Fabrication of Modified Polyurethane Sponge with Excellent Flame Retardant and the Modification Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Methods
3. Results and Discussion
3.1. The Flame-Retardant Performance of the Modified PUS
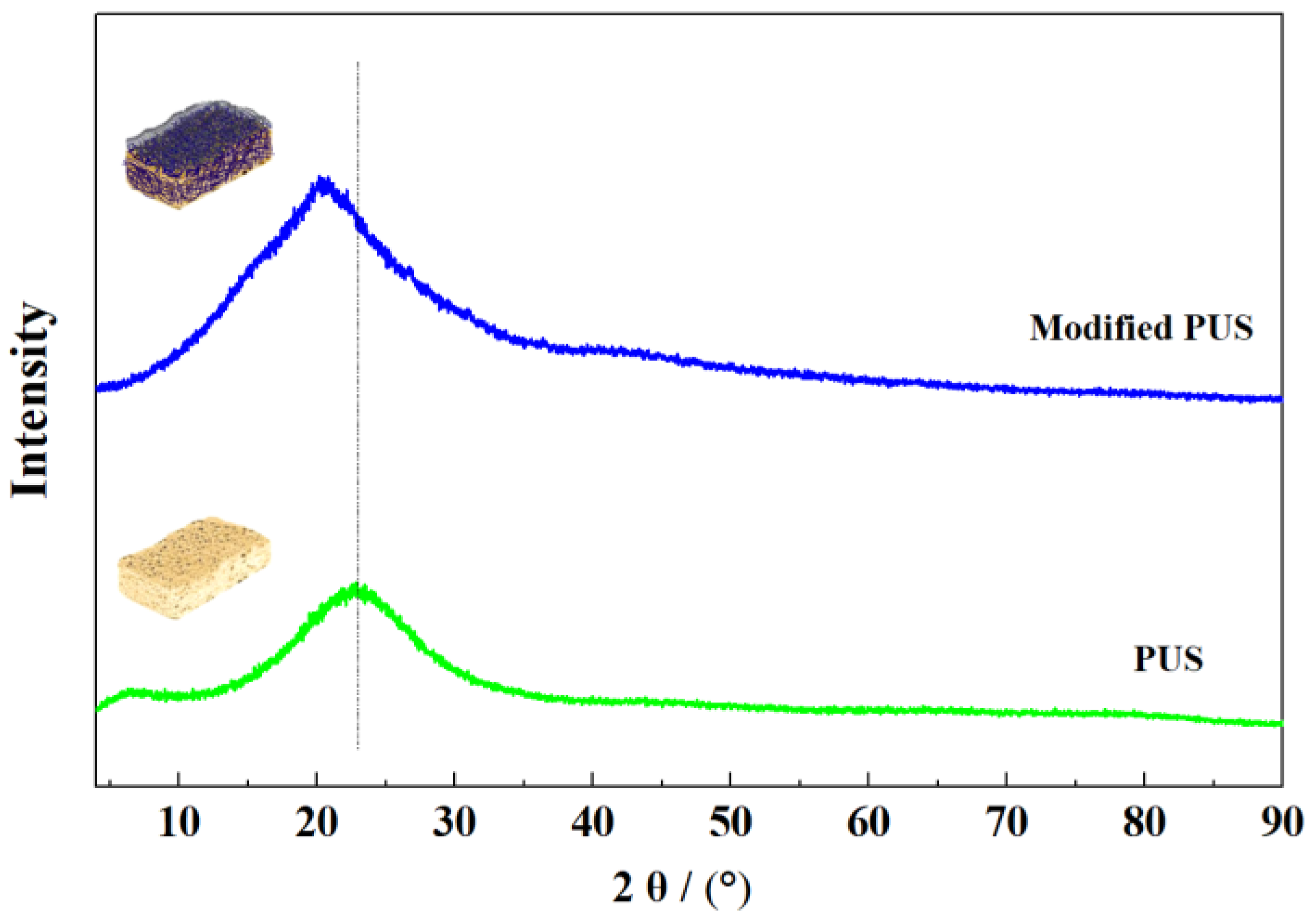
3.2. The Modification Mechanism of the PUS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Li, S.; Ye, Z.; Zhang, Y.; Gao, S.; Rong, H.; Zhang, J.; Deng, L.; Dong, A. Superhydrophobic and superhydrophilic polyurethane sponge for wound healing. Chem. Eng. J. 2022, 446, 136985. [Google Scholar] [CrossRef]
- Jamsaz, A.G.; Elaheh, K. An environmentally friendly superhydrophobic modified polyurethane sponge by seashell for the efficient oil/water separation. Process Safe. Environ. Prot. 2020, 139, 297–304. [Google Scholar] [CrossRef]
- Ma, S.; Chiu, C.P.; Zhu, Y.; Tang, C.Y.; Long, H.; Qarony, W.; Zhao, X.; Zhang, X.; Lo, W.H.; Tsang, Y.H. Recycled waste black polyurethane sponges for solar vapor generation and distillation. Appl. Energy 2017, 206, 63–69. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Ma, Y.; Nie, J.; Sui, G. Super-hydrophobic graphene coated polyurethane (GN@PU) sponge with great oil-water separation performance. Appl. Surf. Sci. 2017, 422, 116–124. [Google Scholar] [CrossRef]
- Olga, G.; Alexandre, B.; Ahmed, A.; Elizaveta, S.; Sabine, S.; Pavel, P.; Rabah, B. Magnetic polyurethane sponge for efficient oil adsorption and separation of oil from oil-in-water emulsions. Separa. Purif. Tech. 2020, 240, 116627. [Google Scholar]
- Sun, R.; Yu, N.; Zhao, J.; Mo, J.; Pan, Y.; Luo, D. Chemically stable superhydrophobic polyurethane sponge coated with ZnO/epoxy resin coating for effective oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125850. [Google Scholar] [CrossRef]
- Dressler, M.; Reinsch, S.; Schadrack, R.; Benemann, S. Burnout behavior of ceramic coated open cell polyurethane (PU) sponges. J. Eur. Ceram. Soc. 2009, 29, 3333–3339. [Google Scholar] [CrossRef]
- Junxiu, P.; Mingjie, L.; Jinyong, R.; Yaofei, W.; Tingting, F.; Yaxuan, W.; Chuanmei, J.; Xilei, C.; Shaoping, K. MOF-derived LDH modified flame-retardant polyurethane sponge for high-performance oil-water separation: Interface engineering design based on bioinspiration. J. Hazard. Mater. 2023, 444, 130398. [Google Scholar]
