New Approach to Improving the Efficiency of Disinfectants against Biofilms
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Action of Disinfectants on Planktonic Bacterial Cultures
3.2. Effect of Disinfectants on Single-Species and Binary Biofilms Formed on Glass Fiber Filters
3.3. Action of Disinfectants on Binary Biofilms (St. aureus + S. typhimurium) Formed on Paper Filters
3.4. Cumulative Effect of Disinfectants and Adjuvants on Binary Biofilms (St. aureus + S. typhimurium)
3.5. Comparative Sensitivity to Disinfectants of the Biofilms Developing on Glass Fiber and Paper Filters
3.6. A Study of the Mechanisms of Action of the Adjuvants
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Combinations of Disinfectants | Viable Cell Titer, CFU/mL | ||||
|---|---|---|---|---|---|
| Paper Filters | Paper Filters | ||||
| Age of the BP | |||||
| 2 Days | 7 Days | 2 Days | 7 Days | ||
| Control without Disinfectants | 1.3 × 109 | 2.1 × 108 | 1.5 × 109 | 3.7 × 108 | |
| Foodlex OXY 0.05% | +isopropyl alcohol 30% | 1.9 × 106 | 1.8 × 108 | 4.2 × 108 | 2.9 × 108 |
| Dimax Chlor 0.038% | +ethyl alcohol 30% | 4.6 × 105 | <103 | 3.9 × 108 | 3.2 × 104 |
| +isopropyl alcohol 30% | 1.8 × 105 | 4.4 × 104 | 2.3 × 107 | 2.7 × 107 | |
| BFR Biocide Enzyme 0.5% | +hexylresorcinol 0.02% | 7.1 × 108 | 6.0 × 104 | 6.1 × 108 | 1.3 × 107 |
| +ethyl alcohol 30% | 3.5 × 105 | 3.8 × 105 | 7.4 × 108 | 3.7 × 105 | |
| +isopropyl alcohol 30% | 1.0 × 103 | <103 | 2.3 × 105 | <103 | |
| +hydrogen peroxide 6% | <103 | <103 | <103 | <103 | |
| Peracetic Acid 0.05% | +hexylresorcinol 0.02% | 4.4 × 108 | 5.3 × 107 | 1.9 × 108 | 2.4 × 107 |
| +ethyl alcohol 30% | 3.6 × 108 | 1.6 × 108 | 5.3 × 108 | 2.7 × 108 | |
| +isopropyl alcohol 30% | 8.1 × 106 | 2.2 × 107 | 4.6 × 108 | 3.0 × 107 | |
| +hydrogen peroxide 6% | 8.2 × 108 | 3.6 × 106 | 8.3 × 109 | 3.5 × 107 | |
References
