Investigation of the Dependence of Electrocatalytic Activity of Copper and Palladium Nanoparticles on Morphology and Shape Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Copper and Palladium Particles
- 1
- AR—analytical reagent.
- 2
- CP reagent—chemically pure reagent.
2.2. Electrochemical Measurements
3. Results and Discussion
3.1. Electrochemical Study of Synthesized Particles in Ethanol Oxidation Processes
3.2. Investigation of the Influence of Synthesis Parameters of Particles in the Composition of Catalysts on Electrocatalytic Activity
3.2.1. Influence of Deposition Time
3.2.2. Influence of Current–Voltage Parameters of Deposition
3.2.3. Effect of KBr Concentration in Particle Growth Solution
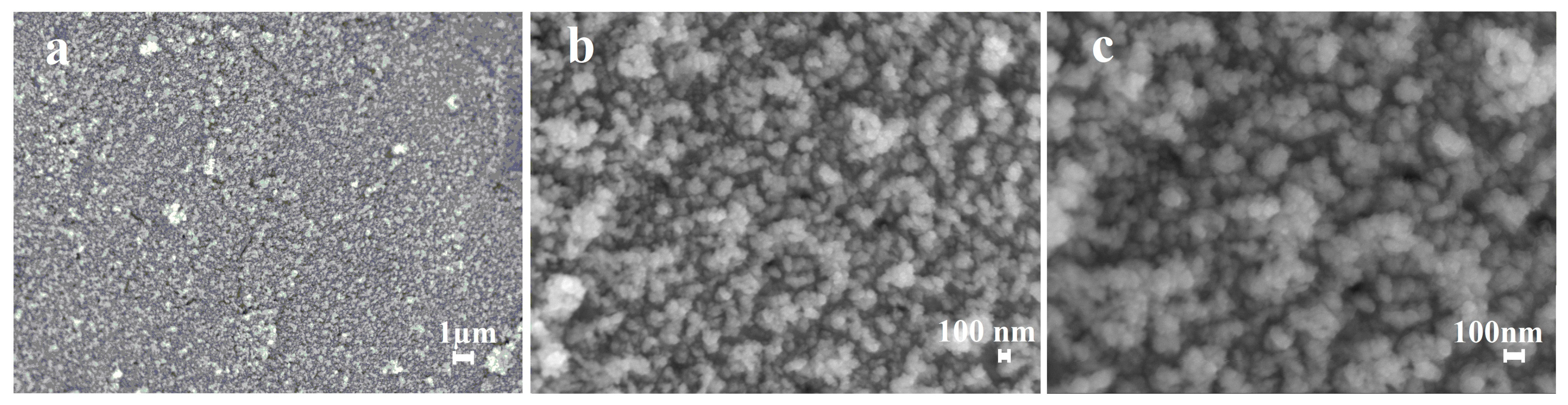
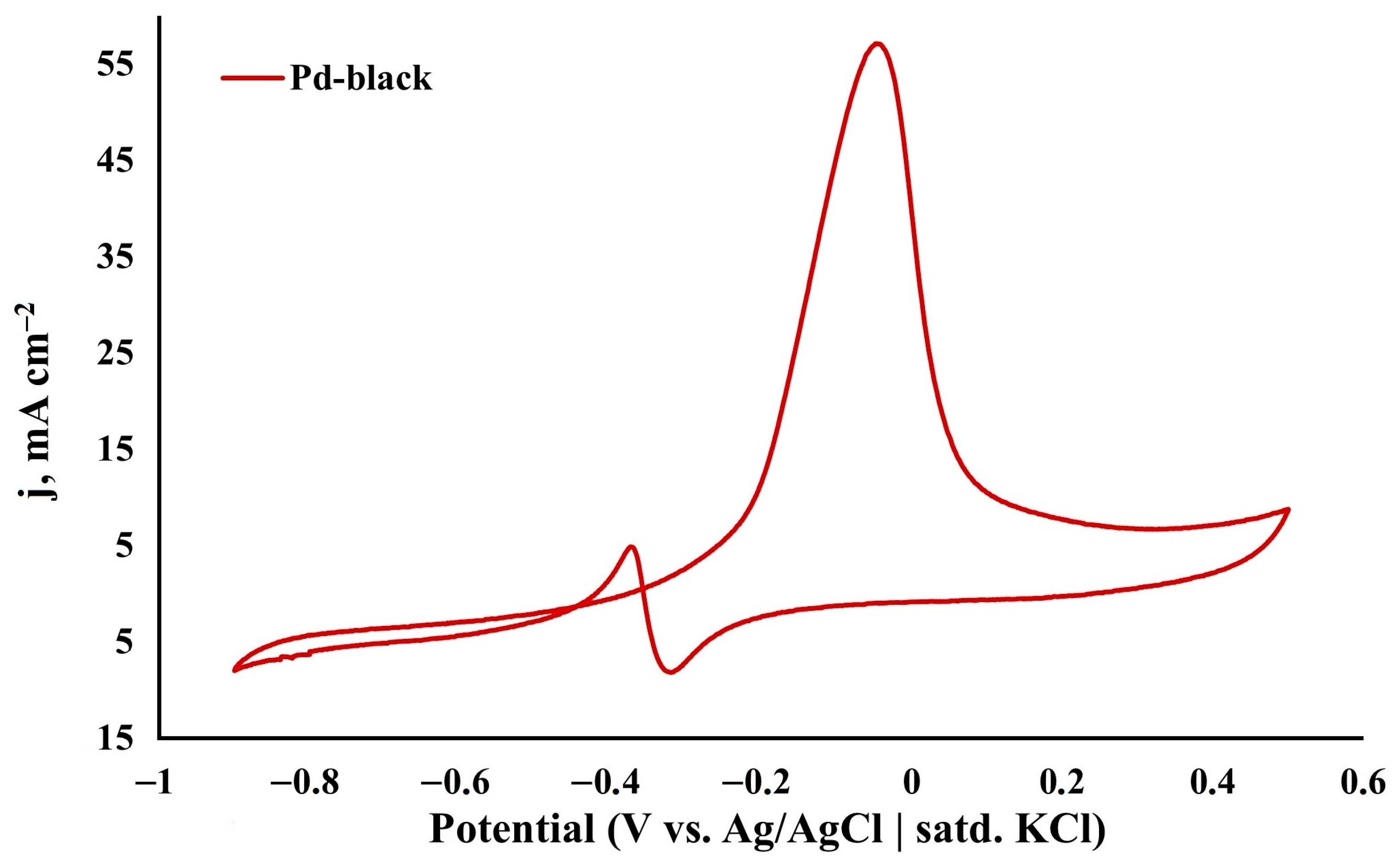
3.3. Morphology and Catalytic Characteristics of the Synthesized Palladium Catalyst
3.4. Comparison of Characteristics of Obtained Copper Catalysts with Analogues
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robertson, D.D.; Personick, M.L. Growing nanoscale model surfaces to enable correlation of catalytic behavior across dissimilar reaction environments. Chem. Mater. 2019, 31, 1121–1141. [Google Scholar] [CrossRef]
- Pushankina, P.; Baryshev, M.; Petriev, I. Synthesis and Study of Palladium Mono- and Bimetallic (with Ag and Pt) Nanoparticles in Catalytic and Membrane Hydrogen Processes. Nanomaterials 2022, 12, 4178. [Google Scholar] [CrossRef]
- Smith, A.F.; Weiner, R.G.; Skrabalak, S.E. Symmetry-dependent optical properties of stellated nanocrystals. J. Phys. Chem. 2016, 120, 20563–20571. [Google Scholar] [CrossRef]
- Basov, A.; Dzhimak, S.; Sokolov, M.; Malyshko, V.; Moiseev, A.; Butina, E.; Elkina, A.; Baryshev, M. Changes in Number and Antibacterial Activity of Silver Nanoparticles on the Surface of Suture Materials during Cyclic Freezing. Nanomaterials 2022, 12, 1164. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.A.; Elward, J.M.; Chakraborty, A. Shape matters: Effect of 1D, 2D, and 3D isovolumetric quantum confinement in semiconductor nanoparticles. J. Phys. Chem. 2016, 120, 24999–25009. [Google Scholar] [CrossRef]
