Piezo-Enhanced Photocatalytic Performance of Bismuth Ferrite-Based Thin Film for Organic Pollutants Degradation
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Thin Films Characterization
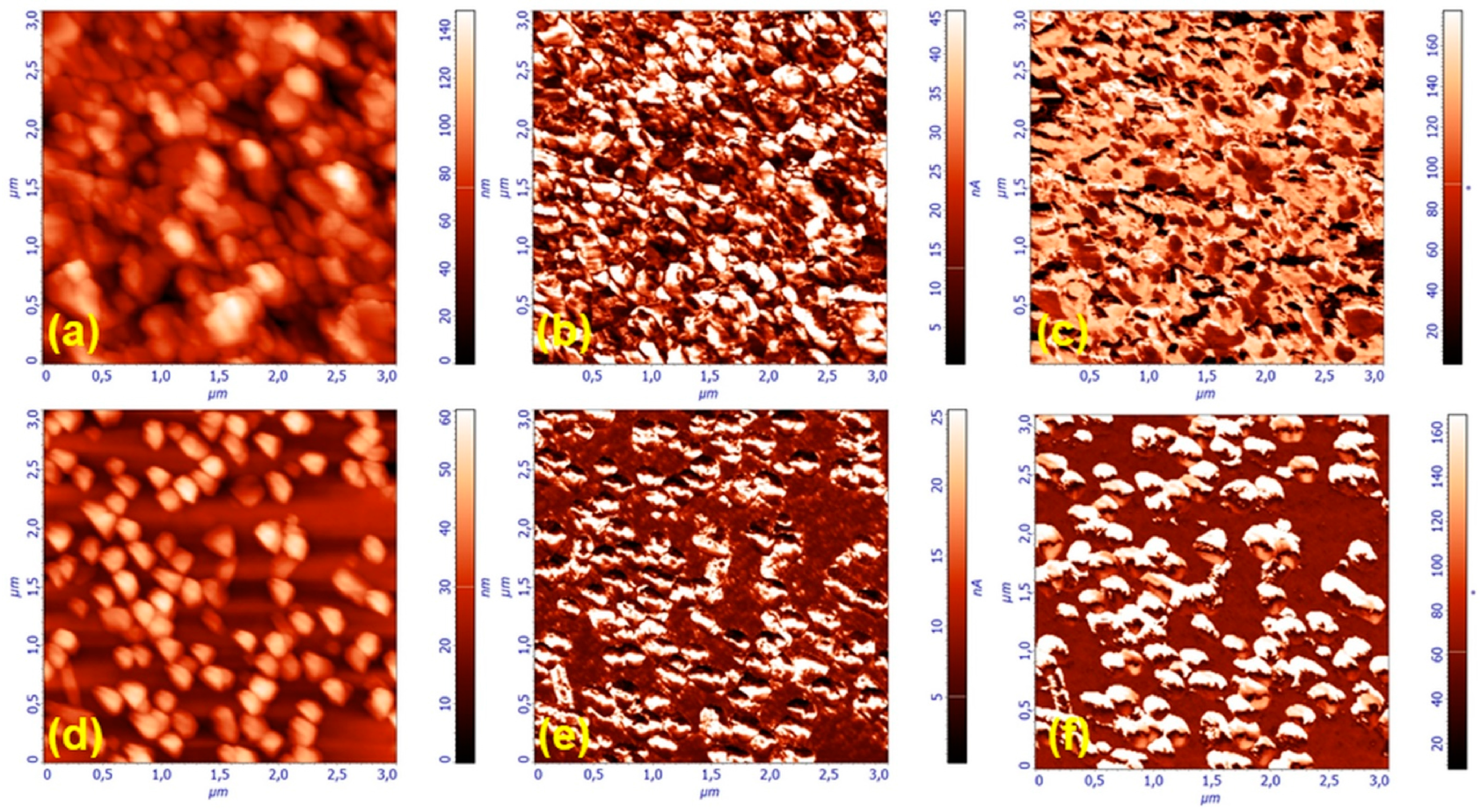
3.1.1. Thin Films Microstructure
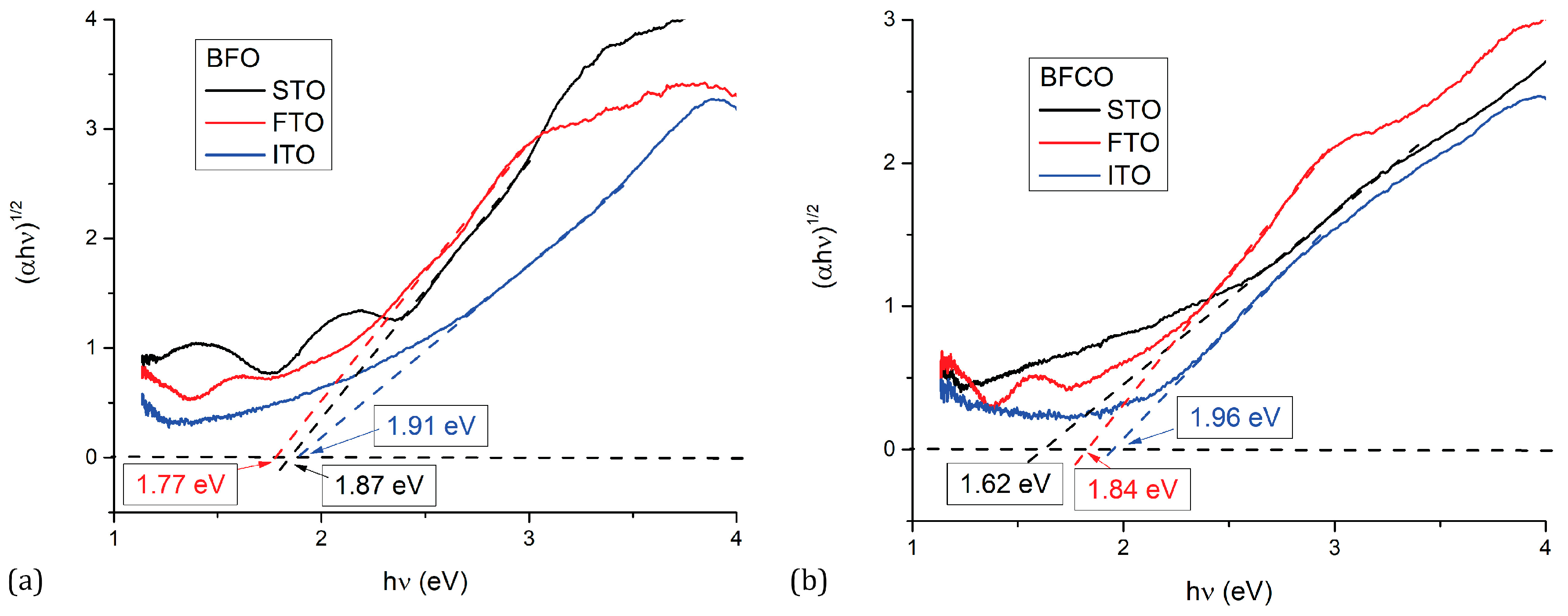
3.1.2. Optical Properties
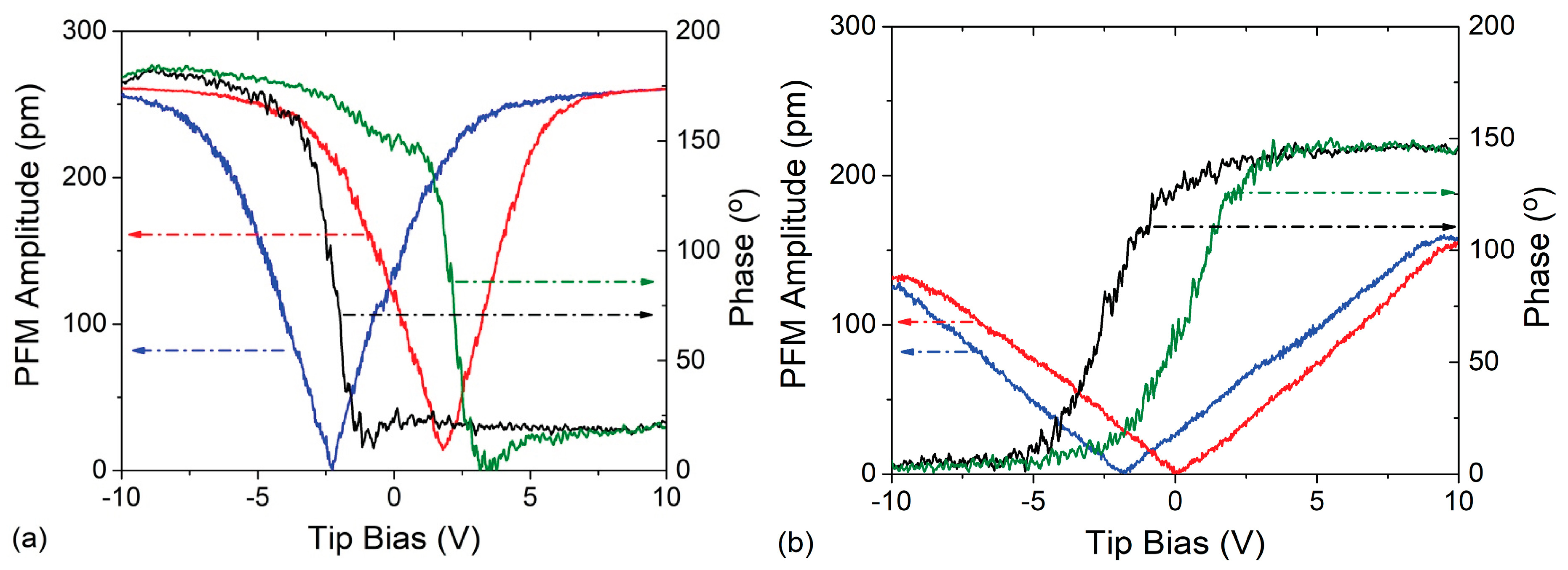
3.1.3. Piezo-Ferroelectric Properties
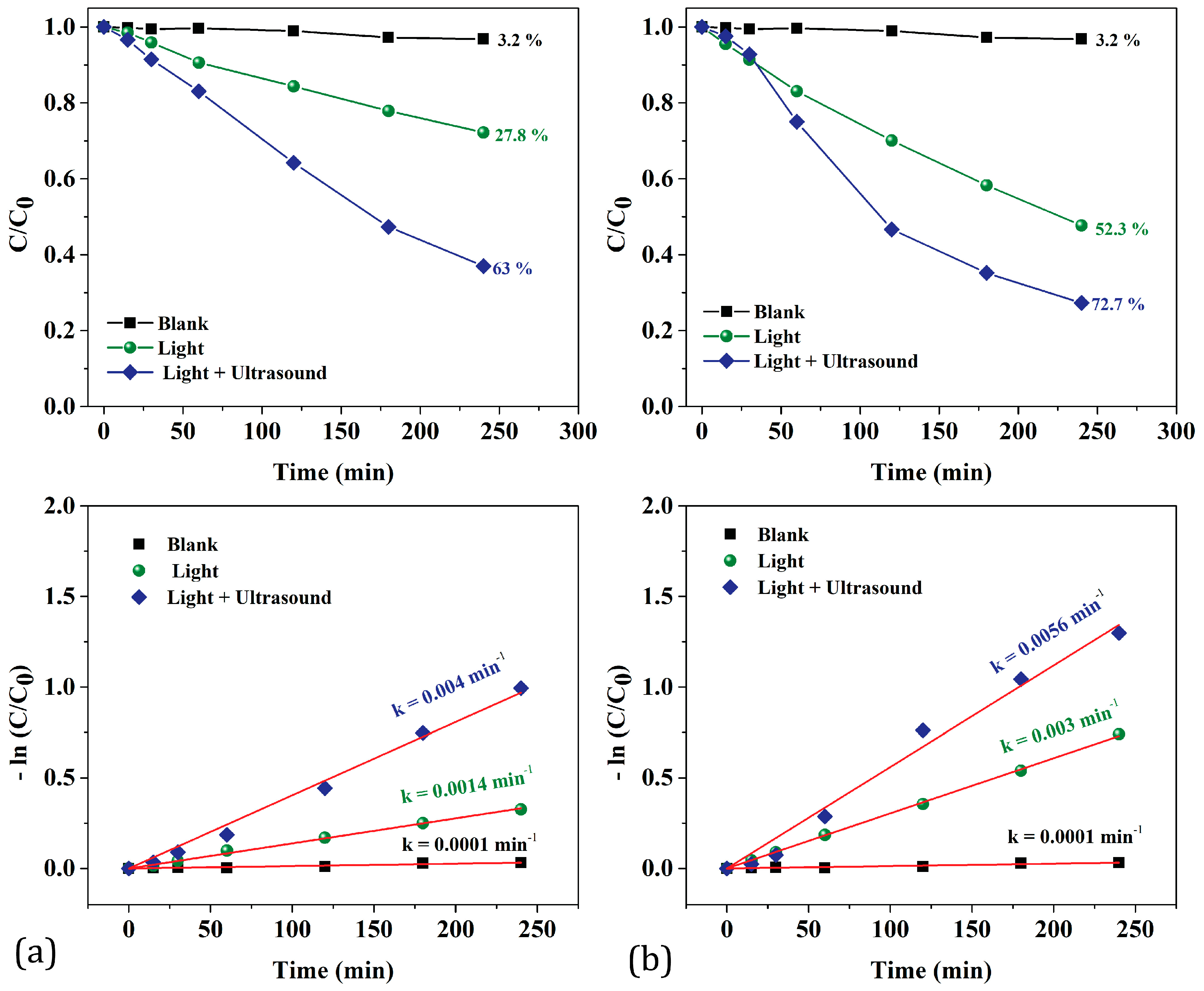
3.2. Piezo-Photodegradation Efficiency Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nechache, R.; Harnagea, C.; Li, S.; Cardenas, L.; Huang, W.; Chakrabartty, J.; Rosei, F. Bandgap Tuning of Multiferroic Oxide Solar Cells. Nat. Photonics 2014, 61, 61–67. [Google Scholar] [CrossRef]
- Alexe, M.; Hesse, D. Tip-Enhanced Photovoltaic Effects in Bismuth Ferrite. Nat. Commun. 2011, 2, 256. [Google Scholar] [CrossRef]
- Qin, M.; Yao, K.; Liang, Y.C. High Efficient Photovoltaics in Nanoscaled Ferroelectric Thin Films. Appl. Phys. Lett. 2008, 93, 122904. [Google Scholar] [CrossRef]
- Kreisel, J.; Alexe, M.; Thomas, P.A. A Photoferroelectric Material Is More than the Sum of Its Parts. Nat. Mater. 2012, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Seidel, J.; Byrnes, S.J.; Shafer, P.; Yang, C.H.; Rossell, M.D.; Yu, P.; Chu, Y.H.; Scott, J.F.; Ager, J.W.; et al. Above-Bandgap Voltages from Ferroelectric Photovoltaic Devices. Nat. Nanotechnol. 2010, 5, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Seidel, J.; Fu, D.; Yang, S.Y.; Alarcón-Lladó, E.; Wu, J.; Ramesh, R.; Ager, J.W. Efficient Photovoltaic Current Generation at Ferroelectric Domain Walls. Phys. Rev. Lett. 2011, 107, 126805. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Roy Chaudhuri, A.; Heon Kim, Y.; Hesse, D.; Alexe, M. Role of Domain Walls in the Abnormal Photovoltaic Effect in BiFeO3. Nat. Commun. 2013, 4, 2835. [Google Scholar] [CrossRef]
