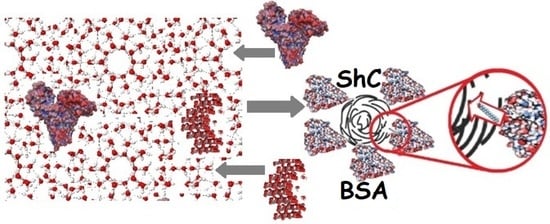
The Role of Water Hydrogen Bonds in the Formation of Associates and Condensates in Dispersions of Serum Albumin with Shungite Carbon and Quartz Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Aqueous Dispersions of Shungite Carbon Nanoparticles
2.1.2. Aqueous Dispersions of Quartz Nanoparticles
2.2. Methods
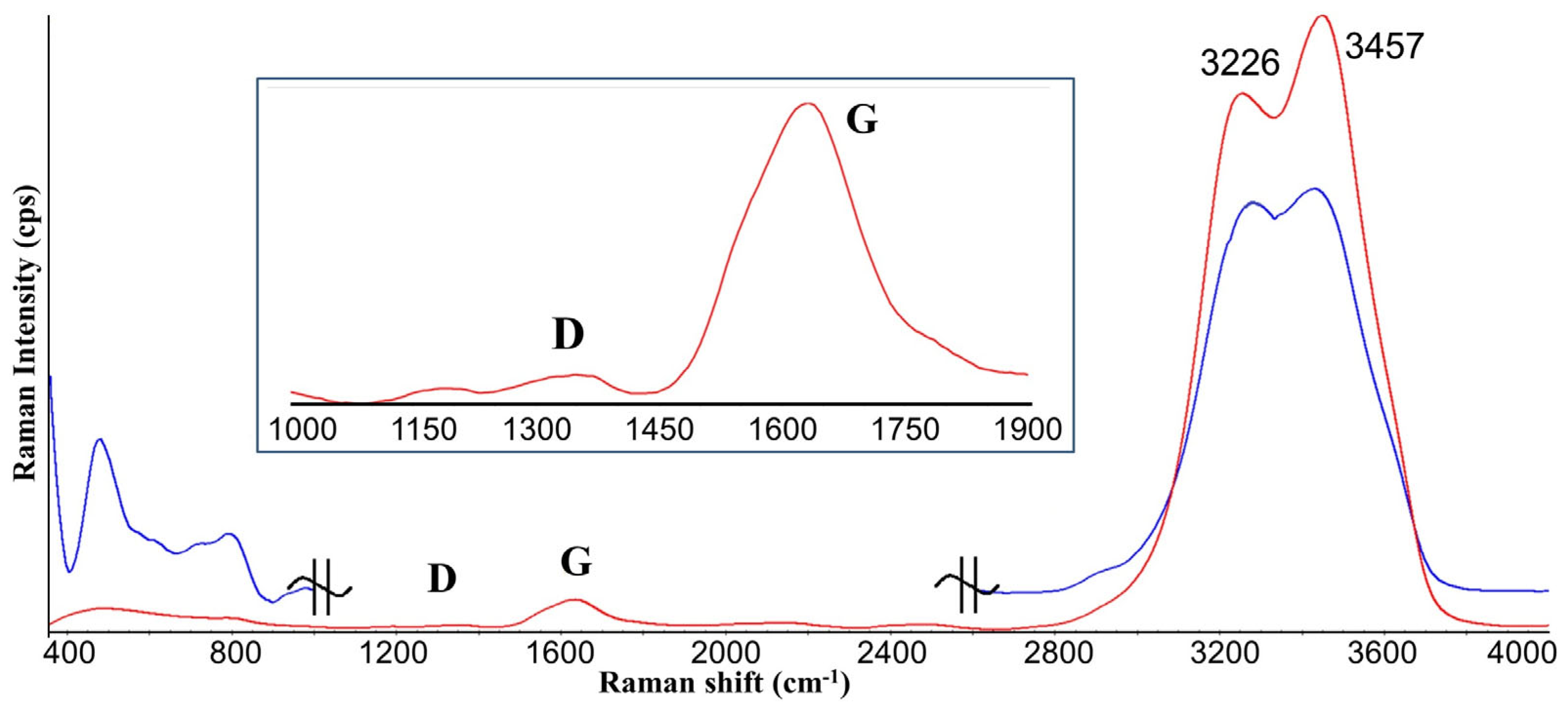
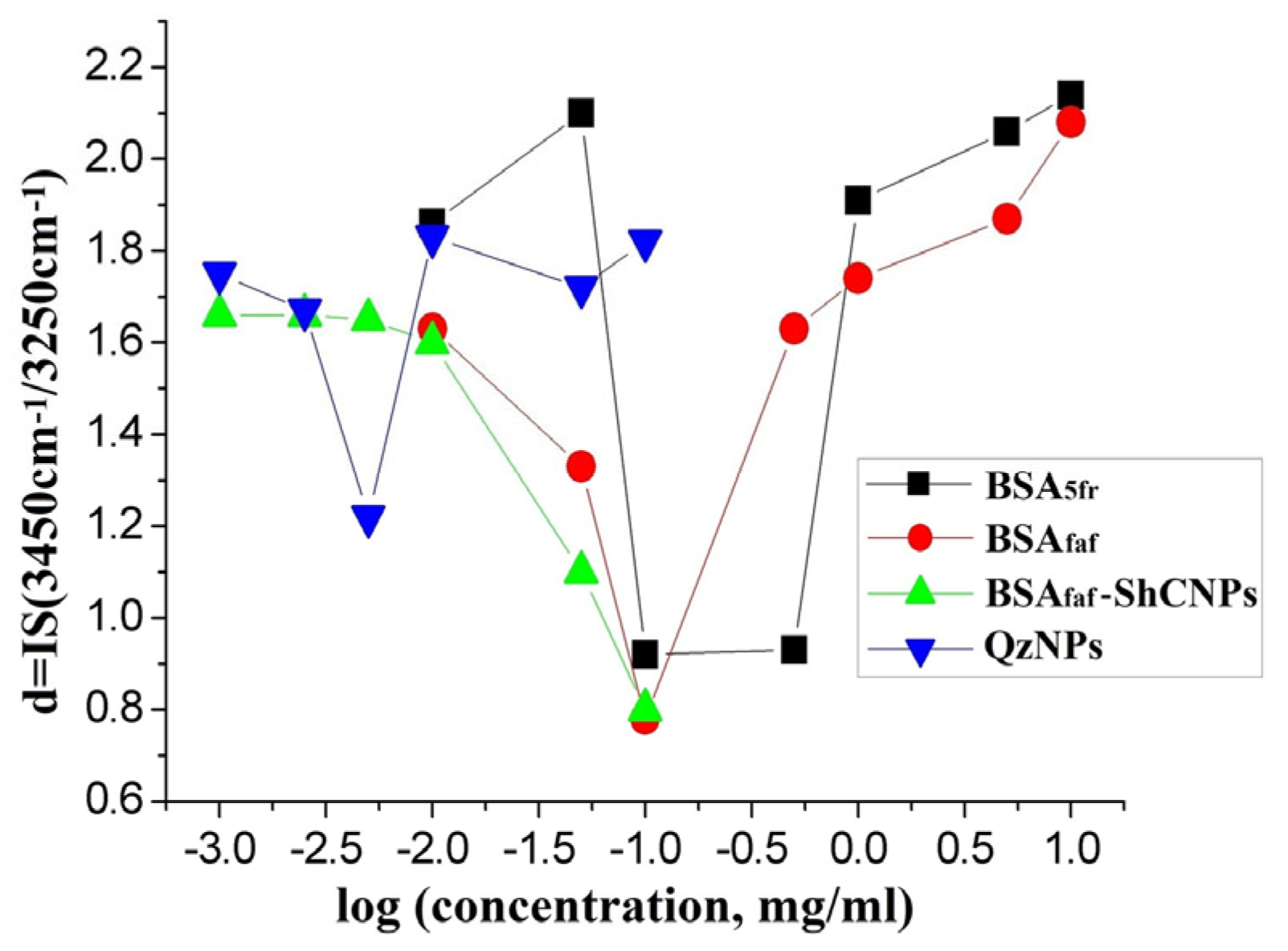
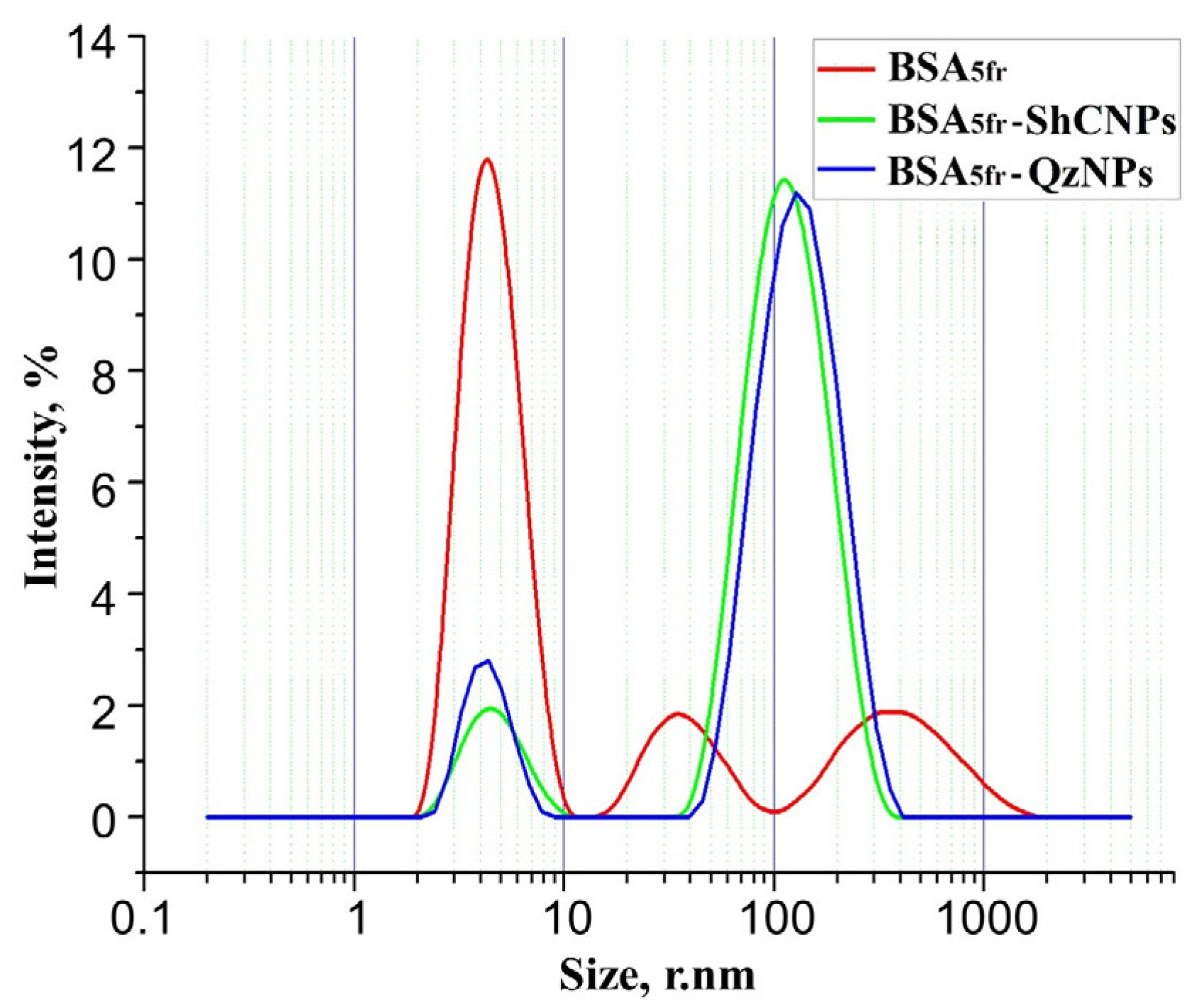
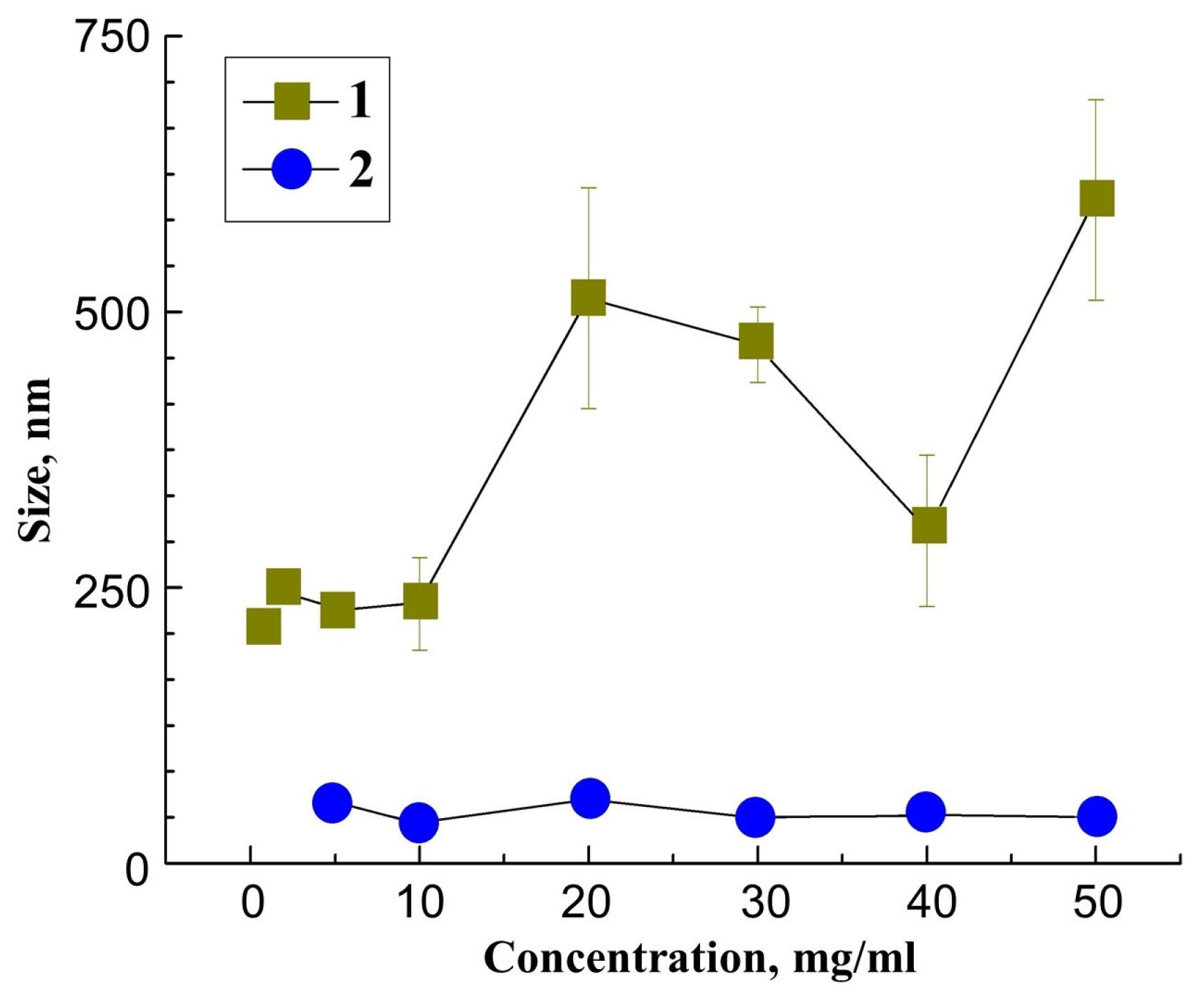
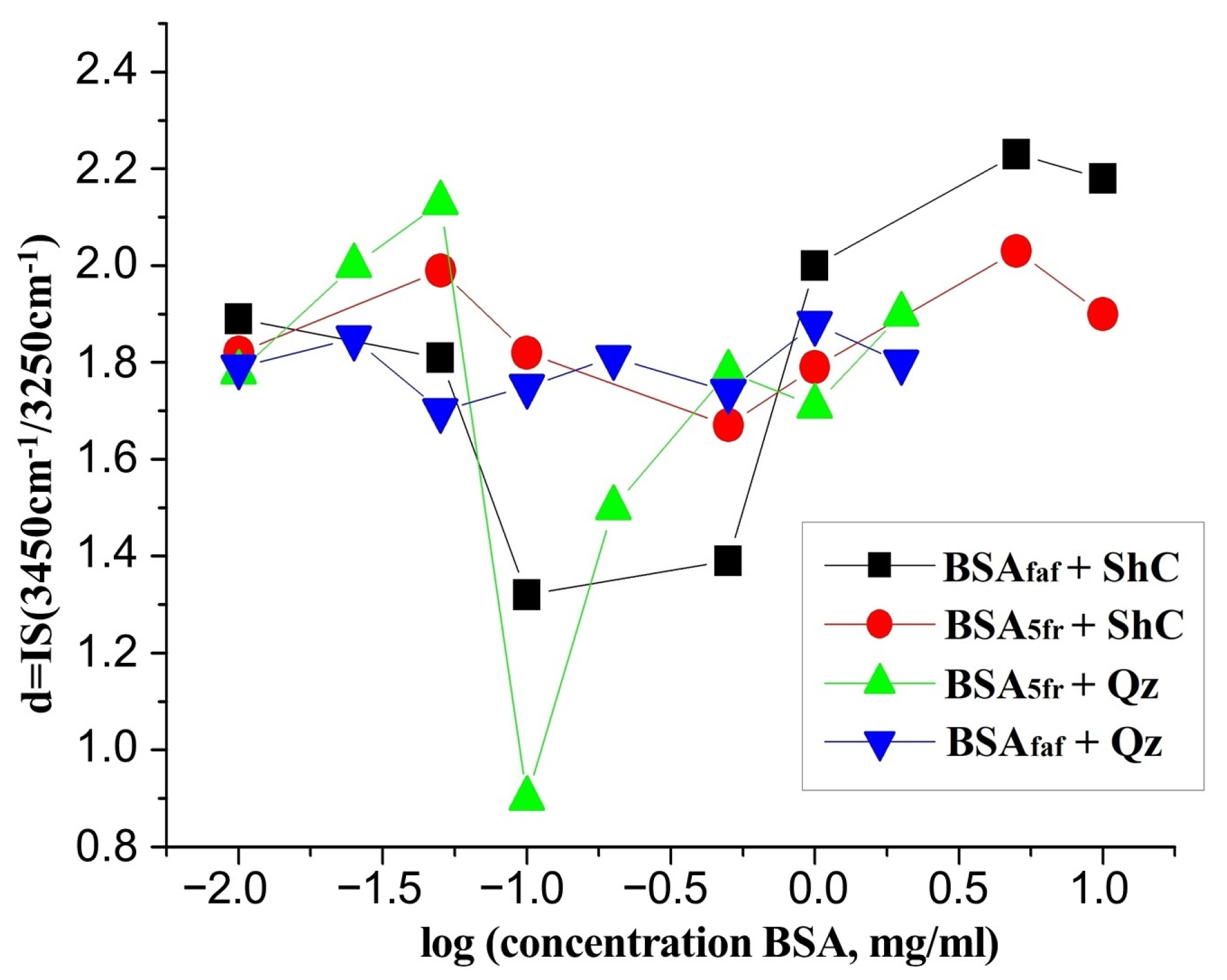
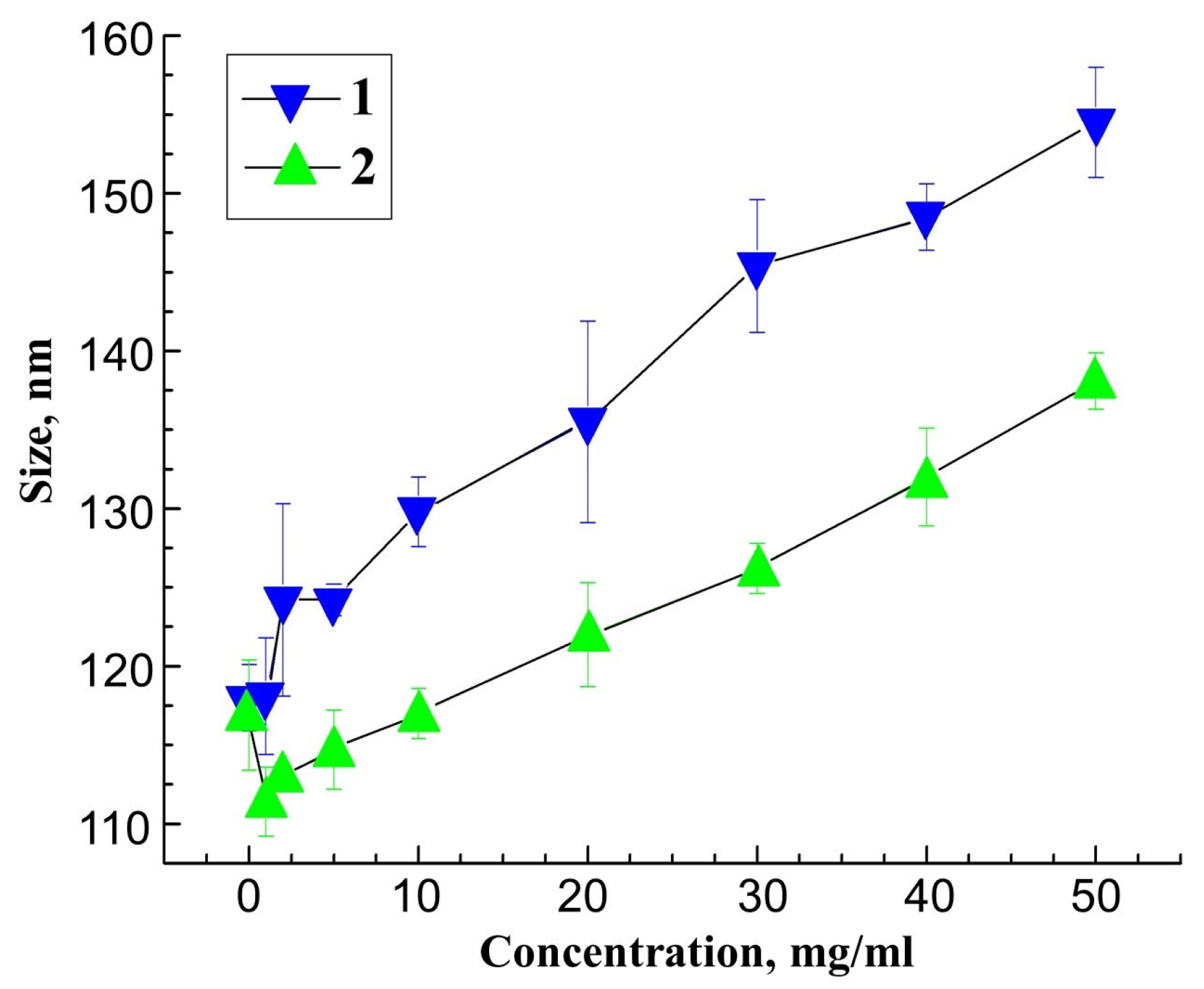
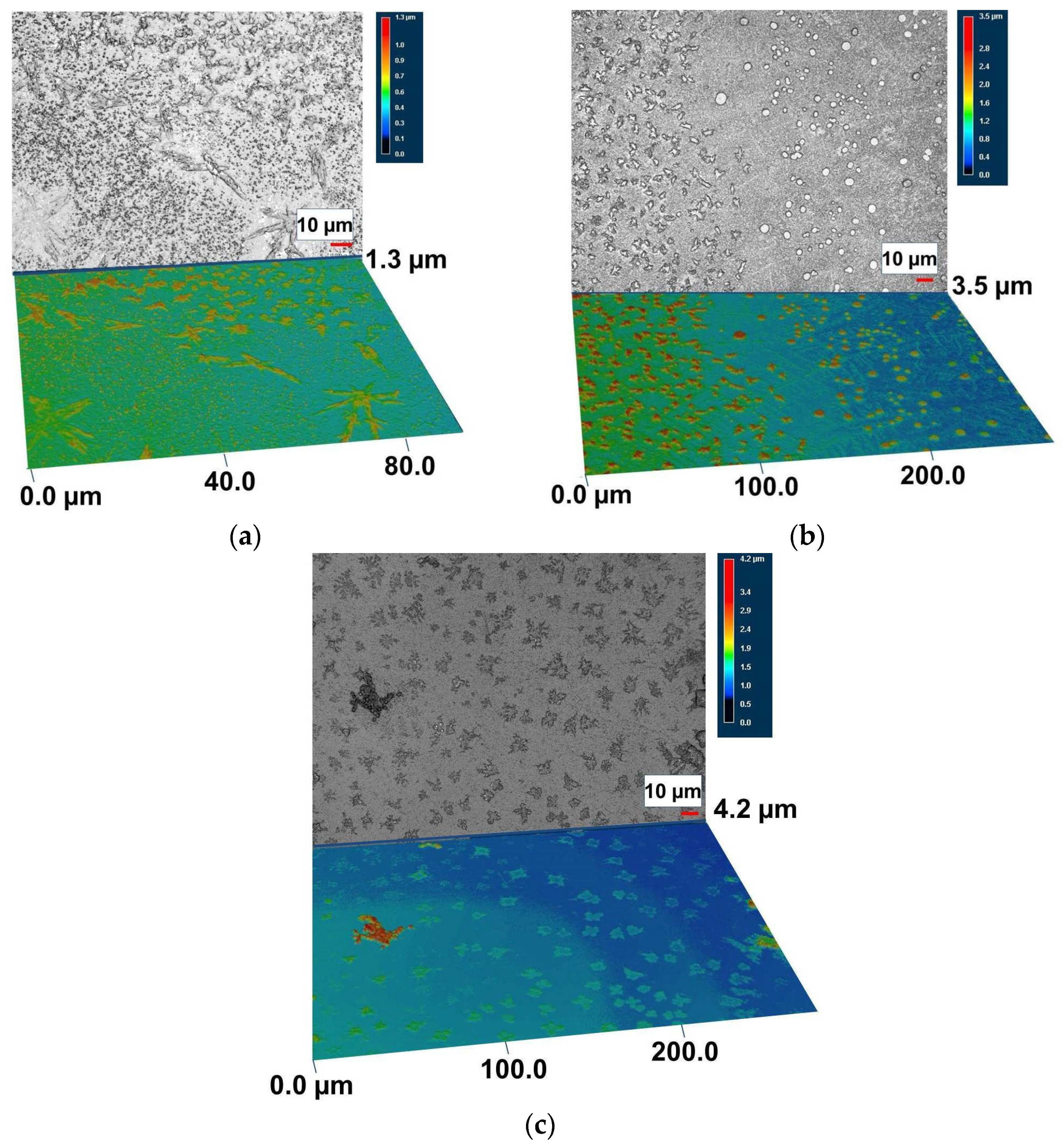
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Cai, R.; Chen, C. The Nano-Bio Interactions of Nanomedicines: Understanding the Biochemical Driving Forces and Redox Reactions. Acc. Chem. Res. 2019, 52, 1507–1518. [Google Scholar] [CrossRef]
- Kopac, T. Protein corona, understanding the nanoparticle-protein interactions and future perspectives: A critical review. Int. J. Biol. Macromol. 2021, 169, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Marichal, L.; Giraudon-Colas, G.; Cousin, F.; Thill, A.; Labarre, J.; Boulard, Y.; Aude, J.C.; Pin, S.; Renault, J.P. Protein-Nanoparticle Interactions: What Are the Protein-Corona Thickness and Organization? Langmuir 2019, 35, 10831–10837. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Lynch, I.; Ejtehadi, M.R.; Monopoli, M.P.; Baldelli Bombelli, F.; Laurent, S. Protein-Nanoparticle Interactions: Opportunities and Challenges. Chem. Rev. 2011, 111, 5610–5637. [Google Scholar] [CrossRef] [PubMed]
