Coupled Modeling of Anisotropic Stress-Induced Diffusion and Trapping of Nitrogen in Austenitic Stainless Steel during Nitriding and Thermal Annealing
Abstract
1. Introduction
2. The Governing Equations of the Nitrogen Transport Model
3. Results and Discussion
4. Conclusions
- During the nitriding and post-nitriding annealing of austenitic stainless steels, the same diffusion mechanisms take place: trapping/detrapping and lattice stress-induced diffusion.
- The anisotropic nature of mass transport processes, i.e., dependence on crystallographic lattice orientation, is observed during both processes of nitriding and post-nitriding isothermal annealing.
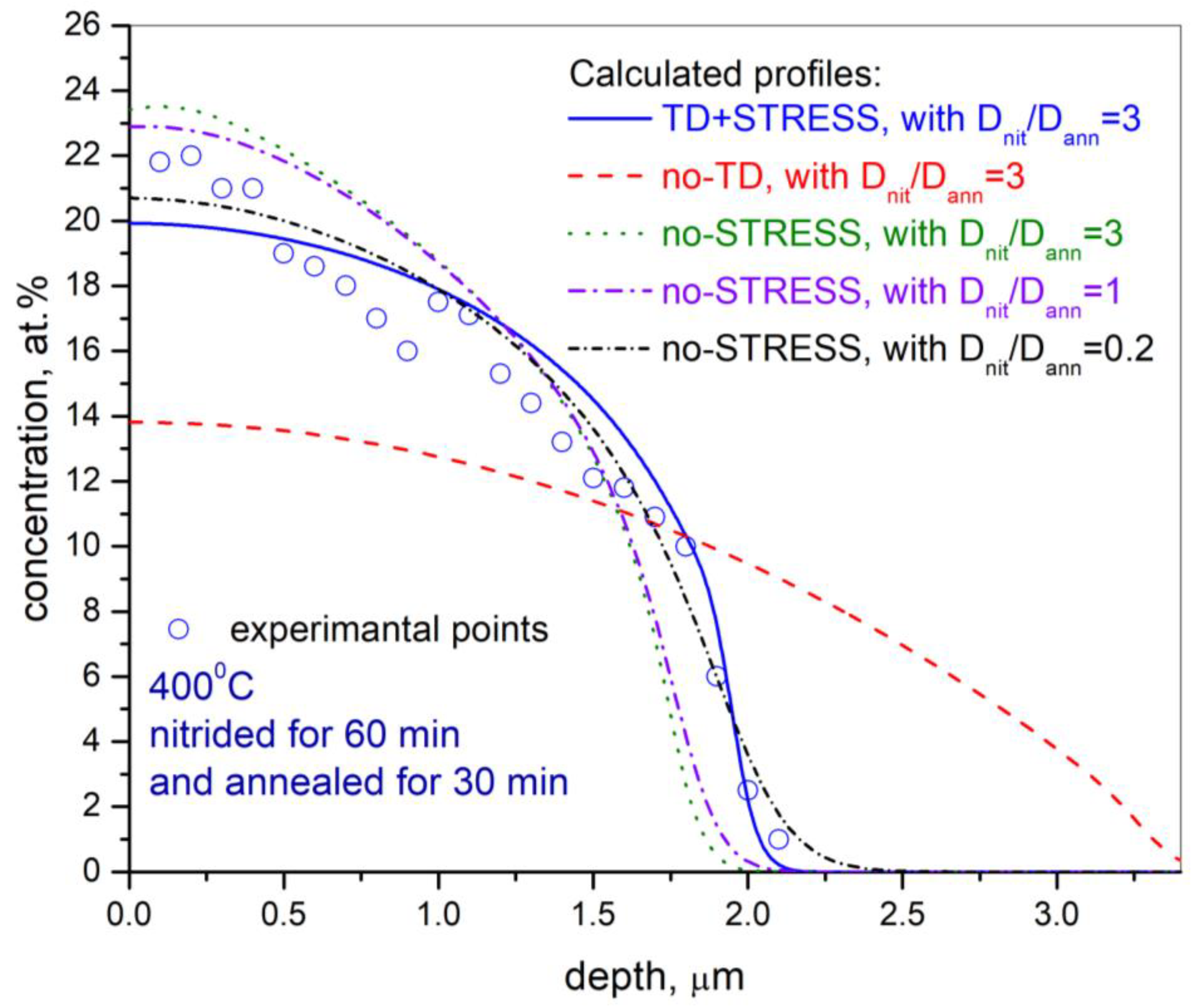
- The trapping/detrapping process has a main influence on the specific distribution depth profile of nitrogen in post-nitrided and post-annealed ASS. However, lattice stress-induced diffusion cannot be excluded.
