Effect of Surface Roughness on Static Corrosion Behavior of J55 Carbon Steel in CO2-Containing Geothermal Water at 65 °C
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
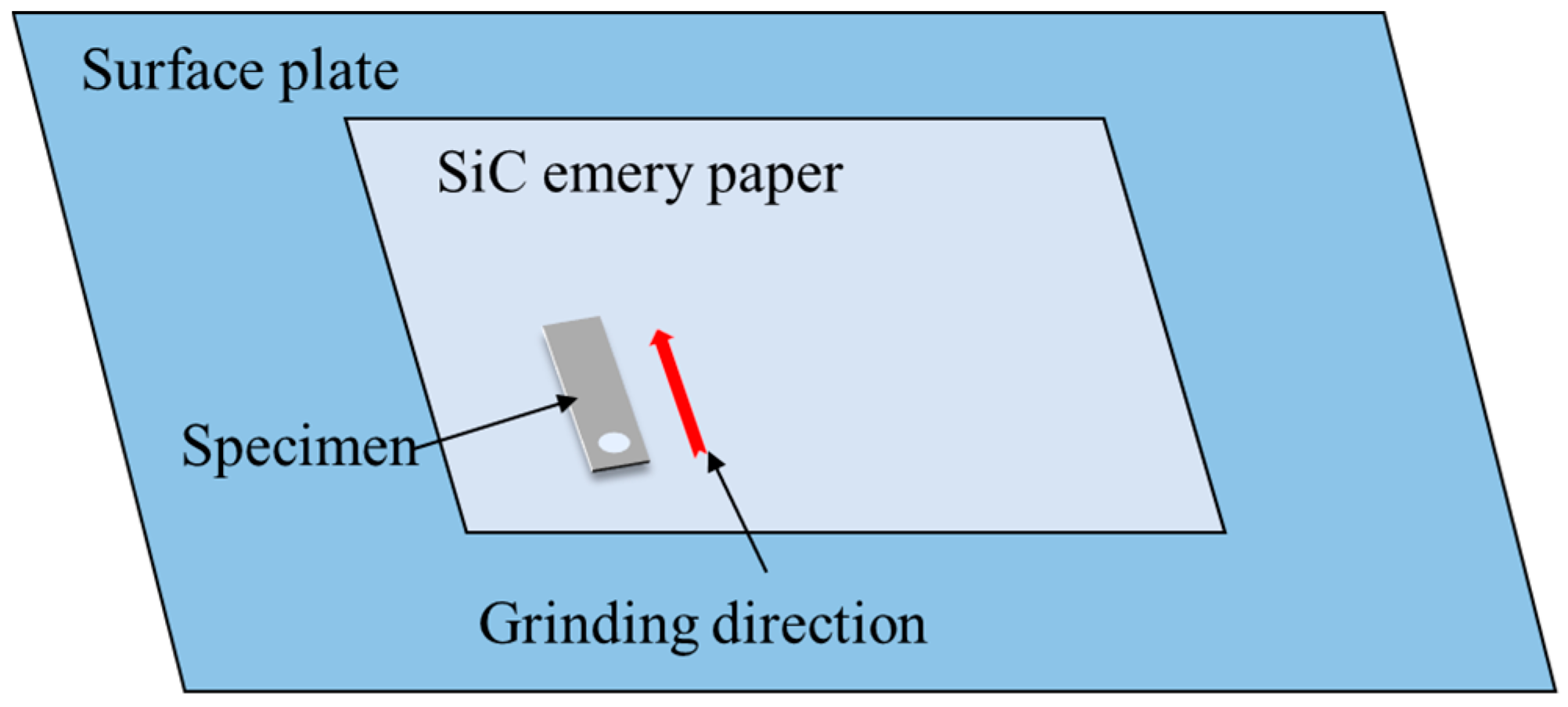
2.2. Surface Finish
2.3. Surface Characterization