- Ma, S.; Sha, Z.; Xia, F.; Jia, R.; Tian, J.; Fu, Y.; Yu, L.; Dong, L. Boosting the Photoresponse Speed of Visible-Light-Active Bismuth Ferrite Thin Films Based on Fe-Site Substitution Strategy and Favorable Heterostructure Design. Appl. Surf. Sci. 2022, 590, 153054. [Google Scholar] [CrossRef]
- Man, S.; Leng, X.; Bai, J.; Kan, S.; Cui, Y.; Wang, J.; Xu, L. Enhancement of Photoelectrochemical Performance of BiFeO3 by Sm3+ Doping. Ceram. Int. 2023, 49, 10255–10264. [Google Scholar] [CrossRef]
- Anand, P.; Jaihindh, D.P.; Chang, W.-K.; Fu, Y.-P. Tailoring the Ca-doped bismuth ferrite for electrochemical oxygen evolution reaction and photocatalytic activity. Appl. Surf. Sci. 2021, 540, 148387. [Google Scholar] [CrossRef]
- Meng, D.; Xiao, Y.; He, H.; Liao, Y.; Zhang, H.; Zhai, J.; Chen, Z.; Martin, L.W.; Bai, F. Enhanced Spontaneous Polarization in Double Perovskite Bi2FeCrO6 Films. J. Am. Ceram. Soc. 2019, 102, 5234–5242. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Kokovkin, V.V.; Volchek, V.V.; Zhivulin, V.E.; Abramov, P.A.; Cherkasova, N.A.; Sun, Z.; Sayyed, M.I.; Tishkevich, D.I.; Trukhanov, A.V. Electrocatalytic Activity of Various Hexagonal Ferrites in OER Process. Mater. Chem. Phys. 2021, 270, 124818. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Turchenko, V.A.; Kostishin, V.G.; Damay, F.; Porcher, F.; Lupu, N.; Bozzo, B.; Fina, I.; Polosan, S.; Silibin, M.V.; et al. The Origin of the Dual Ferroic Properties in Quasi-Centrosymmetrical SrFe12−xInxO19 Hexaferrites. J. Alloys Compd. 2021, 886, 161249. [Google Scholar] [CrossRef]
- Nur-E-Alam, M.; Vasiliev, M.; Belotelov, V.; Alameh, K. Properties of Ferrite Garnet (Bi, Lu, Y)3(Fe, Ga)5O12 Thin Film Materials Prepared by RF Magnetron Sputtering. Nanomaterials 2018, 8, 355. [Google Scholar] [CrossRef]
- Li, H.; Li, F.; Shen, Z.; Han, S.-T.; Chen, J.; Dong, C.; Chen, C.; Zhou, Y.; Wang, M. Photoferroelectric Perovskite Solar Cells: Principles, Advances and Insights. Nano Today 2021, 37, 101062. [Google Scholar] [CrossRef]
