Antimicrobial Activity and Transparency of Polyvinyl Butyral Paint Containing Heated Scallop-Shell Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Paint Containing Scallop HSP
2.2. Characterization of Scallop HSP–PVB Paint
2.3. Antibacterial and Antiviral Efficacies of Scallop HSP–PVB Paint
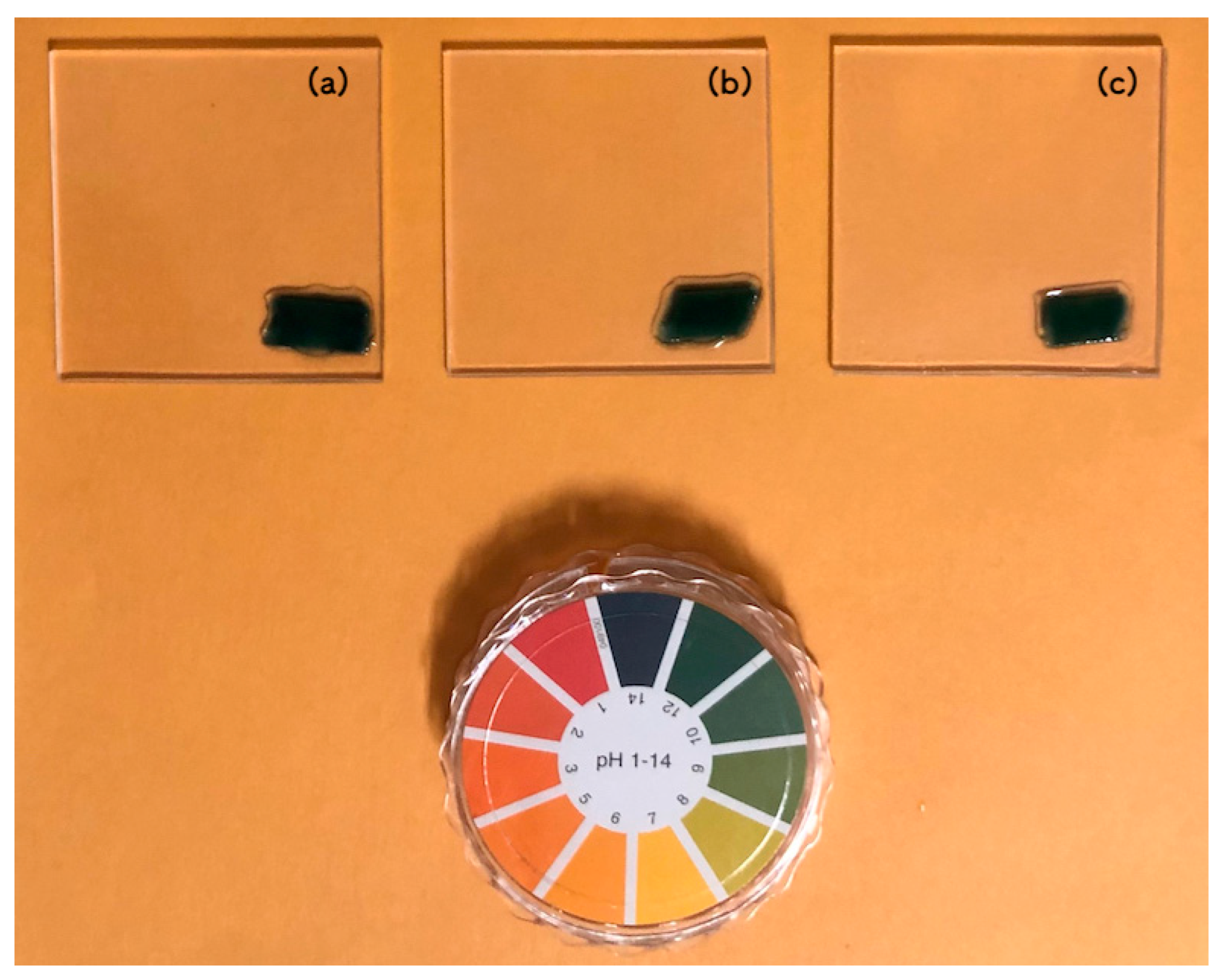
2.4. pH Measurement of Scallop HSP–PVB Paint
3. Results and Discussion
3.1. Properties of Scallop HSP–PVB Paint
3.2. Antibacterial and Antiviral Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HSP | Heated shell powder |
| PVB | Polyvinyl butyral |
| SEM | Scanning electron microscope |
| ISO | International Organization for Standardization |
| JIS | Japanese Industrial Standard |
| SCDLP | Soybean-casein digest broth with lecithin and polysorbate 80 |
| ROS | Reactive oxygen species |
References
- Sawai, J.; Shiga, H.; Kojima, H. Kinetic analysis of the bactericidal action of heated scallop-shell powder. Int. J. Food Microbiol. 2001, 71, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K.N.; Kai, H.; Inoue, K.; Ohto, K.; Kawakita, H.; Harada, H.; Morita, M. Heavy metal removal from contaminated scallop waste for feed and fertilizer application. Bioresour. Technol. 2008, 99, 2436–2441. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.P.; Backeljau, T.; Chapelle, G. Shells from aquaculture: A valuable biomaterial, not a nuisance waste product. Rev. Aquacult. 2019, 11, 42–57. [Google Scholar] [CrossRef]
- Tongwanichniyom, S.; Kitjaruwankul, S.; Phornphisutthimas, S. Production of biomaterials from seafood waste for application as vegetable wash disinfectant. Heliyon 2022, 8, e09357. [Google Scholar] [CrossRef] [PubMed]
- Widakdo, J.; Chen, T.M.; Lin, M.C.; Wu, J.H.; Lin, T.L.; Yu, P.J.; Hung, W.S.; Lee, K.R. Evaluation of the antibacterial activity of eco-friendly hybrid composites on the base of oyster shell powder modified by metal Ions and LLDPE. Polymers 2022, 14, 3001. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, K.; Asada, T.; Yamamoto, K.; Wakabayashi, H.; Sasaki, M.; Sato, M.; Matsuda, J. Antibacterial activity of calcined shell calcium prepared from wild surf clam. J. Health Sci. 2000, 46, 98–103. [Google Scholar] [CrossRef]
- Li, M.; Yao, Z.T.; Chen, T.; Lou, Z.H.; Xia, M. The antibacterial activity and mechanism of mussel shell waste derived material. Powder Technol. 2014, 264, 577–582. [Google Scholar] [CrossRef]
- Rusdaryanti, A.F.; Amalia, U.; Suharto, S. Antibacterial activity of CaO from blood cockle shells (Anadara granosa) calcination against Escherichia coli. Biodiversitas 2020, 21, 2827–2831. [Google Scholar]
- Bae, D.H.; Yeon, J.H.; Park, S.Y.; Lee, D.H.; Ha, S.D. Bactericidal effects of CaO (scallop-shell powder) on foodborne pathogenic bacteria. Arch. Pharm. Res. 2006, 29, 298–301. [Google Scholar] [CrossRef]