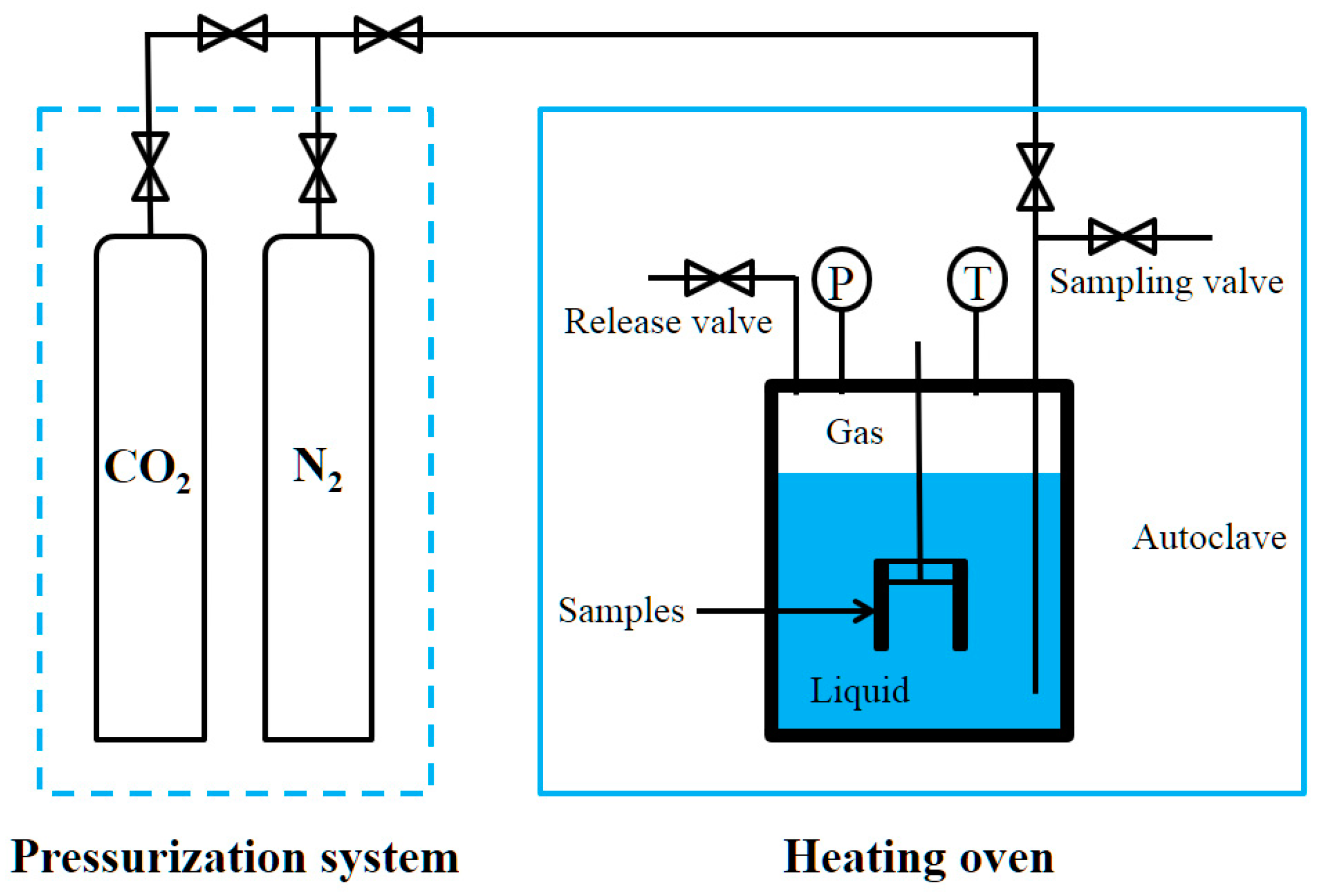
2.4. Weight-Loss Method
2.5. Surface Analysis
3. Results
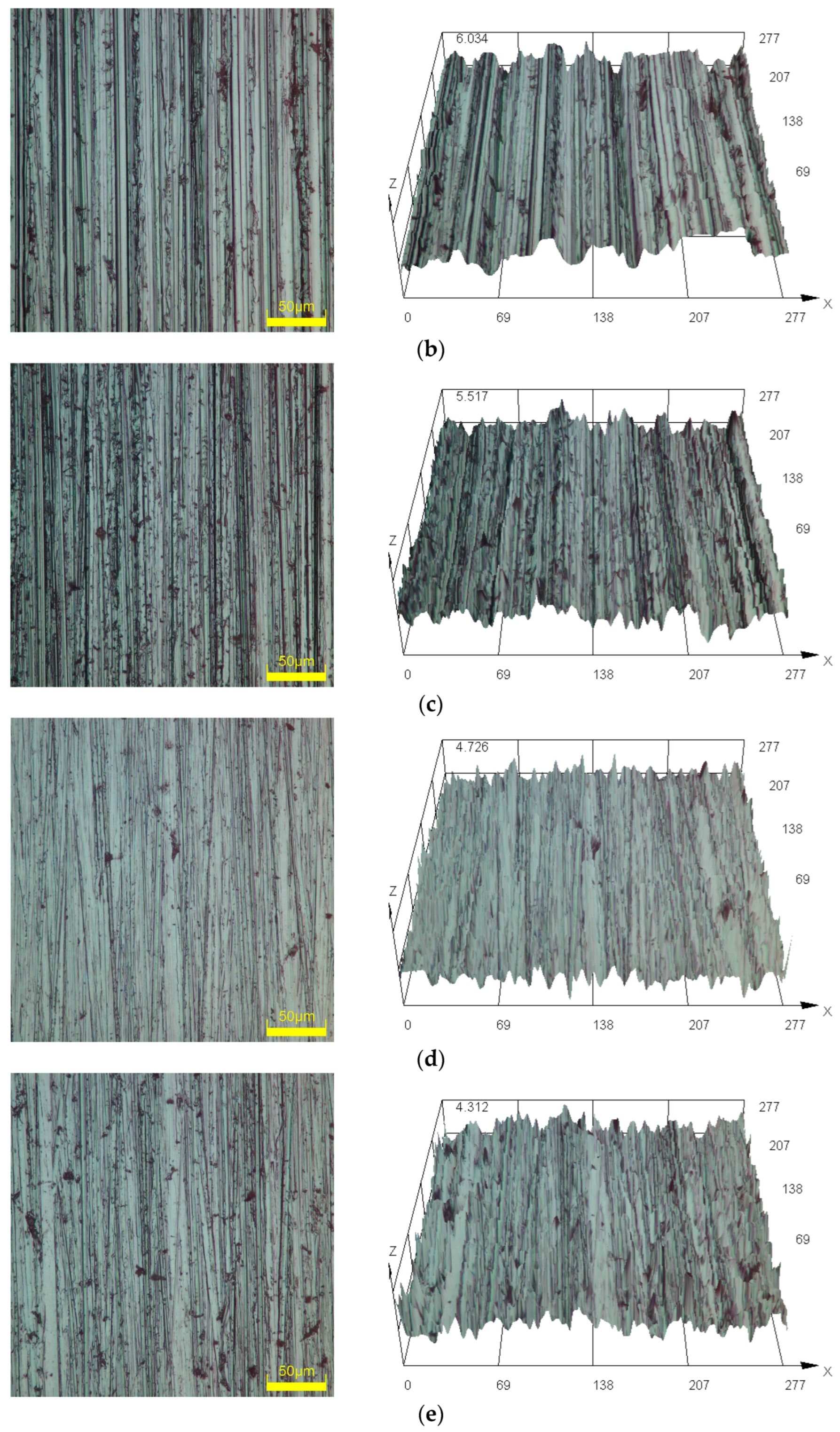
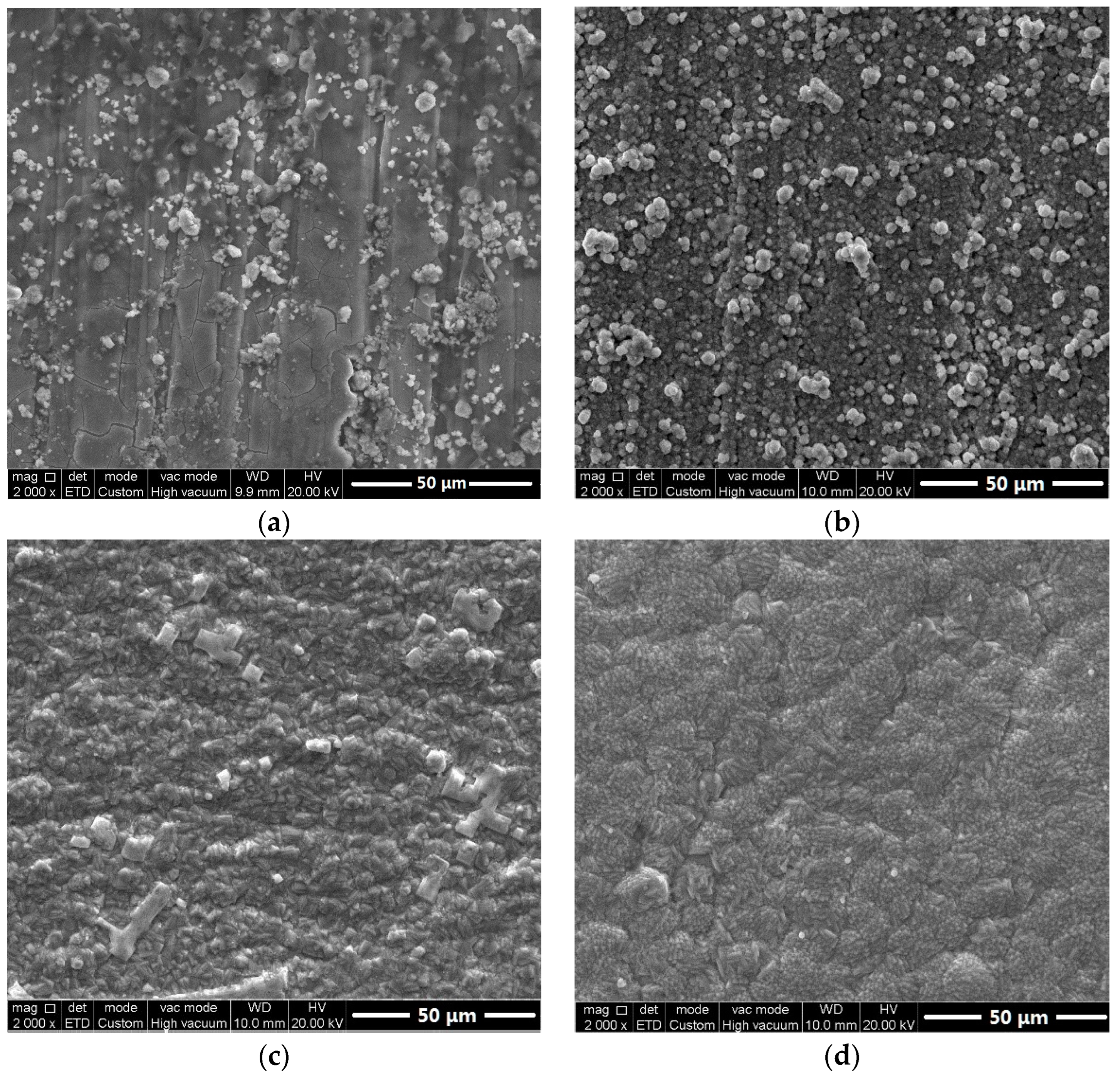
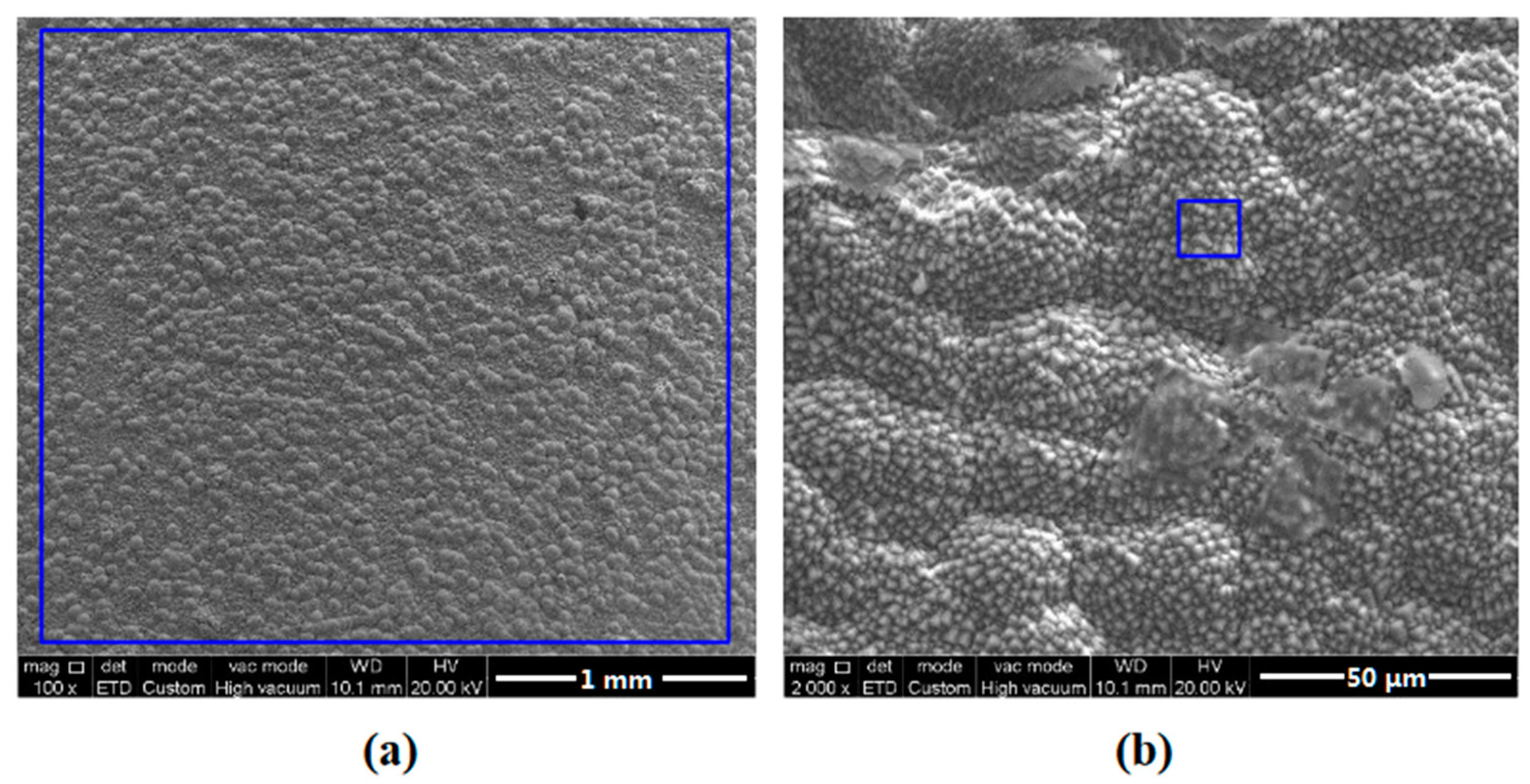
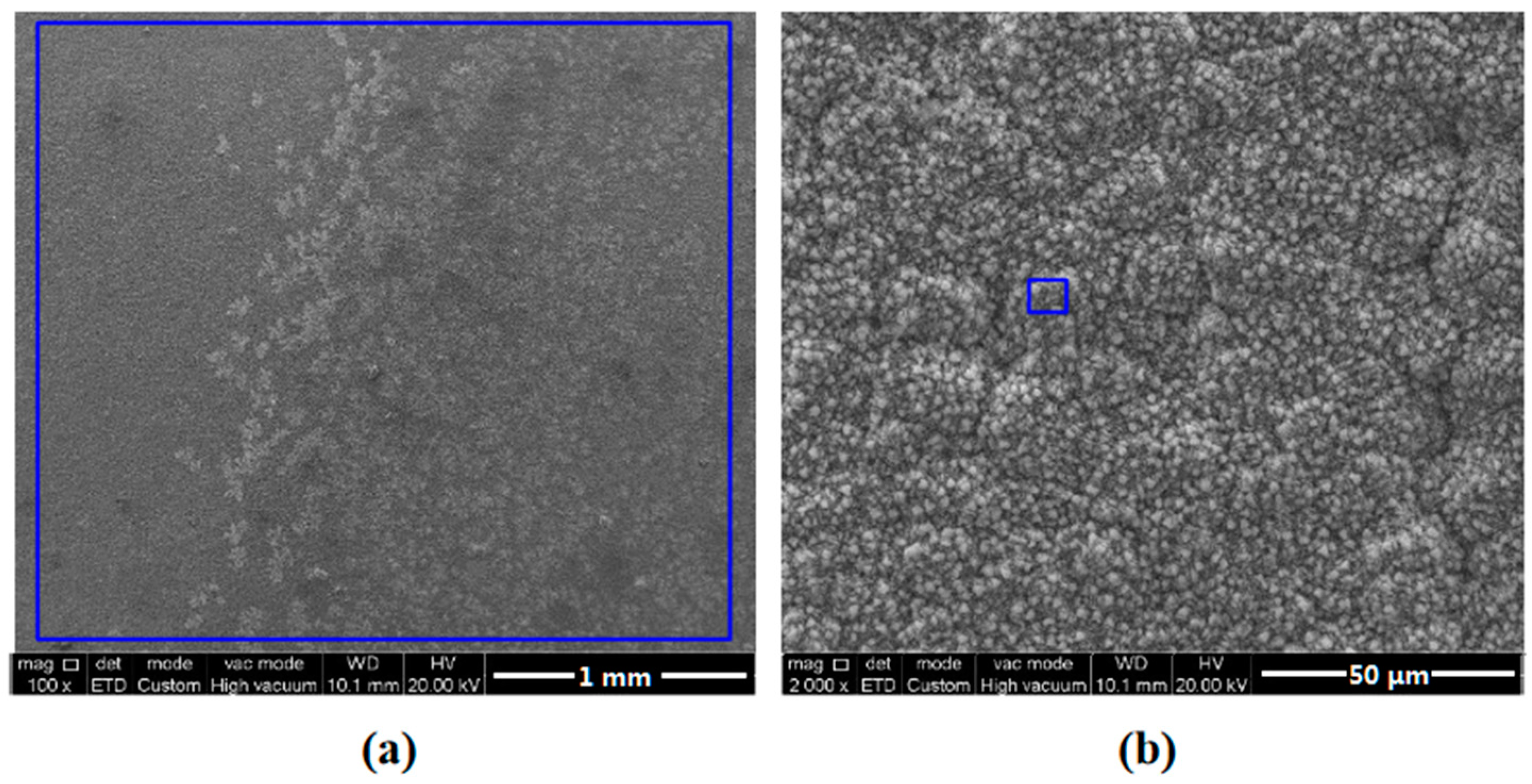
