Abstract
The corrosion resistance of FeCoNiCr high-entropy alloy deposits was investigated upon being prepared by current electrodeposition. The coatings were co-deposited in an electrolyte of an aqueous ferrous, cobalt, nickel, and chromium sulfates solution. Energy dispersive spectrometry analysis demonstrated that all four elements were co-deposited successfully. At the same time, the results from SEM indicate that the surface of the coating exhibits a granular morphology, with uniform density and no presence of cracks, with sizes ranging from 500 nm to 5 μm. Furthermore, X-ray diffraction patterns enunciated that the as-deposited coatings were amorphous. The polarization curves of the FeCoNiCr high-entropy alloy coating were measured by an electrochemical workstation in 3.5 wt.% NaCl, 1 mol·L−1 H2SO4 and 1 mol·L−1 NaOH solutions. The results revealed that the coating exhibited excellent corrosion resistance. The corrosion mechanism of the FeCoNiCr high-entropy alloy coating was analyzed in different environments. Moreover, the scratch testing method was employed to determine the alloy adhesion on the substrate, with higher values obtained for the FeCoNiCr alloy.
1. Introduction
High entropy alloys (HEAs) are a new class of metallic materials that have been widely studied in the scientific community in recent years. They are based on an innovative fabrication method. Unlike traditional alloys, these multicomponent materials have no predominant components and are composed of an isoatomic mixture of five or more elements. By classical metallurgical theory, it can be inferred that at the microstructural level, these materials are comprised of numerous intermetallic compounds. In contrast, due to the high mixing entropy of HEAs, they often exhibit the formation of simple solid solution structures, single-phase crystal structures, or even amorphous structures. Single-phase crystal structures are also very common. Not only the high-entropy effect, the high-entropy alloy exhibits slow diffusion and severe lattice distortion, significantly influencing its structure and properties. Therefore, HEAs have defined a research field that has attracted considerable attention in materials science and engineering [1,2,3,4].
In recent years, HEAs have been extensively studied, and numerous alloy systems with excellent hardness, strength, annealing softening resistance, corrosion resistance, wear resistance, and magnetoelectric properties have been developed. The structures of these alloy systems make them applicable to functional materials. A distinguishing feature of high entropy alloys is their remarkable capability to maintain their properties even under elevated temperatures [5,6,7,8,9].
The common practice for preparing HEAs involves the utilization of physical methods, with melting-casting techniques such as arc and induction furnace smelting being the prevailing choice [10,11]. In addition to melting-casting, mechanical alloying and rapid solidification are alternative synthetic techniques employed to produce bulk materials [12,13].Meanwhile, magnetron sputtering and laser cladding are employed to develop HEA coatings [14,15,16,17].
FeCoNiCr-type high-entropy alloys are less prone to phase transformation or the formation of precipitates. Enhancing their corrosion resistance poses a challenging task. The current general solutions include alloying and introducing new manufacturing techniques [18]. The superior corrosion resistance of FeCoNiCr MPEAs (Multi-Principal Element Alloys) is attributed to the inclusion of the Cr (Chromium) element [19]. However, Y. Qiu et al. [20]. found that FeCoNiCr MPEAs prepared using Spark Plasma Sintering (SPS) exhibited reduced corrosion resistance due to chromium segregation in the matrix. Nevertheless, high-entropy alloys mixed in the same molar ratio exhibit better corrosion resistance [21].
Electroless plating holds great potential in promoting the application of metal nanomaterials. However, due to its reliance on chemical reducing agents and more complex bath control, electroless plating is more costly compared to similar electrodeposition reactions. Additionally, the plating rate of electroless plating is relatively slower [22].
Electrodeposition, on the other hand, is an efficient and rapid process for producing coatings [23], and it is a low-cost method for fabricating high-entropy alloy thin films. This convenient method can also be applied to deposit coatings on substrates with complex geometries. Conventional metallurgical methods for fabricating alloys with large differences in melting points can be cost-intensive, whereas electrodeposition allows for codeposition at lower temperatures through the selection of appropriate solution systems, leading to energy savings [24]. However, due to the varying degrees of attraction between different ions and charges, the preparation process becomes challenging. Therefore, it is important to choose suitable complexing agents, appropriately adjust the metal ion concentrations, and optimize electrodeposition parameters [25].
