Study on Adhesion Properties and Process Parameters of Electroless Deposited Ni-P Alloy for PEEK and Its Modified Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Roughening of the PEEK Substrates
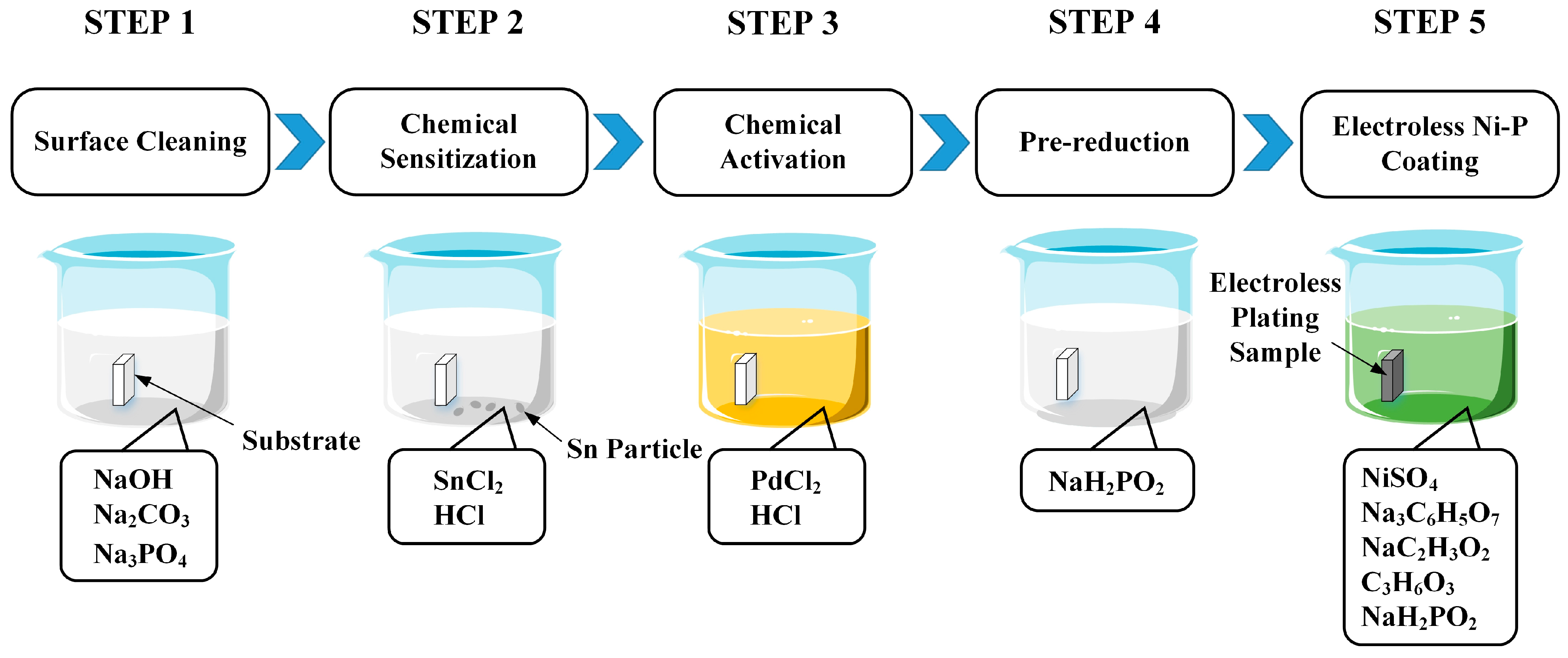
2.3. Electroless Ni–P Alloy on the PEEK Substrate
- (1)
- In order to remove the residual grease and dirt in the process of transportation, storage, and experiment, and obtain a clean sample surface, the sample was cleaned with an alkaline solution (40 g/L NaOH + 30 g/L Na2CO3 + 23.5 g/L Na3PO4) at 70 °C for 15 min. Then the sample was rinsed with water.
- (2)
- In order to generate a layer of reducing substances on the surface of the sample, so as to reduce the metal ions introduced during the activation treatment, the sample was immersed in the sensitizer solution (30 g/L SnCl2 + 50 mL/L HCl) for 20 min, taken out and dried for 5 min, and finally, the surface of the sample was rinsed with a small flow of water.
- (3)
- In order to reduce the catalytic metal ions in the activation solution into metal particles under the action of the reducing agent and adsorb them on the surface of the sample:so that the particles could be used as the catalyst for electroless plating, the sensitized sample was immersed in the activation solution (0.5 g/L PdCl2 + 8 mL/L HCl) for 20 min.Sn2+ + Pd2+ → Sn4+ + Pd↓
- (1)
- To facilitate the chemical reaction of electroless nickel plating, the activated sample was immersed in the pre-reduction solution (25 g/L NaH2PO2·H2O) for 30 s.