- Dong, F.; Wang, Y.; Wang, S.; Shaghaleh, H.; Sun, P.; Huang, X.; Xu, X.; Wang, S.; Liu, H. Flame-retarded polyurethane foam conferred by a bio-based nitrogen-phosphorus-containing flame retardant. React. Funct. Polym. 2021, 168, 105057. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.; Shen, M.; Xu, X.; Liu, H.; Wang, D.; Wang, H.; Shang, S. Biobased Phosphorus Siloxane-Containing Polyurethane Foam with Flame-Retardant and Smoke-Suppressant Performances. ACS Sustain. Chem. Eng. 2021, 9, 8623–8634. [Google Scholar] [CrossRef]
- Jiang, Y.; Pang, X.; Deng, Y.; Sun, X.; Zhao, X.; Xu, P.; Shao, P.; Zhang, L.; Li, Q.; Li, Z. An Alginate Hybrid Sponge with High Thermal Stability: Its Flame Retardant Properties and Mechanism. Polymers 2019, 11, 1973. [Google Scholar] [CrossRef]
- He, W.; Song, P.; Yu, B.; Fang, Z.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Prog. Mater. Sci. 2020, 114, 100687.1–100687.49. [Google Scholar] [CrossRef]
- Liu, C.; Fang, Y.; Miao, X.; Pei, Y.; Yan, Y.; Xiao, W.; Wu, L. Facile fabrication of superhydrophobic polyurethane sponge towards oil-water separation with exceptional flame-retardant performance. Sep. Purif. Technol. 2019, 229, 115801. [Google Scholar] [CrossRef]
- Miao, J.; Fang, Y.; Guo, Y.; Zhu, Y.; Hu, A.; Wang, G. Interpenetrating Polymer Networks of Porous Organic Polymers and Polyurethanes for Flame Resistance and High Mechanical Properties. Appl. Polym. Mater. 2019, 1, 2692–2702. [Google Scholar] [CrossRef]
- Jamsaz, A.G.; Elaheh, K. Flame retardant, superhydrophobic, and superoleophilic reduced graphene oxide/orthoaminophenol polyurethane sponge for efficient oil/water separation. J. Mol. Liq. 2020, 307, 112979. [Google Scholar] [CrossRef]
- Chai, H.; Duan, Q.; Jiang, L.; Sun, J. Effect of inorganic additive flame retardant on fire hazard of polyurethane exterior insulation material. J Therm. Anal. Calorim. 2019, 135, 2857–2868. [Google Scholar] [CrossRef]
- Pang, X.; Chang, R.; Weng, M. Halogen-free flame retarded rigid polyurethane foam: The influence of titanium dioxide modified expandable graphite and ammonium polyphosphate on flame retardancy and thermal stability. Polym. Eng. Sci. 2018, 58, 2008–2018. [Google Scholar] [CrossRef]