- Ciofu, O.; Moser, C.; Østrup, P.; Høiby, J.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, S.; Sen, S.K.; Pattanaik, S.; Raut, S. Review on bacterial biofilm: An universal cause of contamination. Biocatal. Agric. Biotechnol. 2016, 7, 56–66. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nature Reviews. Microbiology 2016, 14, 563–575. [Google Scholar] [PubMed]
- Akinbobola, A.B.; Sherrya, L.; Mckay, W.G.; Ramage, G.; Williams, C. Tolerance of Pseudomonas aeruginosa in in-vitro biofilms to high-level peracetic acid disinfection. J. Hosp. Infect. 2017, 97, 162–168. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A.; Coughlan, L.M.; Briandet, R.; Cotter, P.D. Biofilms in food processing environments: Challenges and opportunities. Annu. Rev. Food Sci. Technol. 2019, 10, 173–195. [Google Scholar] [CrossRef]
- Cai, L.; Li, Y.; Wang, H.; Xu, X.; Zhou, G. Biofilm formation by meat-borne Pseudomonas fluorescens on stainless steel and its resistance to disinfectants. Food Control 2018, 91, 397–403. [Google Scholar]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.K.; Ha, S.D. Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Chitlapilly, D.S.; Wang, R. Biofilm through the Looking Glass: A Microbial Food Safety Perspective. Pathogens 2022, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivas, F.; Ripolles-Avila, C.; Fontecha-Umaña, F.; Ríos-Castillo, A.G.; Rodríguez-Jerez, J.J. Biofims in the spotlight: Detection, quantification and removal methods. Compr. Rev. Food Sci. Food Saf. 2018, 217, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.; Franklin, M. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Wimpenny, J.; Manz, W.; Szewzyk, U. Heterogeneity in biofilms. FEMS Microbiol. Rev. 2000, 24, 661–671. [Google Scholar] [CrossRef]
- Yuan, L.; Burmølle, M.; Sadiq, F.A.; Wang, N.; He, G. Interspecies variation in biofilm-forming capacity of psychrotrophic bacterial isolates from Chinese raw milk. Food Control 2018, 91, 47–57. [Google Scholar] [CrossRef]