Currently, non-aqueous solvents are preferred as the solvent of choice for metal and alloy electrodeposition. This is because of their excellent chemical and thermal stability, wide operating temperature range, wide electrochemical window, high conductivity, and their characteristic of not generating hydrogen or forming hydroxides [26,27,28]. However, non-aqueous solvents are hazardous, toxic, and flammable.
Tong et al. prepared BiFeCoNiMn, MgMnFeCoNiGd, and TmFeCoNiMn multi-component HEA coatings by electrodeposition in DMF (or dimethyl sulfoxide–DMSO) and CH3CN. These alloys have potential applications as magnetic, photoelectric, thermoelectric, and fuel cell materials [29,30,31]. Guo et al. utilized electrodeposition to fabricate FeCoNiCr high-entropy alloy coatings and investigated the effects of current density, bath temperature, and pH on the coating’s properties [21]. To date, there is a need for more knowledge regarding the electrodeposition preparation of high-entropy alloys. Furthermore, the corrosion resistance and corrosion mechanisms of the FeCoNiCr high-entropy alloy coatings in different aqueous solutions have not been reported yet [19].
In this study, FeCoNiCr high-entropy alloy (HEA) coatings were prepared using electrochemical deposition in aqueous solutions, and their corrosion resistance and corrosion mechanisms were investigated. It was found that these coatings exhibit promising anti-corrosion properties, suggesting their potential for corrosion protection applications.
2. Materials and Methods
The electrodeposition of high-entropy alloy coatings and electrochemical studies were performed at 298 K using a CHI660E electrochemical workstation (ARTISAN, Shanghai, China). Plating uses Cu as cathode material, and the FeCoNiCr HEA coatings were deposited onto Cu substrates (12 mm × 12 mm × 1 mm) using current electrodeposition in an electrolyte based on an H2SO4 aqueous solvent system containing FeSO4, CoSO4, NiSO4, and Cr2(SO4)3. Citric acid and sodium citrate were used as complexing agents. The electrolyte’s composition are listed in Table 1.

Table 1.
Chemical composition of the electrolyte (g/L).
Electrodeposition was performed for 30 min at 25 A·dm−2. The pH of the electrodeposition was 2.5. Prior to plating, the Cu substrates were polished with abrasive paper and etched in an H2SO4 (160 mL·L−1) and H2O2 (80 mL·L−1) solution, followed by rinsing with double-distilled water.
A three-electrode setup was employed in the electrochemical cell. A platinum foil (17 mm × 17 mm × 0.2 mm) was utilized as the counter electrode, while a standard saturated calomel electrode worked as the reference electrode (SCE, 0.241 V versus RHE). In this study, all potentials were measured versus the SCE.
The morphology and chemical composition of the FeCoNiCr HEA coating were examined using an HS-4800 field emission electron microscope (SEM, Hitachi, Ltd., Hitachi, Japan) equipped with an INC 250 spectrometer (EDS).
The crystal structure of the coatings was determined using X-ray diffractometry (XRD, D/MAX2500HB+/PC, Rigaku, Akishima, Tokyo, Japan) with Cu Kα radiation (λ = 0.154178 nm) at a scanning rate of 2°/min and a scanning step of 0.02°. Using a wire-cutting machine J19 (HZHF, HangZhou, China)., the alloy coatings were cut into 10 mm × 10 mm square pieces for XRD measurements. The thickness of the deposits was measured with a Filmetrics F20-UVX instrument (Filmetrics, Quezon City, Philippines).
The scratch adhesion testing method was employed to measure the adhesion of the FeCoNiCr HEA coatings electrodeposited on the Cu substrates [32]. A diamond indenter with a standard geometric shape is used to apply a constant force at a predetermined distance on the surface of the coating. The indenter has a standard Rockwell C geometry with a 120° angle and a radius of 200 μm. The determined critical load (FNC) stands for the load at which complete removal of the film is achieved, and it is related to the actual adhesion and resistance to damage of the coating/substrate. The scratch test was conducted with a normal load force that increased from 0 to 100 N at a rate of 10 mm·min−1 and a distance of 10 mm.
3. Results and Discussion
Figure 1 depicts the XRD patterns of the FeCoNiCr alloy coating. For the as-deposited layers, Cu (with a cubic structure Fm-3m) diffraction peaks present due to the deposition substrate were identified as indicated by peaks at 2θ = 50.447°, 74.123°, 89.933°. The FeCoNiCr alloy coating shows a broad diffraction peak near 2θ = 45°. This result suggests that the as-deposited alloys were amorphous.