- Vasudevan, S.; Fullerton-Shirey, S.K. Effect of nanoparticle shape on the electrical and thermal properties of solid polymer electrolytes. J. Phys. Chem. 2019, 123, 10720–10726. [Google Scholar] [CrossRef]
- Cushing, S.K.; Wu, N. Progress and perspectives of plasmon-enhanced solar energy conversion. J. Phys. Chem. Lett. 2016, 7, 666–675. [Google Scholar] [CrossRef]
- Tang, G.; Song, X.; Gao, S.; Wang, Y.; Sun, X.; Chen, F. Cobalt sulfide nanoparticles supported on beryllium copper needles as advanced catalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 28773–28781. [Google Scholar] [CrossRef]
- Petriev, I.; Pushankina, P.; Shostak, N.; Baryshev, M. Gas-Transport Characteristics of PdCu–Nb–PdCu Membranes Modified with Nanostructured Palladium Coating. Int. J. Mol. Sci. 2022, 23, 228. [Google Scholar] [CrossRef]
- Petriev, I.S.; Bolotin, S.N.; Frolov, V.Y.; Barishev, M.G.; Isaev, V.A.; Kopytov, G.F. Modifying the surface of a hydrogen permselective palladium–silver membrane. Bull. Russ. Acad. Sci. Phys. 2016, 80, 624–626. [Google Scholar] [CrossRef]
- Petriev, I.S.; Frolov, V.Y.; Bolotin, S.N.; Baryshev, M.G.; Kopytov, G.F. A Surface-Modified Hydrogen-Permeable Palladium-Silver Plate. Russ. Phys. J. 2015, 58, 1044–1048. [Google Scholar] [CrossRef]
- Vollath, D.; Fischer, F.D.; Beilstein, D.H. Surface energy of nanoparticles—Influence of particle size and structure. J. Nanotechnol. 2018, 9, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- Petriev, I.S.; Pushankina, P.D.; Lutsenko, I.S.; Baryshev, M.G. The Influence of a Crystallographically Atypical Pentagonal Nanostructured Coating on the Limiting Stage of Low-Temperature Hydrogen Transport through Pd–Cu Membranes. Dokl. Phys. 2021, 66, 209–213. [Google Scholar] [CrossRef]
- Petriev, I.S.; Baryshev, M.G.; Voronin, K.A.; Lutsenko, I.S.; Pushankina, P.D.; Kopytov, G.F. Gas Transmission Properties of Pd–Ag Membranes Coated with Modifying Layer. Russ. Phys. J. 2020, 63, 457–461. [Google Scholar] [CrossRef]