- Zhurina, M.V.; Nikolaev, Y.A.; Plakunov, V.K. Role of the extracellular polymer matrix in azithromycin protection of Chromobacterium violaceum biofilms. Microbiology 2019, 88, 505–508. [Google Scholar] [CrossRef]
- Plakunov, V.K.; Nikolaev, Y.A.; Gannesen, A.V.; Chemaeva, D.S.; Zhurina, M.V. A New Approach to Detection of the Protective Effect of Escherichia coli on Gram-Positive Bacteria in Binary Biofilms in the Presence of Antibiotics. Microbiology 2019, 88, 275–281. [Google Scholar] [CrossRef]
- Sanchez-Vizuete, P.; Orgaz, B.; Aymerich, S.; Le Coq, D.; Briandet, R. Pathogens protection against the action of disinfectants in multispecies biofilms. Food Microbiol. 2015, 6, 705. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Brown, M.R.W.; Gilbert, P. Sensitivity of biofilms to antimicrobial agents. J. Appl. Bacteriol. 1993, 74, 875–975. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ikram, S. Surface modification of polysaccharide nanocrystals. In Innovation in Nano-Polysaccharides for Eco-Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 133–161. [Google Scholar]
- Gómez de Saravia, S.G.; Lorenzo de Mele, M.F. Non-invasive methods for monitoring biofilm growth in industrial water systems. Lat. Am. Appl. Res. 2003, 33, 353–359. [Google Scholar]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Smolik, N.A.; Rusznak, L.H.; Jenson, D.A. Biocidal Blends of Quaternary Ammonium Compounds and Chlorine Dioxide. U.S. Patent US5611938A, 18 March 1997. [Google Scholar]
- Delhalle, L.; Taminiau, B.; Fastrez, S.; Fall, A.; Ballesteros, M.; Burteau, S.; Daube, G. Evaluation of Enzymatic Cleaning on Food Processing Installations and Food Products Bacterial Microflora. Front. Microbiol. 2020, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Alpatova, N.A.; Autovia, Z.L.; Lysikova, S.L.; Gelovinskaya, O.V.; Gaydereva, L.A. General Characteristics of Adjuvants and Their Mechanism of Action (Part 1). Bioprep. Prev. Diagn. Treat. 2020, 20, 245–256. [Google Scholar] [CrossRef]
