Physiochemical Analysis of Manilkara zapota (Sapota) Coated with Aloe Vera Gel and Enriched with Ajwain and Oregano Essential Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Collection
2.2. Preparation of Aloe Vera Gel and Treatment Groups
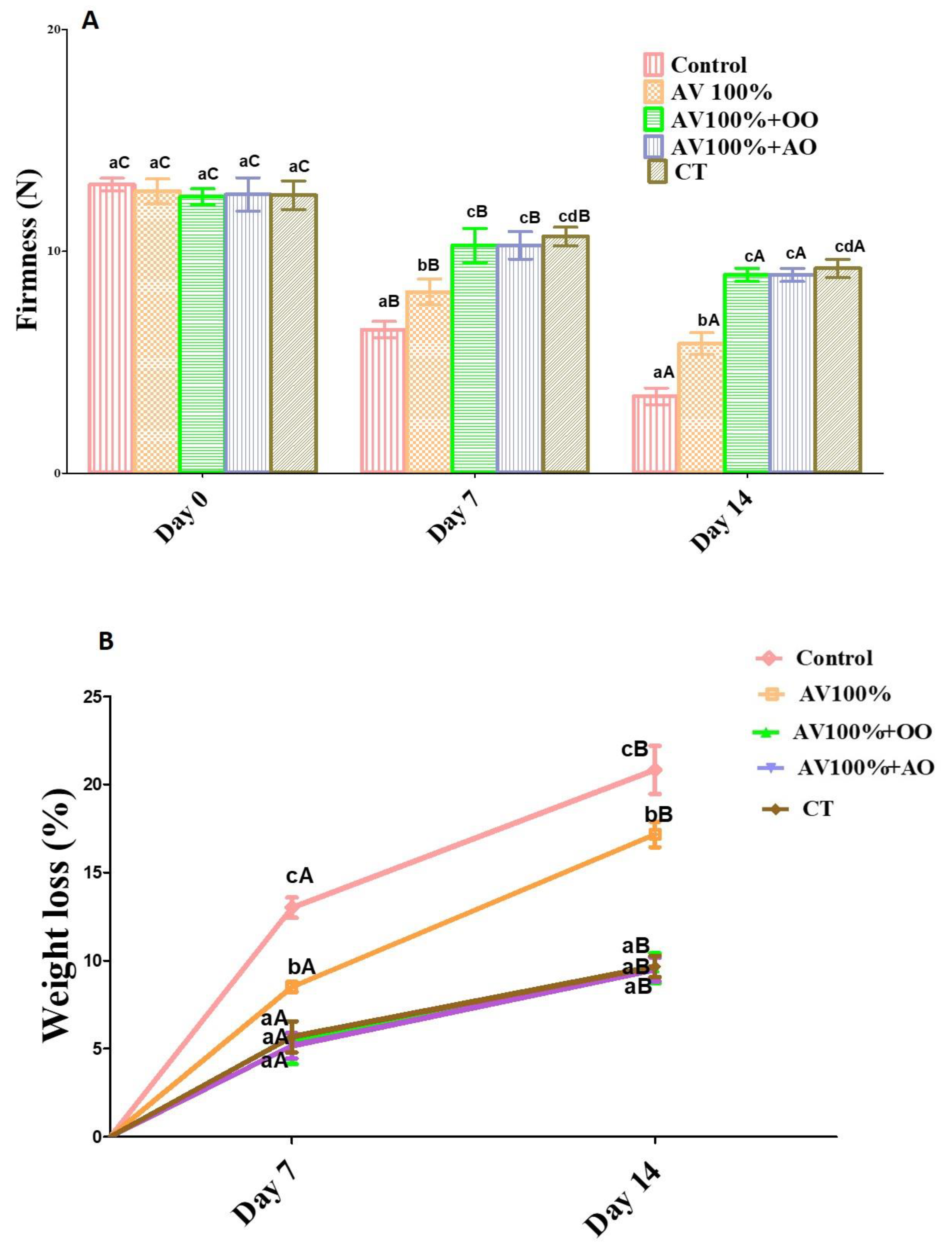
2.3. Weight Loss
2.4. Firmness of Fruit
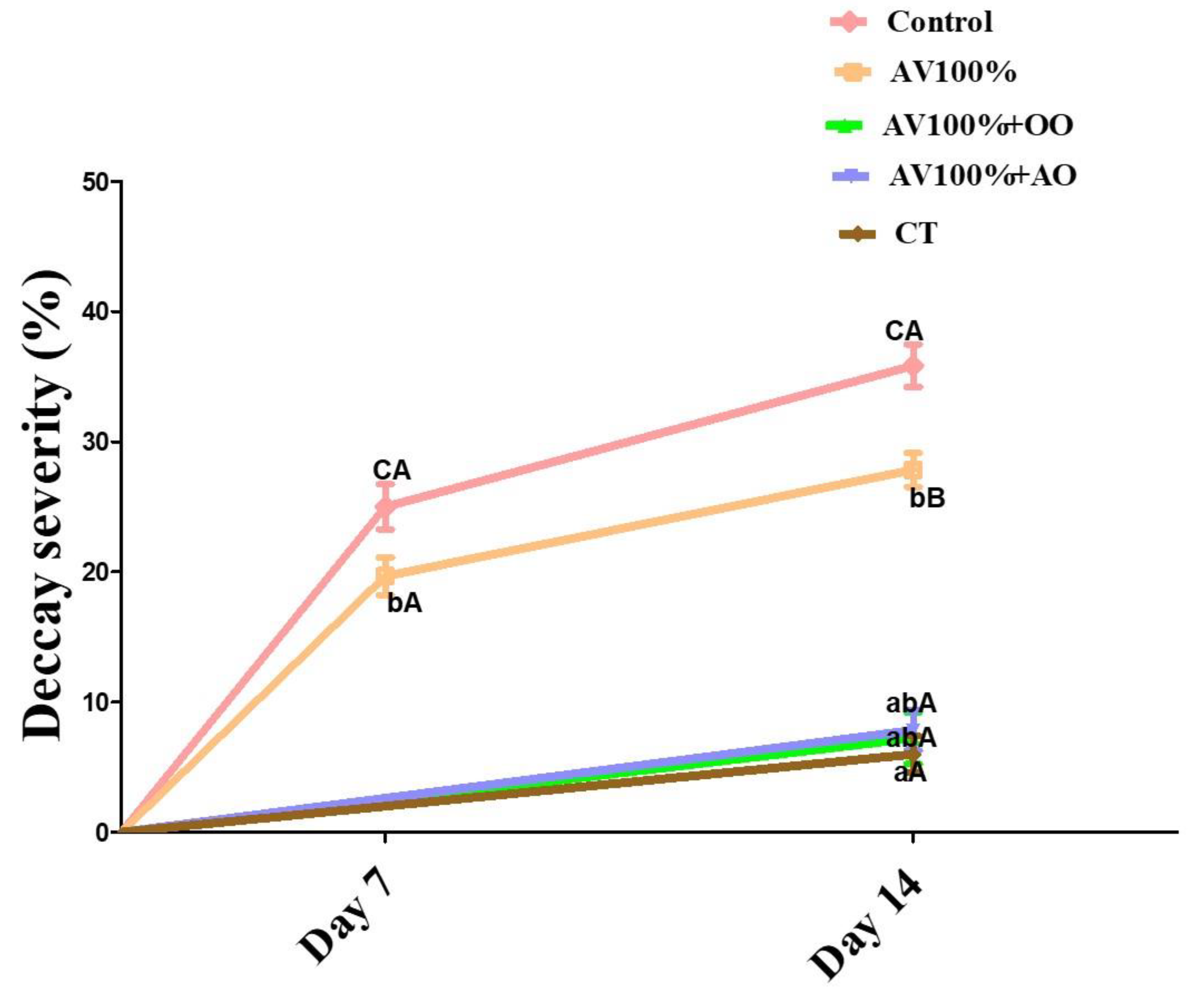
2.5. Decay Incidence
2.6. Total Soluble Solids (TSS)
2.7. Titratable Acidity (TA)
2.8. Ascorbic Acid
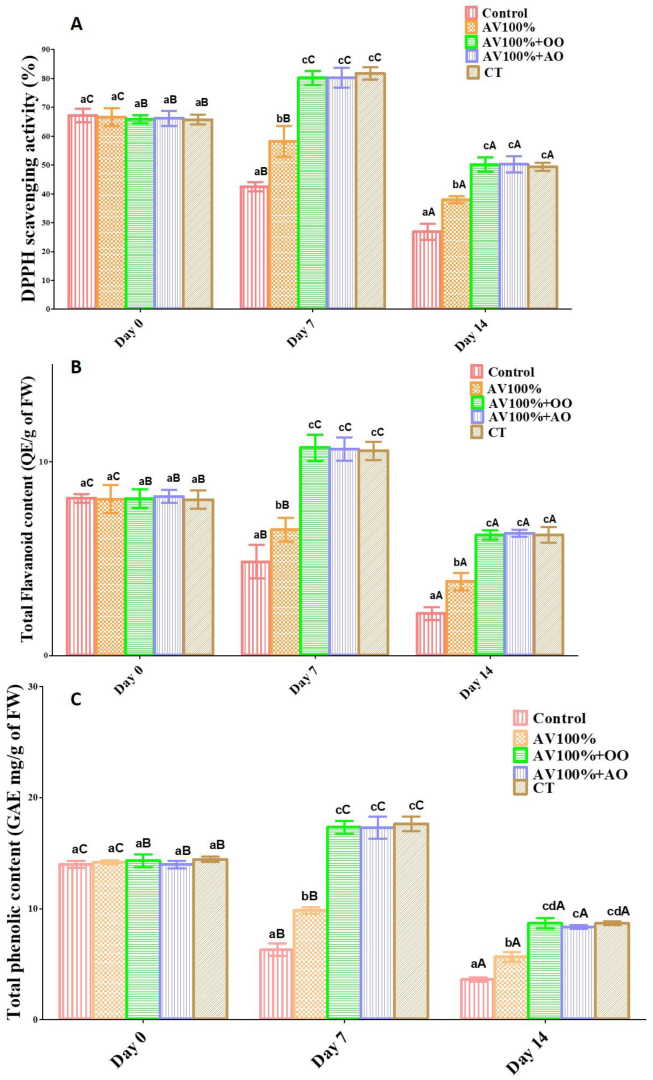
2.9. Total Phenolic Content
2.10. Antioxidant Activity
2.11. Total Flavonoid Content
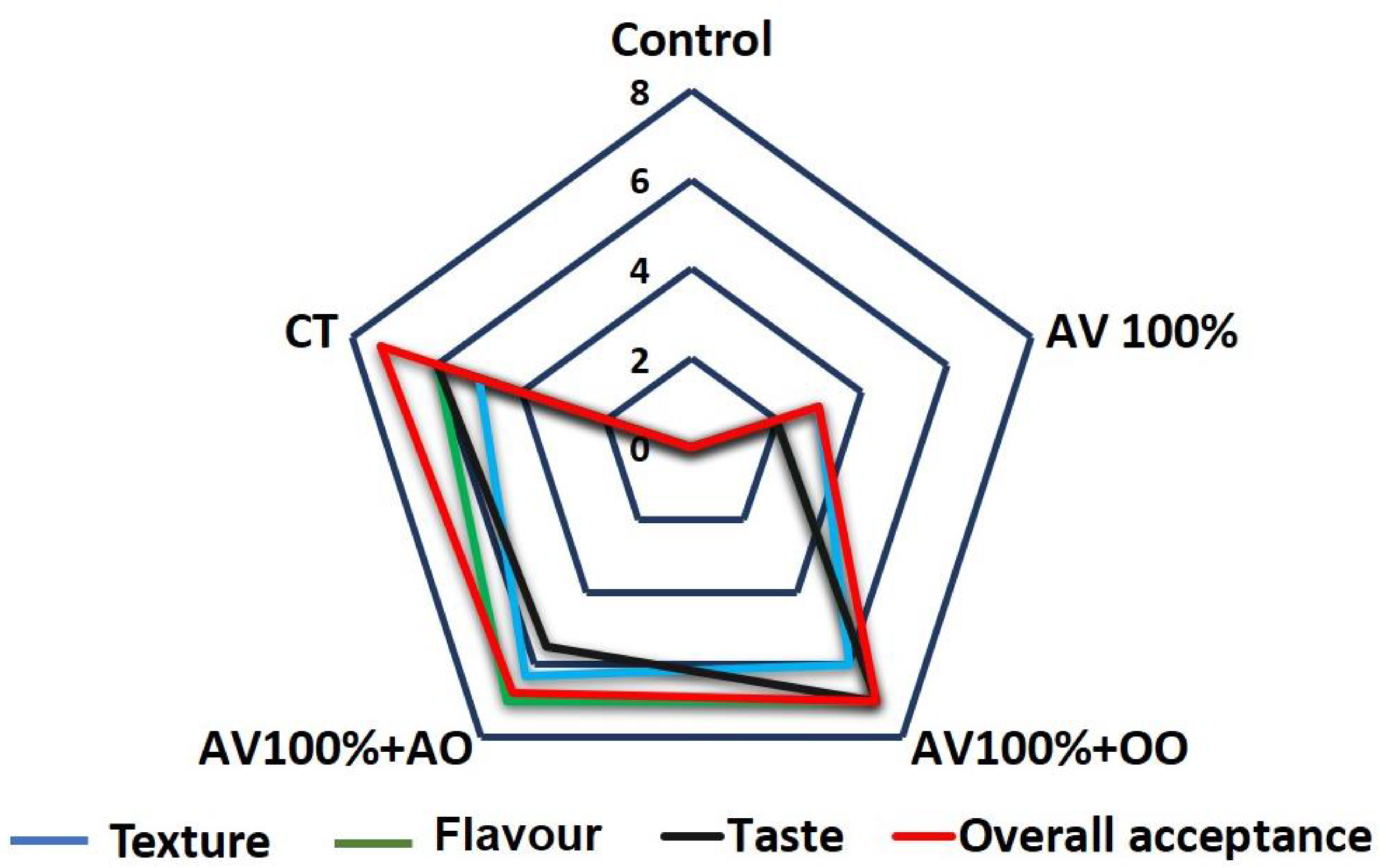
2.12. Sensory Evaluation
2.13. Statistical Analysis
3. Results and Discussion
3.1. TAA, TFC, and TPC of Sapota Fruits
3.2. TSS, TA, and AA of Sapota Fruits
3.3. Weight Loss and Firmness
3.4. Decay Incidence
3.5. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yahia, E.M.; Gutiérrez-Orozco, F.; Arvizu-de Leon, C. Phytochemical and Antioxidant Characterization of Mamey (Pouteria Sapota Jacq. H.E. Moore & Stearn) Fruit. Food Res. Int. 2011, 44, 2175–2181. [Google Scholar]
- Padmaja, N.; Don Bosco, S.J.; Rao, J.S. Physico Chemical Analysis of Sapota (Manilkara zapota) Coated by Edible Aloe Vera Gel. Int. J. Appl. Sci. Biotechnol. 2015, 3, 20–25. [Google Scholar] [CrossRef]
- Bhutia, W.; Pal, R.K.; Sen, S.; Jha, S.K. Response of Different Maturity Stages of Sapota (Manilkara Achras Mill.) Cv. Kallipatti to in-Package Ethylene Absorbent. J. Food Sci. Technol. 2011, 48, 763–768. [Google Scholar] [CrossRef]
- Padmaja, N.; John, D.S. Preservation of Sapota (Manilkara Zapota) by Edible Aloe Vera Gel Coating to Maintain Its Quality. Int. J. Sci. Res. 2012, 3, 177–179. [Google Scholar] [CrossRef]
- Mirshekari, A.; Madani, B.; Yahia, E.M.; Golding, J.B.; Vand, S.H. Postharvest Melatonin Treatment Reduces Chilling Injury in Sapota Fruit. J. Sci. Food Agric. 2020, 100, 1897–1903. [Google Scholar] [CrossRef]
- Ganjyal, G.M.; Hanna, M.A.; Devadattam, D.S.k. Processing of Sapota (Sapodilla): Drying. J. Food Sci. 2003, 68, 517–520. [Google Scholar] [CrossRef]