3.1. Surface Morphology Observation
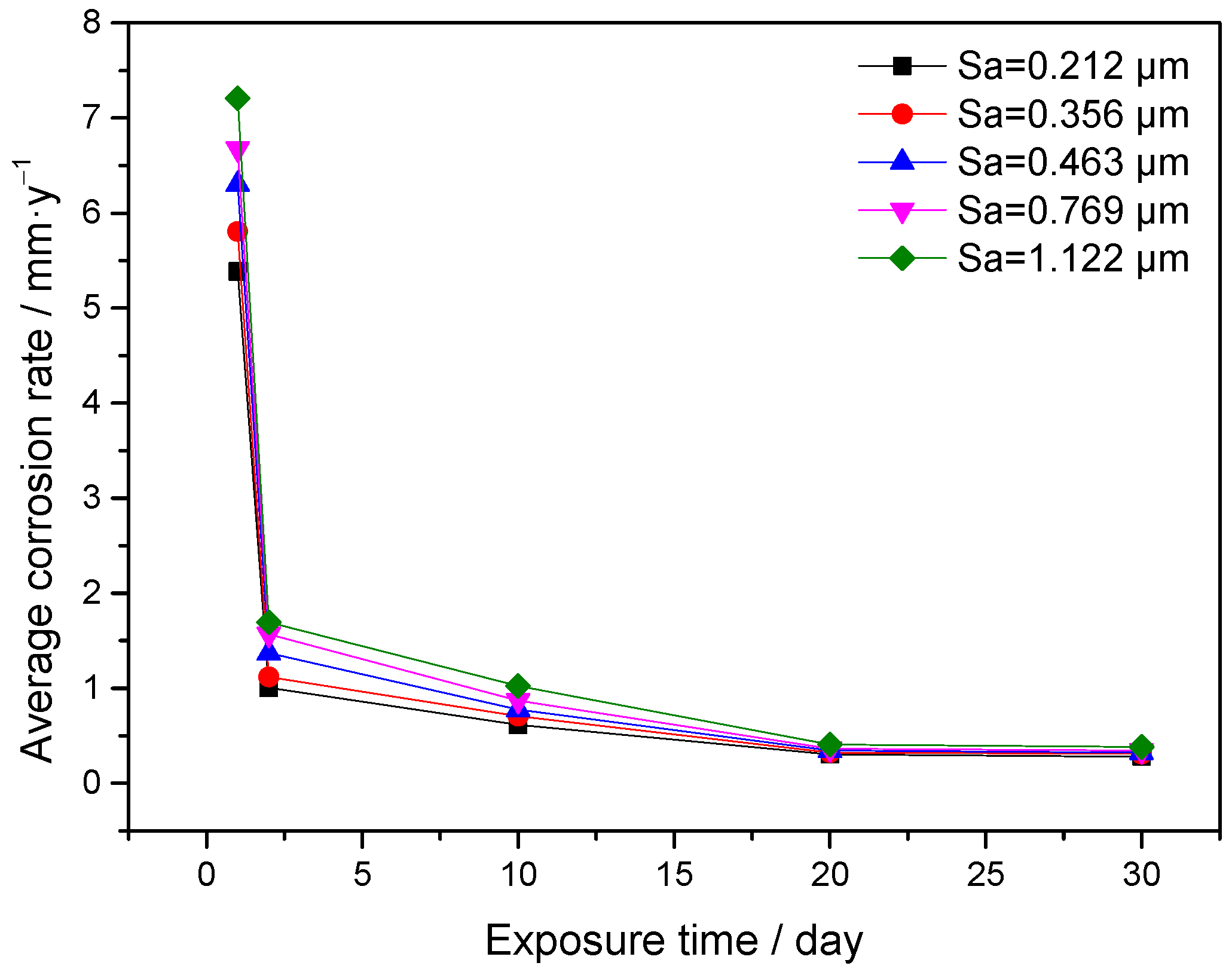
3.2. Weight Loss Tests
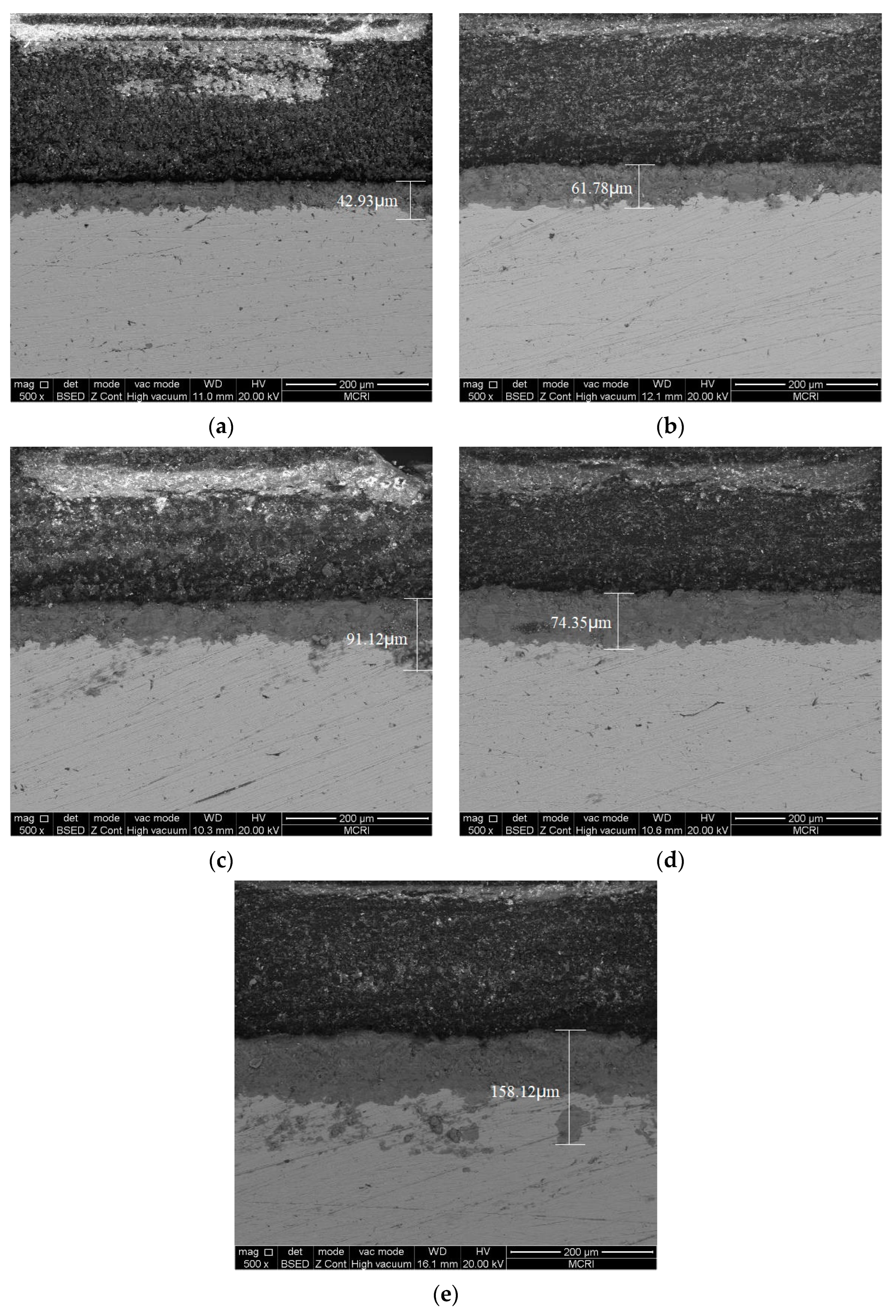
3.3. Characterization of Corrosion Scales Developed on the Surface
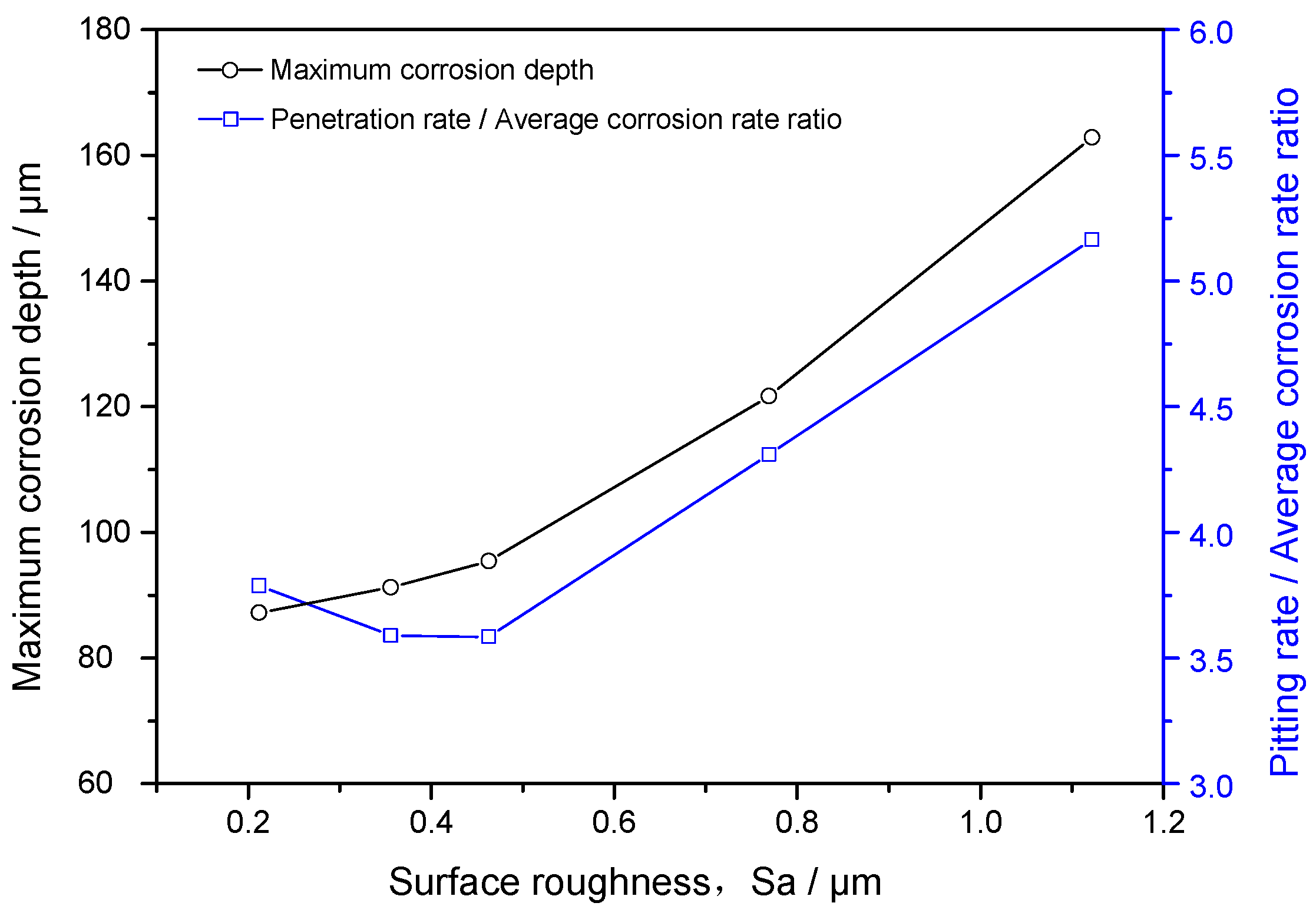
3.4. Maximum Corrosion Depth Tests