- dos Santos, C.A.M.; do Nascimento, J.; Gonçalves, K.C.; Smaniotto, G.; de Freitas Zechin, L.; da Costa Ferreira, M.; Polanczyk, R.A. Compatibility of Bt biopesticides and adjuvants for Spodoptera frugiperda control. Sci. Rep. 2021, 11, 5271. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Prakash, O.; Tiwari, S.; Maiti, P.; Ghosh, S.; Singh, R.K.; Maiti, P. Efficient Herbicide Delivery through a Conjugate Gel Formulation for the Mortality of Broad Leaf Weeds. ACS Omega 2022, 7, 19964–19978. [Google Scholar] [CrossRef] [PubMed]
- Palma-Bautista, C.; Vazquez-Garcia, J.G.; Travlos, I.; Tataridas, A.; Kanatas, P.; Domínguez-Valenzuela, J.A.; Prado, R. Effect of Adjuvant on Glyphosate Effectiveness, Retention, Absorption and Translocation in Lolium rigidum and Conyza canadensis. Plants 2020, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, Y.A.; Tutel’yan, A.V.; Loiko, N.G.; Buck, J.; Sidorenko, S.V.; Lazareva, I.; Gostev, V.; Manzen’yuk, O.Y.; Shemyakin, I.G.; Abramovich, R.A.; et al. The use of 4-Hexylresorcinol as antibiotic adjuvant. PLoS ONE 2020, 15, e0239147. [Google Scholar] [CrossRef]
- Douafer, H.; Andrieu, V.; Phanstiel, O., 4th; Brunel, J.M. Antibiotic Adjuvants: Make Antibiotics Great Again! J. Med. Chem. 2019, 62, 8665–8681. [Google Scholar] [CrossRef]
- Mohapatra, S. Sterilization and Disinfection. In Essentials of Neuroanesthesia; Academic Press: Cambridge, MA, USA, 2017; pp. 929–944. [Google Scholar] [CrossRef]
- Plakunov, V.K.; Martyanov, S.V.; Teteneva, N.A.; Zhurina, M.V. Universal method for quantitative characterization of growth and metabolic activityof microbial biofilms in static models. Microbiology 2016, 85, 484–489. [Google Scholar] [CrossRef]
- Bas, S.; Kramer, M.; Stopar, D. Biofilm Surface Density Determines Biocide Effectiveness. Front. Microbiol. 2017, 8, 2443. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Denyer, S.P.; Hugo, W.B.; Harding, V.D. Synergy in preservative combinations. Int. J. Pharm. 1985, 25, 245–253. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Johnston, M.D.; Hanlon, G.W.; Denyer, S.P. Theory of antimicrobial combinations: Biocidemixtures—Synergy or addition? J. Appl. Microbiol. 2003, 94, 747–759. [Google Scholar] [CrossRef]