- Feng, B.; Hao, L.; Deng, C.; Wang, J.; Song, H.; Xiao, M.; Huang, T.; Zhu, Q.; Gai, H. A highly hydrothermal stable copper-based catalyst for catalytic wet air oxidation of m-cresol in coal chemical wastewater. Chin. J. Chem. Eng. 2022; in press. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Murthy, A.P.; Madhavan, J.; Choi, M.Y. Fundamental aspects and recent advances in transition metal nitrides as electrocatalysts for hydrogen evolution reaction: A review. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100805. [Google Scholar] [CrossRef]
- Jeong, D.-W.; Na, H.-S.; Shim, J.-O.; Jang, W.-J.; Roh, H.-S.; Jung, U.H.; Yoon, W.L. Hydrogen production from low temperature WGS reaction on co-precipitated Cu–CeO2 catalysts: An optimization of Cu loading. Int. J. Hydrogen Energy 2014, 39, 9135–9142. [Google Scholar] [CrossRef]
- An, J.-W.; Wang, G.-C. Coordination-number-determined activity of copper catalyst in water-gas shift reaction. Fuel 2023, 343, 127850. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Y.; Wang, Q. Fabrication, activity and mechanism studies of transition metal molybdate/molybdenum trioxide hybrids as novel CWAO catalysts. Sep. Purif. Technol. 2018, 191, 354–363. [Google Scholar] [CrossRef]
- Yao, Y.; Yu, Z.; Lu, C.; Sun, F.; Wang, Y.; Sun, Z.; Liu, Y.; Wang, A. Highly efficient Cu-based catalysts for selective hydrogenation of furfural: A key role of copper carbide. Renew. Energy 2022, 197, 69–78. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.; Xu, L.; Tian, Y.; Cui, Q.; Liu, X.; Chen, M. Cu2O nanocubes–grafted highly dense Au nanoparticles with modulated electronic structures for improving peroxidase catalytic performances. Talanta 2021, 225, 121990. [Google Scholar] [CrossRef] [PubMed]
- Giziński, D.; Brudzisz, A.; Santos, J.S.; Trivinho-Strixino, F.; Stępniowski, W.J.; Czujko, T. Nanostructured Anodic Copper Oxides as Catalysts in Electrochemical and Photoelectrochemical Reactions. Catalysts 2020, 10, 1338. [Google Scholar] [CrossRef]
- Rai, R.; Chand, D.K. Multicomponent click reactions catalysed by copper(I) oxide nanoparticles (Cu2ONPs) derived using Oryza sativa. J. Chem. Sci. 2020, 132, 83. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Chanda, K.; Chu, Y.-T.; Chiu, C.-Y.; Huang, M.H. Direct formation of small Cu2O nanocubes, octahedra, and octapods for efficient synthesis of triazoles. Nanoscale 2014, 6, 8704–8709. [Google Scholar] [CrossRef]
