Mapping the Accouterment Effects of Plasma Nitriding on AISI 316L in Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
Heat Treatment: Plasma Nitriding
3. Experimentation
3.1. Specimen Preparation for Immersion Test
3.1.1. Immersion Test
3.1.2. Ultrasonic Cleaning
4. Plan of Experiments:
Corrosion Rate by Weight Loss Method
- Weight loss (W): the amount of metal that is lost due to corrosion, expressed in milligrams.
- Density (D): the mass per unit volume of the metal, expressed in grams per cubic centimetre.
- Exposed surface area (A): the amount of surface area of the metal exposed to the corrosive environment, expressed in square inches.
- Exposure time (T): the length of time the metal is exposed to the corrosive environment, expressed in hours.
5. Results and Discussion
5.1. Results of Statistical Analysis of Experiments
5.2. ANOVA of Corrosion Test (Immersion Test) Results
5.3. Multiple Linear Regression Models
6. Confirmation Experiment
7. Electrochemical Behaviour of AISI 316L in Different Simulated Body Fluids
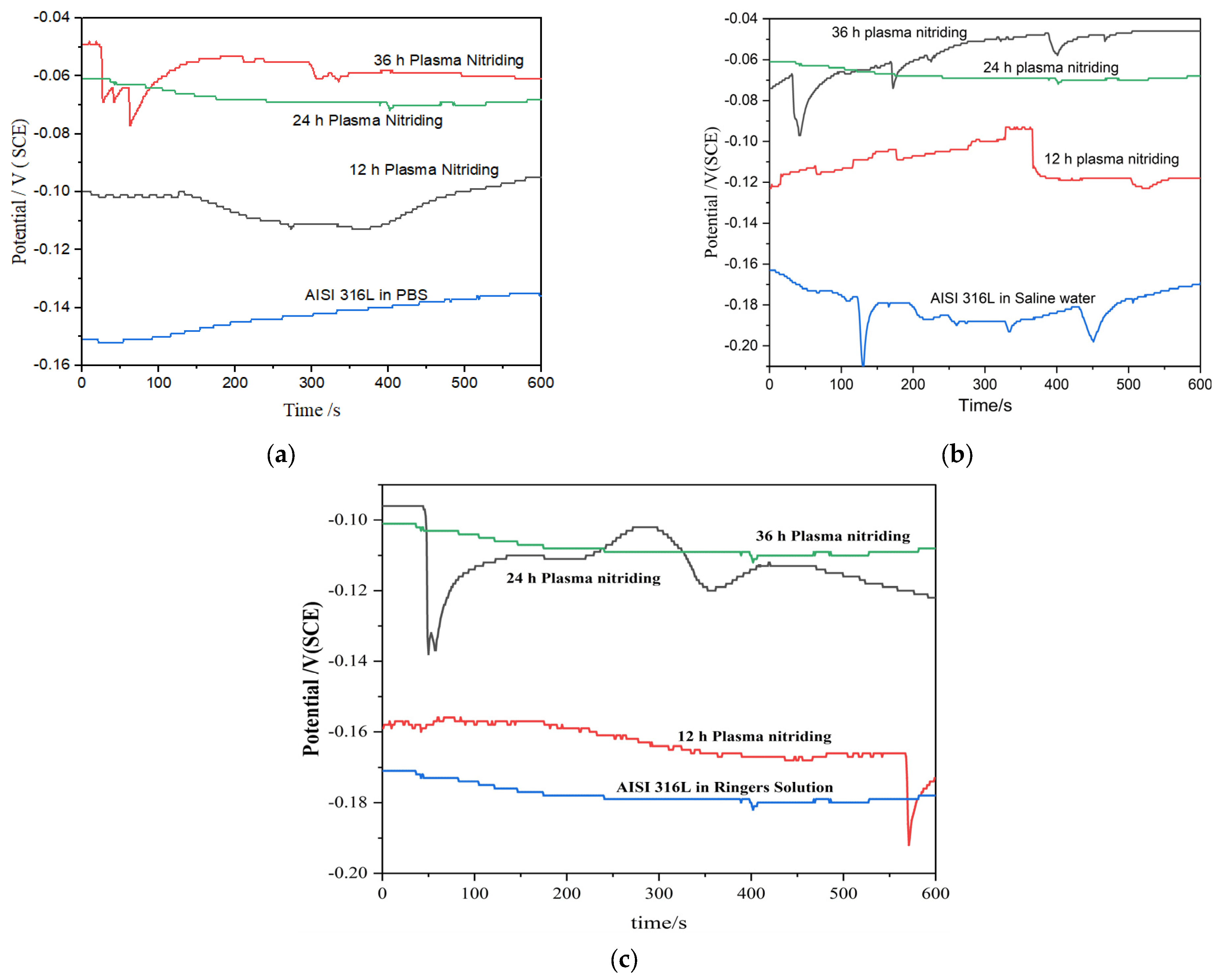
Open Circuit Potential
8. Surface Morphology
8.1. SEM Analysis of Plasma-Nitrided AISI 316L
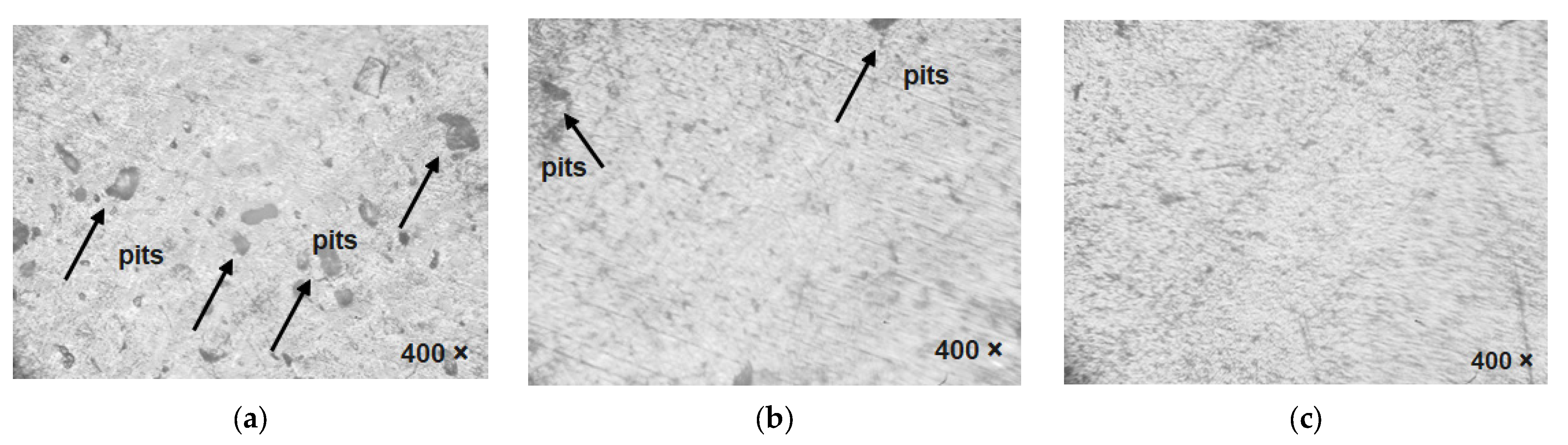
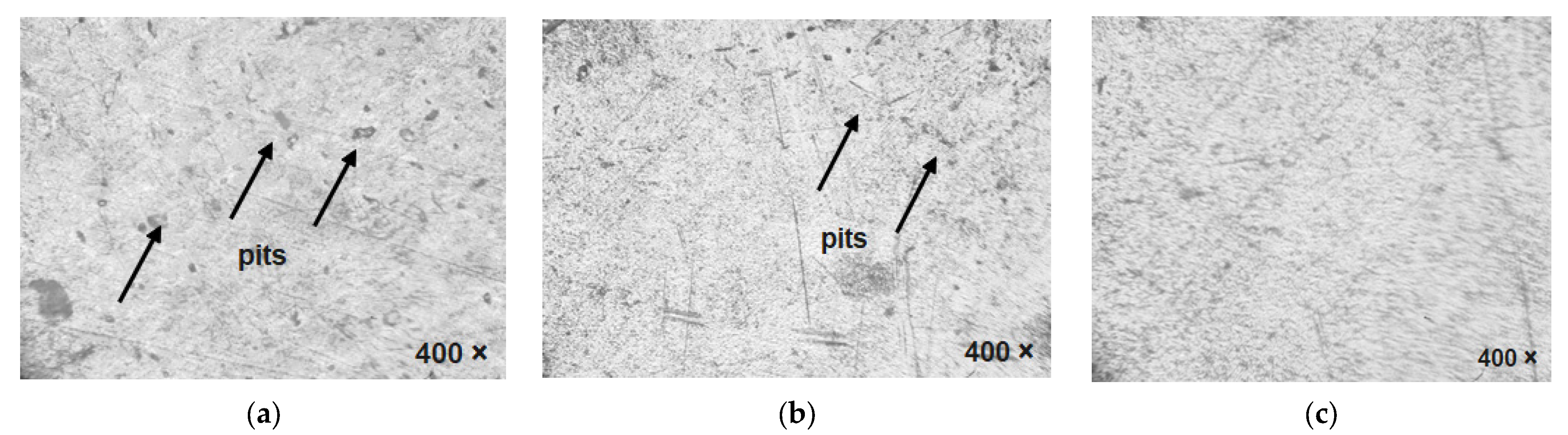
8.2. Optical Microscopic Analysis of Plasma-Nitrided AISI 316L in Various Simulated Body Fluids after the Immersion Test