- Suryawanshi, S.D.S.; Sunita, J.; Magar, A.P. Postharvest Diseases of Minor Fruits and Their Management. In Postharvest Handling and Diseases of Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-00-304550-2. [Google Scholar]
- Mahajan, B.; Tandon, R.; Kapoor, S.; Sidhu, M.K. Natural Coatings for Shelf-Life Enhancement and Quality Maintenance of Fresh Fruits and Vegetables—A Review. J. Postharvest Technol. 2018, 1, 12–26. [Google Scholar]
- da Cruz Cabral, L.; Fernández Pinto, V.; Patriarca, A. Application of Plant Derived Compounds to Control Fungal Spoilage and Mycotoxin Production in Foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef]
- Blancas-Benitez, F.J.; Montaño-Leyva, B.; Aguirre-Güitrón, L.; Moreno-Hernández, C.L.; Fonseca-Cantabrana, Á.; Romero-Islas, L.d.C.; González-Estrada, R.R. Impact of Edible Coatings on Quality of Fruits: A Review. Food Control 2022, 139, 109063. [Google Scholar] [CrossRef]
- Pandey, V.K.; Islam, R.U.; Shams, R.; Dar, A.H. A Comprehensive Review on the Application of Essential Oils as Bioactive Compounds in Nano-Emulsion Based Edible Coatings of Fruits and Vegetables. Appl. Food Res. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Nicolau-Lapeña, I.; Colàs-Medà, P.; Alegre, I.; Aguiló-Aguayo, I.; Muranyi, P.; Viñas, I. Aloe Vera Gel: An Update on Its Use as a Functional Edible Coating to Preserve Fruits and Vegetables. Prog. Org. Coat. 2021, 151, 106007. [Google Scholar] [CrossRef]
- Al-Hilifi, S.A.; Al-Ali, R.M.; Al-Ibresam, O.T.; Kumar, N.; Paidari, S.; Trajkovska Petkoska, A.; Agarwal, V. Physicochemical, Morphological, and Functional Characterization of Edible Anthocyanin-Enriched Aloevera Coatings on Fresh Figs (Ficus carica L.). Gels 2022, 8, 645. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Pujolà, M.; Sepulcre, F. Aloe Vera as an Alternative to Traditional Edible Coatings Used in Fresh-Cut Fruits: A Case of Study with Kiwifruit Slices. LWT–Food Sci. Technol. 2015, 61, 184–193. [Google Scholar] [CrossRef]