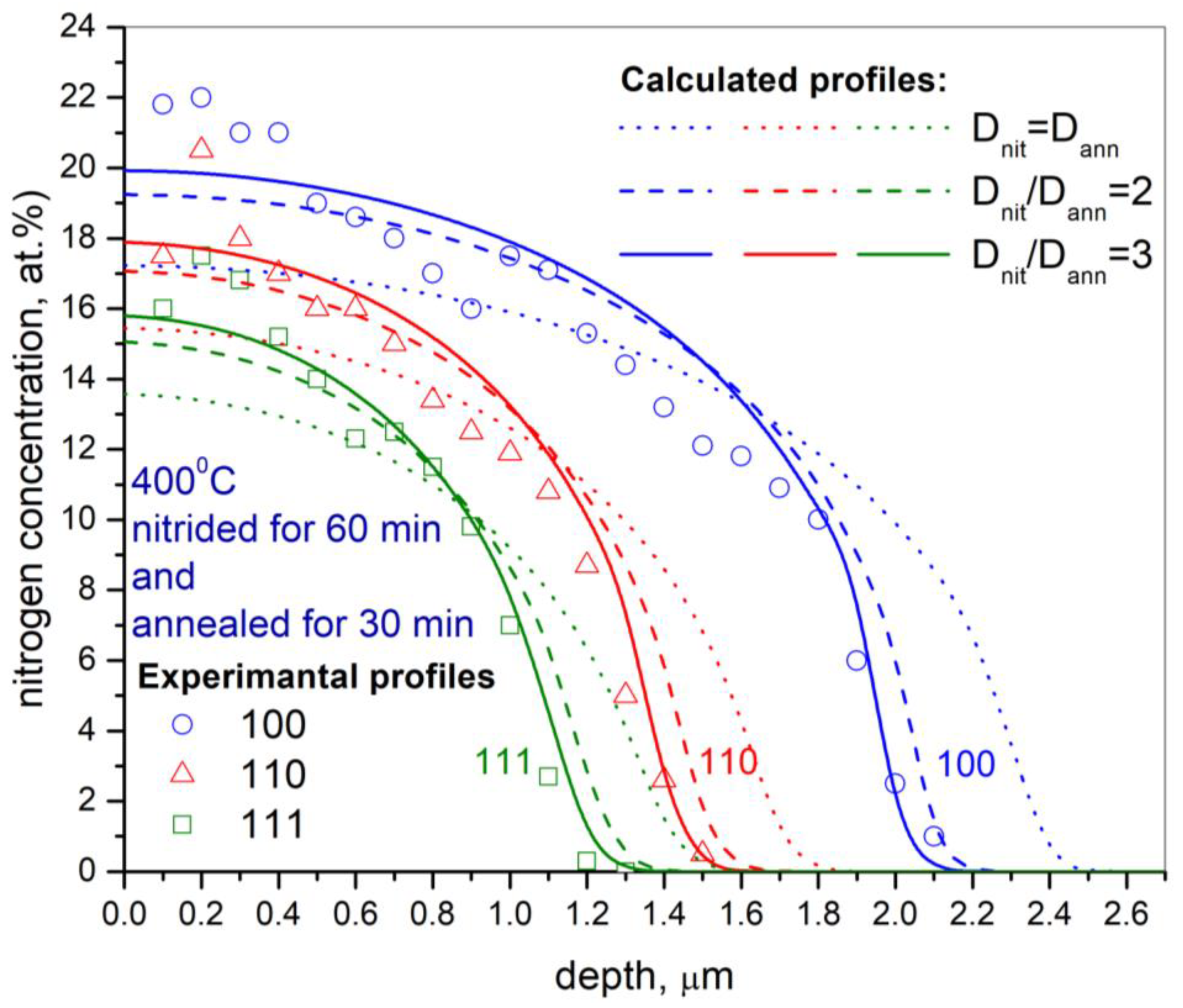
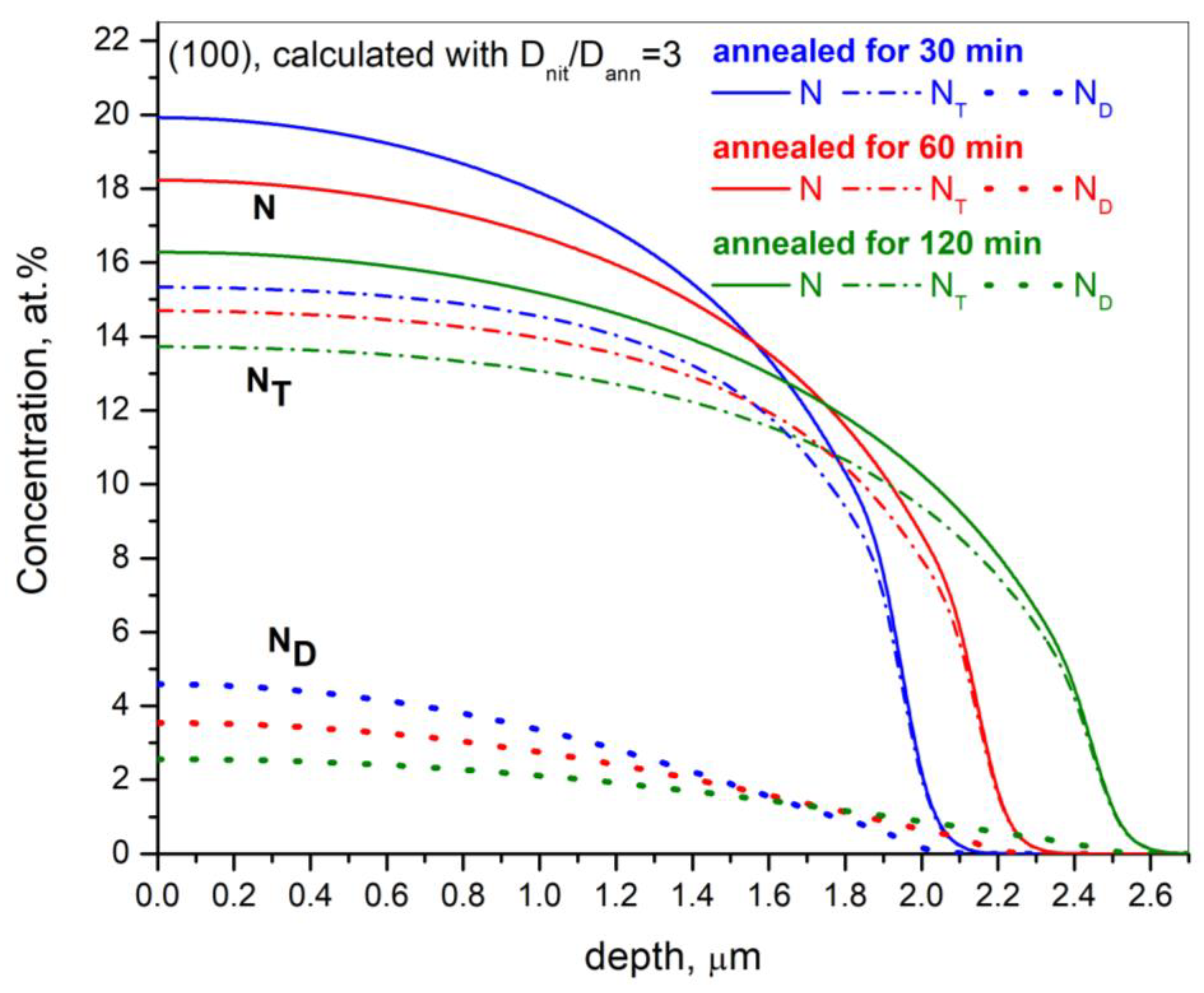
- Nitrogen diffusivity during post-nitriding thermal annealing is significantly lower than during nitriding. The diffusion coefficient during the annealing process, compared with the nitriding process, decreases by factors of 4.3, 3.3, and 2.5 for the orientations (001), (011), and (111), respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menthe, E.; Rie, K.T.; Schultze, J.W.; Simson, S. Structure and properties of plasma-nitrided stainless steel. Surf. Coat. Technol. 1995, 74–75, 412. [Google Scholar] [CrossRef]
- De Las Heras, E.; Ybarra, G.; Lamas, D.; Cabo, A.; Dalibon, E.L.; Brühl, S.P. Plasma nitriding of 316L stainless steel in two different N2-H2 atmospheres—influence on microstructure and corrosion resistance. Surf. Coat. Technol. 2017, 313, 47–54. [Google Scholar] [CrossRef]
- Menthe, E.; Rie, K.T. Further investigation of the structure and properties of austenitic stainless steel after plasma nitriding. Surf. Coat. Technol. 1999, 116–119, 199–204. [Google Scholar] [CrossRef]
- Mingolo, N.; Tschiptschin, A.P.; Pinedo, C.E. On the formation of expanded austenite during plasma nitriding of an AISI 316L austenitic stainless steel. Surf. Coat. Technol. 2006, 201, 4215–4218. [Google Scholar] [CrossRef]
- Keddam, M.; Marcos, G.; Thiriet, T.; Czerwiec, T.; Michel, H. Microstructural characterization of the expanded austenite formed on the plasma nitrided AISI 316 L steel. Mater. Tech. 2013, 101, 204. [Google Scholar] [CrossRef]
- Baldwin, M.J.; Fewell, M.P.; Haydon, S.C.; Kumar, S.; Collins, G.A.; Short, K.T.; Tendys, J. Rf-plasma nitriding of stainless steel. Surf. Coat. Technol. 1998, 98, 1187. [Google Scholar] [CrossRef]
- Fewell, M.P.; Mitchell, D.R.G.; Priest, J.M.; Short, K.T.; Collins, G.A. The nature of expanded austenite. Surf. Coat. Technol. 2000, 131, 300–306. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Hummelshøj, T.S.; Somers, M.A.J. Expanded austenite, crystallography and residual stress. Surf. Eng. 2010, 26, 242–247. [Google Scholar] [CrossRef]