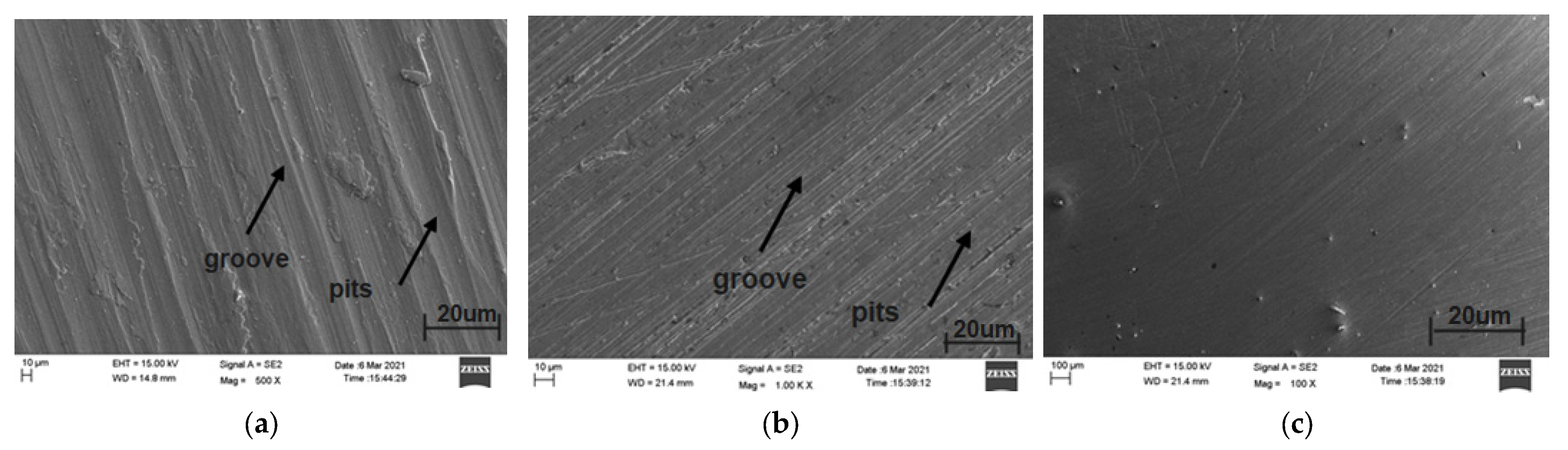
8.3. Scanning Electron Microscopy Analysis of Plasma-Nitrided AISI 316L in Various Simulated Body Fluids after Immersion Test
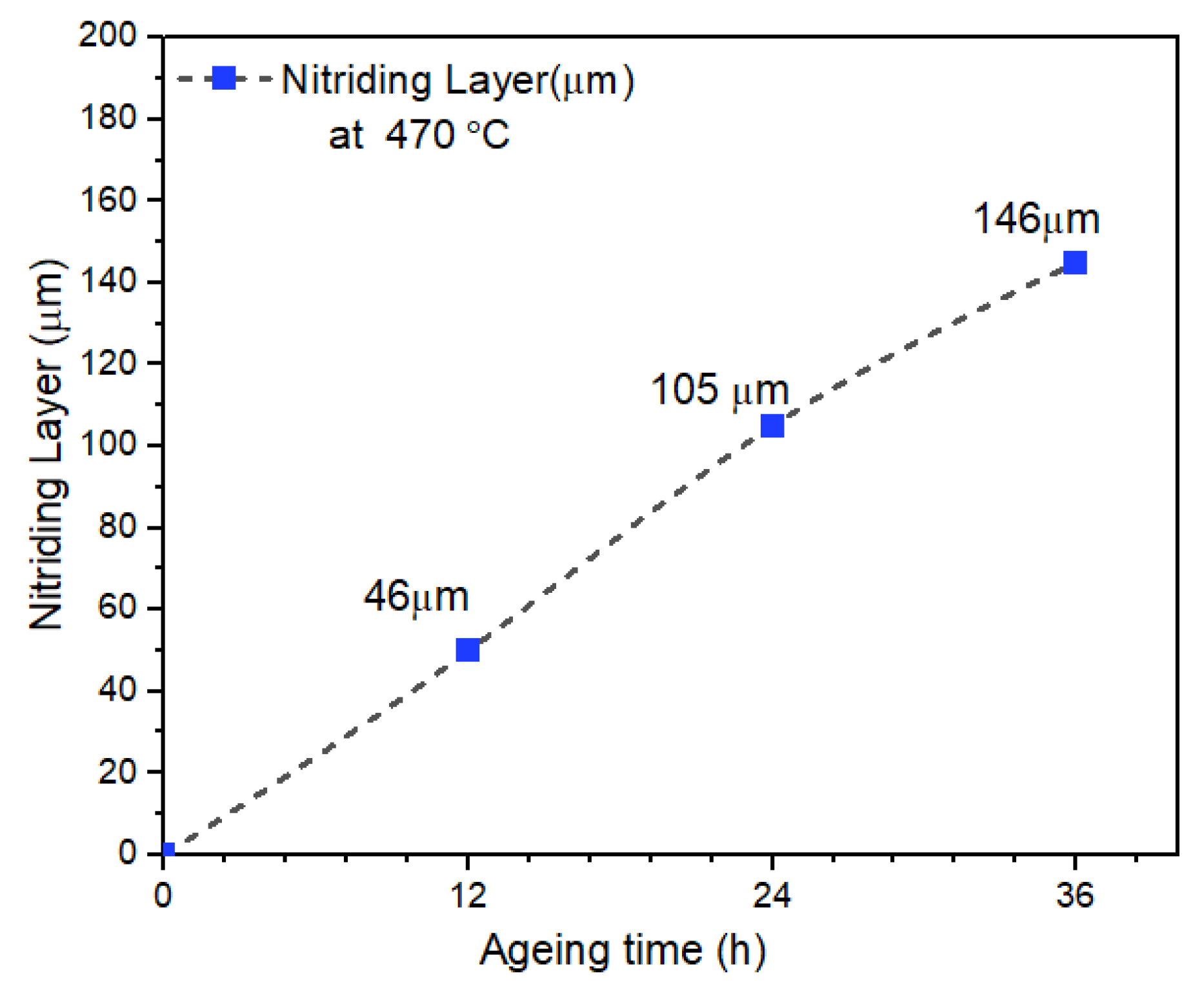
8.4. Microhardness and Case Depth of Plasma-Nitrided AISI 316L
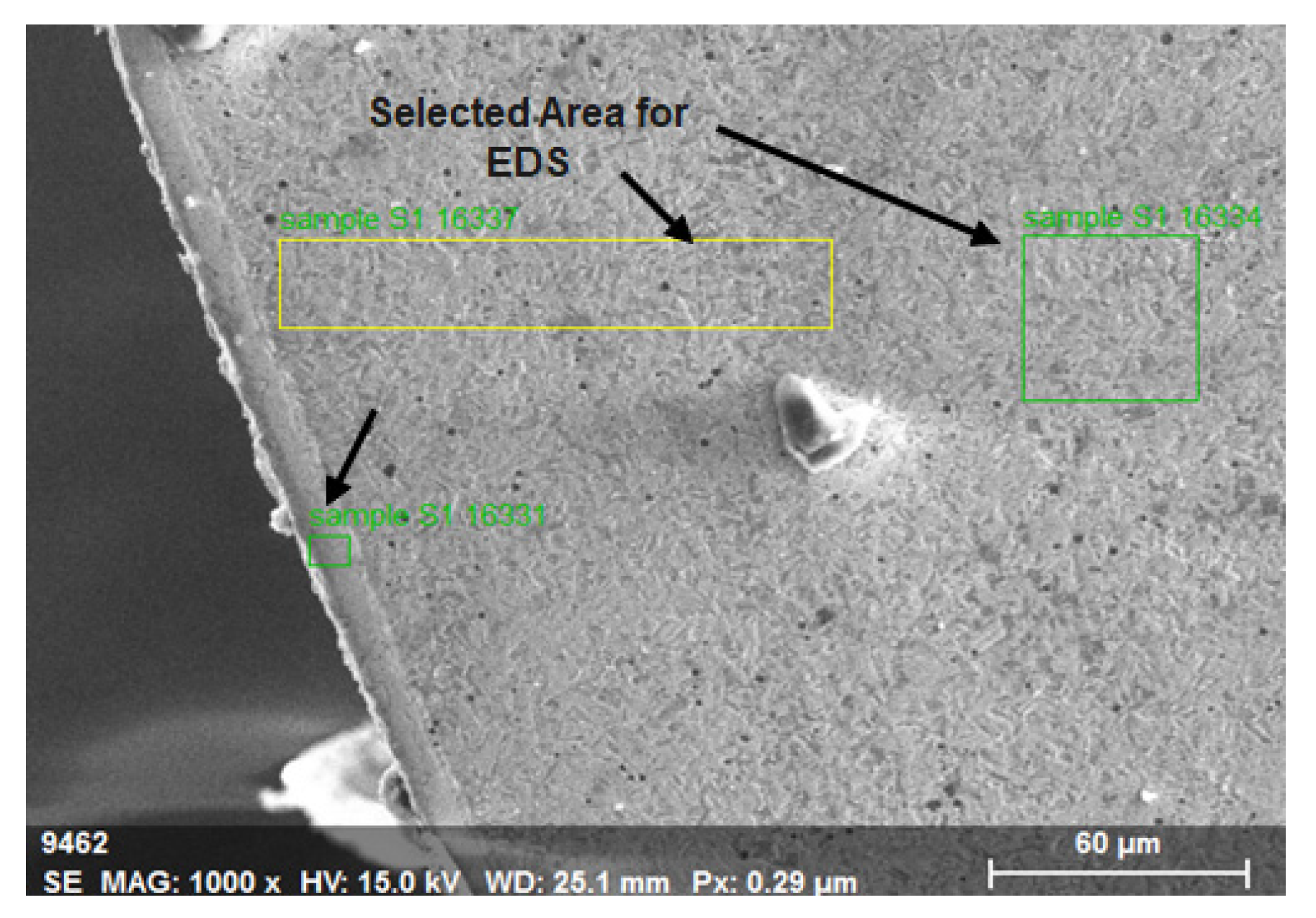
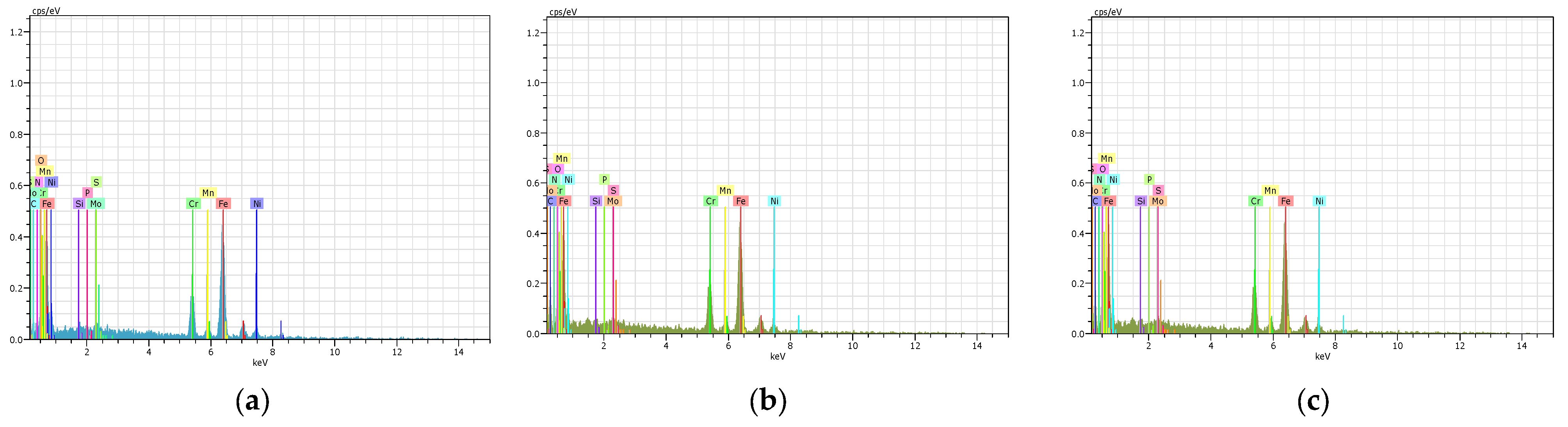
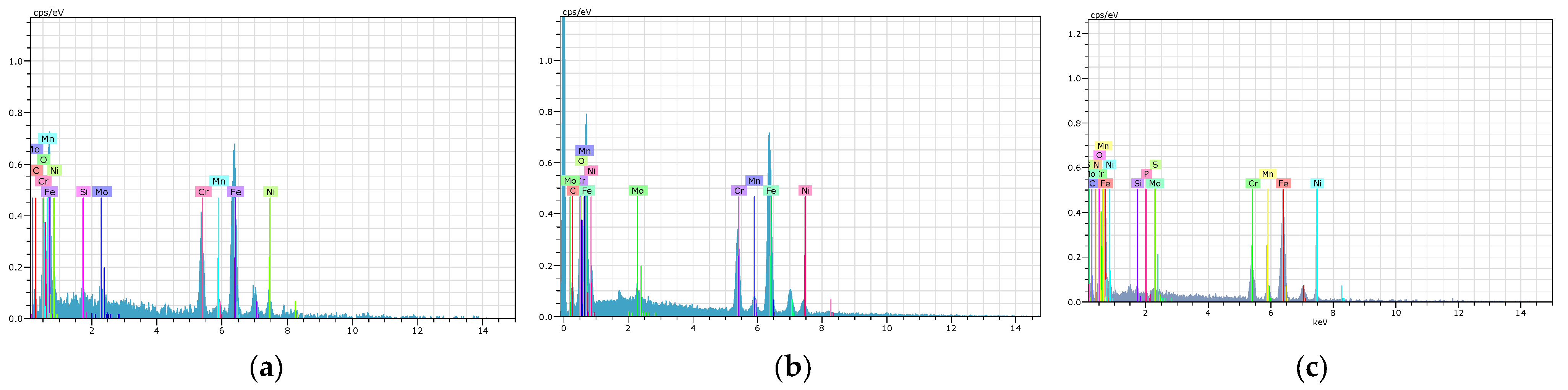
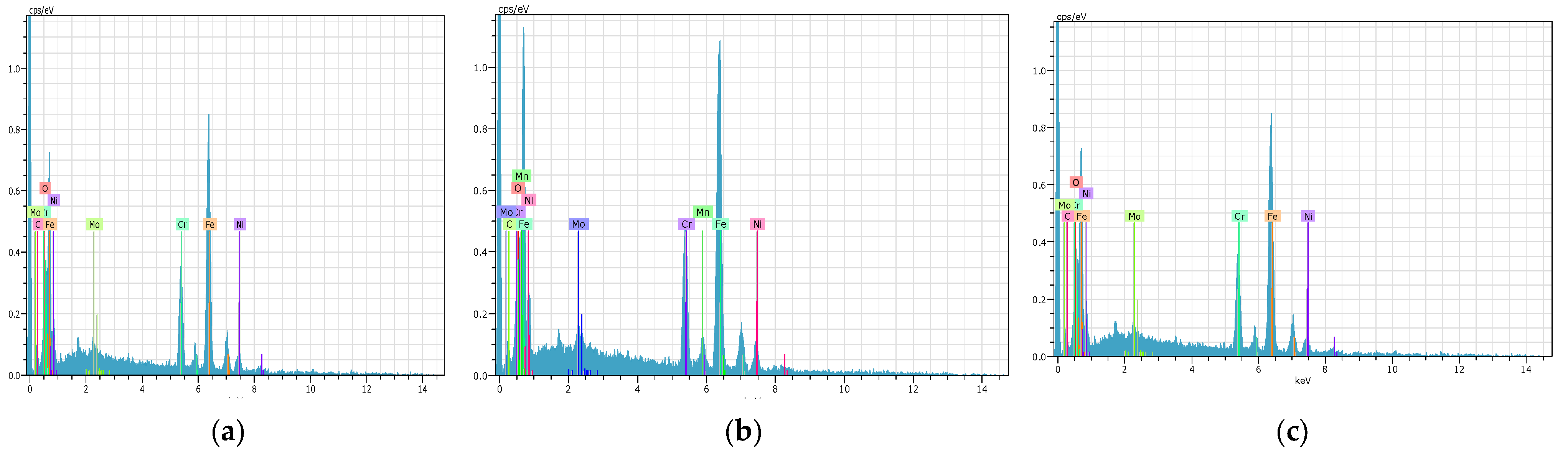
9. Energy-Dispersive X-Ray Spectroscopy(EDS) Analysis
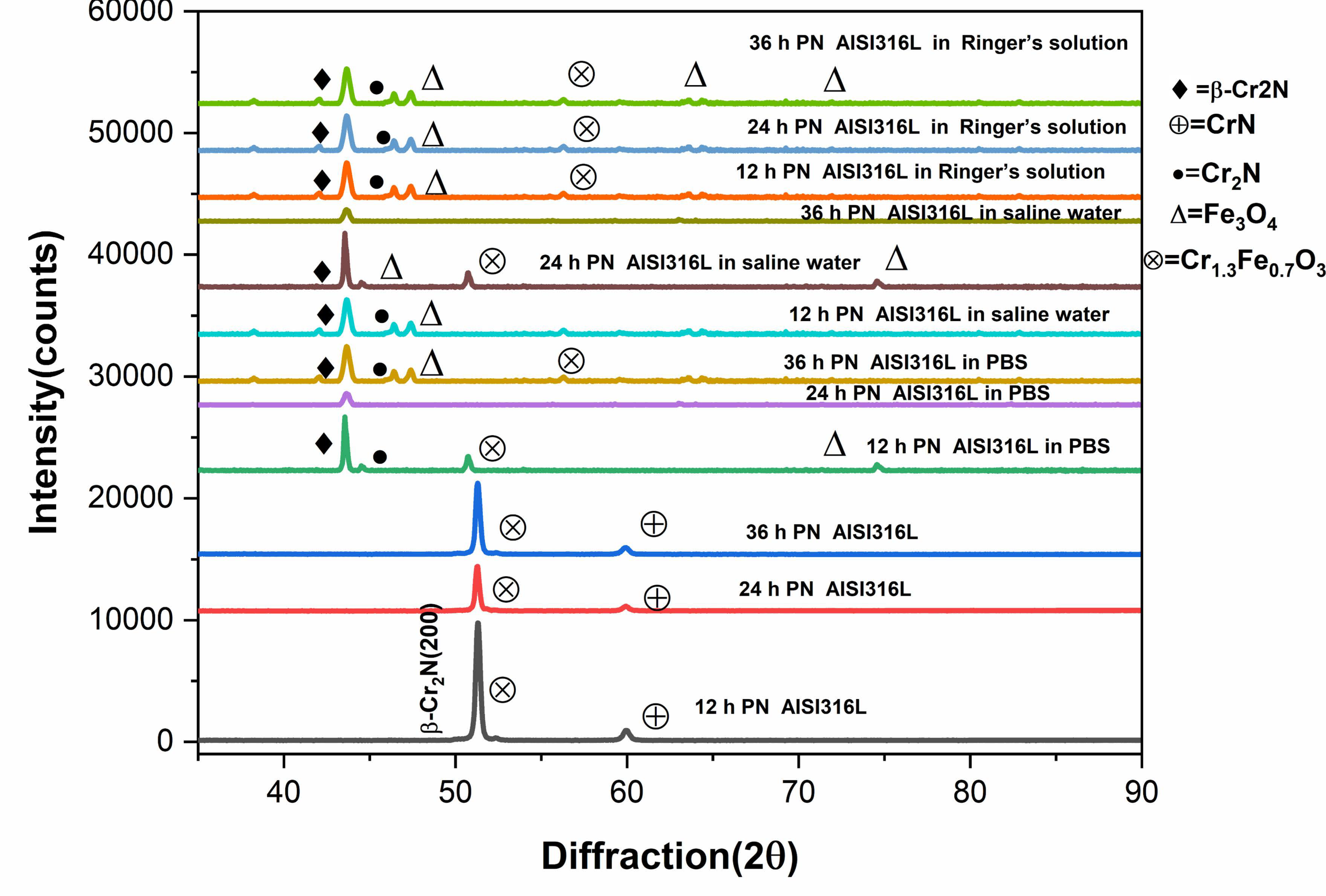
10. X-Ray Diffraction Analysis
11. Conclusions
