Abstract
MnO2 has advantages such as the simple and diverse preparation methods, low cost and high theoretical capacity, but its industrial application is affected by its poor conductivity and fast attenuation of cycle performance. In order to improve its conductivity, battery capacity and performance, MnO2/carbon nanofibers (MnO2/CNFs) are obtained by using electrospinning technology, and the electrochemical performance was confirmed by XRD, SEM, TEM. Confirmed by comparison, the 20% MnO2/CNFs exhibit superior and excellent long cycling performance with a reversible capacity of 835 mA h g−1 at 0.1 A g−1 after the 133th cycle and a high initial specific capacity of 1094 mA h g−1 at a current density of 0.1 A g−1. The MnO2/CNFs have notable specific capacities with a coulombic efficiency of 99.5%, which greatly improve the reaction rate. This can also be used as a flexible electrode material because of its good flexibility. Due to the fact that carbon has better electron/ion conductivity, it shows better kinetics.
1. Introduction
Anode material is one of the core materials of a battery. It is an important factor in determining the safety, cycling stability and other performance of battery [1,2,3]. The development of high-capacity anode materials is very important to further improve the energy density of Li-ion batteries [4,5,6]. With unique outer valence electrons and special physical and chemical properties, transition metal manganese compounds are one of the high-capacity lithium-ion anode materials that have attracted more attention in recent years [7], among which MnO2 is viewed as one of the most valuable electrode materials for lithium battery due to its advantages such as simple and diverse preparation methods, low cost and high capacity (theoretical capacity is 1232 mA h g−1) [8,9]. However, MnO2 also has some disadvantages, involving volume change during the repeated process of charge/discharge and poor electrical conductivity, which result in poor performance of rate and inferior stability of cycle [10,11,12].
To solve the problems and improve the electrochemical performance, many effective strategies have been researched, such as materials, porous structure, and conductive polymer/metallic material coating [13,14,15,16]. Recently, significant effort has been devoted to nanowires, nanosheets, and porous nanostructures to boost the lithium-ion concentration [17,18,19]. Sui et al. synthesized semiconducting polypyrrole coated-δ-MnO2 nanosheet arrays on nickel foam by hydrothermal and electro-deposition. Coated-δ-MnO2 nanosheet arrays can afford ∼430 mA h g−1 after 120 cycles [20]. Single crystalline α-MnO2 nanowires, investigated by Ahmad Umar et al., provide a stable capacity of 330 mA h g −1 for 60 cycles [21]. Special nanostructures have high specific surface area and a great number of active sites, which could enhance electrochemical performance [22,23]. However, because these designs could buffer the anode material of lithium-ion batteries, it is difficult to apply this design directly to the anode material of lithium-ion batteries and the cyclic stability of the anode material is still not ideal. This is because the electrical conductivity of MnO2 is poor, which causes larger volume expansion. Therefore, it is necessary to design a unique anode of lithium-ion battery [24,25,26]. The embedding of MnO2 nanoparticles into carbon nanofibers (CNFS) as independent substrates by electrospinning has been extensively studied, but there has been little research on the embedding of MnO2 nanotubes into carbon nanofibers as independent electrodes.
Herein, MnO2/carbon nanofibers (MnO2/CNFs) were designed by electrospinning technology to enhance the electrochemical properties. This design has several advantages. Firstly, it effectively improves the conductivity and limits the volume expansion, which ensures the stability of the electrode during electrochemical cycling [27,28]. Secondly, MnO2/CNFs has large specific surface area and high porosity, which can improve the adsorption, catalysis and transfer properties of the material [29,30]. Thirdly, the fracture strength and elastic modulus of the fiber are significantly improved, and it has good flexibility, which can be used as the precursor of flexible materials [31,32]. The results show that MnO2/CNFs exhibit superior storage behavior and excellent long cycling performance with a reversible capacity of 835 mA h g−1 at 0.1 A g−1 after the 133th cycle. MnO2/CNFs exhibit notable specific capacities with a coulombic efficiency of 99.5%, which greatly improves the reaction rate and shows better kinetics due to the fact that carbon has better electron/ion conductivity.
2. Experimental Section
2.1. Preparation of MnO2
The transition metal Oxide MnO2 was prepared by hydrothermal method. Firstly, 0.2 g KMnO4 was mixed with 25 mL H2O and stirred for 10 min. Then, 1.25 mL HNO3 was added, stirred thoroughly, and transferred into a 50 mL Teflon lined stainless steel reactor for hydrothermal reaction at 120 °C for 12 h, cooling naturally. Centrifugal washing was performed at 10,000 rates, then drying. MnO2 powder was prepared.
2.2. Preparation of MnO2 NTs
MnO2 was calcined to produce MnO2 NTs. MnO2 powder was put into a tubular furnace and heat treatment was applied in 400 °C air for 4 h to obtain MnO2 NTs.
2.3. Preparation of MnO2 Nanofibers (NFs)
The polymer containing 5% MnO2 NFs and 20% MnO2 NFs was prepared by electrospinning. Weighed out required amounts of MnO2 NTs, N, N dimethylformamide (Tianjin Kaitong Chemical Reagent Co., Ltd., Tianjin, China) were mixed, put in the ultrasonic cleaning machine until fully dissolved, then the appropriate amount of polyacrylonitrile (PAN) was added. The mixture was continuously put in the ultrasonic cleaning machine until all was dissolved. Then, it was mixed into the required slurry in the electrostatic spinning machine for spinning. After re-coating by electrospinning, the polymer containing 5% MnO2/NFs and 20% MnO2/NFs was prepared.
2.4. Preparation of MnO2/CNFs
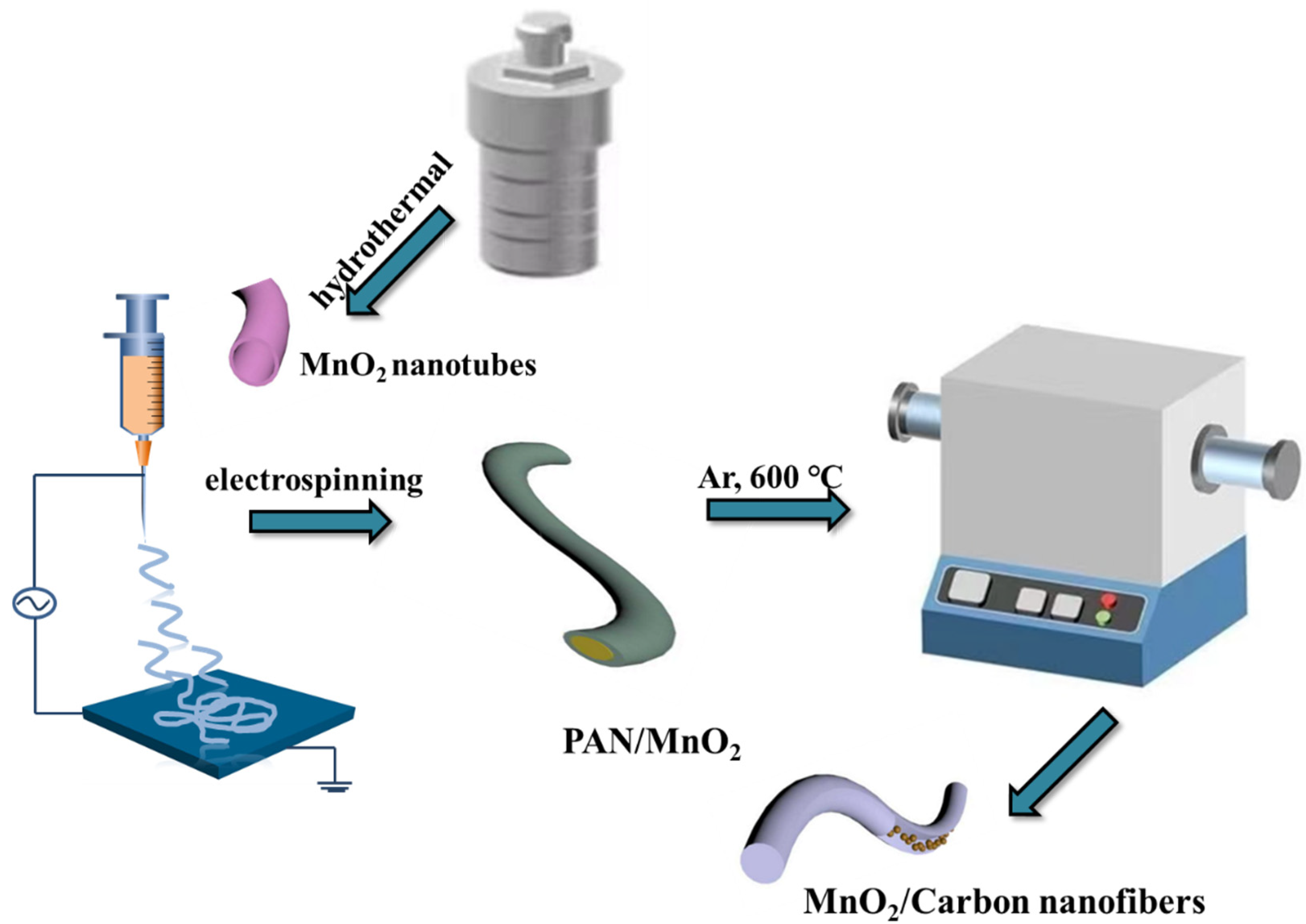
MnO2 NFs polymer was calcined to produce MnO2/CNFs. First, 5% MnO2 /NFs polymer and 20% MnO2/NFs polymer were placed in tubular furnace, respectively, and carbonized in Ar atmosphere at 600 °C for 4 h to obtain 5% MnO2/CNFs and 20% MnO2/CNFs. Schematic of the synthesis procedure of MnO2/CNFs is provided in Figure 1.
