Improving the Electrochemical Performance of LiNi1/3Co1/3Mn1/3O2 Cathode Material by LiF Modification
Abstract
1. Introduction
2. Experimental
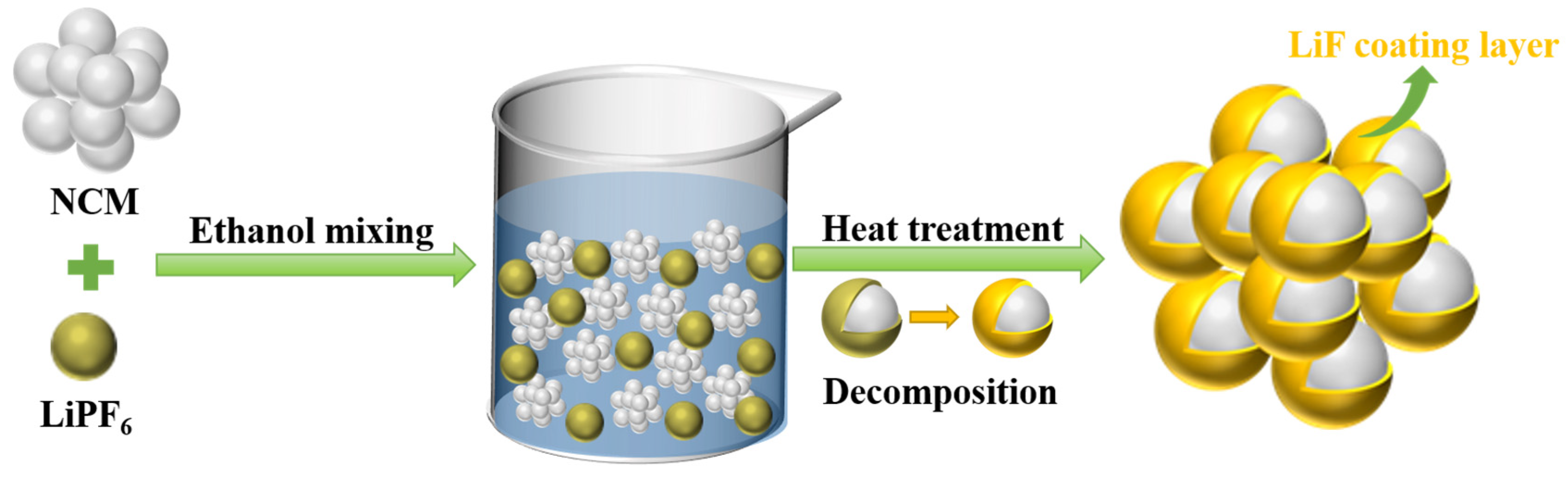
2.1. Sample Preparation
2.2. Characterization of Materials
2.3. Electrochemical Characterization
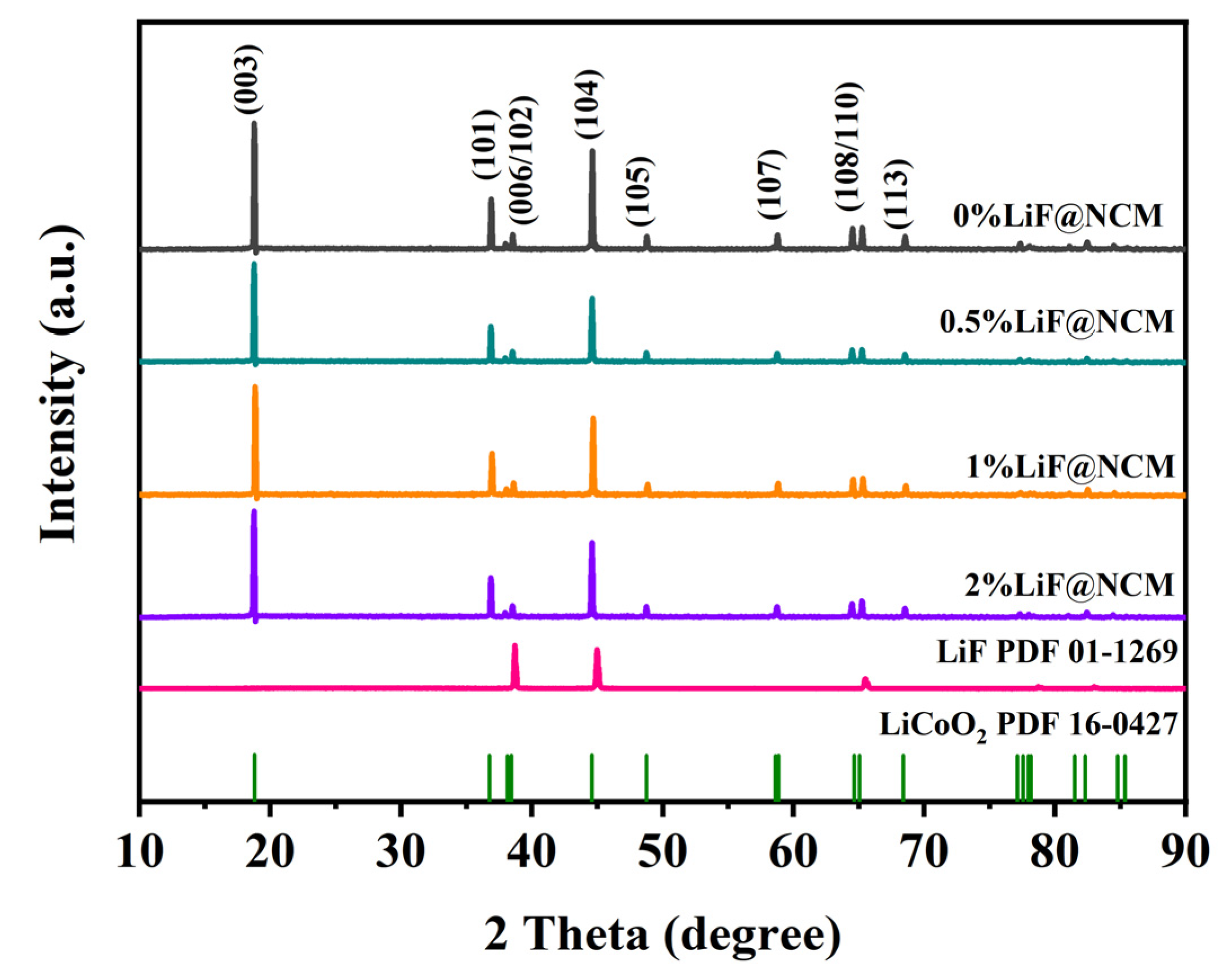
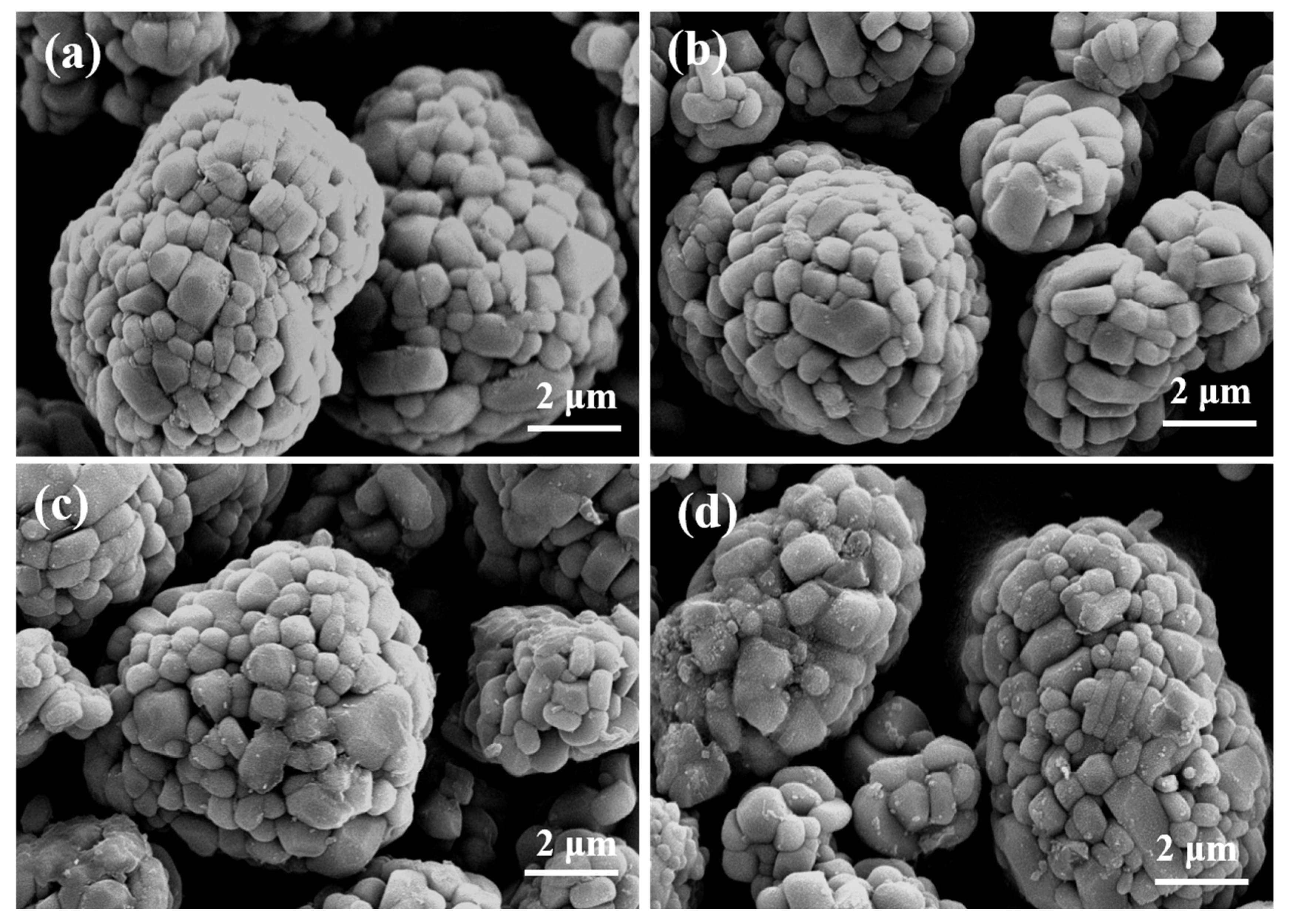
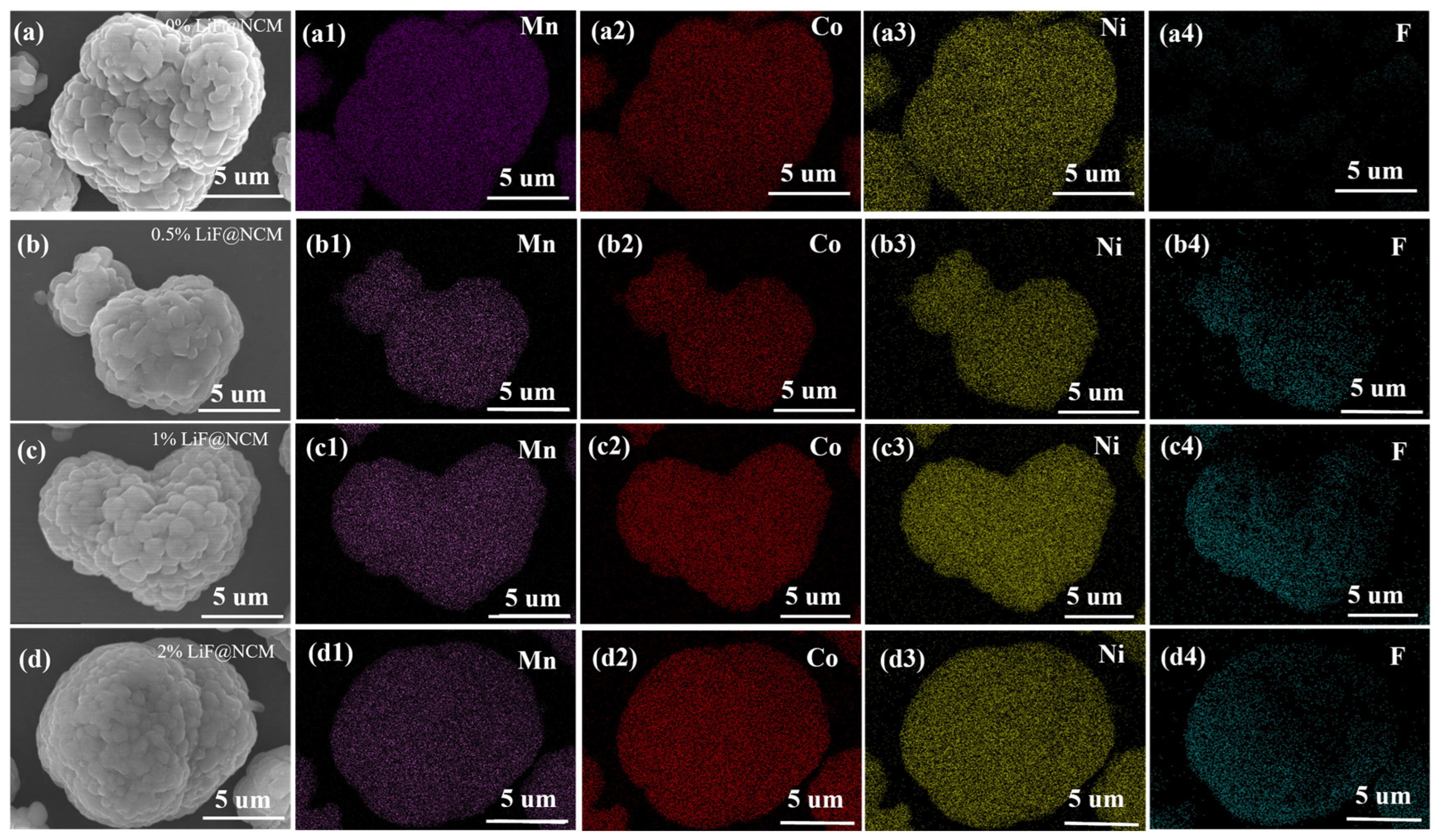
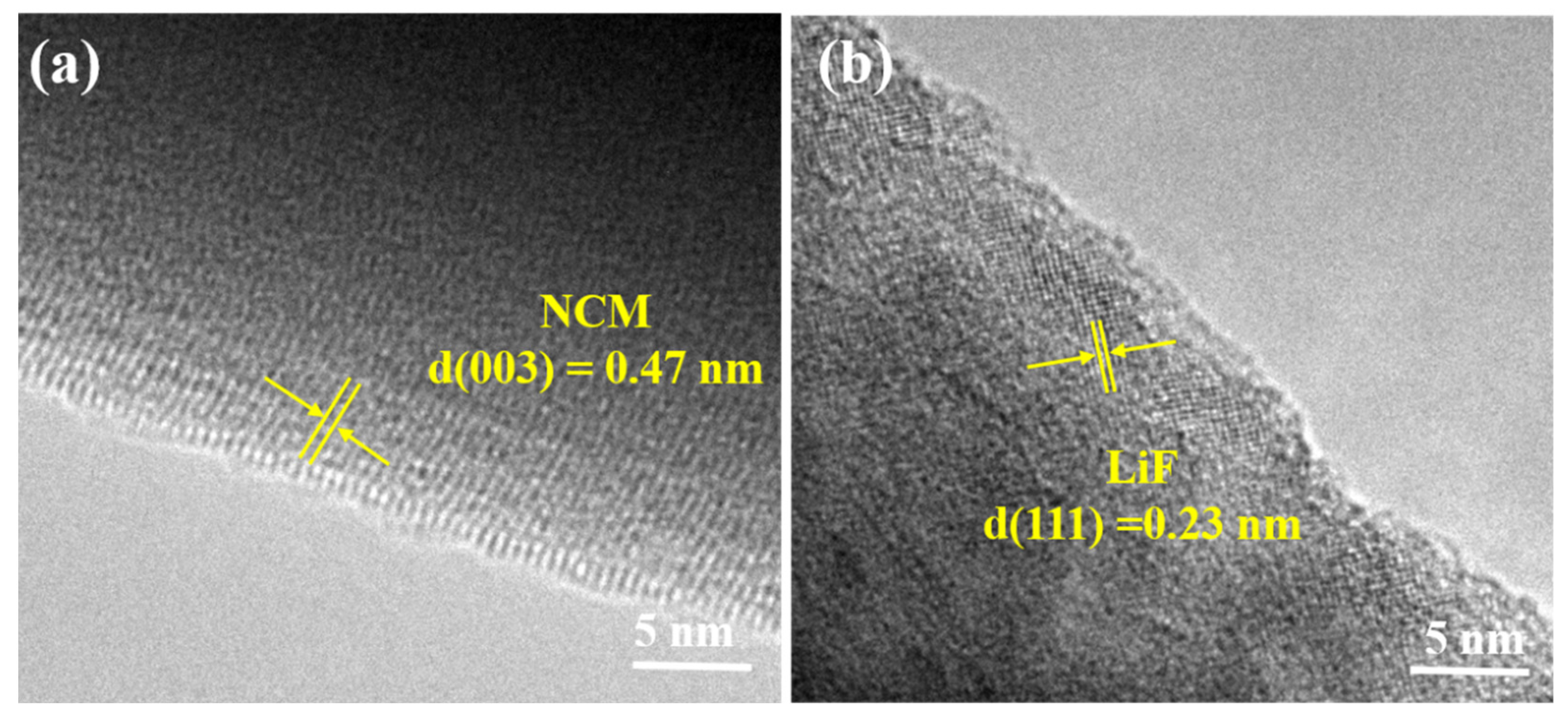
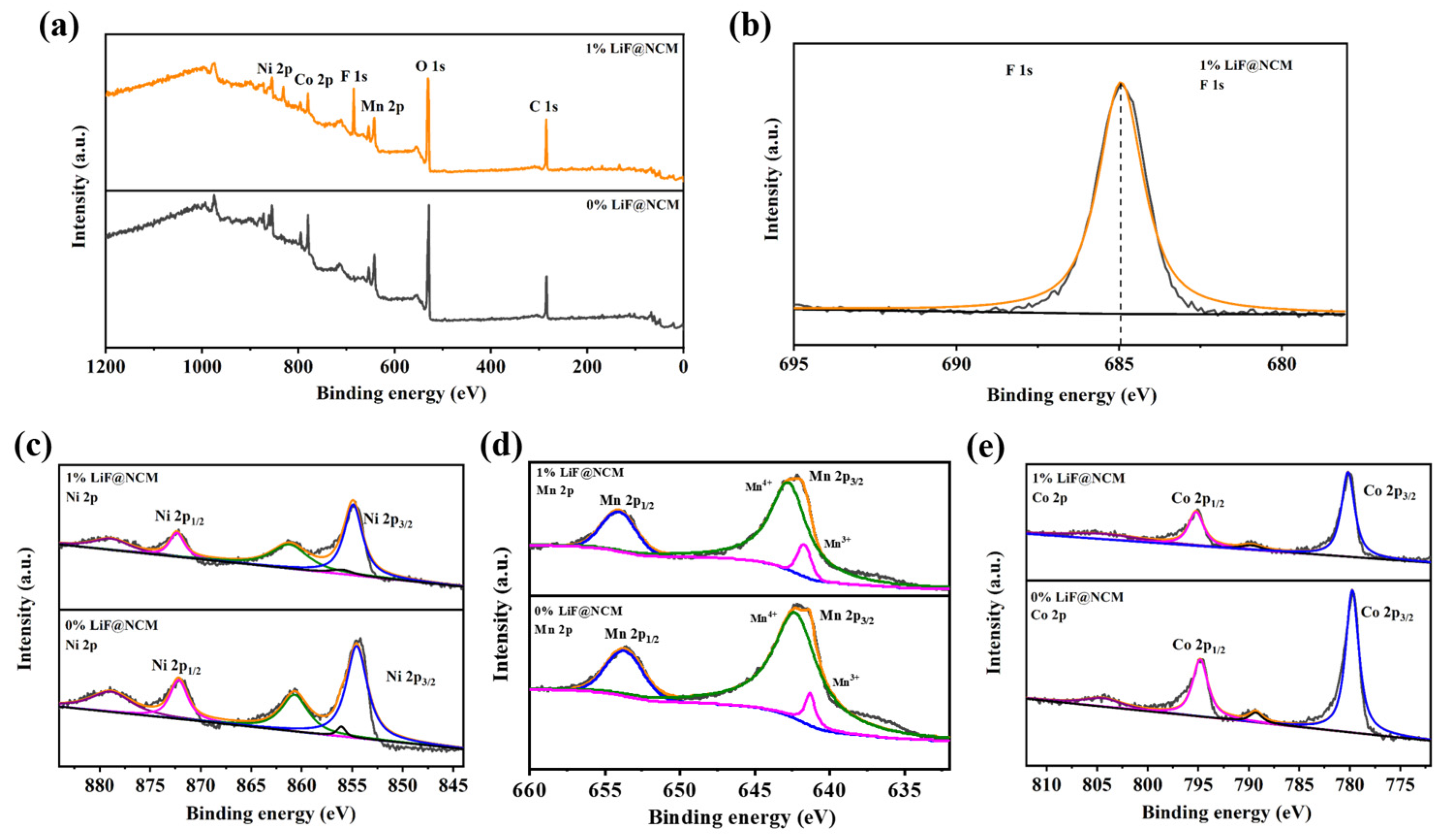
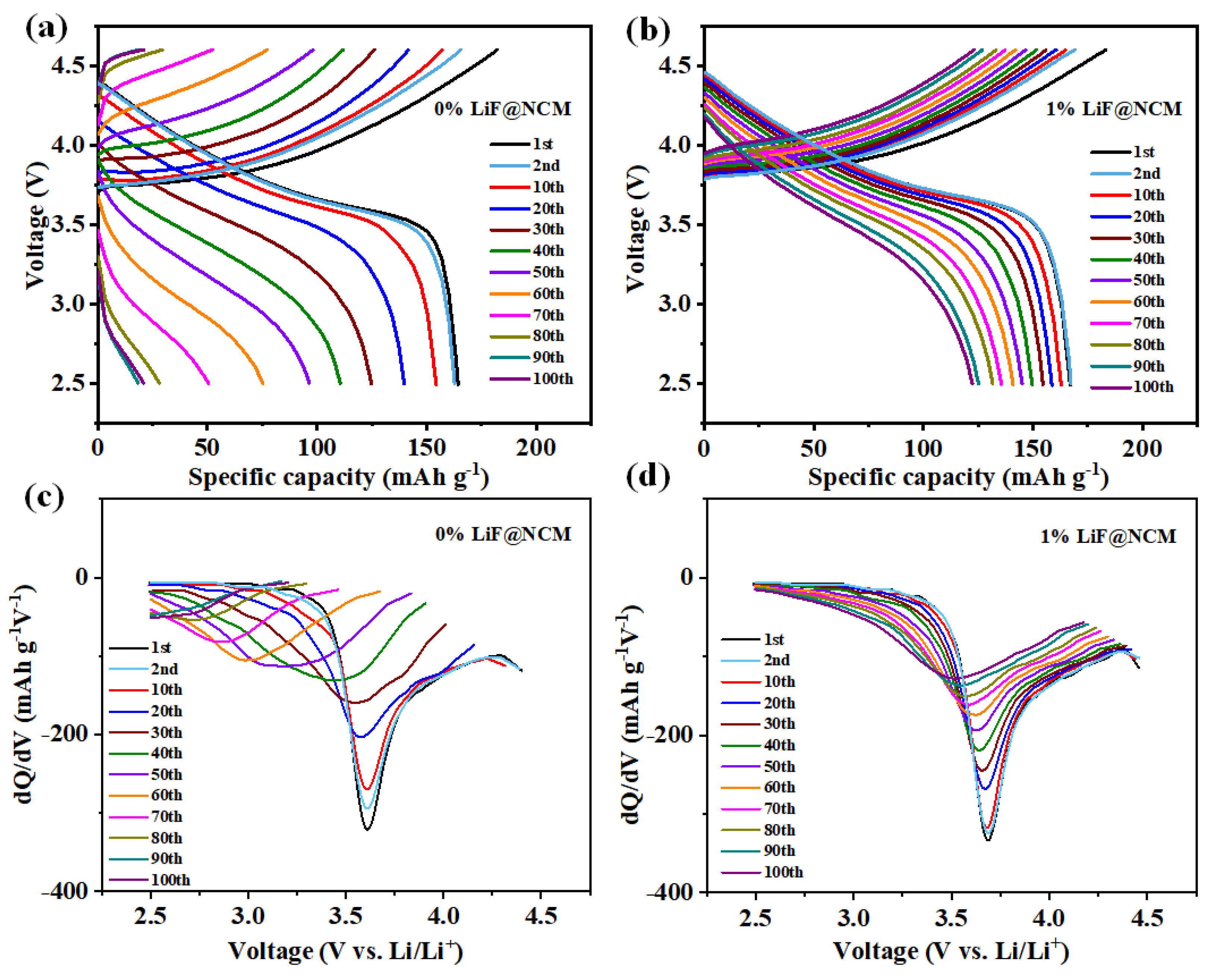
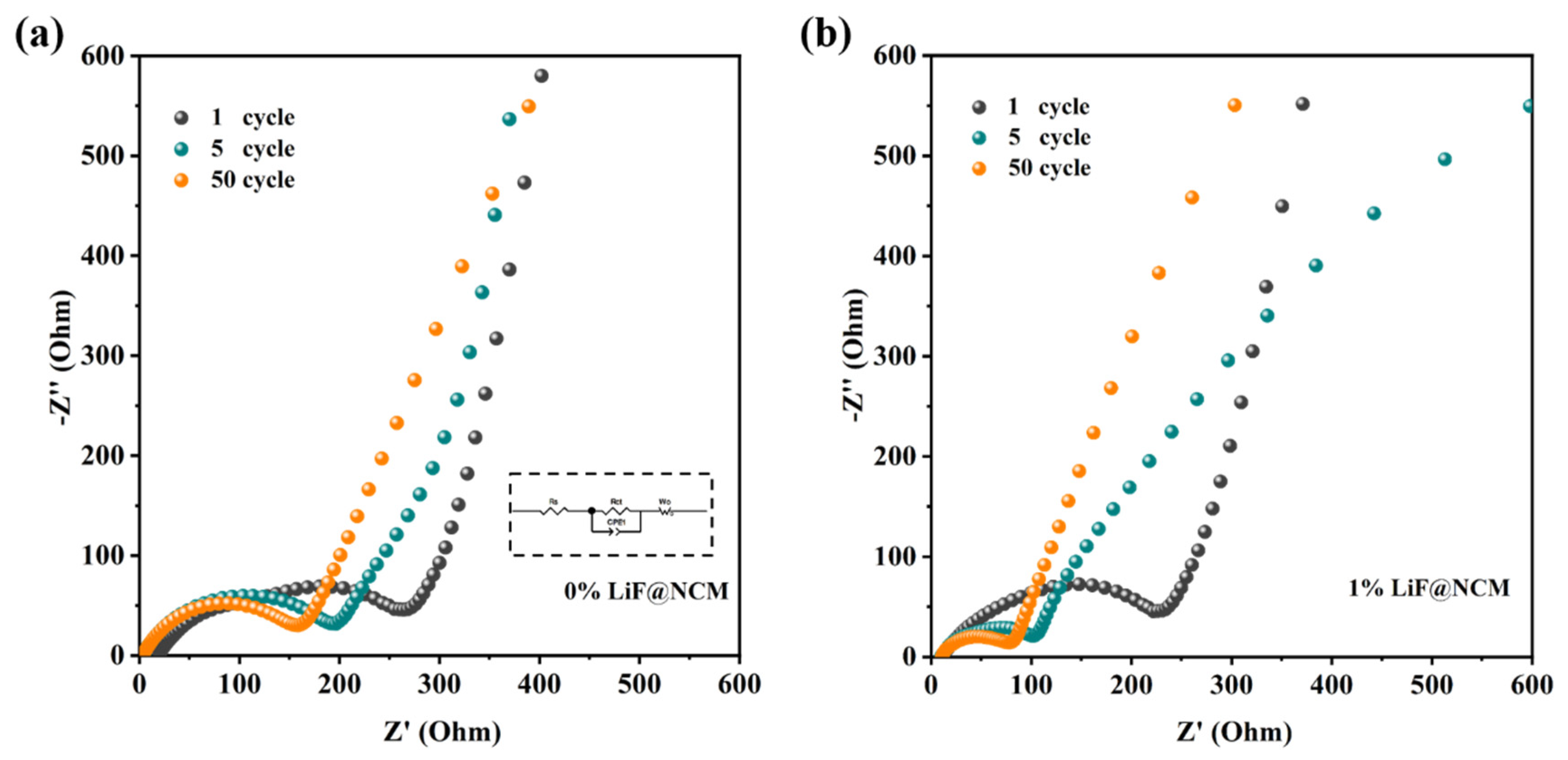
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodenough, J.; Park, K. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, B.; Qu, Z.; Gong, Y.; He, B.; Wang, H. Research on the kinetic properties of the cation disordered rock-salt Li-excess Li1.25Nb0.25Mn0.5O2 material. Solid State Ion. 2019, 339, 114999. [Google Scholar] [CrossRef]
- Mauger, A.; Armand, M.; Julien, C.; Zaghib, K. Challenges and issues facing lithium metal for solid-state rechargeable batteries. J. Power Sources 2017, 353, 333–342. [Google Scholar] [CrossRef]
- de Villers, B.T.; Bak, S.; Yang, J.; Han, S. In Situ ATR-FTIR Study of the Cathode–Electrolyte Interphase: Electrolyte Solution Structure, Transition Metal Redox, and Surface Layer Evolution. Batter. Supercaps. 2021, 4, 778–784. [Google Scholar] [CrossRef]
- Yang, J.; Muhammad, S.; Jo, M.; Kim, H.; Song, K.; Agyeman, D.; Kim, Y.I.; Yoon, W.; Kang, Y. In situ analyses for ion storage materials. Chem. Soc. Rev. 2016, 45, 5717–5770. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Park, S.; Lee, S.; Kim, J.; Huang, D.; Gim, J.; Lee, E.; Kim, G.; Park, K.; Kang, Y.-M.; et al. High-voltage deprotonation of layered-type materials as a newly identified cause of electrode degradation. J. Mater. Chem. A. 2023, 11, 3018–3027. [Google Scholar] [CrossRef]
