Abstract
Biosensors use biological materials, such as enzymes, antibodies, or DNA, to detect specific analytes. These devices have numerous applications in the health and food industries, such as disease diagnosis, food safety monitoring, and environmental monitoring. However, the production of biosensors can result in the generation of chemical waste, which is an environmental concern for the developed world. To address this issue, researchers have been exploring eco-friendly alternatives for immobilising biomolecules on biosensors. One solution uses bio-coatings derived from nanoparticles synthesised via green chemistry and biopolymers. These materials offer several advantages over traditional chemical coatings, such as improved sensitivity, stability, and biocompatibility. In conclusion, the use of bio-coatings derived from green-chemistry synthesised nanoparticles and biopolymers is a promising solution to the problem of chemical waste generated from the production of biosensors. This review provides an overview of these materials and their applications in the health and food industries, highlighting their potential to improve the performance and sustainability of biosensors.
1. Introduction
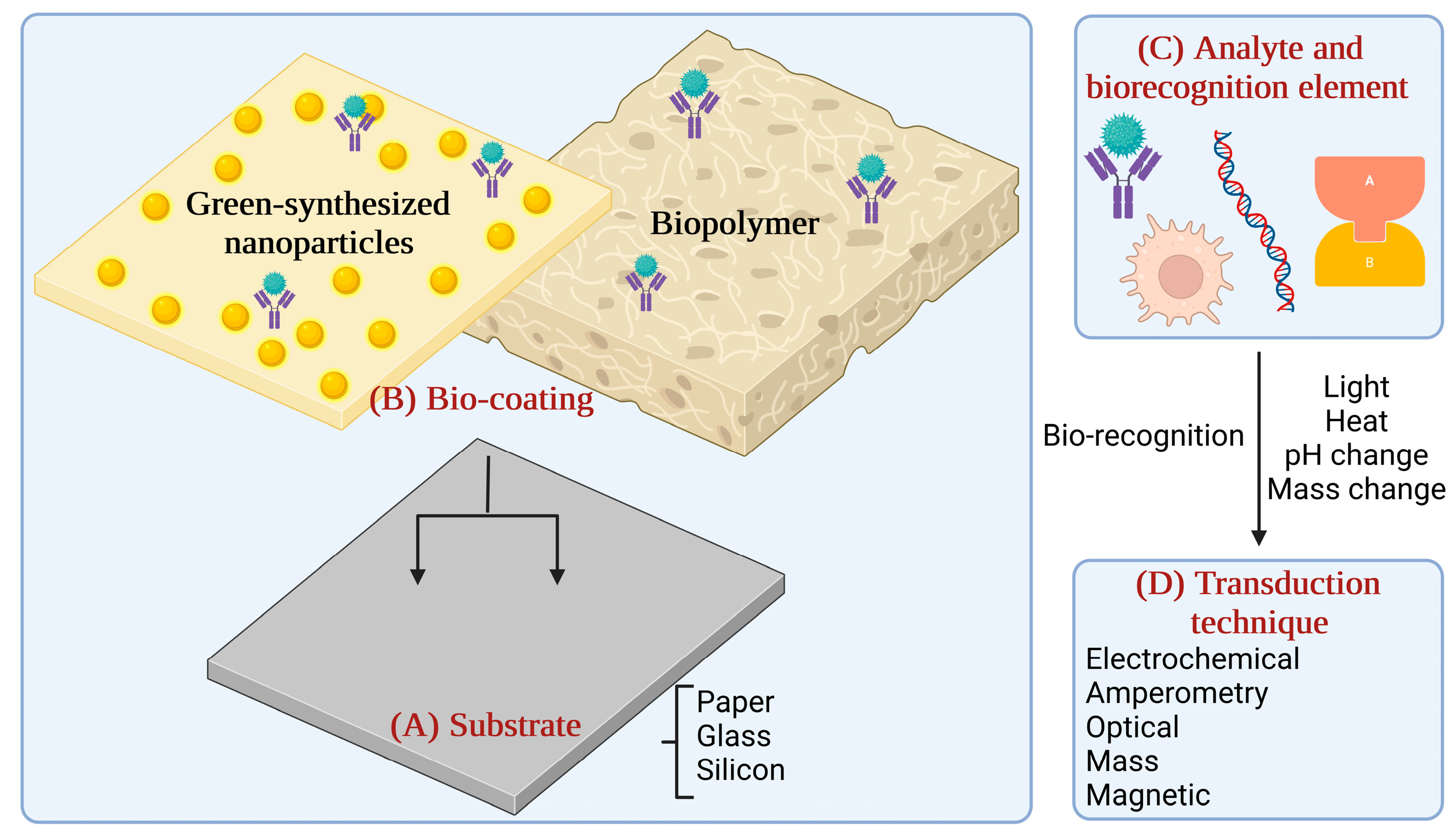
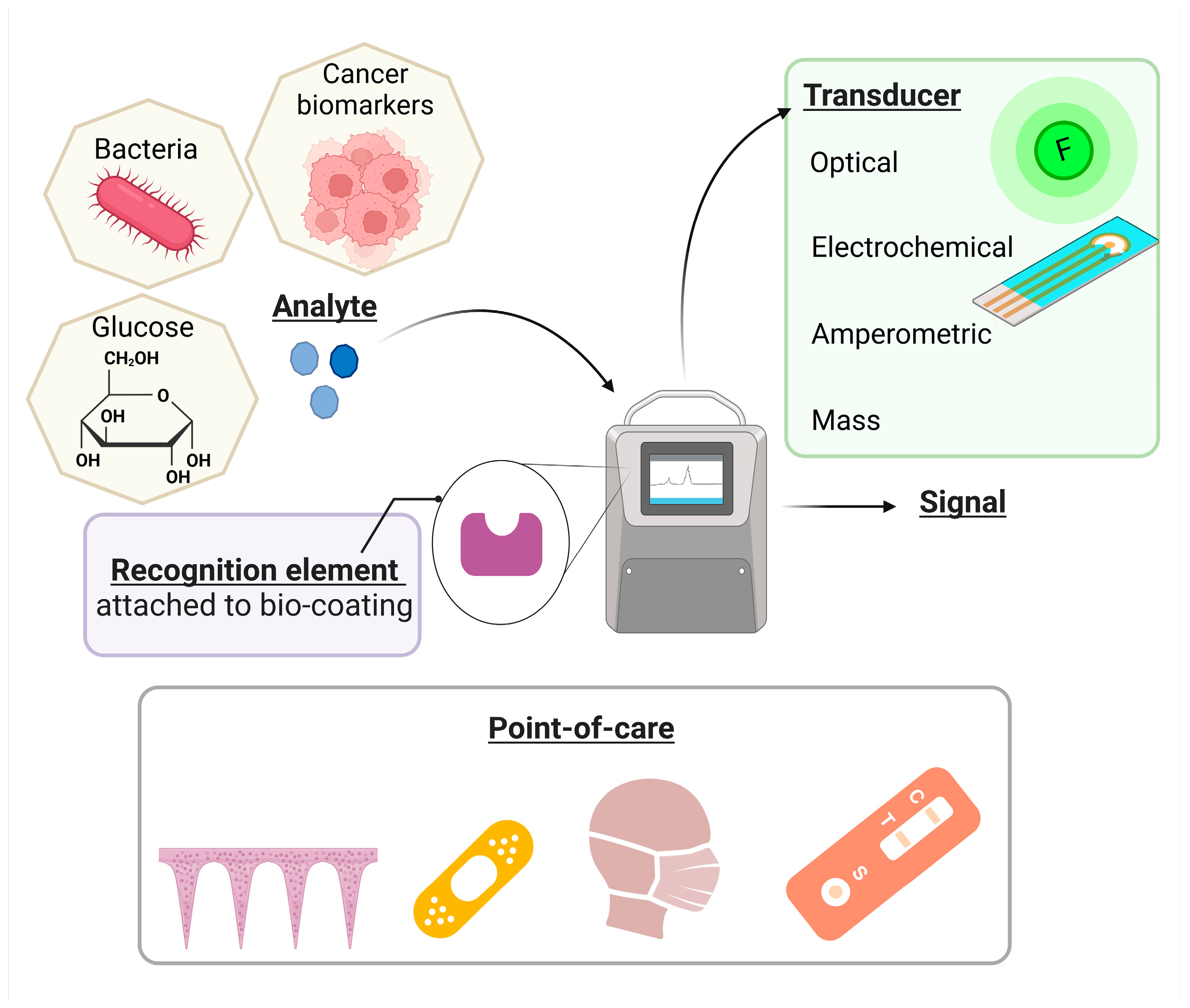
In the digital era, regardless of all the scientific advancements, we are witnessing profound climate change, the emergence of new diseases, and the extinction of many animal and plant species due to household and industrial pollution [1,2]. The use of nanoparticles and polymers in developing biosensors has improved their performance and sensitivity [3,4]. However, their chemical synthesis generates by-products in the environment [5,6]. The green-chemistry and white biotechnology domains explore alternatives to reduce the environmental impact across the whole value chain, including food and medical industries [7,8]. Figure 1 presents the elements of a biosensor, with emphasis on the coating choice, which is the main subject of this review.
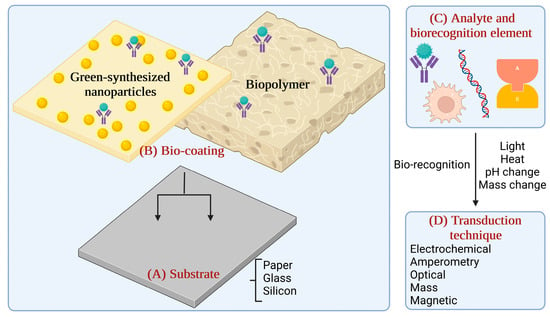
Figure 1.
Schematic of biosensing elements. (A) Substrate; (B) Bio-coating; (C) Analyte and biorecognition element, and (D) Transduction technique (the graphical illustration has been created with BioRender software—BioRender Company, Toronto, ON, Canada).
Briefly, biosensors are devices used to analyse the concentration of a specific target component with the help of a sensitive biological element. They can detect, record, and transmit selective, quantitative, or semi-quantitative analytical information about biochemical reactions [9]. A biosensor includes a substrate, typically paper, glass, or silicon. The substrate must be chemically modified to ensure an effective immobilisation of the bio-recognition elements. The biorecognition elements can be organic components (enzymes, antibodies, hormones, or nucleic acids), biological material (microorganisms, cellular organelles, tissues, or receptor cells), biologically derived material, or biomimetic components. Analyte detection is possible with a transducer which translates the biological response into a quantifiable signal (optical, electrochemical, amperometry) [10,11].
One of the critical challenges in biosensor development is the immobilisation and stabilisation of the biological component on the transducer surface, which can affect the sensor’s sensitivity, selectivity, stability, and reproducibility [12,13]. To address these challenges, bio-coatings have emerged as a promising approach for the functionalisation of biosensors. Bio-coatings refer to a wide range of natural and synthetic materials that can be utilised to immobilise and protect the biological component, enhance its performance, and prevent non-specific interactions with the sample matrix [14]. In this regard, green-synthesised metallic nanoparticles, and biopolymers, in which our group has significant expertise [15,16,17,18,19,20,21,22,23], were explored as ecologically safe alternatives for bio-coatings, and the role they adopt in biosensing applications in the health and food industries. In healthcare, bio-coatings can be employed to modify the surface of biosensors for specific binding to disease markers or pathogens. For example, bio-coatings containing antibodies or peptides can be used to detect specific proteins in the blood, saliva, or urine, enabling early diagnosis of various diseases. Additionally, bio-coatings can be used to enhance the biocompatibility and stability of implantable biosensors for long-term monitoring of health parameters [24].
In the food industry, bio-coatings can be utilised to modify the surface of biosensors for the detecting food contaminants (e.g., bacteria, toxins, chemicals), or can serve as food spoilage indicators (e.g., volatile organic compounds, pH). Bio-coatings can also improve biosensors’ stability and shelf-life, allowing for more reliable and accurate food quality control [25].
The biological synthesis (Table 1) of NPs is a bottom-up approach that involves utilising bacteria, fungi, algae, and plant-derived materials [26,27]. The microbial-mediated synthesis of nanoparticles involves extracellular or intracellular culture filtrates employed as a reducing agent for nanoparticles production. Members of the Monera and Fungi kingdoms possess metallothioneins which make them capable of tolerating, accumulating, and converting metals into metal ions [28]. For instance, Beveridge and Murray (1980) were the first to synthesise gold nanoparticles from Bacillus subtilis [29]. Microorganisms were also employed to synthesise Ag, Pt, Pd, Cu, Fe, Ni, Zn, and Se nanoparticles [27,30]. Table 1 presents the utilised microorganism and the type of metallic nanoparticle obtained.

Table 1.
Nanoparticles synthesised by green synthesis.
Biopolymers are ecologically safe alternatives to conventional polymers thanks to their biodegradable nature and their origin from renewable resources [52]. They can successfully be used as bio-coatings for developing new biosensors, as they act as immobilisation matrices for including the biorecognition elements [53]. Depending on the sources of origin and synthesis routes, they can be classified into natural polymers, biosynthetic polymers, or biopolymer composites.
The most significant amounts of natural biopolymers are industrially extracted from plant and animal sources (e.g., chitosan obtained from shrimp shells, cellulose extracted from wood, cotton, collagen, gelatine extracted from pig and cow), and can play a considerable role in solving the environmental problems raised using polymeric materials [54,55].
Biosynthetic polymers, also known as bioplastics, are categorised into three divisions: non-biodegradable, biodegradable-petroleum, and biodegradable based on natural polymer [12,13]. The last two categories are of interest in this review. Bio-based biodegradable polymers can be produced by microbial fermentation processes starting from biomass or organic waste from agriculture, food processing, and landfills [52,56]. Biodegradable polymers can be obtained through bacterial biosynthesis from natural materials (polysaccharide polyesters) or through chemical synthesis from renewable natural materials (lactic acid polyesters—obtained by fermentation starting from starch [57]).
Biopolymer composites (Table 2) with metal nanoparticles, silica, metal oxides, carbon-based materials, and polymers were developed to overcome the ‘biopolymers’ drawbacks, such as low mechanical and low chemical resistance or hygroscopicity [53].

Table 2.
Biopolymer types—used as a coating for food and health application.