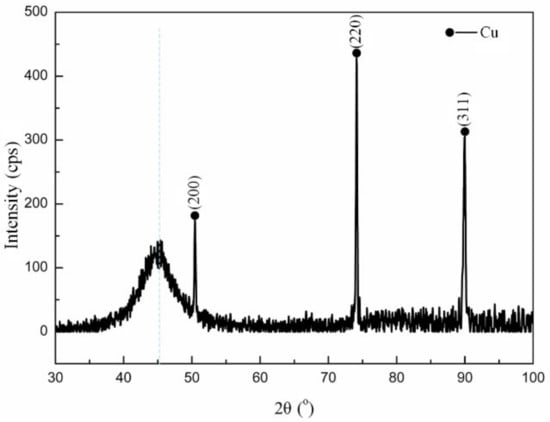
Figure 1.
XRD pattern of the as-deposited FeCoNiCr HEA coating.
Figure 2 displays the SEM image illustrating the morphology of the FeCoNiCr HEA coating deposited at 25 A·dm−2 for 30 min. The deposits on the Cu substrate consisted of spherical particles with sizes ranging from ∼500 nm to 5 μm (Figure 2). The coating was homogenous and free of cracks.
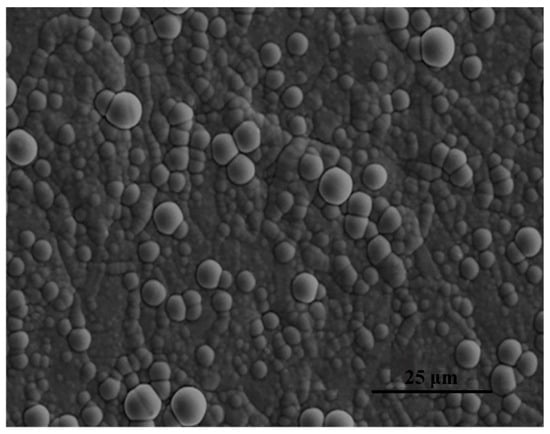
Figure 2.
SEM image of the FeCoNiCr HEA coating deposited at 25 A·dm−2 for 30 min.
The SEM micrograph at high magnification of the FeCoNiCr HEA coating and the elemental mapping of Fe, Co, Ni, Cr are illustrated in Figure 3 and Table 2, revealing the chemical composition of the coating and confirming the presence of Fe, Co, Ni and Cr elements in the deposits. The deposits on the Cu substrate are composed of spherical, the distribution is relatively uniform, and the surface is smooth and shiny without cracks and voids.
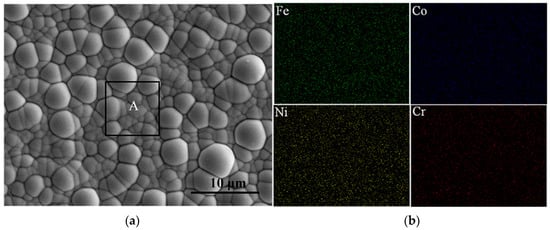
Figure 3.
(a) The SEM micrograph of the FeCoNiCr HEA coating and (b) the elemental mapping of Fe, Co, Ni and Cr from the black rectangle(A) in (a).

Table 2.
The chemical compositions, molar entropies of mixing, and thickness of the FeCoNiCr HEA coating electrodeposited at 25 A·dm−2 for 30 min.
The molar entropies of mixing and thickness of the FeCoNiCr HEA coating deposited at 25 A·dm−2 are also shown in Table 2. The Fe, Co, Ni, and Cr atomic percentages in the FeCoNiCr alloy were 23.84, 28.41, 24.69, and 23.06 at.%, respectively.
The usual expression for the mixed molar entropy of a solid solution is given by the following Equation (1):
where Ci is the molar percentage of a component, n represents the number of elements that make up the solution, R is the gas constant (8.314 J·K−1·mol−1), and . The molar entropies of mixing of the FeCoNiCr HEA coating reached 11.50 J·K−1·mol−1 at 25 A·dm−2.
From the critical load, FNC, values determined by the scratch test method, and displayed in Table 3, it was observed that the current density (DK) had a significant influence on the adhesion, with the maximum adhesion of 35 N being achieved at a DK of 25A·dm−2.

Table 3.
The thickness and critical load of electrodeposited FeCoNiCr HEA coating at different current densities.