- Nasser, F.; Constantinou, J.; Lynch, I. Nanomaterials in the Environment Acquire an “Eco-Corona” Impacting their Toxicity to Daphnia Magna—A Call for Updating Toxicity Testing Policies. Proteomics 2020, 20, 1800412. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.; Rampazzo, E.; Hiebner, D.; Devlin, H.; Quinn, L.; Prodi, L.; Casey, E. Interaction between Engineered Pluronic Silica Nanoparticles and Bacterial Biofilms: Elucidating the Role of Nanoparticle Surface Chemistry and EPS Matrix. ACS Appl. Mater. Interfaces 2022, 14, 34502–34512. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Baldelli Bombelli, F.; Dawson, K.A. Physical-Chemical Aspects of Protein Corona: Relevance to in Vitro and in Vivo Biological Impacts of Nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef]
- Philippe, A.; Schaumann, G.E. Interactions of Dissolved Organic Matter with Natural and Engineered Inorganic Colloids: A Review. Environ. Sci. Technol. 2014, 48, 8946–8962. [Google Scholar] [CrossRef]
- He, J.Z.; Li, C.C.; Wang, D.J.; Zhou, D.M. Biofilms and extracellular polymeric substances mediate the transport of graphene oxide nanoparticles in saturated porous media. J. Hazard. Mater. 2015, 300, 467–474. [Google Scholar]
- Treuel, L.; Brandholt, S.; Maffre, P. Impact of Protein Modification on the Protein Corona on Nanoparticles and Nanoparticle-Cell Interactions. ACS Nano 2014, 8, 503–513. [Google Scholar] [CrossRef]