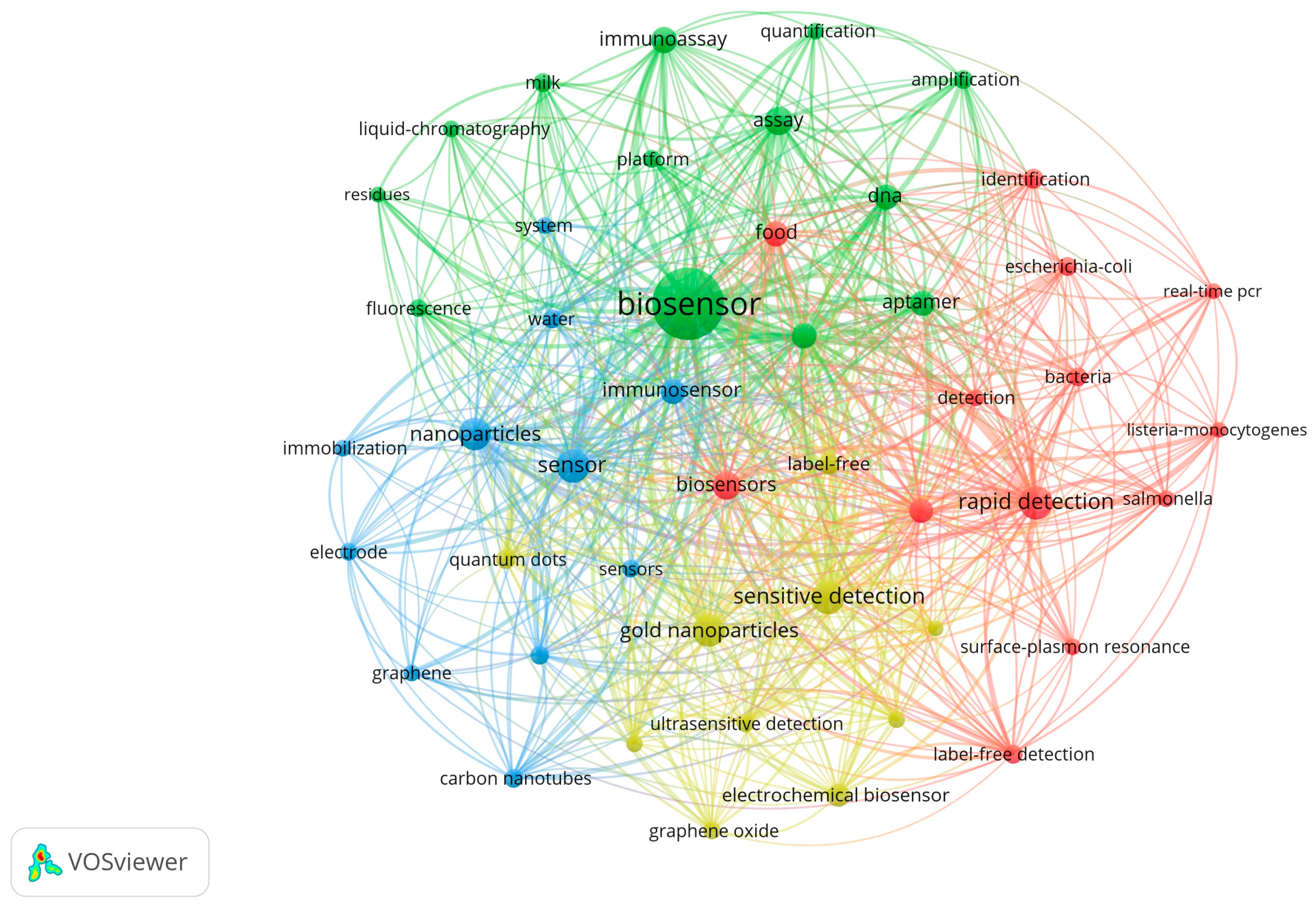
Nanoparticles and biopolymers can be incorporated into chemical or/and biological sensors to improve the analytical performance of selectivity, sensitivity, response time, and accuracy. It is a complex and ramified domain, as seen in Figure 2.
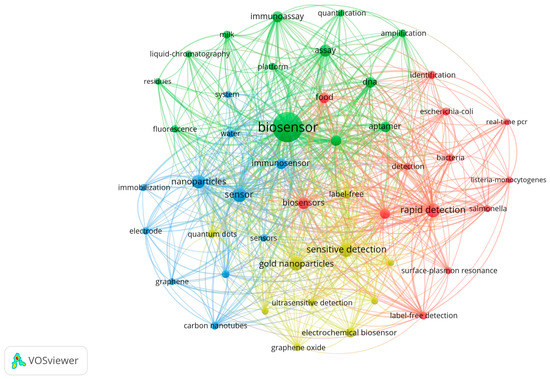
Figure 2.
The bibliometric analysis of data extracted from the ISI Web of Science database using the keywords ”biosensors in food industries” and “biosensors in health industries”.
The analysis of the biosensor’s domain has been performed using a tool for the construction and visualisation of bibliometric elements, namely VOS viewer 1.6.18 [71]. Figure 2 shows a bibliometric analysis of the data extracted from the ISI Web of Science (www.webofscience.com) database, using the following keywords: “biosensors in food industries” and “biosensors in health industries”.
The analysis of bibliometric networks has received considerable attention during the last five years. Figure 2 shows the complexity of the domain, and it is practically impossible to cover all these aspects within a single review. It is a multidisciplinary domain at the confluence of chemistry, physics, biochemistry, and biotechnology, and the list is still open. For these reasons, we have chosen to discuss only biosensors from the food industry and medical applications, namely those that have bio-coating from biopolymers, nanoparticles, or combinations from these categories.
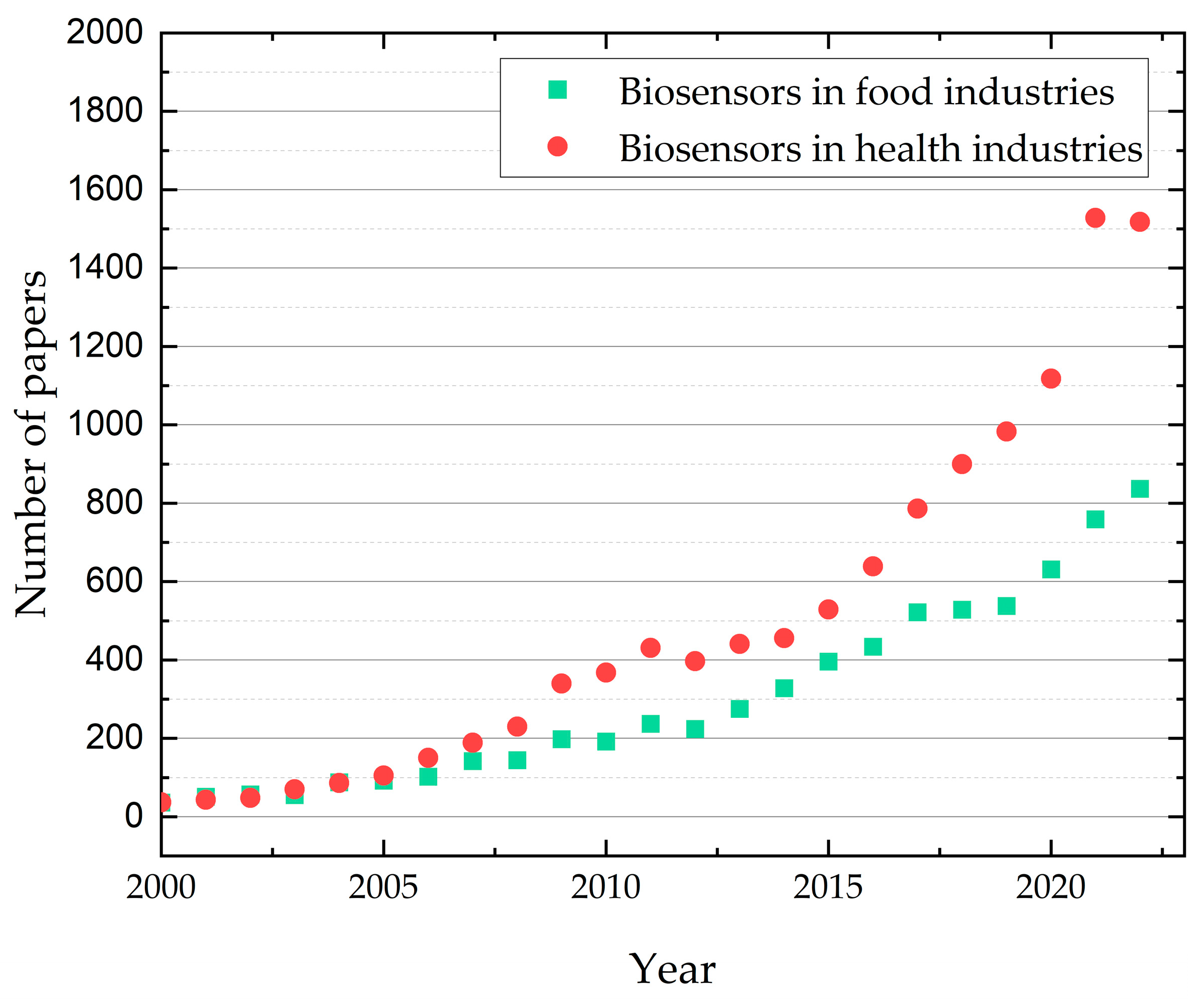
In this regard, Figure 3 shows the search on ISI Web of Science (using the keywords “biosensors in food industries” and “biosensors in health industries”) of the number of papers published through the last 22 years in the biosensors domain. This graph shows an increasing number of articles describing biosensors’ applications in medicine and the food industry. The exponential growth during the last 10 years is probably correlated with the importance of food safety and prevention in the medical domain. An increase in publications in the last two years for applications in healthcare can be observed, probably related to the COVID pandemic.
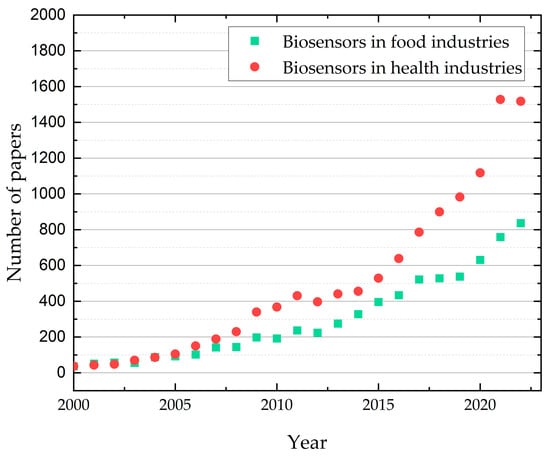
Figure 3.
Publication trend (2000–2022) in the application of biosensors—applications in health and food industries (Source of raw data: ISI Web of Science; search keywords: biosensors in food industries, biosensors in health industries).
While there are reviews addressing biosensors in food and health [72,73,74,75,76,77,78], there are few publications on biosensors based on sustainable materials with applications in these sectors. For instance, one review focused on developing hydrogels for biosensing applications in diagnostics [79], while another concentrated on biodegradable sensors for invasive and non-invasive health monitoring [7]. Concerning the food sector, a complex review addressed the application of eco-friendly biopolymer composites for food packaging [80].
Aside from the ecological consequences of mass farming, agriculture, and food production processes in the food industry, there are concerns related to the increasing levels of food waste and the usual usage of plastics in food packaging. Food packaging is the third-largest global industry and the single-largest contributor to solid waste. Thus, food packaging and waste management are two critical elements in the race to address climate change. In this respect, several recent studies present innovative solutions for intelligent, sustainable food packaging alternatives to petroleum-based plastics and synthetic dyes [25,81].
Correspondingly, in the healthcare sector, there are also rising concerns regarding the clinical use and disposal of biosensors, especially after the COVID pandemic [5]. The emergence of biodegradable materials represents a legitimate solution to the fast-depleting fossil-based materials. This represents a solution to reducing environmental pollution, as nowadays, the trend is towards developing wearable or implantable biosensors that naturally degrade [7]. Several state-of-the-art studies present sustainable biosensors based on biopolymers, green-synthesized NPs, or biopolymer nanocomposites with increased performance [82,83].
The novelty of this review lies in its comprehensive analysis of the latest research and development in biosensors from the food and healthcare sectors that use bio-coatings based on green-synthesized nanoparticles and biopolymers. Such an approach can help identify the current challenges and opportunities in the development of bio-coatings for specific applications, which can further guide research and development efforts. We explored the potential impact of bio-coatings in enhancing biosensors’ sensitivity, selectivity, stability, and durability, which could ultimately improve the safety and quality of food and healthcare products. Viewed from another angle, this review also aims to highlight the eco-friendly and sustainable bio-coatings which can be employed in the biosensor’s development. As the world becomes more environmentally conscious, there is a growing need for sustainable solutions in various industries, including food and healthcare. Thus, a review that emphasizes the sustainability and potential impact of bio-coatings could be of great interest to policymakers, industry professionals, and the general public.