- Anjum, M.A.; Akram, H.; Zaidi, M.; Ali, S. Effect of Gum Arabic and Aloe Vera Gel Based Edible Coatings in Combination with Plant Extracts on Postharvest Quality and Storability of ‘Gola’ Guava Fruits. Sci. Hortic. 2020, 271, 109506. [Google Scholar] [CrossRef]
- Alkaabi, S.; Sobti, B.; Mudgil, P.; Hasan, F.; Ali, A.; Nazir, A. Lemongrass Essential Oil and Aloe Vera Gel Based Antimicrobial Coatings for Date Fruits. Appl. Food Res. 2022, 2, 100127. [Google Scholar] [CrossRef]
- Jaishankar, H.; Laxman, K. Effect of Post Harvest Treatments on Physiological Changes of Sapota cv. Kalipatti at Ambient Storage. Adv. Life Sci. 2016, 5, 3732–3738. [Google Scholar]
- Ahmed, S.R.; Rabbee, M.F.; Roy, A.; Chowdhury, R.; Banik, A.; Kubra, K.; Hassan Chowdhury, M.M.; Baek, K.-H. Therapeutic Promises of Medicinal Plants in Bangladesh and Their Bioactive Compounds against Ulcers and Inflammatory Diseases. Plants 2021, 10, 1348. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N. Antifungal investigations on plant essential oils: A Review. Int. J. Pharm. Pharm. Sci. 2013, 5, 19–28. [Google Scholar]
- Ebrahimi, L.; Jalali, H.; Etebarian, H.R.; Sahebani, N. Evaluation of Antifungal Activity of Some Plant Essential Oils against Tomato Grey Mould Disease. J. Plant Pathol. 2022, 104, 641–650. [Google Scholar] [CrossRef]
- Khaliq, G.; Ramzan, M.; Baloch, A.H. Effect of Aloe Vera Gel Coating Enriched with Fagonia Indica Plant Extract on Physicochemical and Antioxidant Activity of Sapodilla Fruit during Postharvest Storage. Food Chem. 2019, 286, 346–353. [Google Scholar] [CrossRef]
- Jahanshahi, B.; Jafari, A.; Vazifeshenas, M.R.; Gholamnejad, J. A Novel Edible Coating for Apple Fruits. J. Hortic. Postharvest Res. 2018, 1, 63–72. [Google Scholar] [CrossRef]
- Abebe, Z.; Tola, Y.B.; Mohammed, A. Effects of Edible Coating Materials and Stages of Maturity at Harvest on Storage Life and Quality of Tomato (Lycopersicon Esculentum Mill.) Fruits. Afr. J. Agric. Res. 2017, 12, 550–565. [Google Scholar] [CrossRef]