- Manthiram, A.; Song, B.; Li, W. A perspective on nickel-rich layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 2017, 6, 125–139. [Google Scholar] [CrossRef]
- Wang, R.; He, X.; He, L.; Wang, F.; Xiao, R.; Gu, L.; Li, H.; Chen, L. Atomic Structure of Li2MnO3 after Partial Delithiation and Re-Lithiation. Adv. Energy Mater. 2013, 3, 1358–1367. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, Z.; Yin, X.; Guo, H.; Li, X. A modified LiF coating process to enhance the electrochemical performance characteristics of LiNi0.8Co0.1Mn0.1O2 cathode materials. Mater. Lett. 2013, 110, 4–9. [Google Scholar] [CrossRef]
- Ohzuku, T.; Makimura, Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries. Chem. Lett. 2001, 7, 642–643. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Zhu, C.; Sun, Z.; Cheng, W.; Lv, D.; Ren, W.; Bian, L.; Xu, J.; Chang, A. Enhanced Electrochemical Capability of LiNi1/3Co1/3Mn1/3O2 Cathode Materials Coated with Fluoroborate Glass for Lithium-Ion Batteries. ChemElectroChem 2017, 4, 1199–1204. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, J.; Chang, F.; Xu, Z.; Wang, Z.; Hall, P.; Cheng, Y.; Xia, Y. Sol/Antisolvent Coating for High Initial Coulombic Efficiency and Ultra-stable Mechanical Integrity of Ni-Rich Cathode Materials. ACS Appl. Mater. Interfaces 2022, 14, 45272–45288. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chin, C.-T.; Li, Y.; Cheng, C.-H. Sustainable Low-Temperature Coating of LATP/C Solid Electrolyte Composites on LiNi1/3Co1/3Mn1/3O2 Cathode Based on Lithium Iodide Solvent-Recrystallization for Li-Ion Batteries. J. Electrochem. Soc. 2022, 169, 113505. [Google Scholar] [CrossRef]