- The microhardness of plasma nitride AISI 316L increased with increasing nitriding time (h). Microhardness was observed for plasma nitriding samples with ageing times of 12, 24, and 36 h at 1060, 1150, and 1220 HV0.1. The microhardness of plasma-nitrided samples improved by four times that of bare AISI 316L.
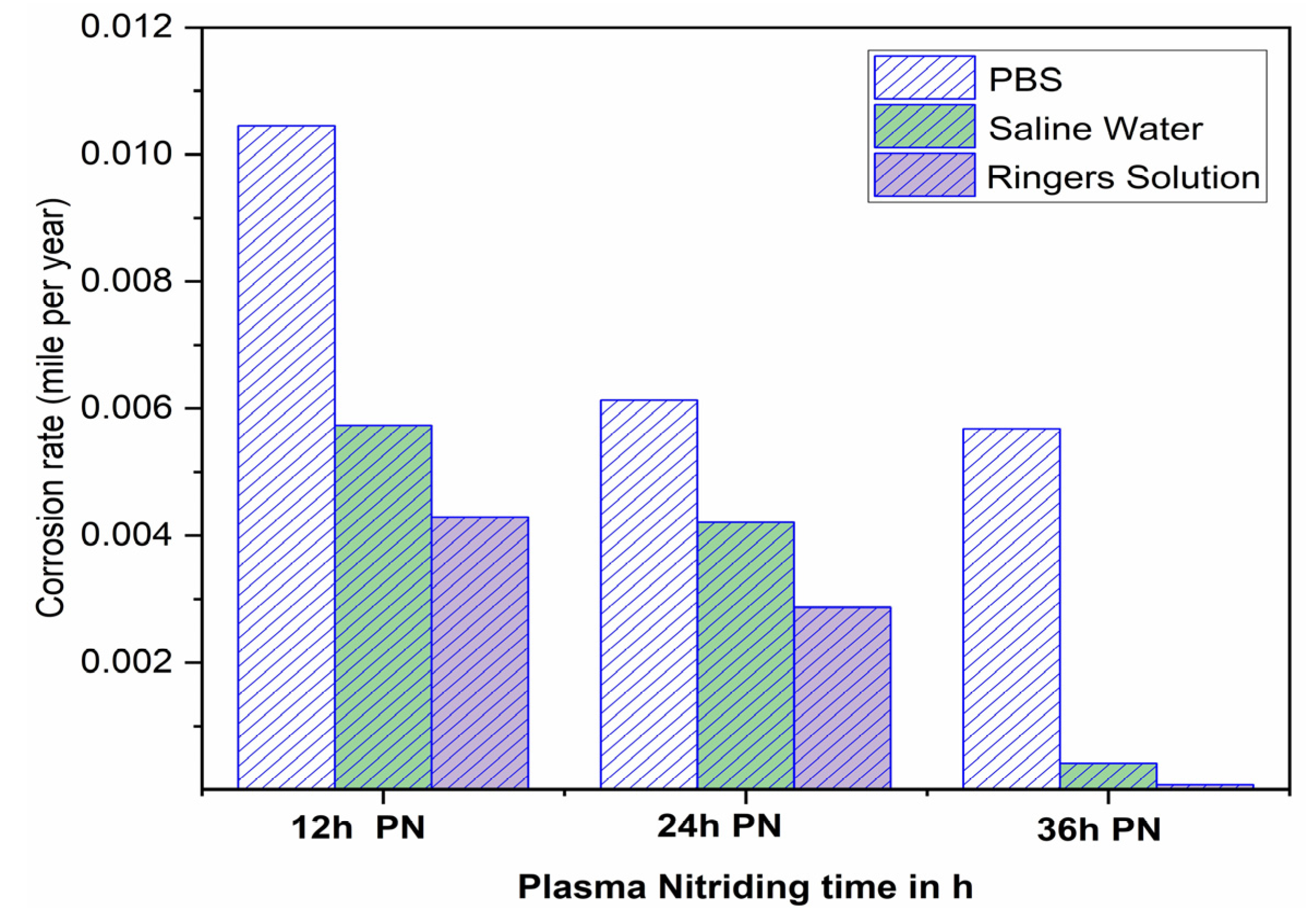
- The corrosion rate (mpy) of plasma nitride AISI 316L was analysed using Taguchi’s Design of experiments. Corrosion resistance plasma nitride AISI 316L increased with increasing ageing times (h) in various simulated body fluids, specifically in Ringer’s solution, and showed less corrosion resistance. PBS > Saline water > Ringer’s solution.