- Danalache, F.; Carvalho, C.Y.; Alves, V.D.; Moldão-Martins, M.; Mata, P. Optimisation of Gellan Gum Edible Coating for Ready-to-Eat Mango (Mangifera indica L.) Bars. Int. J. Biol. Macromol. 2016, 84, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Chettri, S.; Sharma, N.; Mohite, A.M. Utilization of Lima Bean Starch as an Edible Coating Base Material for Sapota Fruit Shelf-Life Enhancement. J. Agric. Food Res. 2023, 12, 100615. [Google Scholar] [CrossRef]
- Vishwasrao, C.; Ananthanarayan, L. Delayed Post-Harvest Ripening-Associated Changes in Manilkara zapota L. Var. Kalipatti with Composite Edible Coating. J. Sci. Food Agric. 2017, 97, 536–542. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Sellamuthu, P.S.; Emmanuel, R.S. Use of Modified Atmosphere Packaging Combined with Essential Oils for Prolonging Post-Harvest Shelf Life of Mango (Cv. Banganapalli and Cv. Totapuri). LWT 2021, 148, 111662. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Mafune, M.; Sivakumar, D.; Soundy, P. Thyme Oil Vapour and Modified Atmosphere Packaging Reduce Anthracnose Incidence and Maintain Fruit Quality in Avocado. J. Sci. Food Agric. 2013, 93, 3024–3031. [Google Scholar] [CrossRef]
- Song, X.; Zhu, L.; Geng, X.; Li, Q.; Zheng, F.; Zhao, Q.; Ji, J.; Sun, J.; Li, H.; Wu, J.; et al. Analysis, Occurrence, and Potential Sensory Significance of Tropical Fruit Aroma Thiols, 3-Mercaptohexanol and 4-Methyl-4-Mercapto-2-Pentanone, in Chinese Baijiu. Food Chem. 2021, 363, 130232. [Google Scholar] [CrossRef]
- Alali, A.A.; Awad, M.A.; Al-Qurashi, A.D.; Mohamed, S.A. Postharvest Gum Arabic and Salicylic Acid Dipping Affect Quality and Biochemical Changes of ‘Grand Nain’ Bananas during Shelf Life. Sci. Hortic. 2018, 237, 51–58. [Google Scholar] [CrossRef]
- Khaliq, G.; Abbas, H.T.; Ali, I.; Waseem, M. Aloe Vera Gel Enriched with Garlic Essential Oil Effectively Controls Anthracnose Disease and Maintains Postharvest Quality of Banana Fruit during Storage. Hortic. Environ. Biotechnol. 2019, 60, 659–669. [Google Scholar] [CrossRef]
- Hosseinifarahi, M.; Jamshidi, E.; Amiri, S.; Kamyab, F.; Radi, M. Quality, Phenolic Content, Antioxidant Activity, and the Degradation Kinetic of Some Quality Parameters in Strawberry Fruit Coated with Salicylic Acid and Aloe Vera Gel. J. Food Process. Preserv. 2020, 44, e14647. [Google Scholar] [CrossRef]