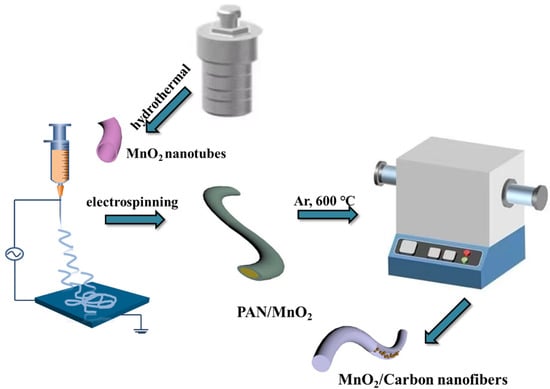
Figure 1.
Schematic of the synthesis procedure of the MnO2/Carbon nanofibers.
2.5. Material Characterization
The compositions of MnO2 NTs, 5% MnO2/CNFs and 20% MnO2/CNFs were characterized by X-ray diffraction (XRD, D8Advance, Bruker AXS, Karlsruhe, Germany), scanning electron microscope (SEM, JSM-6360LV, JEOL, Tokyo, Japan) and transmission electron microscope (TEM, Fei Tecnai G2 F20S-Twin, Hillsboro, OR, USA), then the morphology was further observed. The batteries were assembled in the glove box using 5% MnO2/CNFs and 20% MnO2/CNFs as anode materials, and dried at room temperature for 24 h.
3. Results and Discussion
3.1. Morphology and Structure
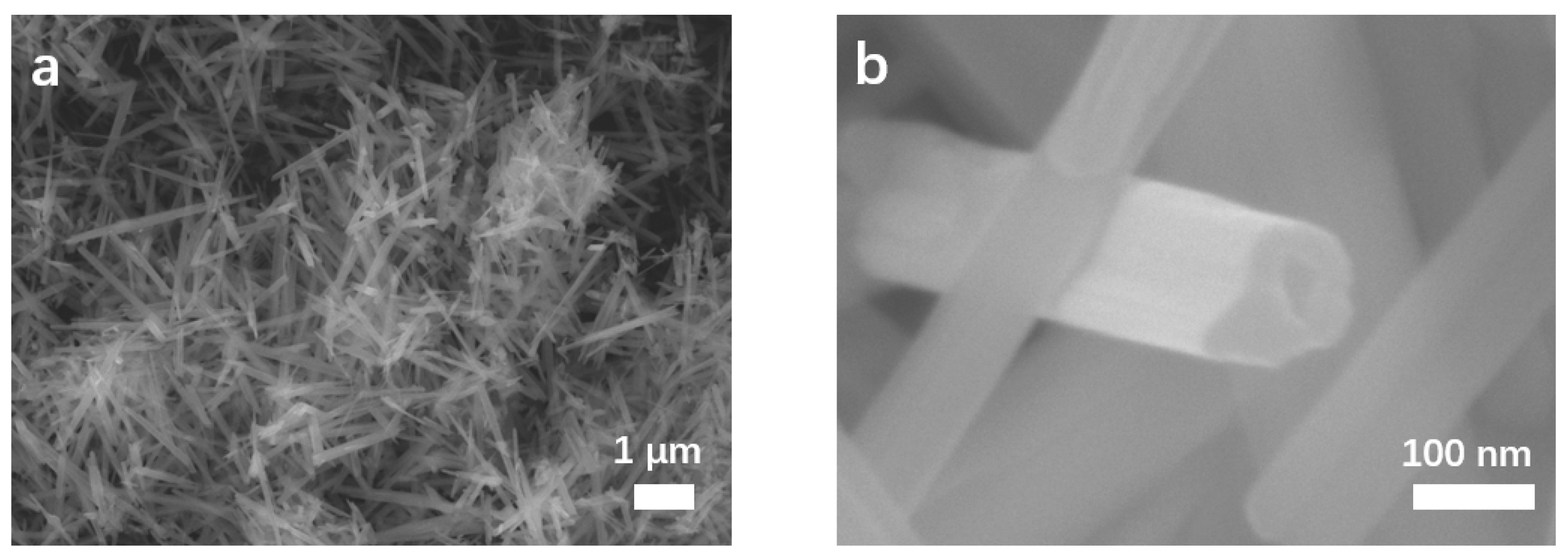
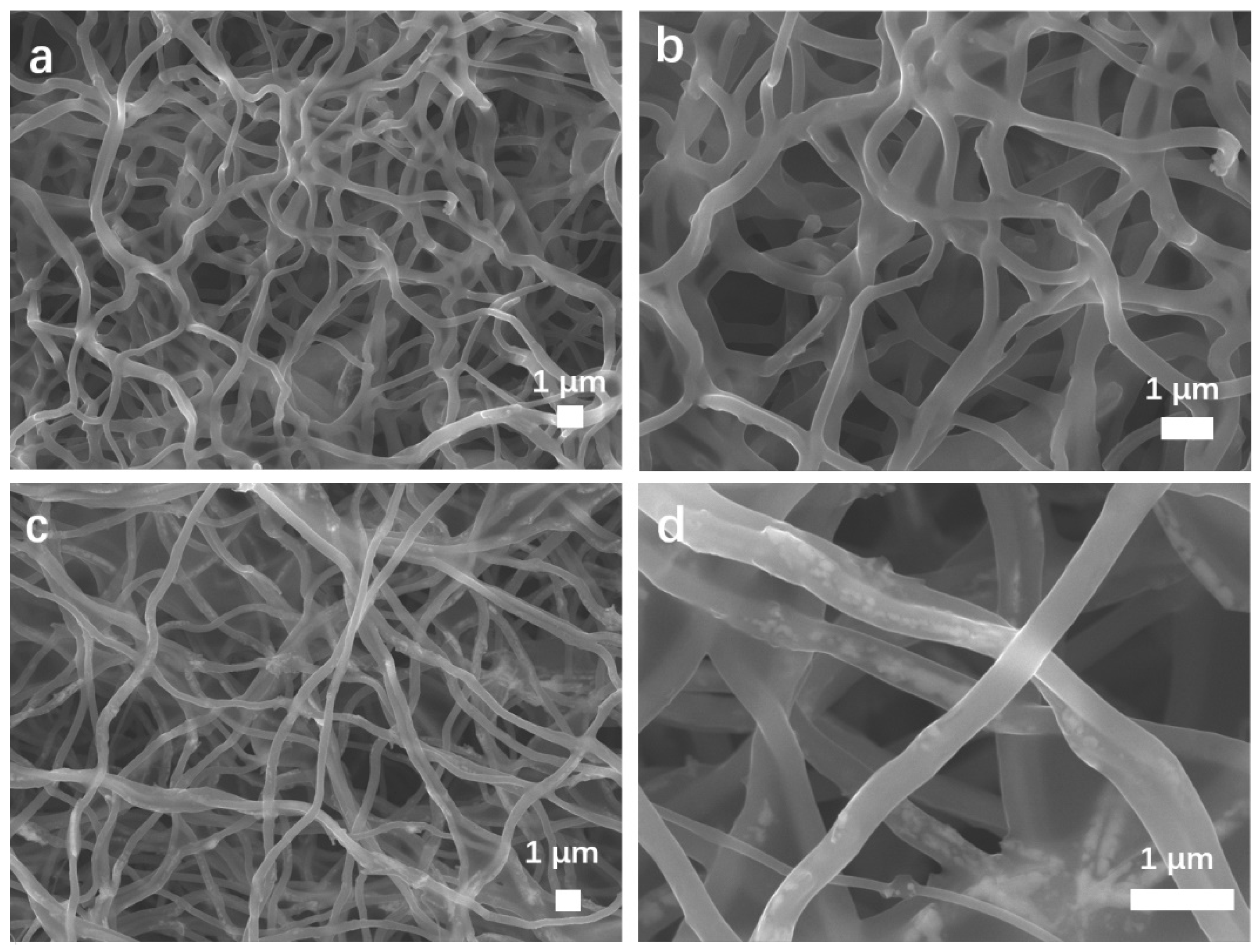
The morphologies and structures of MnO2 NTs, 5% MnO2/CNFs and 20% MnO2/CNFs were obtained. Figure 2 characterizes the morphology of MnO2 NTs by scanning electron microscopy (SEM) at different magnifications. Figure 2a displays the tubular structure of MnO2 NTs with the longest morphology of about 3.3 μm. The morphologies of MnO2 NTs have better uniformity and contain almost no impurity particles. As shown in Figure 3b, hollow tubes are gained with an average diameter of 100 nm (±100). Figure 3a,b shows the 5% MnO2/CNFs, and Figure 3c,d shows the 20% MnO2/CNFs; both of them are uniform nanofibers with a diameter of approximately 300–500 nm. Their uniformly distributed nanoparticles are in high concentration, which reveals that the uniform MnO2/CNFs can be prepared [33,34].
