Quality Evaluation System of Monolayer Brazed Diamond Tools: A Brief Review
Abstract
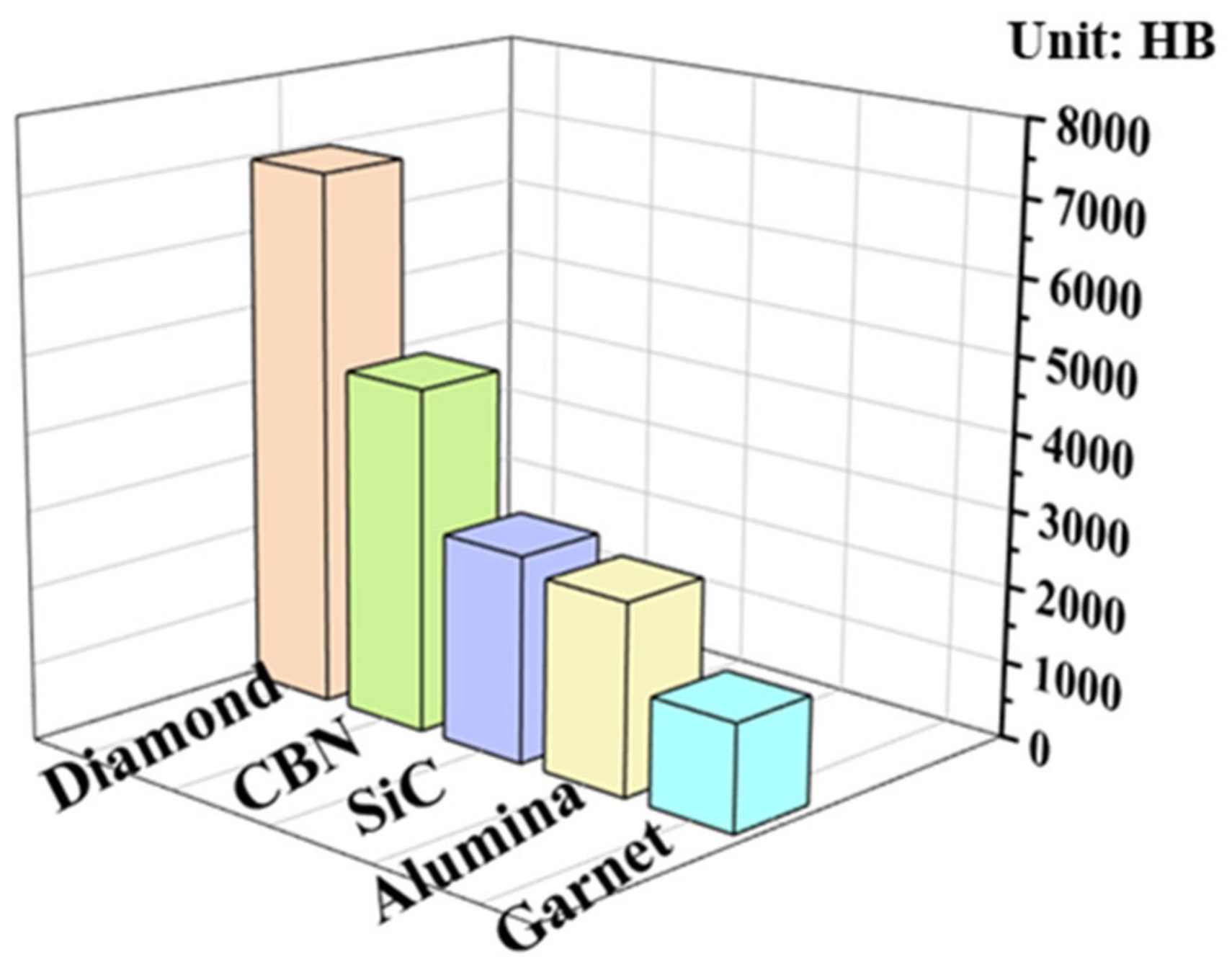
1. Introduction
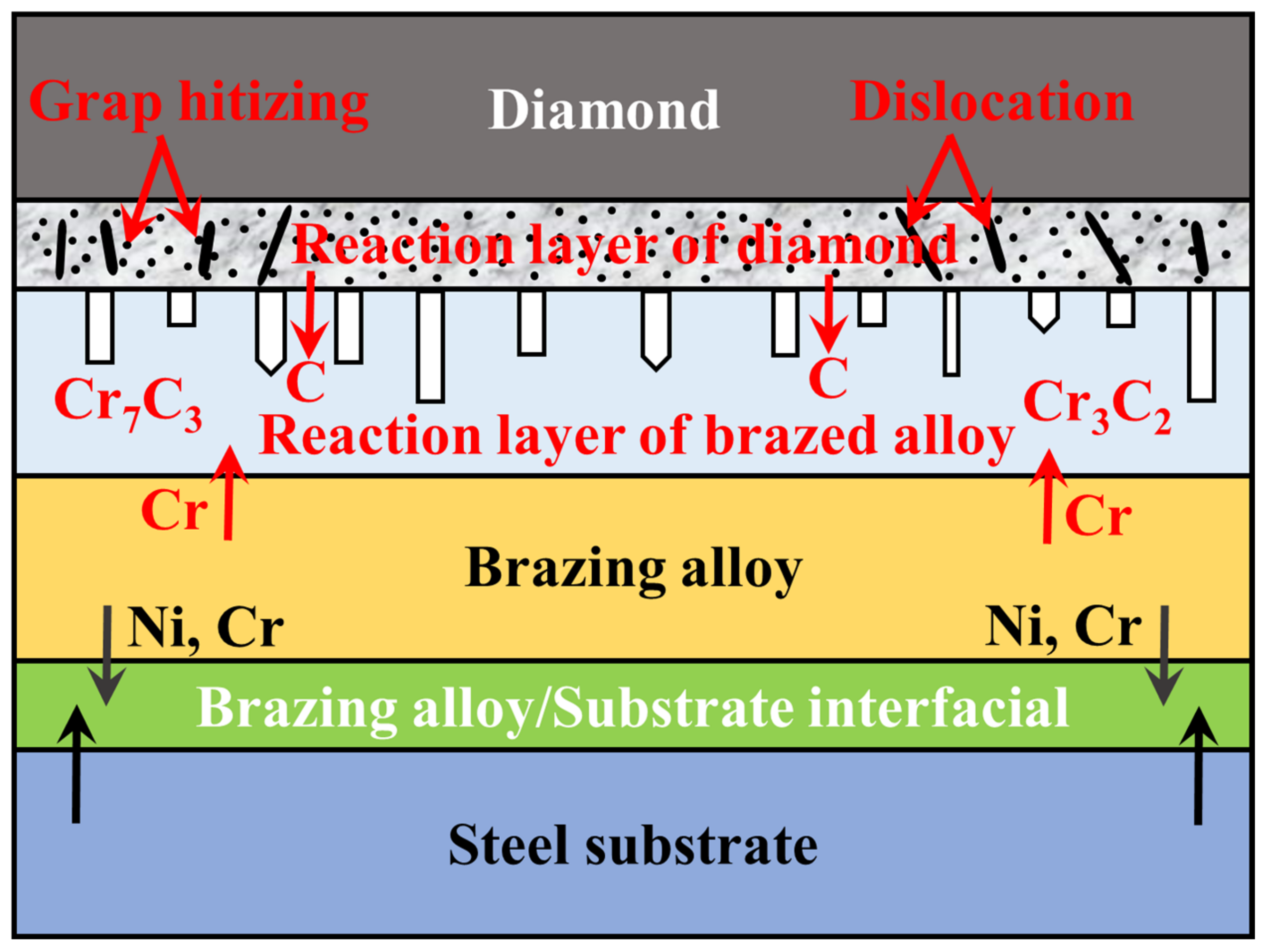
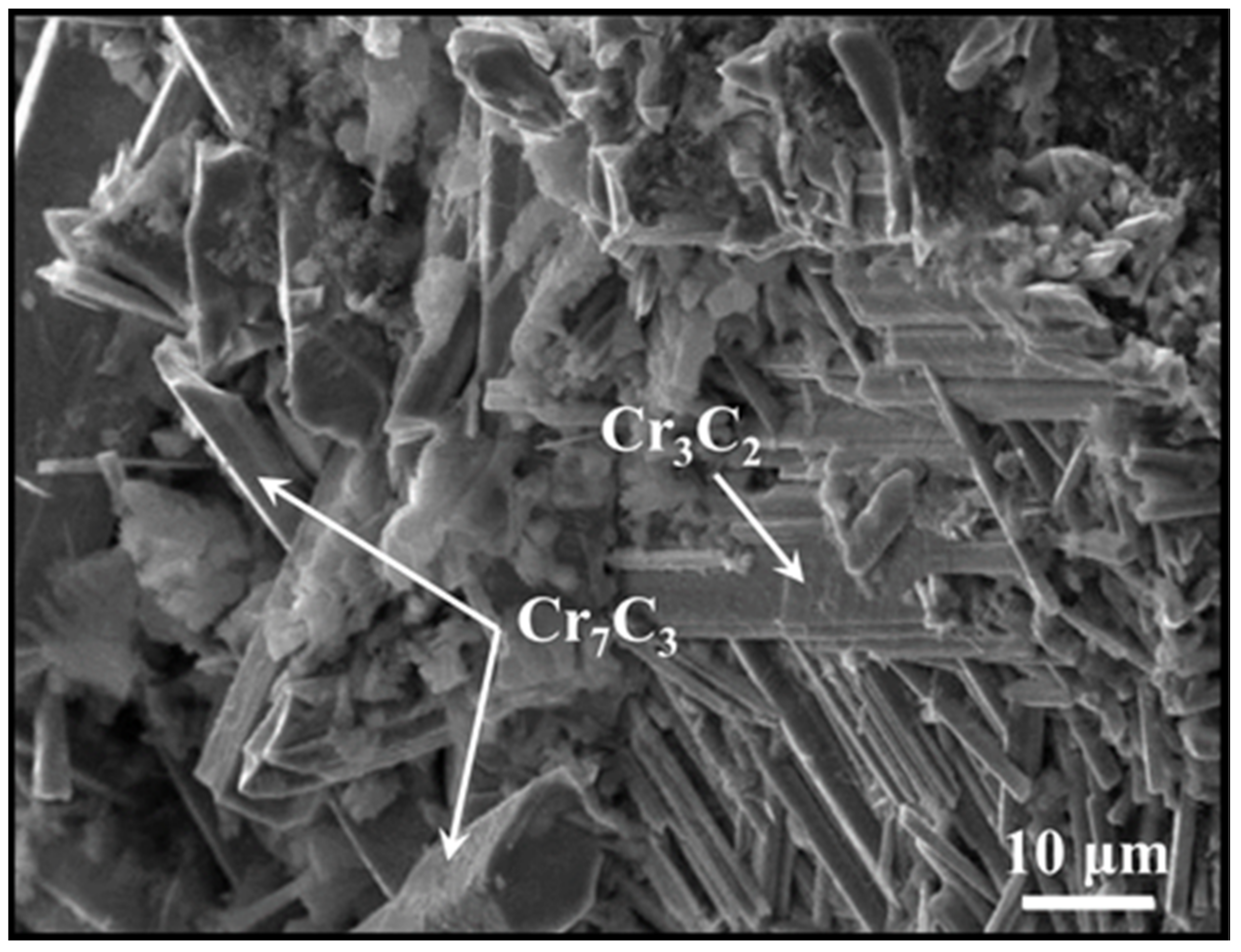
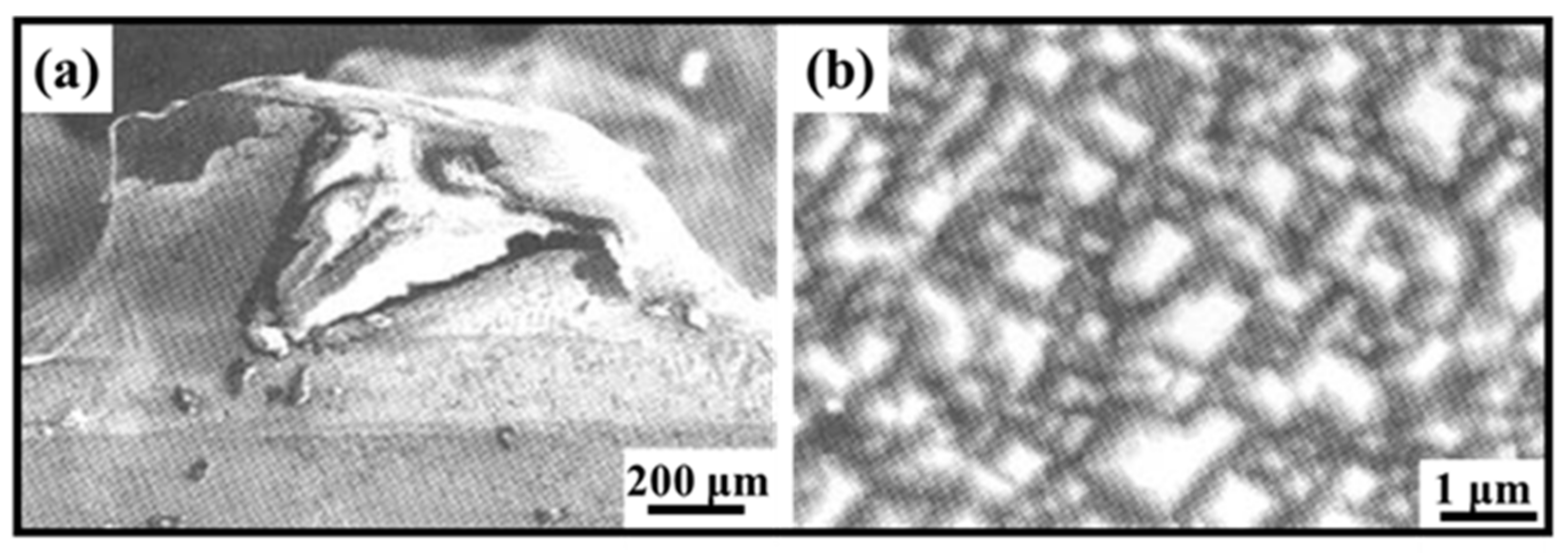
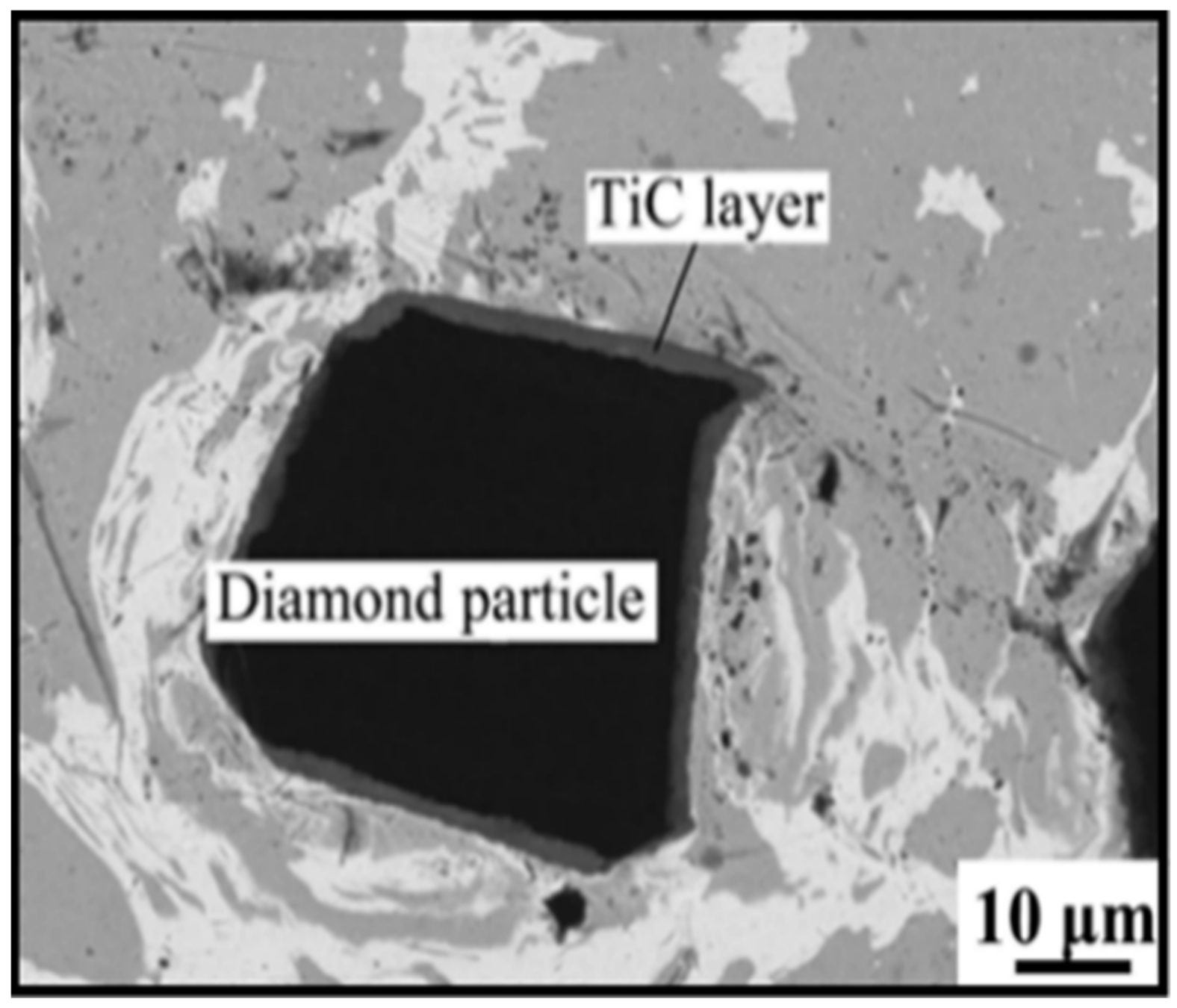
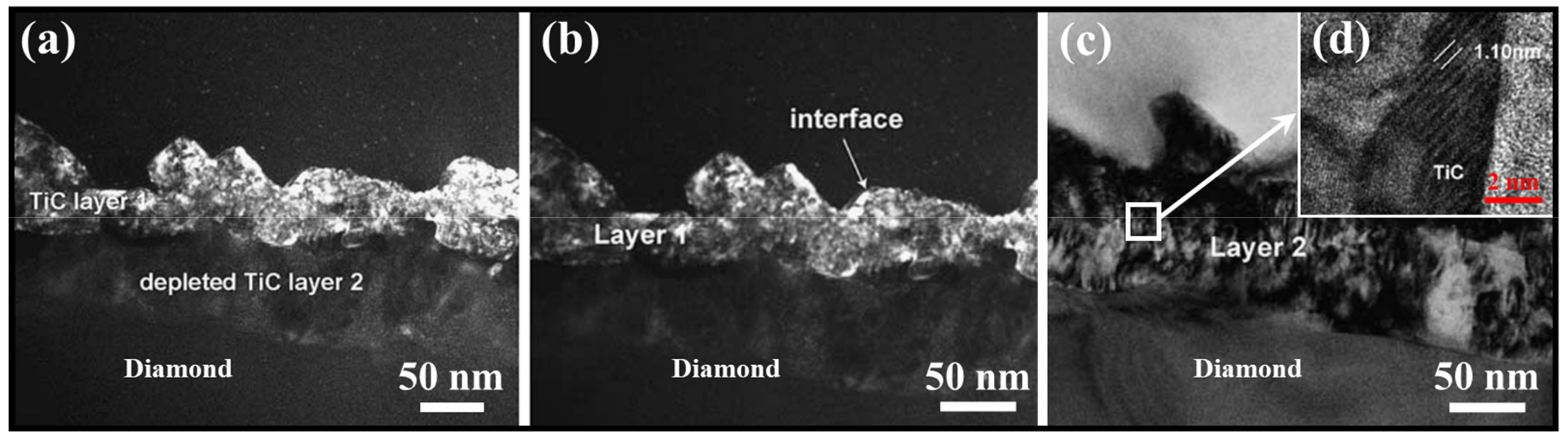
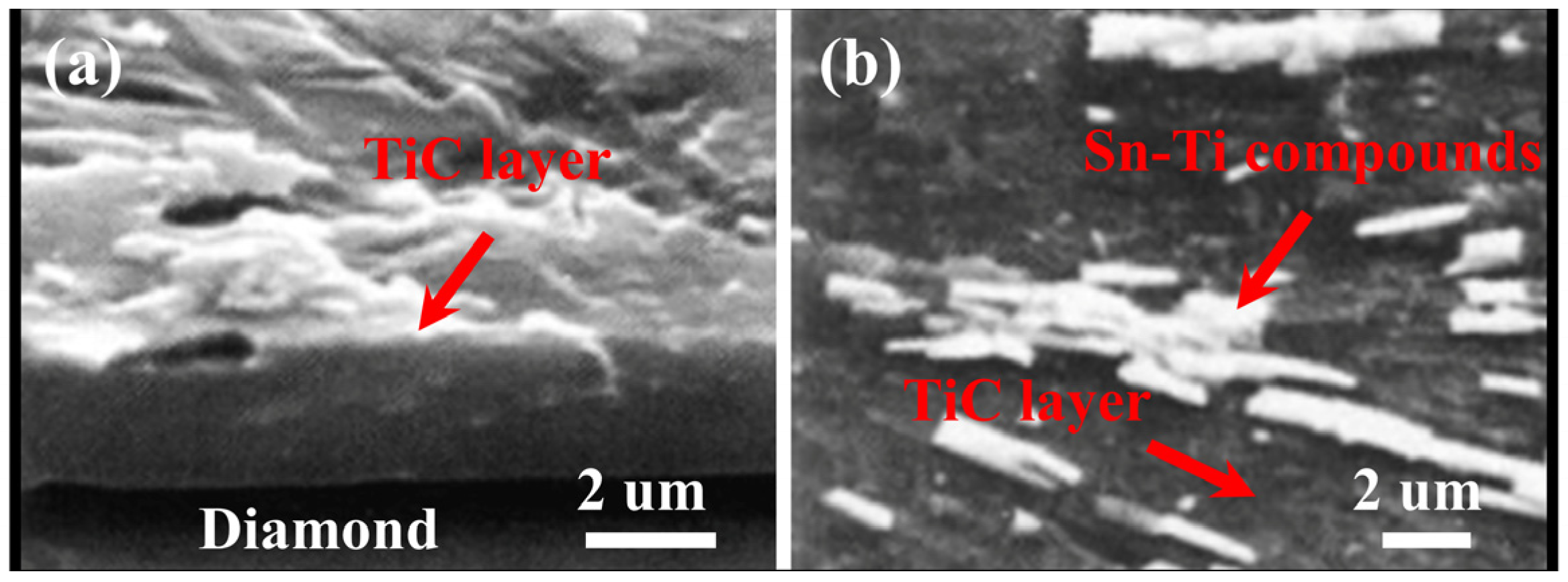
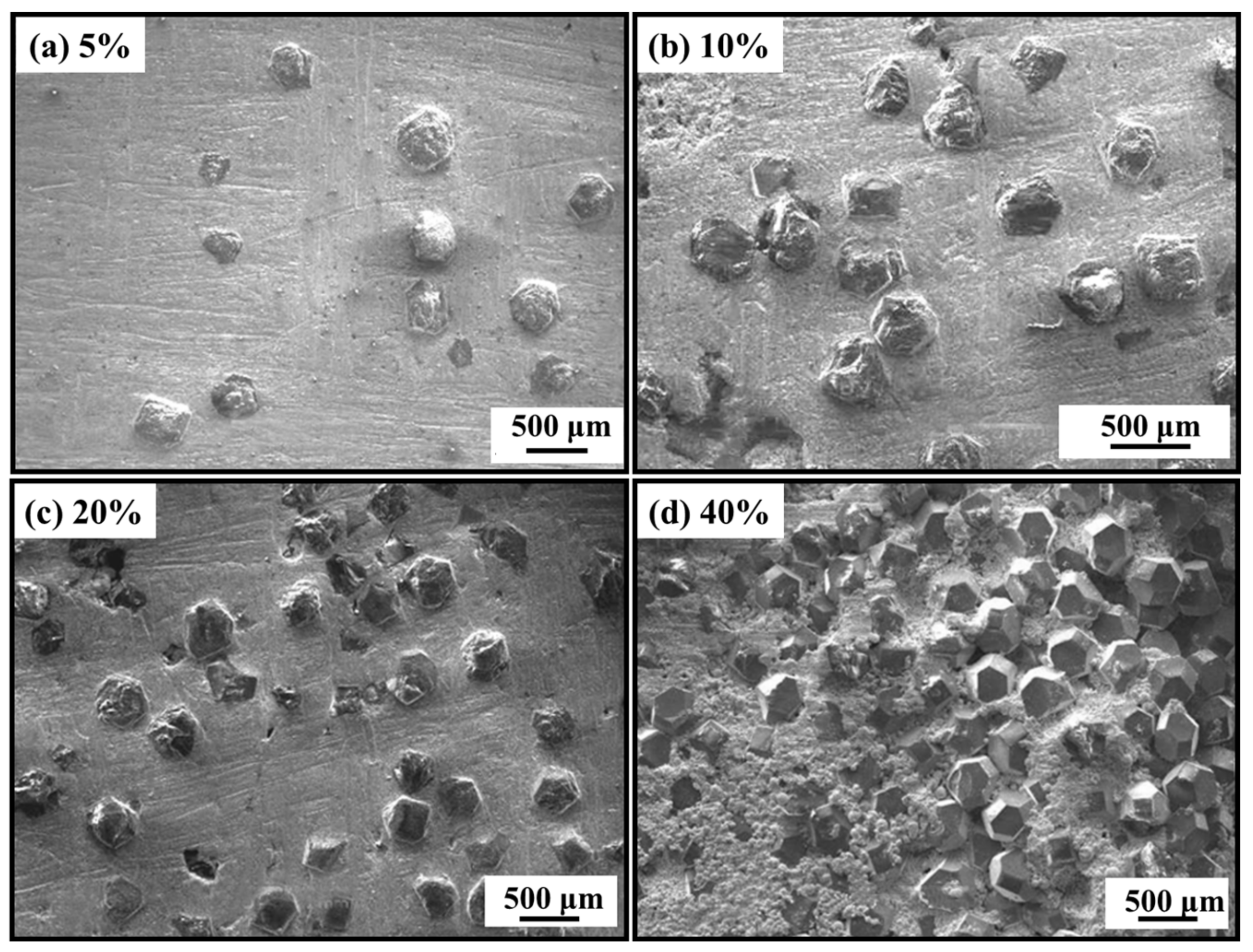
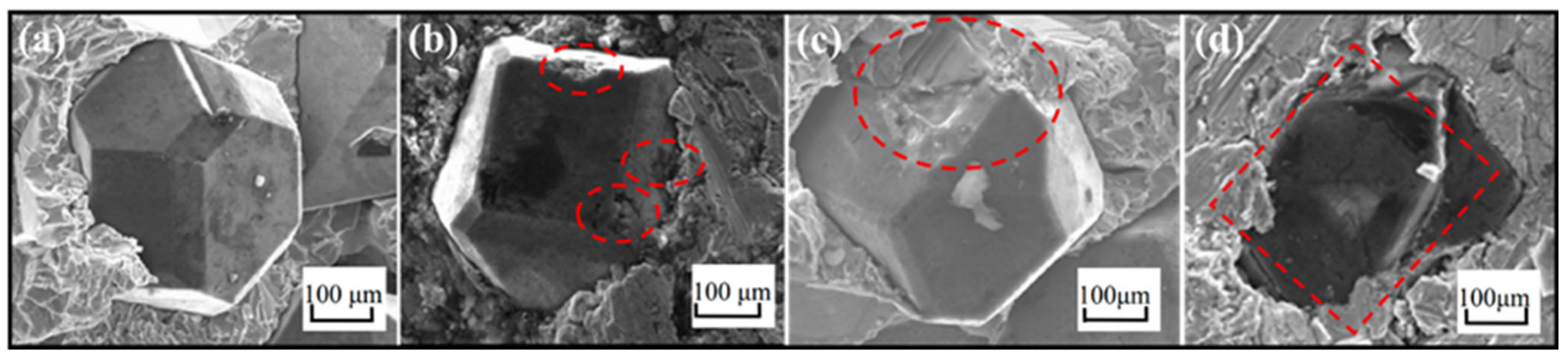
2. Micromorphology of Carbide
2.1. Ni-Based Brazing Alloys
2.2. Ag-Based Brazing Alloys
2.3. Cu-Based Brazing Alloys
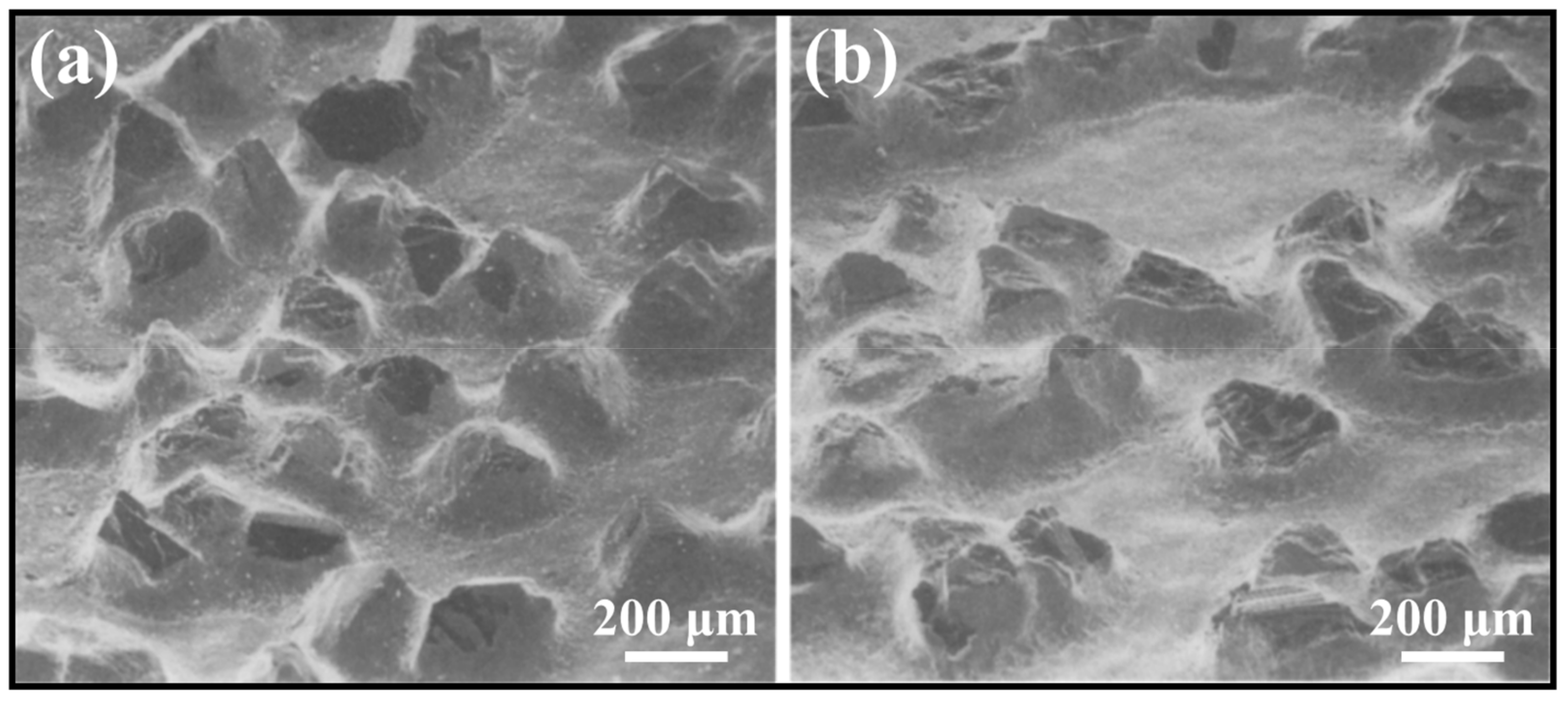
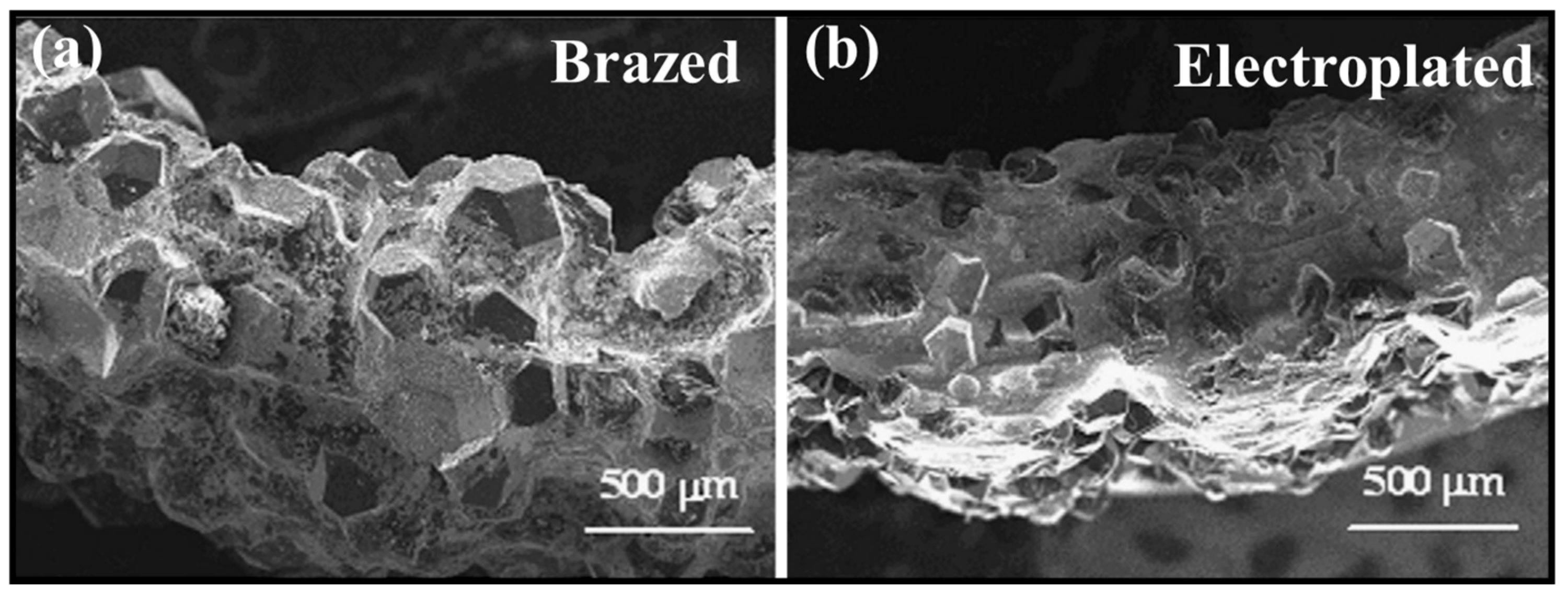
3. Friction and Wear Performance
3.1. Ni-Based Brazing Alloys
3.2. Ag-Based Brazing Alloys
3.3. Cu-Based Brazing Alloys