- Schuberta, J.; Chanana, M. Coating Matters: Review on Colloidal Stability of Nanoparticles with Biocompatible Coatings in Biological Media, Living Cells and Organisms. Curr. Med. Chem. 2018, 25, 4553–4586. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jackman, J.A.; Ferhan, A.R.; Ma, G.J.; Yoon, B.K.; Cho, N.J. Temperature-Induced Denaturation of BSA Protein Molecules for Improved Surface Passivation Coatings. ACS Appl. Mater. Interfaces 2018, 10, 32047–32057. [Google Scholar] [CrossRef] [PubMed]
- Otzen, D.E. Proteins in a brave new surfactant world. Curr. Opin. Colloid Interface Sci. 2015, 20, 161–169. [Google Scholar] [CrossRef]
- Li, D.; Zhang, W.; Yu, X.; Wang, Z.; Su, Z.; Wei, G. When biomolecules meet graphene: From molecule-level interactions to material design and applications. Nanoscale 2016, 8, 19491–19509. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Ahmad, E.; Qadeer, A. Nanoparticles in relation to peptide and protein aggregation. Int. J. Nanomed. 2014, 9, 899–912. [Google Scholar]
- Rosenoer, V.M.; Oratz, M.; Rothschild, M.A. (Eds.) Albumin: Structure, Function and Uses; Elsevier: Amsterdam, The Netherlands, 2014; 412p. [Google Scholar]
- Peters, T., Jr. All about Albumin: Biochemistry, Genetics and Medical Applications; Academic Press, Inc.: San Diego, CA, USA, 1996; 432p. [Google Scholar]
- Sun, B.; Zhang, Y.; Chen, W.; Wang, K.; Zhu, L. Concentration Dependent Effects of Bovine Serum Albumin on Graphene Oxide Colloidal Stability in Aquatic Environment. Environ. Sci. Technol. 2018, 52, 7212–7219. [Google Scholar] [CrossRef] [PubMed]