- Picard, S.; Memet, J.B.; Sabot, R.; Grosseau-Poussard, J.L.; Rivière, J.P.; Meilland, R. Corrosion behaviour, microhardness and surface characterisation of low energy, high current ion implanted austenitic stainless steel. Mater. Sci. Eng. A 2001, 303, 163–172. [Google Scholar] [CrossRef]
- Baranowska, J. Characteristic of the nitrided layers on the stainless steel at low temperature. Surf. Coat. Technol. 2004, 180–181, 145–149. [Google Scholar] [CrossRef]
- Williamson, D.L.; Davis, J.A.; Wilbur, P.J. Effect of austenitic stainless steel composition on low-energy, high-flux, nitrogen ion beam processing. Surf. Coat. Technol. 1998, 103–104, 178. [Google Scholar] [CrossRef]
- He, H.; Czerwiec, T.; Dong, C.; Michel, H. Effect of grain orientation on the nitriding rate of a nickel base alloy studied by electron backscatter diffraction. Surf. Coat. Technol. 2003, 163, 331. [Google Scholar] [CrossRef]
- Czerwiec, T.; Renevier, N.; Michel, H. Low-temperature plasma-assisted nitriding. Surf. Coat. Technol. 2000, 131, 267. [Google Scholar] [CrossRef]
- Dahm, K.L.; Dearnley, P.A. On the nature, properties and wear response of S-phase (nitrogen alloyed stainless steel) coatings on AISI 316L. Proc. Inst. Mech. Eng. 2000, 214, 181–198. [Google Scholar] [CrossRef]
- Saker, A.; He, H.; Czerwiec, T.; Li, X.; Huu, L.T.; Dong, C.; Michel, H.; Frantz, C. Reactive magnetron sputtering of Inconel 690 by Ar–N2 plasma. Thin Solid Films 2008, 516, 1029–1036. [Google Scholar] [CrossRef]
- Terwagne, G.; Colaux, J.; Mitchell, D.R.; Short, K.T. Temperature effect of nitride stainless steel coatings deposited by reactive DC-magnetron sputtering. Thin Solid Films 2004, 469–470, 167–172. [Google Scholar] [CrossRef]
- Baranowska, J.; Fryska, S.; Przekop, J.; Suszko, T. The properties of hard coating composed of S-phase obtained by PVD method. Adv. Manuf. Sci. Technol. 2009, 33, 59–69. [Google Scholar]
- Fryska, S.; Baranowska, J. Microstructure of reactive magnetron sputtered S-phase coatings with a diffusion sub-layer. Vacuum 2017, 142, 72–80. [Google Scholar] [CrossRef]
- Kappaganthu, S.R.; Sun, Y. Influence of sputter deposition conditions on phase evolution in nitrogen-doped stainless steel films. Surf. Coat. Technol. 2005, 198, 59. [Google Scholar] [CrossRef]