2. Biosensors with Bio-Coatings and Applications in the Food Sector
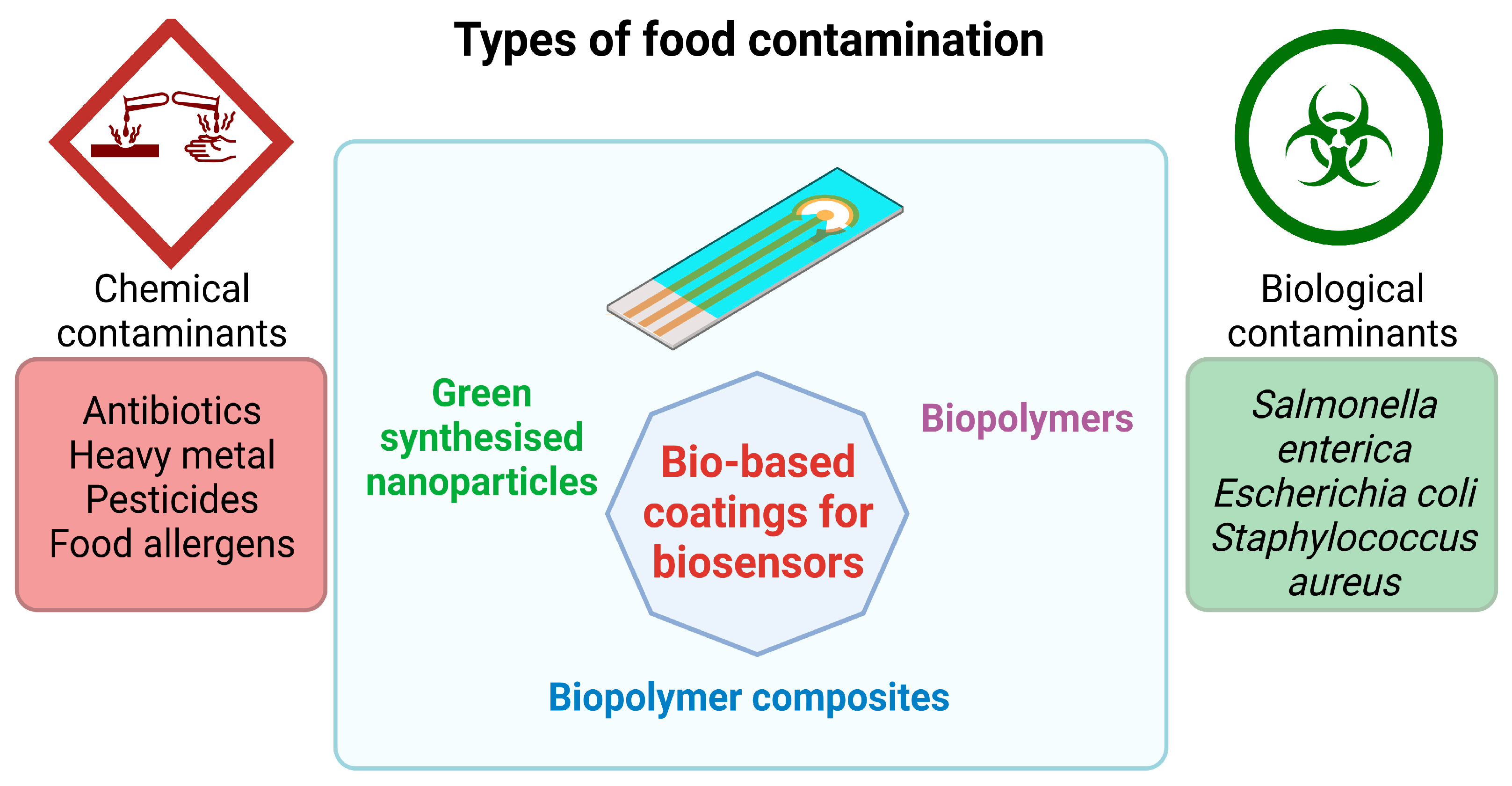
Nanotechnology has brought significant advancements in the food sector by bringing biosensors and food additives in the form of nanoparticles to ensure safety and traceability for packaging food products [84,85] and the development of food production [86]. Despite the extraordinary advantages of food packaging and biosensors in agriculture and the food industry, public opinion expresses concern about toxicity and its effect on the environment. There is slight knowledge of long-term adverse effects on soil, plants, and ultimately humans [87]. Thus, research nowadays focuses more on developing sustainable, non-toxic, and environmentally friendly materials for food packaging and biosensing. In this section, we focus on the development of bio-coatings based on nanoparticles obtained through green synthesis methods and biopolymers with applications in the food sector (Figure 4).
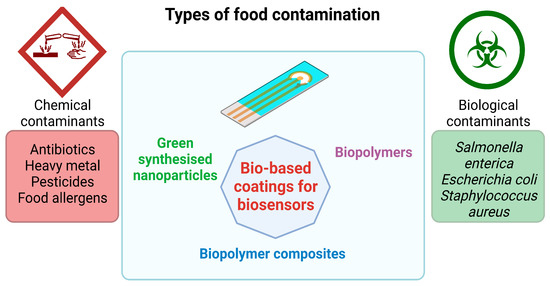
Figure 4.
Applications of bio-based coatings for biosensors used for monitoring food contamination (the graphical illustration has been created with BioRender software—BioRender Company, Toronto, ON, Canada).
Nanotechnology enabled the rapid evolution of biosensors for detecting food components in an easy and timely manner. For example, biosensors were developed to detect external and internal conditions in food packaging, organic compounds, cations, anions, pesticides, antibiotics, heavy metals, microbial cells, and toxins [88]. Biosensors assure consumers that they are purchasing fresh products, reducing the frequency of foodborne infections and food poisoning, contributing thus to food safety [89].
For food analysis, biosensors based on nanoparticles, electrochemical biosensors, and optical biosensors are employed [90]. They can be introduced directly in the packaging material, serving as an “electronic tongue” or “nose” for the identification of the chemical substances released during food alteration [91], or indirectly, using microfluidic devices (ex: Si-based microfluidic systems) for the detection of pathogens, in real-time and with high sensitivity [92].
The biosensors containing nanoparticles were developed to detect and neutralise pathogens or other contaminants, most being used for pathogen detection in fish [93]. Gold nanoparticle biosensors use a surface resonance detection method to detect ochratoxin A, a mycotoxin produced by Aspergillus and Penicillium moulds that often contaminate raw materials and food products and have strong toxic effects on the visceral organs of both humans and animals [94]. The applications of nanoparticles in food preservation and packaging are expected to reach USD 125.7 billion by 2024 and a staggering USD 44.8 billion by 2030 [95].
Nanoelectromechanical systems (NEMS) are already in use in the food sector; these systems contain parts with sizes ranging from mm to nm, which could serve to develop sensors used to preserve food [96]. NEMS could also be used in food control, consisting of advanced transducers for detecting specific chemical and biochemical signals [97].
As previously mentioned, biosensors also evaluate food contamination with antibiotics, preservatives, and mycotoxins. Estimating the residual amount of antibiotics in milk, dairy products, and meat is an essential analysis in food technology. Biosensors with gold nanoparticles that use pyrocatechol violet interact with the hydroxyl and amide groups of antibiotics through hydrogen bonding. As a result, the colour changes even for small concentrations, resulting thus in the detection of antibiotics such as kanamycin, neomycin, streptomycin, and bleomycin, etc. Silver biosensors serve to detect clusters of nitrites in food, which is a frequently used preservative, but also a carcinogenic pollutant [98,99].
The purpose of intelligent packaging is the real-time assessment of product quality by monitoring the interactions between the product, the packaging, and the environment, through a series of sensors or indicators. The sensor readings should be translated into a clear message about quality, safety status, or shelf life. The signal must then be communicated to different actors in the supply chain, including the consumer [100]. The main goal of biosensors is to reduce the time for pathogen detection from days to hours or even minutes. Microbial sensors are biosensors used in the food industry to detect and monitor any alteration occurring during packaging and storage [101].
Globally, the use of active and smart packaging systems has expanded as the European Union and international legislation have put an accent on food safety, decreasing foodborne diseases. At the same time, the new intelligent packaging systems became particularly useful to processors, to the extent that new packaging increases the shelf life of products [102,103,104,105].
The advancements of bio-nanotechnology have considerably improved the wrapping/packaging of food, increasing their quality and safety [106,107,108]. Food packaging can be comprised into four types: passive (no interaction with the product), active (capable of interacting with the product), intelligent (reactive to the environment), and smart (involves the use of technology) [109,110].
The use of metal oxides in food packaging metal nanoparticles with their strong antimicrobial properties are used as “active packaging”. The metallic nanoparticles with pronounced biocidal properties are Ag, Cu, Zn, Ti, and Au. They also contribute to an increase in the mechanical resistance, light resistance, and barrier properties of the packaging [111].
Silver nanoparticles (AgNPs) have been proven to have the best antimicrobial activity against various microorganisms [112]. AgNPs showed better antimicrobial activity than metallic silver due to their extensive relative surface area, which can ensure better contact with microorganisms.
The antifungal effect of LDPE (decorated with nanosilver) was also presented against yeasts and moulds; this fact allowed a significant decrease in the pasteurization temperature of orange juice by 10%. The nano-packaging obtained with low-density polyethylene and nano-silver was able to maintain the sensory, physicochemical, and physiological qualities of blueberries and strawberries at a higher level compared to the usual packaging made with polyethylene bags [113].
Mahdi and collaborators [114] evaluated the antimicrobial effect of the PVC-nano Ag nano packaging used for minced beef, stored at refrigeration temperature (+4 °C). After 7 days of study, it was found that this nano packaging inhibited microbial growth. The inhibitory effect is stronger against the growth of Escherichia coli compared to Staphylococcus aureus. Growth bacteria allowed an increase in the shelf life compared to the usual food packaging. The effects of Ag and TiO2 nanoparticles embedded in polyethylene (PE) on the contents of solid, liquid, high-fat, and highly acidic food samples compared to conventional containers were studied by Metak et al [115].
Copper and its compounds have been known as biocidal substances for centuries, being used today as effective antimicrobial and antiviral agents. Unfortunately, the direct use of copper and its compounds can be toxic to fish and other organisms and can cause environmental damage. The copper nanoparticles can substitute the copper and its compounds, allowing to avoid these issues.
Copper nanoparticles can also be used as cheap alternatives for silver nanoparticles [116,117]. Nevertheless, the use of copper nanoparticles in the food industry raises concerns. Copper is one of the micronutrients necessary for the normal functioning of the human body; it contributes to maintaining homeostasis. If copper intake exceeds the limits of human tolerance, it can present toxic effects such as haemolysis, jaundice, and eventually death. Similarly, if the intake of copper nanoparticles enters the human body in excess by any route, such as ingestion or inhalation, it causes toxic effects in the respiratory tract, the gastrointestinal tract, and other tissues. Chen Z. and co-authors demonstrated that copper nanoparticles are more toxic than copper microparticles because the nanoparticles can quickly enter the body. Copper nanoparticles also cause pathological damage to the liver, kidney, and spleen [118].
Zinc oxide (ZnO) nanoparticles can be used in food preservation due to their antimicrobial properties [117]. Espitia P. and co-authors [119] evaluated the antimicrobial activity of ZnO nanocomposites on Gram-negative bacteria such as Escherichia coli, Pseudomonas aeruginosa, Campylobacter jejuni, as well as Gram-positive bacteria such as Bacillus subtilis, Staphylococcus aureus, and Lactobacillus plantarum. ZnO nanoparticles also showed significant antifungal activity against phytopathogenic fungi of fruits in the post-harvest period: Botrytis cinerea and Penicillium expansum in concentrations higher than 3 mmol/L−1. Antimicrobial food packaging manufactured using ZnO nanoparticles represents an impact on consumers. The balance of positive and negative effects on the food safety of ZnO must be thoroughly assessed. Research on the toxicological impact of ZnO nanoparticles on the ‘consumers’ health focused on migrating ZnO nanoparticles from the package into the contained food. This research emphasised toxicity, inflammatory and carcinogenic effects for in vitro experiments on colon cell cultures [119].
Additionally, edible films containing antimicrobial components are crucial for extending product shelf life and reducing the risk of pathogens. Biodegradable polymer films represent an alternative option in food packaging, as they can be obtained at low cost from renewable sources without causing environmental pollution. Polysaccharides such as cellulose, pullulan, agarose, starch, and chitosan are the most used. Covering films with cellulose and silver nanoparticles in their composition have good antimicrobial effect against Escherichia coli and Bacillus sp. [120]. Based on these properties, Muthulaksmi L., and collaborators [121] consider that cellulose/AgNPs composite films can be used for antimicrobial packaging and health applications.
Pullulan (α-1,4-; α-1,6-glucan) is an edible polysaccharide polymer consisting of maltotriose units. This polymer is produced from the starch of the fungus Aureobasidium pullulans. Pullulan films are colourless, tasteless, resistant to oil, and have low permeability to oxygen but are sensitive to high humidity. As a food additive, it is known by the numerical code E1204. It is a non-toxic, water-soluble packaging material to prevent the oxidation of food [122].
Khalaf H.H and collaborators [123] studied the antimicrobial activity of complex pullulan films incorporating silver nanoparticles (100 nm) and zinc oxide nanoparticles (110 nm). These films also contained oil of oregano (OR) 2% and rosemary oil (RO) 2%. Antibacterial activity was determined during meat preparation and storage at 4, 25, 37, and 55 °C. The test was conducted on Listeria monocytogenes and Staphylococcus aureus bacteria. The study’s results demonstrated that the films obtained from pullulan with the addition of Ag nanoparticles, ZnO, and essential oils of rosemary and oregano inactivated pathogens, especially Listeria monocytogens, and Staphylococcus aureus bacteria, cause meat spoilage. The antimicrobial activity was more pronounced in films with Ag nanoparticles and oregano essential oil. The edible films did not change the physical and organoleptic properties of the product. Active packaging of agar hydrogel using silver nanoparticles effectively extends the shelf life of Fior di Latte cheese [124].
Italian researchers Longano D., Ditaranto N., Cioffi N., et al. [125] developed a new antibacterial additive composed of copper nanoparticles incorporated in polylactic acid, thus combining the antimicrobial properties of copper nanoparticles with the biodegradability of the polymer matrix. It has been experimentally demonstrated that this nanocomposite prevents the proliferation of Pseudomonas spp. bacteria, with great potential in smart packaging.
Table 3 summarizes some applications of Biosensors (obtained through green chemistry) in the food industry.

Table 3.
Various sensing nanoplatforms utilised in the food industry.
Bollella et al. [126] described the green synthesis and characterisation of gold and silver nanoparticles and their application for developing a third-generation lactose biosensor. The researchers developed a lactose biosensor using synthesised nanoparticles, which showed high sensitivity and selectivity for lactose detection. The biosensor had a linear range from 10 to 300 mM, a high sensitivity (5.4 μA mM−1 cm−2) and was stable and reproducible, indicating its potential for practical applications.
Santhosh and co-workers [127] discussed the green synthesis of silver nanoparticles using onion peels, which are a waste product. The authors of the study used a simple, cost-effective, and eco-friendly method to synthesise the nanoparticles. The study found that the silver nanoparticles showed intense antimicrobial activity against various bacterial strains and antiproliferative activity against cancer cells. Overall, the study highlighted the development of a biosensor for the determination of mercury.
Another article [129] presented a novel method for synthesising chitosan-gold nanoparticles (CS-GNPs) and their application in optical biosensing. The CS-GNPs were synthesised using a green chemistry approach, which avoids the use of toxic chemicals typically used in nanoparticle synthesis. The green synthesis of the CS-GNPs was achieved using chitosan, a natural polymer derived from the shells of crustaceans. The results showed that the sensor had high sensitivity and specificity towards the target antigen, with a detection limit of 1 µg/mL. The sensor also showed good stability and reproducibility over time. The authors conclude that their green synthesis approach for CS-GNP synthesis, combined with the use of a specific antibody, provides a promising platform for developing optical biosensors to detect various analytes.
Yu et al. [130] studied a green synthesis approach to produce gold nanoclusters (AuNCs) using papaya juice as a reducing agent. The AuNCs were synthesised in a one-step process, using chloroauric acid as the precursor and papaya juice as the reducing agent. The results showed that the synthesised AuNCs exhibited strong fluorescence properties with a maximum emission wavelength of 440 nm. The fluorescence properties of the AuNCs were afterwards used to develop a sensing platform for detecting L-lysine, an amino acid commonly found in food products.
