1. Introduction
From building materials to kitchenware, wood was present in households since the start of human civilization. Its abundance and ease of workability make it an easy choice for everyday items such as cutting boards and cooking spoons. That wood persisted in the kitchen despite the advent of modern materials such as plastic, which is likely due to its unique physical-mechanical properties which are directly linked to wood’s microanatomy.
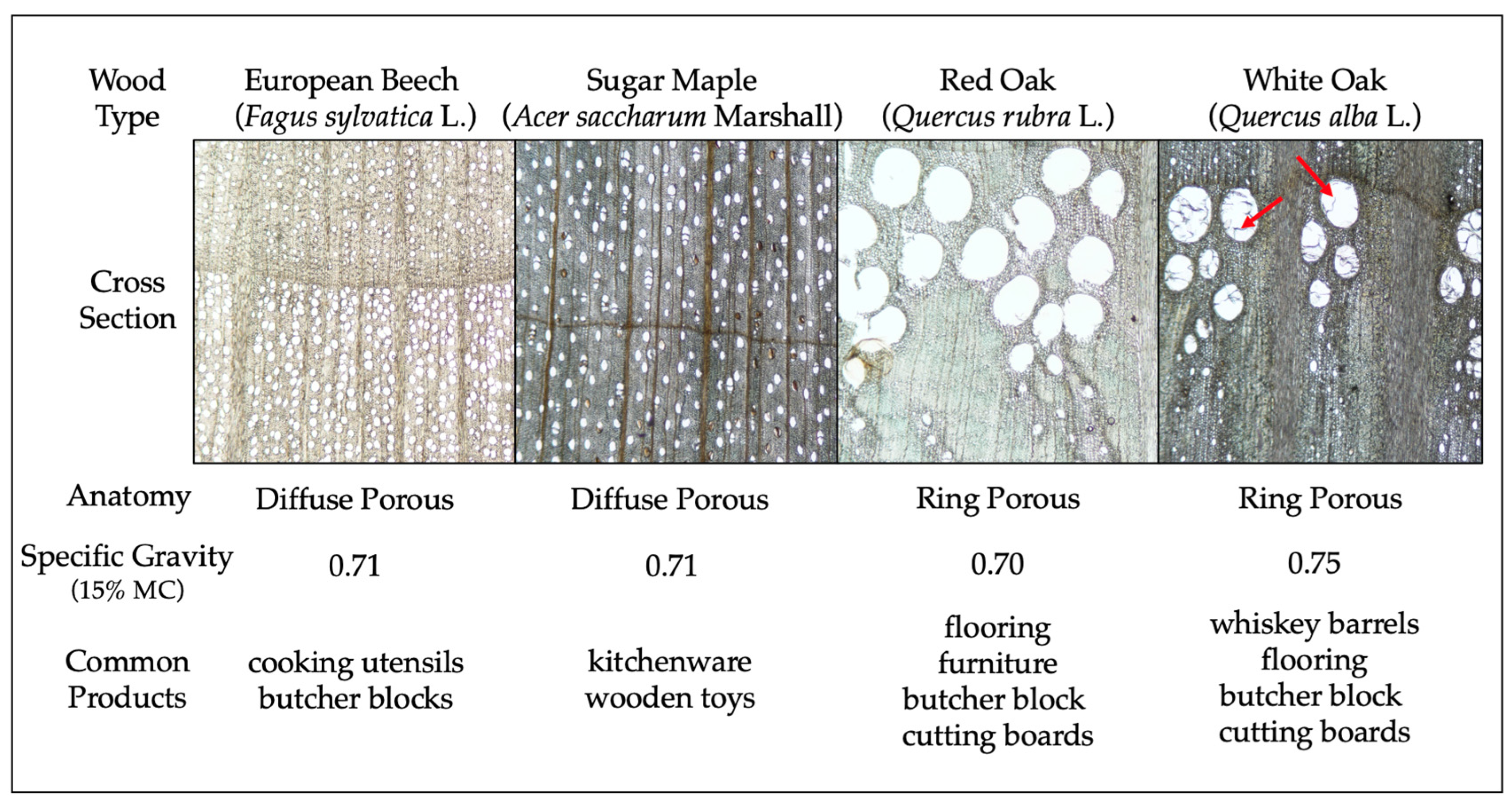
At the most basic level, dichot wood can be broken down into softwoods (angiosperms) and hardwoods (gymnosperms). Softwoods possess mainly tracheids as vertical elements, while hardwoods have vessels and fibers. What these elements have in common is a lumen (hollow space) and a straw-like shape. While the tree is alive, tracheids (softwoods) and vessels (hardwoods) perform an important role—their shapes are optimized for water flow within the trunk via capillary action. This characteristic is also present when the lumber is kiln-dried and one of the reasons why wood is considered a hygroscopic material, as the lumen contains a high number of hydroxyl groups (-OH) that bond easily to water molecules. In addition, wood is constantly absorbing and losing water into the atmosphere as it equilibrates its internal moisture content with the surrounding air.