- Baranowska, J.; Fryska, S.; Suszko, T. The influence of temperature on S-phase coatings deposition by reactive magnetron sputtering. Vacuum 2013, 90, 160–164. [Google Scholar] [CrossRef]
- Czerwiec, T.; Andrieux, A.; Marcos, G.; Michel, H.; Bauer, P. Is “expanded austenite” really a solid solution? Mossbauer observation of an annealed AISI 316L nitrided sample. J. Alloy. Compd. 2019, 811, 151972. [Google Scholar] [CrossRef]
- Jirásková, Y.; Blawert, C.; Schneeweiss, O. Thermal Stability of Stainless Steel Surfaces Nitrided by Plasma Immersion Ion Implantation. Phys. Status Solidi 1999, 175, 537–548. [Google Scholar] [CrossRef]
- Borgioli, F. The “Expanded” Phases in the Low-Temperature Treated Stainless Steels: A Review. Metals 2022, 12, 331. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Wang, D.; Qiu, S.; Ernst, F. Thermal stability of low-temperature-carburized austenitic stainless steel. Acta Mater. 2017, 128, 235–240. [Google Scholar] [CrossRef]
- Molleja, G.; Milanese, M.; Piccoli, M.; Moroso, R.; Niedbalski, J.; Nosei, L.; Feugeas, J. Stability of expanded austenite, generated by ion carburizing and ion nitriding of AISI 316L SS, under high temperature and high energy pulsed ion beam irradiation. Surf. Coat. Technol. 2013, 218, 142–151. [Google Scholar] [CrossRef]
- Tschiptschin, A.P.; Nishikawa, A.S.; Varela, L.B.; Pinedo, C.E. Thermal stability of expanded austenite formed on a DC plasma nitrided 316L austenitic stainless steel. Thin Solid Films 2017, 644, 156–165. [Google Scholar] [CrossRef]
- Djellal, R.; Saker, A.; Bouzabata, B.; Mekki, D.E. Thermal stability and phase decomposition of nitrided layers on 316L and 310 austenitic stainless steels. Surf. Coat. Technol. 2017, 325, 533–538. [Google Scholar] [CrossRef]
- Wu, D.; Kahn, H.; Dalton, J.C.; Michal, G.M.; Ernst, F.; Heuer, A.H. Orientation dependence of nitrogen supersaturation in austenitic stainless steel during low-temperature gas-phase nitriding. Acta Mater. 2014, 79, 339. [Google Scholar] [CrossRef]
- Menéndez, E.; Templier, C.; Garcia-Ramirez, P.; Santiso, J.; Vantomme, A.; Temst, K.; Nogués, J. Magnetic properties of single crystalline expanded austenite obtained by plasma nitriding of austenitic stainless steel single crystals. ACS Appl. Mater. Interfaces 2013, 5, 10118–10126. [Google Scholar] [CrossRef] [PubMed]