- The ranking of control factors using the response table for the S/N ratio obtained for different levels showed simulated body fluids as the dominant factor during the corrosion process, then plasma nitriding time (h), i.e., the thickness of diffusion layer.
- From the ANOVA of the immersion test, it was observed that simulated body fluids significantly influenced corrosion rate, followed by immersion time (days) and nitriding time (h). The minimum corrosion rate was observed for the corrosion rate in the following observed parameters: plasma nitriding (h) = 36 h, simulated body fluids = Ringer’s solution, and immersion time (days) = 27 days.
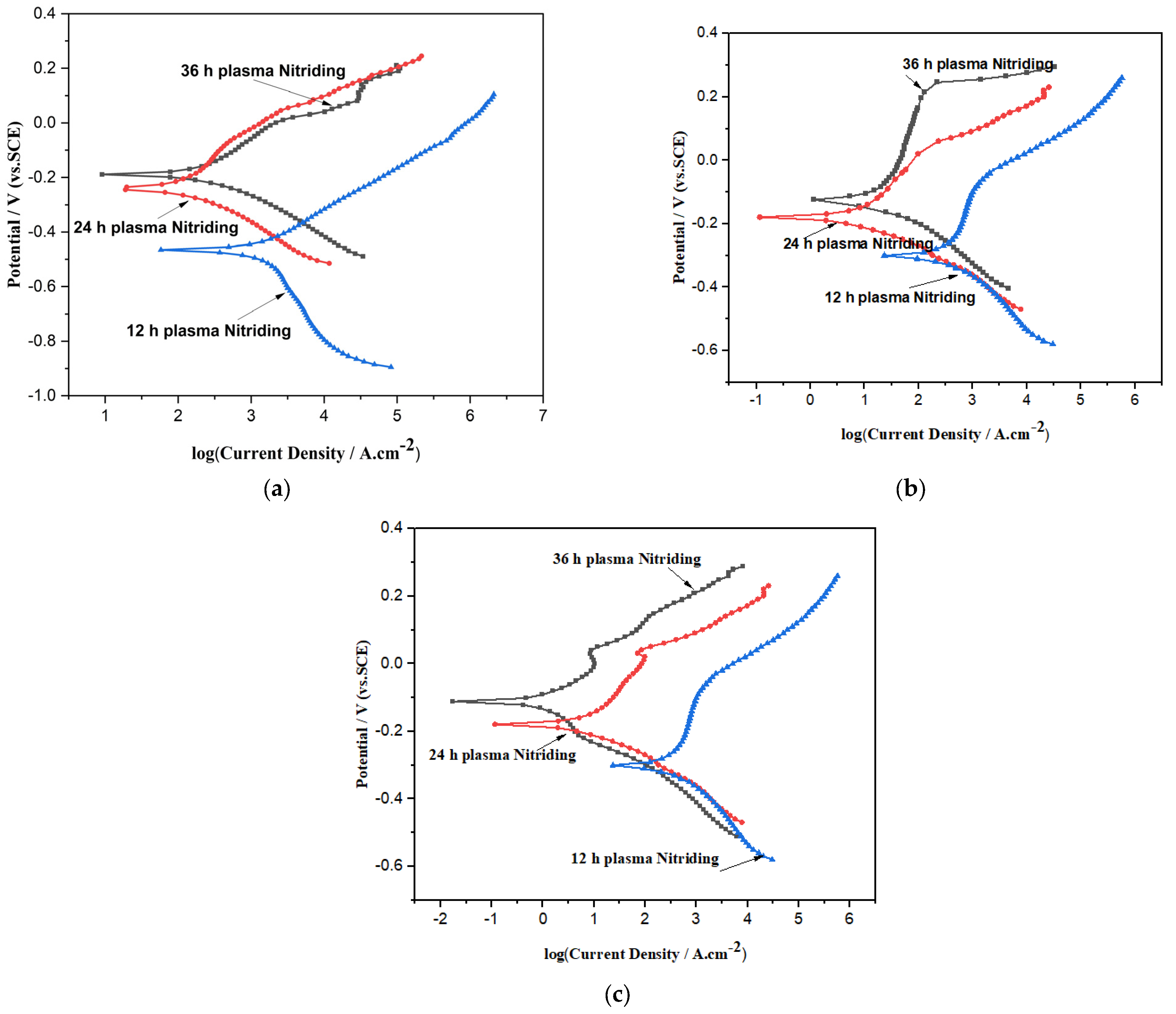
- The Tafel polarization curve showed that the corrosion rate(mpy) was decreased from 12 h plasma nitriding to 36 h plasma nitriding time in different simulated body fluids. In Ringer’s solution, a lower corrosion rate (i.e., 0.0000771 mpy) was observed compared to saline water and phosphate-buffered saline due to the formation of a passive layer on plasma-nitrided AISI 316L.
- A very high stable passivation range for both simulated bodily fluids of saline water and Ringer’s solution was shown at 36 h plasma-nitrided AISI 316L. In the Ringer’s solution, 36 h plasma-nitrided AISI 316L demonstrated much better corrosion resistance (4 times) than 12 h plasma-nitrided AISI 316L SS.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| S/N ratio | Signal-to-noise ratio |
| HV | Vickers’s hardness |
| XRD | X-ray diffraction |
| EDS | Energy-dispersive X-ray spectroscopy |
| ANOVA | Analysis of variance |
| PN | Plasma nitriding |
| IT | Immersion time |
| ED | Environmental details |
| OM | Optical microscopy |
| DOE | Design of experiments |
| SEM | Scanning electron microscope |
References
- Liu, T.; Chen, S.; Cheng, S.; Tian, J.; Chang, X.; Yin, Y. Corrosion behavior of super-hydrophobic surface on copper in seawater. Electrochim. Acta 2007, 52, 8003–8007. [Google Scholar] [CrossRef]
- Wang, K.; Cai, W. Modeling the effects of individual layer thickness and orientation on the tribocorrosion behavior of Al/Cu nanostructured metallic multilayers. Wear 2021, 477, 203849. [Google Scholar] [CrossRef]
- Buciumeanu, M.; Bagheri, A.; Shamsaei, N.; Thompson, S.M.; Silva, F.S.; Henriques, B. Tribocorrosion behavior of additive manufactured Ti-6Al-4V biomedical alloy. Tribol. Int. 2018, 119, 381–388. [Google Scholar] [CrossRef]
- Karimi, S.; Nickchi, T.; Alfantazi, A.M. Long-term corrosion investigation of AISI 316L, Co-28Cr-6Mo, and Ti-6Al-4V alloys in simulated body solutions. Appl. Surf. Sci. 2012, 258, 6087–6096. [Google Scholar] [CrossRef]
- Esfandiari, M.; Dong, H. The corrosion and corrosion–wear behaviour of plasma nitride 17-4PH precipitation hardening stainless steel. Surf. Coat. Technol. 2007, 202, 466–478. [Google Scholar] [CrossRef]
- Albayrak, Ç.; Hacısalihoğlu, İ.; Yenal Vangölü, S.; Alsaran, A. Tribocorrosion behavior of duplex treated pure titanium in Simulated Body Fluid. Wear 2013, 302, 1642–1648. [Google Scholar] [CrossRef]
- Gil, L.; Brühl, S.; Jiménez, L.; Leon, O.; Guevara, R.; Staia, M.H. Corrosion performance of the plasma nitrided 316L stainless steel. Surf. Coat. Technol. 2006, 201, 4424–4429. [Google Scholar] [CrossRef]
- Alsaran, A.; Çelik, A.; Çelik, C. Determination of the optimum conditions for ion nitriding of AISI 5140 steel. Surf. Coat. Technol. 2002, 160, 219–226. [Google Scholar] [CrossRef]
- Kartikasari, R.; Sutrisna; Aziz, I. Corrosion Behavior of Plasma Nitrided SS316L Biomaterial. Open Mater. Sci. J. 2017, 11, 29–37. [Google Scholar] [CrossRef]
- Robino, C.V.; Inal, O.T. Ion nitriding behavior of several low alloy steels. Mater. Sci. Eng. 1983, 59, 79–90. [Google Scholar] [CrossRef]
- Godec, M.; Donik, Č.; Kocijan, A.; Podgornik, B.; Skobir Balantič, D.A. Effect of post-treated low-temperature plasma nitriding on the wear and corrosion resistance of 316L stainless steel manufactured by laser powder-bed fusion. Addit. Manuf. 2020, 32, 101000. [Google Scholar] [CrossRef]
- Sun, Y.; Haruman, E. Effect of electrochemical potential on tribocorrosion behavior of low temperature plasma carburized 316L stainless steel in 1 M H2SO4 solution. Surf. Coat. Technol. 2011, 205, 4280–4290. [Google Scholar] [CrossRef]
- Garg, A.; Aggarwal, P.; Aggarwal, Y.; Belarbi, M.O.; Chalak, H.D.; Tounsi, A.; Gulia, R. Machine learning models for predicting the compressive strength of concrete containing nano silica. Comput. Concr. 2022, 30, 33–42. [Google Scholar] [CrossRef]
- Garg, A.; Belarbi, M.-O.; Tounsi, A.; Li, L.; Singh, A.; Mukhopadhyay, T. Predicting elemental stiffness matrix of FG nanoplates using Gaussian Process Regression based surrogate model in framework of layerwise model. Eng. Anal. Bound. Elem. 2022, 143, 779–795. [Google Scholar] [CrossRef]
- Deshpande, S.; Joshi, A.; Vagge, S.; Anekar, N. Corrosion behavior of nodular cast iron in biodiesel blends. Eng. Fail. Anal. 2019, 105, 1319–1327. [Google Scholar] [CrossRef]