- Fan, L.; Idris, M.A.; Bilyaminu, I.B.; Liu, D. Sonodynamic antimicrobial chemotherapy: An emerging alternative strategy for microbial inactivation. Ultrason. Sonochem. 2021, 75, 105591. [Google Scholar] [CrossRef] [PubMed]
- Ciecholewska-Juśko, D.; Żywicka, A.; Junka, A.; Woroszyło, M.; Wardach, M.; Chodaczek, G.; Szymczyk-Ziółkowska, P.; Migdał, P.; Fijałkowski, K. The effects of rotating magnetic field and antiseptic on in vitro pathogenic biofilm and its milieu. Sci. Rep. 2022, 12, 8836. [Google Scholar] [CrossRef]
- Orazi, G.; Ruoff, K.L.; O’Toole, G.A. Pseudomonas aeruginosa Increases the Sensitivity of Biofilm-Grown Staphylococcus aureus to Membrane-Targeting Antiseptics and Antibiotics. MBio 2019, 10, 15–19. [Google Scholar] [CrossRef]
- Mehta, K.C.; Dargad, R.R.; Borade, D.M.; Swami, O.C. Burden of antibiotic resistance in common infectious diseases: Role of antibiotic combination therapy. J. Clin. Diagn. Res. 2014, 8, ME05. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Singh, A.K.; Singh, S.; Chakravortty, D.; Das, D. Enzymatic dispersion of biofilms: An emerging biocatalytic avenue to combat biofilm-mediated microbial infections. J. Biol. Chem. 2022, 298, 102352. [Google Scholar] [CrossRef]
- Jee, S.C.; Kim, M.; Sung, J.S.; Kadam, A.A. Efficient Biofilms Eradication by Enzymatic-Cocktail of Pancreatic Protease Type-I and Bacterial α-Amylase. Polymers 2020, 12, 3032. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R. β-Lactamase inhibitors and reversal of antibiotic resistance. Trends Pharmacol. Sci. 1991, 12, 227–232. [Google Scholar] [CrossRef] [PubMed]
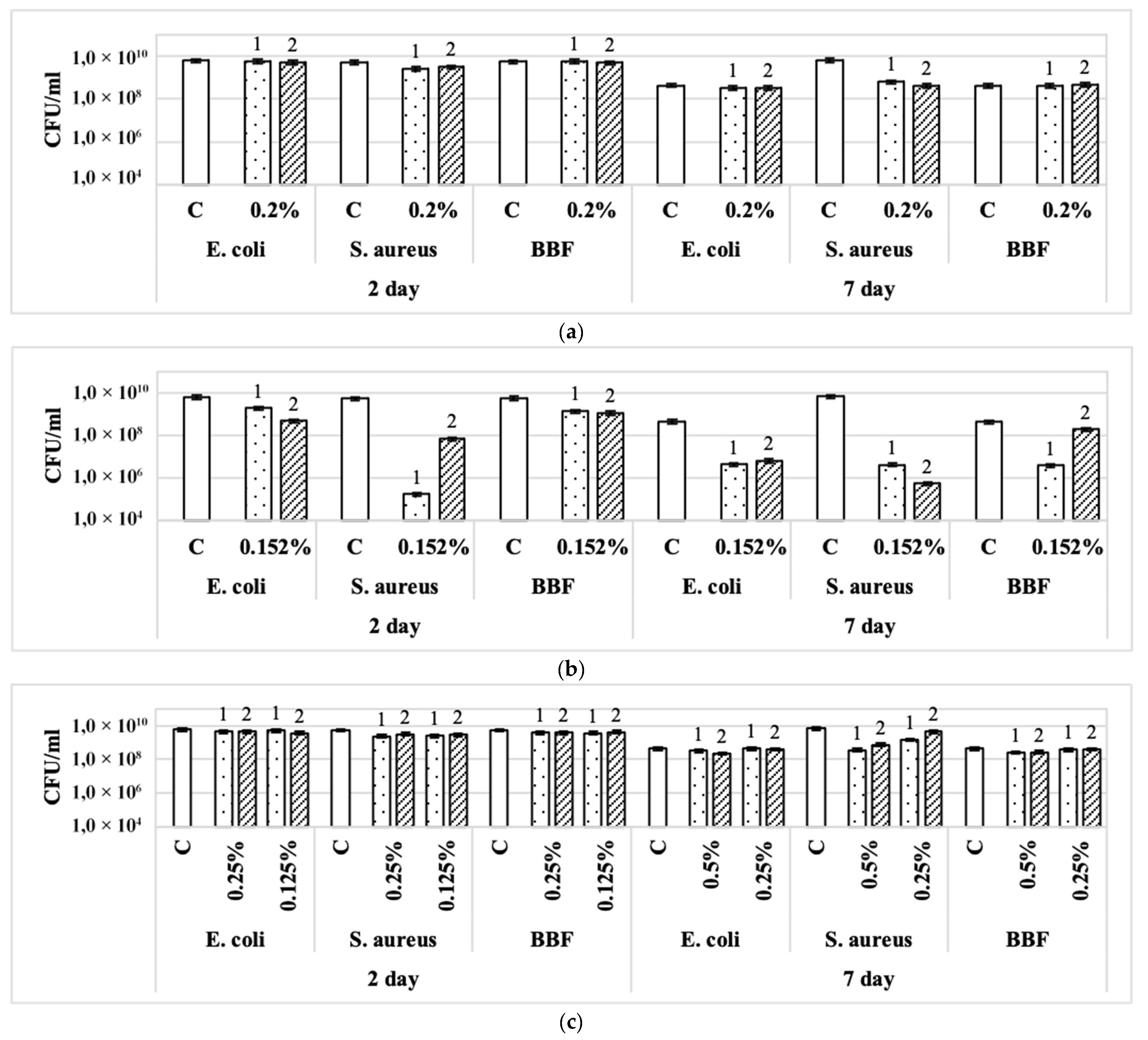

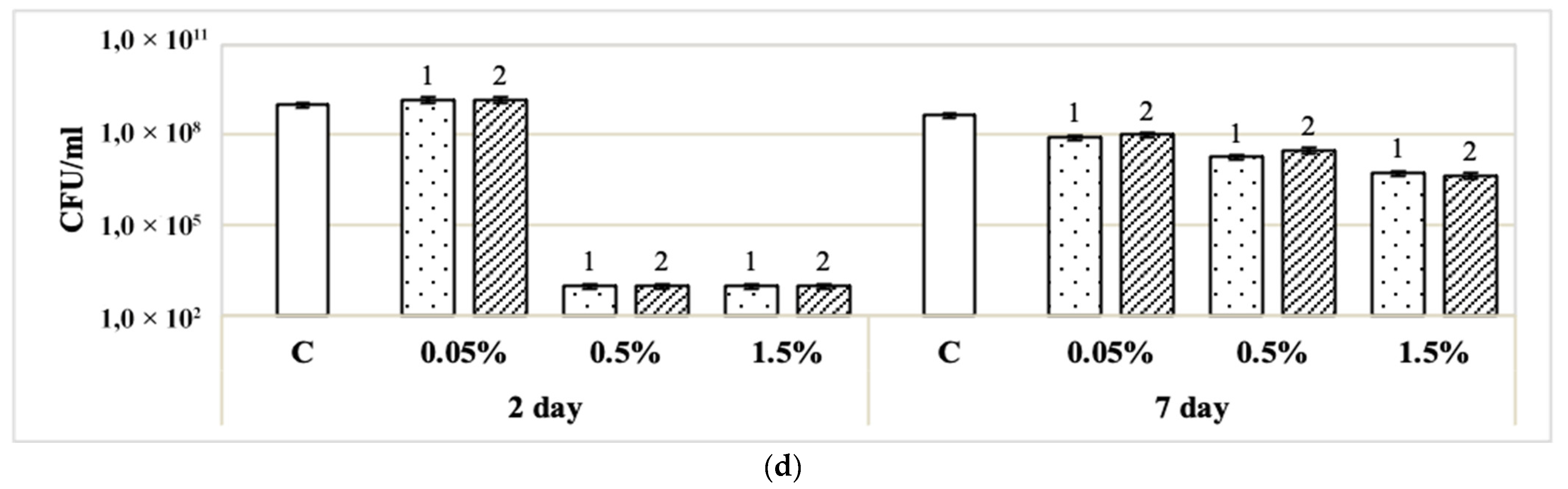
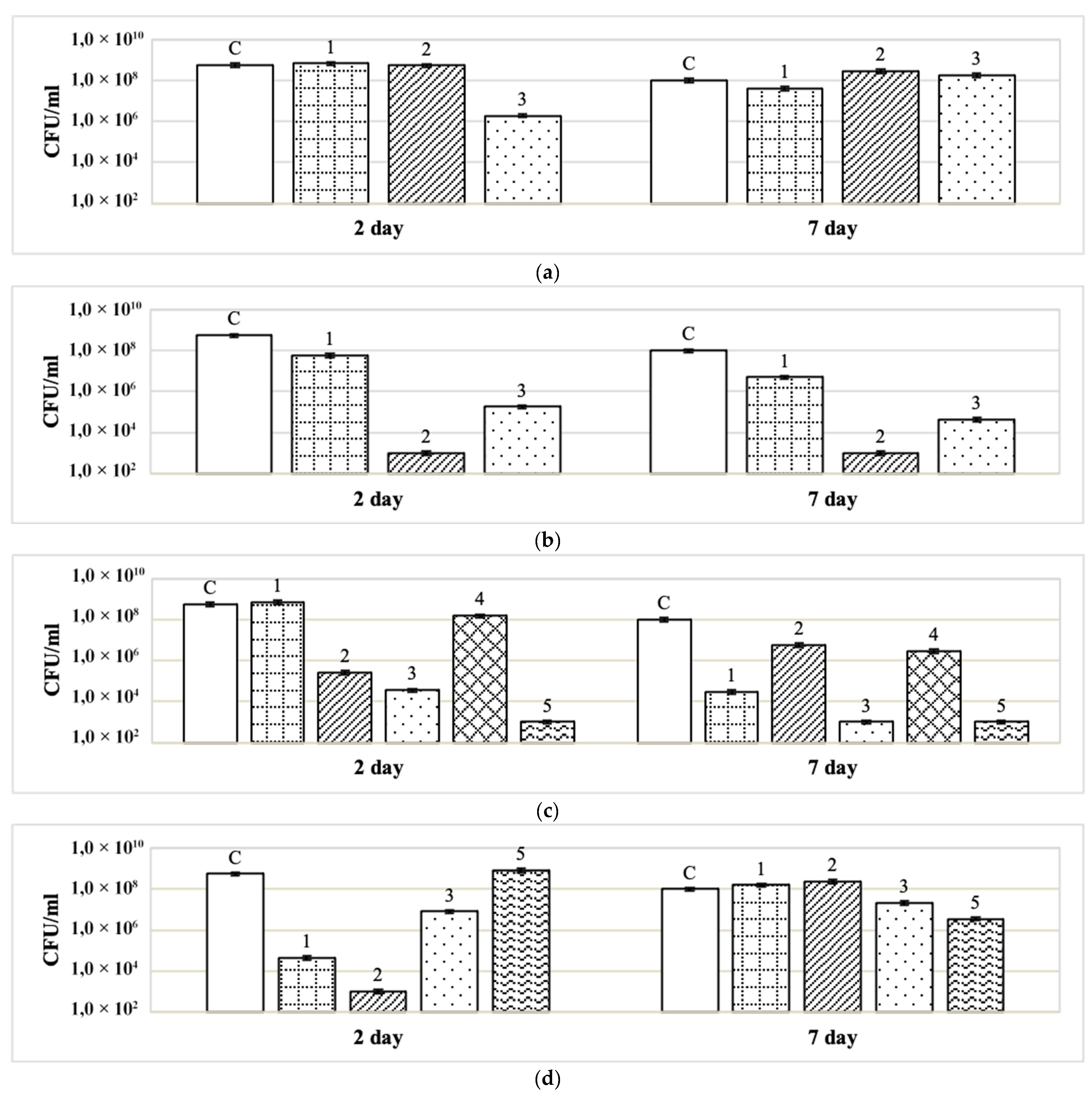