A new method for detecting bismerthiazol [131], a commonly used fungicide in cabbage was developed using protein-capping gold nanoclusters (PC-GNCs). The PC-GNCs were synthesised using a simple, green method, and were then functionalised with the protein from soybeans to enhance their stability and biocompatibility. The sensor’s performance was evaluated by measuring the fluorescence intensity of the PC-GNCs in the presence of different concentrations of bismerthiazol. The results showed that the sensor had a linear response to bismerthiazol concentrations in the range of 5–100 μg/mL with a detection limit of 5 µg/L. The detection time for bismerthiazol was less than 5 minutes, making this approach a fast and efficient method for detecting the fungicide in cabbage.
The same author [132] described a green synthesis approach to produce gold nanoclusters (AuNCs) using soy protein as a template. The synthesised AuNCs were used to develop a sensing platform for detecting copper ions (Cu2+). The sensor’s performance was evaluated using several techniques, including fluorescence spectroscopy and transmission electron microscopy. The fluorescent AuNCs were employed to develop a visual detection method for Cu2+, involving the addition of a Cu2+ solution to the AuNCs, followed by observation of the change in colour of the solution. The detection limit for Cu2+ using this method was 10 μM, below the permissible limit for Cu2+ in drinking water set by the World Health Organization [144].
Bagal-Kestwal et al. [134] developed a fluorescence-based sucrose sensor using plant membranes decorated with invertase-nanogold clusters (INV-NGCs). Invertase is an enzyme that catalyses the hydrolysis of sucrose into glucose and fructose, and nanogold clusters (NGCs) are small clusters of gold nanoparticles. The INV-NGCs were synthesised using a green synthesis method (on the surface of the inner epidermal membranes of onions (Allium cepa L.). The sensor’s performance was evaluated by measuring the fluorescence intensity of the INV-NGC-decorated plant membranes in the presence of different concentrations of sucrose. The results showed that the sensor had a linear response to sucrose concentrations in the range of 2.25 × 10−9 to 4.25 × 10−8 M. The sensor also exhibited good selectivity towards sucrose over other sugars and was stable and reproducible over time.
Another article [135] described the synthesis of reduced graphene oxide (rGO) decorated with gold nanoparticles (AuNPs) using a green chemistry approach. The rGO-AuNP composite was synthesised using rose water as a reducing agent. The synthesised composite was then used as a sensing platform for glucose detection. The sensor’s performance was evaluated by measuring the change in the electrochemical response of the rGO-AuNP composite in the presence of different glucose concentrations. The results showed that the sensor had a linear response to glucose concentrations in the range of 1-8 mM, with a detection limit of 10 µM.
Dayakar and co-workers [136] synthesised pristine copper nanoparticles (CuNPs) (using Ocimum tenuiflorum leaf extract) and studied their use in a non-enzymatic sensing platform for the detection of glucose. The CuNPs were synthesised using a simple, one-pot reduction method and were then characterised using several techniques, including transmission electron microscopy and X-ray diffraction analysis. The sensor’s performance was evaluated by measuring the electrochemical response of the CuNP-modified electrode in the presence of various glucose concentrations. The results showed that the sensor had a linear response to glucose concentrations in the 1–7.2 mM range, with a detection limit of 0.038 µM. Using pristine CuNPs as a sensing platform simplifies the biosensor design and reduces the cost and complexity associated with enzymatic biosensors. The CuNPs synthesised using this approach have the potential to revolutionise the field of biosensing.
The green synthesis of fluorescent carbon nanodots (CNDs) from bamboo leaves was reported by Liu at al. [137] and their use as a sensing platform for the detection of copper (II) ions (Cu2+). The CNDs were synthesised using a one-step hydrothermal method and were then functionalised with sodium hydroxide to enhance their fluorescence properties. The sensor’s performance was evaluated by measuring the change in the fluorescence intensity of the CNDs in the presence of different concentrations of Cu2+. The results showed that the sensor had a linear response to Cu2+ concentrations in the range of 0.333 to 66.6 μM, with a detection limit of 115 nM. Using bamboo leaves as a precursor for CND synthesis reduced the cost and environmental impact of nanoparticle synthesis and provided a new avenue for developing biosensors based on natural materials. The CNDs synthesised using this green synthesis method have the potential to revolutionise the field of biosensing.
Sukumar et al. [138] synthesised rice-shaped copper oxide nanoparticles (CuONPs) using the extract of Caesalpinia bonducella seeds as a reducing and stabilising agent. The synthesised CuONPs were characterised using several techniques, including transmission electron microscopy and X-ray diffraction analysis. The CuONPs exhibited good photocatalytic activity towards the degradation of methylene blue under visible light irradiation and exhibited good antibacterial activity towards Aeromonas and Staphylococcus aureus. The authors concluded that their approach for the green synthesis of rice-shaped CuONPs using Caesalpinia bonducella seed extract is facile and cost-effective. The CuONPs synthesised using this green synthesis method have the potential to revolutionise the field of photocatalysis and antibacterial materials and carry out electrochemical detection of riboflavin.
A new method for the biogenic synthesis of copper oxide nanoparticles (CuONPs) [139] using the aqueous extract of the latex of Ficus religiosa as a reducing and stabilising agent was described by Singh et al. The performance of the CuONPs was evaluated for their biosensing applications, particularly for pesticide detection.
Karthik et al. [140] synthesised gold nanoparticles (AuNPs) decorated graphene oxide (GO) using Bischofia javanica Blume leaves as a reducing and stabilising agent. The synthesised AuNPs-GO composite was used as a sensing platform for detecting chloramphenicol in various food samples (in milk, powdered milk, honey, and eye drops). The sensor’s performance was evaluated by measuring the change in the electrochemical response of the AuNPs-GO composite in the presence of various chloramphenicol concentrations. The results showed that the sensor had a linear response to chloramphenicol concentrations in the range of 1.5–2.95 μM, with a detection limit of 0.25 μM. The sensor also exhibited good selectivity towards chloramphenicol over other antibiotics and was stable and reproducible over time.
A new green synthesis of gold nanoparticles (AuNPs) using the extract of Syzygium aromaticum and their application in enhancing the response of a colourimetric urea biosensor was reported by Kaur and coworkers [141]. The biosensor’s performance was evaluated by measuring the change in the absorbance of the AuNPs in the presence of different concentrations of urease. The biosensor using AuNPs exhibited a significantly enhanced response than the biosensor without AuNPs.
Masibi [142] reported the green synthesis of bimetallic Au-Ag nanoparticles (NPs) using the extract of Citrus × sinensis (L.) Osbeck peels as a reducing and stabilising agent. The Au-Ag NPs exhibited good electrocatalytic activity towards the oxidation of caffeine, with a linear response in the range of 0–59 μM. The electrochemical sensor based on the Au-Ag NPs exhibited good selectivity towards caffeine over other interfering species.
Han et. al. [143] described the green synthesis of reduced gold nanoparticles (AuNPs) using the extract of Radix Pueraria flavonoids as a reducing and stabilising agent. The performance of the synthesised AuNPs was evaluated for their electrochemical sensing applications, particularly for the non-enzymatic detection of cholesterol in food samples. The electrochemical sensor based on the AuNPs exhibited good sensitivity and selectivity towards cholesterol, with two linear ranges of 1–100 and 250–5000 μmol/L. The electrochemical sensor exhibited good stability and reproducibility over time.
The authors concluded that their approach for the green synthesis of reduced AuNPs using Radix Pueraria flavonoids extract is simple and cost-effective. The AuNPs synthesised using this approach exhibited high stability and biocompatibility, making them suitable for developing electrochemical sensors. The reduced AuNPs synthesised using this green synthesis method have the potential to revolutionise the field of electrochemical sensing for the non-enzymatic detection of cholesterol in food samples.
3. Biosensors with Bio-Coatings and Applications in the Health Sector
Humankind experiences a death toll of around 2 million lives yearly from increasing bacterial resistance to antibiotics and viral infections [9]. An increasing need to develop new diagnostic tools for better detection of pathogens was revealed during the COVID-19 pandemic. Moreover, COVID-19 revealed the worrisome facet of the increased environmental impact of biosensors.
In addition, the development of sustainable biosensors proves to be valuable for continuously monitoring body signals and biomarkers and therapeutics and diagnostics purposes (Figure 5). In this respect, the materials must be biocompatible, biodegradable, and offer high detection performance. Consequently, biosensing using sustainable materials will also aid in minimising waste production [7].
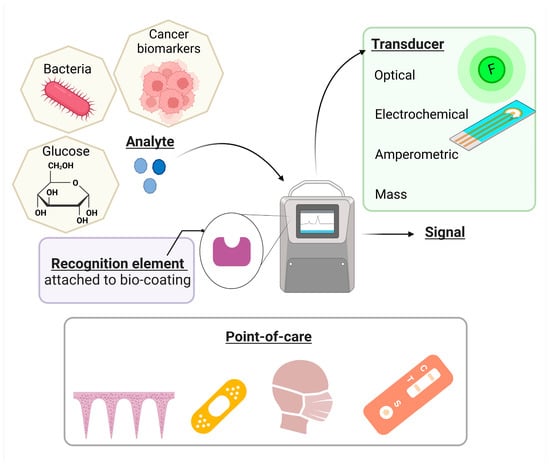
Figure 5.
Potential point-of-care applications for biosensors with bio-based coatings (the graphical illustration has been created with BioRender software—BioRender Company, Toronto, ON, Canada).
In a biosensor, adding functionality to the substrate plays a crucial role in maintaining the ‘biosensor’s stability, increasing operational usage, and regulating the interactions between the sensing surface and analytes. The ‘biosensor’s functional bio coating also plays a significant role in amplifying the detection signal and reducing the non-specific binding, thus increasing the specificity of the analyte detection. Natural polymers were explored as coatings in biomedical applications through their non-toxicity, biodegradability, and biocompatibility. Even though natural polymers offer several advantages, they possess certain drawbacks, such as hygroscopicity, and low mechanical and chemical resistance, which restricts their application in biosensors. These downsides can be overcome by preparing biopolymer composites with nanoparticles, carbon-based materials, metal oxides, polymers, and so on [53].
Cellulose- and paper-based biosensors have the following advantages: (a) rapid diagnosis of infectious diseases due to their safe environmental disposal, (b) availability for mass-production, and (c) simple recycling process [9,145]. They are used for the detection of antibodies, antigens, or nucleic acids from saliva, sputum, and blood, by using colourimetric, fluorescent, or electrochemical detection approaches [146]. Notably, various gold nanoparticle-based tests for SARS-CoV-2 were FDA-approved under EUA designation for large-scale COVID-19 testing. Briefly, they are lateral-flow test strips on which colloidal gold-conjugated recombinant SARS-CoV-2 antigens are dried at the end of the membrane strip. Each test strip contains a pad for the sample addition, a pad containing COVID-19 antigen conjugated with gold nanoparticles, and gold-rabbit IgG, a nitrocellulose membrane with a control line coated with anti-human IgG, an IgM line coated with anti-human IgM, and finally, a pad for absorbing the waste [147,148,149,150]. However, sustainable biosensing can be limited by inadequate storage conditions, which could tamper the shelf-life of sustainable diagnostics, and by the emergence of mutated SARS-CoV-2 variants [9]. Lateral flow assays, which typically consist of a sample pad based on cellulose and a nitrocellulose pad onto which the specific antibodies are immobilised (the test line) were also primarily reported to be used for the detection of antimicrobial-resistant bacteria [151].
Chitosan and carboxymethyl cellulose are other polysaccharides extensively explored for developing new biosensors thanks to their natural abundance, biocompatibility, biodegradability, non-toxicity, and the capability to create adherent thin films on electrochemical surfaces [152]. Real-time detection of glucose in a range of concentrations from 1 to 15 mM was achieved by Kim et al. using an electrochemical biosensor based on graphene oxide/cobalt/chitosan nanocomposite [61]. Ambrosetti et al. reported the fabrication of carboxymethyl-dextran-based protein-patterned surfaces for enhanced biomolecular recognition using SPR [153].
A unique patented electrochemical biosensor was developed by NovioSense BV to be worn under the lower eye lid to continuously monitor glucose levels in the basal tear fluid. The measurements have shown a good correlation for blood glucose values, with clinical feasibility. The polysaccharide material acts as a barrier between the metal surface and the soft tissue of the eye, allowing the free diffusion of the analyte into the coating while stabilising and preventing the enzyme migration out of it. Furthermore, the polysaccharide-coated device did not produce pain or irritation [154].
Heparin was for the first time reported to be used instead of an antibody as a biorecognition element in a single-walled carbon nanotube-based (SWNT) chemiresistive biosensor, for the detection of Dengue virus. This was possible because heparin is a structural homologue of heparan sulphate, a receptor for Dengue virus serotypes [155].
Hasanah et al. reported a pectin-based optical biosensor for the detection of triglycerides for the first time. Pectin was employed for increasing lipase absorption. A detection limit of 15 mg/dL was obtained [156].
Poly-L-lysine (PLL) is biocompatible, stable, soluble in water, and useful for enzyme immobilisation, as the negatively charged proteins can be immobilised with ease onto the positively charged PLL via electrostatic interactions. The functionalisation of the electrodes with PLL can be accomplished through electro-polymerisation. A study involving a graphene field effect transistor modified with PLL was conducted by Gao et al. for ultrasensitive detection of miRNA biomarkers in breast cancer and SARS-CoV2. A detection limit of 1 fM was achieved within 20 minutes by using 2 µL of the sample [157].
A zein/gelatine-based electrochemical biosensor for glucose detection was reported. The gelatine top-coat of the biocompatible sandwich supported the covalent attachment of glucose oxidase (GOx), ensuring the access of the substrate to the enzyme, while the zein base-coat protected from interferents caused erroneous detection of hydrogen peroxide. The group reported a sub-μm detection limit, a long shelf life, and accurate recovery of glucose in model samples [158].
Protein-based biopolymers are regarded as excellent candidates for ‘biosensors’ coating due to their biocompatibility, cross-linking ability, and biodegradability. Of particular interest are enzymes, which are primarily exploited in biosensing because of their catalytic nature, selectivity, and low energy requirements [52,159]. However, the challenge posed by developing enzyme-based biosensors is related to maintaining their catalytic activity. In this respect, electrospray ionisation (ESI) has been employed for the deposition of laccase on a carbon substrate. This deposition method has the advantage of retaining the enzyme activity and the analytical performances in terms of working and storage stability for up to two months, with a limit of catechol detection of 1.7 μM, in the linear range of 2–100 μM [159]. Another study involved the use of a tyrosinase biosensor based on chitosan nanoparticles for catecholamine detection. A detection limit of 0.17μM, with an excellent sensitivity of 0.583 μA μM−1cm−2 was reported in [160].