- Rozhkova, N.N.; Rozhkov, S.P.; Goryunov, A.S. Natural Grapheme-Based Shungite Nanocarbon. In Carbon Nanomaterials Sourcebook: Graphene, Fullerenes, Nanotubes, and Nanodiamonds; Aliofkhazraei, M., Ali, N., Milne, W.I., Eds.; CRC Press Inc. (Taylor and Francis Group): Boka Raton, FL, USA; London, UK; New York, NY, USA, 2016; Volume 1, pp. 153–178. [Google Scholar]
- Rozhkova, N.N. Aggregation and stabilization shungite carbon nanoparticles. Russ. J. Gen. Chem. 2013, 4, 240–251. [Google Scholar] [CrossRef]
- Rozhkova, N.N.; Yemel’yanova, G.I.; Gorlenko, L.E.; Gribanov, A.V.; Lunin, V.V. From stable aqueous dispersion of carbon nanoparticles to the clusters of metastable shungite carbon. Glass Phys. Chem. 2011, 37, 613–618. [Google Scholar] [CrossRef]
- Sheka, E.F.; Popova, N.A. Molecular theory of graphene oxide. Phys. Chem. Chem. Phys. 2013, 15, 13304–13322. [Google Scholar] [CrossRef]
- Razbirin, B.S.; Rozhkova, N.N.; Sheka, E.F. Fractals of graphene quantum dots in photoluminescence of shungite. JETP 2014, 118, 735–746. [Google Scholar] [CrossRef]
- Razbirin, B.S.; Rozhkova, N.N.; Sheka, E.F. Photonics of shungite quantum dots. In Graphene Science Handbook: Electrical and Optical Properties; Aliofkhazraei, M., Ali, N., Milne, W.I., Eds.; CRC Press Inc. (Taylor and Francis Group): Boka Raton, FL, USA; London, UK; New York, NY, USA, 2016; pp. 425–436. [Google Scholar]
- Rozhkov, S.P.; Goryunov, A.S. Structural dynamic effects of protein and other biologically significant molecules interaction with shungite nanocarbon. Trans. Karelian Res. Cent. Russ. Acd. Sci. Exp. Biol. Ser. 2017, 5, 33–44. (In Russian) [Google Scholar]