- Sarakinos, K.; Alami, J.; Konstantinidis, S. High Power Pulsed Magnetron Sputtering: A Review on Scientific and Engineering State of the Art. Surf. Coat. Technol. 2010, 204, 1661–1684. [Google Scholar] [CrossRef]
- Tiron, V.; Velicu, I.; Matei, T.; Cristea, D.; Cunha, L.; Stoian, G. Ultra-Short Pulse HiPIMS: A Strategy to Suppress Arcing during Reactive Deposition of SiO2 Thin Films with Enhanced Mechanical and Optical Properties. Coatings 2020, 10, 633. [Google Scholar] [CrossRef]
- Benetti, D.; Nouar, R.; Nechache, R.; Pepin, H.; Sarkissian, A.; Rosei, F.; MacLeod, J.M. Combined Magnetron Sputtering and Pulsed Laser Deposition of TiO2 and BFCO Thin Films. Sci. Rep. 2017, 7, 2503. [Google Scholar] [CrossRef]
- Dong, B.W.; Miao, J.; Han, J.Z.; Shao, F.; Yuan, J.; Meng, K.K.; Wu, Y.; Xu, X.G.; Jiang, Y. Applied Surface Science High Resistance Ratio of Bipolar Resistive Switching in a Multiferroic/High-K Bi(Fe0.95Cr0.05)O3/ZrO2/Pt Heterostructure. Appl. Surf. Sci. 2018, 434, 687–692. [Google Scholar] [CrossRef]
- Ma, S.; Xia, F.; Jia, R.; Sha, Z.; Tian, J.; Yu, L.; Dong, L. Discovery of a Novel Visible-Light-Active Photodetector Based on Bismuth Ferrite: Constructing and Optimizing the Cr-Doped-BiFeO3/NiO Thin Fi Lm Heterostructure. Mater. Today Chem. 2023, 27, 101309. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.W.; Kim, W.; Kim, M.H.; Song, T.K.; Kim, S.S. Electrical Properties of (Bi0.9Ho0.1)(Fe0.975Cr0.025)O3 Thin Films Prepared by Using a Chemical Solution Deposition. Integr. Ferroelectr. 2012, 140, 37–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, S.; Cheng, J. The Study of BiCrxFe1−xO3 Thin Films Synthesized by Sol–Gel Technique. J. Eur. Ceram. Soc. 2010, 30, 271–275. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.; Shi, T.; Shao, X. Nd-Cr Co-Doped BiFeO3 Thin Films for Photovoltaic Devices with Enhanced Photovoltaic Performance. Thin Solid Films 2020, 698, 137852. [Google Scholar] [CrossRef]