Sartori et al. used botryosphaeran as a matrix for immobilising and maintaining the enzymatic activity of laccase stability onto a glassy carbon electrode (GCE) functionalised with MWCNTs. The electrochemical biosensing platform was applied for dopamine determination. A good selectivity was reported in the presence of uric acid, ascorbic acid, and other phenolic compounds. The limit of detection was 0.94 μmol L−1 [152].
DNA polymers gained lately significant attention as coatings for improved performance biosensors. DNA hydrogels are particularly interesting thanks to their biocompatibility, non-toxicity, programmable assembly, molecular recognition, and high loading capacity [161,162]. DNA hydrogels have been reported to detect small molecules, proteins, viruses, or toxins. For instance, a high affinity-based electrochemiluminescence biosensor for the detection of miRNA let-7a was reported. The biosensor exhibited good sensitivity within 10 fM–10 nM and a detection limit of 1.49 fM [161]. Mao et al. demonstrated the working principle of a DNA hydrogel-based three-dimensional electron transporter. They have shown that, compared to conventional functionalised electrodes, DNA hydrogels improve the efficiency of electron transfer. Moreover, DNA hydrogels allow the incorporation of electroactive molecular elements (e.g., DNAzyme) which further increases the biosensing performance [163].
Due to their superior physicochemical properties, synthetic biodegradable polymers are considered as biocoatings for biosensors. Poly(lactic acid) (PLA), polycaprolactone (PCL), polyhydroxybutyrate (PHB), and polyhydroxyvalerate (PHV) are among the most employed synthetic biodegradable coating polymers in the development of biosensors [7]. Marzo et al. demonstrated a new concept of a 3D-printed enzymatic graphene Poly(lactic acid) electrode for direct electron transfer using peroxidase enzyme for hydrogen peroxide (H2O2) detection. They demonstrated that the detection mechanisms of the 3D-printed biosensors are associated with the direct electron transfer between the horse radish peroxidase (HRP) and the activated electrode, without employing electron mediators. This process proves their future utility for real sample detection of biomarkers [164]. Silva et al. developed a 3D-printed enzymatic reduced graphene oxide-PLA electrode for the detection of serotonin with a detection limit of 0.032 μmol L−1 [66]. Cardoso et al. achieved a detection limit of 0.02 for uric acid and 0.03 μmol L−1 for nitrite within a linear range from 0.5–250 μmol L−1 on a 3D printed graphene-PLA electrode [67].
“Green chemistry” of nanomaterials plays a vital role in developing eco-friendly sensors in biomedical bio-applications (due to their biocompatibility and low toxicity). For example, Zamarchi and Vieira [128] reported that AgNPs synthesised by using the extract Araucaria angustifolia are used to manufacture an electrochemical biosensor to detect paracetamol.
Table 4 summarises the nanomaterial obtained by green chemistry used in the healthcare domain.

Table 4.
Sensing nanoplatforms used in healthcare.
The synthesis of ZnONPs via the green route is possible by employing plants, moulds, bacteria, and algae [168,169]. The most used ZnONPs-based biosensors are the ones that detect small molecules such as glucose, cholesterol, and urea [170]. The synthesis of ZnONPs with the help of Caesalpinia bonducella seed extract is considered for obtaining a biosensor employed in the detection of vitamin B2.
Another type of biosensor [128] obtained by green chemistry is a new sensor for detecting paracetamol, a widely used pain reliever, based on silver nanoparticles synthesised from plant extracts. The plant extract used in this study was obtained from a pine nut (Araucaria angustifolia). The synthesised silver nanoparticles were then used to modify the surface of a glassy carbon electrode, creating a sensor capable of detecting paracetamol in solution. The sensor’s performance was tested by measuring the current response of the modified electrode in the presence of different concentrations of paracetamol. The results showed that the sensor had good sensitivity and selectivity towards paracetamol, with a detection limit of 8.50 × 10−8 mol L−1. The sensor also showed good stability and reproducibility over time.
Bollella and co-workers [126] reported the synthesis of AgNPs and AuNPs by green chemistry using quercetin to make a lactose biosensor (based on cellobiose dehydrogenase from Trametes villosa). Additionally, the gold nanoparticles obtained through green synthesis [171] can be used to obtain biosensors with applications in medicine, such as determining the glucose content in commercial glucose injections.
The research on environmentally friendly and biodegradable sensors for healthcare applications is still in its early stages, as many issues remain to address. These issues are related to detection performances, biocompatibility, and safety when using biopolymer nanocomposites, or nanoparticles, even though they are obtained through green synthesis methods.
4. Conclusions and Future Perspectives
The choice of functionalisation strategy to attach the biorecognition element to the active element is fundamental in biosensing to achieve the desired performance. This strategy also ensures reusability with a limited impact on the environment. Nowadays, alternatives to conventional functionalisation strategies are being explored to obtain ecologically friendly and sustainable biosensors. This review gives an overview of bio-coatings derived from nanoparticles synthesised via green chemistry, biopolymers, and biopolymer composites and their role in biosensing applications with usage in the health and food sectors.
Substantial research efforts have been made to develop environment-friendly biosensors for smart packaging to increase food safety and quality by detecting pathogens promptly and inhibiting the ‘microorganisms’ growth. In this regard, biopolymers, biogenic nanoparticles, and biopolymer composites as bio-coatings in biosensors and smart packaging represents a milestone. However, biosensors must meet the market demands in terms of specificity, sensitivity, and detection limit. Moreover, the potential risks for human health posed by the integration of biosensors into smart packaging will need to be addressed before commercialisation. The possible migration of nanoparticles from packaging into food and their toxic effect on the human body and the environment are of great importance and are given special attention from regulatory bodies.
In healthcare, particularly in developing biosensors, the use of bio-coatings minimises their impact on the surrounding environment. Moreover, the switch towards sustainable, biocompatible, and eco-friendly biosensors opens the path towards wearable biosensors for continuous monitoring of various health parameters. The biosensors will be expected to play a big part in human well-being as they are expected to detect infections and life-threatening diseases in a fast manner. Improvements and tests need to be done to ensure their best performance and safety, especially for biosensors coming directly into contact with the human body. Thus, toxicity assessments for various bio-coatings employed in biosensors must be accomplished before taking them a step forward toward commercialisation.
The future direction of research in this field is likely to be focused on the following areas: (a) improving the sensitivity and stability of bio-coatings: researchers will continue to work on developing bio-coatings that have improved sensitivity and stability, allowing for more accurate and reliable biosensor readings; (b) expanding the range of applications: eco-friendly bio-coatings are expected to expand into new applications, such as wearable biosensors and real-time monitoring systems; (c) developing alternative materials: researchers will continue exploring alternative materials, such as biodegradable polymers and natural products, for synthesising bio-coatings; (d) enhancing biocompatibility: efforts will be made to improve bio-coatings’ biocompatibility further, ensuring their safe and effective use in biomedical applications; (e) improving the sustainability of biosensor production: bio-coatings synthesised via green-chemistry methods are expected to reduce biosensor production’s environmental impact, making it more sustainable.
Overall, future research in this field will likely focus on improving biosensors’ performance and sustainability using eco-friendly bio-coatings.
Author Contributions
Both authors have the same contribution. All authors have read and agreed to the published version of the manuscript.
Funding
M.P. acknowledges the support of a grant of the Ministry of Research, Innovation and Digitization, CCCDI—UEFISCDI, Bio-Iso-Pat project financed by PN-III-P2-2.1-PED-2021-0580 and SPIONNANODET project financed by PN-III-P1-1.1-PD-2021-0516 program; C.U. gratefully acknowledges the support of a grant of the Ministry of Research, Innovation and Digitization, CCCDI—UEFISCDI, project number PN-III-P2-2.1-PED-2021-0042, within PNCDI III.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Au | Gold |
| Ag | Silver |
| WHO | World Health Organization |
| QD | quantum dots |
| ASTM | American Society for Testing and Materials |
| NP | nanoparticle |
| mm | millimetre |
| nm | nanometre |
| NM | nanomaterial |
| HPLC-MS | High-performance liquid chromatography-mass spectrometry |
| PCR | Polymerase chain reaction |
| ELISA | Enzyme-linked immunosorbent assay |
| Pt | Platinum |
| Pd | Palladium |
| Zn | Zinc |
| Cd | Cadmium |
| Cu | Copper |
| Fe | Iron |
| Ni | Nickel |
| Co | Cobalt |
| HAuCl4 | Tetrachloroauric Acid |
| H2PtCl6 | Hexachloroplatinic acid |
| RhCl3 | Rhodium (III) chloride |
| PdCl2 | Palladium (II) chloride |
| cm | centimetre |
| TiO2 | Titanium dioxide |
| RF | radio frequency |
| K | Kelvin |
| kHz | kilohertz |
| MHz | megahertz |
| kW | kilowatt |
| MW | megawatt |
| atm | atmosphere |
| sec | seconds |
| N | Nitrogen |
| DMF | dimethylformamide |
| PEG | polyethylene glycol |
| UV | ultraviolet |
| AuNPs | gold nanoparticles |
| °C | degrees Celsius |
| min | minutes |
| ZnO | Zinc oxide |
| SnO2 | Tin oxide |
| PbO | Lead (II) oxide |
| EC-SPR | Electrochemical—surface plasmon resonance sensor |
| DNA | Deoxyribonucleic Acid. |
| LSPR | Localised surface plasmon resonance |
| SERS | Surface-enhanced Raman scattering |
| E. coli | Escherichia coli |
| PMNCs | polymeric nanocomposites |
| antibodies | ABs |
| GOX | glucose oxidase |
| PDA | polydopamine |
| DA | dopamine |
| CFU | colony-forming unit |
| mL | millilitre |
| PtNPs | platinum nanoparticles |
| PBNCs | polymeric bionanocomposites |
| L. monocytogenes | Listeria monocytogenes |
| μm | micrometre |
| LOD | Limit of detection |
| g | gram |
| β-Gal | β-galactosidase |
| S. typhimurium | Salmonella typhimurium |
| h | hours |
| PBS | phosphate buffered saline |
| EC | Commission Regulation |
| No | Number |
| S. boydii | Shigella boydii |
| ICS | immunochromatographic strip |
| S. aureus | Staphylococcus aureus |
| ATCC | American Type Culture Collection |
| MNPs | metal nanoparticles |
| MOs | metal oxides |
| CuO | copper oxide |
| Ag2O | silver oxide |
| CuNPs | Copper nanoparticles |
| pg | picograms |
| Fe3O4 | Iron oxide |
| SeNP | Selenium nanoparticle |
| FeNP | Iron nanoparticle |
| kg | kilogram |
| K | Potassium |
| Mg | Magnesium |
| Ca | Calcium |
| Hg | Mercury |
| IC | inhibition concentration |
| LC | lethal concentration |
| CMT | maximum permissible concentration |
| FDA | Food and Drug Administration |
| LOx | lactate oxidase |
| BC | Bio-cellulose |
| Co | Collagen |