| Disinfectant | Concentration of Disinfectants | CFU/mL After Exposure to Disinfectant (% of Control in Parentheses) | |
|---|---|---|---|
| 10 min | 30 min | ||
| E. coli | |||
| Control without disinfectants | 5.0 × 109 (100%) | ||
| Foodlex OXY | 0.05% * | 4.5 × 109 (90%) | 3.1 × 109 (62%) |
| 0.2% | 4.5 × 109 (90%) | 1.0 × 108 (2%) | |
| Dimax Chlor | 0.038% * | 5.2 × 108 (10%) | 1.8 × 109 (36%) |
| 0.152% | <102 | 3.5 × 103 (0.0001%) | |
| BFR Biocide Enzyme | 0.5% * | <102 | <102 |
| 0.25% | <102 | <102 | |
| 0.125% | <102 | <102 | |
| S. aureus | |||
| Control without disinfectants | 2.8 × 109 (100%) | ||
| Foodlex OXY | 0.05% * | 7.9 × 108 (28%) | 7.3 × 108 (23%) |
| 0.2% | 1.0 × 109 (36%) | 2.7 × 108 (10%) | |
| Dimax Chlor | 0.038% * | 2.8 × 108 (10%) | 1.3 × 107 (0.5%) |
| 0.152% | <102 | <102 | |
| BFR Biocide Enzyme | 0.5% * | <102 | <102 |
| 0.25% | <102 | <102 | |
| 0.125% | <102 | <102 | |
| Binary culture (S. aureus + E. coli—3:1) | |||
| Control without disinfectants | 1.0 × 1010 (100%) | ||
| Foodlex OXY | 0.05% * | 3.5 × 109 (34%) | 4.2 × 109 (41%) |
| 0.2% | 3.5 × 109 (34%) | 8.1 × 107 (1%) | |
| Dimax Chlor | 0.038% * | 5.1 × 108 (5%) | 4.2 × 108 (4%) |
| 0.152% | <102 | <102 | |
| BFR Biocide Enzyme | 0.5% * | <102 | <102 |
| 0.25% | <102 | <102 | |
| 0.125% | <102 | <102 | |
| Combinations of Disinfectants with Adjuvants | Decrease in Titer after Exposure to Disinfectant (10 min), Orders (% CFU from Baseline Value) | ||
|---|---|---|---|
| 2 Days | 7 Days | ||
| Control without disinfectants | (100%) | (100%) | |
| Foodlex OXY 0.05% | +hexylresorcinol 0.02% | (100%) | >1 order of magnitude (40%) |
| +ethyl alcohol 30% | >1 order of magnitude (97%) | (100%) | |
| +isopropyl alcohol 30% | 3 orders of magnitude (0.1%) | (100%) | |
| Dimax Chlor 0.038% | +hexylresorcinol 0.02% | 1 orders of magnitude (11%) | 1.3 orders of magnitude (5%) |
| +ethyl alcohol 30% | 6 orders of magnitude (<0.0002%) | 6 orders of magnitude (<0.0002%) | |