- Mohammadi, L.; Ramezanian, A.; Tanaka, F.; Tanaka, F. Impact of Aloe Vera Gel Coating Enriched with Basil (Ocimum basilicum L.) Essential Oil on Postharvest Quality of Strawberry Fruit. J. Food Meas. Charact. 2021, 15, 353–362. [Google Scholar] [CrossRef]
- Mohammadi, L.; Tanaka, F.; Tanaka, F. Preservation of Strawberry Fruit with an Aloe Vera Gel and Basil (Ocimum basilicum) Essential Oil Coating at Ambient Temperature. J. Food Process. Preserv. 2021, 45, e15836. [Google Scholar] [CrossRef]
- Sarker, A.; Grift, T.E. Bioactive Properties and Potential Applications of Aloe Vera Gel Edible Coating on Fresh and Minimally Processed Fruits and Vegetables: A Review. J. Food Meas. Charact. 2021, 15, 2119–2134. [Google Scholar] [CrossRef]
- Rahmanzadeh Ishkeh, S.; Asghari, M.; Shirzad, H.; Alirezalu, A.; Ghasemi, G. Lemon Verbena (Lippia citrodora) Essential Oil Effects on Antioxidant Capacity and Phytochemical Content of Raspberry (Rubus ulmifolius Subsp. Sanctus). Sci. Hortic. 2019, 248, 297–304. [Google Scholar] [CrossRef]
- Mandal, D.; Sailo, L.; Hazarika, T.; Shukla, A.C. Effect of Edible Coating on Shelf Life and Quality of Local Mango Cv. Rangkuai of Mizoram. Res. Crops 2018, 19, 419–424. [Google Scholar] [CrossRef]
- Mani, A.; Prasanna, V.; Halder, S.; Praveena, J. Efficacy of Edible Coatings Blended with Aloe Vera in Retaining Post-Harvest Quality and Improving Storage Attributes in Ber (Ziziphus mauritiana Lamk.). Int. J. Chem. Stud. 2018, 6, 1727–1733. [Google Scholar]
- Radi, M.; Akhavan-Darabi, S.; Akhavan, H.-R.; Amiri, S. The Use of Orange Peel Essential Oil Microemulsion and Nanoemulsion in Pectin-Based Coating to Extend the Shelf Life of Fresh-Cut Orange. J. Food Process. Preserv. 2018, 42, e13441. [Google Scholar] [CrossRef]
