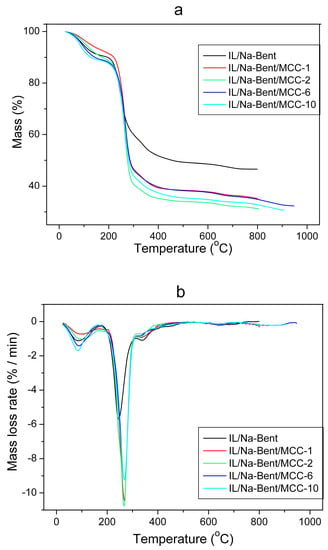
Figure 8.
TG curves (a) and their DTG derivatives (b) for BMImAc/Na-Bent/MCC ionogels.
The second stage is the main stage of the thermal destruction of ionogels. It starts at a temperature of 223–238 °C and ends at a temperature of 488–563 °C depending on the concentration of microcrystalline cellulose in the composition of ionogels. As can be seen from Table 5, in this stage, 40%~60% of the material decomposes. Apparently, the vast majority of the decayed matter corresponds to the BMImAc since the initial IL decomposes in the range of 220~260 °C (Table 4) which is included in that for ionogels. However, the maximum rate of thermal decomposition (the peak magnitude of the DTG curve) for the ionogels is more than two times lower than that for the initial IL. The introduction of sodium bentonite and microcrystalline cellulose into the composition of the ionogel increases the thermal stability of the composite by more than 200 °C, as evidenced by the increase in the temperature range of the second stage of the ionogel (Table 5) in comparison with a pure ionic liquid (Table 4). The presented data indicate that the interaction with bentonite nanoparticles hinders the decomposition of the IL molecules.

Table 5.
Summary of thermal behavior of the IL/Na-Bent/MCC ionogels.
It should be noted that earlier [26], when studying the thermal behavior of the BMImAc/Halloysite composites, we made the opposite conclusion. Namely, we reported that BMImAc confined in the halloysite nanotubes decomposed easier than bulk IL. Thus, it can be assumed that the shape of clay mineral particles affects the heat resistance of the BMImAc/clay composites.
During the second stage of the thermal decomposition of the BMImAc/Na-Bent/MCC ionogels, a rapid mass loss is observed which is related to the decomposition of IL adsorbed on clay particles and the resulting rapidity of the process. Then, as can be seen from Figure 8a, the region of rapid weight loss passes to a slower one associated with the decomposition of the IL in the inter-layer space of Na-Bent. The temperature interval corresponding to slow decomposition includes the temperature intervals for reducing the weight of the initial components of composites such as MCC and Na-bentonite (Table 4). Therefore, it can be concluded that these components (at least MCC) undergo thermal degradation. Note that the introduction of only 1% of MCC into the IL/Na-Bent ionogel results in a significant (more than 10%) decrease in the residual mass of the material (Table 5).
During the third phase (606~917 °C), the mass of ionogels decreases by 2%–3% which can be explained by the removal of chemically bound water.
Thus, the conducted studies demonstrated that the introduction of MCC macromolecules enhanced the thermal stability of IL/Na-Bent ionogels. This is of great practical importance because equipment that uses IL/Na-Bent/MCC ionogels can withstand greater thermal loads than those without MCC.
3.6. Transport Properties of the Materials under Study
For the practical application of ionogels in electrochemical devices, one of the most important physical and chemical properties is electrical conductivity which depends on the composition of the material. In this regard, in the current study, measurements were made of the electrical conductivity of ionogels which differ in terms of the concentration of MCC.
The temperature dependence of the ionic conductivity of the IL in the initial and immobilized state in ionogels with different concentrations of the polymer in the composition is presented in Figure 9. All composites showed a monotonic increase in specific conductivity by three to four orders of magnitude with an increase in temperature from −30 to +80 °C.
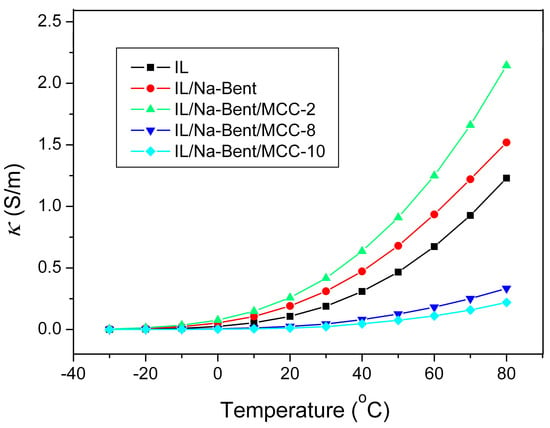
Figure 9.
The effect of temperature on the specific conductivity of ionogels.