- Goryunov, A.; Rozhkov, S.; Rozhkova, N. Fatty acid transfer between serum albumins and shungite carbon nanoparticles and its effect on protein aggregation and association. Eur. Biophys. J. 2020, 49, 85–94. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, H.; Ni, K.; Feng, Y. Water clusters and density fluctuations in liquid water based on extended hierarchical clustering methods. Sci.Rep. 2022, 12, 8036. [Google Scholar] [CrossRef] [PubMed]
- Rozhkov, S.P.; Goryunov, A.S.; Kolodey, V.A.; Pron’kina, L.A.; Rozhkova, N.N. Interaction between serum albumin molecules, fatty acids and graphenes of shungite carbon nanoparticles in aqueous dispersion based on raman spectroscopic analysis of water in the high wavenumber region. Biophysics 2022, 67, 884–890. [Google Scholar]
- Chaban, I.A.; Rodnikova, M.N.; Zhakova, V.V. Concentration interval of destruction of the network of hydrogen bonds of water in aqueous solutions of non-electrolytes. Biophysics 1996, 41, 293–298. (In Russian) [Google Scholar]
- Mizukami, M.; Kobayashi, A.; Kurihara, K. Structuring of interfacial water on silica surface in cyclohexane studied by surface forces measurement and sum frequency generation vibrational spectroscopy. Langmuir 2012, 28, 14284–14290. [Google Scholar] [CrossRef]
- Kim, J.; Cremer, P.S. Elucidating changes in interfacial water structure upon protein adsorption. Chem. Phys. Chem. 2001, 2, 543–546. [Google Scholar] [CrossRef]
- Institute of Geology of the Karelian Research Centre of the Russian Academy of Sciences. Method for Obtaining an Aqueous Dispersion of Carbon Nanoparticles from Shungite. Patent RF No. 2642632, 25 January 2018. [Google Scholar]