| CuONPs | Copper oxide nanoparticles |
| fmol | femtomole |
| COVID-19 | Coronavirus Disease 2019 |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| USD | The United States dollar |
| LDPE | Low-density polyethylene |
| RFID | Frequencies radio |
| EFSA | The European Food Safety Authority |
| MNTS | Micro- and Nanotechnologies |
| LDPE | Low-density polyethylene |
| OR | oil of oregano |
| RO | rosemary oil |
| SWNT | single walled carbon nanotube based |
| PLL | Poly-L-lysine |
| ESI | electrospray ionisation |
| GCE | glassy carbon electrode |
| PCL | polycaprolactone |
| PHB | polyhydroxy butyrate |
| PHV | polyhydroxy valerate |
| PE | polymers polyethylene |
| PVC | polyvinyl chloride |
| EVOH | ethylene vinyl alcohol |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| PBAT | poly (butylene adipate-co-terephthalate |
| TPS | cellulose-based thermoplastic starch |
| PLA | poly lactide |
| PHA | poly-hydroxyalkanoate |
| PHB | poly-hydroxybutyrate |
| PGA | poly-glutamic acid |
| MCF-7 | Michigan Cancer Foundation-7 |
References
- Chu, E.W.; Karr, J.R. Environmental Impact: Concept, Consequences, Measurement. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- McNeely, J.A. Nature and COVID-19: The Pandemic, the Environment, and the Way ahead. Ambio 2021, 50, 767–781. [Google Scholar] [CrossRef]
- Noah, N.M.; Ndangili, P.M. Green Synthesis of Nanomaterials from Sustainable Materials for Biosensors and Drug Delivery. Sens. Int. 2022, 3, 100166. [Google Scholar] [CrossRef]
- Moulahoum, H.; Ghorbanizamani, F.; Celik, E.G.; Timur, S. Nano-Scaled Materials and Polymer Integration in Biosensing Tools. Biosensors 2022, 12, 301. [Google Scholar] [CrossRef]
- Yasri, S.; Wiwanitkit, V. Sustainable Materials and COVID-19 Detection Biosensor: A Brief Review. Sens. Int. 2022, 3, 100171. [Google Scholar] [CrossRef]
- Ungureanu, C. Coatings with Natural Products—One Perspective on the Challenges Related to New Coatings’ Development. Coatings 2022, 12, 941. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Dervin, S.; Ganguly, P.; Dahiya, R. Biodegradable Materials for Sustainable Health Monitoring Devices. ACS Appl. Bio. Mater. 2021, 4, 163–194. [Google Scholar] [CrossRef]
- Valencia, G.A.; Luciano, C.G.; Monteiro Fritz, A.R. Smart and Active Edible Coatings Based on Biopolymers. In Polymers for Agri-Food Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 391–416. [Google Scholar]
- Andriukov, B.G.; Lyapun, I.N.; Matosova, E.V.; Somova, L.M. Biosensor Technologies in Medicine: From Detection of Biochemical Markers to Research into Molecular Targets (Review). Sovrem. Tehnol. V Med. 2020, 12, 70–83. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem. 2016, 60–61, 1–8. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Sharma, B.; Jain, P. Graphene Based Biopolymer Nanocomposites; Springer: Singapore, 2021; pp. 273–286. ISBN 978-981-15-9180-8. [Google Scholar]
- Cader Mhd Haniffa, M.A.; Ching, Y.C.; Abdullah, L.C.; Poh, S.C.; Chuah, C.H. Review of Bionanocomposite Coating Films and Their Applications. Polymers 2016, 8, 246. [Google Scholar] [CrossRef]
- Pradhan, S.; Brooks, A.K.; Yadavalli, V.K. Nature-Derived Materials for the Fabrication of Functional Biodevices. Mater. Today Bio. 2020, 7, 100065. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Badea, N.; Bacalum, M.; Ungureanu, C.; Suica-Bunghez, I.R.; Iordache, S.M.; Pirvu, C.; Zgura, I.; Maraloiu, V.A. 3D Hybrid Structures Based on Biomimetic Membranes and Caryophyllus Aromaticus-“Green” Synthesized Nano-Silver with Improved Bioperformances. Mater. Sci. Eng. C 2019, 101, 120–137. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Ungureanu, C.; Badea, N.; Bacalum, M.; Lazea-Stoyanova, A.; Zgura, I.; Negrila, C.; Enculescu, M.; Burnei, C. Novel Ecogenic Plasmonic Biohybrids as Multifunctional Bioactive Coatings. Coatings 2020, 10, 659. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Ungureanu, C.; Iordache, S.M.; Iordache, A.M.; Bunghez, I.R.; Ghiurea, M.; Badea, N.; Fierascu, R.C.; Stamatin, I. Eco-Designed Biohybrids Based on Liposomes, Mint-Nanosilver and Carbon Nanotubes for Antioxidant and Antimicrobial Coating. Mater. Sci. Eng. C 2014, 39, 177–185. [Google Scholar] [CrossRef]
- Ungureanu, C.; Dumitriu, C.; Popescu, S.; Enculescu, M.; Tofan, V.; Popescu, M.; Pirvu, C. Enhancing Antimicrobial Activity of TiO2/Ti by Torularhodin Bioinspired Surface Modification. Bioelectrochemistry 2016, 107, 14–24. [Google Scholar] [CrossRef]
- Ungureanu, C.; Fierascu, I.; Fierascu, R.C.; Costea, T.; Avramescu, S.M.; Călinescu, M.F.; Somoghi, R.; Pirvu, C. In Vitro and in Vivo Evaluation of Silver Nanoparticles Phytosynthesized Using Raphanus sativus L. Waste Extracts. Materials 2021, 14, 1845. [Google Scholar] [CrossRef]
- Ungureanu, C.; Popescu, S.; Purcel, G.; Tofan, V.; Popescu, M.; Sǎlǎgeanu, A.; Pîrvu, C. Improved Antibacterial Behavior of Titanium Surface with Torularhodin-Polypyrrole Film. Mater. Sci. Eng. C 2014, 42, 726–733. [Google Scholar] [CrossRef]
- Ungureanu, C.; Tihan, G.T.; Zgârian, R.G.; Fierascu, I.; Baroi, A.M.; Răileanu, S.; Fierăscu, R.C. Metallic and Metal Oxides Nanoparticles for Sensing Food Pathogens—An Overview of Recent Findings and Future Prospects. Materials 2022, 15, 5374. [Google Scholar] [CrossRef]
- Boldeiu, A.; Simion, M.; Mihalache, I.; Radoi, A.; Banu, M.; Varasteanu, P.; Nadejde, P.; Vasile, E.; Acasandrei, A.; Popescu, R.C.; et al. Comparative Analysis of Honey and Citrate Stabilized Gold Nanoparticles: In Vitro Interaction with Proteins and Toxicity Studies. J. Photochem. Photobiol. B 2019, 197, 111519. [Google Scholar] [CrossRef]
- Ruta, L.L.; Banu, M.A.; Neagoe, A.D.; Kissen, R.; Bones, A.M.; Farcasanu, I.C. Accumulation of AG(I) by Saccharomyces Cerevisiae Cells Expressing Plant Metallothioneins. Cells 2018, 7, 266. [Google Scholar] [CrossRef]
- Onuki, Y.; Bhardwaj, U.; Papadimitrakopoulos, F.; Burgess, D.J. Biocompatibility of Implanted Diabetes Devices: Part 2: A Review of the Biocompatibility of Implantable Devices: Current Challenges to Overcome Foreign Body Response. J. Diabetes Sci. Technol. 2008, 2, 1003. [Google Scholar] [CrossRef]
- Neethirajan, S.; Ragavan, V.; Weng, X.; Chand, R. Biosensors for Sustainable Food Engineering: Challenges and Perspectives. Biosensors 2018, 8, 23. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” Synthesis of Metals and Their Oxide Nanoparticles: Applications for Environmental Remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Kiew, S.F.; Boakye-Ansah, S.; Lau, S.Y.; Barhoum, A.; Danquah, M.K.; Rodrigues, J. Green Approaches for the Synthesis of Metal and Metal Oxide Nanoparticles Using Microbial and Plant Extracts. Nanoscale 2022, 14, 2534–2571. [Google Scholar] [CrossRef]
- Beveridge, T.J.; Murray, R.G.E. Sites of Metal Deposition in the Cell Wall of Bacillus Subtilis. J. Bacteriol. 1980, 141, 876–887. [Google Scholar] [CrossRef]
- Patil, S.; Chandrasekaran, R. Biogenic Nanoparticles: A Comprehensive Perspective in Synthesis, Characterization, Application and Its Challenges. J. Genet. Eng. Biotechnol. 2020, 18, 67. [Google Scholar] [CrossRef]
- Koul, B.; Poonia, A.K.; Yadav, D.; Jin, J.O. Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects. Biomolecules 2021, 11, 886. [Google Scholar] [CrossRef]
- Johnston, C.W.; Wyatt, M.A.; Li, X.; Ibrahim, A.; Shuster, J.; Southam, G.; Magarvey, N.A. Gold Biomineralization by a Metallophore from a Gold-Associated Microbe. Nat. Chem. Biol. 2013, 9, 241–243. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Yong, P.; Macaskie, L.E. Enzymatic Recovery of Elemental Palladium by Using Sulfate-Reducing Bacteria. Appl. Env. Microbiol. 1998, 64, 4607–4609. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Suresh Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of Silver Nanocrystals by Bacillus Licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef]
- Baco-Carles, V.; Datas, L.; Tailhades, P. Copper Nanoparticles Prepared from Oxalic Precursors. ISRN Nanotechnol. 2011, 2011, 729594. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Microbial Production of Gold Nanoparticles. Gold Bull. 2006, 39, 22–28. [Google Scholar] [CrossRef]
- Kowshik, M.; Ashtaputre, S.; Kharrazi, S.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Extracellular Synthesis of Silver Nanoparticles by a Silver-Tolerant Yeast Strain. Nanotechnology 2002, 14, 95. [Google Scholar] [CrossRef]
- Pimprikar, P.S.; Joshi, S.S.; Kumar, A.R.; Zinjarde, S.S.; Kulkarni, S.K. Influence of Biomass and Gold Salt Concentration on Nanoparticle Synthesis by the Tropical Marine Yeast Yarrowia Lipolytica NCIM 3589. Colloids Surf. B Biointerfaces 2009, 74, 309–316. [Google Scholar] [CrossRef]
- Govender, Y.; Riddin, T.; Gericke, M.; Whiteley, C.G. Bioreduction of Platinum Salts into Nanoparticles: A Mechanistic Perspective. Biotechnol. Lett. 2009, 31, 95–100. [Google Scholar] [CrossRef]
- Velmurugan, P.; Shim, J.; You, Y.; Choi, S.; Kamala-Kannan, S.; Lee, K.J.; Kim, H.J.; Oh, B.T. Removal of Zinc by Live, Dead, and Dried Biomass of Fusarium Spp. Isolated from the Abandoned-Metal Mine in South Korea and Its Perspective of Producing Nanocrystals. J. Hazard. Mater. 2010, 182, 317–324. [Google Scholar] [CrossRef]
- Das, S.K.; Das, A.R.; Guha, A.K. Adsorption Behavior of Mercury on Functionalized Aspergillus Versicolor Mycelia: Atomic Force Microscopic Study. Langmuir 2009, 25, 360–366. [Google Scholar] [CrossRef]
- Das, S.K.; Das, A.R.; Guha, A.K. Gold Nanoparticles: Microbial Synthesis and Application in Water Hygiene Management. Langmuir 2009, 25, 8192–8199. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Cepoi, L.; Rudi, L.; Chiriac, T.; Valuta, A.; Zinicovscaia, I.; Duca, G.H.; Kirkesali, E.; Frontasyeva, M.; Culicov, O.; Pavlov, S.; et al. Biochemical Changes in Cyanobacteria during the Synthesis of Silver Nanoparticles. Can. J. Microbiol. 2014, 61, 13–21. [Google Scholar] [CrossRef]
- Parial, D.; Pal, R. Green Synthesis of Gold Nanoparticles Using Cyanobacteria and Their Characterization. Indian J. Appl. Res. 2011, 4, 69–72. [Google Scholar] [CrossRef]
- Govindaraju, K.; Kiruthiga, V.; Kumar, V.G.; Singaravelu, G. Extracellular Synthesis of Silver Nanoparticles by a Marine Alga, Sargassum Wightii Grevilli and Their Antibacterial Effects. J. Nanosci. Nanotechnol. 2009, 9, 5497–5501. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Yéprémian, C.; Djédiat, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. Improvement of Kinetics, Yield, and Colloidal Stability of Biogenic Gold Nanoparticles Using Living Cells of Euglena Gracilis Microalga. J. Nanoparticle Res. 2016, 18, 79. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid Synthesis of Au, Ag, and Bimetallic Au Core–Ag Shell Nanoparticles Using Neem (Azadirachta Indica) Leaf Broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Lee, H.J.; Song, J.Y.; Kim, B.S. Biological Synthesis of Copper Nanoparticles Using Magnolia Kobus Leaf Extract and Their Antibacterial Activity. J. Chem. Technol. Biotechnol. 2013, 88, 1971–1977. [Google Scholar] [CrossRef]
- Song, J.Y.; Kwon, E.Y.; Kim, B.S. Biological Synthesis of Platinum Nanoparticles Using Diopyros Kaki Leaf Extract. Bioprocess Biosyst. Eng. 2010, 33, 159–164. [Google Scholar] [CrossRef]
- Thangavelu, R.M.; Ganapathy, R.; Ramasamy, P.; Krishnan, K. Fabrication of Virus Metal Hybrid Nanomaterials: An Ideal Reference for Bio Semiconductor. Arab. J. Chem. 2020, 13, 2750–2765. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tomita, S.; Sawada, K.; Shiba, K.; Yanagi, H.; Yamashita, I.; Uraoka, Y.; Smith, D.R.; Padilla, W.J.; Vier, D.C.; et al. Chiral Meta-Molecules Consisting of Gold Nanoparticles and Genetically Engineered Tobacco Mosaic Virus. Opt. Express 2012, 20, 24856–24863. [Google Scholar] [CrossRef]