- Qi, X.; Tsai, P.C.; Chen, Y.C.; Lin, Q.R.; Huang, J.C.A.; Chang, W.C.; Chen, I.G. Optimal Growth Windows of Multiferroic BiFeO3 Films and Characteristics of Ferroelectric Domain Structures. Thin Solid Films 2009, 517, 5862–5866. [Google Scholar] [CrossRef]
- Kartavtseva, M.S.; Gorbenko, O.Y.; Kaul, A.R.; Murzina, T.V.; Savinov, S.A.; Barthélémy, A. BiFeO3 Thin Films Prepared Using Metalorganic Chemical Vapor Deposition. Thin Solid Films 2007, 515, 6416–6421. [Google Scholar] [CrossRef]
- Khare, A.; Singh, A.; Prabhu, S.S.; Rana, D.S. Controlling Magnetism of Multiferroic (Bi0.9La0.1)2FeCrO6 Thin Films by Epitaxial and Crystallographic Orientation Strain. Appl. Phys. Lett. 2013, 102, 192911. [Google Scholar] [CrossRef]
- Irfan, S.; Rizwan, S.; Shen, Y.; Li, L.; Asfandiyar; Butt, S.; Nan, C.W. The Gadolinium (Gd3+) and Tin (Sn4+) Co-Doped BiFeO3 Nanoparticles as New Solar Light Active Photocatalyst. Sci. Rep. 2017, 7, srep42493. [Google Scholar] [CrossRef]
- Sportelli, G.; Boselli, T.; Protti, S.; Serpone, N.; Ravelli, D. Photovoltaic Materials as Heterogeneous Photocatalysts: A Golden Opportunity for Sustainable Organic Syntheses. Sol. RRL 2023, 7, 2201008. [Google Scholar] [CrossRef]
- Gao, F.; Chen, X.; Yin, K.; Dong, S.; Ren, Z.; Yuan, F.; Yu, T.; Zou, Z.; Liu, J.-M. Visible-Light Photocatalytic Properties of Weak Magnetic BiFeO3 Nanoparticles. Adv. Mater. 2007, 19, 2889–2892. [Google Scholar] [CrossRef]
- Fatima, S.; Ali, S.I.; Iqbal, M.Z.; Rizwan, S. The High Photocatalytic Activity and Reduced Band Gap Energy of La and Mn Co-Doped BiFeO3/Graphene Nanoplatelet (GNP) Nanohybrids. RSC Adv. 2017, 7, 35928–35937. [Google Scholar] [CrossRef]
- Sharmin, F.; Basith, M.A. Highly Efficient Photocatalytic Degradation of Hazardous Industrial and Pharmaceutical Pollutants Using Gadolinium Doped BiFeO3 Nanoparticles. J. Alloys Compd. 2022, 901, 163604. [Google Scholar] [CrossRef]