- Siegfried, M.J.; Choi, K.-S. Electrochemical Crystallization of Cuprous Oxide with Systematic Shape Evolution. Adv. Mater. 2004, 16, 1743–1746. [Google Scholar] [CrossRef]
- Ashok, A.; Kumara, A.; Saad, M.A.S.; Al-Marri, M.J. Electrocatalytic conversion of CO2 over in-situ grown Cu microstructures on Cu and Zn foils. J. CO2 Util. 2021, 53, 101749–101759. [Google Scholar] [CrossRef]
- Radi, A.; Pradhan, D.; Sohn, Y.; Leung, K.T. Nanoscale Shape and Size Control of Cubic, Cuboctahedral, and Octahedral Cu-Cu2O Core-Shell Nanoparticles on Si(100) by One-Step, Templateless, Capping-Agent-Free Electrodeposition. ACS Nano 2010, 4, 1553–1560. [Google Scholar] [CrossRef]
- Haba, T.; Ikeda, K.; Uosaki, K. Electrochemical and in situ SERS study of the role of an inhibiting additive in selective electrodeposition of copper in sulfuric acid. Electrochem. Commun. 2019, 98, 19–22. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Y.; Handoko, D.A.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective Electrochemical Reduction of Carbon Dioxide to Ethylene and Ethanol on Copper(I) Oxide Catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- El Attar, A.; Oularbi, L.; Chemchoub, S.; El Rhazi, M. Effect of Electrochemical Activation on the Performance and Stability of Hybrid (PPy/Cu2O nanodendrites) for Efficient Ethanol Oxidation in Alkaline Medium. J. Electroanal. Chem. 2021, 885, 115042. [Google Scholar] [CrossRef]
- Chung, D.Y.; Kim, H.; Chung, Y.-H. Inhibition of CO poisoning on Pt catalyst coupled with the reduction of toxic hexavalent chromium in a dual-functional fuel cell. Sci. Rep. 2014, 4, 7450. [Google Scholar] [CrossRef]
- Aguilar, M.S.; Esparza, R.; Rosas, G. Time-dependent facile synthesis of CuO hedgehog-like nanostructures and their catalytic activity. J. Solid State Chem. 2019, 277, 46–53. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; He, J.; Johnson, M.W.; Stern, I.; Kim, D.H.; Pesika, N.S. Additive-Mediated Electrochemical Synthesis of Platelike Copper Crystals for Methanol Electrooxidation. Langmuir 2013, 29, 13135–13139. [Google Scholar] [CrossRef] [PubMed]