| +isopropyl alcohol 30% | 3 orders of magnitude (0.03%) | 3.5 orders of magnitude (0.04%) | |
| BFR Biocide Enzyme 0.5% | +hexylresorcinol 0.02% | (100%) | 4 orders of magnitude (0.03%) |
| +ethyl alcohol 30% | 3 orders of magnitude (0.05%) | 1.5 orders of magnitude (6%) | |
| +isopropyl alcohol 30% | 4 orders of magnitude (0.01%) | 6 orders of magnitude (<0.0002%) | |
| +hydrogen peroxide 3% | 1.5 × 108 (27%) | 2.9 × 106 (3%) | |
| +hydrogen peroxide 6% | 6 orders of magnitude (0.0002%) | 6 orders of magnitude (<0.0002%) | |
| Peracetic acid 0.05% | +hexylresorcinol 0.02% | 4 orders of magnitude (0.008%) | (100%) |
| +ethyl alcohol 30% | 6 orders of magnitude (0.0002%) | (100%) | |
| +isopropyl alcohol 30% | 2 orders of magnitude (1%) | 1 order of magnitude (22%) | |
| +hydrogen peroxide 6% | (100%) | 2 orders of magnitude (4%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demkina, E.V.; Ilicheva, E.A.; El-Registan, G.I.; Pankratov, T.A.; Yushina, Y.K.; Semenova, A.A.; Nikolaev, Y.A. New Approach to Improving the Efficiency of Disinfectants against Biofilms. Coatings 2023, 13, 582. https://doi.org/10.3390/coatings13030582
Demkina EV, Ilicheva EA, El-Registan GI, Pankratov TA, Yushina YK, Semenova AA, Nikolaev YA. New Approach to Improving the Efficiency of Disinfectants against Biofilms. Coatings. 2023; 13(3):582. https://doi.org/10.3390/coatings13030582
Chicago/Turabian StyleDemkina, Elena V., Ekaterina A. Ilicheva, Galina I. El-Registan, Timofey A. Pankratov, Yulia K. Yushina, Anastasia A. Semenova, and Yuriy A. Nikolaev. 2023. "New Approach to Improving the Efficiency of Disinfectants against Biofilms" Coatings 13, no. 3: 582. https://doi.org/10.3390/coatings13030582
APA StyleDemkina, E. V., Ilicheva, E. A., El-Registan, G. I., Pankratov, T. A., Yushina, Y. K., Semenova, A. A., & Nikolaev, Y. A. (2023). New Approach to Improving the Efficiency of Disinfectants against Biofilms. Coatings, 13(3), 582. https://doi.org/10.3390/coatings13030582