- (2)
- Immerse the pre-reduced sample in the chemical plating solution for reaction for 15 min. The composition and operating conditions of the electroless Ni-P coating solution are shown in Table 1. The reaction formula of electroless deposition of Ni-P alloy is:
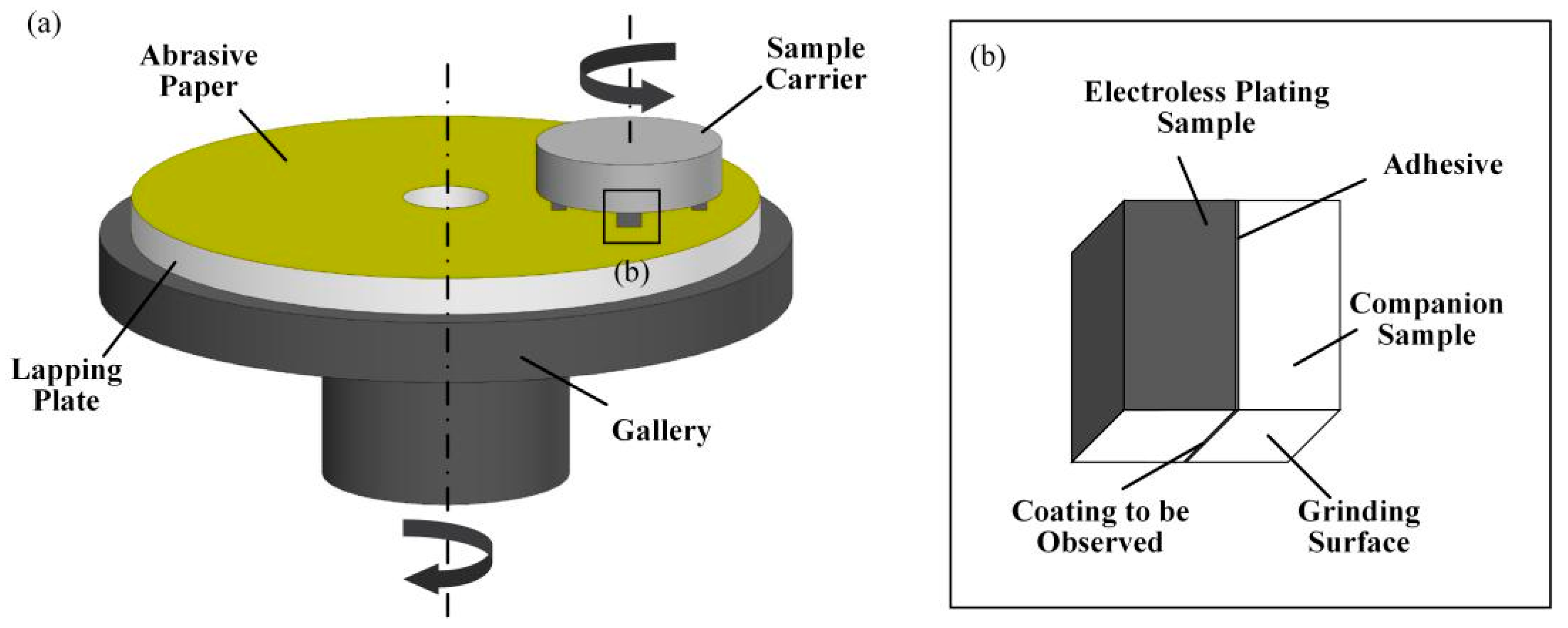
2.4. Adhesion Strength of Electroless Ni-P Alloy Films
2.5. Optimization of Process Parameters of Electroless Ni-P Alloy
3. Results and Discussion
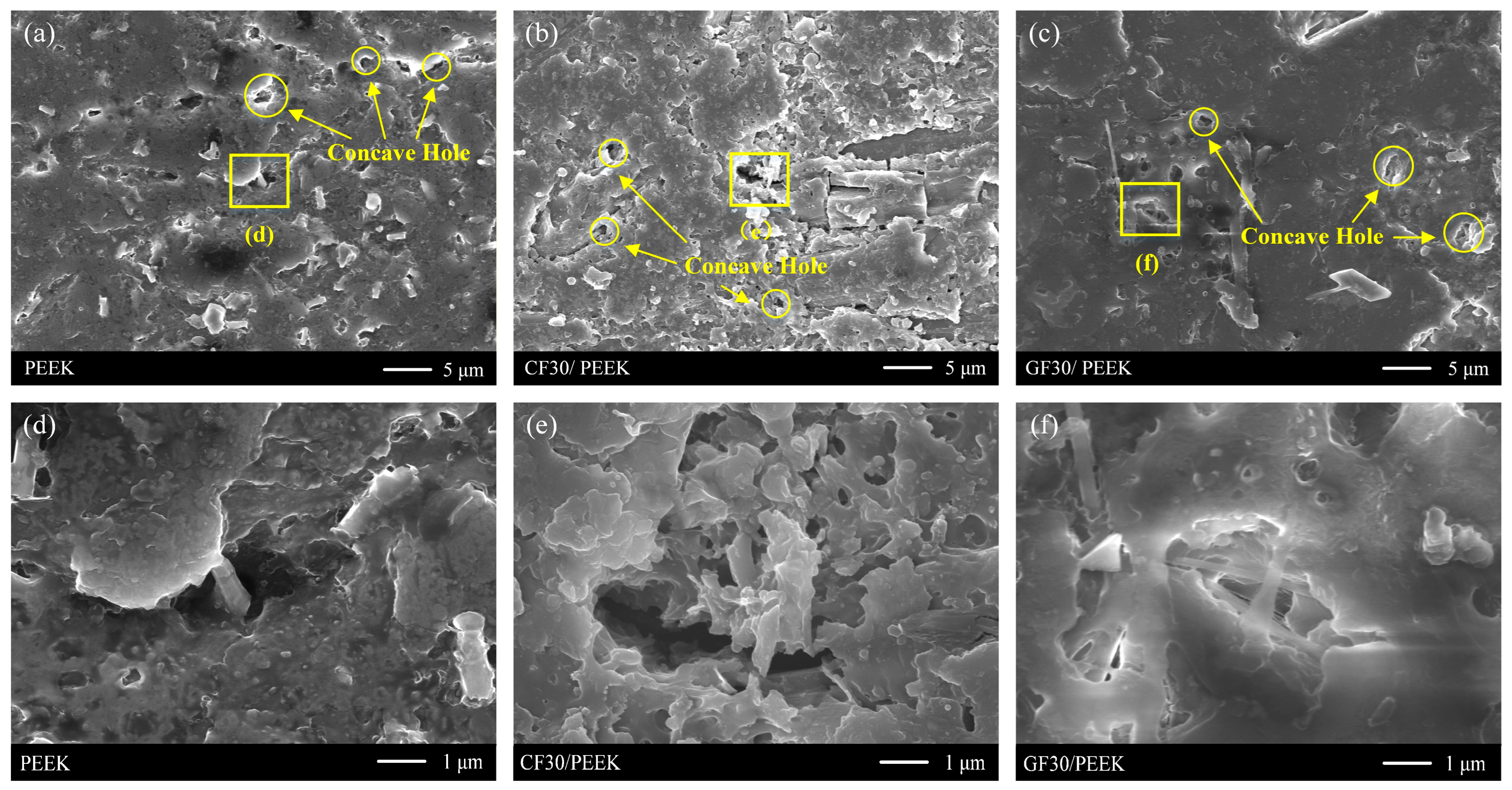
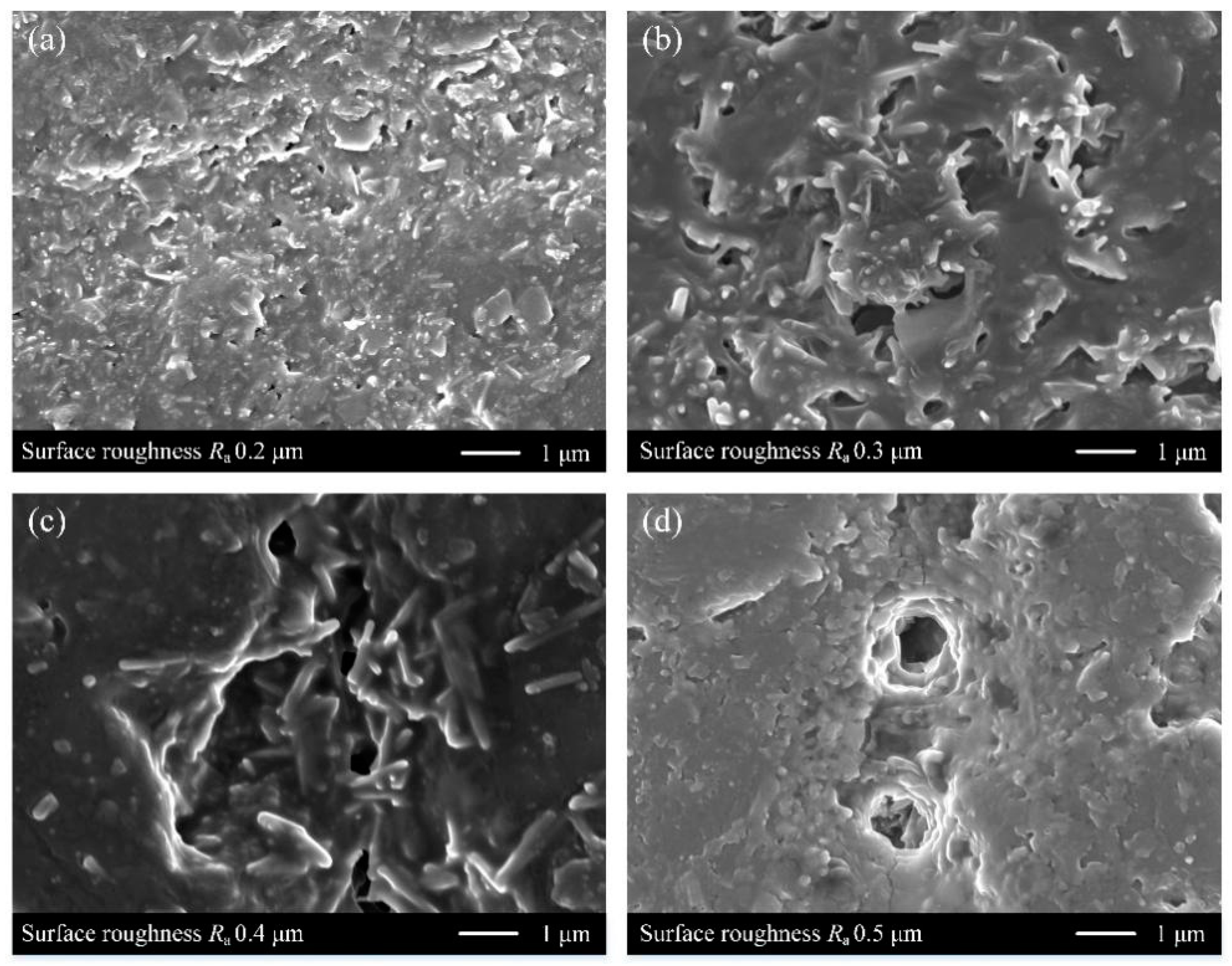
3.1. Sample Surface after Roughening
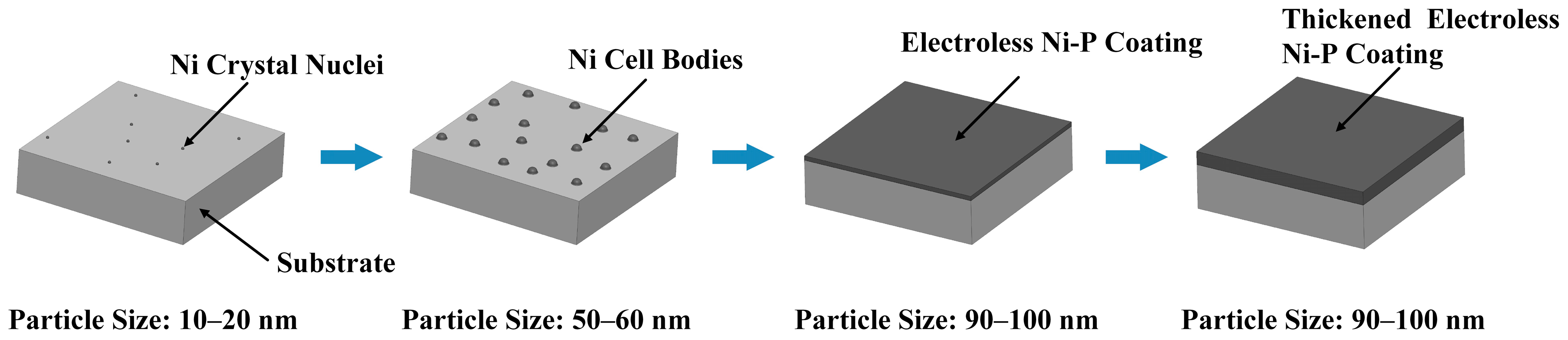
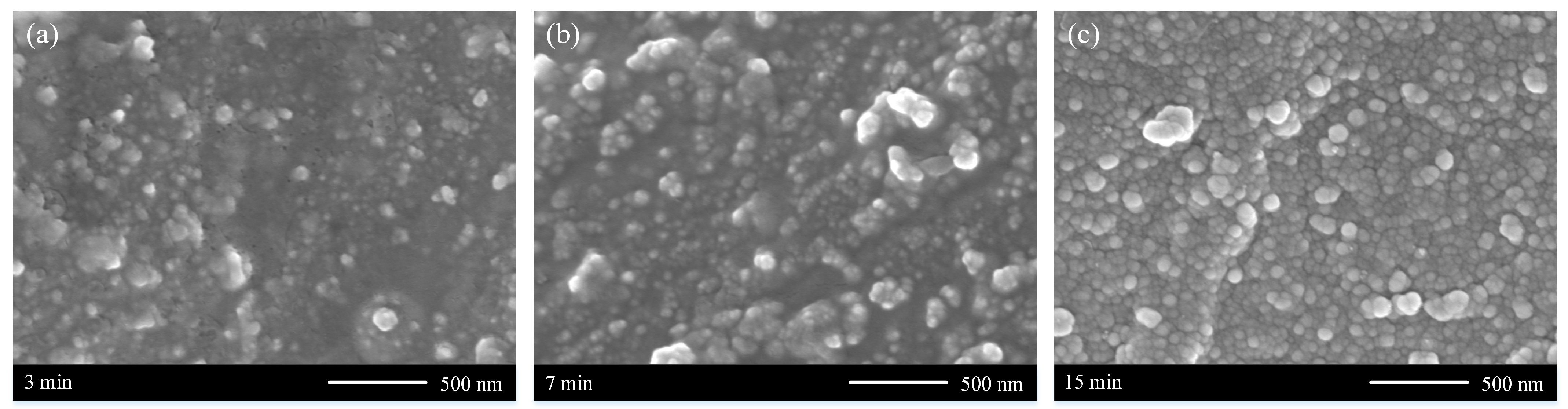
3.2. Coating Formation Process
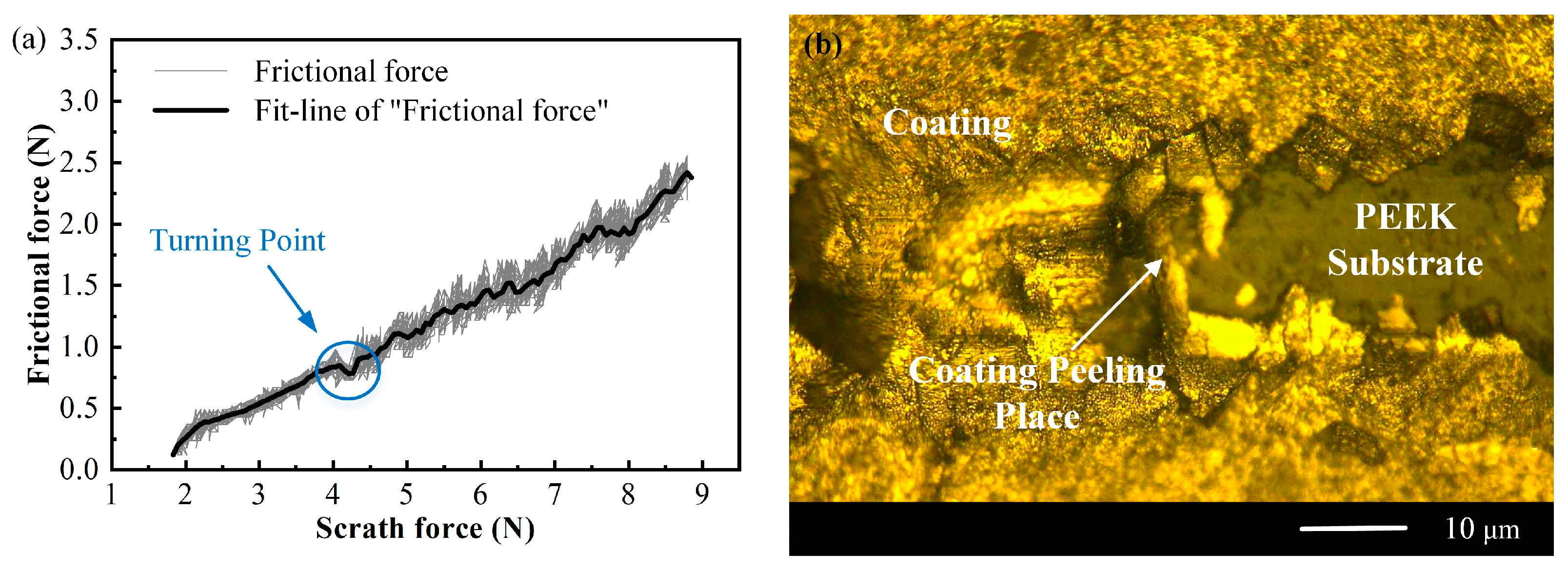
3.3. Coating Adhesion Test
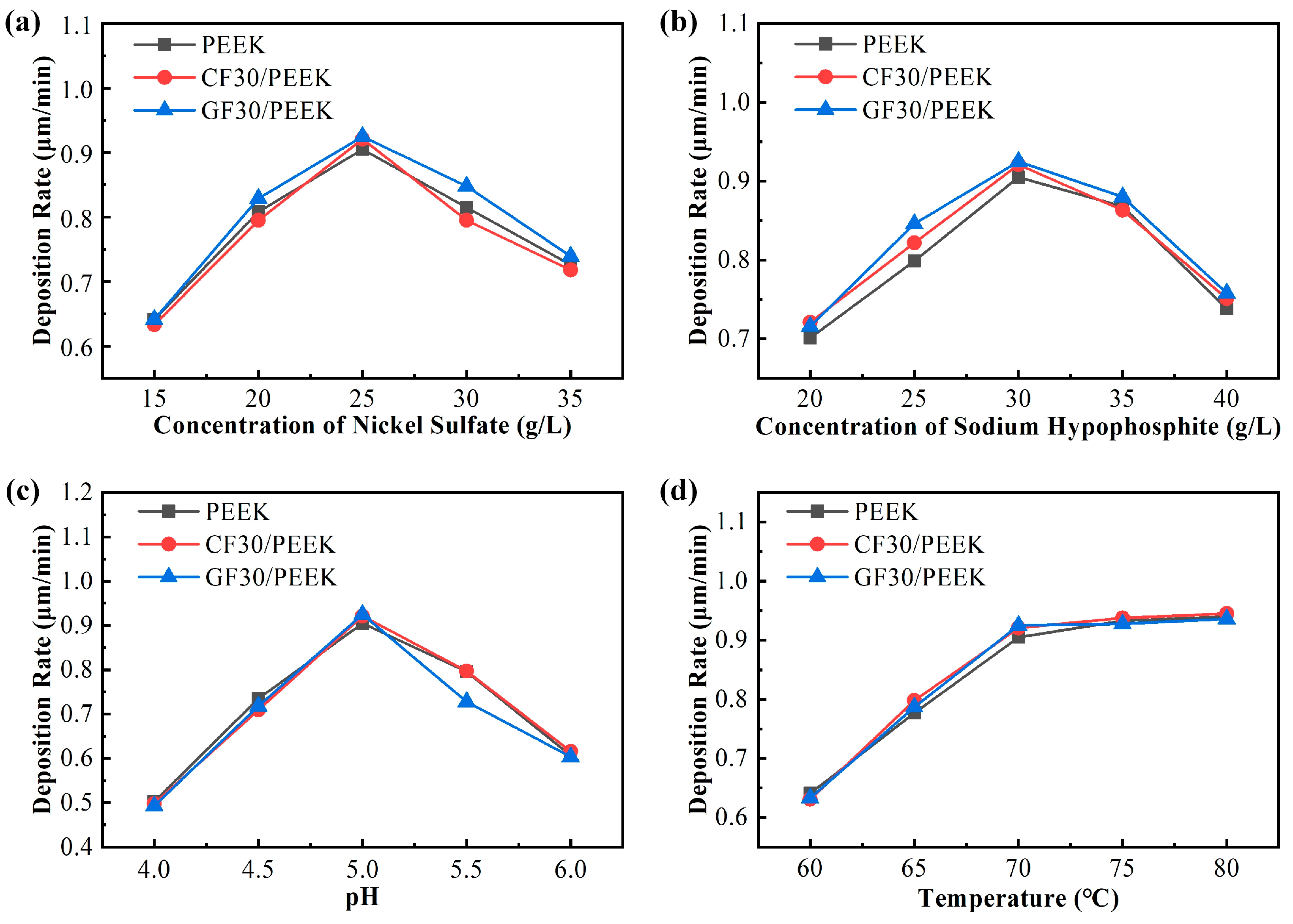
3.4. Parameter Optimization Experiment
4. Conclusions
- (1)
- The mechanism of the effect of substrate surface roughness on the coating adhesion was revealed. Due to the change of area and depth of concave holes after roughening, the coating adhesion between the three materials and the Ni-P alloy coating increased first and then decreased with the increase of substrate surface roughness, and the coating adhesion was highest when the surface roughness Ra was 0.4 μm.
- (2)
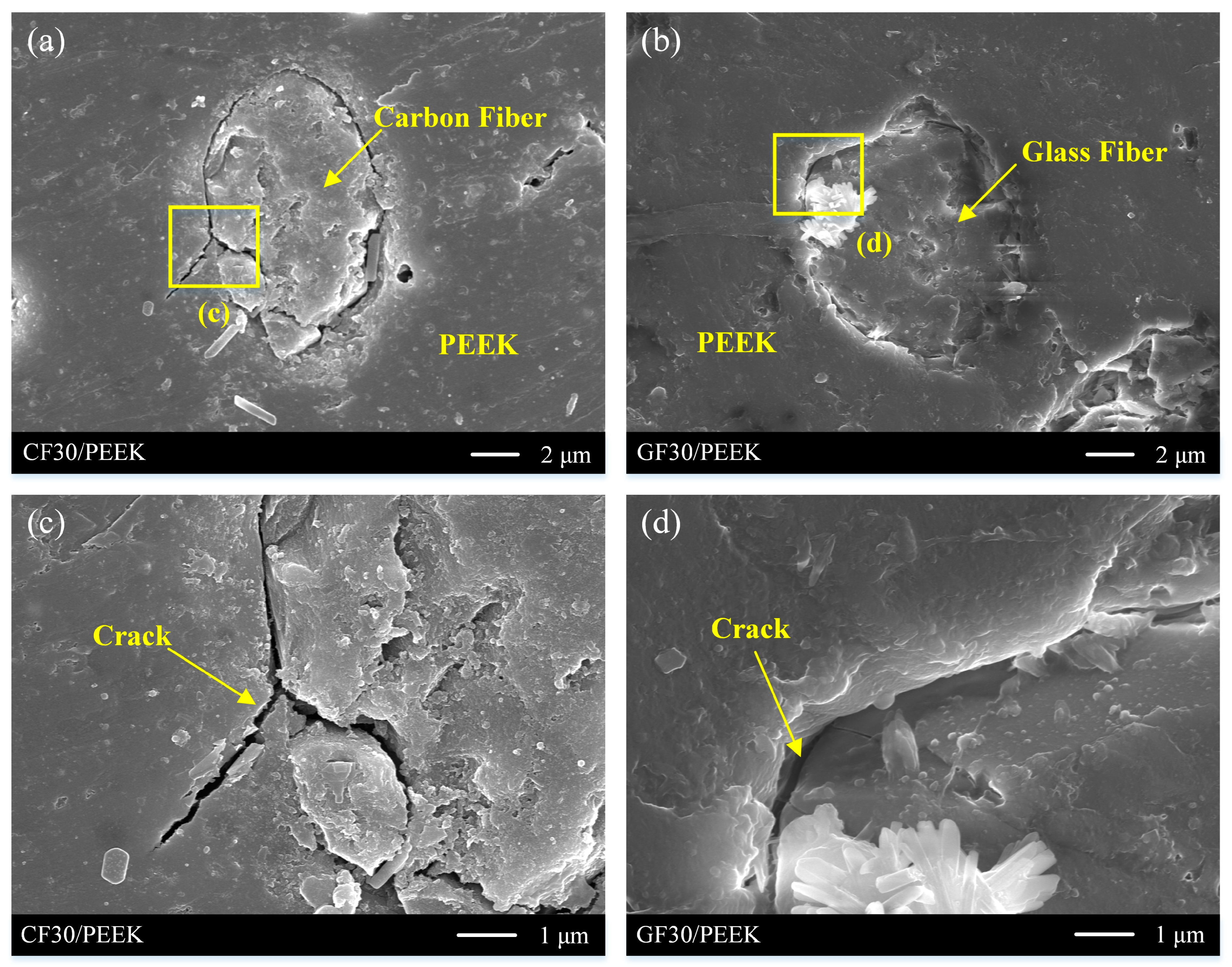
- The mechanism that the modified fiber in the substrate material affects the coating adhesion was revealed. The contact between fiber and resin was corroded and formed cracks when the fiber-reinforced PEEK material was roughened. The coating adhesion between fiber-reinforced PEEK materials and Ni-P alloy was greater than that of pure PEEK materials because coating embedded in cracks increased the mechanical anchoring force between the coating and the substrate.