- Fewell, M.P.; Priest, J.M. High-order diffractometry of expanded austenite using synchrotron radiation. Surf. Coat. Technol. 2008, 202, 802–1815. [Google Scholar] [CrossRef]
- Borgioli, F. From austenitic stainless steel to expanded austenite-S phase: Formation, characteristics and properties of an elusive metastable phase. Metals 2020, 10, 187. [Google Scholar] [CrossRef]
- Martinavičius, A.; Abrasonis, G.; Möller, W. Influence of crystal orientation and ion bombardment on the nitrogen diffusivity in single-crystalline austenitic stainless steel. J. Appl. Phys. 2011, 110, 075907. [Google Scholar] [CrossRef]
- Kahn, H.; Michal, G.M.; Ernst, F.; Heuer, A.H. Poisson effects on X-ray diffraction patterns in low-temperature-carburized austenitic stainless steel. Metall. Mater. Trans. A 2009, 40, 1799–1804. [Google Scholar] [CrossRef]
- He, H.; Zou, J.X.; Dong, C.; Czerwiec, T.; Michel, H. Stress induced anisotropic diffusion during plasma-assisted nitriding of a Ni-based alloy. Mater. Sci. Forum 2005, 475, 3669–3672. [Google Scholar] [CrossRef]
- Moskalioviene, T.; Galdikas, A. Crystallographic Orientation Dependence of Nitrogen Mass Transport in Austenitic Stainless Steel. Metals 2020, 10, 615. [Google Scholar] [CrossRef]
- Somers, M.A.J.; Kücükyildiz, Ö.C.; Ormstrup, C.A.; Alimadadi, H.; Hattel, J.H.; Christiansen, T.L.; Winther, G. Residual stress in expanded austenite on stainless steel; origin, measurement, and prediction. Mater. Perform. Charact. 2018, 7, 693–716. [Google Scholar] [CrossRef]
- Mändl, S.; Rauschenbach, B. Anisotropic strain in nitrided austenitic stainless steel. J. Appl. Phys. 2000, 88, 3323–3329. [Google Scholar] [CrossRef]
- Moskalioviene, T.; Galdikas, A. Kinetic model of anisotropic stress assisted diffusion of nitrogen in nitrided austenitic stainless steel. Surf. Coat. Technol. 2019, 366, 277–285. [Google Scholar] [CrossRef]
- Galdikas, A.; Moskalioviene, T. Modeling of stress induced nitrogen diffusion in nitrided stainless steel. Surf. Coat. Technol. 2011, 205, 3742–3746. [Google Scholar] [CrossRef]