The polarization curve of the FeCoNiCr HEA coating in 3.5 wt.% NaCl solution, 1 mol·L−1 H2SO4 and 1 mol·L−1 NaOH are shown in Figure 4. The coating displayed active dissolution characteristics according to the polarization curve in Figure 4a. The corrosion current density was increased suddenly, indicating that the FeCoNiCr HEA coating was undergoing pitting corrosion in 3.5 wt.% NaCl solution. Meanwhile, the Ecorr and icorr obtained by epitaxy were −0.45 V and 1.73 × 10−6 A·cm−2, respectively. The metal corrosion resistance is inversely related to Ecorr while positively related to icorr. This demonstrates that the metal corrosion resistance improves when the Ecorr value is high. Concurrently, when the icorr value is low, the corrosion resistance is low. The experimental results illustrated the Ecorr of the FeCoNiCr HEA coating in 3.5 wt.% NaCl solution was higher than that of the CoCrFeNi high-entropy alloy coating [33] prepared by vacuum hot-pressing sintering (−1.08 V). However, the icorr of FeCoNiCr HEA coating in 3.5 wt.% NaCl solution was lower than that of CoCrFeNi HEA coating (9.44 × 10−6 A·cm−2). Therefore, the FeCoNiCr HEA coating prepared by electrodeposition exhibited superior corrosion resistance.
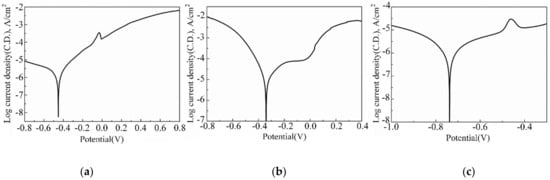
Figure 4.
The Polarization curve of the FeCoNiCr HEA coating in (a) 3.5 wt. % NaCl solution, (b) 1 mol·L−1 H2SO4, (c) 1 mol·L−1 NaOH.
The FeCoNiCr HEA coating exhibited an activation-passivation phenomenon according to the polarization curve in Figure 4b. Meanwhile, the Ecorr and icorr obtained by epitaxy were −0.33 V and 1.25 × 10−5 A·cm−2, respectively. Experimental results have shown that the icorr of FeCoNiCr HEA coating in a 1 mol·L−1 H2SO4 solution is lower than that of FeCoNiCr HEA coating in a 0.5 mol·L−1 H2SO4 solution [19]. Moreover, the icorr of the FeCoNiCr HEA coating in 1 mol·L−1 H2SO4 solution was lower than that of the AlCoCrFeNiCu HEA coating (2.50 × 10−5 A·cm−2) [34] and 304 stainless steel (2.14 × 10−5 A·cm−2) [35]. Therefore, the FeCoNiCr HEA coating exhibited excellent corrosion resistance and a strong passivation tendency.
According to the polarization curve in Figure 4c, the FeCoNiCr HEA coating exhibited active dissolution characteristics. The Ecorr and icorr obtained by epitaxy were -0.74 V and 1.38 × 10−6 A·cm−2, respectively. Furthermore, the icorr of FeCoNiCr HEA coating in 1 mol·L−1 NaOH solution was lower than that of Al0.3CoCrFeNi HEA (5.20 × 10−6 A·cm−2) [36], Zr55Al10Cu30Ni5 amorphous alloy (0.40 × 10−5 A·cm−2) [37], and AZ91D magnesium alloy (1.02 × 10−5 A·cm−2) [38].
In order to further demonstrate the corrosion resistance of FeCoNiCr high-entropy alloy coatings, the average corrosion rate (rcorr) can be calculated using the parameter icorr obtained from the polarization curve, based on Faraday’s Law [39]. The Equation (2) calculation is as follows:
where ρ is the density of the alloy (in g/cm3), icorr (in µA/cm2) is the corrosion current density, and EW (the equivalent weight of the alloy) can be expressed as Equation (3):
where ni is the valence of the ith element of the alloy, fi is the mass fraction of the ith element in the alloy, and Wi is the atomic weight of the ith element in the alloy.
The average corrosion rate rcorr of the FeCoNiCr HEA coating in 3.5 wt.% NaCl solution, 1 mol·L−1 H2SO4 and 1 mol·L−1 NaOH can be calculated by formulae (2), (3); they were 1.57 × 10−2, 1.14 × 10−1 and 1.26 × 10−2 mm·year−1, respectively.