4. Discussions
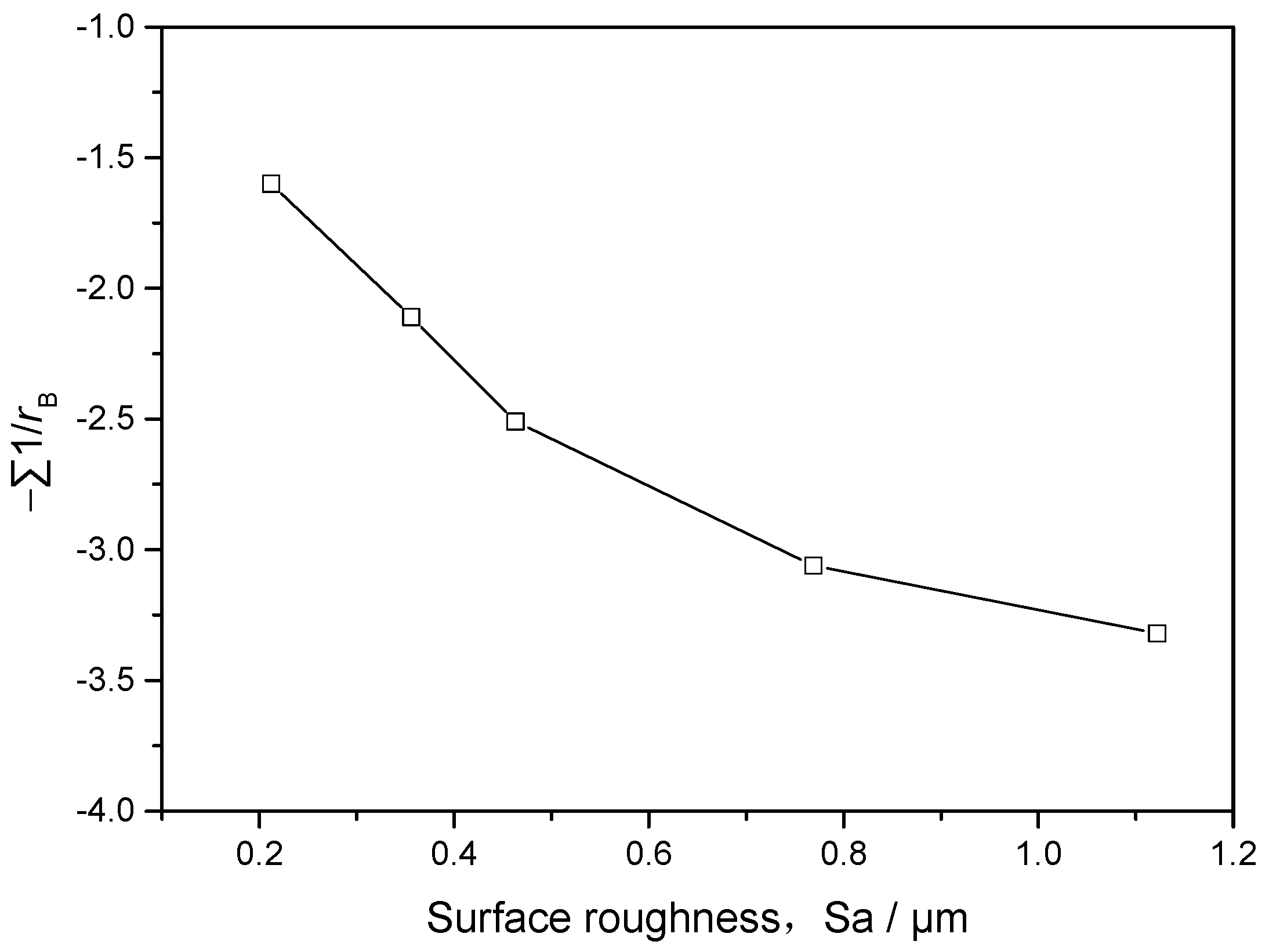
4.1. Influence of Surface Effect
4.2. Effect of Corrosion Product Layer
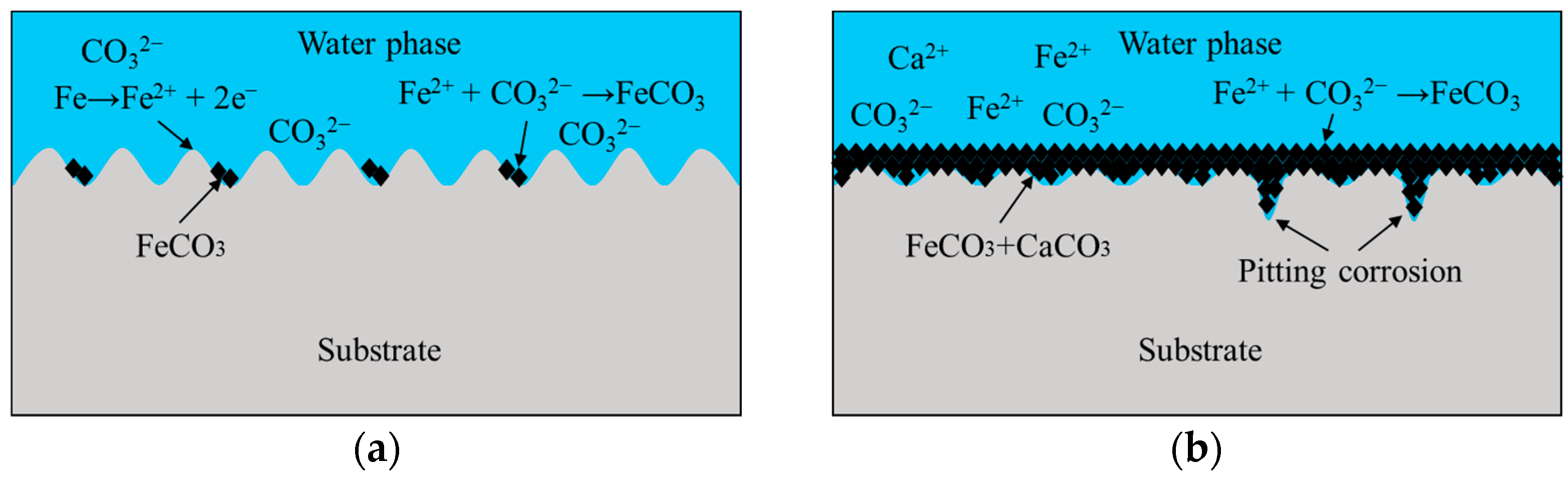
4.3. Corrosion Mechanism
5. Conclusions
- (1)
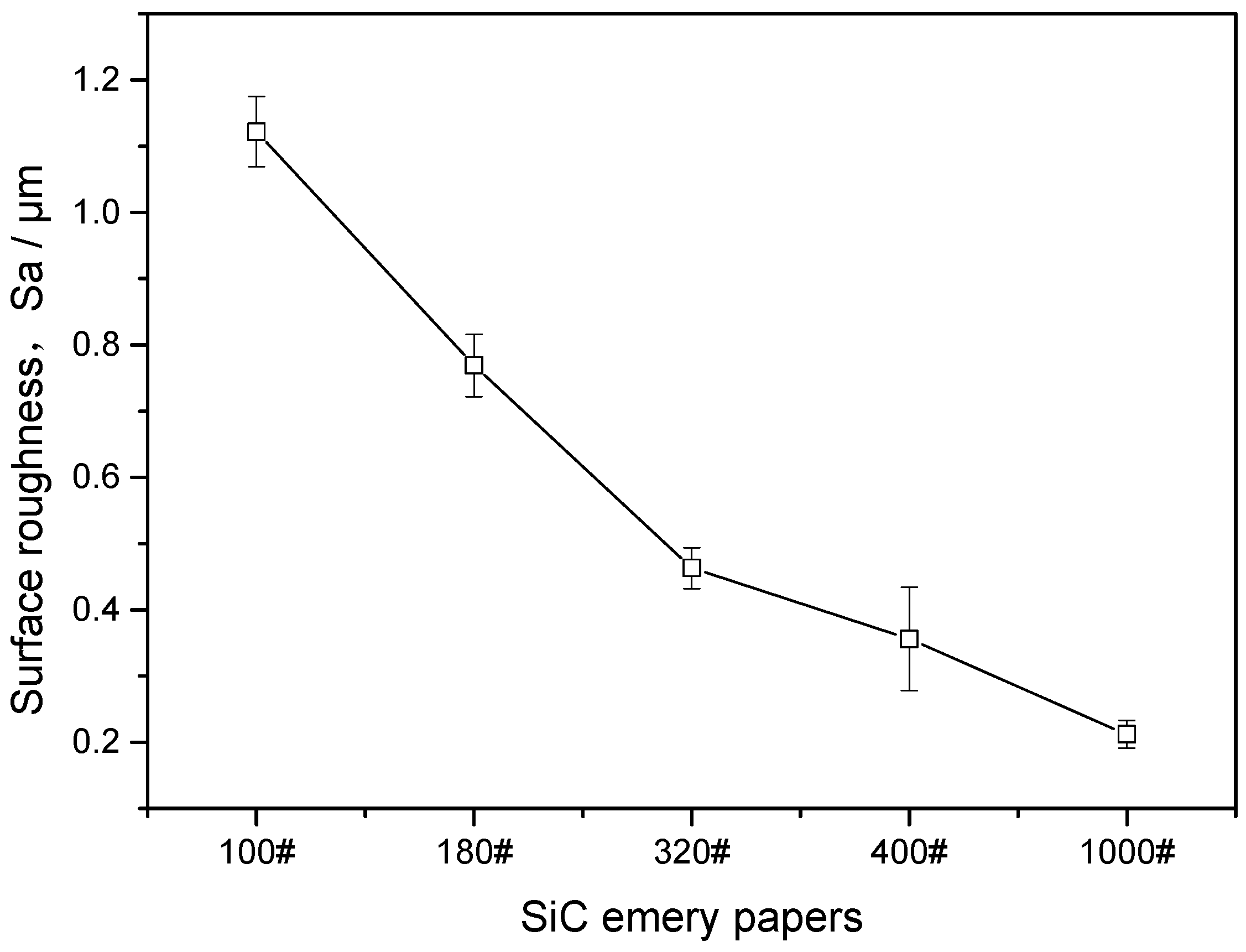
- At 65 °C, the static corrosion rate of J55 carbon steel in CO2-containing geothermal water increases with increasing surface roughness. The surface roughness of J55 carbon steel increases by 5.3-fold and the CO2 corrosion rate increases by 1.4-fold under different exposure times.