The dependence of the electrical conductivity on the composition of the ionogel is complex. According to Figure 9, the triple BMImAc/Na-Bent/MCC−2 ionogel has the highest electrical conductivity. With a further increase in the MCC concentration, the κ value decreases. But there is no steady trend towards a decrease in electrical conductivity when the MCC concentration increases. We think that this is due to the presence of water in the ionogels which significantly affects the electrical conductivity. Obviously, the effect of water is minimal at −30 °C. Therefore, for this temperature, the dependence of the electrical conductivity on the composition of ionogels was plotted (Figure 10).

Figure 10.
Dependence of the specific electrical conductivity on the MCC concentration in ionogels at −30 °C.
Figure 10 shows that if the BMImAc/Na-Bent/MCC-2 is not taken into account, the κ value decreases quite smoothly with an increase in the concentration of MCC in the ionogel. But for BMImAc/Na-Bent/MCC-2, the electrical conductivity is much higher compared to other studied ionogels. However, it can be argued that this maximum is also observed for ionogels without Na-bentonite (Figure 11).

Figure 11.
Temperature dependences of the electrical conductivity of IL solutions depending on the amount of polymer in the composition.
Thus, the electrical conductivity of the studied ionogels (both with and without Na-bentonite) reaches its maximum value when the MCC concentration in the precursor (BMImAc/MCC) is 2%.
In an attempt to explain this maximum and based on Walden’s rule we made rheological measurements. However, it was found that the dynamic viscosity decreased monotonically with an increase in the concentration of MCC (Table 6). The data obtained do not allow explaining the presence of a maximum in electrical conductivity and indicate the need for further studies of the ionogels properties.

Table 6.
Dynamic viscosity of the synthesized iongels.
3.7. Prospects for Applications and Ecological Benefits of the Synthesized Iongels
In the presented article, we proposed an approach for the production of thickened electrolytes which is an alternative to many technologies for the production of cellulose-containing nanocomposites. The novelty of this approach is that we use an ionic liquid that dissolves cellulose very well. This makes it possible to synthesize solid ion-conducting composites that can be used as electrolytes in current sources and energy storage devices. At the operation end of such an electrolyte it is easily processed to obtain the initial components: upon interaction with water, cellulose and clay are released and the ionic liquid is purified from water by distillation. Thus, there is a closed environmentally friendly technology.
4. Conclusions
In this paper, IL/Na-Bent/MCC promising bionanocomposite ionogels with various cellulose concentrations were successfully synthesized and characterized by a combination of physicochemical methods. According to scanning electron microscopy, it was shown that the ionogel is an ionic liquid densely filled with clay particles. With an increase in the concentration of cellulose in the ionogel, there is formation of regions with various densities, which differ in density from the main solution. Based on the WAXS data, an increase in the interlayer space of Na-bentonite in ionogels was revealed due to the intercalation of IL molecules. For BMImAc/Na-Bent ionogels, the glass transition temperature was found to be higher than that of pure BMImAc, and this trend was observed for other similar IL/clay ionogels studied previously. TG analysis showed that the inclusion of MCC led to an increase in the heat resistance of the prepared IL/Na-Bent/MCC ionogels. The highest electrical conductivity of IL/Na-Bent/MCC triple ionogels was achieved at a cellulose concentration of 2%. However, the mechanism and reasons for the occurrence of this maximum are not clarified in the current study. Therefore, in the future, we plan to use other methods and approaches for these purposes. The synthesized and studied triple ionogels are of great practical interest for electrochemical devices, flexible electronics (Figure 1, last snippet), as CO2 absorbers, antibacterial coatings, etc.