- Petriev, I.; Pushankina, P.; Bolotin, S.; Lutsenko, I.; Kukueva, E.; Baryshev, M. The influence of modifying nanoflower and nanostar type Pd coatings on low temperature hydrogen permeability through Pd-containing membranes. J. Membr. Sci. 2021, 620, 118894. [Google Scholar] [CrossRef]
- Petriev, I.S.; Pushankina, P.D.; Lutsenko, I.S.; Baryshev, M.G. Anomalous Kinetic Characteristics of Hydrogen Transport through Pd–Cu Membranes Modified by Pentatwinned Flower-Shaped Palladium Nanocrystallites with High-Index Facets. Tech. Phys. Lett. 2021, 47, 803–806. [Google Scholar] [CrossRef]
- Su, Y.; Li, C.; Yao, C.; Xu, L.; Xue, J.; Yuan, W.; Liu, J.; Cheng, M.; Hou, S. Palladium nanoparticles immobilized in B, N doped porous carbon as electrocatalyst for ethanol oxidation reaction. Mater. Today Energy 2021, 20, 100628. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Henriksson, G.; Cornell, A. Insights on the ethanol oxidation reaction at electrodeposited PdNi catalysts under conditions of increased mass transport. Int. J. Hydrogen Energy 2021, 46, 1615–1626. [Google Scholar] [CrossRef]
- El Attar, A.; Chemchoub, S.; Kalan, M.D.; Oularbi, L.; El Rhazi, M. Designing New Material Based on Functionalized Multi-Walled Carbon Nanotubes and Cu(OH)2–Cu2O/Polypyrrole Catalyst for Ethanol Oxidation in Alkaline Medium. Front. Chem. 2022, 10, 843736. [Google Scholar] [CrossRef]
- Heli, H.; Jafarian, M.; Mahjani, M.G.; Gobal, F. Electro-oxidation of methanol on copper in alkaline solution. Electrochim. Acta 2004, 49, 4999–5006. [Google Scholar] [CrossRef]
- Casella, I.G.; Gatta, M. Anodic electrodeposition of copper oxide/hydroxide films by alkaline solutions containing cuprous cyanide ions. J. Electroanal. Chem. 2000, 494, 12–20. [Google Scholar] [CrossRef]
- Miller, B. Split-Ring Disk Study of the Anodic Processes at a Copper Electrode in Alkaline Solution. J. Electrochem. Soc. 1969, 116, 1675. [Google Scholar] [CrossRef]
- Farrell, S.T.; Breslin, C.B. Oxidation and photo-induced oxidation of glucose at a polyaniline film modified by copper particles. Electrochim. Acta 2004, 49, 4497. [Google Scholar] [CrossRef]