4. Mechanical Performance
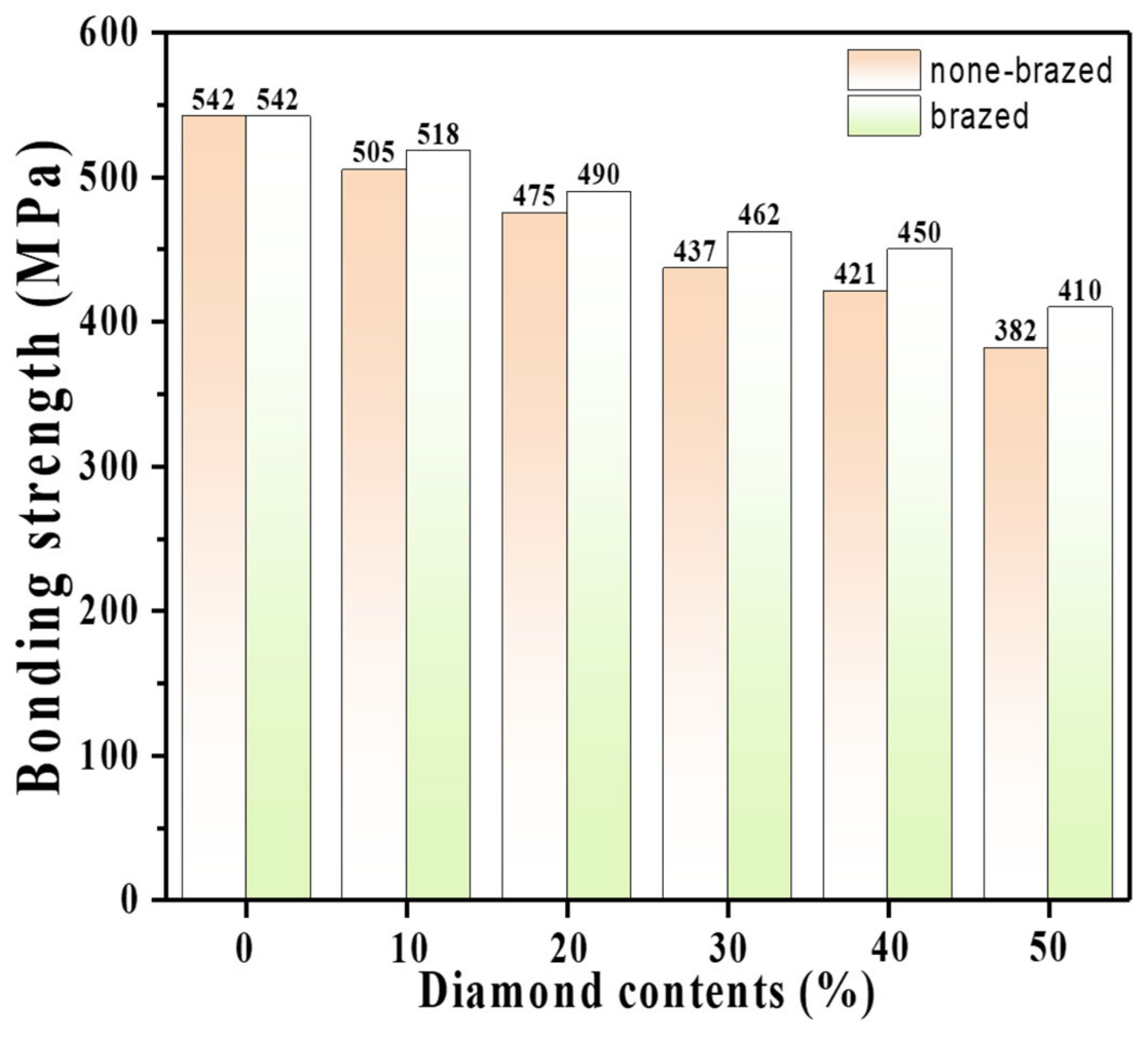
4.1. Strength
4.2. Residual Stress
5. Summary and Prospect
5.1. Summary
- (1)
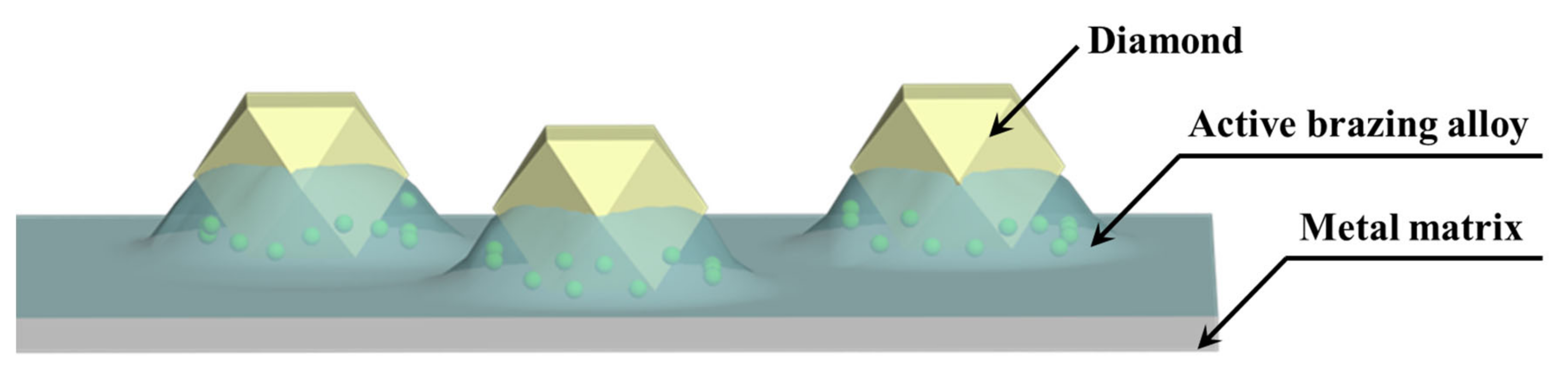
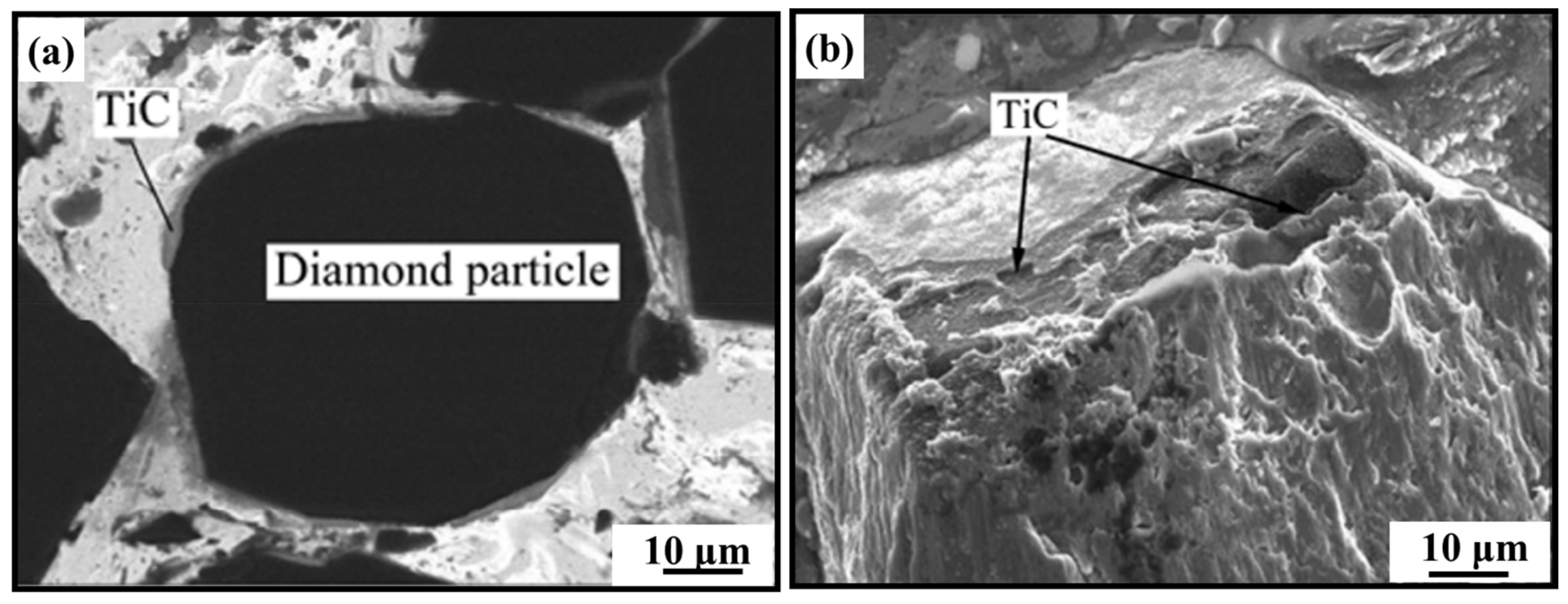
- Micromorphology: Carbide is the key to achieving a high-strength connection between diamond and matrix. The carbide layer formed in the brazing process can act not only as a diffusion barrier to avoid the overreaction between the matrix and diamond particles, but also as a buffer zone to reduce the stress caused by the difference of lattice constant and thermal expansion coefficient between the diamond particles and the bonded matrix, so as to realize the long-term service life of diamond tools. The Cr and Ti elements in the brazing