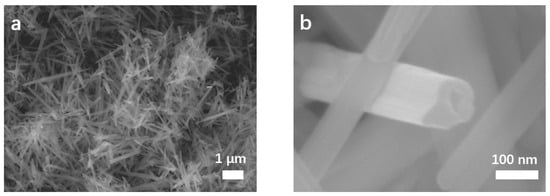
Figure 2.
SEM images of MnO2 NTs (a,b).
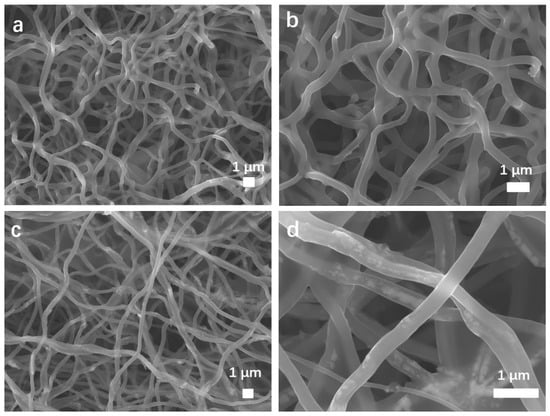
Figure 3.
SEM images of the MnO2/CNFs in different components: 5% MnO2/CNFs (a,b); 20% MnO2/CNFs (c,d).
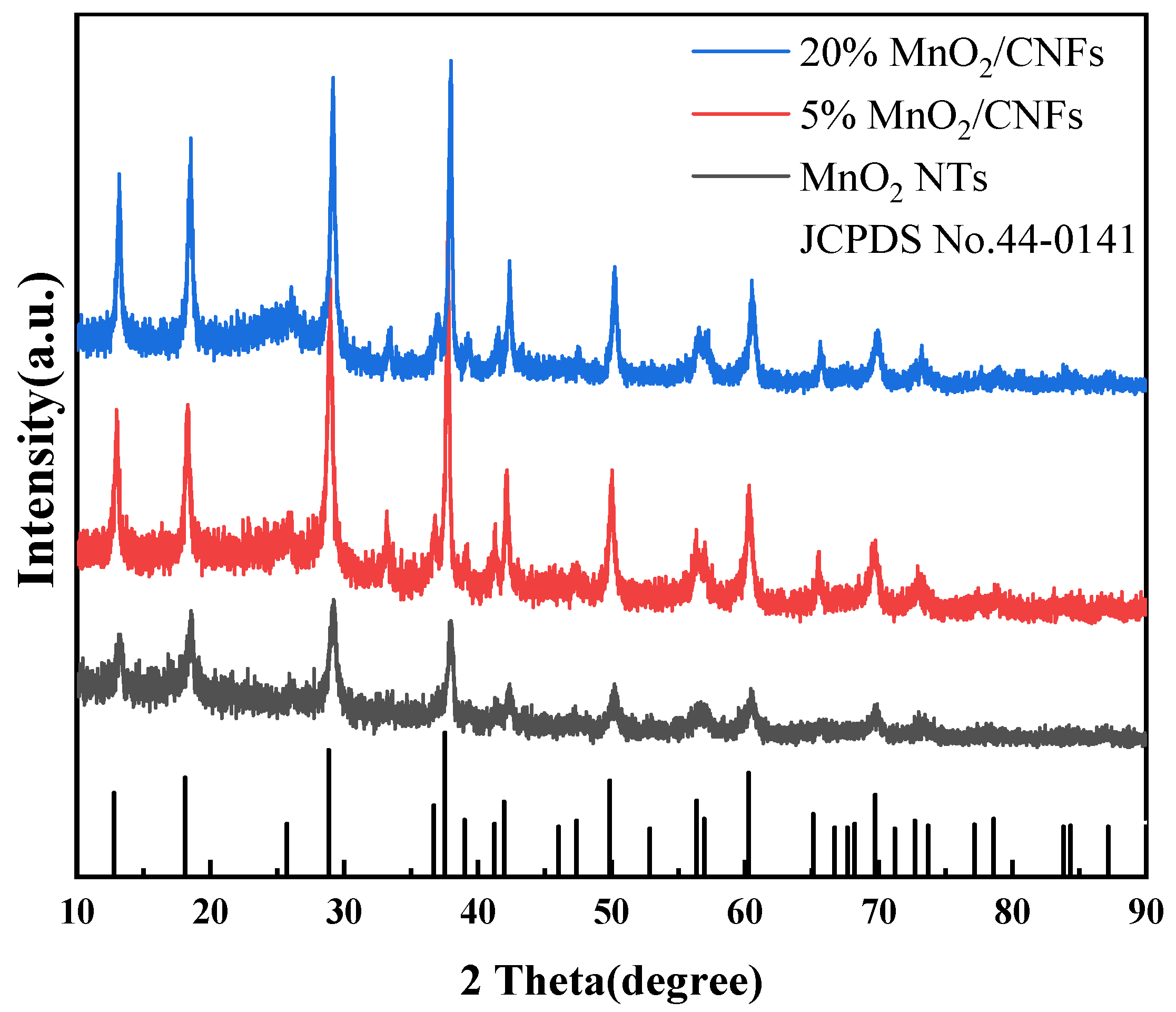
The crystal structures and phase information of MnO2 NTs, 5% MnO2/CNFs and 20% MnO2/CNFs were characterized by X-ray diffraction (XRD). The results are demonstrated in Figure 4. The diffraction peaks of MnO2 NTs, 5% MnO2/CNFs and 20% MnO2/CNFs are in line with the standard card of MnO2 (JCPDS NO. 44-0141). The XRD patterns of MnO2 NTs, 5% MnO2/CNFs and 20% MnO2/CNFs show no noticeable change. The typical diffraction peaks correspond to the (110), (200), (220), (310), (211), (301), (411), (431), (521), (002), (541), (312) and (332) crystal planes of manganese dioxide, respectively. This indicates that manganese dioxide was integrated into the composite material, which presents the successful preparation. The peak of 20% MnO2/CNFs is strong and narrow, which confirms good crystallinity and high purity [35,36].
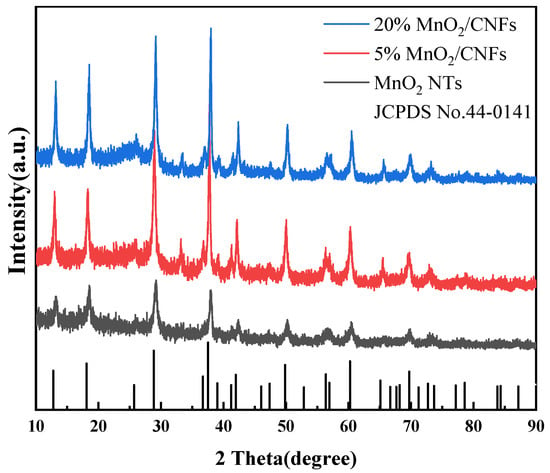
Figure 4.
XRD patterns of MnO2 NTs, 5% MnO2/CNFs and 20% MnO2/CNFs.
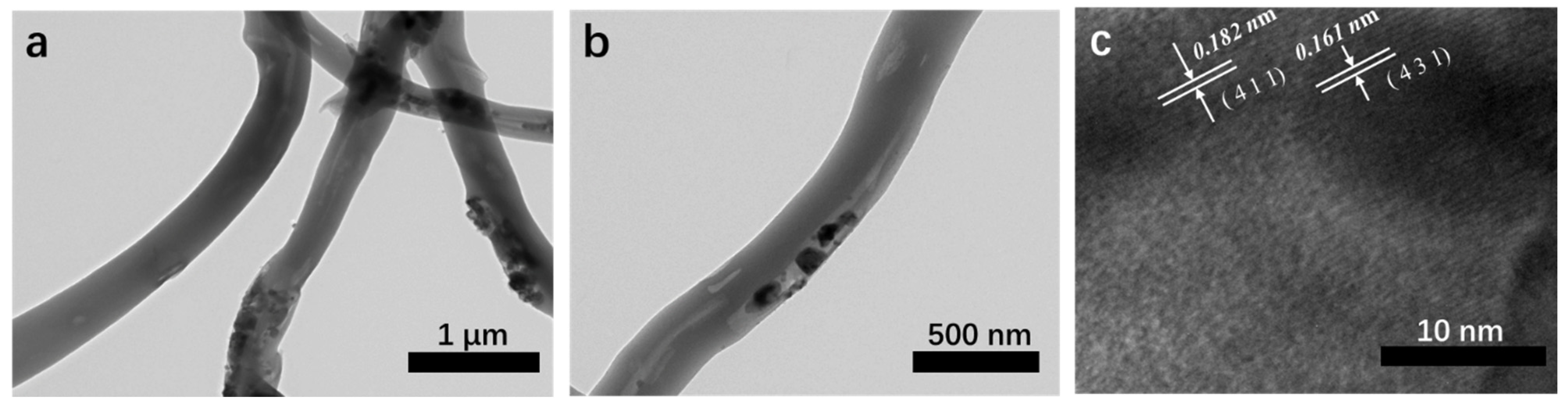
To further study the structure of 20% MnO2/CNFs samples, the TEM characterization is shown in Figure 5. The prepared 20% MnO2/CNFs showed a nanotube structure with the diameter of 250 nm and filled with the MnO2 particles in Figure 5b. In Figure 5c, the high-resolution TEM image of the MnO2/CNFs demonstrates that the lattice spacings are 0.182 and 0.161 nm, which is in line with the spacing of the (411) and (431) planes of MnO2. Obviously, it is proved that the ingredient of the nanofibers is MnO2 again [19,36,37].