- (3)
- The electroless plating process parameters of different PEEK materials based on the highest deposition rate are proposed. The type of substrate had little effect on the deposition rate of the coating. The optimal process parameters were selected as nickel sulfate: 25 g/L, sodium hypophosphite: 30 g/L, pH: 5.0, and temperature: 70 °C.
- (4)
- The workpieces formed by this process, which are based on PEEK and its modified materials, and the surface of which is chemically deposited with Ni-P alloy, can be used in the industry not only for television sets, radio recorders, electronic components, household appliances, and other daily industrial products but also for electronics, aviation, aerospace, machinery, precision instruments, and other sophisticated industries.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davim, J.P.; Cardoso, R. Effect of the reinforcement (carbon or glass fibres) on friction and wear behaviour of the PEEK against steel surface at long dry sliding. Wear 2009, 266, 795–799. [Google Scholar] [CrossRef]
- Song, J.; Liao, Z.; Shi, H.; Xiang, D.; Liu, Y.; Liu, W.; Peng, Z. Fretting Wear Study of PEEK-Based Composites for Bio-implant Application. Tribol. Lett. 2017, 65, 150. [Google Scholar] [CrossRef]
- Ling, X.; Jing, X.; Zhang, C.; Chen, S. Polyether Ether Ketone (PEEK) Properties and Its Application Status. IOP Conf. Series Earth Environ. Sci. 2020, 453, 12080. [Google Scholar] [CrossRef]