- Mabuti, L.A.; Manding, I.K.S.; Mercado, C.C. Photovoltaic and Photocatalytic Properties of Bismuth Oxyiodide-Graphene Nanocomposites. RSC Adv. 2018, 8, 42254–42261. [Google Scholar] [CrossRef]
- Su, C.; Li, C.; Li, R.; Wang, W. Insights into Highly Efficient Piezocatalytic Molecule Oxygen Activation over Bi2Fe4O9: Active Sites and Mechanism. Chem. Eng. J. 2022, 452, 139300. [Google Scholar] [CrossRef]
- Su, C.; Li, R.; Li, C.; Wang, W. Piezo-Promoted Regeneration of Fe2+ Boosts Peroxydisulfate Activation by Bi2Fe4O9 Nanosheets. Appl. Catal. B Environ. 2022, 310, 121330. [Google Scholar] [CrossRef]
- Chang, S.C.; Chen, H.Y.; Chen, P.H.; Lee, J.T.; Wu, J.M. Piezo-Photocatalysts Based on a Ferroelectric High-Entropy Oxide. Appl. Catal. B Environ. 2023, 324, 122204. [Google Scholar] [CrossRef]
- Xu, S.; Qian, W.; Zhang, D.; Zhao, X.; Zhang, X.; Li, C.; Bowen, C.R.; Yang, Y. A Coupled Photo-Piezo-Catalytic Effect in a BST-PDMS Porous Foam for Enhanced Dye Wastewater Degradation. Nano Energy 2020, 77, 105305. [Google Scholar] [CrossRef]
- Mushtaq, F.; Chen, X.; Hoop, M.; Torlakcik, H.; Pellicer, E.; Sort, J.; Gattinoni, C.; Nelson, B.J.; Pané, S. Piezoelectrically Enhanced Photocatalysis with BiFeO3 Nanostructures for Efficient Water Remediation. iScience 2018, 4, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Ye, C.; Wang, K.; Zhao, C.; Wu, Y.; He, Y. Photodeposition of CoOx Nanoparticles on BiFeO3 Nanodisk for Efficiently Piezocatalytic Degradation of Rhodamine B by Utilizing Ultrasonic Vibration Energy. Ultrason. Sonochem. 2021, 80, 105813. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zheng, D.; Jin, C.; Zheng, W.; Bai, H. High-Performance Photovoltaic Readable Ferroelectric Nonvolatile Memory Based on La-Doped BiFeO3 Films. ACS Appl. Mater. Interfaces 2018, 10, 19836–19843. [Google Scholar] [CrossRef]