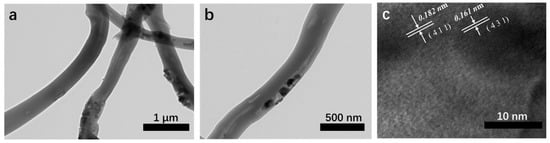
Figure 5.
TEM images of 20% MnO2/CNFs (a,b) and HRTEM image of 20% MnO2/CNFs (c).
3.2. Electrochemical Performance
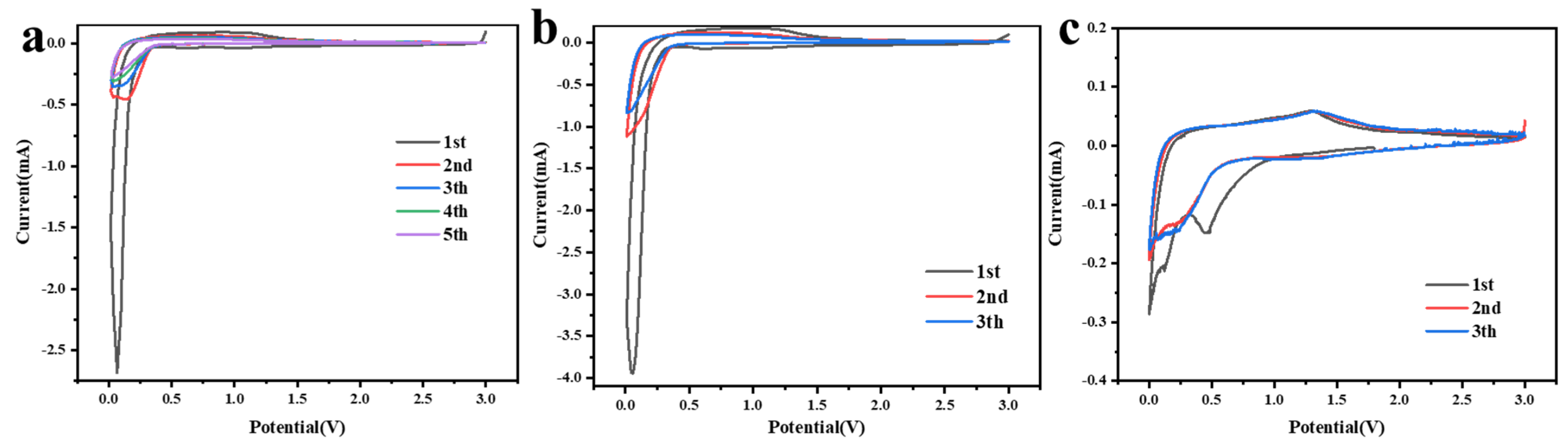
The lithium electrochemical performances of MnO2 NTs, 5% MnO2/CNFs and 20% MnO2/CNFs were evaluated using coin cells. The CV curves of MnO2 NTs, 5% MnO2/CNFs and 20% MnO2/CNFs for the first several cycles at a scan rate of 0.1 mV s−1 in the potential range 0.01–3.0 V are shown, respectively, in Figure 6a–c. Compared with MnO2 NTs and 5% MnO2/CNFs in Figure 6a,b, the first cathodic cycle creates a broad peak at 0.45 V in Figure 6c, which vanishes in the following cathodic cycles. This illustrates that the reaction is irreversible and the energy difference prompts the formation of the solid electrolyte interface (SEI) on the electrode surface [30,31]. In addition to this, the peak decreases sharply below 0.35 V. This occurs because of the reduction in Mn4+ to Mn0 (MnO2 + 4Li+ + 4e− → Mn + 2Li2O) [22,38]. In the first anodic cycles, the strong peak at 1.32 V is corresponding to the dissolution of Li+ from Li2O and the oxication of Mn0 to Mn2+ (Mn + 2Li2O → MnO2 + 4Li+ + 4e−) [22,23,38]. In the other anodic cycles, the reaction is reversible because the peaks have no obvious changes. In addition, almost in the same place, the excellent electrochemical stability was revealed.
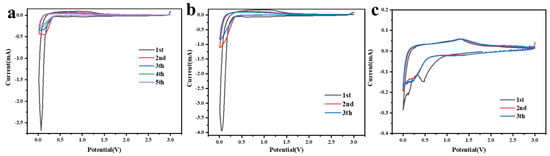
Figure 6.
Cyclic voltammetry curves of MnO2 NTs. (a) 5% MnO2/CNFs (b) and 20% MnO2/CNFs (c) at a scan rate of 0.1 mV s−1 in the potential range of 0.01–3.0 V.
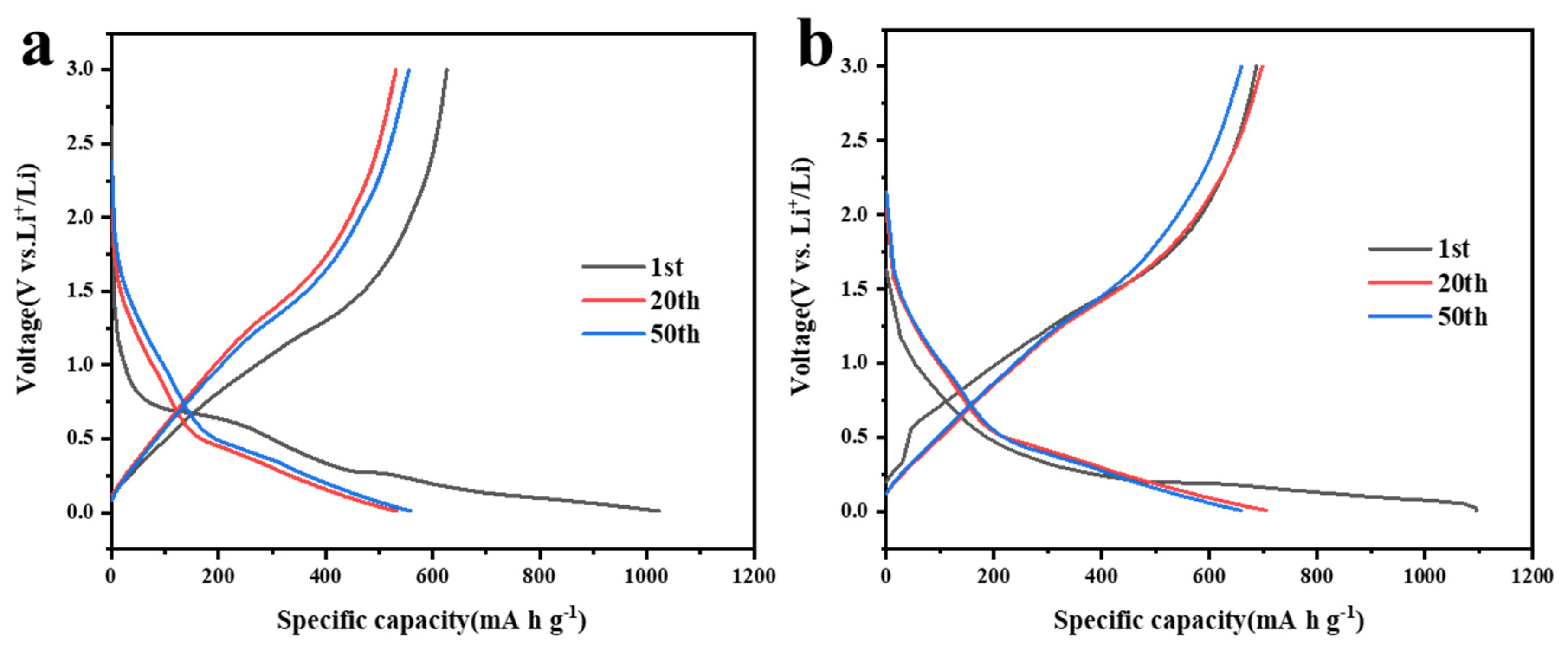
The discharge/charge graphs of 5% MnO2/CNFs and 20% MnO2/CNFs at a current density of 0.1 A g−1 are displayed in Figure 7a,b. The curves of lap 1, lap 20 and lap 50 were regarded as the samples There are obvious discharge voltage plateaus at about 0.76 V (Figure 7a) and 0.57 V (Figure 7b). The specific capacities of the first discharge are 1023 and 1094 mA h g−1, respectively; in the following cycles, the specific capacities are around 531–558 and 658–704 mA h g−1. An SEI film was formed due to the lost capacity, which is irreversible, and it is the same as cyclic voltammetry curves (Figure 7a,b). Thus, the mechanism of alloying lithiation is proved. Meanwhile, the discharge/charge capacity of the 20% MnO2/CNFs remains higher than that of the 5% MnO2/CNFs, which indicates not only high performance, but also excellent battery reversibility [39,40].