- Chen, Y.A.; Shih, P.S.; Chang, F.L.; Huang, J.H. Development of Cu-Cu Side-by-Side Interconnetion Using Controlled Electroless Cu Plating. In Proceedings of the 2022 IEEE 72nd Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 31 May–3 June 2022. [Google Scholar]
- Vitry, V.; Bonin, L. Effect of temperature on ultrasound-assisted electroless nickel-boron plating. Ultrason. Sonochem. 2019, 56, 327–336. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, S. Microwave Assisted Eletroless Copper Plating on Carbon Nanotubes. Adv. Mater. Res. 2011, 399–401, 741–746. [Google Scholar] [CrossRef]
- Yazdani, S.; Vitry, V. RSM models approach for optimization of the mechanical properties of electroless Ni-B-nanodiamond coating: An experimental and molecular dynamic simulation study. Surf. Coat. Technol. 2023, 452, 129133. [Google Scholar] [CrossRef]
- Yazdani, S.; Chapon, P.; Dupont, V.; Vitry, V. Role of CTAB surfactant on electroless Ni–B deposition and its mechanical and corrosion behaviour. Surf. Coat. Technol. 2023, 452, 129133. [Google Scholar] [CrossRef]
- Vitry, V.; Hastir, J.; Mégret, A.; Yazdani, S.; Yunacti, M.; Bonin, L. Recent advances in electroless nickel-boron coatings. Surf. Coat. Technol. 2021, 429, 127937. [Google Scholar]
- Tamilarasan, T.R.; Rajendran, R.; Sanjith, U.; Rajagopal, G.; Sudagar, J. Wear and scratch behaviour of electroless Ni-P-nano-TiO2: Effect of surfactants. Wear 2016, 346, 148–157. [Google Scholar] [CrossRef]
- Tsuru, Y.; Mochinaga, K.; Ooyagi, Y.; Foulkes, F.R. Application of vapor-deposited carbon and zinc as a substitute for palladium catalyst in the electroless plating of nickel. Surf. Coat. Technol. 2003, 169, 116–119. [Google Scholar] [CrossRef]