- (2)
- The static corrosion rate of J55 carbon steel in CO2-containing geothermal water changes with exposure time, and there is little change in the corrosion rate after immersion for 2 days.
- (3)
- After immersion for 2 days, a complete corrosion product layer was formed on the surface of J55 carbon steel, and the corrosion rate was mainly affected by the corrosion product layer. The corrosion rate of J55 carbon steel displays little change.
- (4)
- In CO2-containing geothermal water environment, the surface of J55 carbon steel was covered with FeCO3 and a minute amount of CaCO3.
- (5)
- At the initial stages of corrosion, the surface roughness affects the corrosion rate through the corrosion driving force and the corrosion reaction surface area. After the formation of complete corrosion product layer, the corrosion product layer is the primary factor affecting the corrosion rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faizal, M.; Bouazza, A.; Singh, R.M. An Overview of Ocean Thermal and Geothermal Energy Conversion Technologies and Systems. Int. J. Air-Cond. Refrig. 2016, 24, 1630006. [Google Scholar] [CrossRef]
- Okoroafor, E.R.; Smith, C.M.; Ochie, K.I.; Nwosu, C.J.; Gudmundsdottir, H.; Aljubran, M. Machine learning in subsurface geothermal energy: Two decades in review. Geothermics 2022, 102, 102401. [Google Scholar] [CrossRef]
- Soltani, M.; Kashkooli, F.M.; Dehghani-Sanij, A.; Nokhosteen, A.; Ahmadi-Joughi, A.; Gharali, K.; Mahbaz, S.; Dusseault, M. A comprehensive review of geothermal energy evolution and development. Int. J. Green Energy 2019, 16, 971–1009. [Google Scholar] [CrossRef]
- James, N.; Sadiq, J.Z. Corrosion in geothermal environment Part 1: Fluids and their impact. Renew. Sustain. Energy Rev. 2017, 82, 1333–1346. [Google Scholar]
- Hua, Y.; Yue, X.; Liu, H.; Zhao, Y.; Wen, Z.; Wang, Y.; Zhang, T.; Zhang, L.; Sun, J.; Neville, A. The evolution and characterisation of the corrosion scales formed on 3Cr steel in CO2-containing conditions relevant to geothermal energy production. Corros. Sci. 2021, 183, 109342. [Google Scholar] [CrossRef]
- Ura-Bińczyk, E.; Banaś, J.; Mazurkiewicz, B.; Solarski, W.; Lewandowska, M.; Roguska, A.; Andrzejczuk, M.; Balcer, M.; Kulik, S.; Żarnowiec, P.; et al. On-site monitoring and laboratory characterization of corrosion processes in the geothermal water of Polish Lowland. Geothermics 2018, 77, 267–277. [Google Scholar] [CrossRef]
- Marbun, B.T.H.; Ridwan, R.H.; Sinaga, S.Z.; Pande, B.; Purbantanu, B.A. Casing failure identification of long-abandoned geothermal wells in Field Dieng, Indonesia. Geotherm. Energy 2019, 7, 31. [Google Scholar] [CrossRef]
- Boch, R.; Leis, A.; Haslinger, E.; Goldbrunner, J.E.; Mittermayr, F.; Fröschl, H.; Hippler, D.; Dietzel, M. Scale-fragment formation impairing geothermal energy production: Interacting H2S corrosion and CaCO3 crystal growth. Geotherm. Energy 2017, 5, 4. [Google Scholar] [CrossRef]
- Yanagisawa, N.; Masuda, Y.; Asanuma, H.; Osato, K.; Sakura, K. Estimation of casing material corrosion rates for supercritical geothermal development. Geothermics 2021, 96, 102149. [Google Scholar] [CrossRef]
- Vitaller, A.V.; Angst, U.M.; Elsener, B. Laboratory tests simulating corrosion in geothermal power plants: Influence of service conditions. Geotherm. Energy 2020, 8, 9. [Google Scholar] [CrossRef]
- Walton, C.A.; Horstemeyer, M.F.; Martin, H.J.; Francis, D.K. Formulation of a macroscale corrosion damage internal state variable mode. Int. J. Solids Struct. 2014, 51, 1235–1245. [Google Scholar] [CrossRef]
- Yuan, L.; Frank, C.Y. Effect of surface finishing on early-stage corrosion of a carbon steel studied by electrochemical and atomic force microscope characterizations. Appl. Surf. Sci. 2016, 336, 95–103. [Google Scholar]
- Hansen, N.; Hughes, D.A. Graded Nanostructures Produced by Sliding and Exhibiting Universal Behavior. Phys. Rev. Lett. 2001, 87, 182–184. [Google Scholar] [CrossRef]
- Reddy, U.; Dubey, D.; Panda, S.S.; Ireddy, N.; Jain, J.; Mondal, K.; Singh, S.S. Effect of Surface Roughness Induced by Milling Operation on the Corrosion Behavior of Magnesium Alloys. J. Mater. Eng. Perform. 2021, 30, 7354–7364. [Google Scholar] [CrossRef]
- Paknahad, H.; Nogorani, F.S. Corrosion Behavior of Low-Temperature Nickel and Iron Aluminized Martensitic Stainless Steel Substrates with Different Surface Roughness in Acidic Medium. J. Mater. Eng. Perform. 2022, 32, 1874–1882. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Abd-El-Nabey, B.A. Corrosion performance of a steel surface modified by a robust graphene-based superhydrophobic film with hierarchical roughness. J. Mater. Sci. 2022, 57, 11376–11391. [Google Scholar] [CrossRef]