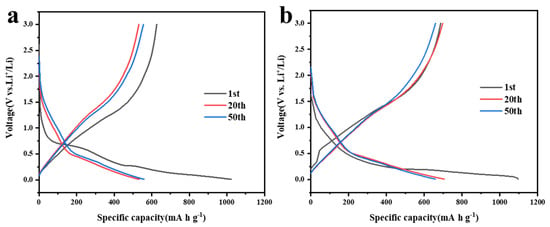
Figure 7.
Discharge/charge curves in selected cycles for 5% MnO2/CNFs (a) and 20% MnO2/CNFs (b).
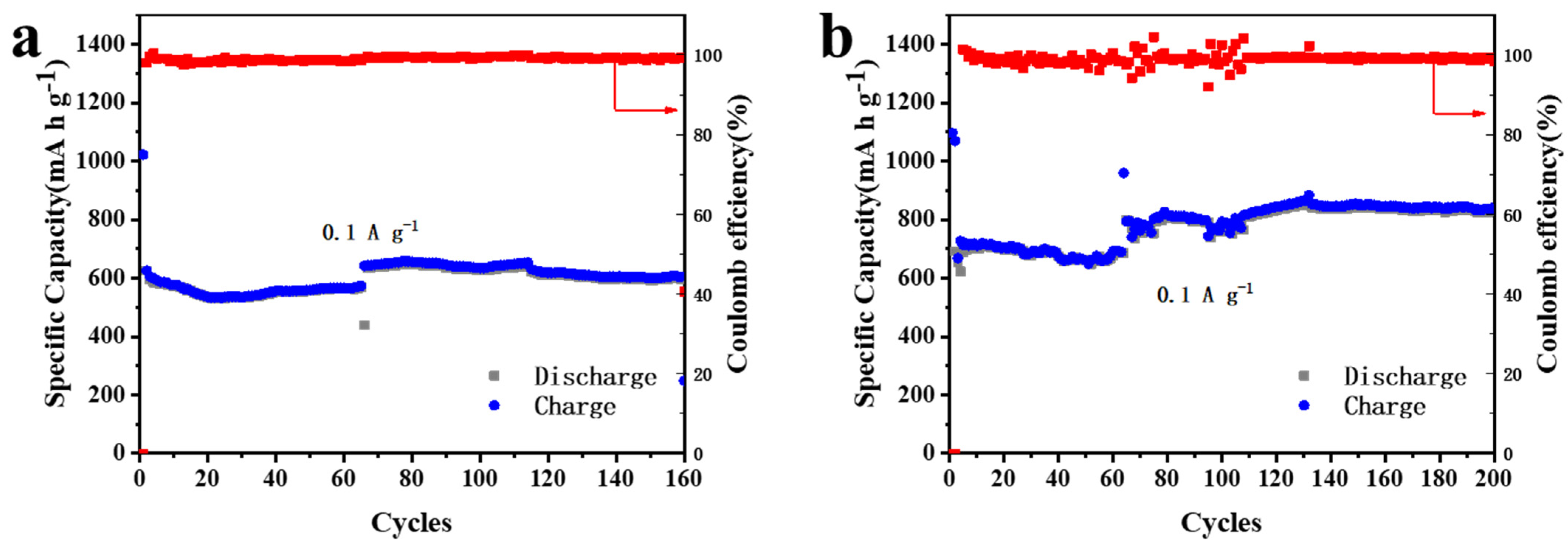
The cycle performances of 5% MnO2/CNFs for 160 cycles and of 20% MnO2/CNFs for 200 cycles at a current density of 0.1 A g−1 are shown in Figure 8a,b. The initial discharge/charge specific capacities of 20% MnO2/CNFs are 1094 and 687 mA h g−1, higher than those of 5% MnO2/CNFs. As the cycles continue, the specific capacities 5% MnO2/CNFs maintain at around 610 mA h g−1 from the 117th cycle to the 160th cycle, while those of 20% MnO2/CNFs remain at around 835 mA h g−1 from the 133th cycle to the 200th cycle. During the whole process, their coulombic efficiency holds at around 98.9% and 99.5%, respectively; 20% MnO2/CNFs owns the higher coulombic efficiency. The higher the percentage of MnO2/CNFs, the better reversibility and structural stability [41,42,43].
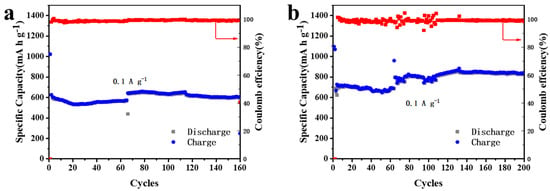
Figure 8.
Cycling performance and coulombic efficiency of 5% MnO2/CNFs for 160 cycles (a) and of 20% MnO2/CNFs for 200 cycles (b) at a current density of 0.1 A g−1.
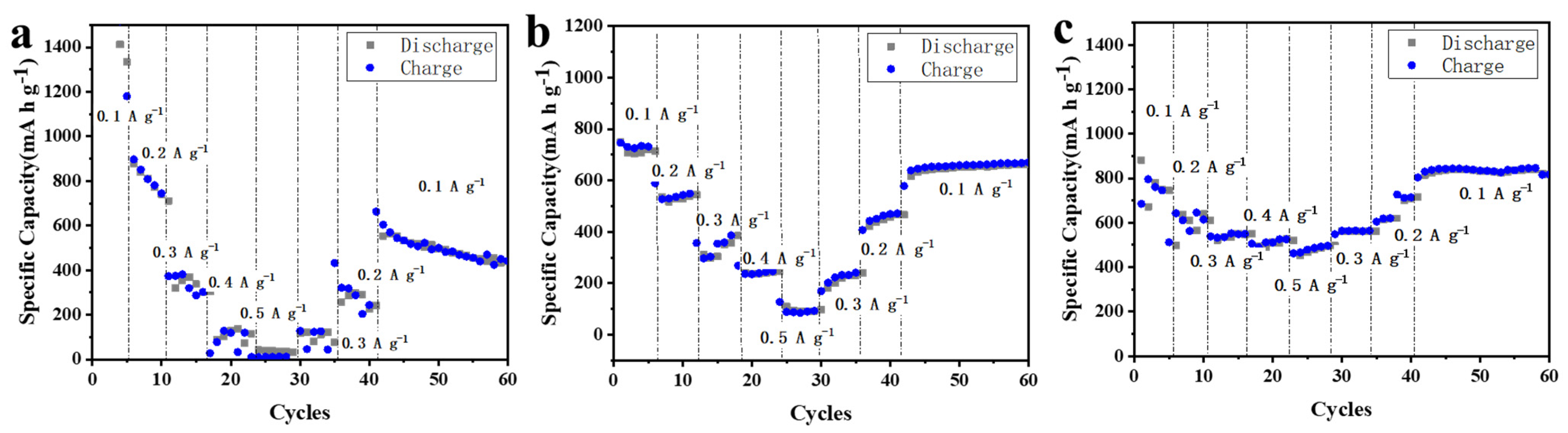
To compare the discharge/charge capacity, the rate performances of MnO2 NTs, 5% MnO2/CNFs and 20% MnO2/CNFs are tested at different current rates ranging from 0.1 to 0.5 A g−1 (Figure 9a–c). MnO2 NTs do not contain the MnO2/CNFs, so the structure is damaged easily and the cycle is unstable (Figure 9a). On the contrary, because of the carbon nanofibers, 5% MnO2/CNFs and 20% MnO2/CNFs have good structural stability (Figure 9b,c), but 20% MnO2/CNFs show superior capacity, the corresponding invertible capacities are 796, 611, 552, 486 mA h g−1 at 0.1, 0.2, 0.3, 0.5 A g−1. When tested again at 0.1, 0.2, 0.3 A g−1, the reversible capacities of 835, 620,565 mA h g−1 are reached. In addition, the MnO2/CNFs show better rate capability compared to the MnO2 NTs nanoparticle electrode at different rates from 0.1 to 0.5 A g−1 (Figure 9) [44,45].
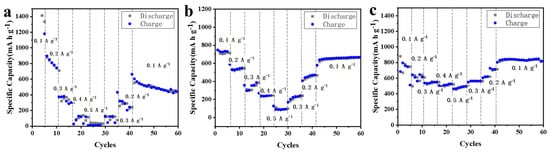
Figure 9.