- Mahr, M.S.; Hübert, T.; Sabel, M.; Schartel, B.; Bahr, H.; Militz, H. Fire retardancy of sol–gel derived titania wood-inorganic composites. J. Mater. Sci. 2012, 47, 6849–6861. [Google Scholar] [CrossRef]
- Zheng, X.; Dong, Q.; Wang, X.; Yu, P.; Wang, W.; Zhang, J.; Ren, L. Improvement of Flame Retardancy of Polyurethane Foam Using DOPO-Immobilized Silica Aerogel. Front. Mater. 2021, 8, 673906. [Google Scholar] [CrossRef]
- Brannum, D.J.; Price, E.J.; Villamil, D.; Kozawa, S.; Brannum, M.; Berry, C.; Semco, R.; Wnek, G.E. Flame-Retardant Polyurethane Foams: One-Pot, Bioinspired Silica Nanoparticle Coating. Appl. Polym. Mater. 2019, 1, 2015–2022. [Google Scholar] [CrossRef]
- Yan, M.; Fu, Y.; Pan, Y.; Cheng, X.; Gong, L.; Zhou, Y.; Ahmed, H.; Zhang, H. Highly elastic and fatigue resistant wood/silica composite aerogel operated at extremely low temperature. Compos. Part B Eng. 2022, 230, 109496. [Google Scholar] [CrossRef]
- Furuno, T.; Fujisawa, M. Carbonization of wood-silica composites and formation of silicon carbide in the cell wall. Wood Fiber Sci. 2004, 36, 269–277. [Google Scholar]
- Wang, Z.; Li, X. Mechanical Properties and Flame Retardancy of Rigid Polyurethane Foams Containing SiO2 Nanospheres/Graphene Oxide Hybrid and Dimethyl Methylphosphonate. Polym.-Plast. Technol. Eng. 2018, 57, 884–892. [Google Scholar] [CrossRef]
- Lei, Y.; Hu, Z.; Cao, B.; Chen, X.; Song, H. Enhancements of thermal insulation and mechanical property of silica aerogel monoliths by mixing graphene oxide. Mater. Chem. Phys. 2017, 187, 183–190. [Google Scholar] [CrossRef]
- Singh, V.K.; Elomaa, O.; Johansson, L.; Hannula, S.; Koskinen, J. Lubricating properties of silica/graphene oxide composite powders. Carbon 2014, 79, 227–235. [Google Scholar] [CrossRef]
- Du, Y.; Huang, L.; Wang, Y.; Yang, K.; Zhang, Z.; Wang, Y.; Kipper, M.J.; Belfiore, L.A.; Tang, J. Preparation of graphene oxide/silica hybrid composite membranes and performance studies in water treatment. J. Mater. Sci. 2020, 55, 11188–11202. [Google Scholar] [CrossRef]
- Zhou, X.X.; Song, W.F.; Zhu, G.Z. A facile approach for fabricating silica dioxide/reduced graphene oxide coated cotton fabrics with multifunctional properties. Cellulose 2020, 27, 2927–2938. [Google Scholar] [CrossRef]