- Khan, A.; Singh, Z.; Abbasi, N.; Swinny, E. Pre-or Postharvest Application of Putrescine and Low Temperature Storage Affect Fruit Ripening and Quality of “Angelino” Plum. J. Sci. Food Agric. 2008, 88, 1686–1695. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Xylia, P.; Chrysargyris, A. Sage Essential Oil Improves the Effectiveness of Aloe Vera Gel on Postharvest Quality of Tomato Fruit. Agronomy 2019, 9, 635. [Google Scholar] [CrossRef]
- Abdollahi, M.; Bazargani-Gilani, B.; Aghajani, N.; Daraei Garmakhany, A. Response Surface Optimization of the Effect of Aloe Vera Gel Coating Enriched with Golpar Essential Oil on the Shelf Life, Postharvest Quality, Color Change and Sensory Attributes of Fresh-Cut Orange Fruit. J. Food Process. Preserv. 2022, 46, e17019. [Google Scholar] [CrossRef]
- Mohammadi, L.; Hassanzadeh Khankahdani, H.; Tanaka, F.; Tanaka, F. Effect of Aloe Vera Gel Combined with Basil (Ocimum basilicum L.) Essential Oil as a Natural Coating on Maintaining Post-Harvest Quality of Peach (Prunus persica L.) during Storage. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 594, p. 012008. [Google Scholar] [CrossRef]
- Passafiume, R.; Gaglio, R.; Sortino, G.; Farina, V. Effect of Three Different Aloe Vera Gel-Based Edible Coatings on the Quality of Fresh-Cut “Hayward” Kiwifruits. Foods 2020, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Benítez, S.; Achaerandio, I.; Sepulcre, F.; Pujolà, M. Aloe Vera Based Edible Coatings Improve the Quality of Minimally Processed ‘Hayward’ Kiwifruit. Postharvest Biol. Technol. 2013, 81, 29–36. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Longkumer, M.; Ahmad, M.S.; Barman, K.; Thakur, P.K.; Kabir, J. Postharvest Biology and Technology of Sapota: A Concise Review. Acta Physiol. Plant 2014, 36, 3115–3122. [Google Scholar] [CrossRef]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit Ripening Phenomena—An Overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hajebi Seyed, R.; Rastegar, S.; Faramarzi, S. Impact of Edible Coating Derived from a Combination of Aloe Vera Gel, Chitosan and Calcium Chloride on Maintain the Quality of Mango Fruit at Ambient Temperature. J. Food Meas. Charact. 2021, 15, 2932–2942. [Google Scholar] [CrossRef]