The post-corrosion morphology of the FeCoNiCr HEA coating in 3.5 wt.% NaCl solution, 1 mol·L−1 H2SO4 and 1 mol·L−1 NaOH were show in Figure 5. The polarization curve results were consistent with the coating morphology after corrosion. Corrosion pits were observed on the surface of the FeCoNiCr HEA coating after corrosion in 3.5 wt.% NaCl solution, as illustrated in Figure 5a. According to the theory of oxide films [40], Cl− has a high permeability, allowing it to penetrate and destroy the oxide film on the FeCoNiCr HEA coating surface. Cl− is enriched in the area where the FeCoNiCr HEA coating surface is connected to the electrolyte. In addition, Cl− readily forms complexes with the metal elements in the high-entropy alloy coating, destroying the oxide film on the coating surface. According to adsorption theory [41], the corrosion mechanism of the FeCoNiCr HEA coating in 3.5 wt.% NaCl solution can be expressed as follows: (1) M + Cl− → (MCl−), (2) (MCl−) + H2O → (MOH) + HCl + e−, (3) (MOH) + H2O → (MOH)2 + H⁺ + e−, and (4) M → Mn⁺ + ne−. When the FeCoNiCr HEA coating was exposed to the NaCl solution, the metal element (M) in the coating dissolved into the solution under the action of Cl−, causing pitting corrosion on the coating surface. Therefore, pitting corrosion induced by the co-action of oxygen depolarization and Cl− was the failure mode of the FeCoNiCr HEA coating in 3.5 wt.% NaCl solution.
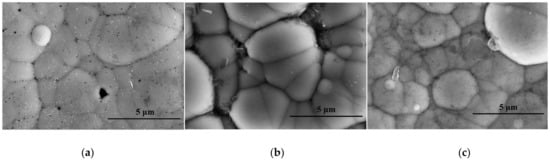
Figure 5.
The post-corrosion morphology of the FeCoNiCr HEA coating in (a) 3.5 wt. % NaCl solution, (b) 1 mol·L−1 H2SO4 and (c) 1 mol·L−1 NaOH.
As illustrated in Figure 5b, there was no corrosion on the grain surface of the FeCoNiCr HEA coating. Corrosion was only limited to the grain boundary and cracks and took the form of relatively uniform corrosion pits. Therefore, the corrosion mechanism of the FeCoNiCr HEA coating in a 1 mol·L−1 H2SO4 solution was uniform corrosion. The electrochemical corrosion process of the coating in 1 mol·L−1 H2SO4 solution can be expressed as follows: 2H⁺ + 2e− → H2 and M → Mn⁺ + ne−. Therefore, the FeCoNiCr HEA coating exhibited a passivation phenomenon in the H2SO4 solution, forming some stable compounds, such as MSO4 and M2O3 [42], on the surface. These compounds had a dense structure, hence inhibiting the corrosion of the coating. Therefore, uniform corrosion caused by hydrogen depolarization was the failure mode of the FeCoNiCr HEA coating in a 1 mol·L−1 H2SO4 solution.
Figure 5c depicts that the FeCoNiCr HEA coating surface after corrosion was relatively flat, with only a few corrosion pits evenly distributed. Therefore, the corrosion mechanism of the FeCoNiCr HEA coating in a 1 mol·L−1 NaOH solution was uniform corrosion. The electrochemical corrosion process of the FeCoNiCr HEA coating in a 1 mol·L−1 NaOH solution can be expressed as follows: 2M + O2 + 2H2O → 2M2⁺ + 4OH− and M2⁺ + 4OH− → MO22⁺ + 2H2O. The metal element (M) on the surface of the FeCoNiCr HEA coating reacts with OH− in a 1 mol·L−1 NaOH solution to form NaMO2 or Na2MO2 [43]. Therefore, the uniform corrosion caused by the oxygen absorption reaction was the failure mode of the FeCoNiCr HEA coating in 1 mol·L−1 NaOH solution.
4. Conclusions
FeCoNiCr HEA coatings were prepared by electrochemical deposition at 25 A·dm−2 in an aqueous ferrous, cobalt, nickel, and chromium sulfates solution. The EDS results illustrated that the four elements, Fe, Co, Ni, and Cr, were successfully deposited. Meanwhile, the results from SEM indicate that the surface of the coating exhibits a granular morphology, with uniform density and no presence of cracks, with sizes ranging from 500 nm to 5 μm. The XRD patterns demonstrated that the as-deposited coatings were amorphous. Furthermore, the scratch testing method was employed to determine the alloy adhesion on the substrate, yielding higher values of 35 N.