- ASTM G31; Standard Practice for Laboratory Immersion Corrosion Testing of Metals. ASM Standards: West Conshohocken, PA, USA, 2019. [CrossRef]
- Chamane, L.; Surnam, B.Y.R. Corrosion Behaviour of Mild Steel in Atmospheric Exposure and Immersion Tests. Int. J. Innov. Res. Sci. Eng. Technol. 2017, 6. [Google Scholar]
- Gabryelczyk, A.; Ivanov, S.; Bund, A.; Lota, G. Taguchi method in experimental procedures focused on corrosion process of positive current collector in lithium-ion batteries. Electrochim. Acta 2020, 360, 137011. [Google Scholar] [CrossRef]
- Yousefieh, M.; Shamanian, M.; Saatchi, A. Optimization of the pulsed current gas tungsten arc welding (PCGTAW) parameters for corrosion resistance of super duplex stainless steel (UNS S32760) welds using the Taguchi method. J. Alloy. Compd. 2011, 509, 782–788. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, H.; Northwood, D.; Nie, X. Optimization of the electrolytic plasma oxidation processes for corrosion protection of magnesium alloy AM50 using the Taguchi method. J. Mater. Process. Technol. 2007, 182, 58–64. [Google Scholar] [CrossRef]
- Mali, A.S.; Vagge, S.T.; Kolekar, A. Tribological behaviour of LM25 hybrid metal matrix composites by using Taguchi’s techniques. Mater. Today Proc. 2022, 50, 1827–1834. [Google Scholar] [CrossRef]
- Radhika, N.; Subramaniam, R. Wear behaviour of aluminium/alumina/graphite hybrid metal matrix composites using Taguchi’s techniques. Ind. Lubr. Tribol. 2013, 65, 166–174. [Google Scholar] [CrossRef]
- Palanisamy, K.L.; Devabharathi, V.; Sundaram, N.M. Corrosion inhibition studies of mild steel with carrier oil stabilized of iron oxide nanoparticles incorporated into a paint. Int. J. ChemTech Res. 2014, 7, 1661–1664. [Google Scholar]
- Rathi, V.R.; Nirmal, S.D.; Kokate, S.J. Nirmal Corrosion study of mild steel, tor steel and CRS steel by weight loss method. J. Chem. Pharm. Res. 2010, 2, 97–100. [Google Scholar]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Monteiro, W.A.; Pereira, S.A.L.; Vatavuk, J. Nitriding Process Characterization of Cold Worked AISI 304 and 316 Austenitic Stainless Steels. J. Metall. 2017, 2017, 1052706. [Google Scholar] [CrossRef]
- Alves, C., Jr.; Lima, J.A.; Hajek, V.; da Cunha, J.B.M.; dos Santos, C.A. Effect of cooling rate on properties of plasma nitrided AISI 1010 steel. Surf. Coat. Technol. 2007, 201, 7566–7573. [Google Scholar] [CrossRef]
- Abrasonis, G.; Riviere, J.P.; Templier, C.; Declemy, A.; Pranevicius, L.; Milhet, X. Ion beam nitriding of single and polycrystalline austenitic stainless steel. J. Appl. Phys. 2005, 97, 083531. [Google Scholar] [CrossRef]
- Singh, V.; Marchev, K.; Cooper, C.V.; Meletis, E.I. Intensified plasma-assisted nitriding of AISI 316L stainless steel. Surf. Coat. Technol. 2002, 160, 249–258. [Google Scholar] [CrossRef]
- Fossati, A.; Borgioli, F.; Galvanetto, E.; Bacci, T. Glow-discharge nitriding of AISI 316L austenitic stainless steel: Influence of treatment time. Surf. Coat. Technol. 2006, 200, 3511–3517. [Google Scholar] [CrossRef]
- Feng, K. Corrosion behavior of SS316L in simulated and accelerated PEMFC environments. Int. J. Hydrog. Energy 2011, 36, 13032–13042. [Google Scholar] [CrossRef]
- Nosei, L.; Farina, S.; Ávalos, M.; Náchez, L.; Gómez, B.J.; Feugeas, J. Corrosion behavior of ion nitrided AISI 316L stainless steel. Thin Solid Film. 2008, 516, 1044–1050. [Google Scholar] [CrossRef]
- Vats, V.; Baskaran, T.; Arya, S.B. Tribo-corrosion study of nickel-free, high nitrogen and high manganese austenitic stainless steel. Tribol. Int. 2018, 119, 659–666. [Google Scholar] [CrossRef]
- Rastkar, A.R.; Akbari, S. Surface Characteristics of Silicon Nitride Compounds Deposited on Plasma Nitrided Austenitic Stainless Steels 316L. Chem. Mater. Eng. 2014, 2, 179–189. [Google Scholar] [CrossRef]
- Sun, Y.; Rana, V. Tribocorrosion behaviour of AISI 304 stainless steel in 0.5 M NaCl solution. Mater. Chem. Phys. 2011, 129, 138–147. [Google Scholar] [CrossRef]
- Sun, Y.; Bailey, R. Comparison of Wear Performance of Low Temperature Nitrided and Carburized 316L Stainless Steel under Dry Sliding and Corrosive-Wear Conditions. J. Mater. Eng. Perform. 2022, 32, 1238–1247. [Google Scholar] [CrossRef]
- Zhao, H.; Duan, L. High corrosion resistance performance of 304 stainless steel after liquid nitrocarburization. Compos. Eng. 2018, 155, 173–177. [Google Scholar] [CrossRef]
- De Frutos, A.; Arenas, M.A.; Fuentes, G.G.; Rodríguez, R.J.; Martínez, R.; Avelar-Batista, J.C.; de Damborenea, J.J. Tribocorrosion behaviour of duplex surface treated AISI 304 stainless steel. Surf. Coat. Technol. 2010, 204, 1623–1630. [Google Scholar] [CrossRef]
- Díaz-Guillén, J.C.; Granda-Gutiérrez, E.E.; Vargas-Gutiérrez, G.; Díaz-Guillén, M.R.; Aguilar-Martínez, J.A.; Álvarez-Contreras, L. Effect of Nitriding Current Density on the Surface Properties and Crystallite Size of Pulsed Plasma-Nitrided AISI 316L. J. Mater. Sci. Chem. Eng. 2015, 3, 45–51. [Google Scholar] [CrossRef]