alloys will present segregated preferentially to the diamond surface and form the Cr-rich and Ti-rich reaction product layer. This carbide layer can promote wetting and bonding behaviors by interacting with Cr, Ti, and C atoms on the diamond surface at elevated temperatures. Finally, the high-strength combination of diamond and active brazing alloy and matrix is realized.
- (2)
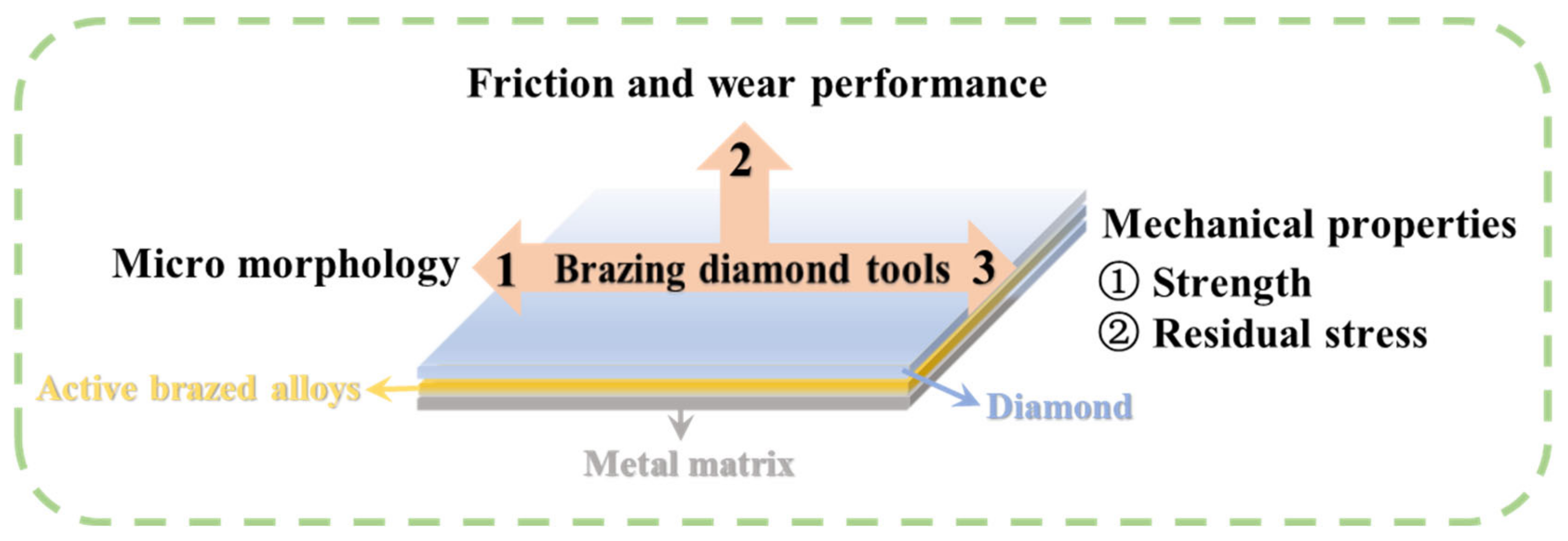
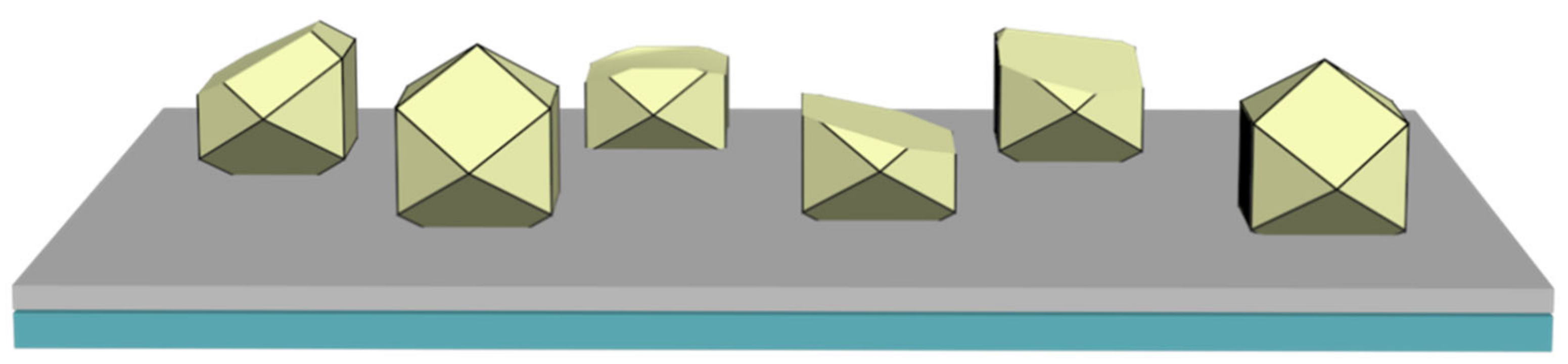
- Friction and wear performance: Wear performance is one of the key evaluation criteria of diamond tools. Wear not only refers to the wear of the diamond itself but also includes the wear of the active brazing alloy. If the wear failure of the diamond is too high, it indicates that the binding force between the diamond and the matrix is weak, and the tool life is too short. Too much wear on the active brazing alloy will cause the tool to break directly from the brazed joint, which is more fatal. Moreover, the protrusion height of a diamond determines the space of its heat dissipation during diamond processing. Too high protrusion will cause premature diamond failure, and too low protrusion will significantly reduce the working efficiency of the tool.
- (3)
- Mechanical performance: Compared with other diamond tools, the distance between adjacent diamond abrasive grains of the brazed diamond is large, which provides more heat dissipation space for the forced convection heat exchange that occurs during the use of this tool, and at the same time, makes the wear resistance of the brazed diamond tools better. Therefore, it is essential to improve the diamond itself to avoid the probability of fatigue cracks and crack propagation when diamond tools are subjected to alternating thermal stress and impact stress, reducing the failure rate of diamond tools. In conclusion, in the future, the mechanized operation should be realized as far as possible to keep the diamond exposure height as consistent as possible. In addition, to avoid severe wear caused by excessive exposure, diamonds must be kept at a certain distance from each other to avoid too small spacing so that the heat convection cannot be fully released. If the spacing is too large, the number of diamond abrasive particles will be reduced, leading to a decrease in the working efficiency of the tool.