- Gruverman, A.; Alexe, M.; Meier, D. Piezoresponse Force Microscopy and Nanoferroic Phenomena. Nat. Commun. 2019, 10, 1661. [Google Scholar] [CrossRef]
- Puli, V.S.; Pradhan, D.K.; Katiyar, R.K.; Coondoo, I.; Panwar, N.; Misra, P.; Chrisey, D.B.; Scott, J.F.; Katiyar, R.S. Photovoltaic Effect in Transition Metal Modified Polycrystalline BiFeO3 Thin Films. J. Phys. D Appl. Phys. 2014, 47, 075502. [Google Scholar] [CrossRef]
- Paillard, C.; Bai, X.; Infante, I.C.; Guennou, M.; Geneste, G.; Alexe, M.; Kreisel, J.; Dkhil, B. Photovoltaics with Ferroelectrics: Current Status and Beyond. Adv. Mater. 2016, 28, 5153–5168. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Sharma, Y.; Hong, S.; Katiyar, R.S. Modulation of Oxygen Vacancies Assisted Ferroelectric and Photovoltaic Properties of (Nd, V) Co-Doped BiFeO3 Thin Films. J. Phys. D Appl. Phys. 2018, 51, 275303. [Google Scholar] [CrossRef]
- Racles, C.; Ursu, C.; Dascalu, M.; Asandulesa, M.; Tiron, V.; Bele, A.; Tugui, C.; Teodoro, S. Multi-Stimuli Responsive Free-Standing Fi Lms of DR1- Grafted Silicones. Chem. Eng. J. 2020, 401, 126087. [Google Scholar] [CrossRef]
- Tiron, V.; Jijie, R.; Dumitru, I.; Cimpoesu, N.; Burducea, I.; Iancu, D.; Borhan, A.; Gurlui, S.; Bulai, G. Piezo-Ferroelectric Response of Bismuth Ferrite Based Thin Films and Their Related Photo/Piezocatalytic Performance. Ceram. Int. 2023, 49, 20304–20314. [Google Scholar] [CrossRef]
- Tiron, V.; Ciolan, M.A.; Bulai, G.; Mihalache, G.; Lipsa, F.D.; Jijie, R. Efficient Removal of Methylene Blue and Ciprofloxacin from Aqueous Solution Using Flower-like, Nanostructured ZnO Coating under UV Irradiation. Nanomaterials 2022, 12, 2193. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of Lattice Strain in ZnO Nanoparticles: X-Ray Peak Profile Analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Cai, M.Q.; Huang, H.; Chan, H.L.W. First-Principles Study on the Electronic and Optical Properties of BiFeO3. Solid State Commun. 2009, 149, 641–644. [Google Scholar] [CrossRef]