- Gurunathan, S.; Han, J.W.; Dayem, A.A. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Kim, E.; Han, J. A Novel Biomolecule-Mediated Reduction of Graphene Oxide: A Multifunctional Anti-Cancer Agent. Molecules 2016, 21, 375. [Google Scholar] [CrossRef]
- Goryunov, A.S.; Borisova, A.G.; Rozhkov, S.S.; Rozhkova, N.N. Structural and Colloid Effects of Interaction between Shungite Carbon Nanoparticles and Linoleic Fatty Acid. Curr. Nanosci. 2023, 19, 68–75. [Google Scholar] [CrossRef]
- Institute of Geology of the Karelian Research Centre of the Russian Academy of Sciences. Nanosized Quartz and Method of Its Production. Patent RF No. 2778691, 23 August 2022. [Google Scholar]
- Michnik, A.; Michalik, K.; Drzazga, Z. Stability of bovine serum albumin at different pH. J. Thermal Anal. Calorim. 2005, 80, 399–406. [Google Scholar] [CrossRef]
- Masson, L.E.; O’Brien, C.M.; Pence, I.J.; Herington, J.L.; Reese, J.; van Leeuwenf, T.G.; Mahadevan-Jansen, A. Dual excitation wavelength system for combined fingerprint and high wavenumber Raman spectroscopy. Analyst 2018, 143, 6049–6060. [Google Scholar] [CrossRef] [PubMed]
- Baschenko, S.M.; Marchenko, L.S. On Raman spectra of water, its structure and dependence on temperature. Semicond. Phys. QuantumElectron. Optoelectron. 2011, 14, 77–79. [Google Scholar] [CrossRef]
- Korepanov, V.I.; Hamaguchi, H. Ordered structures in liquid water as studied by Raman spectroscopy and the phonon confinement model. Bull. Chem. Soc. Jpn. 2019, 92, 1127–1130. [Google Scholar] [CrossRef]