- Li, G.; Feng, X.; Ding, Y.; Ye, S.; Gao, X. AlF3-coated Li(Li0.17Ni0.25Mn0.58)O2 as cathode material for Li-ion batteries. Electrochim. Acta 2012, 78, 308–315. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Huang, T.; Yu, A. CaF2-coated Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials for Li-ionbatteries. Electrochim. Acta 2013, 109, 52–58. [Google Scholar] [CrossRef]
- Chong, S.; Chen, Y.; Yan, W.; Guo, S.; Tan, Q.; Wu, Y.; Jiang, T.; Liu, Y. Suppressing capacity fading and voltage decay of Li-rich layered cathode material by a surface nano-protective layer of CoF2 for lithium-ion batteries. J. Power Sources 2016, 332, 230–239. [Google Scholar] [CrossRef]
- Zhang, X.; Belharouak, I.; Li, L.; Lei, Y.; Elam, J.W.; Nie, A.; Yassar, R.S.; Axelbaum, R.L. Structural and Electrochemical Study of Al2O3 and TiO2 coated Li1.2Ni0.13Mn0.54Co0.13O2 Cathode Material Using ALD. Adv. Energy Mater. 2013, 3, 1299–1307. [Google Scholar] [CrossRef]
- Huang, B.; Wang, R.; Gong, Y.; He, B.; Wang, H. Enhanced Cycling Stability of Cation Disordered Rock-Salt Li1.2Ti0.4Mn0.4O2 Material by Surface Modification With Al2O3. Front. Chem. 2019, 7, 107. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, F.; Li, L.; Chen, M.; Zhong, X.; Li, W.; Lu, L. Effect of Li3PO4 coating of layered lithium-rich oxide on electrochemical performance. J. Power Sources 2017, 341, 147–155. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, X.; Zhao, T.; Li, L.; Xie, M.; Chen, R. Multifunctional AlPO4 coating for improving electrochemical properties of low-cost Li[Li0.2Fe0.1Ni0.15Mn0.55]O2 cathode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 3773–3781. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, E.; He, C.; Shi, C.; Li, J.; Zhao, N. Effect of amorphous FePO4 coating on structure and electrochemical performance of Li1.2Ni0.13Co0.13Mn0.54O2 as cathode material for Li-ion batteries. J. Power Sources 2013, 236, 25–32. [Google Scholar] [CrossRef]
- Zhang, M. Enhanced Electrochemical Performance of LiNi1/3Co1/3Mn1/3O2 at a High Cut-Off Voltage of 4.6 V by Li1.3Al0.3Ti1.7(PO4)3 Coating. Coatings 2022, 12, 1964. [Google Scholar] [CrossRef]
- Qu, Z.; Liu, S.; Zhang, P.; Wang, R.; Wang, H.; He, B.; Gong, Y.; Jin, J.; Li, S. Enhanced eletrochemical performances of LiCoO2 at high cut-off voltage by introducing LiF additive. Solid State Ion. 2021, 365, 115654. [Google Scholar] [CrossRef]
- Yu, K.; Chen, J.; Lin, K.; Li, J.; Shi, Z. Constructing LiF-rich artificial SEI at a two-dimensional copper net current collector in anode-free lithium metal batteries. Surf. Interfaces 2022, 34, 102326. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Q.; Dai, S.; Li, W.; Liu, X.; Ding, F.; Zhang, J. Effect of F– doping and LiF coating on improving the high-voltage cycling stability and rate capacity of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 34153. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, Y.; Chen, F.; He, X.; Yasmin, A.; Hu, Q.; Wen, Z.; Chen, C. In situ formation of LiF decoration on a Li-rich material for long-cycle life and superb low-temperature performance. J. Mater. Chem. A 2019, 7, 11513–11519. [Google Scholar] [CrossRef]