- Neaton, J.B.; Ederer, C.; Waghmare, U.V.; Spaldin, N.A.; Rabe, K.M. First-Principles Study of Spontaneous Polarization in Multiferroic BiFeO3. Phys. Rev. B-Condens. Matter Mater. Phys. 2005, 71, 014113. [Google Scholar] [CrossRef]
- Clark, S.J.; Robertson, J. Band Gap and Schottky Barrier Heights of Multiferroic BiFeO3. Appl. Phys. Lett. 2007, 90, 132903. [Google Scholar] [CrossRef]
- McDonnell, K.; Wadnerkar, N.; English, N.J.; Rahman, M.; Dowling, D. Photo-Active and Optical Properties of Bismuth Ferrite (BiFeO3): An Experimental and Theoretical Study. Chem. Phys. Lett. 2013, 572, 78–84. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.L.; Díaz, D.; Zumeta-Dubé, I.; Arriola-Santamaría, H.; Betancourt, I.; Santiago-Jacinto, P.; Nava-Etzana, N. Easy Synthesis of High-Purity BiFeO3 Nanoparticles: New Insights Derived from the Structural, Optical, and Magnetic Characterization. Inorg. Chem. 2013, 52, 10306–10317. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Yeo, D.; Chang, H.; Loh, L.; Dunn, S. Perovskite BiFeO3 Thin Film Photocathode Performance with Visible Light Activity. Nanotechnology 2016, 27, 345402. [Google Scholar] [CrossRef] [PubMed]
- Himcinschi, C.; Vrejoiu, I.; Friedrich, M.; Nikulina, E.; Ding, L.; Cobet, C.; Esser, N.; Alexe, M.; Rafaja, D.; Zahn, D.R.T. Substrate Influence on the Optical and Structural Properties of Pulsed Laser Deposited BiFeO3 Epitaxial Films. J. Appl. Phys. 2010, 107, 123524. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A.; Pradhan, S. Phytostimulatory Effect of Silver Nanoparticles (AgNPs) on Rice Seedling Growth: An Insight from Antioxidative Enzyme Activities and Gene Expression Patterns. Ecotoxicol. Environ. Saf. 2018, 161, 624–633. [Google Scholar] [CrossRef]
- Liang, Z.; Yan, C.F.; Rtimi, S.; Bandara, J. Piezoelectric Materials for Catalytic/Photocatalytic Removal of Pollutants: Recent Advances and Outlook. Appl. Catal. B Environ. 2019, 241, 256–269. [Google Scholar] [CrossRef]