- Ojani, R.; Raoof, J.-B.; Ahmady-Khanghah, Y. Copper-poly(2-aminodiphenylamine) as a novel and low cost electrocatalyst for electrocatalytic oxidation of methanol in alkaline solution. Electrochim. Acta 2011, 56, 3380–3386. [Google Scholar] [CrossRef]
- Hameed, R.M.A.; Fahim, A.E.; Allam, N.K. Tin oxide as a promoter for copper@palladium nanoparticles on graphene sheets during ethanol electro-oxidation in NaOH solution. J. Mol. Liq. 2020, 297, 111816. [Google Scholar] [CrossRef]
- Wang, M.; He, Y.; Li, R.; Ma, Z.; Zhang, Z.; Wang, X. Electrochemical activated PtAuCu alloy nanoparticle catalysts for formic acid, methanol and ethanol electro-oxidation. Electrochim. Acta 2015, 178, 259–269. [Google Scholar] [CrossRef]
- Yasmin, S.; Roy, N.; Kabir, M.H.; Jeon, S. Nitrogen-functionalized carbon nanotube based palladium nanoparticles as an efficient catalyst for oxygen reduction and ethanol oxidation reaction. Appl. Surf. Sci. Adv. 2022, 9, 100235. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; El-Newehy, M.; Al-Deyab, S. Cobalt/copper-decorated carbon nanofibers as novel non-precious electrocatalyst for methanol electrooxidation. Nanoscale Res. Lett. 2014, 9, 2. [Google Scholar] [CrossRef]
- Cherevko, S.; Kulyk, N.; Chung, C.-H. Nanoporous Pt@AuxCu100–x by Hydrogen Evolution Assisted Electrodeposition of AuxCu100–x and Galvanic Replacement of Cu with Pt: Electrocatalytic Properties. Langmuir 2012, 28, 3306–3315. [Google Scholar] [CrossRef]
- Hsieh, M.-W.; Whang, T.-J. Electrodeposition of PdCu alloy and its application in methanol electro-oxidation. Appl. Surf. Sci. 2013, 270, 252–259. [Google Scholar] [CrossRef]
- Tsui, L.-S.; Zafferoni, C.; Lavacchi, A.; Innocenti, M.; Vizza, F.; Zangaria, G. Electrocatalytic activity and operational stability of electrodeposited Pd–Co films towards ethanol oxidation in alkaline electrolytes. J. Power Sources 2015, 293, 815–822. [Google Scholar] [CrossRef]
- Maya-Cornejo, J.; Diaz-Real, J.A.; Lopez-Miranda, J.L.; Alvarez-Contreras, L.; Esparza, R.; Arjona, N.; Esteveza, M. Formation of Cu@Pd core@shell nanocatalysts with high activity for ethanol electro-oxidation in alkaline medium. Appl. Surf. Sci. 2021, 538, 148119. [Google Scholar] [CrossRef]