- Moskalioviene, T.; Galdikas, A. Stress induced and concentration dependent diffusion of nitrogen in plasma nitrided austenitic stainless steel. Vacuum 2012, 86, 1552–1557. [Google Scholar] [CrossRef]
- Jespersen, F.N.; Hattel, J.H.; Somers, M.A.J. Modelling the evolution of composition-and stress-depth profiles in austenitic stainless steels during low-temperature nitriding. Model. Simul. Mat. Sci. Eng. 2016, 24, 025003. [Google Scholar] [CrossRef]
- Kücükyildiz, Ö.C.; Grumsen, F.B.; Christiansen, T.L.; Winther, G.; Somers, M.A.J. Anisotropy effects on gaseous nitriding of austenitic stainless steel single crystals. Acta Mater. 2020, 194, 168–177. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Somers, M.A.J. The Influence of Stress on Interstitial Diffusion—Carbon Diffusion Data in Austenite Revisited. Defect Diffus. Forum 2010, 297–301, 1408–1413. [Google Scholar] [CrossRef]
- Parascandola, S.; Moller, W.; Williamson, D.L. The nitrogen transport in austenitic stainless steel at moderate temperatures. Appl. Phys. Lett. 2000, 76, 2194–2196. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Drouet, M.; Martinavičius, A.; Somers, M.A.J. Isotope exchange investigation of nitrogen redistribution in expanded austenite. Scr. Mater. 2013, 69, 582–585. [Google Scholar] [CrossRef]
- Galdikas, A.; Moskalioviene, T. The Anisotropic Stress-Induced Diffusion and Trapping of Nitrogen in Austenitic Stainless Steel during Nitriding. Metals 2020, 10, 1319. [Google Scholar] [CrossRef]
- Mändl, S.; Scholze, F.; Neumann, H.; Rauschenbach, B. Nitrogen diffusivity in expanded austenite. Surf. Coat. Technol. 2003, 174–175, 1191–1195. [Google Scholar] [CrossRef]
- Oriani, R. The diffusion and trapping of hydrogen in steel. Acta Metall. 1970, 18, 147–157. [Google Scholar] [CrossRef]
- Moskalioviene, T.; Galdikas, A.; Riviere, J.P.; Pichon, L. Modeling of nitrogen penetration in polycrystalline AISI 316L austenitic stainless steel during plasma nitriding. Surf. Coat. Technol. 2011, 205, 3301–3306. [Google Scholar] [CrossRef]
- Möller, W.; Parascandola, S.; Telbizova, T.; Günzel, R.; Richter, E. Surface processes and diffusion mechanisms of ion nitriding of stainless steel and aluminium. Surf. Coat. Technol. 2001, 136, 73–79. [Google Scholar] [CrossRef]