Usually, when wood is used in the household, a coating (finish) is utilized to slow the absorption of water (vapor or liquid) into the material, minimizing dimensional change. Coatings are common on furniture and flooring, and increasingly in cookware such as cooking spoons and butcher block boards/cutting boards. This trend was aided by the increase in ‘specialized’ finishes marketed for such applications, which often promise to keep wood cutting boards from cracking (which occurs usually only when a board has been improperly washed via extended submersion) and to keep ‘harmful bacteria’ from being absorbed into the wood. Kitchen tools, in general, are exposed to a wide array of bacteria that are natural members of the microbiota of diverse food types, but also may contact foodborne pathogenic bacteria, such as Shiga toxin-producing Escherichia coli (STEC), Salmonella enterica, and Listeria monocytogenes.
Cutting boards are a common surface used to cut many of these food items. After the cutting boards are washed, which introduces liquid water onto what is likely a dry piece of wood, the wood pulls the water into the wood, and the bacteria along with it, as it equilibrates the moisture. This keeps the surface of the wood clean, and the bacteria remain trapped inside the board, where they eventually die [
1,
2,
3,
4]. This remains true no matter how scarred the wooden board becomes over time, the same of which is not true of plastic boards.
There is very little research on wood cutting boards and their movement of bacteria, with most of the available research coming from a single research group. Other studies that compared wood to plastic boards did not consider wood anatomy or the mechanics of the moment. There were studies on wood’s ability to move bacteria outside of cutting boards, with a prominent example being oak’s use in healthcare facilities [
5]; however, this deals with a different type of bacteria and environment. To make matters more difficult, there is little to no literature on the role of finishes/coatings on cutting boards, and how this might affect bacterial movement [
6]. Many research articles that tested wood cutting boards against plastic boards did not note if any coating was on the wood at all, leaving the reader to infer the wood was raw. It is reasonable to infer that blocking the vessels and tracheids of wood would slow or inhibit bacterial absorption, giving the board properties closer to plastic boards, wherein the bacteria must be physically washed away [
7]. This can be seen to a lesser extent in studies that used bacteria carried in chicken broth, as the fat in the broth was shown to inhibit wood absorption of the bacteria, leaving more recoverable bacteria on the wood surface [
7]. Whether finishing cutting boards is actually any safer than raw wood cutting boards is unknown, as the aforementioned study noted that washing any board with soap and water removed the bacteria sufficiently, regardless of material. One of the few studies that did explore how plant-based coatings affect wood-in-use and bacterial growth was tied to instruments (Tran-Ly et al. 2022), but was tied more to the role of fungal melanin. Another recent study investigated how coatings
encourage bacterial growth on wood, specifically wood furniture, and found linseed oil the worst offender (Bohinic et al. 2019).
In order to begin to answer the question of whether it is reasonable to coat wood cutting boards for use in the home kitchen, this study focused on the effect of two coatings on the capillarity of wooden cutting boards, in an attempt to determine if coatings affect the recovery of bacteria from the surface of different wood species. Results from this study can be used to guide appropriate cutting board care to reduce the risk of survival and cross-contamination of foodborne pathogens on wooden butcher blocks and cutting boards and will hopefully serve as a springboard for future research.