Author Contributions
Conceptualization, O.A. and A.A.; Data curation, A.A., O.A. and A.N.; Formal analysis, O.A., A.N. and V.S.; Investigation, V.S.; Methodology, A.A.; Project Administration, A.A.; Writing—original draft, O.A., A.N. and V.S.; Writing—Review and Editing, O.A. and A.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed by the State Assignment (subject No. 122040500044-4).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The measurements were performed in the center for joint use of scientific equipment “The upper Volga region centre of physico-chemical research”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kanmani, P.; Aravind, J.; Kamaraj, M.; Sureshbabu, P.; Karthikeyan, S. Environmental applications of chitosan and cellulosic biopolymers: A comprehensive outlook. Bioresour. Technol. 2017, 242, 295–303. [Google Scholar] [CrossRef]
- Santra, D.; Sen, K. Ionic liquid-modified cellulosic hydrogels for loading and sustained release of selenourea: An ensuing inhibition of tyrosinas activity. Mater. Today Chem. 2020, 18, 100365. [Google Scholar] [CrossRef]
- Neves, R.M.; Ornaghi, H.L., Jr.; Alves, F.C.; Ademir, A.J.; Tom, M.; Mayookh, H.; Uthaman, A.; Thoma, S. Creep and stress relaxation behavior of functionalized microcrystalline cellulose/epoxy composites. Cellulose 2023, 30, 2197–2216. [Google Scholar] [CrossRef]
- Chaerunisaa, A.Y.; Sriwidodo, S.; Abdassah, M. Microcrystalline cellulose as pharmaceutical excipient. In Pharmaceutical Formulation Design—Recent Practices; Ahmad, U., Akhtar, U., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Trachea, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.R.N.; Taiwo, O.F.A.; Hassand, T.M.; Haafiz, M.K.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.; Jawaid, M.; Karim, Z.; Abdullah, L.C. Morphological, physiochemical and thermal properties of microcrystalline cellulose (MCC) extracted from bamboo Fiber. Molecules 2020, 25, 2824. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.P.; Weigel, P.; Purz, H.J.; Ganster, J. Structure formation of regenerated cellulose materials from nmmo-solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Shuai, J.; Wang, X. Novel solvents for cellulose. Bioresources 2021, 16, 2192–2195. [Google Scholar] [CrossRef]
- Pang, J.; Liu, X.; Zhang, X.; Wu, Y.; Sun, R. Fabrication of cellulose film with enhanced mechanical properties in ionic liquid 1-allyl-3-methylimidaxolium chloride (AmimCl). Materials 2013, 6, 1270–1284. [Google Scholar] [CrossRef]
- Chiappe, C.; Pieraccini, D. Ionic liquids: Solvent properties and organic reactivity. J. Phys. Org. Chem. 2005, 18, 275. [Google Scholar] [CrossRef]
- Galińnski, M.; Lewandowski, A.; Stępniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567. [Google Scholar] [CrossRef]
- Grishina, E.P.; Kudryakova, N.O.; Ramenskaya, L.M.; Fadeeva, Y.A. The effect of the nature of cation on transport properties of bis(trifluoromethylsulfonyl)imide ionic liquids. Russ. J. Electrochem. 2019, 55, 970–977. [Google Scholar] [CrossRef]
- Singh, M.P.; Singh, R.K.; Chandra, S. Ionic liquids confined in porous matrices: Physicochemical properties and applications. Prog. Mater. Sci. 2014, 64, 73–120. [Google Scholar] [CrossRef]
- Kunz, W.; Häckl, K. The hype with ionic liquids as solvents. Chem. Phys. Lett. 2016, 661, 6–12. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic liquids toxicity—Benefits and threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Gigante, V.; Bagherzadeh, R.; Mezzetta, A.; Milazzo, M.; Guazzelli, L.; Cinelli, P.; Lazzeri, A.; Danti, S. Cellulose based faber spinning processes using ionic liquids. Cellulose 2022, 29, 3079–3129. [Google Scholar] [CrossRef]
- Xu, A.; Guo, X.; Xu, R. Understanding the dissolution of cellulose in 1-butyl-3-methylimidazolium acetate+ DMAc solvent. Int. J. Biol. Macromol. 2015, 81, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Zhang, J.; Hao, S.; Duan, X.; Song, H.; Zhang, J. Novel chemically cross-linked chitosan-cellulose based ionogel with self-healability, high ionic conductivity, and high thermo-mechanical stability. Cellulose 2020, 27, 5121–5133. [Google Scholar] [CrossRef]
- Lu, X.; Xu, S.; Chen, J.; Ni, L.; Ma, X.; Cao, S.; Gao, H. Cellulose dissolution in ionic liquid from hydrogen bonding perspective: First principles calculations. Cellulose 2023, 30, 4181–4195. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Abdan, K.; Kaneko, T. A Concise review on the physicochemical properties of biopolymer blends prepared in ionic Liquids. Molecules 2021, 26, 216. [Google Scholar] [CrossRef] [PubMed]