- Fu, C.; Wang, J.; Wang, J.; Meng, L.; Zhang, W.; Li, X.; Li, L. A LiPF6-electrolyte-solvothermal route for the synthesis of LiF/LixPFyOz-coated Li-rich cathode materials with enhanced cycling stability. J. Mater. Chem. A 2019, 7, 23149–23161. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Huang, M.; Huang, J.; Fang, Z. Preparation and Rate Capability of Carbon Coated LiNi1/3Co1/3Mn1/3O2 as Cathode Material in Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 12408–12415. [Google Scholar] [CrossRef]
- Deng, R.; Tao, J.; Zhong, W.; Wen, L.; Yang, Y.; Li, J.; Lin, Y. Tri-functionalized Li2B4O7 coated LiNi0.5Co0.2Mn0.3O2 for boosted performance lithium-ion batteries. J. Alloys Compd. 2023, 940, 168767. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhao, Y.; Bao, Y.; Zhang, W.; Guan, M. Research on the electrochemical properties of vanadium boride coated on the surface of NCM811. J. Alloys Compd. 2022, 927, 166967. [Google Scholar] [CrossRef]
- Dunlap, N.; Sulas-Kern, D.; Weddle, P.; Usseglio-Viretta, F.; Walker, P.; Todd, P.; Boone, D.; Colclasure, A.; Smith, K.; de Villers, B.T.; et al. Laser ablation of Li-ion electrodes for fast charging: Material properties, rate capability, Li plating, and wetting. J. Power Sour. 2022, 537, 231464. [Google Scholar] [CrossRef]
- Yi, H.; Tan, L.; Xia, L.; Li, L.; Li, H.; Liu, Z.; Wang, C.; Zhao, Z.; Duan, J.; Chen, Z. Ce-modified LiNi0.5Co0.2Mn0.3O2 cathode with enhanced surface and structural stability for Li ion batteries. Adv. Powder Technol. 2021, 32, 2493–2501. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, X.; Li, S.; Cui, Y.; Cui, Y.; Zhao, Y.; Cui, Y. In situ formed LiNi0.8Co0.1Mn0.1O2@LiF composite cathode material with high rate capability and long cycling stability for lithium-ion batteries. Ionics 2020, 26, 2165–2176. [Google Scholar] [CrossRef]