3. Results
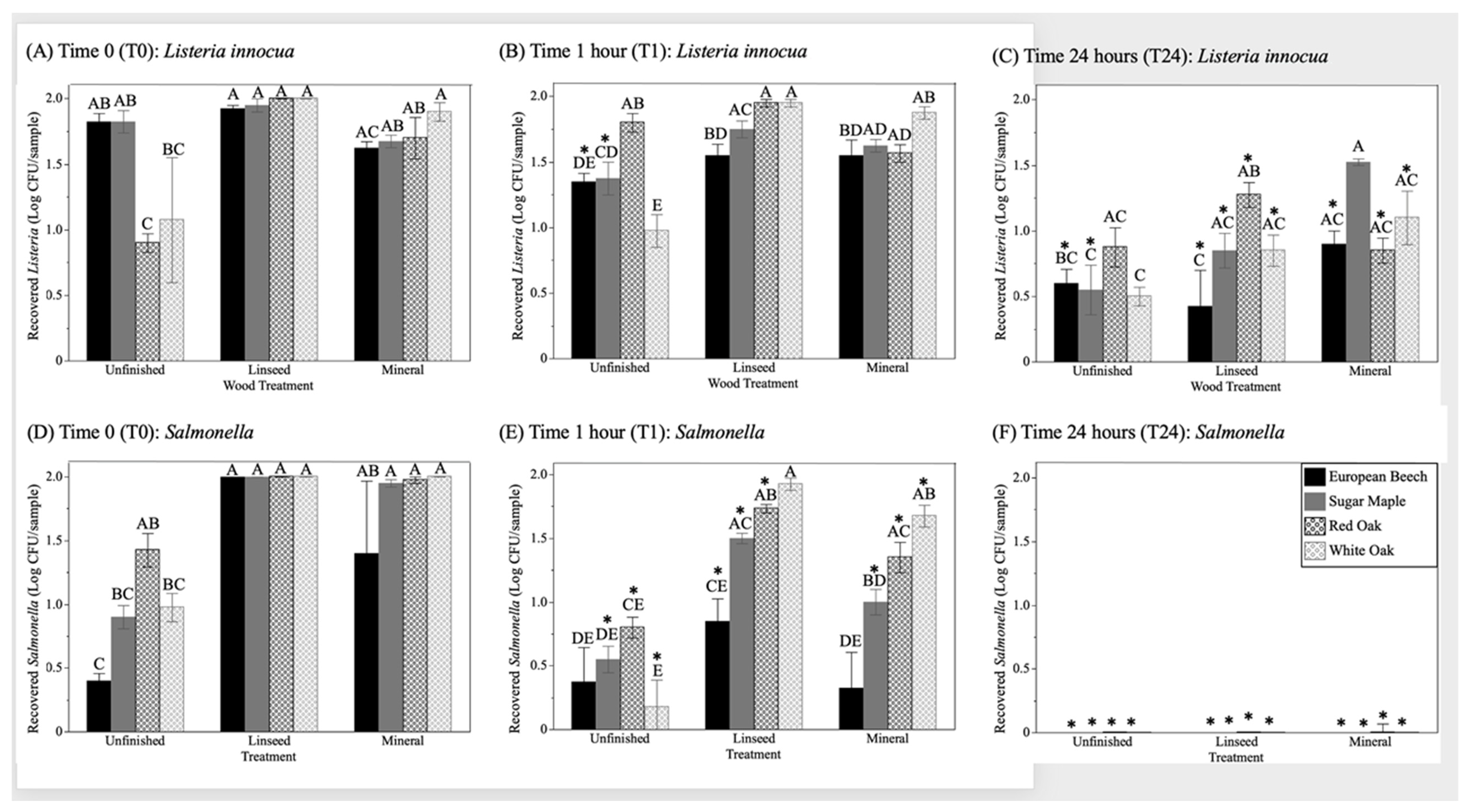
Samples with five coats of either linseed or mineral oil were not able to absorb the bacterial solution, which merely ran off the sample. As such, these samples were removed from the analysis.
Quantitative recovery of
Listeria and
Salmonella from wood samples of different surface treatments are shown in
Figure 2. Wood species (
p = 0.0006), wood surface treatment (untreated, linseed oil, mineral oil) (
p <0.0001), and time (
p <0.0001) had statistically significant impacts on the recovery of inoculated bacteria from wood samples. Significantly lower recovery of bacteria were for European beech samples, whereas all other wood species were comparable across the study. The application of an oil coating (either linseed or mineral) resulted in significantly higher levels of bacterial recovery from the wood surface compared to untreated wood (
p < 0.0001). Bacterial recovery significantly decreased with increasing time since inoculation (
p < 0.0001).