- Drouet, M.; Stinville, J.C.; Villechaise, P.; Rivière, J.P.; Templier, C. Surface evolution during low-temperature plasma-assisted nitriding of austenitic stainless steel. Eur. Phys. J. Appl. Phys. 2008, 43, 349–351. [Google Scholar] [CrossRef]








| Solution | Compositions |
|---|---|
| PBS | NaCl (8 g/L) KCl (0.2 g/L) Na2HPO4 (1.44 g/L) KH2PO4 (0.254 g/L) |
| Saline Water | Dextrose (5% w/v) Sodium Chloride (0.9% w/v) |
| Ringer’s solution | NaCl (8.6 g/L) KCl (0.3 g/L) CaCl2 (0.33 g/L) |
| Level | Nitriding Time (h) | Simulated Body Fluids | Immersion Time (days) |
|---|---|---|---|
| 1 | 12 | PBS | 9 |
| 2 | 24 | Saline Water | 18 |
| 3 | 36 | Ringer’s solution | 27 |
| Input Parameters | Response | |||
|---|---|---|---|---|
| Sr. No. | Plasma Nitriding (h) | Environmental Details | Immersion Time (days) | Corrosion Rate (mpy) |
| 1 | 12 | PBS | 9 | 0.100 |
| 2 | 12 | PBS | 9 | 0.120 |
| 3 | 12 | PBS | 9 | 0.110 |
| 4 | 12 | Saline Water | 18 | 0.130 |
| 5 | 12 | Saline Water | 18 | 0.123 |
| 6 | 12 | Saline Water | 18 | 0.132 |
| 7 | 12 | Ringer’s solution | 27 | 0.440 |
| 8 | 12 | Ringer’s solution | 27 | 0.430 |
| 9 | 12 | Ringer’s solution | 27 | 0.440 |
| 10 | 24 | PBS | 18 | 0.230 |
| 11 | 24 | PBS | 18 | 0.240 |
| 12 | 24 | PBS | 18 | 0.241 |
| 13 | 24 | Saline Water | 27 | 0.231 |
| 14 | 24 | Saline Water | 27 | 0.232 |
| 15 | 24 | Saline Water | 27 | 0.211 |
| 16 | 24 | Ringer’s solution | 9 | 0.441 |
| 17 | 24 | Ringer’s solution | 9 | 0.423 |
| 18 | 24 | Ringer’s solution | 9 | 0.415 |
| 19 | 36 | PBS | 27 | 0.312 |
| 20 | 36 | PBS | 27 | 0.321 |
| 21 | 36 | PBS | 27 | 0.303 |
| 22 | 36 | Saline Water | 9 | 0.311 |
| 23 | 36 | Saline Water | 9 | 0.302 |
| 24 | 36 | Saline Water | 9 | 0.311 |
| 25 | 36 | Ringer’s solution | 18 | 0.451 |
| 26 | 36 | Ringer’s solution | 18 | 0.405 |
| 27 | 36 | Ringer’s solution | 18 | 0.435 |
| Exp. No. | Plasma Nitriding (h) | Environmental Details | Immersion Time (Days) | Corrosion Rate (mpy) | S/N Ratio | Corrosion Rate (mpy) |
|---|---|---|---|---|---|---|
| 1 | 12 | PBS | 9 | 0.100 | 19.1483 | 0.100 |
| 2 | 12 | PBS | 9 | 0.120 | 19.1483 | 0.120 |
| 3 | 12 | PBS | 9 | 0.110 | 19.1483 | 0.110 |
| 4 | 12 | Saline Water | 18 | 0.130 | 17.8293 | 0.130 |
| 5 | 12 | Saline Water | 18 | 0.123 | 17.8293 | 0.123 |
| 6 | 12 | Saline Water | 18 | 0.132 | 17.8293 | 0.132 |
| 7 | 12 | Ringer’s solution | 27 | 0.440 | 7.1965 | 0.440 |
| 8 | 12 | Ringer’s solution | 27 | 0.430 | 7.1965 | 0.430 |
| 9 | 12 | Ringer’s solution | 27 | 0.440 | 7.1965 | 0.440 |
| 10 | 24 | PBS | 18 | 0.230 | 12.5031 | 0.230 |
| 11 | 24 | PBS | 18 | 0.240 | 12.5031 | 0.240 |
| 12 | 24 | PBS | 18 | 0.241 | 12.5031 | 0.241 |
| 13 | 24 | Saline Water | 27 | 0.231 | 12.9612 | 0.231 |
| 14 | 24 | Saline Water | 27 | 0.232 | 12.9612 | 0.232 |
| 15 | 24 | Saline Water | 27 | 0.211 | 12.9612 | 0.211 |
| 16 | 24 | Ringer’s solution | 9 | 0.441 | 7.4022 | 0.441 |
| 17 | 24 | Ringer’s solution | 9 | 0.423 | 7.4022 | 0.423 |
| 18 | 24 | Ringer’s solution | 9 | 0.415 | 7.4022 | 0.415 |
| 19 | 36 | PBS | 27 | 0.312 | 10.1145 | 0.312 |
| 20 | 36 | PBS | 27 | 0.321 | 10.1145 | 0.321 |
| 21 | 36 | PBS | 27 | 0.303 | 10.1145 | 0.303 |
| 22 | 36 | Saline Water | 9 | 0.311 | 10.2282 | 0.311 |
| 23 | 36 | Saline Water | 9 | 0.302 | 10.2282 | 0.302 |
| 24 | 36 | Saline Water | 9 | 0.311 | 10.2282 | 0.311 |
| 25 | 36 | Ringer’s solution | 18 | 0.451 | 7.3154 | 0.451 |
| 26 | 36 | Ringer’s solution | 18 | 0.405 | 7.3154 | 0.405 |
| 27 | 36 | Ringer’s solution | 18 | 0.435 | 7.3154 | 0.435 |
| Level | PN (h) | ED | IT (Days) |
|---|---|---|---|
| 1 | 14.725 | 13.992 | 12.260 |
| 2 | 10.956 | 13.673 | 12.549 |
| 3 | 9.219 | 7.305 | 10.091 |
| Delta | 5.505 | 6.617 | 2.459 |
| Rank | 2 | 1 | 3 |
| Source | DF | Seq. SS | Adj. SS | Adj. MS | F | P | P% |
|---|---|---|---|---|---|---|---|
| Plasma Nitriding (h) | 2 | 0.02362 | 0.02362 | 0.01181 | 3.02 | 0.249 | 18.73% |
| Environmental Details | 2 | 0.89136 | 0.89136 | 0.04456 | 11.38 | 0.089 | 70.62% |
| Immersion time (days) | 2 | 0.00561 | 0.00561 | 0.002810 | 0.72 | 0.582 | 4.44% |
| Residual Error | 2 | 0.00783 | 0.00783 | 0.003917 | 6.20% | ||
| Total | 8 | 0.12621 |
| Exp. No. | Nitriding Time (h) | Simulated Body Fluids | Immersion Time (Days) |
|---|---|---|---|
| 1 | 13 | PBS | 8 |
| 2 | 28 | Saline Water | 16 |
| 3 | 35 | Ringer’s solution | 25 |
| Regression Model Equation (3) | |||
|---|---|---|---|
| Exp. No. | Exp. Corrosion Rate (mpy) | Corrosion Rate (mpy) | % Error |
| 1 | 0.162 | 0.148 | 8.61 |
| 2 | 0.249 | 0.235 | 5.30 |
| 3 | 0.312 | 0.293 | 5.83 |
| Regression model Equation (4) | |||
| 1 | 0.154 | 0.148 | 3.59 |
| 2 | 0.249 | 0.236 | 5.32 |
| 3 | 0.462 | 0.447 | 3.30 |
| Regression model Equation (5) | |||
| 1 | 0.374 | 0.359 | 3.90 |
| 2 | 0.463 | 0.447 | 3.41 |
| 3 | 0.542 | 0.505 | 6.78 |
| 12 h PN | 24 h PN | 36 h PN | |
|---|---|---|---|
| Ecorr (V SCE) | −4410 ± 0.002 | −2241 ± 0.002 | −1981 |
| icorr (μA/cm2) | 0.0232 | 0.01277 | 0.009566 |
| Rp Ohm | 4.944 × 104 | 1.235 × 105 | 3.823 × 105 |
| βa (V/dec) | 0.131 | 0.258 | 0.160 |
| βc (V/dec) | 0.044 | 0.040 | 0.083 |