5.2. Prospect
- (1)
- Active brazing alloy: For the Ni-based filler metal, the primary function of chromium is to increase the oxidation resistance, corrosion resistance, and high-temperature strength. However, the brazing temperature of the Ni-based filler metal is too high, so other elements, such as silicon, can be considered to reduce the melting point of the filler metal and increase the fluidity.
- (2)
- Micromorphology: The shape of carbide affects the contact area between diamond and brazed alloy and thus affects the bonding strength. All these are attributed to the brazing process parameters. By realizing the automation of the brazing process, the carbide quantity and the carbide layer’s thickness can be controlled to achieve relatively accurate carbide relative values.
- (3)
- Friction and wear performance: According to the difference in wear performance between diamond and active brazing alloy, the matching of substrate material and diamond wear performance can be realized by selecting active brazing alloy and optimizing the formula, so that the diamond can be exposed automatically and evenly during grinding without dressing. In addition, the brazing process should be adjusted to ensure not only the combination of diamond and brazing alloy, but also the strength and wear resistance of the brazing alloy itself.
- (4)
- Mechanical performance: (1) From the perspective of processing, with the increasingly high requirements of efficient and precision processing, the brazing strength of the diamond is not better. When the brazing strength is too large, the abrasive particles of the diamond will not be able to fall off, and the abrasive particles will bring grinding burns, grinding cracks, and other quality problems in processing. Therefore, after determining the range of optimum bonding strength in reverse, more appropriate brazing alloy and brazing process parameters can be further determined according to the application occasions of the tools, so as to satisfy the use breadth and depth of brazed diamond tools from multiple angles. (2) In order to maximize the chemical bonding between diamond and matrix achieved by carbide, the quantity, morphology and thickness of the carbide layer should be optimized by adjusting the brazing parameters. In order to avoid cracking of brazed diamond tools caused by residual stress, the difference in thermal expansion coefficient and lattice parameters between diamond and matrix should be reduced as much as possible to achieve high-strength brazed diamond tool joints under a low-stress state.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riley, D.P. Lattice constant of diamond and the C-C single bond. Nature 1944, 153, 587–588. [Google Scholar] [CrossRef]
- Shen, X.T. Fabrication and evaluation of monolayer diamond grinding tools by hot filament chemical vapor deposition method. J. Mater. Process. Technol. 2019, 265, 1–11. [Google Scholar] [CrossRef]
- Artini, C.; Muolo, M.C.; Passeronr, A. Diamond-metal interfaces in cutting tools: A review. J. Mater. Sci. 2012, 47, 3252–3264. [Google Scholar] [CrossRef]
- Oliveira, L.; Bobrovnitchii, G.S.; Filgueira, M. Processing and characterization of impregnated diamond cutting tools using a ferrous metal matrix. Int. J. Refract. Met. Hard Mater. 2007, 25, 328–335. [Google Scholar] [CrossRef]
- Hwang, T.W.; Evans, C.J.; Malkin, S. High speed grinding of silicon nitride with electroplated diamond wheels, Part 2: Wheel topography and grinding mechanisms. J. Manuf. Sci. Eng. 2000, 122, 42–50. [Google Scholar] [CrossRef]
- Xiao, H.Z.; Xiao, B.; Liu, S.X.; Wu, H.H. Interfacial analysis of vacuum brazing diamond/WC mixed abrasives with Ni-Cr-B-Si active filler. Vacuum 2019, 164, 158–164. [Google Scholar] [CrossRef]
- Flegner, P.; Ján, K.; Durdán, M.; Marek, L.; Beáta, S.; Marcel, P. Significant damages of core diamond bits in the process of rocks drilling. Eng. Fail. Anal. 2016, 59, 354–365. [Google Scholar] [CrossRef]
- Zheng, L.; Huan, H.; Zeng, Y.; Song, S.Y.; Cheng, S.L.; Zhang, C.W. A study on the failure mechanism and wear loss of impregnated diamond bits during machining process of armor ceramics. J. Mech. Sci. Technol. 2018, 32, 261–268. [Google Scholar] [CrossRef]
- Xiao, B.; Xu, H.J.; Fu, Y.C.; Xu, X.P.; Huang, H. Form and distribution characterization of reaction products at the brazing interface between Ni-Cr alloy and diamond. Key Eng. Mater. 2004, 259–260, 151–153. [Google Scholar] [CrossRef]
- Cui, B.; Chen, J.; Li, H.; Zuo, R.Z.; Cheng, Z.; Sun, Z.P.; Li, Y.J.; Wang, B.; Xu, D. Effect of holding time on interfacial reaction layer characteristics and mechanical performance of brazed diamonds with Cu-Sn-Ti-Ga filler metals. Diam. Relat. Mater. 2022, 123, 108826. [Google Scholar] [CrossRef]
- Yin, X.H.; Xu, F.; Min, C.Y.; Dong, Y.F.; Cui, X.; Dong, B.X. Promoting the bonding strength and abrasion resistance of brazed diamond using Cu-Sn-Ti composite alloys reinforced with tungsten carbide. Diam. Relat. Mater. 2021, 112, 108239. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Mao, C.; Hu, Y.; Li, K.; Zhang, J.; Tang, K.; Bi, Z. Microstructure and properties at bonds of diamond grains and Ni Cr filler alloy by fiber laser brazing. Diam. Relat. Mater. 2022, 125, 108969. [Google Scholar] [CrossRef]
- Wang, S.Y.; Xiao, B.; Su, S.C.; Wang, J.L.; Xiao, Y. Interfacial characteristics and thermal damage of brazed W-coated diamond with Ni- based filler alloy. Diam. Relat. Mater. 2021, 116, 108401. [Google Scholar] [CrossRef]
- Liao, X.J.; Mu, D.K.; Fu, W.; Hui, H.A.; Han, H.C. Low-temperature wetting mechanisms of polycrystalline chemical vapor deposition (CVD) diamond by Sn-Ti solder alloys. Mater. Des. 2019, 182, 108039. [Google Scholar] [CrossRef]
- Fu, Y.C.; Xiao, B.; Xu, J.H.; Xu, H.J. Machining performance of monolayer brazed diamond tools. Key Eng. Mater. 2004, 259–260, 73–77. [Google Scholar] [CrossRef]
- Trenker, A.; Seidemann, H. High-vacuum brazing of diamond tools. Ind. Diam. Rev. 2002, 62, 49–51. [Google Scholar]
- Chen, Y.; Fu, Y.; Su, H.; Xu, J.; Xu, H. The effects of solder alloys on the morphologies and mechanical properties of brazed diamond grits. Int. J. Refract. Met. Hard Mater. 2014, 42, 23–29. [Google Scholar] [CrossRef]
- Khalid, F.A.; Klotz, U.E.; Elsener, H.R.; Zigerlig, B.; Gasser, P. On the interfacial nanostructure of brazed diamond grits. Scr. Mater. 2004, 50, 1139–1143. [Google Scholar] [CrossRef]
- Naidich, Y.V.; Kolesnichenko, G.A. Investigation of the wetting of diamond and graphite by molten metals and alloys. Sov. Powder Metall. Met. Ceram. 1966, 5, 156–158. [Google Scholar] [CrossRef]
- Huang, S.F.; Tsai, H.L.; Lin, S.T. Effects of brazing route and brazing alloy on the interfacial structure between diamond and bonding matrix. Mater. Chem. Phys. 2004, 84, 251–258. [Google Scholar] [CrossRef]
- Elsener, K.R.; Khalid, F.A.; Elsener, H.R. Nanocrystalline phases and epitaxial interface reactions during brazing of diamond grits with silver based Incusil-ABA alloy. Diam. Relat. Mater. 2006, 15, 1520–1524. [Google Scholar] [CrossRef]
- Chattopadhyay, A.K.; Chollet, L.; Hintermann, H.E. On performance of brazed bonded monolayer diamond grinding wheel. CIRP Ann. -Manuf. Technol. 1991, 40, 347–350. [Google Scholar] [CrossRef]
- Sung, M.; Sung, J.C. The brazing of diamond. Diam. Abras. Eng. 2008, 27, 134–142. [Google Scholar] [CrossRef]
- Li, W.C.; Liang, C.; Lin, S.T. Epitaxial interface of nanocrystalline TiC formed between Cu-10Sn-15Ti alloy and diamond. Diam. Relat. Mater. 2002, 11, 1366–1373. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhou, Y.M.; Zhang, F.L.; Xu, Z.C. Interfacial microstructure and performance of brazed diamond grits with Ni–Cr–P alloy. J. Alloy. Compd. 2009, 476, 884–888. [Google Scholar] [CrossRef]