The Ecorr, icorr and rcorr of the FeCoNiCr HEA coating in 3.5 wt.% NaCl solution were −0.45 V, 1.73 × 10−6 A·cm−2 and 1.57 × 10−2 mm·year−1, respectively. As a result, the corrosion mechanism was pitting caused by the co-action of oxygen depolarization and Cl−. The FeCoNiCr HEA coatings demonstrated superior corrosion resistance, outperforming some amorphous and five-element high-entropy alloys because of their higher Ecorr and lower icorr.
Moreover, the Ecorr, icorr and rcorr of the FeCoNiCr HEA coating in 1 mol·L−1 H2SO4 solutions were −0.33 V,1.25 × 10−5 A·cm−2 and 1.14 × 10−1 mm·year−1, respectively. Therefore, the corrosion mechanism was uniform corrosion caused by hydrogen depolarization.
Finally, the Ecorr, icorr and rcorr of FeCoNiCr HEA coating in 1 mol·L−1 NaOH solution were −0.74 V, 1.38 × 10−6 A·cm−2 and 1.26 × 10−2 mm·year−1, respectively. As a result, uniform corrosion caused by the oxygen absorption reaction was the corrosion mechanism. The icorr was low in 1 mol·L−1 H2SO4 acidic solution and 1 mol·L−1 NaOH alkaline solution, implying improved corrosion resistance.
Consequently, the FeCoNiCr HEA coating prepared in this study has excellent corrosion resistance, which provides a theoretical basis and practical basis for its application in engineering practice. These results demonstrate that electrodeposition is a promising method for synthesizing FeCoNiCr HEAs.
Author Contributions
Conceptualization, Z.X.; Methodology, Z.X., Y.W., X.G., Q.Q. and F.G.; Software, J.X. and F.G.; Validation, Y.W.; Formal analysis, L.P.; Investigation, Y.W., X.G. and L.P.; Resources, Z.X., Y.W., R.W. and J.Y.; Data curation, Z.X. and Y.W.; Writing—original draft, Z.X.; Writing—review & editing, J.X.; Supervision, Z.X., Q.Q. and R.W.; Project administration, J.Y.; Funding acquisition, Z.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Hebei Natural Science Foundation of China under Grants No. E2020203158, Hebei Province Department of Human Resources and Social Security under Grants No. E2020100006 and the Hebei Province Innovation Ability Promotion Project under Grants No. 22567609H.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- MacDonald, B.E.; Fu, Z.; Zheng, B.; Chen, W.; Lin, Y.; Chen, F.; Zhang, L.; Ivanisenko, J.; Zhou, Y.; Hahn, H.; et al. Recent Progress in High Entropy Alloy Research. JOM 2017, 69, 2024–2031. [Google Scholar] [CrossRef]
- Gao, M.C. Progress in High Entropy Alloys. JOM 2015, 67, 2251–2253. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, M.C.; Lin, S.K. Progress in High-Entropy Alloys. JOM 2019, 71, 3417–3418. [Google Scholar] [CrossRef]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and Technology in High-Entropy Alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and Properties of High-Entropy Alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical Properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 Refractory High Entropy Alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Zhang, T.W.; Ma, S.G.; Zhao, D.; Wu, Y.C.; Zhang, Y.; Wang, Z.H.; Qiao, J.W. Simultaneous Enhancement of Strength and Ductility in a NiCoCrFe High-Entropy Alloy upon Dynamic Tension: Micromechanism and Constitutive Modeling. Int. J. Plast. 2020, 124, 226–246. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, L.; He, W.; Liaw, P. Alloying and Processing Effects on the Aqueous Corrosion Behavior of High-Entropy Alloys. Entropy 2014, 16, 895–911. [Google Scholar] [CrossRef]