Rate performance of MnO2 NTs (a), 5% MnO2/ CNFs (b) and of 20% MnO2/ CNFs (c) at various current rates ranging from 0.1 to 0.5 A g−1.
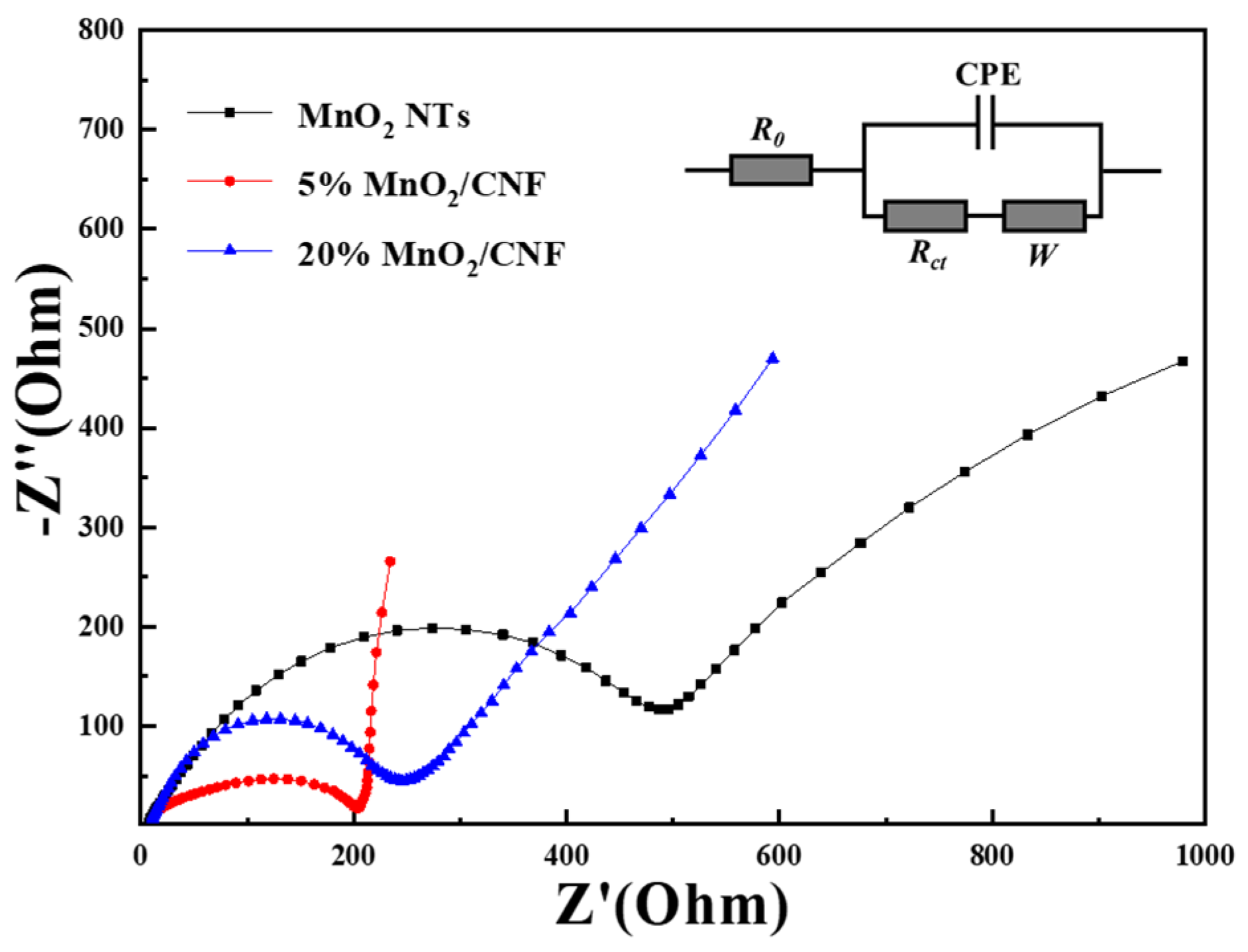
To understand the state of the electrode process and the influence of mass transfer process based on the three different electrodes, EIS (electrochemical impedance spectra) were executed. As shown in Figure 10, the EIS of MnO2 NTs, 5% MnO2/CNF and 20% MnO2/CNF are composed of semicircles and oblique lines, the high-middle frequency regions of the semicircles represent the charge transfer impedance of the electrode. The smaller the diameter of the arc, the lower the charge transfer impedance [46,47]. It is obvious that 5% MnO2/CNF and 20% MnO2/CNF have a smaller semicircle and the similar impedance values, indicating that both of them have a lower charge transfer impedance compared with MnO2 NTs. The charge transfer resistances of MnO2 NTs, 5% MnO2/CNF and 20% MnO2/CNF are 205, 245, and 495 Ω, respectively. The low frequency regions of the oblique lines are caused by the Warburg impedance of the ion diffusion; the larger the angle between the oblique line and the real axis, the better the ion diffusion of the capacitor, the higher electrochemical capacitance [35,46]. In Figure 10, the slope of the impedance lines of 5% MnO2/CNF and 20% MnO2/CNF is larger than that of MnO2 NTs, and 5% MnO2/CNF is the best of them, but the impedance semicircle of 5% MnO2/CNF is rotated. The rotation of the impedance semicircle of 5% MnO2/CNF is related to the inhomogeneity of the electrode/electrolyte interface. Because of the inhomogeneity of the electrode surface, the electrochemical activation energy of each point on the electrode surface may be different, which produces different charge transfer resistance values, leading to poor electrical conductivity [48,49]. To sum up, 20% MnO2/CNF improves the conductivity on account of the unique structure with the carbon and has the best comprehensive performance.
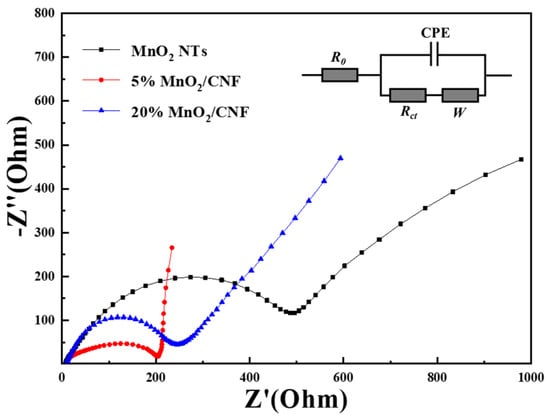
Figure 10.
Electrochemical impedance spectra with selected equivalent circuit (inset) for MnO2 NTs, 5% MnO2/CNF and 20% MnO2/CNF.
3.3. Analysis of Electrode Structure after Charge/Discharge
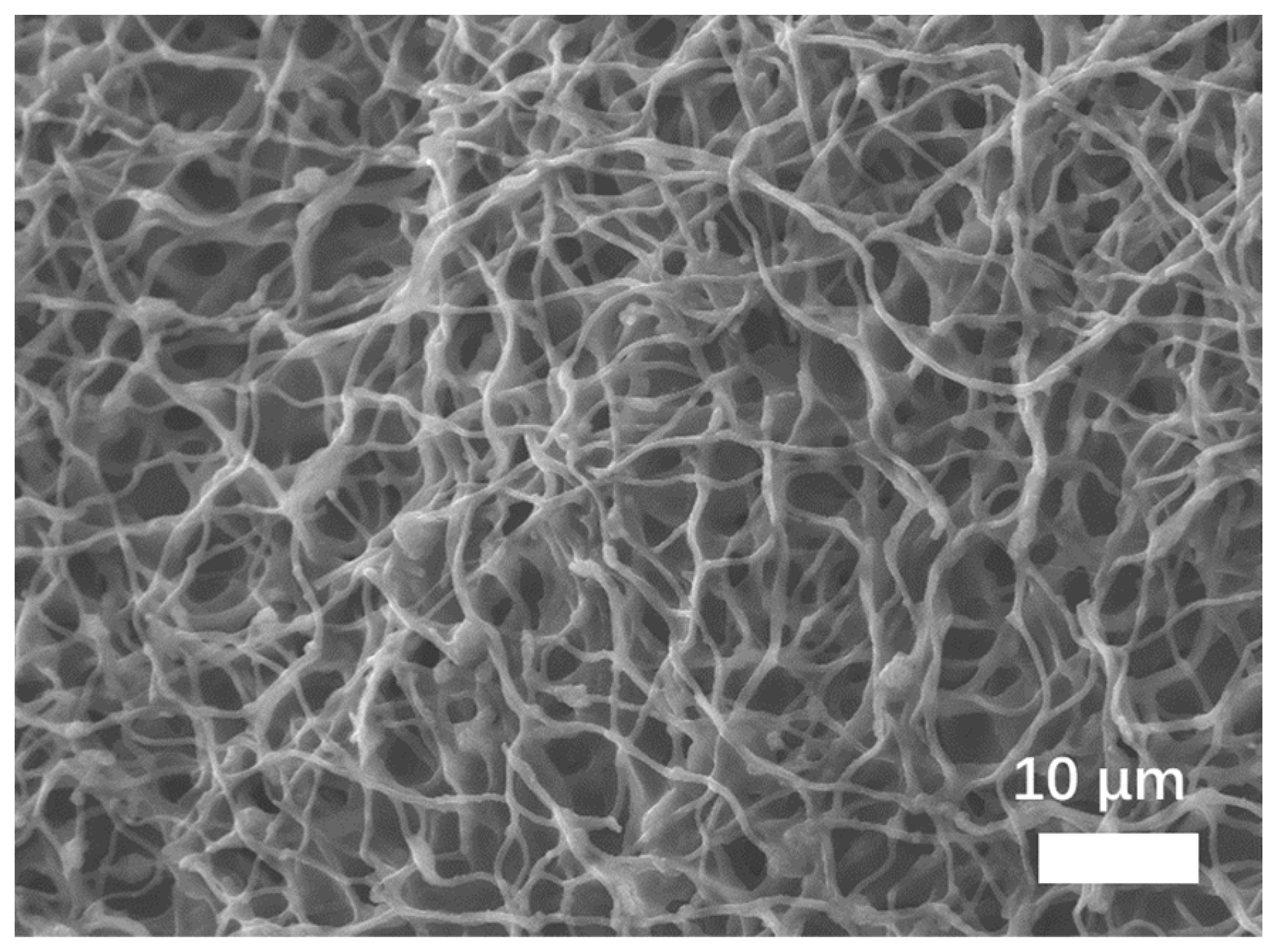
To further confirm the relationship between fiber structure and cycling stability, the electrode was disassembled after the cycling; the structure and morphology are shown in Figure 11. It may be very clearly seen that 20% MnO2/CNFs still maintain the fiber structure, and the fiber structure is not broken. The structure of fiber can effectively release the expanded volume of the alloy during the lithium process, thus maintaining the structure stability and improving the stability and efficiency of the cycle.