Listeria and
Salmonella differed significantly in recovery across the experiment with
Listeria having significantly higher recovery compared to
Salmonella across the experimental design (
p < 0.0001).
Bacterial recovery from wood samples immediately after inoculation (T0) and drying (T0) varied significantly depending on the wood species and surface treatment for both
Listeria (
Figure 2A) and
Salmonella (
Figure 2D). For both bacteria, the highest levels of recovery (~100% of inoculum) were, for wood samples, treated with 1 coating of linseed oil with variability between individual samples regardless of wood species. Similarly, wood samples treated with 1 coating of mineral oil also resulted in high rates of recovery of bacteria from all wood species. These findings suggest that one coating of may slow the absorption of bacteria into the wood grain, keeping the bacteria available on the surface of the wood.
The recovery of bacteria at T0 from unfinished wood samples was more variable, particularly between wood species.
Listeria recovery from the unfinished diffuse porous wood species (European beech and sugar maple) was comparable to wood samples treated with one coating of linseed oil, whereas the recovery of
Listeria was significantly reduced from unfinished ring porous species (red oak and white oak) (
Figure 2A), which have larger vessel elements and, in theory, a faster ability to equilibrate liquids. The recovery of
Listeria from white oak, a species where tyloses clog the vessel elements, had a high level of variability. The recovery of
Salmonella was significantly lower for unfinished European beech compared with unfinished red oak (
Figure 2D). The reduced recovery of bacteria from unfinished wood samples is a well-understood function of wood hygroscopicity and, along with it, the absorption of bacteria into the wood grain; the lack of mobility of
Listeria compared to
Salmonella in diffuse porous woods was unclear, and may be due to cell surface charge and/or relative hydrophobicity.
The recovery of
Listeria and
Salmonella decreased with increasing time post-inoculation and drying.
Listeria recovery from untreated diffuse porous woods (European beech and sugar maple) was significantly lower at the 1 h timepoint (T1) compared to T0 (
Figure 2B). There was not a significant difference in recovery of
Listeria between any other treatments between T0 and T1. The recovery of
Listeria from wood samples continued to decrease over time with significantly lower recovery at T24 from all oil-treated samples with the exception of sugar maple treated with mineral oil. Nearly all (98%) of wood samples had recoverable
Listeria at the T24 timepoint indicating strong survival of
Listeria and/or a lack of penetration into the wood surface (
Table 1).
Salmonella recovery was significantly reduced between the T0 and T1 timepoint for nearly all woods and wood treatments with the exception of white oak treated with linseed oil.
Salmonella recovery declined precipitously between T1 and T24, with detection (1–2 CFU/sample) only occurring in wood samples treated with mineral oil (
Figure 2F and
Table 1). This low level of detection of
Salmonella from mineral oil-treated samples occurred across all wood species (1–2 samples out of 4 per species) with the exception of white oak (0/4). The reduced recovery of
Salmonella from wood samples suggest either the inability of
Salmonella to survive on these surfaces and/or that these cells are adsorbed deeper within the wood structure preventing them from being recovered.
4. Discussion
Many studies investigating the survival of bacteria on kitchen surfaces, in particular cutting boards, inoculated extremely high contamination levels (>7 log CFU/sample) and often in a solution with high organic load (e.g., media, chicken fat) [
1,
6,
7]. These methodological decisions assist in the modeling of microbial inactivation and/or mimic a worst-case scenario for contamination using a food system; however, they can overwhelm the wood system, making it difficult to identify impacts of the wood system on microbial survival. Previous studies recovered bacteria from cutting board surfaces by agitating in an isotonic diluent prior to further dilution and plating onto agar media [
7,
8]. This standard approach is a necessity for high inoculum studies (e.g., those that require dilution for enumeration purposes) and would likely result in high recovery of bacteria from the test sample; however, it will recover bacteria not only from the surface, but also from the interior crevices, cracks, and microstructure of the wood—thereby giving a false inference about how much bacteria are actually ‘available.’ Methods used in the study were intended to mimic pathogen levels (
Listeria and
Salmonella) that may transfer from contaminated food onto cutting boards with normal use (100 cells). Using this low level of inoculation, we could effectively sample and directly enumerate viable cells only from the top food contact surface of the wood without confounding results due to the recovery of bacteria that may have been adsorbed more deeply in the wood tissue. Our inoculation and recovery approach is comparable to that previously described by Ak et al. [
7] and appropriately mimicked contact with a home kitchen cutting board.