- Tsai, M.H.; Chang, K.C.; Li, J.H.; Tsai, R.C.; Cheng, A.H. A Second Criterion for Sigma Phase Formation in High-Entropy Alloys. Mater. Res. Lett. 2016, 4, 90–95. [Google Scholar] [CrossRef]
- He, J.Y.; Wang, H.; Wu, Y.; Liu, X.J.; Mao, H.H.; Nieh, T.G.; Lu, Z.P. Precipitation Behavior and Its Effects on Tensile Properties of FeCoNiCr High-Entropy Alloys. Intermetallics 2016, 79, 41–52. [Google Scholar] [CrossRef]
- Li, Z.; Tasan, C.C.; Pradeep, K.G.; Raabe, D. A TRIP-Assisted Dual-Phase High-Entropy Alloy: Grain Size and Phase Fraction Effects on Deformation Behavior. Acta Mater. 2017, 131, 323–335. [Google Scholar] [CrossRef]
- Anand Sekhar, R.; Samal, S.; Nayan, N.; Bakshi, S.R. Microstructure and Mechanical Properties of Ti-Al-Ni-Co-Fe Based High Entropy Alloys Prepared by Powder Metallurgy Route. J. Alloys Compd. 2019, 787, 123–132. [Google Scholar] [CrossRef]
- Kao, S.W.; Yeh, J.W.; Chin, T.S. Rapidly Solidified Structure of Alloys with up to Eight Equal-Molar Elements—A Simulation by Molecular Dynamics. J. Phys. Condens. Matter 2008, 20, 145214. [Google Scholar] [CrossRef]
- Tang, W.Y.; Yeh, J.W. Effect of Aluminum Content on Plasma-Nitrided Al x CoCrCuFeNi High-Entropy Alloys. Met. Mat Trans. A 2009, 40, 1479–1486. [Google Scholar] [CrossRef]
- Tsai, D.C.; Deng, M.J.; Chang, Z.C.; Kuo, B.H.; Chen, E.C.; Chang, S.Y.; Shieu, F.S. Oxidation Resistance and Characterization of (AlCrMoTaTi)-Six-N Coating Deposited via Magnetron Sputtering. J. Alloys Compd. 2015, 647, 179–188. [Google Scholar] [CrossRef]
- Xie, L.; Brault, P.; Thomann, A.L.; Bauchire, J.M. AlCoCrCuFeNi High Entropy Alloy Cluster Growth and Annealing on Silicon: A Classical Molecular Dynamics Simulation Study. Appl. Surf. Sci. 2013, 285, 810–816. [Google Scholar] [CrossRef]
- Yu, L.; Hao, L.; Wei, L. Hierarchical FeCoNiCr high entropy alloy thin films with combined high strength and excellent corrosion resistance. Mater. Des. 2023, 231, 112049. [Google Scholar]
- Zhang, C.; Zhu, J.K.; Zhang, G.Q. Laser powder bed fusion of nano-TiB2 reinforced FeCoNiCr high-entropy alloy with enhanced strength and firm corrosion resistance. J. Alloys Compd. 2022, 927, 167110. [Google Scholar] [CrossRef]
- Chai, W.K.; Lu, T.; Pan, Y. Corrosion behaviors of FeCoNiCrx (x = 0, 0.5, 1.0) multi-principal element alloys: Role of Cr-induced segregation. Intermetallics 2020, 116, 106654. [Google Scholar] [CrossRef]
- Qiu, Y.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion characteristics of high entropy alloys. Mater. Sci. Technol. 2015, 31, 1235–1243. [Google Scholar] [CrossRef]
- Guo, F.Y.; YU, J.K.; Xiao, J.J. Preparation of FeCoNiCr High Entropy Alloy Coatings and Optimization of Process Parameters. Rare Met. Mater. Eng. 2021, 50, 2337–2342. [Google Scholar]
- Muench, F. Electroless plating of metal nanomaterials. ChemElectroChem 2021, 8, 2993–3012. [Google Scholar] [CrossRef]
- Yazdani, S.; Mesbah, M.; Dupont, V. Microstructure, wear and crack propagation evolution of electrodeposited nickel-nano diamond composite coatings: Molecular dynamic modeling and experimental study. Surf. Coat. Technol. 2023, 462, 129500. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, W.; Li, B. Influences of Co and process parameters on structure and corrosion properties of nanocrystalline Ni-W-Co ternary alloy film fabricated by electrodeposition at low current density. Surf. Coat. Technol. 2022, 439, 128457. [Google Scholar] [CrossRef]
- Bian, H.; Wang, R.; Zhang, K. Facile electrodeposition synthesis and super performance of nano-porous Ni-Fe-Cu-Co-W high entropy alloy electrocatalyst. Surf. Coat. Technol. 2023, 459, 129407. [Google Scholar] [CrossRef]