- Mane, P.V.; Shinde, N.B.; Mulla, I.M.; Koli, R.R.; Shelke, A.R.; Karanjkar, M.M.; Gosavi, S.R.; Deshpande, N.G. Bismuth Ferrite Thin Film as an Efficient Electrode for Photocatalytic Degradation of Methylene Blue Dye. Mater. Res. Express 2018, 6, 026426. [Google Scholar] [CrossRef]
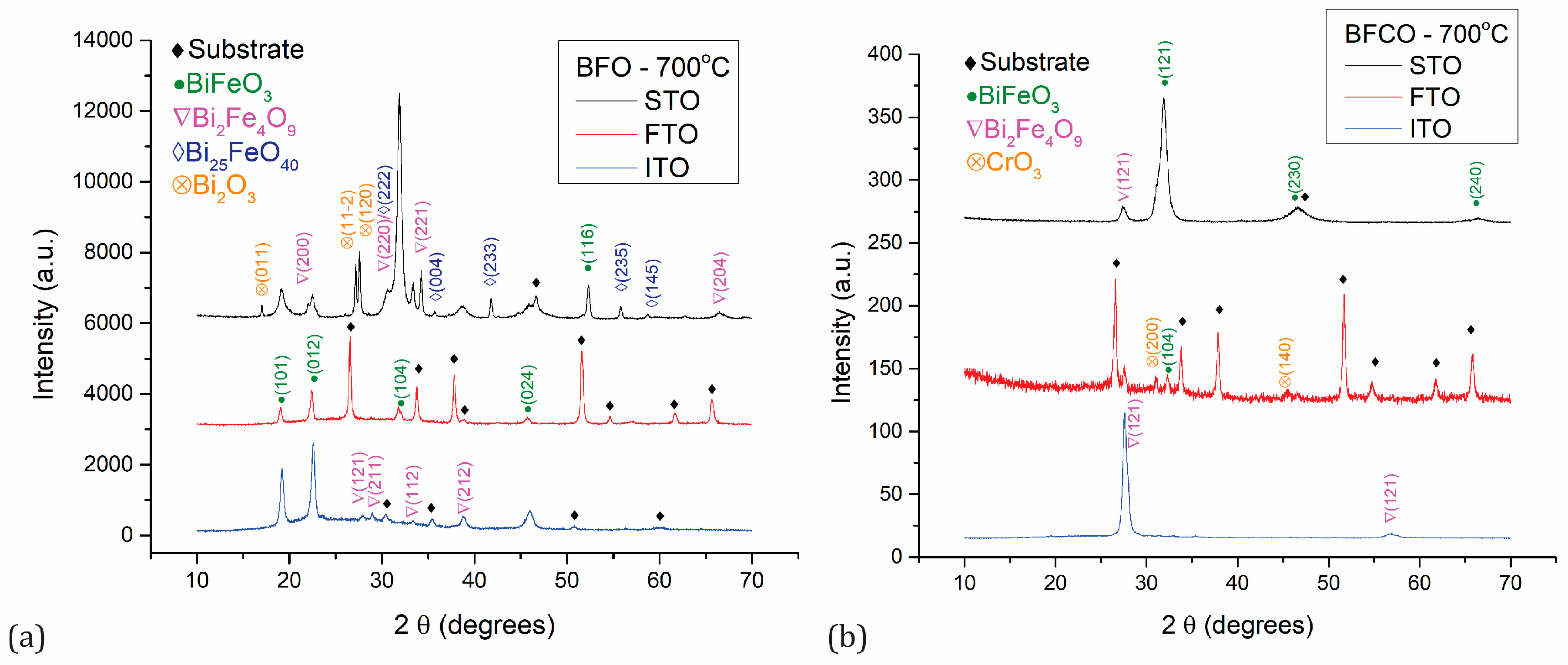
| Sample | Phase | Crystallite Size (±1 nm) | Strain (±0.02%) |
|---|---|---|---|
| BFO/STO | BiFeO3 | 14 | 1.3 |
| Bi25FeO40 | 36 | 0.24 | |
| Bi2O3 | 50 | 0.34 | |
| BFO/FTO | BiFeO3 | 22 | 0.78 |
| BFO/ITO | BiFeO3 | 17 | 0.93 |
| Bi2Fe4O9 | 22 | 0.61 | |
| BFCO/STO | BiFeO3 | 6 | 1.5 |
| Bi2Fe4O9 | 12 | 1.2 | |
| BFCO/FTO | BiFeO3 | 24 | 0.54 |
| Bi2Fe4O9 | 21 | 0.7 | |
| CrO3 | 31 | 0.43 | |
| BFCO/ITO | Bi2Fe4O9 | 10 | 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiron, V.; Jijie, R.; Matei, T.; Velicu, I.-L.; Gurlui, S.; Bulai, G. Piezo-Enhanced Photocatalytic Performance of Bismuth Ferrite-Based Thin Film for Organic Pollutants Degradation. Coatings 2023, 13, 1416. https://doi.org/10.3390/coatings13081416
Tiron V, Jijie R, Matei T, Velicu I-L, Gurlui S, Bulai G. Piezo-Enhanced Photocatalytic Performance of Bismuth Ferrite-Based Thin Film for Organic Pollutants Degradation. Coatings. 2023; 13(8):1416. https://doi.org/10.3390/coatings13081416
Chicago/Turabian StyleTiron, Vasile, Roxana Jijie, Teodora Matei, Ioana-Laura Velicu, Silviu Gurlui, and Georgiana Bulai. 2023. "Piezo-Enhanced Photocatalytic Performance of Bismuth Ferrite-Based Thin Film for Organic Pollutants Degradation" Coatings 13, no. 8: 1416. https://doi.org/10.3390/coatings13081416
APA StyleTiron, V., Jijie, R., Matei, T., Velicu, I.-L., Gurlui, S., & Bulai, G. (2023). Piezo-Enhanced Photocatalytic Performance of Bismuth Ferrite-Based Thin Film for Organic Pollutants Degradation. Coatings, 13(8), 1416. https://doi.org/10.3390/coatings13081416