- Li, W.; Li, D. Influence of surface morphology on corrosion and electronic behavior. Acta Mater. 2006, 54, 445–452. [Google Scholar] [CrossRef]
- Pistorius, P.C.; Burstein, G.T. Metastable pitting corrosion of stainless steel and the transition to stability. Philos. Trans. R. Soc. Lond. Ser. A Phys. Eng. Sci. 1992, 341, 531–559. [Google Scholar] [CrossRef]
- Sang, M.L.; Wang, L.; Kim, Y.H.; Jang, H. Surface roughness and the corrosion resistance of 21Cr ferritic stainless steel. Corros. Sci. 2012, 63, 404–409. [Google Scholar]
- Lee, S.M.; Lee, W.G.; Kim, Y.H.; Jang, H. Effect of surface roughness on early stages of pitting corrosion of Type 301 stainless steel. Corros. Sci. 1997, 39, 1665–1672. [Google Scholar]
- Sasaki, K.; Burstein, G. The generation of surface roughness during slurry erosion-corrosion and its effect on the pitting potential. Corros. Sci. 1996, 38, 2111–2120. [Google Scholar] [CrossRef]
- Asma, R.N.; Yuli, P.; Mokhtar, C. Study on the Effect of Surface Finish on Corrosion of Carbon Steel in CO2 Environment. J. Appl. Sci. 2011, 11, 2053–2057. [Google Scholar] [CrossRef]
- James, N.; Sadiq, J.Z. Corrosion in geothermal environment Part 2: Metals and alloys. Renew. Sustain. Energ. Rev. 2018, 82, 1347–1363. [Google Scholar]
- Tavares, L.M.; da Costa, E.M.; Andrade, J.J.D.O.; Hubler, R.; Huet, B. Effect of calcium carbonate on low carbon steel corrosion behavior in saline CO2 high pressure environments. Appl. Surf. Sci. 2015, 359, 143–152. [Google Scholar] [CrossRef]
- Okafor, P.C.; Liu, C.B.; Zhu, Y.J.; Zheng, Y.G. Corrosion and Corrosion Inhibition Behavior of N80 and P110 Carbon Steels in CO2-Saturated Simulated Formation Water by Rosin Amide Imidazoline. Ind. Eng. Chem. Res. 2011, 50, 7273–7281. [Google Scholar] [CrossRef]
- Yin, Z.; Zhao, W.; Bai, Z.; Feng, Y. Characteristics of CO2 corrosion scale formed on P110 steel in simulant solution with saturated CO2. Surf. Interface. Anal. 2010, 40, 1231–1236. [Google Scholar] [CrossRef]
- Zhu, S.; Fu, A.; Miao, J.; Yin, Z.; Zhou, G.; Wei, J. Corrosion of N80 carbon steel in oil field formation water containing CO2 in the absence and presence of acetic acid. Corros. Sci. 2011, 53, 3156–3165. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, Y. Electrochemical characterization and computational fluid dynamics simulation of flow-accelerated corrosion of X65 steel in a CO2-saturated oilfield formation water. Corros. Sci. 2010, 52, 2716–2724. [Google Scholar] [CrossRef]
- Dugstad, A.; Hemmer, H.; Seiersten, M. Effect of Steel Microstructure on Corrosion Rate and Protective Iron Carbonate Film Formation. Corrosion 2001, 57, 369–378. [Google Scholar] [CrossRef]
- Li, J.L.; Zhu, S.D.; Qun, C.T. Abrasion resistances of CO2 corrosion scales formed at different temperatures and their relationship to corrosion behavior. Br. Corros. J. 2014, 49, 73–79. [Google Scholar]
- Wei, L.; Pang, X.; Gao, K. Effects of crude oil on the corrosion behavior of pipeline steel under wet CO2 conditions. Mater. Perform. 2015, 54, 58–62. [Google Scholar]
- Nešić, S.; Lunde, L. Carbon Dioxide Corrosion of Carbon Steel in Two-Phase Flow. Corrosion 1994, 50, 717–727. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Farelas, F.; Nešić, S.; Magalhães, A.A.O.; Andrade, C.D.A. Corrosion Behavior of Deep Water Oil Production Tubing Material Under Supercritical CO2 Environment: Part 1—Effect of Pressure and Temperature. Corrosion 2014, 70, 38–47. [Google Scholar] [CrossRef]
- Zhang, G.A.; Liu, D.; Li, Y.Z.; Guo, X.P. Corrosion behavior of N80 carbon steel in formation water under dynamic supercritical CO2 condition. Corros. Sci. 2017, 120, 107–120. [Google Scholar] [CrossRef]
- ASTM Standard G1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2011.
- Bai, H.; Wang, Y.; Ma, Y.; Zhang, Q.; Zhang, N. Effect of CO2 Partial Pressure on the Corrosion Behavior of J55 Carbon Steel in 30% Crude Oil/Brine Mixture. Materials 2018, 11, 1765. [Google Scholar] [CrossRef]
- Al-Khateeb, M.; Barker, R.; Neville, A.; Thompson, H. An experimental and theoretical investigation of the influence of surface roughness on corrosion in CO2 environments. J. Corros. Sci. Eng. 2018, 20, 1–29. [Google Scholar]
- Eyu, G.D.; Will, G.; Dekkers, W.; MacLeod, J. Effect of hydrodynamics and surface roughness on the electrochemical behaviour of carbon steel in CSG produced water. Appl. Surf. Sci. 2015, 357, 506–515. [Google Scholar] [CrossRef]