- Pen, H.; Ren, J.; Wang, Y.; Xiong, Y.; Wang, Q.; Li, Q.; Zhao, X.; Zhan, L.; Zheng, L.; Tang, Y.; et al. One-stone, two birds: Alloying effect and surface defects induced by Pt on Cu2−xSe nanowires to boost C-C bond cleavage for electrocatalytic ethanol oxidation. Nano Energy 2021, 88, 106307. [Google Scholar] [CrossRef]
- Wang, H.; Guan, A.; Zhang, J.; Mi, Y.; Li, S.; Yuan, T.; Jing, C.; Zhang, L.; Zhang, L.; Zheng, G. Copper-doped nickel oxyhydroxide for efficient electrocatalytic ethanol oxidation. Chin. J. Catal. 2022, 43, 1478–1484. [Google Scholar] [CrossRef]
- Panah, N.B.; Danaee, I.; Ghamsari, Z.G. Effect of Electrochemical Surface Pretreatment on Electro-Catalytic Activity of Copper for Ethanol Oxidation in Alkaline Media. Surf. Eng. Appl. Electrochem. 2019, 55, 630–637. [Google Scholar] [CrossRef]
- Miao, Z.; Xu, C.; Zhan, J.; Xua, Z. Morphology-control and template-free fabrication of bimetallic Cu–Ni alloy rods for ethanol electro-oxidation in alkaline media. J. Alloys Compd. 2021, 855, 157438. [Google Scholar] [CrossRef]
- Zare, B.; Roushani, M. Electrocatalytic oxidation of ethanol on the copper, carbon paste and glassy carbon electrode modified with Cu-BDC MOF. Front. Chem. Res. 2020, 2, 38–41. [Google Scholar] [CrossRef]
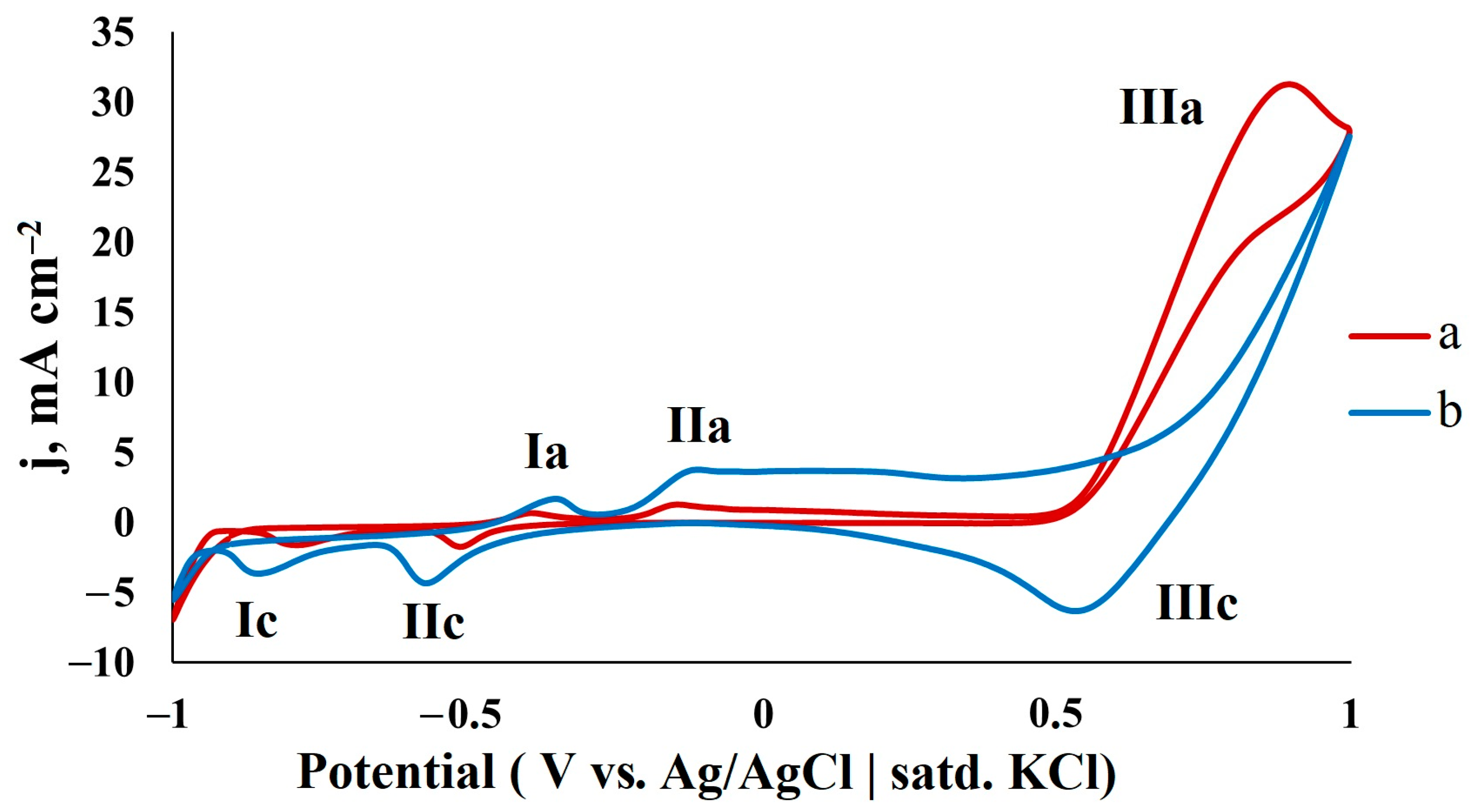
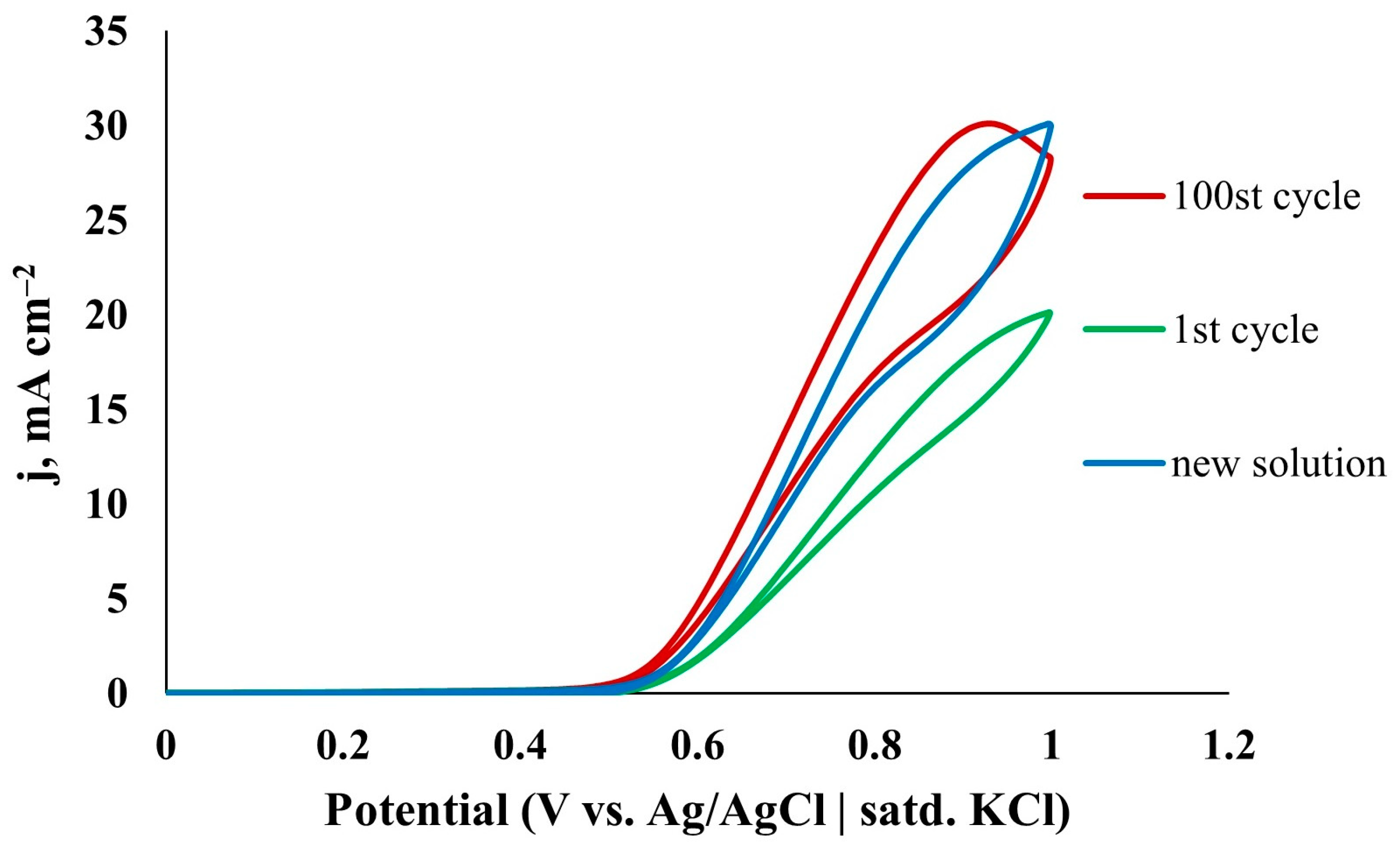
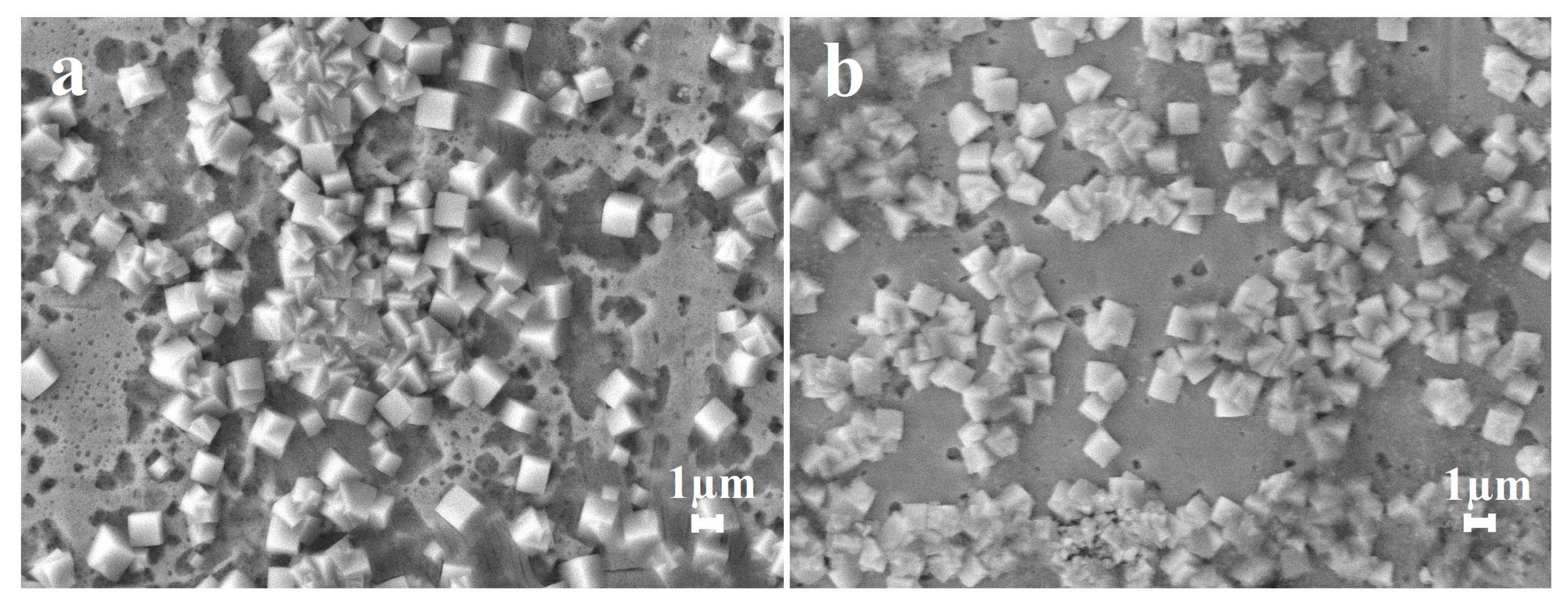
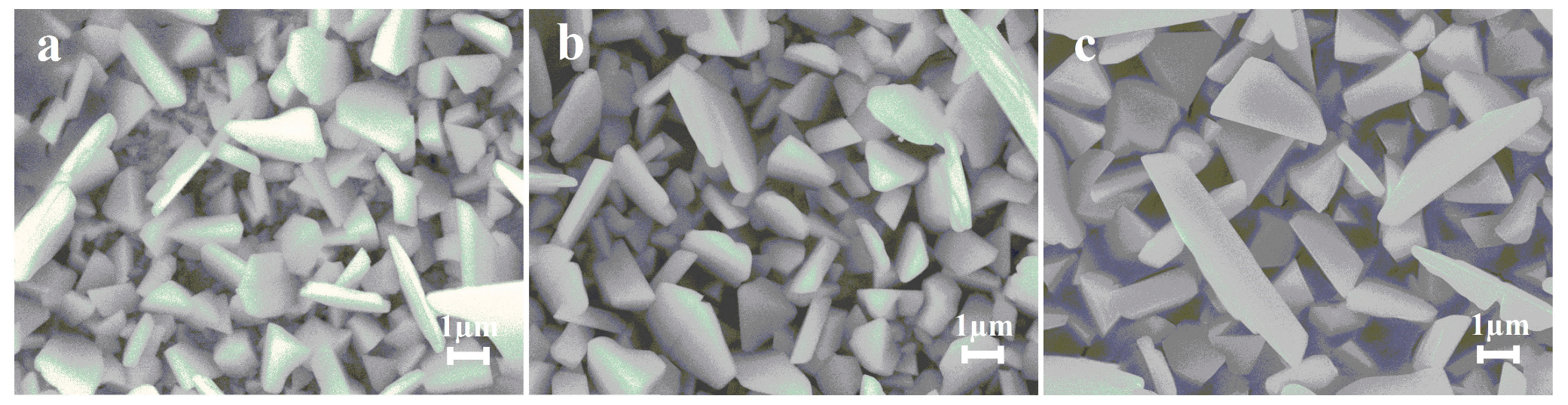
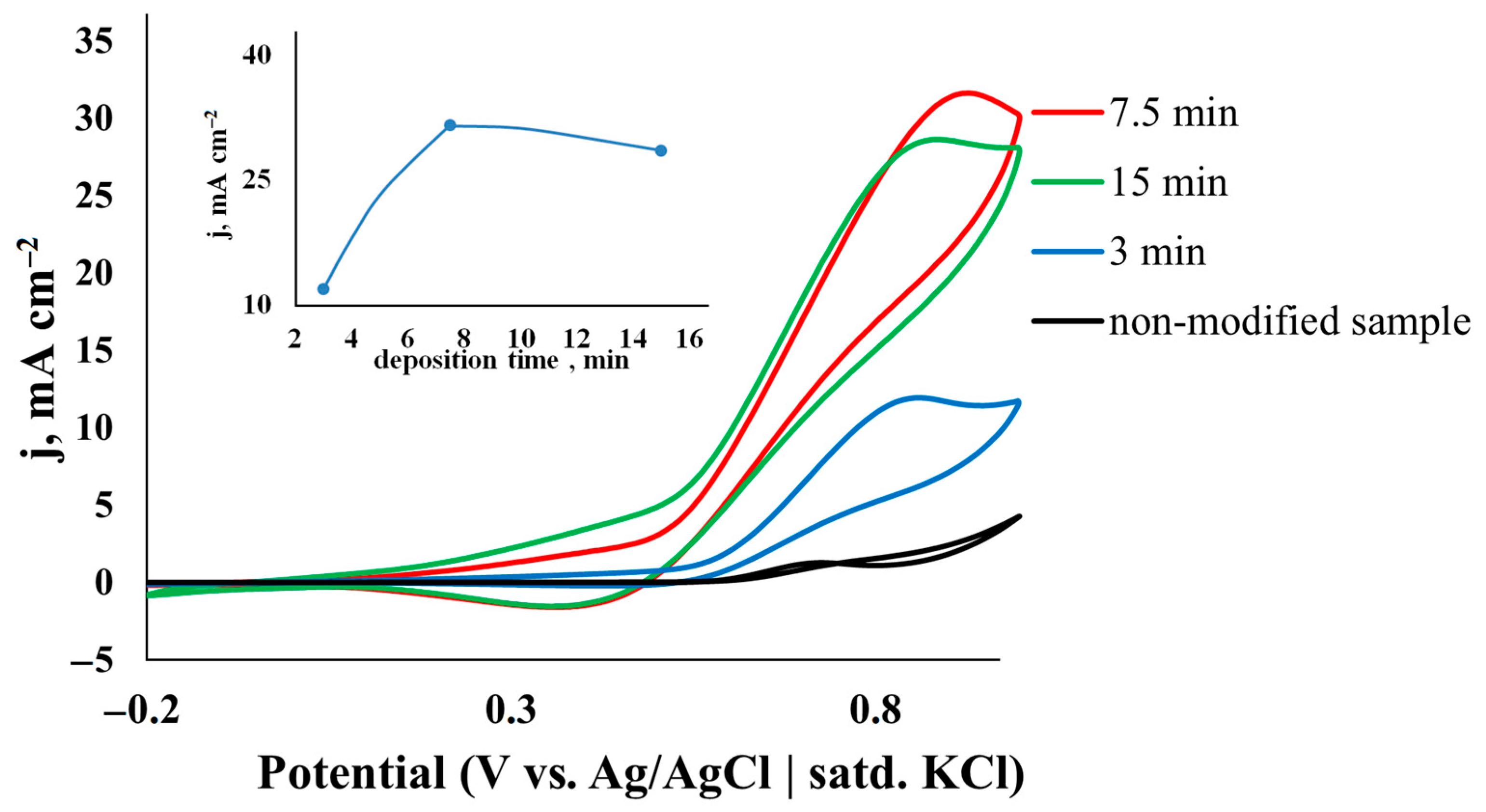

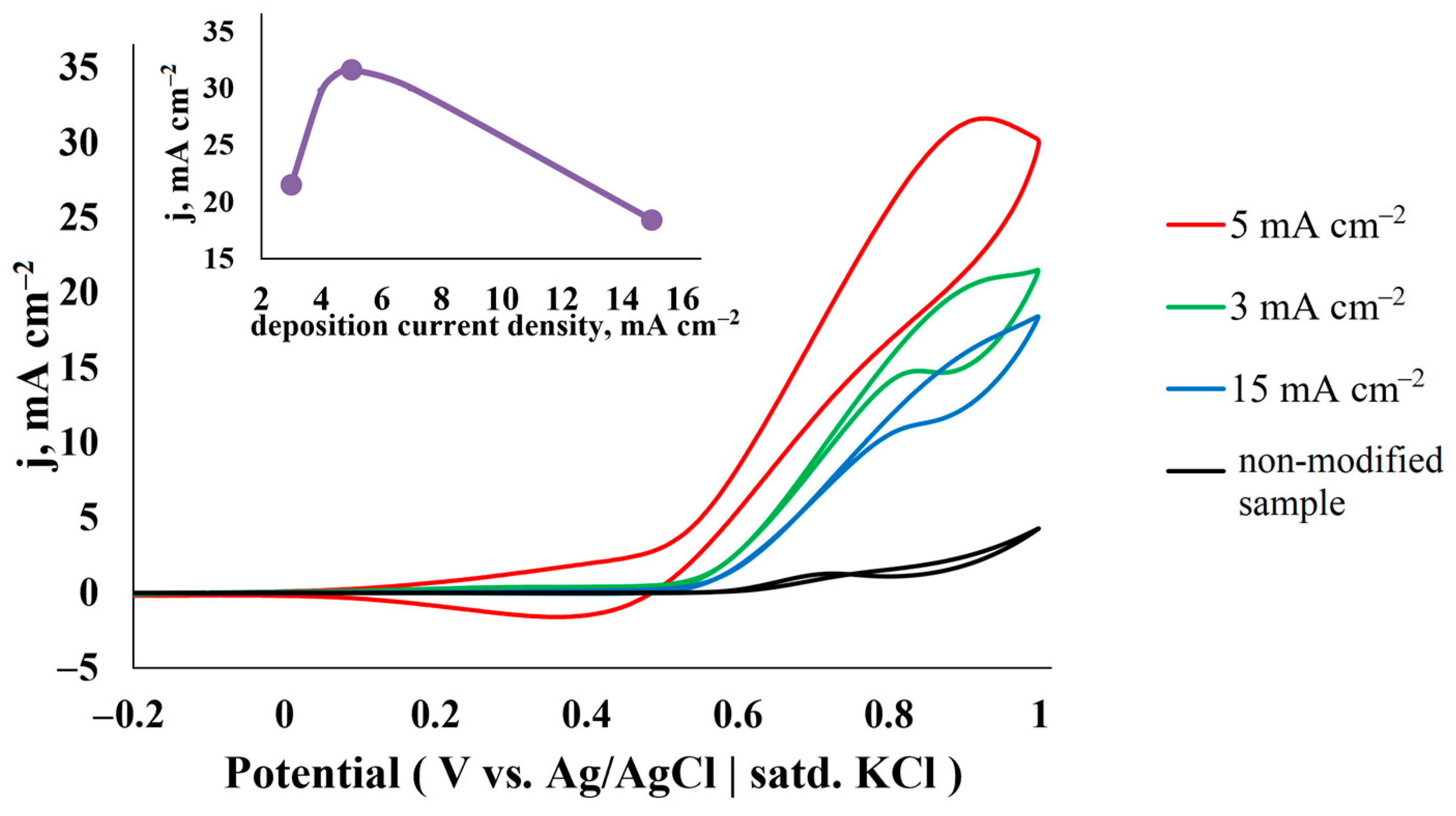
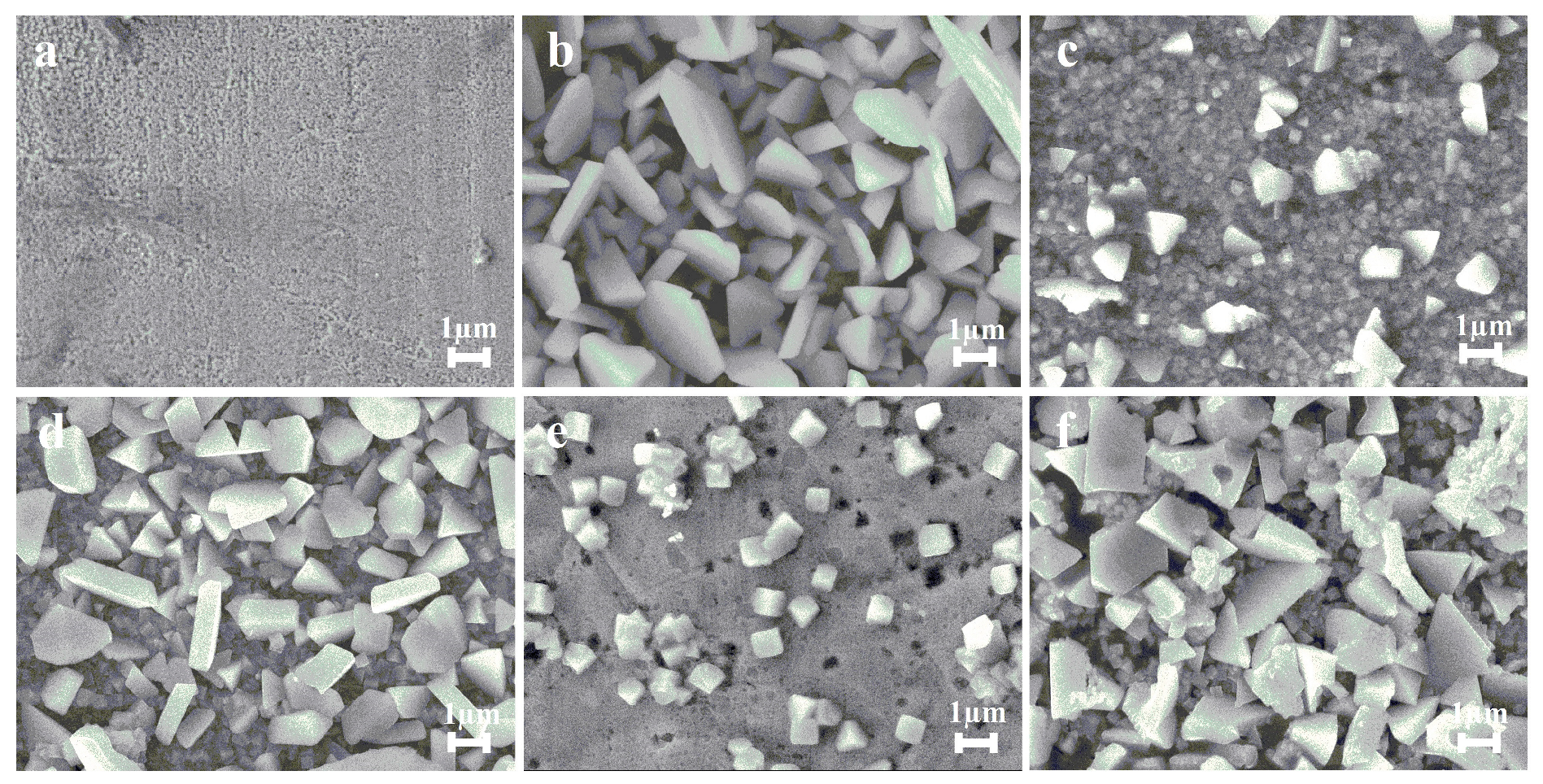
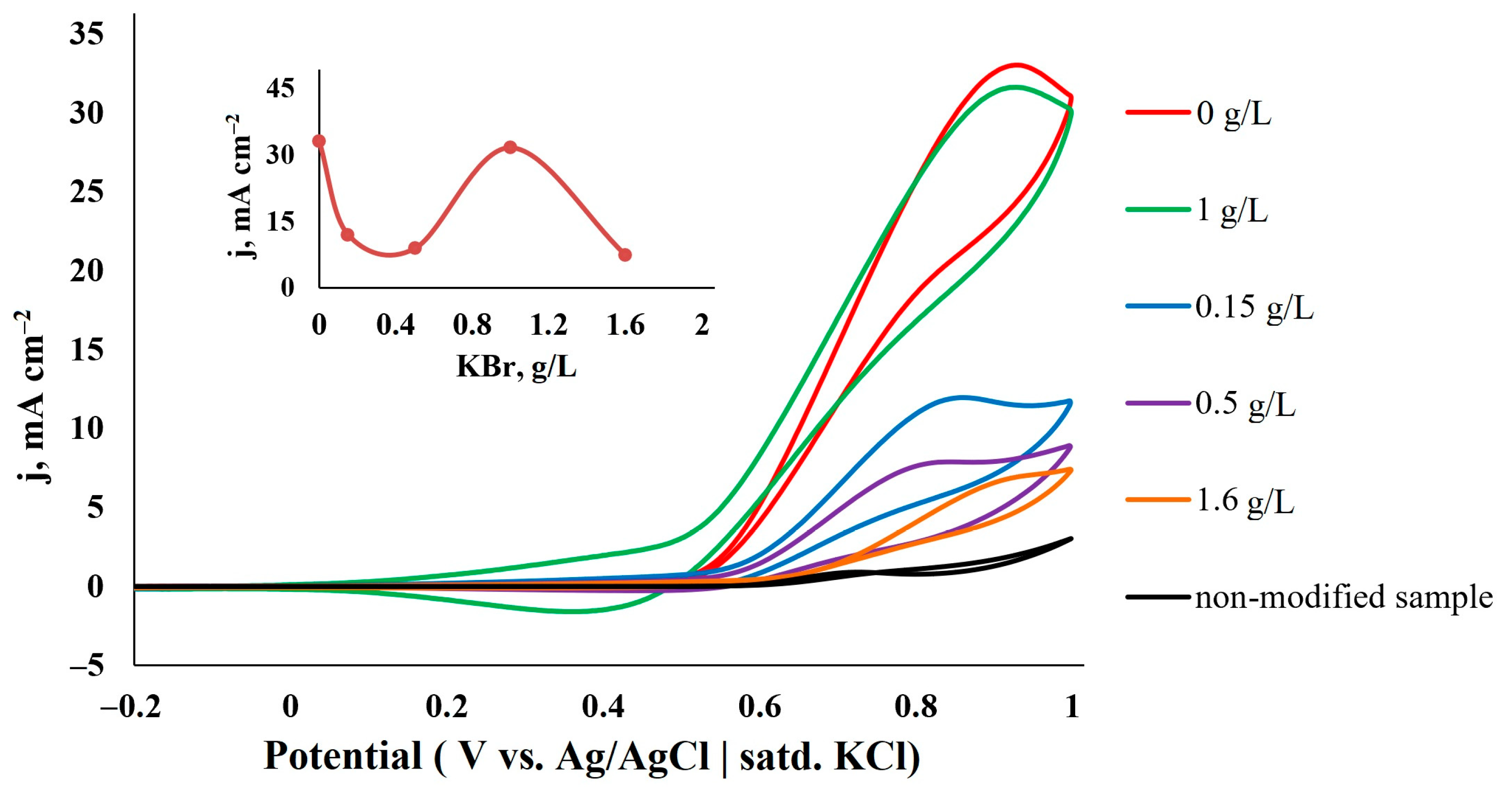
| Catalyst | C, M | v, mV s−1 | j, mA cm−2 | Ref. |
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 |
| Cu lamellar nanoparticles Cu octahedra | 0.25 CH3OH 0.1 NaOH | 10 | 8.3 4.3 | [35] |
| Cu-CeO2/Cu nanofibers decorated with copper particles | 2.0 CH3OH 1.0 KOH | 50 | 12.0 | [49] |
| Pt@Au honeycomb particles Pt@Au50Cu50 nanodendrites | 1.0 CH3OH 1.0 NaOH | 50 | 0.82 0.4 | [50] |
| Pd80Cu20 needle particles | 1.0 CH3OH 1.0 KOH | 25 | 11.81 | [51] |