- Betiha, M.A.; Mohamed, G.G.; Negm, N.A.; Hussein, M.F.; Ahmed, H.E. Fabrication of ionic liquid-cellulose-silica hydrogels with appropriate thermal stability and good salt tolerance as potential drilling fluid. Arab. J. Chem. 2020, 13, 6201–6220. [Google Scholar] [CrossRef]
- Soheilmoghaddam, M.; Wahit, M.U.; Whye, W.T.; Akos, N.I.; Pour, R.H.; Yussuf, A.A. Bionanocomposites of regenerated cellulose/zeolite prepared using environmentally benign ionic liquid solvent. Carbohydr. Polym. 2014, 106, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.K.; Wesley, O.N.; Koech, L.; Nelana, S.M.; Rutto, H.L. Structural features of cellulose and cellulose nanocrystals via in situ incorporation of magnetic iron oxide nanoparticles: Modification and characterization. Coatings 2023, 13, 39. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, H.J.; Kim, M.H.; Kim, J.S.; Kang, N.; Lee, J.Y.; Kim, K.T.; Lee, J.I.; Kim, D.D. Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. J. Pharm. Investig. 2016, 46, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhao, K.; Feng, Z.; Hou, Y.; Li, H.; Zhao, J.; Tiana, Y.; Song, H. High performance liquid crystalline bionanocomposite ionogels prepared by in situ crosslinking of cellulose/halloysite nanotubes/ionic liquid dispersions and its application in supercapacitors. Appl. Surf. Sci. 2018, 455, 599–607. [Google Scholar] [CrossRef]
- Alekseeva, O.V.; Shibaeva, V.D.; Noskov, A.V.; Ivanov, V.K.; Agafonov, A.V. Enhancing the thermal stability of ionogels: Synthesis and properties of triple ionic liquid/halloysite/MCC ionogels. Molecules 2021, 26, 6198. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Kuznetsova, S.A.; Levdansky, V.A.; Levdansky, A.V.; Vasil’eva, N.Y.; Chesnokov, N.V.; Ivanchenko, N.M.; Djakovitch, L.; Pinel, C. Optimized methods for obtaining cellulose and cellulose sulfates from birch wood. Wood Sci. Technol. 2015, 49, 825–843. [Google Scholar] [CrossRef]
- Ardizzone, S.; Dioguardi, F.S.; Mussini, T.; Mussini, P.R.; Rondinini, S.; Vercelli, B.; Vertova, A. Microcrystalline cellulose powders: Structure, surface features and water sorption capability. Cellulose 1999, 6, 57–69. [Google Scholar] [CrossRef]
- Fontana, J.P.; Camilo, F.F.; Bizeto, M.A.; Faez, R. Evaluation of the role of an ionic liquid as organophilization agent into montmorillonite for NBR rubber nanocomposite production. Appl. Clay Sci. 2013, 83–84, 203–209. [Google Scholar] [CrossRef]
- Agafonov, A.V.; Shibaeva, V.D.; Kraev, A.S.; Guseinov, S.S.; Ramenskaya, L.M.; Kudryakova, N.O.; Grishina, E.P. Effect of synthesis conditions on the properties of an ionic liquid in the 1-butyl-3-methylimidazolium acetate-Na-bentonite ionogel. Steric stabilization and confinement. J. Mol. Liq. 2020, 315, 113703. [Google Scholar] [CrossRef]
- Marekha, B.A.; Bria, M.; Moreau, M.; Waele, I.D.; Miannay, F.-A.; Smortsova, Y.; Takamuku, T.; Kalugin, O.N.; Kiselev, M.; Idrissi, A. Inter molecular interactions in mixtures of 1-n-butyl-3-methylimidazolium acetate and water: Insights from IR, Raman, NMR spectroscopy and quantum chemistry calculations. J. Mol. Liq. 2015, 210, 227–237. [Google Scholar] [CrossRef]
- Das, K.; Ray, D.; Bandyopadhyay, N.R.; Sengupta, S. Study of the properties of microcrystalline cellulose particles from different renewable resources by XRD, FTIR, Nanoindentation, TGA and SEM. J. Polym. Environ. 2010, 18, 355–363. [Google Scholar] [CrossRef]
- Mahmoudian, S.; Wahit, M.U.; Ismail, A.F.; Balakrishnan, H.; Imran, M. Bionanocomposite fibers based on cellulose and montmorillonite using ionic liquid 1-ethyl-3-methylimidazolium acetate. J. Mater. Sci. 2015, 50, 1228–1236. [Google Scholar] [CrossRef]
- Zielinska, D.; Skrzypczak, A.; Peplinska, B.; Borysiak, S. Nanocellulose-based polymer composites functionalized with new gemini ionic liquids. Int. J. Mol. Sci. 2022, 23, 15807. [Google Scholar] [CrossRef] [PubMed]
- Noskov, A.V.; Alekseeva, O.V.; Shibaeva, V.D.; Agafonov, A.V. Synthesis, structure and thermal properties of montmorillonite/ionic liquid ionogels. RSC Adv. 2020, 10, 34885–34894. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, O.V.; Noskov, A.V.; Grishina, E.P.; Ramenskaya, L.M.; Kudryakova, N.O.; Ivanov, V.K.; Agafonov, A.V. Structure and thermal properties of the montmorillonite/ionic liquid composites. Materials 2019, 12, 2578. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).