- Chen, Y.; Howdyshell, R.; Howdyshell, S.; Ju, L.-K. Characterizing Pitting Corrosion Caused by a Long-Term Starving Sulfate-Reducing Bacterium Surviving on Carbon Steel and Effects of Surface Roughness. Corrosion 2014, 70, 767–780. [Google Scholar] [CrossRef]
- Esmaeely, S.N.; Choi, Y.-S.; Young, D.; Nešić, S. Effect of Calcium on the Formation and Protectiveness of Iron Carbonate Layer in CO2 Corrosion. Corrosion 2013, 69, 912–920. [Google Scholar] [CrossRef]
- Esmaeely, S.N.; Young, D.; Brown, B.; Nešić, S. Effect of Incorporation of Calcium into Iron Carbonate Protective Layers in CO2 Corrosion of Mild Steel. Corrosion 2016, 73, 238–246. [Google Scholar] [CrossRef]
- Farelas, F.; Choi, Y.; Nešić, S.; Magalhães, A.A.O.; Andrade, C.D.A. Corrosion Behavior of Deep Water Oil Production Tubing Material Under Supercritical CO2 Environment: Part 2—Effect of Crude Oil and Flow. Corrosion 2013, 70, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhao, B.; Yu, Y.X.; Zhang, X.T.; Xu, T.; Shou, B.A. Effect of surface roughness on corrosion behavior of 4130X steel in 0.5% NaCl solution. China Spec. Equip. Saf. 2017, 33, 25–33. [Google Scholar]
- Xue, Y.Q.; Fan, J.C. Thermodynamic analysis of rough electrochemical corrosion. Hardw. Sci. Technol. 1998, 26, 21–23. [Google Scholar]
- Davies, D.H.; Burstein, C.T. The effects of bicarbonate on the corrosion and passivation of iron. Corrosion 1980, 36, 416–422. [Google Scholar] [CrossRef]
- Ogundele, G.I.; White, W.E. Some Observations on Corrosion of Carbon Steel in Aqueous Environments Containing Carbon Dioxide. Corrosion 1986, 42, 71–78. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Han, E.-H.; Ke, W. Characterization of Different Surface States and Its Effects on the Oxidation Behaviours of Alloy 690TT. J. Mater. Sci. Technol. 2012, 28, 353–361. [Google Scholar] [CrossRef]
- Yin, Q.; Yang, J.; Xiu, H.; Liu, S.; Li, Z.; Tan, C.; Li, T.; Zhao, S.; Sun, T.; Li, W.; et al. Material qualification of a 13Cr-L80 casing for sour conditions. Mater. Test. 2019, 61, 833–841. [Google Scholar] [CrossRef]

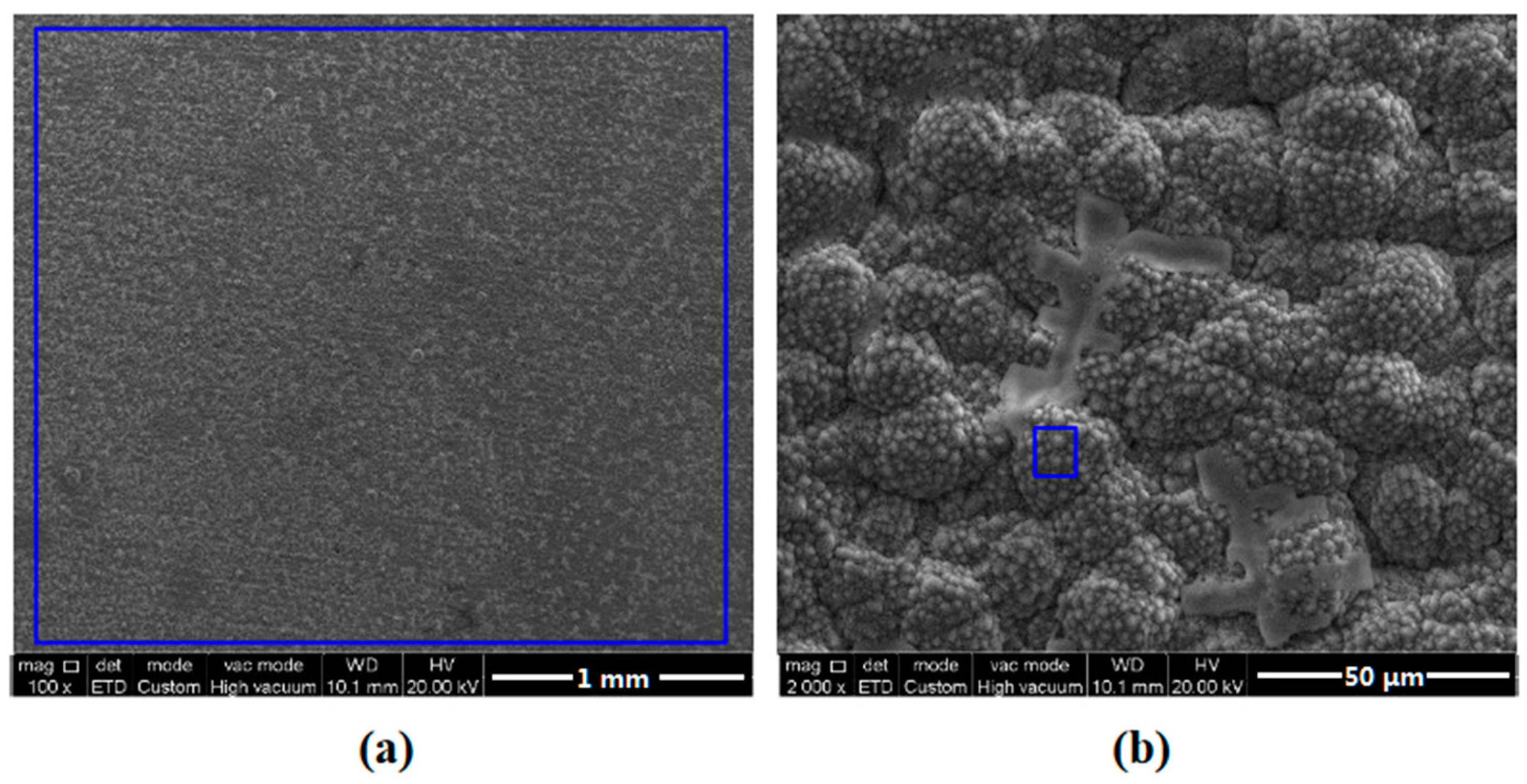

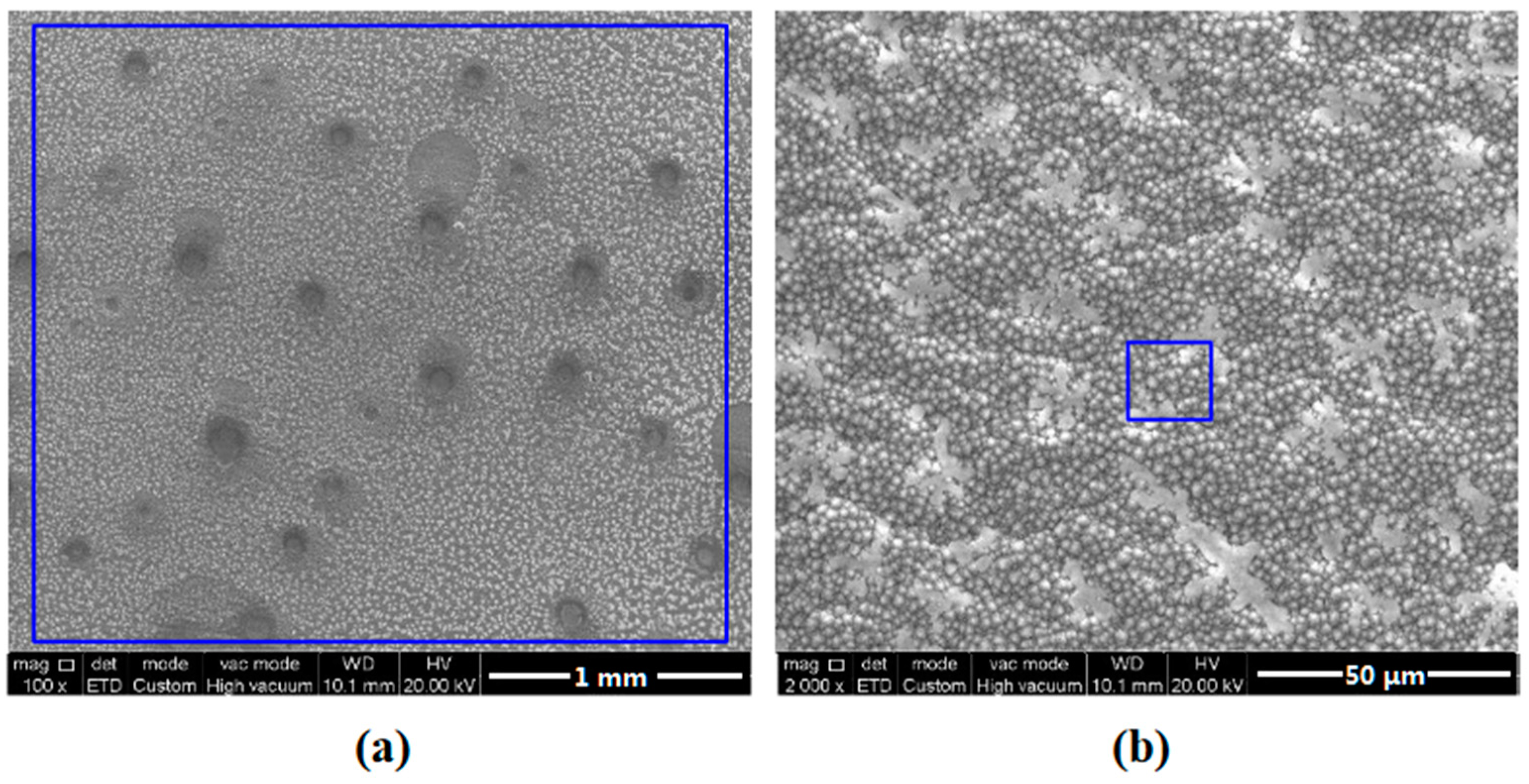


| Element | C | Si | Mn | P | S | Cr | Ni | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Concentration (wt.%) | 0.36 | 0.30 | 1.45 | 0.016 | 0.004 | 0.051 | 0.009 | 0.07 | balance |
| Ion | Cl− | HCO3− | CO32− | SO42− | Ca2+ | Mg2+ | Na+ |
|---|---|---|---|---|---|---|---|
| Concentration (mg·L−1) | 408.40 | 282.33 | 153.47 | 3775.74 | 126.07 | 25.69 | 2105.21 |
| Elements (At%) | Sa = 0.212 μm | Sa = 0.356 μm | Sa = 0.463 μm | Sa = 0.769 μm | Sa = 1.122 μm | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Whole | Local | Whole | Local | Whole | Local | Whole | Local | Whole | Local | |
| C K | 20.78 | 21.13 | 23.84 | 15.29 | 19.52 | 17.56 | 20.00 | 24.85 | 20.96 | 18.04 |
| O K | 48.47 | 51.13 | 45.84 | 55.02 | 46.11 | 51.38 | 49.26 | 39.34 | 48.89 | 50.46 |
| Cl K | 1.61 | 0.53 | 0.55 | / | 0.45 | 0.21 | 1.19 | 1.61 | 1.57 | / |
| Ca K | 2.33 | 6.08 | 2.64 | 6.54 | 2.09 | 1.99 | 2.00 | 4.42 | 2.30 | 2.13 |
| Mn K | / | / | / | / | / | / | 0.46 | 0.75 | 0.58 | 0.30 |
| Fe K | 26.81 | 21.13 | 27.13 | 23.15 | 31.83 | 28.86 | 27.09 | 29.03 | 25.70 | 29.07 |
| total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Sa (μm) | 0 | 0.212 | 0.356 | 0.463 | 0.769 | 1.122 |
| Length (μm) | 277 | 422.26 | 467.64 | 500.91 | 594.20 | 621.42 |
| RA | 1.00 | 1.52 | 1.69 | 1.81 | 2.15 | 2.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, H.; Cui, X.; Wang, R.; Lv, N.; Yang, X.; Li, R.; Ma, Y. Effect of Surface Roughness on Static Corrosion Behavior of J55 Carbon Steel in CO2-Containing Geothermal Water at 65 °C. Coatings 2023, 13, 821. https://doi.org/10.3390/coatings13050821
Bai H, Cui X, Wang R, Lv N, Yang X, Li R, Ma Y. Effect of Surface Roughness on Static Corrosion Behavior of J55 Carbon Steel in CO2-Containing Geothermal Water at 65 °C. Coatings. 2023; 13(5):821. https://doi.org/10.3390/coatings13050821
Chicago/Turabian StyleBai, Haitao, Xing Cui, Rui Wang, Naixin Lv, Xupeng Yang, Ruixuan Li, and Yun Ma. 2023. "Effect of Surface Roughness on Static Corrosion Behavior of J55 Carbon Steel in CO2-Containing Geothermal Water at 65 °C" Coatings 13, no. 5: 821. https://doi.org/10.3390/coatings13050821
APA StyleBai, H., Cui, X., Wang, R., Lv, N., Yang, X., Li, R., & Ma, Y. (2023). Effect of Surface Roughness on Static Corrosion Behavior of J55 Carbon Steel in CO2-Containing Geothermal Water at 65 °C. Coatings, 13(5), 821. https://doi.org/10.3390/coatings13050821