Most of the prior research on wood cutting boards prioritized comparisons between wood and plastic surfaces as opposed to comparing wood species. Of the wood cutting board specific research to date, there was no significant difference found between tested wood species (1,2,3,4,7). Ak et al. [
1,
7] evaluated the survival of
Escherichia coli on commercial wood cutting boards made from ash, basswood, beech, birch, butternut, cherry, hard maple (sugar maple), oak, and American black walnut. Within 3 min of inoculation, the recovery of
E. coli was reduced to between 1 and 20% of the initial inoculum level (3–4 log CFU/sample) with no significant differences between wood species (7). In a second study, Ak et al. [
1] also demonstrated a rapid loss of recovery from wooden cutting boards inoculated at >7 log CFU/sample with no difference between these same wood species. Schonwalder et al. [
9] tested the penetration and survival of
E. coli and
Enterococcus faecium on wood boards and blocks of Scots pine, Norway spruce, European larch, beech, and black poplar using destructive sampling methods (sawing and grinding) with the collective results of various experiments pointing to pine wood having the lowest microbial recovery. Given that differences in microbial survival to wood species are likely smaller than the contribution of inoculation level and time of sampling, an accurate comparison between wood species differences will require a large sample size as well as a thoughtful experimental design to capture biological differences between individual trees as well as wood species. In particular, comparisons between hardwoods (deciduous trees) and conifers (coniferous trees) need to be very well designed, as fundamental cellular differences (vessels versus no vessels, resin canals, gum ducts, etc.) between the two groups can make comparisons difficult.
Scientific studies aside, the results presented herein are in direct contrast to popular/internet knowledge. Frequent claims are made on nonscientific platforms about how wood that is less porous (likely meaning diffuse porous, or ‘closed grain’) is ‘safer’ than wood that is more porous (likely meaning ring porous) ([
10,
11,
12] among many others). Many sites discuss maples, walnuts, and cherries as being ideal, with woods such as oak, especially red oak, not being ‘safe’. These statements are based on a misunderstanding of anatomy and physical properties of wood and do not recognize the hygroscopicity of all wood. This study used two diffuse porous woods (sugar maple and European beech) and two ring porous woods (red oak and white oak), one of which had occluded vessels (white oak) and should, in theory, function more like a diffuse porous wood. Bacterial recovery from European beech wood was significantly lower compared to the other three wood species tested, although the oaks performed better with
Listeria when left uncoated (in that they had less recoverable bacteria on their surface). Recovery from European beech was amongst the lowest across nearly all treatments with the exception of significantly higher
Listeria recovery at T0 for untreated wood samples. European beech is a diffuse porous (‘closed grain’) wood, but so is sugar maple, which did not perform in a similar manner.
In contrast to evaluating different wood species, there is not a significant body of work on how coatings may affect the adsorption and/or penetration of bacteria to wood cutting boards. Ak et al. [
7] reported comparable and very low recovery (<11% of initial inoculum) of
E. coli K12 from basswood and maple/walnut cutting boards with and without mineral oil coating. Our research demonstrated wood treated with linseed or mineral oil significantly increased the recovery of bacteria from the wood surface for at least 1 h after contamination. The treatment of wood with either oil did not have a significant impact on bacterial recovery 24 h after contamination. It is interesting to note that there was not a significant difference between the types of coatings, as their properties and behavior in the wood were quite different—and noting that the study by Bohinic et al. [
13] found a significant increase in bacterial growth with linseed oil over other coatings. Linseed oil is a hardening oil, in that once it cures, it forms a film inside the wood. Mineral oil is a non-hardening oil that remains liquid inside the wood and it would be reasonable to assume that it would have less effect on the board’s hygroscopic nature. However, both types of finishes are absorptive finishes (in contrast to top coating acrylic finishes); therefore, some aspects of the wood vessels were invariably blocked and are apt to impair wood’s ability to move water and, potentially, bacteria (
Figure 3).