- Maeda, Y.; Kitano, H. The structure of water in polymer systems as revealed by Raman spectroscopy. Spectrochim. Acta Part A 1995, 51, 2433–2446. [Google Scholar] [CrossRef]
- Burikov, S.; Dolenko, S.; Dolenko, T.; Patsaeva, S.; Yuzhakov, V. Decomposition of water Raman stretching band with a combination of optimization methods. Mol. Phys. 2010, 108, 739–747. [Google Scholar] [CrossRef]
- Unal, M.; Akkus, O. Shortwave-infrared Raman spectroscopic classification of water fractions inarticular cartilage ex vivo. J. Biomed. Opt. 2018, 23, 1–11. [Google Scholar] [CrossRef]
- Borzova, V.A.; Markossian, K.A.; Chebotareva, N.A.; Kleymenov, S.Y.; Poliansky, N.B.; Muranov, K.O.; Stein-Margolina, V.A.; Shubin, V.V.; Markov, D.I.; Kurganov, B.I. Kinetics of Thermal Denaturation and Aggregation of Bovine Serum Albumin. PLoS ONE 2016, 11, e0153495. [Google Scholar] [CrossRef]
- Shrake, A.J.; Ross, P.D. Biphasic denaturation of human albumin due to ligand redistribution during unfolding. J. Biol. Chem. 1988, 263, 15392–15399. [Google Scholar] [CrossRef]
- Rozhkov, S.P.; Goryunov, A.S. Possible phase effects in dispersion of a globular protein in the temperature range of the protein’s native state. Biophysics 2022, 67, 876–883. [Google Scholar]
- Bulone, D.; Martorana, V.; SanBiagio, P.L. Effects of intermediates on aggregation of native bovine serum albumin. Biophys. Chem. 2001, 91, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergstrom, L.; Cölfen, H. Pre-nucleation clusters as solute precursors in crystallization. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.; Roosen-Runge, F.; Zhang, F.; Lotze, G.; Feoktystov, A.; Jacobs, R.; Schreiber, F. On the question of two-step nucleation in protein crystallization. Faraday Discuss. 2015, 179, 41–58. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar]