| Pd48Co52 lamellar particles Pd23Co77 lamellar particles | 0.1 C2H5OH 2.0 KOH | 10 | 15.0 22.5 | [52] |
| Cu@Pd IV particles smaller than 10 nm | 1.0 C2H5OH 2.0 KOH | 20 | 3.0 | [53] |
| PtCu/Cu2-xSe nanowires with a large number of defects | 1.0 C2H5OH 1.0 KOH | 50 | 5.03 | [54] |
| Cu-Ni spherical particles doped with Cu atoms | 0.05 C2H5OH 1.0 NaOH | 10 | 30.0 | [55] |
| PPy/Cu2O/CPE octahedra nanodendrites | 0.2 C2H5OH 0.1 NaOH | 10 | 2.25 4.0 | [30] |
| Cu dendritic and spherical particles | 1.0 C2H5OH 0.5 NaOH | 10 | 6.0 | [56] |
| Cu-Ni porous nanorods | 0.05 C2H5OH 1.0 NaOH | 20 | 30.0 | [57] |
| Cu@Pd/SnO2-Gr-5 spherical particles on graphene sheets | 1.0 C2H5OH 0.5 NaOH | 10 | 90.0 | [46] |
| Cu-BDC/CE octahedrons | 0.01 C2H5OH 1.0 NaOH | 50 | 0.7 | [58] |
| Pd-black | 0.5 C2H5OH 0.1 NaOH | 50 | 56.51 | This work |
| Cu cubic particles Cu lamellar and prismatic particles | 0.5 C2H5OH 0.1 NaOH | 10 | 33.01 31.59 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petriev, I.; Pushankina, P.; Glazkova, Y.; Andreev, G.; Baryshev, M. Investigation of the Dependence of Electrocatalytic Activity of Copper and Palladium Nanoparticles on Morphology and Shape Formation. Coatings 2023, 13, 621. https://doi.org/10.3390/coatings13030621
Petriev I, Pushankina P, Glazkova Y, Andreev G, Baryshev M. Investigation of the Dependence of Electrocatalytic Activity of Copper and Palladium Nanoparticles on Morphology and Shape Formation. Coatings. 2023; 13(3):621. https://doi.org/10.3390/coatings13030621
Chicago/Turabian StylePetriev, Iliya, Polina Pushankina, Yuliya Glazkova, Georgy Andreev, and Mikhail Baryshev. 2023. "Investigation of the Dependence of Electrocatalytic Activity of Copper and Palladium Nanoparticles on Morphology and Shape Formation" Coatings 13, no. 3: 621. https://doi.org/10.3390/coatings13030621
APA StylePetriev, I., Pushankina, P., Glazkova, Y., Andreev, G., & Baryshev, M. (2023). Investigation of the Dependence of Electrocatalytic Activity of Copper and Palladium Nanoparticles on Morphology and Shape Formation. Coatings, 13(3), 621. https://doi.org/10.3390/coatings13030621