- Chaabouni, E.; Gassara, F.; Brar, S.K. Biopolymers Synthesis and Application. In Biotransformation of Waste Biomass into High Value Biochemicals; Springer: New York, NY, USA, 2014; pp. 415–443. ISBN 9781461480051. [Google Scholar]
- Sawant, S.N. Development of Biosensors from Biopolymer Composites. In Biopolymer Composites in Electronics; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 353–383. ISBN 9780081009741. [Google Scholar]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Song, J.; Winkeljann, B.; Lieleg, O. Biopolymer-Based Coatings: Promising Strategies to Improve the Biocompatibility and Functionality of Materials Used in Biomedical Engineering. Adv. Mater. Interfaces 2020, 7, 2000850. [Google Scholar] [CrossRef]
- Pagliano, G.; Ventorino, V.; Panico, A.; Pepe, O. Integrated Systems for Biopolymers and Bioenergy Production from Organic Waste and By-Products: A Review of Microbial Processes. Biotechnol. Biofuels 2017, 10, 1–24. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307. [Google Scholar] [CrossRef]
- Kim, D.S.; Yang, X.; Lee, J.H.; Yoo, H.Y.; Park, C.; Kim, S.W.; Lee, J. Development of GO/Co/Chitosan-Based Nano-Biosensor for Real-Time Detection of D-Glucose. Biosensors 2022, 12, 464. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Ansari, A.A.; Tiwari, A.; Malhotra, B.D. Multi-Walled Carbon Nanotubes/Sol–Gel-Derived Silica/Chitosan Nanobiocomposite for Total Cholesterol Sensor. Sens. Actuators B Chem. 2009, 137, 727–735. [Google Scholar] [CrossRef]
- Leva-Bueno, J.; Meuskens, I.; Linke, D.; Millner, P.A.; Peyman, S.A. A Novel, Proof-of-Concept Electrochemical Impedimetric Biosensor Based on Extracellular Matrix Protein–Adhesin Interaction. Sens. Diagn. 2022, 1, 1003–1013. [Google Scholar] [CrossRef]
- Gomes, N.O.; Carrilho, E.; Machado, S.A.S.; Sgobbi, L.F. Bacterial Cellulose-Based Electrochemical Sensing Platform: A Smart Material for Miniaturized Biosensors. Electrochim. Acta 2020, 349, 136341. [Google Scholar] [CrossRef]
- Bougadi, E.T.; Kalogianni, D.P. Paper-Based DNA Biosensor for Food Authenticity Testing. Food Chem. 2020, 322, 126758. [Google Scholar] [CrossRef]
- Kaushik, A.; Solanki, P.R.; Ansari, A.A.; Sumana, G.; Ahmad, S.; Malhotra, B.D. Iron Oxide-Chitosan Nanobiocomposite for Urea Sensor. Sens. Actuators B Chem. 2009, 138, 572–580. [Google Scholar] [CrossRef]
- Zhao, L.; Wen, Z.; Jiang, F.; Zheng, Z.; Lu, S. Silk/Polyols/GOD Microneedle Based Electrochemical Biosensor for Continuous Glucose Monitoring. RSC Adv. 2020, 10, 6163–6171. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Chakaravarthy, S.; Hernandez-Rangel, A.; Prokhorov, E.; Luna-Bárcenas, G.; Esparza, R.; Meyyappan, M. Chitosan Supported Silver Nanowires as a Platform for Direct Electrochemistry and Highly Sensitive Electrochemical Glucose Biosensing. RSC Adv. 2016, 6, 20102–20108. [Google Scholar] [CrossRef]
- Silva, V.A.O.P.; Fernandes-Junior, W.S.; Rocha, D.P.; Stefano, J.S.; Munoz, R.A.A.; Bonacin, J.A.; Janegitz, B.C. 3D-Printed Reduced Graphene Oxide/Polylactic Acid Electrodes: A New Prototyped Platform for Sensing and Biosensing Applications. Biosens. Bioelectron. 2020, 170, 112684. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Silva, P.R.L.; Lima, A.P.; Rocha, D.P.; Oliveira, T.C.; do Prado, T.M.; Fava, E.L.; Fatibello-Filho, O.; Richter, E.M.; Muñoz, R.A.A. 3D-Printed Graphene/Polylactic Acid Electrode for Bioanalysis: Biosensing of Glucose and Simultaneous Determination of Uric Acid and Nitrite in Biological Fluids. Sens. Actuators B Chem. 2020, 307, 127621. [Google Scholar] [CrossRef]
- Yang, B.; Kong, J.; Fang, X. Bandage-like Wearable Flexible Microfluidic Recombinase Polymerase Amplification Sensor for the Rapid Visual Detection of Nucleic Acids. Talanta 2019, 204, 685–692. [Google Scholar] [CrossRef]
- Park, T.J.; Yoo, S.M.; Keum, K.C.; Lee, S.Y. Microarray of DNA-Protein Complexes on Poly-3-Hydroxybutyrate Surface for Pathogen Detection. Anal. Bioanal. Chem. 2009, 393, 1639–1647. [Google Scholar] [CrossRef]
- Yazdanparast, S.; Benvidi, A.; Banaei, M.; Nikukar, H.; Tezerjani, M.D.; Azimzadeh, M. Dual-Aptamer Based Electrochemical Sandwich Biosensor for MCF-7 Human Breast Cancer Cells Using Silver Nanoparticle Labels and a Poly(Glutamic Acid)/MWNT Nanocomposite. Microchim. Acta 2018, 185, 405. [Google Scholar] [CrossRef]
- Perianes-Rodriguez, A.; Waltman, L.; van Eck, N.J. Constructing Bibliometric Networks: A Comparison between Full and Fractional Counting. J. Inf. 2016, 10, 1178–1195. [Google Scholar] [CrossRef]
- Deshpande, K.; Kanungo, L. Chemiluminescence and Fluorescence Biosensors for Food Application: A Review. Sens. Actuators Rep. 2023, 5, 100137. [Google Scholar] [CrossRef]
- Abdulhussein, S.K.; Fadhel, F.; Al-Kazazz, M.; Rheima, A.M. The Role of Nanomaterials in the Recent Development of Electrochemical Biosensors. Port. Electrochim. Acta 2023, 41, 211–221. [Google Scholar] [CrossRef]
- Khan, A.; Haque, M.N.; Kabiraz, D.C.; Yeasin, A.; al Rashid, H.; Sarker, A.C.; Hossain, G. A Review on Advanced Nanocomposites Materials Based Smart Textile Biosensor for Healthcare Monitoring from Human Sweat. Sens. Actuators A Phys. 2023, 350, 114093. [Google Scholar] [CrossRef]
- Deyasi, A.; Basak, A.; Sarkar, A. Application of Nanomaterial-Based Biosensors for Healthcare Diagnostics. Smart Innov. Syst. Technol. 2023, 322, 103–122. [Google Scholar] [CrossRef]
- Naghdi, T.; Ardalan, S.; Asghari Adib, Z.; Sharifi, A.R.; Golmohammadi, H. Moving toward Smart Biomedical Sensing. Biosens. Bioelectron. 2023, 223, 115009. [Google Scholar] [CrossRef]
- Yau, A.; Wang, Z.; Ponthempilly, N.; Zhang, Y.; Wang, X.; Chen, Y. Biosensor Integrated Tissue Chips and Their Applications on Earth and in Space. Biosens. Bioelectron. 2023, 222, 114820. [Google Scholar] [CrossRef]
- Soozanipour, A.; Ejeian, F.; Boroumand, Y.; Rezayat, A.; Moradi, S. Biotechnological Advancements towards Water, Food and Medical Healthcare: A Review. Chemosphere 2023, 312, 137185. [Google Scholar] [CrossRef]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthc Mater. 2021, 10, 2100062. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U. Novel Biopolymer-Based Sustainable Composites for Food Packaging Applications: A Narrative Review. Food Packag. Shelf Life 2022, 33, 100892. [Google Scholar] [CrossRef]
- Food Packaging Is (Naturally) Getting Smarter-ACS Axial|ACS Publications. Available online: https://axial.acs.org/2022/11/18/food-packaging-is-naturally-getting-smarter/ (accessed on 6 January 2023).
- Purohit, J.; Chattopadhyay, A.; Singh, N.K. Green Synthesis of Microbial Nanoparticle: Approaches to Application. In Nanotechnology in the Life Sciences; Springer Science and Business Media B.V.: Berlin/Heidelberg, Germany, 2019; pp. 35–60. [Google Scholar]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Pectin Modified Metal Nanoparticles and Their Application in Property Modification of Biosensors. Carbohydr. Polym. Technol. Appl. 2021, 2, 100164. [Google Scholar] [CrossRef]
- Ashfaq, A.; Khursheed, N.; Fatima, S.; Anjum, Z.; Younis, K. Application of Nanotechnology in Food Packaging: Pros and Cons. J. Agric. Food Res. 2022, 7, 100270. [Google Scholar] [CrossRef]
- Biswas, R.; Alam, M.; Sarkar, A.; Haque, M.I.; Hasan, M.M.; Hoque, M. Application of Nanotechnology in Food: Processing, Preservation, Packaging and Safety Assessment. Heliyon 2022, 8, e11795. [Google Scholar] [CrossRef]
- Stewart, C.M.; Busta, F.F. Global Harmonization of the Control of Microbiological Risks. Ensuring Glob. Food Saf. 2010, 177, 461–474. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, V.; Sharma, M.M.M.; Patra, A.; Singh, B.; Mehta, S.; Husen, A. A Review on Biosensors and Nanosensors Application in Agroecosystems. Nanoscale Res. Lett. 2021, 16, 136. [Google Scholar] [CrossRef]
- Kaur, H.; Siwal, S.S.; Saini, R.V.; Singh, N.; Thakur, V.K. Significance of an Electrochemical Sensor and Nanocomposites: Toward the Electrocatalytic Detection of Neurotransmitters and Their Importance within the Physiological System. ACS Nanosci. Au 2022, 3, 1–27. [Google Scholar] [CrossRef]
- Ameta, S.K.; Rai, A.K.; Hiran, D.; Ameta, R.; Ameta, S.C. Use of Nanomaterials in Food Science. In Biogenic Nano-Particles and Their Use in Agro-Ecosystems; Springer: Singapore, 2020; pp. 457–488. [Google Scholar] [CrossRef]
- Toydemir, G.; Cekic, S.D.; Ozkan, G.; Uzunboy, S.; Avan, A.N.; Capanoglu, E.; Apak, R. Nanosensors for Foods. Food Eng. Ser. 2020, 327–375. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of Electronic Nose (e-Nose) and Electronic Tongue (e-Tongue) in Food Quality-Related Properties Determination: A Review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Mairhofer, J.; Roppert, K.; Ertl, P. Microfluidic Systems for Pathogen Sensing: A Review. Sensors 2009, 9, 4804. [Google Scholar] [CrossRef]
- Chelliah, R.; Wei, S.; Daliri, E.B.M.; Rubab, M.; Elahi, F.; Yeon, S.J.; Jo, K.H.; Yan, P.; Liu, S.; Oh, D.H. Development of Nanosensors Based Intelligent Packaging Systems: Food Quality and Medicine. Nanomaterials 2021, 11, 1515. [Google Scholar] [CrossRef]
- Rhouati, A.; Bulbul, G.; Latif, U.; Hayat, A.; Li, Z.H.; Marty, J.L. Nano-Aptasensing in Mycotoxin Analysis: Recent Updates and Progress. Toxins 2017, 9, 349. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Singh, S.; Melnik, R. Coupled Multiphysics Modelling of Sensors for Chemical, Biomedical, and Environmental Applications with Focus on Smart Materials and Low-Dimensional Nanostructures. Chemosensors 2022, 10, 157. [Google Scholar] [CrossRef]
- Staples, M.; Daniel, K.; Cima, M.J.; Langer, R. Application of Micro- and Nano-Electromechanical Devices to Drug Delivery. Pharm. Res. 2006, 23, 847–863. [Google Scholar] [CrossRef]
- Kivirand, K.; Kagan, M.; Rinken, T.; Kivirand, K.; Kagan, M.; Rinken, T. Biosensors for the Detection of Antibiotic Residues in Milk. In Biosensors-Micro and Nanoscale Applications; BoD—Books on Demand: Paris, France, 2015. [Google Scholar] [CrossRef]
- Adrian, J.; Pasche, S.; Diserens, J.M.; Sánchez-Baeza, F.; Gao, H.; Marco, M.P.; Voirin, G. Waveguide Interrogated Optical Immunosensor (WIOS) for Detection of Sulfonamide Antibiotics in Milk. Biosens. Bioelectron. 2009, 24, 3340–3346. [Google Scholar] [CrossRef]
- Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef]
- Taj, A.; Zia, R.; Iftikhar, M.; Younis, S.; Bajwa, S.Z. Nanosensors for Food Inspection. Nanosens. Smart Agric. 2022, 685–703. [Google Scholar] [CrossRef]
- The Regulation of Active and Intelligent Food Packaging in the U.S. and the EU*|PackagingLaw.com. Available online: https://www.packaginglaw.com/special-focus/regulation-active-and-intelligent-food-packaging-us-and-eu (accessed on 28 November 2022).
- Sustainable Management of Food Basics|US EPA. Available online: https://www.epa.gov/sustainable-management-food/sustainable-management-food-basics (accessed on 28 November 2022).
- EUR-Lex-32009R0450-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32009R0450&qid=1669628352163 (accessed on 28 November 2022).
- Guidelines on Submission of a Dossier for Safety Evaluation by the EFSA of Active or Intelligent Substances Present in Active and Intelligent Materials and Articles Intended to Come into Contact with Food. EFSA J. 2009, 7, 1208.
- The Potential Risks Arising from Nanoscience and Nanotechnologies on Food and Feed Safety. EFSA J. 2009, 7, 958. [CrossRef]
- Guidance on the Risk Assessment of the Application of Nanoscience and Nanotechnologies in the Food and Feed Chain. EFSA J. 2011, 9, 2140. [CrossRef]
- Astuto, M.C. Overview of the EFSA Guidance on Particle-Technical Requirements and Proposed Appraisal Routes to Establish the Presence of Small Particles in Food and Feed Applications; European Food Safety Authority (EFSA): Parma, Italy, 2022.