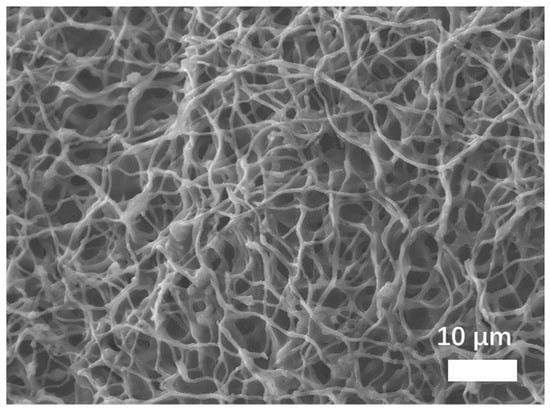
Figure 11.
SEM images of the 20% MnO2/CNFs after 200 cycles.
4. Conclusions
This study provides a simple method to prepare MnO2/CNFs material as high-performance anode. MnO2/CNFs were prepared by electrospinning, and the electrochemical properties of MnO2 NTs, 5% MnO2/CNFs and 20% MnO2/CNFs were evaluated. SEM images proved that both of MnO2/CNFs are the uniform nanofibers with a diameter of approximately 300–500 nm, which reveals that the uniform MnO2/CNFs can be prepared. In comparison with them, 20% MnO2/CNFs anode can provide the initial reversible capacity of 1094 mA h g−1 at 0.1 mA g−1 and remains at 835 mA h g−1 after 133 cycles. It is proved that the presence of CNFs improves the electrical conductivity of the composites by impedance spectrum. As a result, 20% MnO2/CNFs had the better structural stability, high specific capacity, excellent reversibility, long cycling performance, and superior ionic conductivity, which provided ideas and prospect for designing a high-performance anode for LIBs.
Author Contributions
Conceptualization, D.M. and X.M.; methodology, M.Q.; software, D.M. and X.M.; validation, M.Q., X.Q. and G.Z.; formal analysis, D.M. and X.M.; investigation, D.M.; resources, M.Q.; data curation, D.M.; writing—original draft preparation, D.M.; writing—review and editing, D.M.; visualization, M.Q.; supervision, M.Q.; project administration, X.Q.; funding acquisition, M.Q. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge financial support from Fundamental Research Funds for the Central Universities of Heilongjiang Province of China (2022-KYYWF-0603), Research Fund for the Doctoral Program of Higher Education of China (JMSUBZ 2020-02) and Fundamental Research Funds for the Central Universities of Heilongjiang Province of China (2021-KYYWF-0554).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Takeuchi, E.S.; Takeuchi, K.J.; Marschilok, A.C. The Ongoing Importance of Lithium Primary Batteries: 50+ Years and Going Strong. In Electrochemical Society Meeting Abstracts 242; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2022; p. 102. [Google Scholar]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef]
- Zeng, X.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of lithium battery technologies for electric vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Evarts, E.C. Lithium batteries: To the limits of lithium. Nature 2015, 526, S93–S95. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W.; Lei, T.; Jiao, Y.; Huang, J.; Hu, A.; Gong, C.; Yan, C.; Wang, X.; Xiong, J. Strategies toward high-loading lithium–sulfur battery. Adv. Energy Mater. 2020, 10, 2000082. [Google Scholar] [CrossRef]
- Roselin, L.S.; Juang, R.-S.; Hsieh, C.-T.; Sagadevan, S.; Umar, A.; Selvin, R.; Hegazy, H.H. Recent advances and perspectives of carbon-based nanostructures as anode materials for Li-ion batteries. Materials 2019, 12, 1229. [Google Scholar] [CrossRef] [PubMed]
- Kour, S.; Tanwar, S.; Sharma, A. A review on challenges to remedies of MnO2 based transition-metal oxide, hydroxide, and layered double hydroxide composites for supercapacitor applications. Mater. Today Commun. 2022, 32, 104033. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. Hybrid biochar supported transition metal doped MnO2 composites: Efficient contenders for lithium adsorption and recovery from aqueous solutions. Desalination 2022, 522, 115387. [Google Scholar] [CrossRef]
- Gu, X.; Yue, J.; Li, L.; Xue, H.; Yang, J.; Zhao, X. General synthesis of MnOx (MnO2, Mn2O3, Mn3O4, MnO) hierarchical microspheres as lithium-ion battery anodes. Electrochim. Acta 2015, 184, 250–256. [Google Scholar] [CrossRef]
- Feng, L.; Xuan, Z.; Zhao, H.; Bai, Y.; Guo, J.; Su, C.-W.; Chen, X. MnO2 prepared by hydrothermal method and electrochemical performance as anode for lithium-ion battery. Nanoscale Res. Lett. 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- George, N.S.; Jose, L.M.; Aravind, A. Review on Transition Metal Oxides and Their Composites for Energy Storage Application; IntechOpen: London, UK, 2022. [Google Scholar]
- Ashokkumar, K.; Dhanapandian, S.; Suthakaran, S.; Krishnakumar, N.; Anandan, M. Synthesis of MnO2 nanoparticles and its effective utilization as high-performance of supercapacitor. Mater. Today Proc. 2022, 49, 2675–2678. [Google Scholar] [CrossRef]
- Bach-Toledo, L.; Hryniewicz, B.M.; Marchesi, L.F.; Dall’Antonia, L.H.; Vidotti, M.; Wolfart, F. Conducting polymers and composites nanowires for energy devices: A brief review. Mater. Sci. Energy Technol. 2020, 3, 78–90. [Google Scholar] [CrossRef]
- Voskanyan, A.A.; Ho, C.-K.; Chan, K.Y. 3D δ-MnO2 nanostructure with ultralarge mesopores as high-performance lithium-ion battery anode fabricated via colloidal solution combustion synthesis. J. Power Sources 2019, 421, 162–168. [Google Scholar] [CrossRef]
- Abdah, M.A.A.M.; Mokhtar, M.; Khoon, L.T.; Sopian, K.; Dzulkurnain, N.A.; Ahmad, A.; Sulaiman, Y.; Bella, F.; Su’ait, M.S. Synthesis and electrochemical characterizations of poly (3, 4-ethylenedioxythiophene/manganese oxide coated on porous carbon nanofibers as a potential anode for lithium-ion batteries. Energy Rep. 2021, 7, 8677–8687. [Google Scholar] [CrossRef]
- Agobi, A.U.; Louis, H.; Magu, T.O.; Dass, P.M. A review on conducting polymers-based composites for energy storage application. J. Chem. Rev. 2019, 1, 19–34. [Google Scholar]
- Sohal, N.; Maity, B.; Basu, S. Morphology effect of one-dimensional MnO2 nanostructures on heteroatom-doped carbon dot-based biosensors for selective detection of glutathione. ACS Appl. Bio Mater. 2022, 5, 2355–2364. [Google Scholar] [CrossRef]
- Mohammed, M.K.; Hassan, D.; Hashim, A. Exploring the optical properties of MnO2 nanoparticles doped PVP for optoelectronics devices. World J. Adv. Res. Rev. 2023, 17, 1178–1185. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, K.; Lyu, Y.; Liu, P.; Zhang, Q.; Zhang, B. Hollow nitrogen-doped carbon nanofibers filled with MnO2 nanoparticles/nanosheets as high-performance microwave absorbing materials. Carbon 2022, 196, 49–58. [Google Scholar] [CrossRef]
- Sui, Y.; Liu, C.; Zou, P.; Zhan, H.; Cui, Y.; Yang, C.; Cao, G. Polypyrrole coated δ-MnO2 nanosheet arrays as a highly stable lithium-ion-storage anode. Dalton Trans. 2020, 49, 7903–7913. [Google Scholar] [CrossRef]
- Umar, A.; Ahmed, F.; Algadi, H.; Ibrahim, A.A.; Alhamami, M.A.; Qasem, H.; Akbar, S. Single crystalline α-manganese dioxide (α-MnO2) nanowires as anode materials for lithium-ion batteries. Mater. Express 2022, 12, 1560–1565. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, L.; Xiao, J.; Liu, H. MnO/C cubo-polyhedrons derived from α-MnO2@ZIF-8 as anode materials for high-performance lithium-ion batteries. Sustain. Energy Fuels 2020, 4, 633–642. [Google Scholar] [CrossRef]