- Hassan, H.S.; EL-Hefny, M.; Ghoneim, I.M.; El-Lahot, M.S.R.A.; Akrami, M.; Al-Huqail, A.A.; Ali, H.M.; Abd-Elkader, D.Y. Assessing the Use of Aloe Vera Gel Alone and in Combination with Lemongrass Essential Oil as a Coating Material for Strawberry Fruits: HPLC and EDX Analyses. Coatings 2022, 12, 489. [Google Scholar] [CrossRef]
- Rasouli, M.; Koushesh Saba, M.; Ramezanian, A. Inhibitory Effect of Salicylic Acid and Aloe Vera Gel Edible Coating on Microbial Load and Chilling Injury of Orange Fruit. Sci. Hortic. 2019, 247, 27–34. [Google Scholar] [CrossRef]
- Valverde, J.M.; Valero, D.; Martínez-Romero, D.; Guillén, F.; Castillo, S.; Serrano, M. Novel Edible Coating Based on Aloe Vera Gel To Maintain Table Grape Quality and Safety. J. Agric. Food Chem. 2005, 53, 7807. [Google Scholar] [CrossRef]
- Hooda, P.K.; Singh, J. Effect of Edible Coatings on Jujube (Ziziphus mauritiana Lamk.) Fruits: A Review. Pharma Innov. J. 2022, 11, 2231–2235. [Google Scholar]
- Kumar, N.; Neeraj; Pratibha; Singla, M. Enhancement of Storage Life and Quality Maintenance of Litchi (Litchi chinensis Sonn.) Fruit Using Chitosan:Pullulan Blend Antimicrobial Edible Coating. Int. J. Fruit Sci. 2020, 20, S1662–S1680. [Google Scholar] [CrossRef]
- Zare, M.R.; Khorram, M.; Barzegar, S.; Asadian, F.; Zareshahrabadi, Z.; Saharkhiz, M.J.; Ahadian, S.; Zomorodian, K. Antimicrobial Core–Shell Electrospun Nanofibers Containing Ajwain Essential Oil for Accelerating Infected Wound Healing. Int. J. Pharm. 2021, 603, 120698. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C. Evaluation of Phytochemical Composition in Selected Medicinal Plants and Potential Application as Antimicrobial Agent. Master’s Thesis, Delaware State University, Dover, DE, USA, 2019. [Google Scholar]

| Treatment | TSS (°Brix) | Titratable Acidity (%) | Ascorbic Acid (mg/100 g) | TSS (°Brix) | Titratable Acidity (%) | Ascorbic Acid (mg/100 g) | TSS (°Brix) | Titratable Acidity (%) | Ascorbic Acid (mg/100 g) |
|---|---|---|---|---|---|---|---|---|---|
| Storage Days | Day 0 | Day 7 | Day 14 | ||||||
| Control | 13.8 ± 0.44 aA | 0.59 ± 0.01 aC | 22.09 ± 0.85 abC | 21.8 ± 0.45 dB | 0.19 ± 0.06 aB | 15.17 ± 0.85 aB | 27.8 ± 0.59 cC | 0.07 ± 0.002 aA | 8.21 ± 0.54 aA |