- Ahmed, I.; Lin, H.; Zou, L.; Li, Z.; Brody, A.L.; Qazi, I.M.; Lv, L.; Pavase, T.R.; Khan, M.U.; Khan, S.; et al. An Overview of Smart Packaging Technologies for Monitoring Safety and Quality of Meat and Meat Products. Packag. Technol. Sci. 2018, 31, 449–471. [Google Scholar] [CrossRef]
- Nicoletti, M.; del Serrone, P. Intelligent and Smart Packaging. In Future Foods; InTech: London, UK, 2017. [Google Scholar]
- Onyeaka, H.; Passaretti, P.; Miri, T.; Al-Sharify, Z.T. The Safety of Nanomaterials in Food Production and Packaging. Curr. Res. Food Sci. 2022, 5, 763–774. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Valipoor Motlagh, N.; Hamed Mosavian, M.T.; Mortazavi, S.A. Effect of Polyethylene Packaging Modified with Silver Particles on the Microbial, Sensory and Appearance of Dried Barberry. Packag. Technol. Sci. 2013, 26, 39–49. [Google Scholar] [CrossRef]
- Soltani, S.; Nourdahr, R. Study on the Antimicrobial Effect of Nanosilver Tray Packaging of Minced Beef at Refrigerator Temperature. Glob. Vet. 2012, 9, 284–289. [Google Scholar] [CrossRef]
- Metak, A.M. Effects of Nanocomposite Based Nano-Silver and Nano-Titanium Dioxideon Food Packaging Materials. Int. J. Appl. Sci. Technol. 2015, 5, 26–40. [Google Scholar]
- Durak, J.; Rokoszak, T.; Skiba, A.; Furman, P.; Styszko, K. Environmental Risk Assessment of Priority Biocidal Substances on Polish Surface Water Sample. Env. Sci. Pollut. Res. Int. 2021, 28, 1254. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper as a Biocidal Tool. Curr. Med. Chem. 2005, 12, 2163–2175. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute Toxicological Effects of Copper Nanoparticles in Vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.d.F.F.; Coimbra, J.S.d.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioproc. Tech. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Anvar, A.A.; Ahari, H.; Ataee, M. Antimicrobial Properties of Food Nanopackaging: A New Focus on Foodborne Pathogens. Front. Microbiol. 2021, 12, 690706. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Rajini, N.; Nellaiah, H.; Kathiresan, T.; Jawaid, M.; Varada Rajulu, A. Experimental Investigation of Cellulose/Silver Nanocomposites Using In Situ Generation Method. J. Polym. Environ. 2017, 25, 1021–1032. [Google Scholar] [CrossRef]
- Rai, M.; Wypij, M.; Ingle, A.P.; Trzcińska-Wencel, J.; Golińska, P. Emerging Trends in Pullulan-Based Antimicrobial Systems for Various Applications. Int. J. Mol. Sci. 2021, 22, 13596. [Google Scholar] [CrossRef]
- Khalaf, H.H.; Sharoba, A.M.; El-Tanahi, H.H.; Morsy, M.K.; Morsy, M.K. Stability of antimicrobial activity of pullulan edible films incorporated with nanoparticles and essential oils and their impact on turkey deli meat quality. J. Food Dairy Sci. 2013, 4, 557–573. [Google Scholar] [CrossRef]
- Incoronato, A.L.; Conte, A.; Buonocore, G.G.; del Nobile, M.A. Agar Hydrogel with Silver Nanoparticles to Prolong the Shelf Life of Fior Di Latte Cheese. J. Dairy Sci. 2011, 94, 1697–1704. [Google Scholar] [CrossRef]
- Longano, D.; Ditaranto, N.; Cioffi, N.; di Niso, F.; Sibillano, T.; Ancona, A.; Conte, A.; del Nobile, M.A.; Sabbatini, L.; Torsi, L. Analytical Characterization of Laser-Generated Copper Nanoparticles for Antibacterial Composite Food Packaging. Anal. Bioanal. Chem. 2012, 403, 1179–1186. [Google Scholar] [CrossRef]
- Bollella, P.; Schulz, C.; Favero, G.; Mazzei, F.; Ludwig, R.; Gorton, L.; Antiochia, R. Green Synthesis and Characterization of Gold and Silver Nanoparticles and Their Application for Development of a Third Generation Lactose Biosensor. Electroanalysis 2017, 29, 77–86. [Google Scholar] [CrossRef]
- Santhosh, A.; Theertha, V.; Prakash, P.; Smitha Chandran, S. From Waste to a Value Added Product: Green Synthesis of Silver Nanoparticles from Onion Peels Together with Its Diverse Applications. Mater. Today Proc. 2021, 46, 4460–4463. [Google Scholar] [CrossRef]
- Zamarchi, F.; Vieira, I.C. Determination of Paracetamol Using a Sensor Based on Green Synthesis of Silver Nanoparticles in Plant Extract. J. Pharm. Biomed. Anal. 2021, 196, 113912. [Google Scholar] [CrossRef]
- Majdi, H.; Salehi, R.; Pourhassan-Moghaddam, M.; Mahmoodi, S.; Poursalehi, Z.; Vasilescu, S. Antibody Conjugated Green Synthesized Chitosan-Gold Nanoparticles for Optical Biosensing. Colloid Interface Sci. Commun. 2019, 33, 100207. [Google Scholar] [CrossRef]
- Yu, T.; Xu, C.; Qiao, J.; Zhang, R.; Qi, L. Green Synthesis of Gold Nanoclusters Using Papaya Juice for Detection of L-Lysine. Chin. Chem. Lett. 2019, 30, 660–663. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Pei, R.; Xie, Y.; Yao, W.; Guo, Y.; Qian, H. Fast Detection of Bismerthiazol in Cabbage Based on Fluorescence Quenching of Protein-Capping Gold Nanoclusters. Anal. Sci. 2018, 34, 415–419. [Google Scholar] [CrossRef]
- Cheng, Y.; Kang, W.; Guo, Y.; Du, C.; Xie, Y.; Chen, Y.; Yao, W.; Qian, H. Visual Detection of Cu2+ Based on Fluorescence Quenching of Green-Synthesized Gold Nanoclusters Using Soy Protein as Template. Food Agric. Immunol. 2017, 28, 848–858. [Google Scholar] [CrossRef]
- Gayda, G.Z.; Demkiv, O.M.; Stasyuk, N.Y.; Serkiz, R.Y.; Lootsik, M.D.; Errachid, A.; Gonchar, M.V.; Nisnevitch, M. Metallic Nanoparticles Obtained via “Green” Synthesis as a Platform for Biosensor Construction. Appl. Sci. 2019, 9, 720. [Google Scholar] [CrossRef]
- Bagal-Kestwal, D.; Kestwal, R.M.; Chiang, B.H. Invertase-Nanogold Clusters Decorated Plant Membranes for Fluorescence-Based Sucrose Sensor. J. Nanobiotechnol. 2015, 13, 30. [Google Scholar] [CrossRef]
- Amouzadeh Tabrizi, M.; Varkani, J.N. Green Synthesis of Reduced Graphene Oxide Decorated with Gold Nanoparticles and Its Glucose Sensing Application. Sens. Actuators B Chem. 2014, 202, 475–482. [Google Scholar] [CrossRef]
- Dayakar, T.; Rao, K.V.; Bikshalu, K.; Rajendar, V.; Park, S.H. Novel Synthesis and Characterization of Pristine Cu Nanoparticles for the Non-Enzymatic Glucose Biosensor. J. Mater. Sci. Mater. Med. 2017, 28, 109. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhang, Y. One-Step Green Synthesized Fluorescent Carbon Nanodots from Bamboo Leaves for Copper(II) Ion Detection. Sens. Actuators B Chem. 2014, 196, 647–652. [Google Scholar] [CrossRef]
- Sukumar, S.; Rudrasenan, A.; Padmanabhan Nambiar, D. Green-Synthesized Rice-Shaped Copper Oxide Nanoparticles Using Caesalpinia Bonducella Seed Extract and Their Applications. ACS Omega 2020, 5, 1040–1051. [Google Scholar] [CrossRef]
- Singh, P.; Singh, K.R.; Singh, J.; Singh, R.P. Biogenic Synthesis of Copper Oxide Nanoparticles: Characterization and Biosensing Application. ECS Trans. 2022, 107, 20127–20133. [Google Scholar] [CrossRef]
- Karthik, R.; Govindasamy, M.; Chen, S.M.; Mani, V.; Lou, B.S.; Devasenathipathy, R.; Hou, Y.S.; Elangovan, A. Green Synthesized Gold Nanoparticles Decorated Graphene Oxide for Sensitive Determination of Chloramphenicol in Milk, Powdered Milk, Honey and Eye Drops. J. Colloid Interface Sci. 2016, 475, 46–56. [Google Scholar] [CrossRef]
- Kaur, B.; Markan, M.; Singh, M. Green Synthesis of Gold Nanoparticles from Syzygium Aromaticum Extract and Its Use in Enhancing the Response of a Colorimetric Urea Biosensor. Bionanoscience 2012, 2, 251–258. [Google Scholar] [CrossRef]
- Masibi, K.K.; Fayemi, O.E.; Adekunle, A.S.; Sherif, E.S.M.; Ebenso, E.E. Electrochemical Determination of Caffeine Using Bimetallic Au−Ag Nanoparticles Obtained from Low-Cost Green Synthesis. Electroanalysis 2020, 32, 2745–2755. [Google Scholar] [CrossRef]
- Han, B.; Guan, H.; Song, Y.; Liu, Y. Radix Pueraria Flavonoids Assisted Green Synthesis of Reduced Gold Nanoparticles: Application for Electrochemical Nonenzymatic Detection of Cholesterol in Food Samples. ACS Omega 2022, 7, 43045–43054. [Google Scholar] [CrossRef]
- Manne, R.; Kumaradoss, M.M.R.M.; Iska, R.S.R.; Devarajan, A.; Mekala, N. Water Quality and Risk Assessment of Copper Content in Drinking Water Stored in Copper Container. Appl. Water Sci. 2022, 12, 127. [Google Scholar] [CrossRef]
- Pinheiro, T.; Cardoso, A.R.; Sousa, C.E.A.; Marques, A.C.; Tavares, A.P.M.; Matos, A.M.; Cruz, M.T.; Moreira, F.T.C.; Martins, R.; Fortunato, E.; et al. Paper-Based Biosensors for COVID-19: A Review of Innovative Tools for Controlling the Pandemic. ACS Omega 2021, 6, 29268–29290. [Google Scholar] [CrossRef]
- Choi, J.R. Development of Point-of-Care Biosensors for COVID-19. Front Chem. 2020, 8, 517. [Google Scholar] [CrossRef]
- Wang, J.; Drelich, A.J.; Hopkins, C.M.; Mecozzi, S.; Li, L.; Kwon, G.; Hong, S. Gold Nanoparticles in Virus Detection: Recent Advances and Potential Considerations for SARS-CoV-2 Testing Development. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1754. [Google Scholar] [CrossRef]
- Ardekani, L.S.; Thulstrup, P.W. Gold Nanoparticle-Mediated Lateral Flow Assays for Detection of Host Antibodies and COVID-19 Proteins. Nanomaterials 2022, 12, 1456. [Google Scholar] [CrossRef]
- WANTAI SARS-CoV-2 Ab Rapid Test-Letter of Authorization|Enhanced Reader. Available online: https://www.fda.gov/media/140035/download (accessed on 16 January 2023).
- Xu, L.; Li, D.; Ramadan, S.; Li, Y.; Klein, N. Facile Biosensors for Rapid Detection of COVID-19. Biosens. Bioelectron. 2020, 170, 112673. [Google Scholar] [CrossRef]
- Boutal, H.; Moguet, C.; Pommiès, L.; Simon, S.; Naas, T.; Volland, H. The Revolution of Lateral Flow Assay in the Field of AMR Detection. Diagnostics 2022, 12, 1744. [Google Scholar] [CrossRef]
- Coelho, J.H.; Eisele, A.P.P.; Valezi, C.F.; Mattos, G.J.; Schirmann, J.G.; Dekker, R.F.H.; Barbosa-Dekker, A.M.; Sartori, E.R. Exploring the Exocellular Fungal Biopolymer Botryosphaeran for Laccase-Biosensor Architecture and Application to Determine Dopamine and Spironolactone. Talanta 2019, 204, 475–483. [Google Scholar] [CrossRef]
- Ambrosetti, E.; Conti, M.; Teixeira, A.I.; Zilio, S.D. Patterned Carboxymethyl-Dextran Functionalized Surfaces Using Organic Mixed Monolayers for Biosensing Applications. ACS Appl. Bio. Mater. 2022, 5, 3310–3319. [Google Scholar] [CrossRef]
- Kownacka, A.E.; Vegelyte, D.; Joosse, M.; Anton, N.; Toebes, B.J.; Lauko, J.; Buzzacchera, I.; Lipinska, K.; Wilson, D.A.; Geelhoed-Duijvestijn, N.; et al. Clinical Evidence for Use of a Noninvasive Biosensor for Tear Glucose as an Alternative to Painful Finger-Prick for Diabetes Management Utilizing a Biopolymer Coating. Biomacromolecules 2018, 19, 4504–4511. [Google Scholar] [CrossRef]
- Wasik, D.; Mulchandani, A.; Yates, M.V. A Heparin-Functionalized Carbon Nanotube-Based Affinity Biosensor for Dengue Virus. Biosens. Bioelectron. 2017, 91, 811–816. [Google Scholar] [CrossRef]
- Hasanah, U.; Md Sani, N.D.; Heng, L.Y.; Idroes, R.; Safitri, E. Construction of a Hydrogel Pectin-Based Triglyceride Optical Biosensor with Immobilized Lipase Enzymes. Biosensors 2019, 9, 135. [Google Scholar] [CrossRef]
- Gao, J.; Wang, C.; Wang, C.; Chu, Y.; Wang, S.; Sun, M.Y.; Ji, H.; Gao, Y.; Wang, Y.; Han, Y.; et al. Poly-l-Lysine-Modified Graphene Field-Effect Transistor Biosensors for Ultrasensitive Breast Cancer MiRNAs and SARS-CoV-2 RNA Detection. Anal. Chem. 2022, 94, 1626–1636. [Google Scholar] [CrossRef]
- Soysa, H.S.M.; Rattanopas, S.; Teanphonkrang, S.; Quek, T.; Phomphrai, K.; Schulte, A. Biopolymer Cooperation for Sustainable High-Performance Oxidase-Based Biosensing with the Simplest Possible Readout of Substrate Conversion. Adv. Mater. Technol. 2021, 6, 2100096. [Google Scholar] [CrossRef]
- Castrovilli, M.C.; Tempesta, E.; Cartoni, A.; Plescia, P.; Bolognesi, P.; Chiarinelli, J.; Calandra, P.; Cicco, N.; Verrastro, M.F.; Centonze, D.; et al. Fabrication of a New, Low-Cost, and Environment-Friendly Laccase-Based Biosensor by Electrospray Immobilization with Unprecedented Reuse and Storage Performances. ACS Sustain. Chem. Eng. 2022, 10, 1888–1898. [Google Scholar] [CrossRef]
- Gigli, V.; Tortolini, C.; Capecchi, E.; Angeloni, A.; Lenzi, A.; Antiochia, R. Novel Amperometric Biosensor Based on Tyrosinase/Chitosan Nanoparticles for Sensitive and Interference-Free Detection of Total Catecholamine. Biosensors 2022, 12, 519. [Google Scholar] [CrossRef]
- Zhao, M.L.; Zeng, W.J.; Chai, Y.Q.; Yuan, R.; Zhuo, Y.; Zhuo, Y. An Affinity-Enhanced DNA Intercalator with Intense ECL Embedded in DNA Hydrogel for Biosensing Applications. Anal. Chem. 2020, 92, 11044–11052. [Google Scholar] [CrossRef]
- Li, F.; Lyu, D.; Liu, S.; Guo, W. DNA Hydrogels and Microgels for Biosensing and Biomedical Applications. Adv. Mater. 2020, 32, e1806538. [Google Scholar] [CrossRef]
- Mao, X.; Mao, D.; Chen, T.; Jalalah, M.; Al-Assiri, M.S.; Harraz, F.A.; Zhu, X.; Li, G. DNA Hydrogel-Based Three-Dimensional Electron Transporter and Its Application in Electrochemical Biosensing. ACS Appl. Mater. Interfaces 2020, 12, 36851–36859. [Google Scholar] [CrossRef]
- López Marzo, A.M.; Mayorga-Martinez, C.C.; Pumera, M. 3D-Printed Graphene Direct Electron Transfer Enzyme Biosensors. Biosens. Bioelectron. 2020, 151, 111980. [Google Scholar] [CrossRef]
- Muthuchamy, N.; Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Lee, Y.R. High-Performance Glucose Biosensor Based on Green Synthesized Zinc Oxide Nanoparticle Embedded Nitrogen-Doped Carbon Sheet. J. Electroanal. Chem. 2018, 816, 195–204. [Google Scholar] [CrossRef]
- Hojjati-Najafabadi, A.; Salmanpour, S.; Sen, F.; Asrami, P.N.; Mahdavian, M.; Khalilzadeh, M.A. A Tramadol Drug Electrochemical Sensor Amplified by Biosynthesized Au Nanoparticle Using Mentha Aquatic Extract and Ionic Liquid. Top. Catal. 2022, 65, 587–594. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, M.; Singh, G. Recent Advancements in Urea Biosensors for Biomedical Applications. IET Nanobiotechnol. 2021, 15, 358. [Google Scholar] [CrossRef]
- Anbuvannan, M.; Ramesh, M.; Viruthagiri, G.; Shanmugam, N.; Kannadasan, N. Synthesis, Characterization and Photocatalytic Activity of ZnO Nanoparticles Prepared by Biological Method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 143, 304–308. [Google Scholar] [CrossRef]
- Rajiv, P.; Rajeshwari, S.; Venckatesh, R. Bio-Fabrication of Zinc Oxide Nanoparticles Using Leaf Extract of Parthenium Hysterophorus L. and Its Size-Dependent Antifungal Activity against Plant Fungal Pathogens. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 112, 384–387. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Rajeswari, V.D.; Fanizzi, F.P. A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 3569758. [Google Scholar] [CrossRef]
- Zheng, B.; Qian, L.; Yuan, H.; Xiao, D.; Yang, X.; Paau, M.C.; Choi, M.M.F. Preparation of Gold Nanoparticles on Eggshell Membrane and Their Biosensing Application. Talanta 2010, 82, 177–183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).