- Zheng, H.; Mo, Y.; Liu, W. Fabrication of Nano-Al2O3/Ni Composite Particle with Core-Shell Structure by a Modified Electroless Plating Process. Adv. Mater. Res. 2012, 455, 49–54. [Google Scholar]
- Rajaguru, J.C.; Duke, M.; Au, C. Investigation of electroless nickel plating on rapid prototyping material of acrylic resin. Rapid Prototyp. J. 2016, 22, 162–169. [Google Scholar] [CrossRef]
- Hieu, D.N.; My, D.N.H.; Thu, V.T.; Hung, N.Q. Selected-area growth of nickel micropillars on aluminum thin films by electroless plating for applications in microbolometers. J. Sci. Adv. Mater. Devices 2017, 2, 192–198. [Google Scholar] [CrossRef]
- Gharde, S.; Kandasubramanian, B. The Importance of Electroless Metallic Build-Up on Surface Modified Substrates for Multifunctional Engineering Applications: A Recent Progress Update. Trans. Indian Inst. Met. 2018, 71, 2873–2892. [Google Scholar] [CrossRef]
- Jiang, S.Q.; Kan, C.W.; Yuen, C.W.M.; Wong, W.K. Electroless nickel plating of polyester fiber. J. Appl. Polym. Sci. 2008, 108, 2630–2637. [Google Scholar] [CrossRef]
- Guo, R.H.; Jiang, S.X.; Yuen, C.W.M.; Ng, M.C.F.; Lan, J.W. Optimization of electroless nickel plating on polyester fabric. Fibers Polym. 2013, 14, 459–464. [Google Scholar] [CrossRef]
- Su, Y.; Zhou, B.; Liu, L.; Lian, J.; Li, G. Electromagnetic shielding and corrosion resistance of electroless Ni-P and Ni-P-Cu coatings on polymer/carbon fiber composites. Polym. Compos. 2014, 36, 923–930. [Google Scholar] [CrossRef]