- Zhang, L.; Barrett, R.; Cloetens, P.; Detlefs, C.; Rio, M.S. Anisotropic elasticity of silicon and its application to the modelling of X-ray optics. J. Synchrotron. Radiat. 2014, 21, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Zener, C. Contributions to the Theory of Beta-Phase Alloys. Phys. Rev. 1947, 71, 846–851. [Google Scholar] [CrossRef]
- Martinavičius, A.; Abrasonis, G.; Möller, W.; Templier, C.; Rivière, J.P.; Declémy, A.; Chumlyakov, Y. Anisotropic ion-enhanced diffusion during ion nitriding of single crystalline austenitic stainless steel. J. Appl. Phys. 2009, 105, 93502. [Google Scholar] [CrossRef]
- Juul, N.Y.; Oddershede, J.; Beaudoin, A.; Chatterjee, K.; Koker, M.K.A.; Dale, D.; Shade, P.; Winther, G. Measured resolved shear stresses and Bishop-Hill stress states in individual grains of austenitic stainless steel. Acta Mater. 2017, 141, 388–404. [Google Scholar] [CrossRef]
- Gressmann, T.; Wohlschlogel, M.; Shang, S.; Welzel, U.; Leineweber, A.; Mittemeijer, E.J.; Liu, Z.K. Elastic anisotropy of γ’-Fe4N and elastic grain interaction in γ’-Fe4N1−y layers on α-Fe: First-principles calculations and diffraction stress measurements. Acta Mater. 2007, 55, 5833–5843. [Google Scholar] [CrossRef]
- Galdikas, A.; Pranevičius, L. Surface composition changes of ternary alloys in the non-steady state regime of preferential sputtering. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2000, 164, 868–872. [Google Scholar] [CrossRef]
- Abrasonis, G.; Möller, W.; Ma, X.X. Anomalous Ion Accelerated Bulk Diffusion of Interstitial Nitrogen. Phys. Rev. Lett. 2006, 96, 065901. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Jung, M.; Meka, S.R.; Fonović, M.; Leineweber, A.; Mittemeijer, E.J. Dependence of the nitriding rate of ferritic and austenitic substrates on the crystallographic orientation of surface grains; gaseous nitriding of Fe-Cr and Ni-Ti alloys. Philos. Mag. 2015, 95, 4143–4160. [Google Scholar] [CrossRef]
- Christiansen, T.; Dahl, K.V.; Somers, M.A.J. Nitrogen diffusion and nitrogen depth profiles in expanded austenite: Experimental assessment, numerical simulation and role of stress. Mater. Sci. Technol. 2008, 24, 159–167. [Google Scholar] [CrossRef]