Our findings suggest that a minimal coating of wood with oil delays/prevents the absorption of bacteria into the wood grain and/or protects the bacteria from stresses associated with the wood surface (antimicrobial compounds, rapid desiccation due to hygroscopicity). Prior cutting board studies attempted to improve recovery of bacteria that may have adsorbed deeper into the wood structure by using sonication [
7], grinding [
9], or planing [
14]; however, these more aggressive methods yielded only minimal increases (0 to 30%) in microbial recovery. Schonwalder et al. [
9] demonstrated that bacterial contaminants penetrated at least 3 mm into wood; however, there is very limited evidence that they would return to the surface [
15,
16]. Collective results from several wood studies seemed to demonstrate that numerous and diverse bacteria (pathogens, surrogates, and native food microbiota) rapidly lose viability (regardless of initial inoculum level and humidity) upon contact with wood [
1,
7,
9]. Ak et al. [
1] attempted several methods to provide evidence of antimicrobial properties of wood that might explain the reduced recovery of bacteria from wood surfaces. These methods yielded inconsistent results beyond demonstrating that any antimicrobial activity associated with the wood was unlikely to be water soluble. Polyphenolic compounds extracted from wood, specifically stilbenes and their esters, were shown to have antimicrobial activity against
L. monocytogenes [
17]. The preparation of methanolic extracts from different woods may be a valid strategy for recovering antimicrobial substances from wood [
18]. Wood typically has an acidic pH (pH 4.3–5.2) and the combination acid and desiccation stress (a function of moisture content and hygroscopicity) likely contribute to any antimicrobial activity of wood surfaces [
15,
19]. Additional research is necessary to identify and characterize the antimicrobial properties of wood.
In the study, wood samples were coated with a minimal amount (single coat) of linseed or mineral oil prior to microbial challenge. Attempts were made to increase the oil application to be more substantial (five coats/applications); however, this resulted in an extreme change in the surface properties of the wood samples. The inoculum could not adsorb or dry on the heavily coated surfaces and, instead, beaded or flowed off the sample, making data associated with these treatments unreliable (data not shown). Further investigation of microbial behavior on heavily coated surfaces would require an adjustment to the inoculum preparation to reduce surface tension (e.g., emulsifier or surfactant), or an adjustment that made the oil itself antimicrobial. A few examples of this include Lu and Chang [
20], where a waterborne urethane oil (WUO) made from linseed oil had various antimicrobial agents added to it; Sailer et al. [
21], where linseed oil-treated wood blocks developed a protective fungal biofilm; and a study that combined linseed oil with fungal melanin to inhibit antimicrobial growth on wooden instruments [
22].
Listeria and
Salmonella differed significantly in their survival on the wood surfaces tested in this study. While both bacteria declined in their recovery over the 24 h test period,
Listeria was significantly more stable on the wood surface compared to
Salmonella; this was particularly evident for unfinished wood samples.
Salmonella recovery was substantially reduced on unfinished wood samples immediately after inoculation (T0), whereas
Listeria recovery remained high on unfinished diffuse porous wood types and reduced on unfinished ring porous wood types (ring porous having a higher porosity and greater ability to ‘move’ bacteria). Other studies, including Schonwalder et al. [
9] and Chen et al. [
5], found improved survival of Gram-positive bacteria on wood compared to Gram-negative bacteria. It is important to note that single bacterial strains were used in most studies, and so, care should be taken in overgeneralizing these findings. However, diverse studies on wood cutting boards consistently demonstrated that low levels of microbial recovery with a few minutes following inoculation and recovery consistently decreased as time increased (12–24 h post-inoculation) and did not seem to be impacted by humidity [
1,
7]. What is perhaps most interesting about this study is that wood species did matter: the species best able to decontaminate the wood surface for
Salmonella was diffuse porous European beech, while the best species for
Listeria removal was either of the oaks (ring porous woods). There was no ‘one best wood’. It is also important to note that the authors recognize that microorganisms could differ in their affinity/adsorption to the wood surface based on wood species and oil treatment, and that this could lead to differences in recovery of the bacteria. However, the authors considered the chemical (e.g., complex protein/carbohydrate mixture, hydrophobicity) and physical qualities (e.g., pH, water activity, etc.) of the agar media and determined them to be sufficiently comparable to food systems that the cell transfer would be comparable to the conditions in the “real world” kitchen environment. As the purpose of this study was to mimic real world environments of modern kitchens, these methods worked well.