- Meng, W.; Xu, K.; Nan, J.; He, L. Monolayer diamond tools brazed with active filler. Rare Met. Mater. Eng. 2004, 7, 771–774. (In Chinese) [Google Scholar]
- Chattopadhyay, A.K.; Roy, P.; Sarangi, S.K. Study of wettability test of pure aluminum against uncoated and coated carbide inserts. Surf. Coat. Technol. 2009, 204, 410–417. [Google Scholar] [CrossRef]
- Chen, N.Y.Q. Effects of brazing temperature and holding time on wettability of brazing diamond and brazing interface analysis. Weld. World 2020, 64, 1763–1770. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Xu, H.J.; Fu, Y.C.; Xiao, B.; Xu, J.H. Induction brazing diamond grinding wheel with Ni-Cr filler alloy. Mater. Sci. Forum. Trans. Tech. Publ. 2006, 532, 377–380. [Google Scholar] [CrossRef]
- Zhan, Y.J.; Li, Y.; Huang, H.; Xu, X.P. Effects of the wear characteristics of brazed diamond grits on grinding forces. Adv. Mater. Res. 2007, 24–25, 233–238. [Google Scholar] [CrossRef]
- Bing, K.; Eigenmann, B.; Scholtes, B.; Macherauch, E. Brazing residual stresses in components of different metallic materials. Mater. Sci. Eng. A 1994, 174, 95–101. [Google Scholar] [CrossRef]
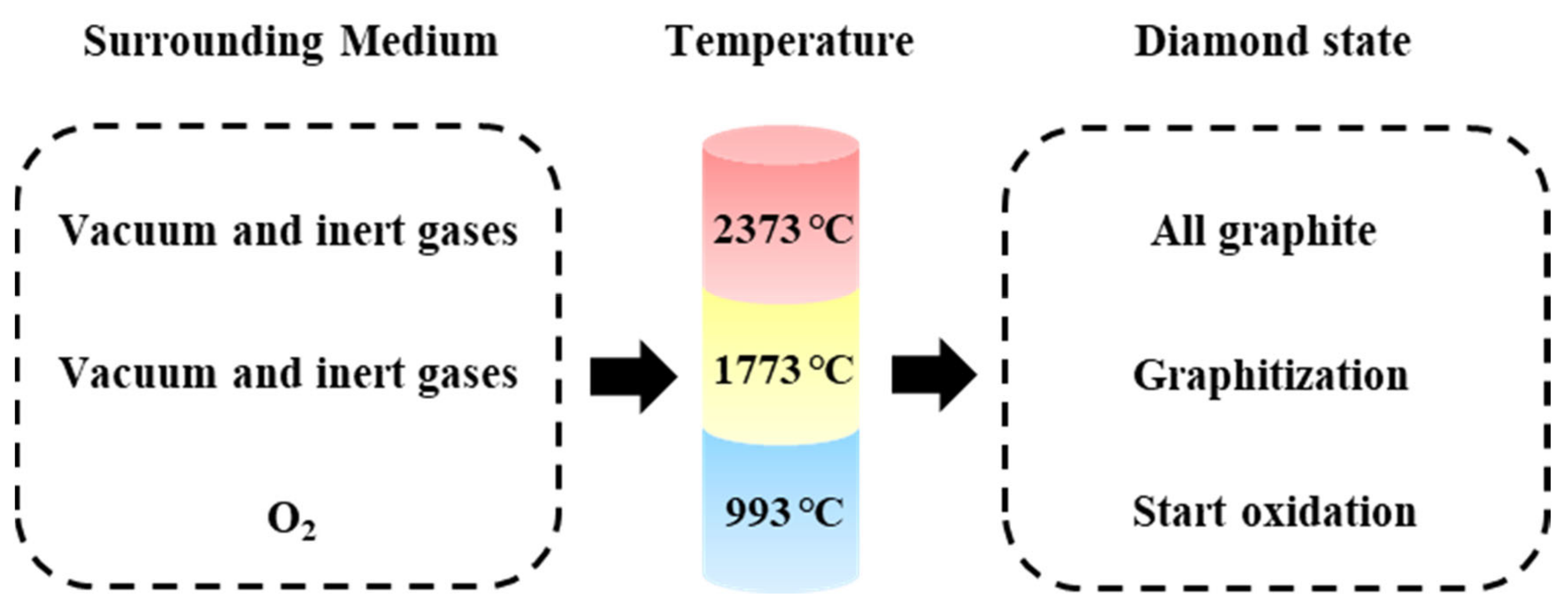
- Gippius, A.A.; Khmelnitsky, R.A.; Dravin, V.A.; Khomich, A.V. Diamond-graphite transformation induced by light ions implantation. Diam. Relat. Mater. 2003, 12, 538–541. [Google Scholar] [CrossRef]
- Dbs, A.; Fb, B.; Jp, B.; Felix, P.; Jan, G.; Juan, M.S.; Hwirim, F.; Saleh, B.; Andreas, V.; Kerstin, P.; et al. Monitoring the thermally induced transition from sp3-hybridized into sp2 hybridized carbons. Carbon 2020, 172, 214–227. [Google Scholar] [CrossRef]
- Sun, B.; Meng, P.; Lu, J.B.; Zhao, B.; Liu, J.J. Influence of brazing time on microstructure of high temperature brazing diamond with Ni-Cr alloy. Adv. Mater. Res. 2012, 452–453, 628–632. [Google Scholar] [CrossRef]
- Lu, J.; Cao, Z.; Qi, F.; Qian, M.; Zhang, W. Evolution of interface carbide diamond brazed with filler alloy containing Cr. Diam. Relat. Mater. 2018, 90, 116–125. [Google Scholar] [CrossRef]
- Onzawa, T.; Suzumura, A.; Ko, M.W. Microstructure of titanium joint made with Ti-Zr base brazing fillers. Q. J. Jpn. Weld. Soc. 1989, 7, 455–460. [Google Scholar] [CrossRef]
- Huang, G.Q.; Chen, H.J.; Huang, H.; Xu, X.P. Wear of a brazed diamond grinding wheel with diamond grits covered with brazing alloy. Adv. Mater. Res. 2009, 76–78, 125–130. [Google Scholar] [CrossRef]
- Song, W.Y.; Liu, F.B.; Bu, C.G.; Zhou, G.D. Analysis on the thermal stress of brazed diamond bit during cooling process. Procedia Eng. 2014, 73, 48–54. [Google Scholar] [CrossRef]
- Zaharinie, T.; Aliff, A.; Hamdi, M.; Ariga, T. Brazing diamond grits onto stainless steel using active filler metal and porous nickel as an interlayer: Analysis of the porous nickel/stainless steel interface. IOP Conf. Ser. Mater. Sci. Eng. 2019, 473, 012005. [Google Scholar] [CrossRef]
- Yang, Z.B.; Xu, J.H.; Liu, A.J. Analysis on microstructure of laser brazing diamond grits with a Ni-based filler alloy. Adv. Mater. Res. 2010, 97–101, 3879–3883. [Google Scholar] [CrossRef]
- Ma, B.J.; Xu, Z.A. Research on high-powered brazing diamond saw for cutting vehicle tyre. Adv. Mater. Res. 2010, 87–88, 52–57. [Google Scholar] [CrossRef]
- Lu, J.B.; Xi, Y.J.; Wang, Z.X. Carbide growth and orientation relationship of vacuum brazing diamond with Ni-Cr alloy. Chin. J. Nonferrous Met. 2010, 20, 137–142. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.H.; Fu, Y.C.; Su, H.H. Thermal damage of diamond grits during the brazing process with Ni-Cr alloy. Solid State Phenom. 2011, 175, 22–26. [Google Scholar] [CrossRef]
- Ewels, C.P.; Wilson, N.T.; Heggie, M.I.; Jones, R.; Briddon, P.R. Graphitization at diamond dislocation cores. J. Phys. Condens. Matter 2001, 13, 8965–8972. [Google Scholar] [CrossRef]
- Yang, Z.B.; Xu, J.H.; Liu, A.J. Delaminating behavior and formation mechanism of the interfacial microstructure in the laser brazing diamond with Ni-Cr alloys. Chin. J. Lasers 2009, 36, 3079–3083. [Google Scholar] [CrossRef]
- Xiao, H.Z.; Xiao, B.; Wu, H.H. Interfacial characteristics and mechanical properties of the vacuum brazing diamond grains segment with Ni-Cr composite active filler and tungsten carbide reinforcement. J. Alloy. Compd. 2019, 781, 226–234. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhang, J.; Zhang, M.J.; Peng, P. First-principles calculations on brazed diamond with FeCoCrNi high entropy alloys doped with strong carbide-forming elements. Solid State Commun. 2022, 357, 114980. [Google Scholar] [CrossRef]
- Barin, I.; Knacke, O. Thermochemical properties of inorganic substances, Forsch. Ingenieurwes 1992, 58, 103. [Google Scholar] [CrossRef]
- Jin, H.X.; Ji, H.H.; Hua, Z.; Xing, K.Z. Brazing of carbon fiber reinforced SiC composite and TC4 using Ag-Cu-Ti active brazing alloy. Mater. Sci. Eng. A 2010, 527, 1096–1101. [Google Scholar] [CrossRef]
- Li, W.C.; Lin, S.T.; Liang, C. Interfacial segregation of Ti in the brazing of diamond grits onto a steel substrate using a Cu-Sn-Ti brazing alloy. Metall. Mater. Trans. A 2002, 33, 2163–2172. [Google Scholar] [CrossRef]
- Fujihana, T.; Okabe, Y.; Iwaki, M. Crystal structure of carbon-implanted titanium, vanadium and chromium. Nucl. Instrum. Methods Phys. Res. 1997, 127, 660–663. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Ghosh, A. Development and quality assessment of multi-point brazed diamond dressers produced by active brazing under high vacuum. Int. J. Adv. Manuf. Technol. 2018, 99, 647–662. [Google Scholar] [CrossRef]
- Palavra, A.; Fernandes, J.A.S.; Serra, C.; Costa, F.M.; Rocha, L.A.; Silva, R.F. Wettability studies of reactive brazing alloys on CVD diamond plates. Diam. Relat. Mater. 2001, 10, 775–780. [Google Scholar] [CrossRef]
- Lu, J.B.; Xu, J.H. Microstructure of interface bet ween Ag-Cu-Ti brazing filler metal and diamond. Trans. China Weld. Inst. 2007, 28, 4. (In Chinese) [Google Scholar] [CrossRef]
- Lu, J.; Xu, J. Interface Microstructure and Thermal Stress of Diamond Brazing with Ag-Cu-Ti Filler. Rare Met. Mater. Eng. 2009, 38, 642–646. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Zhang, F.L.; Fu, K.X.; Wang, C.Y. Brazing of diamond grits with Ag-Cu-Zn alloy activated by Cr or Ti powder under different environments. Int. J. Precis. Technol. 2009, 1, 139–146. [Google Scholar] [CrossRef]
- Wu, M.; Cao, C.Z.; He, X.B.; Qu, X.H. Brazing diamond/Cu composite to alumina using reactive Ag-Cu-Ti alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 1701–1708. [Google Scholar] [CrossRef]
- Qilin, L.; Honghua, S.; Jiuhua, X.; Lei, W.N. Interfacial microstructure and residual stress of continuously brazed diamond with Ag-Cu-Ti alloy using ultra-high frequency induction. Rare Met. Mater. Eng. 2016, 45, 3250–3254. (In Chinese) [Google Scholar]
- Chen, Y.; Su, H.H.; Fu, Y.C.; Guo, Z.C. Investigation of interface microstructure of diamond and ti coated diamond brazed with Cu-Sn-Ti Alloy. Key Eng. Mater. 2011, 487, 199–203. [Google Scholar] [CrossRef]
- Guo, Z.C.; Chen, Y.; Hong-Hua, S.U.; Fu, Y.C. Microstructure for Interface between Diamond and 45 Steel Vacuum-Brazed by Cu-Sn-Ti Alloy. Front. Environ. Sci. Eng. 2012, 36, 20–23. [Google Scholar] [CrossRef]
- Duan, D.Z.; Xiao, B.; Wang, B.; Han, P.; Li, W.J.; Xia, S.W. Microstructure and mechanical properties of pre-brazed diamond abrasive grains using Cu-Sn-Ti alloy. Int. J. Refract. Met. Hard Mater. 2015, 48, 427–432. [Google Scholar] [CrossRef]