- Li, R.; Gao, Y.; Gu, Y. Study on New Surface Modification Method of ABS before Electroless Nickel Plating. China Plast. Ind. 2008, 36, 66–68. [Google Scholar]
- Fujii, S.; Hamasaki, H.; Takeoka, H.; Tsuruoka, T.; Akamatsu, K.; Nakamura, Y. Electroless nickel plating on polymer particles. J. Colloid Interface Sci. 2014, 430, 47–55. [Google Scholar] [CrossRef]
- Furuno, M.; Kitajima, K.; Tsukuda, Y.; Akamatsu, T. Effect of Ground Surface Roughness of Tool on Adhesion Characteristics of PVD Coating. Adv. Mater. Res. 2010, 126–128, 609–614. [Google Scholar] [CrossRef]
- Bharathidasan, T.; Kumar, S.V.; Bobji, M.; Chakradhar, R.; Basu, B.J. Effect of wettability and surface roughness on ice-adhesion strength of hydrophilic, hydrophobic and superhydrophobic surfaces. Appl. Surf. Sci. 2014, 314, 241–250. [Google Scholar] [CrossRef]
- Kleinbichler, A.; Pfeifenberger, M.; Zechner, J.; Wöhlert, S.; Cordill, M. Scratch induced thin film buckling for quantitative adhesion measurements. Mater. Des. 2018, 155, 203–211. [Google Scholar] [CrossRef]
- Wang, H.L.; Liu, L.Y.; Jiang, W.F. Effect of process parameters on deposition rate of electroless Ni-P plating on AZ91D magnesium alloy. Adv. Mater. Res. 2012, 418–420, 936–939. [Google Scholar] [CrossRef]
- Xu, F.C.; Kusukawa, K. Adhesion Assessment of BNT Films on Titanium Substrates Using a Scratch Test. Adv. Mater. Res. 2014, 875-877, 584–587. [Google Scholar] [CrossRef]
- Lu, G.J.; Zangari, G. Study of the electroless deposition process of Ni-P-based ternary alloys. J. Electrochem. Soc. 2003, 150, 777–786. [Google Scholar] [CrossRef]