| Corrosion Rate (mile/year) | 0.01045 | 0.005734 | 0.00429 |
| 12 h PN | 24 h PN | 36 h PN | |
|---|---|---|---|
| Ecorr (V SCE) | −4622 ± 0.002 | −2211 ± 0.002 | −1181 ± 0.002 |
| icorr (μA/cm2) | 0.0136 | 0.0009393 | 0.00064 |
| Rp Ohm | 3.823 × 105 | 1.235 × 105 | 4.944 × 104 |
| βa (V/dec) | 0.160 | 0.198 | 0.131 |
| βc (V/dec) | 0.083 | 0.040 | 0.044 |
| Corrosion Rate (mile/year) | 0.00613 | 0.00421 | 0.00287 |
| 12 h PN | 24 h PN | 36 h PN | |
|---|---|---|---|
| Ecorr (V SCE) | −3112 ± 0.002 | −1911 ± 0.002 | −1081 ± 0.002 |
| icorr (μA/cm2) | 0.0126 | 9.293 × 10−5 | 0.0001718 |
| Rp Ohm | 4.944 × 104 | 1.235 × 105 | 3.823 × 105 |
| βa (V/dec) | 0.132 | 0.186 | 0.158 |
| βc (V/dec) | 0.053 | 0.040 | 0.045 |
| Corrosion Rate (mile/year) | 0.00568 | 0.000417 | 0.0000771 |
| Sr. No. | Plasma Nitriding Period (h) | Micro-Hardness HV0.1 |
|---|---|---|
| 1. | 0 | 334 |
| 2. | 12 | 1026 |
| 3. | 24 | 1150 |
| 4. | 36 | 1220 |
| Elements | Series | Weight% | Atomic% | Weight% | Atomic% | Weight% | Atomic% |
|---|---|---|---|---|---|---|---|
| PN 12 h AISI 316L | PN 24 h AISI 316L | PN 36 h AISI 316L | |||||
| Fe | K | 60.41 | 44.31 | 58.4 | 41.84 | 62 | 44.52 |
| Cr | K | 11.49 | 9.46 | 13.2 | 8.22 | 10.2 | 8.52 |
| Ni | K | 8.53 | 6.54 | 5.51 | 6.51 | 8.62 | 5.5 |
| C | K | 5.18 | 17.39 | 6.27 | 14.13 | 5.19 | 16 |
| N | K | 8.94 | 15.21 | 9.14 | 16.75 | 9.95 | 19.05 |
| Mn | K | 1.78 | 1.3 | 1.4 | 1.03 | 0.46 | 0.33 |
| O | K | 1.69 | 4.26 | 4.11 | 10.15 | 1.96 | 4.89 |
| Mo | L | 1.25 | 0.53 | 1.42 | 0.45 | 1.12 | 0.47 |
| Si | K | 0.4 | 0.57 | 0.33 | 0.56 | 0.3 | 0.45 |
| P | K | 0.18 | 0.24 | 0.12 | 0.17 | 0.1 | 0.15 |
| S | K | 0.15 | 0.19 | 0.1 | 0.19 | 0.1 | 0.12 |
| Elements | Series | Weight% | Atomic% | Weight% | Atomic% | Weight% | Atomic% |
|---|---|---|---|---|---|---|---|
| PN 12 h AISI 316L in PBS | PN 24 h AISI 316L in PBS | PN 36 h AISI 316L in PBS | |||||
| Fe | K | 61.41 | 45.33 | 59.4 | 41.77 | 61.5 | 43.45 |
| Cr | K | 10.66 | 10.4 | 12.26 | 8.31 | 10.42 | 8.4 |
| Ni | K | 8.44 | 6.01 | 5.22 | 6.26 | 8.45 | 4.53 |
| C | K | 4.99 | 16.12 | 6.33 | 14.33 | 5.22 | 12.5 |
| N | K | 9.01 | 15 | 9.22 | 16.75 | 9.96 | 18.07 |
| Mn | K | 1.79 | 1.3 | 1.39 | 1.03 | 0.47 | 0.34 |
| O | K | 1.7 | 4.26 | 4.15 | 10.15 | 1.96 | 11.45 |
| Mo | L | 1.23 | 0.54 | 1.45 | 0.46 | 1.45 | 0.49 |
| Si | K | 0.44 | 0.59 | 0.34 | 0.56 | 0.35 | 0.48 |
| P | K | 0.19 | 0.25 | 0.13 | 0.18 | 0.12 | 0.16 |
| S | K | 0.14 | 0.2 | 0.11 | 0.2 | 0.1 | 0.13 |
| Elements | Series | Weight% | Atomic% | Weight% | Atomic% | Weight% | Atomic% |
|---|---|---|---|---|---|---|---|
| PN 12 h AISI 316L in Saline Water | PN 24 h AISI 316L in Saline Water | PN 36 h AISI 316L in Saline Water | |||||
| Fe | K | 61.31 | 44.31 | 58.4 | 41.01 | 62.18 | 42.5 |
| Cr | K | 10.01 | 9.46 | 13.2 | 8.23 | 10.1 | 7.53 |
| Ni | K | 8.58 | 6.54 | 5.51 | 6.1 | 8.3 | 3.33 |
| C | K | 5.13 | 17.39 | 6.27 | 14.13 | 5.5 | 16 |
| N | K | 8.99 | 15.21 | 9.14 | 16.7 | 9.95 | 18.04 |
| Mn | K | 1.75 | 1.3 | 1.4 | 1.19 | 1.1 | 0.32 |
| O | K | 1.64 | 4.26 | 4.11 | 11.3 | 1.25 | 11.88 |
| Mo | L | 1.65 | 0.53 | 1.42 | 0.4 | 1.07 | 0.48 |
| Si | K | 0.6 | 0.57 | 0.33 | 0.57 | 0.31 | 0.46 |
| P | K | 0.18 | 0.24 | 0.12 | 0.18 | 0.12 | 0.14 |
| S | K | 0.16 | 0.19 | 0.1 | 0.19 | 0.12 | 0.13 |
| Elements | Series | Weight% | Atomic% | Weight% | Atomic% | Weight% | Atomic% |
|---|---|---|---|---|---|---|---|
| PN 12 h AISI 316L in Ringer’s Solution | PN 24 h AISI 316L in Ringer’s Solution | PN 36 h AISI 316L in Ringer’s Solution | |||||
| Fe | K | 60.9 | 43.5 | 58.98 | 42 | 61.5 | 42.72 |
| Cr | K | 11.4 | 9.46 | 13.2 | 8.22 | 10.22 | 6.57 |
| Ni | K | 8.13 | 6.54 | 4.93 | 6.5 | 8.8 | 5.22 |
| C | K | 5.01 | 17.35 | 6.27 | 14.13 | 5.19 | 15 |
| N | K | 8.94 | 16 | 9.1 | 16.53 | 9.95 | 18.05 |
| Mn | K | 1.78 | 1.3 | 1.4 | 1.04 | 0.46 | 0.33 |
| O | K | 1.69 | 4.26 | 4.11 | 10.14 | 1.96 | 10.9 |
| Mo | L | 1.35 | 0.55 | 1.45 | 0.49 | 1.45 | 0.47 |
| Si | K | 0.45 | 0.59 | 0.32 | 0.58 | 0.25 | 0.46 |
| P | K | 0.19 | 0.25 | 0.13 | 0.17 | 0.11 | 0.15 |
| S | K | 0.16 | 0.2 | 0.11 | 0.2 | 0.11 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mali, A.S.; Vagge, S.T.; Rathod, M.J. Mapping the Accouterment Effects of Plasma Nitriding on AISI 316L in Biomedical Applications. Coatings 2023, 13, 839. https://doi.org/10.3390/coatings13050839
Mali AS, Vagge ST, Rathod MJ. Mapping the Accouterment Effects of Plasma Nitriding on AISI 316L in Biomedical Applications. Coatings. 2023; 13(5):839. https://doi.org/10.3390/coatings13050839
Chicago/Turabian StyleMali, Amol Shivaji, Shashikant Tukaram Vagge, and Manoj Jagannath Rathod. 2023. "Mapping the Accouterment Effects of Plasma Nitriding on AISI 316L in Biomedical Applications" Coatings 13, no. 5: 839. https://doi.org/10.3390/coatings13050839
APA StyleMali, A. S., Vagge, S. T., & Rathod, M. J. (2023). Mapping the Accouterment Effects of Plasma Nitriding on AISI 316L in Biomedical Applications. Coatings, 13(5), 839. https://doi.org/10.3390/coatings13050839