- Zhang, Y.; Self, E.; Thapaliya, B.; Giovine, R.; Meyer, H.; Li, L.; Yue, Y.; Chen, D.; Tong, W.; Chen, G.; et al. Formation of LiF Surface Layer during Direct Fluorination of High-Capacity Co-Free Disordered Rocksalt Cathodes. ACS Appl. Mater. Interfaces 2021, 13, 38221–38228. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.; Huang, X.; Liu, D.; Dou, A.; Su, M.; Chu, D. Electrochemical performance of Li1.2Ni0.2Mn0.6O2 coated with a facilely synthesized Li1.3Al0.3Ti1.7(PO4)3. J. Power Sources 2018, 403, 27–37. [Google Scholar] [CrossRef]
- Xue, L.; Li, Y.; Xu, B.; Chen, Y.; Cao, G.; Li, J.; Deng, S.; Chen, Y.; Chen, J. Effect of Mo doping on the structure and electrochemical performances of LiNi0.6Co0.2Mn0.2O2 cathode material at high cut-off voltage. J. Alloys Compd. 2018, 748, 561–568. [Google Scholar] [CrossRef]
- Wang, H.; Hashem, A.; Abdel-Ghany, A.; Abbas, S.; El-Tawil, R.; Li, T.; Li, X.; El-Mounayri, H.; Tovar, A.; Zhu, L.; et al. Effect of Cationic (Na+) and Anionic (F−) Co-Doping on the Structural and Electrochemical Properties of LiNi1/3Mn1/3Co1/3O2 Cathode Material for Lithium-Ion Batteries. Int. J. Mol. Sci. 2022, 23, 6755. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, X.; Liu, L.; Lee, J.; Seo, D.-H.; Bo, S.-H.; Urban, A.; Ceder, G. A disordered rock-salt Li-excess cathode material with high capacity and substantial oxygen redox activity: Li1.25Nb0.25Mn0.5O2. Electrochem. Commun. 2015, 60, 70–73. [Google Scholar] [CrossRef]
- Zhang, X.; Sui, X.; Zhou, S.; Tang, C.; Wang, R. Li-B alloy with artificial solid electrolyte interphase layer for long-life lithium metal batteries. Solid State Ion. 2020, 354, 115408. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Evertz, M.; Streipert, B.; Wagner, R.; Klöpsch, R.; Vortmann, B.; Hahn, H.; Nowak, S.; Amereller, M.; Gentschev, A.; et al. The truth about the 1st cycle Coulombic efficiency of LiNi1/3Co1/3Mn1/3O2 (NCM) cathodes. Phys. Chem. Chem. Phys. 2016, 18, 3956–3965.24. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Guo, Y.; Liu, C.; Dan, L. Electrochemical characterization of AlPO4 coated LiNi1/3Co1/3Mn1/3O2 cathode materials for high temperature lithium battery application. Rare Met. 2021, 40, 78. [Google Scholar] [CrossRef]
- Bai, Y.; Chang, Q.; Yu, Q.; Zhao, S.; Jiang, K. A novel approach to improve the electrochemical performances of layered LiNi1/3Co1/3Mn1/3O2 cathode by YPO4 surface coating. Electrochim. Acta 2013, 112, 414. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Wang, J.-Q.; Yang, X.-L.; Liang, G.; Li, T.; Yu, P.-L.; Ma, D. Enhanced Electrochemical Performance of Fast Ionic Conductor LiTi2(PO4)3-Coated LiNi1/3Co1/3Mn1/3O2 Cathode Material. ACS Appl. Mater. Interfaces 2018, 10, 11663. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, L.; Bian, L.; Xu, J.; Ren, W.; Hu, P.; Chang, A. Highly enhanced low temperature discharge capacity of LiNi1/3Co1/3Mn1/3O2 with lithium boron oxide glass modification. J. Power Sources 2015, 277, 139. [Google Scholar] [CrossRef]
- Shen, D.; Zhang, D.; Wen, J.; Chen, D.; He, X.; Yao, Y.; Li, X.; Duger, C. LiNi1/3Co1/3Mn1/3O2 coated by Al2O3 from urea homogeneous precipitation method: Improved Li storage performance and mechanism exploring. J. Solid State Electrochem. 2015, 19, 1523. [Google Scholar] [CrossRef]
- Wang, W.; Yin, Z.; Wang, J.; Wang, Z.; Li, X.; Guo, H. Effect of heat-treatment on Li2ZrO3-coated LiNi1/3Co1/3Mn1/3O2 and its high voltage electrochemical performance. J. Alloys Compd. 2015, 651, 737. [Google Scholar] [CrossRef]