- Hoogendoorn, B.W.; Parra, M.; Capezza, A.J.; Li, Y.; Forsberg, K.; Xiao, X.; Olsson, R.T. Cellulose-assisted electrodeposition of zinc for morphological control in battery metal recycling. Mater. Adv. 2022, 3, 5304–5314. [Google Scholar] [CrossRef]
- Zirignon, J.-C.; Capezza, A.J.; Xiao, X.; Andersson, R.L.; Forslund, M.; Diner, P.; Olsson, R.T. Experimental review of PEI electrodeposition onto copper substrates for insulation of complex geometries. RSC Adv. 2021, 11, 34599–34604. [Google Scholar] [CrossRef]
- Yakovlev, E.V.; Markov, A.B.; Neiman, A.A. Adhesive Strength of Ni-Cu Surface Alloy Formation by Low-Energy High-Current Electron Beam. Russ. Phys. J. 2021, 63, 1804–1809. [Google Scholar] [CrossRef]
- Stefánsson, A.; Arnórsson, S.; Sveinbjörnsdóttir, Á.E. Redox reactions and potentials in natural waters at disequilibrium. Chem. Geol. 2005, 221, 289–311. [Google Scholar] [CrossRef]
- Navabpour, P.; Teer, D.G.; Hitt, D.J.; Gilbert, M. Evaluation of non-stick properties of magnetron-sputtered coatings for moulds used for the processing of polymers. Surf. Coatings Technol. 2006, 201, 3802–3809. [Google Scholar] [CrossRef]
- Gerth, J.; Wiklund, U. The influence of metallic interlayers on the adhesion of PVD TiN coatings on high-speed steel. Wear 2008, 264, 885–892. [Google Scholar] [CrossRef]
- Wang, X.M.; Duo, J. Effect on Electroless Nickel Coating on Magnesium Alloy by Nickel Electroless Plating Parameters. Adv. Mater. Res. 2011, 295–297, 1522–1525. [Google Scholar] [CrossRef]
- Karadaglioglu, O.I.; Alagoz, L.G.; Caliskan, A.; Vaizoglu, G.A. The Effect of Different Surface Roughening Systems on the Micro-Shear Bond Strength of Aged Resin Composites. Niger. J. Clin. Pract. 2022, 25, 37. [Google Scholar] [CrossRef]