- Cao, Z.; Yang, Y.; Qin, J.; Su, Z. A core-shell porous MnO2/Carbon nanosphere composite as the anode of lithium-ion batteries. J. Power Sources 2021, 491, 229577. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Huang, C.; Wu, Y.; Hou, J.; Situ, Y.; Huang, H. Electrochemical charge/discharge cycling and morphological effects in MnO2/PANC nanostructures for supercapacitors. Electrochim. Acta 2022, 428, 140929. [Google Scholar] [CrossRef]
- Ji, W.; Miao, X.; Zhang, T.C.; Wang, Y.; Yuan, S. Fe-metal-organic-framework/MnO2 nanowire/granular activated carbon nanostructured composites for enhanced As (III) removal from aqueous solutions. Appl. Surf. Sci. 2022, 606, 155011. [Google Scholar] [CrossRef]
- Kumar, R.R.; Thanigaivel, S.; Dey, N.; Priya, A.; Karthick, A.; Mohanavel, V.; Kannadhasan, S.; Muhibbullah, M.; Osman, S.M. Performance evaluation of cyclic stability and capacitance of manganese oxide modified graphene oxide nanocomposite for potential supercapacitor applications. J. Nanomater. 2022, 2022, 7352246. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, M.I. Carbon nanotubes/nanofibers (CNTs/CNFs): A review on state of the art synthesis methods. Microsyst. Technol. 2022, 28, 885–901. [Google Scholar] [CrossRef]
- Soltani, S.; Khanian, N.; Shojaei, T.R.; Choong, T.S.Y.; Asim, N. Fundamental and recent progress on the strengthening strategies for fabrication of polyacrylonitrile (PAN)-derived electrospun CNFs: Precursors, spinning and collection, and post-treatments. J. Ind. Eng. Chem. 2022, 110, 329–344. [Google Scholar] [CrossRef]
- Li, C.; Bi, A.T.; Chen, H.L.; Pei, Y.R.; Zhao, M.; Yang, C.C.; Jiang, Q. Rational design of porous Sn nanospheres/N-doped carbon nanofibers as an ultra-stable potassium-ion battery anode material. J. Mater. Chem. A 2021, 9, 5740–5750. [Google Scholar] [CrossRef]
- Azam, M.A.; Safie, N.E.; Ahmad, A.S.; Yuza, N.A.; Zulkifli, N.S.A. Recent advances of silicon, carbon composites and tin oxide as new anode materials for lithium-ion battery: A comprehensive review. J. Energy Storage 2021, 33, 102096. [Google Scholar] [CrossRef]
- Zhang, N.; Yan, C.; Li, L.; Khan, M. Assessment of fiber factor for the fracture toughness of polyethylene fiber reinforced geopolymer. Constr. Build. Mater. 2022, 319, 126130. [Google Scholar] [CrossRef]
- Zhao, X.; Zou, D.; Wang, S. Flexible phase change materials: Preparation, properties and application. Chem. Eng. J. 2022, 431, 134231. [Google Scholar] [CrossRef]
- Zhang, X.; Su, X.; Zhang, B.; Wang, J. Preparation of MnO2@ CNFs composites and their tunable microwave absorption properties. Mater. Res. Express 2019, 6, 075005. [Google Scholar] [CrossRef]
- Wang, Z.; Han, Y.; Fan, W.; Wang, Y.; Huang, L. Shell-core MnO2/Carbon@ Carbon nanotubes synthesized by a facile one-pot method for peroxymonosulfate oxidation of tetracycline. Sep. Purif. Technol. 2021, 278, 119558. [Google Scholar] [CrossRef]
- Kim, E.S.; Lee, H.-J.; Kim, B.-H. Sandwich-structured carbon nanofiber/MnO2/carbon nanofiber composites for high-performance supercapacitor. Electrochim. Acta 2022, 406, 139883. [Google Scholar] [CrossRef]
- Huang, C.-L.; Chiang, L.-M.; Su, C.-A.; Li, Y.-Y. MnO2/carbon nanotube-embedded carbon nanofibers as core–shell cables for high performing asymmetric flexible supercapacitors. J. Ind. Eng. Chem. 2021, 103, 142–153. [Google Scholar] [CrossRef]
- Dirican, M.; Yanilmaz, M.; Asiri, A.M.; Zhang, X. Polyaniline/MnO2/porous carbon nanofiber electrodes for supercapacitors. J. Electroanal. Chem. 2020, 861, 113995. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, W.Y.; Lee, S.; Kim, T.-S.; Lee, J.W. Graphene intercalated free-standing carbon paper coated with MnO2 for anode materials of lithium ion batteries. Electrochim. Acta 2020, 348, 136310. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, L.; He, W.; Xu, S.; Liu, K.; Huang, T.; Li, Y.; Cui, M.; Xie, J. Improved supercapacitive performances of manganese dioxide on acid-treated carbon nanofibers derived from polyaniline. J. Energy Storage 2023, 60, 106596. [Google Scholar] [CrossRef]
- Yan, S.; Tang, C.; Wang, X.; Zhang, H.; Yang, Z.; Zhang, C.; Liu, S. Hierarchical MnO2 nanowire arrays consisting of multitripod structures grown on porous carbon nanofibers for high-performance supercapacitor electrode. J. Electroanal. Chem. 2020, 856, 113475. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, Y.A.; Kim, B.-H. Electrospun polyacrylonitrile/cyclodextrin-derived hierarchical porous carbon nanofiber/MnO2 composites for supercapacitor applications. Carbon 2020, 164, 296–304. [Google Scholar] [CrossRef]
- Liu, C.-S.; Huang, C.-L.; Fang, H.-C.; Hung, K.-Y.; Su, C.-A.; Li, Y.-Y. MnO2-based carbon nanofiber cable for supercapacitor applications. J. Energy Storage 2021, 33, 102130. [Google Scholar] [CrossRef]
- Rao, T.P.; Kumar, A.; Naik, V.M.; Naik, R. Effect of carbon nanofibers on electrode performance of symmetric supercapcitors with composite α-MnO2 nanorods. J. Alloys Compd. 2019, 789, 518–527. [Google Scholar] [CrossRef]
- Yoon, J.; Choi, W.; Kim, T.; Kim, H.; Choi, Y.S.; Kim, J.M.; Yoon, W.-S. Reaction mechanism and additional lithium storage of mesoporous MnO2 anode in Li batteries. J. Energy Chem. 2021, 53, 276–284. [Google Scholar] [CrossRef]
- Ette, P.M.; Bosubabu, D.; Ramesha, K. Graphene anchored mesoporous MnO2 nanostructures as stable and high-performance anode materials for Li-ion batteries. Electrochim. Acta 2022, 414, 140164. [Google Scholar] [CrossRef]
- Ko, W.Y.; Sitindaon, R.S.; Lubis, A.L.; Yang, Y.R.; Wang, H.Y.; Lin, S.T.; Lin, K.J. Vertically-oriented zinc-doped γ-MnO2 nanowalls as high-rate anode materials for li-ion batteries. J. Energy Storage 2022, 54, 105329. [Google Scholar] [CrossRef]
- Huyan, Y.; Chen, J.; Yang, K.; Zhang, Q.; Zhang, B. Tailoring carboxyl tubular carbon nanofibers/MnO2 composites for high-performance lithium-ion battery anodes. J. Am. Ceram. Soc. 2021, 104, 1402–1414. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; He, J.; Wang, Q.; Zhu, D.; Yan, Y.; Chen, Y. Is proton a charge carrier for δ-MnO2 cathode in aqueous rechargeable magnesium-ion batteries? J. Energy Chem. 2022, 68, 572–579. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, C.; Qiao, W.; Ling, L.; Wang, J. Nanoarchitectured MnO2 Confined to Mesoporous Carbon Microspheres as Bifunctional Electrodes for High-Performance Supercapacitors and Lithium-Ion Capacitors. Ind. Eng. Chem. Res. 2022, 61, 1748–1760. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).