| Modification | Voltage Range (V) | Initial Specific Discharge Capacity (mAh g−1) | Coulombic Efficiency (%) | Test Conditions Specific Current (mA g−1) | Ref. |
|---|---|---|---|---|---|
| LiF | 2.5–4.6 | 185 | 88.9 | 40 | This work |
| AlPO4 | 2.75–4.2 | 159 | 87.2 | 20 | [41] |
| YPO4 | 2.5–4.4 | 161.3 | 86.3 | 16 | [42] |
| Li1.3Al0.3Ti1.7(PO4)3/C | 2.75–4.4 | 171 | 87.9 | 20 | [13] |
| active carbon | 2.5–4.5 | 191.2 | 91.1 | 85 | [28] |
| fluoroborate glass | 2.5–4.5 | 207.5 | 88.2 | 40 | [11] |
| LiTi2(PO4)3 | 2.8–4.5 | 191.5 | 91.1 | 85 | [43] |
| lithium boron oxide | 2.5–4.5 | 175.8 | 85.1 | 40 | [44] |
| Al2O3 | 2.75–4.5 | 204.8 | 86.1 | 27.8 | [45] |
| Li2ZrO3 | 3.0–4.6 | 197.8 | 86.0 | 16 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Zhang, X.; Zhang, Z.; Liu, S.; Wang, R. Improving the Electrochemical Performance of LiNi1/3Co1/3Mn1/3O2 Cathode Material by LiF Modification. Coatings 2023, 13, 727. https://doi.org/10.3390/coatings13040727
Zhou S, Zhang X, Zhang Z, Liu S, Wang R. Improving the Electrochemical Performance of LiNi1/3Co1/3Mn1/3O2 Cathode Material by LiF Modification. Coatings. 2023; 13(4):727. https://doi.org/10.3390/coatings13040727
Chicago/Turabian StyleZhou, Sisi, Xianggong Zhang, Zhihao Zhang, Songting Liu, and Rui Wang. 2023. "Improving the Electrochemical Performance of LiNi1/3Co1/3Mn1/3O2 Cathode Material by LiF Modification" Coatings 13, no. 4: 727. https://doi.org/10.3390/coatings13040727
APA StyleZhou, S., Zhang, X., Zhang, Z., Liu, S., & Wang, R. (2023). Improving the Electrochemical Performance of LiNi1/3Co1/3Mn1/3O2 Cathode Material by LiF Modification. Coatings, 13(4), 727. https://doi.org/10.3390/coatings13040727