- Askhabov, A.M. Pre-nucleation clusters and non-classical crystal formation. Proc. Russ. Mineral. Soc. 2019, 148, 1–13. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozhkov, S.; Goryunov, A.; Kolodey, V.; Pron’kina, L.; Rozhkova, N. The Role of Water Hydrogen Bonds in the Formation of Associates and Condensates in Dispersions of Serum Albumin with Shungite Carbon and Quartz Nanoparticles. Coatings 2023, 13, 471. https://doi.org/10.3390/coatings13020471
Rozhkov S, Goryunov A, Kolodey V, Pron’kina L, Rozhkova N. The Role of Water Hydrogen Bonds in the Formation of Associates and Condensates in Dispersions of Serum Albumin with Shungite Carbon and Quartz Nanoparticles. Coatings. 2023; 13(2):471. https://doi.org/10.3390/coatings13020471
Chicago/Turabian StyleRozhkov, Sergey, Andrey Goryunov, Vladimir Kolodey, Lyubov Pron’kina, and Natalia Rozhkova. 2023. "The Role of Water Hydrogen Bonds in the Formation of Associates and Condensates in Dispersions of Serum Albumin with Shungite Carbon and Quartz Nanoparticles" Coatings 13, no. 2: 471. https://doi.org/10.3390/coatings13020471
APA StyleRozhkov, S., Goryunov, A., Kolodey, V., Pron’kina, L., & Rozhkova, N. (2023). The Role of Water Hydrogen Bonds in the Formation of Associates and Condensates in Dispersions of Serum Albumin with Shungite Carbon and Quartz Nanoparticles. Coatings, 13(2), 471. https://doi.org/10.3390/coatings13020471