| AV 100% | 14.0 ± 0.28 aA | 0.53 ± 0.04 aC | 21.45 ± 0.97 aC | 19.8 ± 0.52 cB | 0.33 ± 0.07 bB | 17.23 ± 0.95 bB | 24.0 ± 0.46 bC | 0.13 ± 0.04 bA | 11.05 ± 0.76 bA |
| AV 100% + OO | 14.9 ± 0.21 abA | 0.55 ± 0.03 aC | 21.98 ± 0.49 aC | 15.9 ± 0.26 abB | 0.50 ± 0.05 cB | 20.27 ± 0.49 cB | 19.5 ± 0.56 aC | 0.26 ± 0.02 cA | 17.26 ± 1.09 cdA |
| AV 100% + AO | 14.3 ± 0.25 aA | 0.48 ± 0.01 aC | 21.33 ± 0.96 aC | 16.0 ± 0.31 abB | 0.46 ± 0.04 cB | 20.15 ± 0.96 cB | 19.4 ± 0.39 aC | 0.30 ± 0.02 cA | 17.14 ± 0.89 cdA |
| CT | 13.9 ± 0.29 aA | 0.52 ± 0.02 aC | 20.96 ± 0.93 aC | 15.4 ± 0.29 aB | 0.50 ± 0.03 cB | 19.89 ± 0.93 cB | 19.0 ± 0.41 aC | 0.28 ± 0.01 cA | 16.91 ± 0.69 cA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poongavanam, S.S.; Subramaniyan, V.; Rajendra, A.B.; Sellamuthu, P.S.; Jarugala, J.; Sadiku, E.R. Physiochemical Analysis of Manilkara zapota (Sapota) Coated with Aloe Vera Gel and Enriched with Ajwain and Oregano Essential Oils. Coatings 2023, 13, 1358. https://doi.org/10.3390/coatings13081358
Poongavanam SS, Subramaniyan V, Rajendra AB, Sellamuthu PS, Jarugala J, Sadiku ER. Physiochemical Analysis of Manilkara zapota (Sapota) Coated with Aloe Vera Gel and Enriched with Ajwain and Oregano Essential Oils. Coatings. 2023; 13(8):1358. https://doi.org/10.3390/coatings13081358
Chicago/Turabian StylePoongavanam, Senthamil Selvi, Vishnupriya Subramaniyan, Abhishek Biswal Rajendra, Periyar Selvam Sellamuthu, Jayaramudu Jarugala, and Emmanuel Rotimi Sadiku. 2023. "Physiochemical Analysis of Manilkara zapota (Sapota) Coated with Aloe Vera Gel and Enriched with Ajwain and Oregano Essential Oils" Coatings 13, no. 8: 1358. https://doi.org/10.3390/coatings13081358
APA StylePoongavanam, S. S., Subramaniyan, V., Rajendra, A. B., Sellamuthu, P. S., Jarugala, J., & Sadiku, E. R. (2023). Physiochemical Analysis of Manilkara zapota (Sapota) Coated with Aloe Vera Gel and Enriched with Ajwain and Oregano Essential Oils. Coatings, 13(8), 1358. https://doi.org/10.3390/coatings13081358