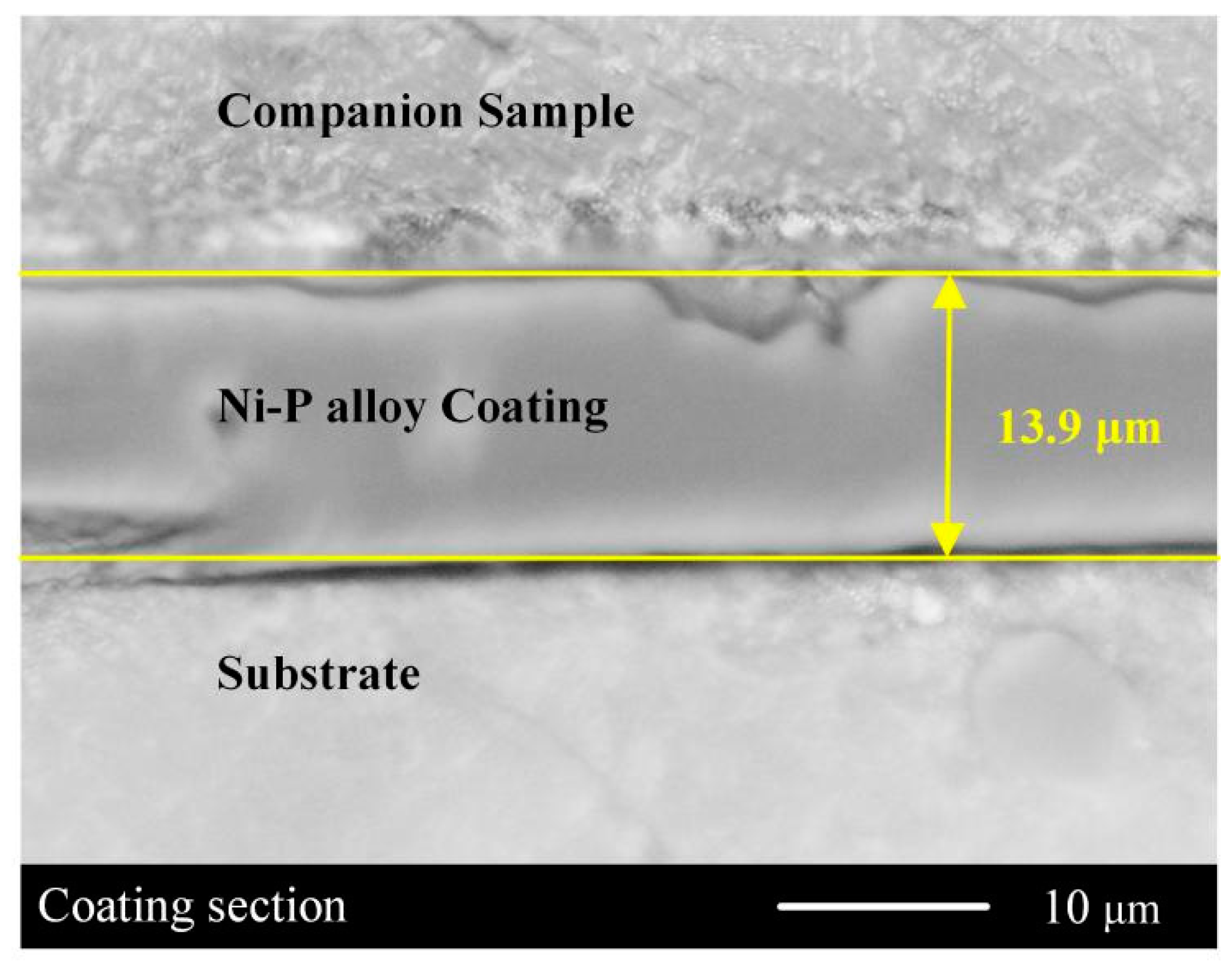
| Parameters | NiSO4·6H2O | NaH2PO2·H2O | Na3C6H5O7 | CH3COONa | C3H6O3 | Temperature | pH |
|---|---|---|---|---|---|---|---|
| Value | 25 g/L | 30 g/L | 10 g/L | 20 g/L | 30 g/L | 70 °C | 5.0 |
| Parameters | Value | ||||
|---|---|---|---|---|---|
| Nickel sulfate (g/L) | 15 | 20 | 25 | 30 | 35 |
| Sodium hypophosphite (g/L) | 20 | 25 | 30 | 35 | 40 |
| pH | 4.0 | 4.5 | 5.0 | 5.5 | 6.0 |
| Temperature (°C) | 60 | 65 | 70 | 75 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Wu, C.; Yang, X.; Cheng, J.; Kang, R. Study on Adhesion Properties and Process Parameters of Electroless Deposited Ni-P Alloy for PEEK and Its Modified Materials. Coatings 2023, 13, 388. https://doi.org/10.3390/coatings13020388
Gao S, Wu C, Yang X, Cheng J, Kang R. Study on Adhesion Properties and Process Parameters of Electroless Deposited Ni-P Alloy for PEEK and Its Modified Materials. Coatings. 2023; 13(2):388. https://doi.org/10.3390/coatings13020388
Chicago/Turabian StyleGao, Shang, Chongyao Wu, Xin Yang, Jirui Cheng, and Renke Kang. 2023. "Study on Adhesion Properties and Process Parameters of Electroless Deposited Ni-P Alloy for PEEK and Its Modified Materials" Coatings 13, no. 2: 388. https://doi.org/10.3390/coatings13020388
APA StyleGao, S., Wu, C., Yang, X., Cheng, J., & Kang, R. (2023). Study on Adhesion Properties and Process Parameters of Electroless Deposited Ni-P Alloy for PEEK and Its Modified Materials. Coatings, 13(2), 388. https://doi.org/10.3390/coatings13020388