- Liu, S.X.; Xiao, B.; Zhang, Z.Y.; Duan, D.Z. Microstructural characterization of diamond/CBN grains steel braze joint interface using Cu-Sn-Ti active filler alloy. Int. J. Refract. Met. Hard Mater. 2016, 54, 54–59. [Google Scholar] [CrossRef]
- Si, S.H.; Ding, Z.H.; Zuo, R.C.; Bing, C.A.; Dong, X.A. Adding Hf element to improve the strength and wear resistance of diamond brazed with Ni-based boron-free brazing filler metal. Diam. Relat. Mater. 2022, 121, 108723. [Google Scholar] [CrossRef]
- Shen, J.; Li, L.; Wu, X.; Chen, X.; Huang, H.K.; Mu, W.Z.; De, K. Interfacial characteristics of titanium coated micro-powder diamond abrasive tools fabricated by electroforming-brazing composite process. Int. J. Refract. Met. Hard Mater. 2019, 84, 104973. [Google Scholar] [CrossRef]
- Tönshoff, H.K.; Denkena, B.; Apmann, H.H.; Asche, J. Diamond tools in stone and civil engineering industry: Cutting principles, wear and applications. Diam. Relat. Mater. 2003, 11, 736–741. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Simhan, D.R.; Ghosh, A. Challenges in brazing large synthetic diamond grit by Ni-based filler alloy. J. Mater. Process. Technol. 2017, 250, 390–400. [Google Scholar] [CrossRef]
- Brinksmeier, E.; Gläbe, R.; Osmer, J. Ultra-Precision Diamond Cutting of Steel Molds. CIRP Ann.-Manuf. Technol. 2006, 55, 551–554. [Google Scholar] [CrossRef]
- Klotz, U.E.; Liu, C.; Khalid, F.A.; Elsener, H.R. Influence of brazing parameters and alloy composition on interface morphology of brazed diamond. Mater. Sci. Eng. A 2008, 495, 265–270. [Google Scholar] [CrossRef]
- Kizikov, É.D.; Kebko, V.P. Microadditions to alloys of the system Cu-Sn-Ti. Met. Sci. Heat Treat. 1987, 29, 68–71. [Google Scholar] [CrossRef]
- Sun, B.J.; Jiang, C.J.; Zong, F.L. Performance and wear of brazing diamond grinding disc in machining gray cast iron. Diam. Relat. Mater. 2020, 106, 107820. [Google Scholar] [CrossRef]
- Lane, B.M.; Dow, T.A.; Scattergood, R. Thermo-chemical wear model and worn tool shapes for single-crystal diamond tools cutting steel. Wear 2013, 300, 216–224. [Google Scholar] [CrossRef]
- Dda, B.; Feng, H.A.; Ding, J.J.; Lin, Q.J.; Li, C.S.; Wang, C.Y.; Jiang, Z.D. Microstructure and performance of brazed diamonds with multilayer graphene-modified Cu–Sn–Ti solder alloys. Ceram. Int. 2021, 47, 22854–22863. [Google Scholar] [CrossRef]
- Chattopadhyay, A.K.; Chollet, L.; Hintermann, H.E. Experimental investigation on induction brazing of diamond with Ni-Cr hardfacing alloy under argon atmosphere. J. Mater. Sci. 1991, 26, 5093–5100. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Zhang, F.L.; He, M.J.; Huang, H.P. Effect of arraying patterns of diamond grits on the wear of the mono-layer brazed diamond tool. Solid State Phenom. 2011, 175, 47–51. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Ghosh, A. On bond wear, grit-alloy interfacial chemistry and joint strength of synthetic diamond brazed with Ni-Cr-B-Si-Fe and Ti activated Ag-Cu filler alloys. Int. J. Refract. Met. Hard Mater. 2017, 72, 236–243. [Google Scholar] [CrossRef]
- Sun, F.; Feng, J.; Dan, L. Bonding of CVD diamond thick films using an Ag-Cu-Ti brazing alloy. J. Mater. Process. Technol. 2001, 115, 333–337. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, Y.; Xu, H. Experimental research on wear resistance of brazed diamond with Ag-base solder. Diam. Abras. Eng. 2008, 166, 35–39. (In Chinese) [Google Scholar] [CrossRef]
- Yang, Z. Effect of diamond content on abrasive resistance of brazing filler metal. Hot Work. Technol. 2019, 48, 226–228. (In Chinese) [Google Scholar]
- Lu, J.B.; Li, H.; Li, Y.; Zheng, G. Study on diamond vacuum brazed with Cu-based filler metal containing Cr. Int. J. Adv. Manuf. Technol. 2017, 91, 1453–1460. [Google Scholar] [CrossRef]
- Huang, S.F.; Tsai, H.L.; Lin, S.T. Laser brazing of diamond grits using a Cu-15Ti-10Sn brazing alloy. Mater. Trans. 2002, 43, 2604–2608. [Google Scholar] [CrossRef]
- Wu, Q.P. Effect of brazing atmosphere on brazing performance of diamond. J. Mech. Eng. 2012, 48, 51. [Google Scholar] [CrossRef]
- Zhu, W.J.; Wang, J.; Liu, L.B.; Liu, S.H. Modeling and simulation of the TiC reaction layer growth during active brazing of diamond using DICTRA. Comput. Mater. Sci. 2013, 78, 74–82. [Google Scholar] [CrossRef]
- Feng, X.U. Thermal residual stress of polycrystalline diamond compacts. Trans. Nonferrous Met. Soc. China 2010, 20, 227–232. [Google Scholar] [CrossRef]
- Kim, J.G.; Jin, Y. Behavior of residual stress on CVD diamond films. Mater. Sci. Eng. B 1998, 57, 24–27. [Google Scholar] [CrossRef]
- Buhl, S.; Leinenbach, C.; Spolenak, R.; Konrad, W. Microstructure, residual stresses and shear strength of diamond-steel-joints brazed with a Cu-Sn-based active filler alloy. Int. J. Refract. Met. Hard Mater. 2012, 30, 16–24. [Google Scholar] [CrossRef]
- Buhl, S.; Leinenbach, C.; Spolenak, R.; Konrad, W. Influence of the brazing parameters on microstructure, residual stresses and shear strength of diamond–metal joints. J. Mater. Sci. 2010, 45, 4358–4368. [Google Scholar] [CrossRef]
- Mao, Y.M.; Gao, J.; Zheng, K.; Bai, J.; Xue, Y.P.; Yu, S.W.; Wang, Y.; Hei, H.J.; Wang, W.B. Optimization for solderability of large-size single-crystal diamond with heterogeneity alloys by tantalum coating. Surf. Coat. Technol. 2022, 437, 128382. [Google Scholar] [CrossRef]
- Duan, Z.; Duan, B.; Xiao, B.; Wang, W.; Zhang, Z.Y.; Wang, B.; Han, P.; Ding, X.Y. Interface characteristics and performance of pre-brazed diamond grains with Ni–Cr composite alloy. J. Alloy. Compd. 2015, 644, 626–631. [Google Scholar] [CrossRef]
- Qi, W.; Lu, J.; Li, Y.; Qi, W.C.; Lu, J.B.; Yang, L.; Xu, S. Vacuum brazing diamond grits with Cu-based or Ni-based filler metal. J. Mater. Eng. Perform. 2017, 26, 4112–4120. [Google Scholar] [CrossRef]
- Akbari, M.; Buhl, S.; Leinenbach, C.; Spolenak, R.; Wegener, K. Thermomechanical analysis of residual stresses in brazed diamond metal joints using Raman spectroscopy and finite element simulation. Mech. Mater. 2012, 52, 69–77. [Google Scholar] [CrossRef]









| Elements | Measuring Temperature/ °C | Contact Angle/° | |
|---|---|---|---|
| Elementary substance | Contact angle of common metals on the diamond surface | ||
| Ag | 1000 | 120 | |
| Cu | 1150 | 145 | |
| Alloys containing active elements | Wetting angle of the alloys containing active elements on the diamond surface | ||
| Ag + 0.5%Ti | 1000 | 45 | |
| Ag + 2Ti | 1000 | 5 | |
| Cu + 6%Ti | 1150 | 30 | |
| Cu + 8%Ti | 1150 | 15 | |
| Cu + 10%Ti | 1150 | 0 | |
| Cu + 0.5%Cr | 1150 | 22 | |
| Cu + 2%Cr | 1150 | 20 | |
| Cu + 5%Cr | 1150 | 0 | |
| Cu + 10%Cr | 1250 | 0 | |
| Cu + 50%Cr | 1150 | 22 | |
| Response Equation | ∆G0/(kJ·mol−1) |
|---|---|
| C(Diamond)→C(Graphite) | −6.028 |
| 3Cr + 2C(Diamond)→Cr3C2 | −109.929 |
| 3Cr + 2C(Graphite)→Cr3C2 | −97.874 |
| 7Cr + 3C(Diamond)→Cr7C3 | −221.455 |
| 7Cr + 3C(Graphite)→Cr7C3 | −203.372 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Mao, Y.; Zhang, M.; Ye, N.; Dai, S.; Zhu, L. Quality Evaluation System of Monolayer Brazed Diamond Tools: A Brief Review. Coatings 2023, 13, 565. https://doi.org/10.3390/coatings13030565
Wang J, Mao Y, Zhang M, Ye N, Dai S, Zhu L. Quality Evaluation System of Monolayer Brazed Diamond Tools: A Brief Review. Coatings. 2023; 13(3):565. https://doi.org/10.3390/coatings13030565
Chicago/Turabian StyleWang, Jinfang, Yamei Mao, Meng Zhang, Nengyong Ye, Sheng Dai, and Liu Zhu. 2023. "Quality Evaluation System of Monolayer Brazed Diamond Tools: A Brief Review" Coatings 13, no. 3: 565. https://doi.org/10.3390/coatings13030565
APA StyleWang, J., Mao, Y., Zhang, M., Ye, N., Dai, S., & Zhu, L. (2023). Quality Evaluation System of Monolayer Brazed Diamond Tools: A Brief Review. Coatings, 13(3), 565. https://doi.org/10.3390/coatings13030565