- Simka, W.; Puszczyk, D.; Nawrat, G. Electrodeposition of Metals from Non-Aqueous Solutions. Electrochim. Acta 2009, 54, 5307–5319. [Google Scholar] [CrossRef]
- Li, R.; Dong, Q.; Xia, J.; Luo, C.; Sheng, L.; Cheng, F.; Liang, J. Electrodeposition of Composition Controllable Zn Ni Coating from Water Modified Deep Eutectic Solvent. Surf. Coat. Technol. 2019, 366, 138–145. [Google Scholar] [CrossRef]
- Soare, V.; Burada, M.; Constantin, I.; Mitrică, D.; Bădiliţă, V.; Caragea, A.; Târcolea, M. Electrochemical Deposition and Microstructural Characterization of AlCrFeMnNi and AlCrCuFeMnNi High Entropy Alloy Thin Films. Appl. Surf. Sci. 2015, 358, 533–539. [Google Scholar] [CrossRef]
- Yao, C.Z.; Zhang, P.; Liu, M.; Li, G.R.; Ye, J.Q.; Liu, P.; Tong, Y.X. Electrochemical Preparation and Magnetic Study of Bi–Fe–Co–Ni–Mn High Entropy Alloy. Electrochim. Acta 2008, 53, 8359–8365. [Google Scholar] [CrossRef]
- Li, H.; Sun, H.; Wang, C.; Wei, B.; Yao, C.; Tong, Y.; Ma, H. Controllable Electrochemical Synthesis and Magnetic Behaviors of Mg–Mn–Fe–Co–Ni–Gd Alloy Films. J. Alloys Compd. 2014, 598, 161–165. [Google Scholar] [CrossRef]
- Yao, C.; Wei, B.; Zhang, P.; Lu, X.; Liu, P.; Tong, Y. Facile Preparation and Magnetic Study of Amorphous Tm-Fe-Co-Ni-Mn Multicomponent Alloy Nanofilm. J. Rare Earths 2011, 29, 133–137. [Google Scholar] [CrossRef]
- Sergici, A.O.; Randall, N.X. Scratch Testing of Coatings. Adv. Mater. Process. 2006, 164, 41–44. [Google Scholar]
- Shang, C.Y. The Synthesis, Micro-Characterization and Properties of CoCrFeNi (Cu, W, W0.5Mo0.5, Mo, WC) High Entropy Alloy Coatings. Master’s Thesis, Jinan University, Guangzhou, China, 2017. [Google Scholar]
- Shi, Y.G.; Zhang, T.B.; Kou, H.C.; Li, J. Study on corrosion properties of AlCoCrFeNiCu high entropy alloy in different media. Hot Work. Technol. 2011, 18, 1–3. [Google Scholar]
- Niu, X.L.; Wang, L.J.; Sun, D.; Julius, J. Research on microstructure and electrochemical properties of AlxFeCoCrNiCu (x = 0.25, 0.5, 1.0) high-entropy alloys. J. Funct. Mater. 2013, 44, 532–535. [Google Scholar]
- Tang, Q.H.; Dai, P.Q.; Hua, N.B. Electrochemical properties of nanocrystalline Al0.3CoCrFeNi high-entropy alloy in alkaline solution. Mater. Mechan. Eng. 2015, 39, 1–4. [Google Scholar]
- Wang, C.; Zhang, Q.S.; Jiang, F.; Zhang, H.F.; Hu, Z.Q. Corrosion behavior of Zr55Al10Cu30Ni5 amorphous alloy in NaOH solution. Rare Metal Mater. Eng. 2003, 32, 814–817. [Google Scholar]
- Zhou, Q.Y.; Sheng, M.Q.; Zhong, Q.D.; Lin, H.; Niu, X.B.; Wang, Y. Electrochemical study for magnesium alloy passivating behavior in the NaOH solution with F−. Acta Chim. Sin. 2010, 68, 1487–1493. [Google Scholar]
- Hsu, Y.J.; Chiang, W.C.; Wu, J.K. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005, 92, 112–117. [Google Scholar] [CrossRef]
- Cao, C.N. Principle of Corrosion Electrochemistry; Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- Zhang, S.H.; Lian, J.; Feng, L.; Guo, Y.L. Reseach Progress on electrochemical and photo-electrochemical properties of passive film metal surface. Corr. Prot. 2011, 32, 381–384. [Google Scholar]
- Ismail, K.M.; Fathi, A.M.; Badawy, W.A. Effect of Nickel Content on the Corrosion and Passivation of Copper-Nickel Alloys in Sodium Sulfate Solutions. Corrosion 2004, 60, 795–803. [Google Scholar] [CrossRef]
- Yu, F.Z. Corrosion Resistance of Metal Materials; Science Press: Beijing, China, 1982. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).