- Wang, M.; Ma, L.; Li, B.; Zhang, W.; Zheng, H.; Wu, G.; Huang, Y.; Song, G. One-step generation of silica particles onto graphene oxide sheets for superior mechanical properties of epoxy composite and scale application. Compos. Commun. 2020, 22, 100514. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Li, H.; Lai, X.; Zeng, X. Superhydrophobic, flame-retardant and magnetic polyurethane sponge for oil-water separation. J. Environ. Chem. Eng. 2022, 10, 107580. [Google Scholar] [CrossRef]












| Samples | TTI (s) | ITF (s) | CO-Y (kg/kg) | CO2-Y (kg/kg) |
|---|---|---|---|---|
| PUS | 2 | 98 | 2.56 | 222.83 |
| Modified PUS | 29 | 154 | 0.16 | 1.51 |
| Element | Ratio of Atom/% |
|---|---|
| O | 67.36 |
| Si | 32.64 |
| Parameter | PUS | Modified PUS |
|---|---|---|
| Solid residue rate (wt%) | 5.65 | 57.29 |
| DTGmax (wt%/min) | 1.18 | 0.16 |
| Tmax (℃) | 306 | 354 |
| Samples | THR(kJ/g) | HRR (W/g) | Temperature (℃) | ||
|---|---|---|---|---|---|
| P1 | P2 | P1 | P2 | ||
| PUS | 33 | 150 | 617 | 293 | 407 |
| Modified PUS | 9 | 21 | 40 | 274 | 392 |
| Element | PUS | Modified PUS |
|---|---|---|
| C | 55.91 | 29.01 |
| N | 19.05 | 4.68 |
| O | 29.92 | 46.48 |
| Si | -- | 19.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zhang, C.-Y.; Yu, Y.-L.; Liang, C.-J.; Yuan, G.-M.; Yang, H.; Wu, Y.-Y.; Lin, S.-M. Fabrication of Modified Polyurethane Sponge with Excellent Flame Retardant and the Modification Mechanism. Coatings 2023, 13, 807. https://doi.org/10.3390/coatings13040807
Li H, Zhang C-Y, Yu Y-L, Liang C-J, Yuan G-M, Yang H, Wu Y-Y, Lin S-M. Fabrication of Modified Polyurethane Sponge with Excellent Flame Retardant and the Modification Mechanism. Coatings. 2023; 13(4):807. https://doi.org/10.3390/coatings13040807
Chicago/Turabian StyleLi, Hang, Chen-Yang Zhang, Ya-Ling Yu, Chang-Jin Liang, Guang-Ming Yuan, Huan Yang, Yun-Ying Wu, and Shao-Min Lin. 2023. "Fabrication of Modified Polyurethane Sponge with Excellent Flame Retardant and the Modification Mechanism" Coatings 13, no. 4: 807. https://doi.org/10.3390/coatings13040807
APA StyleLi, H., Zhang, C.-Y., Yu, Y.-L., Liang, C.-J., Yuan, G.-M., Yang, H., Wu, Y.-Y., & Lin, S.-M. (2023). Fabrication of Modified Polyurethane Sponge with Excellent Flame Retardant and the Modification Mechanism. Coatings, 13(4), 807. https://doi.org/10.3390/coatings13040807