- Kücükyildiz, Ö.C.; Sonne, M.R.; Thorborg, J.; Somers, M.A.J.; Hattel, J.H. Thermo-chemical-mechanical simulation of low temperature nitriding of austenitic stainless steel; inverse modelling of surface reaction rates. Surf. Coat. Technol. 2020, 381, 125145. [Google Scholar] [CrossRef]



| S11, 1/GPa | S12, 1/GPa | S44, 1/GPa | Zener Ratio | |
|---|---|---|---|---|
| AISI 316L γ phase [54] | 9.84 × 10−3 | −3.86 × 10−3 | 8.40 × 10−3 | 3.26 |
| AISI 316L γN phase [55] | 4.43 × 10−3 | −1.35 × 10−3 | 21.7 × 10−3 | 0.53 |
| vsp, cm/s [53] | iN, s−1 | EA, eV [49] | EB, eV [49] | Do, cm2s−1 |
| 1.3 × 10−8 | 11.8 × 10−4 | 1.1 | 0.23 | 1.12 × 10–4 |
| N0, cm−3 [49] | Ht, cm−3 [49] | VN, m3/mol | Rt, cm [49] | αN |
| 7.29 × 1022 | 1.31 × 1022 | 3.9 × 10−5 | 0.38 × 10−7 | 0.8 |
| βN, cm/at.% [46] | a0, cm [46] | Xstress(hkl), MPa/at.% | ||
| 7.5 × 10−11 | 0.36 × 10−7 | Xstress values for each of the three considered crystallographic orientations were taken from our previous work [46] (see Figure 2 in [46]) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moskaliovienė, T.; Andriūnas, P.; Galdikas, A. Coupled Modeling of Anisotropic Stress-Induced Diffusion and Trapping of Nitrogen in Austenitic Stainless Steel during Nitriding and Thermal Annealing. Coatings 2023, 13, 415. https://doi.org/10.3390/coatings13020415
Moskaliovienė T, Andriūnas P, Galdikas A. Coupled Modeling of Anisotropic Stress-Induced Diffusion and Trapping of Nitrogen in Austenitic Stainless Steel during Nitriding and Thermal Annealing. Coatings. 2023; 13(2):415. https://doi.org/10.3390/coatings13020415
Chicago/Turabian StyleMoskaliovienė, Teresa, Paulius Andriūnas, and Arvaidas Galdikas. 2023. "Coupled Modeling of Anisotropic Stress-Induced Diffusion and Trapping of Nitrogen in Austenitic Stainless Steel during Nitriding and Thermal Annealing" Coatings 13, no. 2: 415. https://doi.org/10.3390/coatings13020415
APA StyleMoskaliovienė, T., Andriūnas, P., & Galdikas, A. (2023). Coupled Modeling of Anisotropic Stress-Induced Diffusion and Trapping of Nitrogen in Austenitic Stainless Steel during Nitriding and Thermal Annealing. Coatings, 13(2), 415. https://doi.org/10.3390/coatings13020415