- Nakamura, S.; Ishihara, M.; Sato, Y.; Takayama, T.; Hiruma, S.; Ando, N.; Fukuda, K.; Murakami, K.; Yokoe, H. Concentrated bioshell calcium oxide (BiSCaO) water kills pathogenic microbes: Characterization and activity. Molecules 2020, 25, 3001. [Google Scholar] [CrossRef]
- Sawai, J.; Shiga, H. Kinetic analysis of antifungal activity of heated scallop-shell powder against Trichophyton and its possible application to the treatment of dermatophytosis. Biocontrol Sci. 2006, 11, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Qin, Y.; Guan, X.; Liu, S.; Yu, H.; Li, P. Comparison of antifungal activities of scallop shell, oyster shell and their pyrolyzed products. Egypt. J. Aquat. Res. 2013, 39, 83–90. [Google Scholar] [CrossRef]
- Sawai, J.; Miyoshi, H.; Kojima, H. Sporicidal kinetics of Bacillus subtilis spores by heated scallop shell powder. J. Food Prot. 2003, 66, 1482–1485. [Google Scholar] [CrossRef] [PubMed]
- Thammakarn, C.; Satoh, K.; Suguro, A.; Hakim, H.; Ruenphet, S.; Takehara, K. Inactivation of avian influenza virus, Newcastle disease virus and goose parvovirus using solution of nano-sized scallop shell powder. J. Vet. Med. Sci. 2014, 76, 1277–1280. [Google Scholar] [CrossRef]
- Thammakarn, C.; Tsujimura, M.; Satoh, K.; Hasegawa, T.; Tamura, M.; Kawamura, A.; Ishida, Y.; Suguro, A.; Hakim, H.; Ruenphet, S.; et al. Efficacy of scallop shell powders and slaked lime for inactivating avian influenza virus under harsh conditions. Arch. Virol. 2015, 160, 2577–2581. [Google Scholar] [CrossRef]
- Bodur, T.; Cagri-Mehmetoglu, A. Removal of Listeria monocytogenes, Staphylococcus aureus and Escherichia coli O157:H7 biofilms on stainless steel using scallop shell powder. Food Control 2012, 25, 1–9. [Google Scholar] [CrossRef]
- Kubo, M.; Ohshima, Y.; Irie, F.; Kikuchi, M.; Sawai, J. Disinfection treatment of heated scallop-shell powder on biofilm of Escherichia coli ATCC 25922 surrogated for E. coli O157:H7. J. Biomater. Nanobiotechnol. 2013, 4, 10–19. [Google Scholar] [CrossRef]
- Shimamura, N.; Irie, F.; Yamakawa, T.; Kikuchi, M.; Sawai, J. Heated scallop-shell powder treatment for deactivation and removal of Listeria sp. biofilm formed at a low temperature. Biocontrol Sci. 2015, 20, 153–157. [Google Scholar] [CrossRef]
- Tsukuda, H.; Akimoto, T.; Fukikoshi, N.; Wada, R.; Sawai, J. Antibiofilm effects of heated scallop shell powder on Campylobacter jejuni biofilms. Membranes 2021, 12, 43. [Google Scholar] [CrossRef]
- Sawai, J.; Nagasawa, K.; Kikuchi, M. Ability of heated scallop-shell powder to disinfect Staphylococcus aureus biofilm. Food Sci. Technol. Res. 2013, 19, 561–568. [Google Scholar] [CrossRef]
- Bari, M.L.; Inatsu, Y.; Kawasaki, S.; Nazuka, E.; Isshiki, K. Calcinated calcium killing of Escherichia coli O157: H7, Salmonella, and Listeria monocytogenes on the surface of tomatoes. J. Food Prot. 2002, 65, 1706–1711. [Google Scholar] [CrossRef]
- Chen, X.; Tango, C.N.; Daliri, E.B.M.; Oh, S.Y.; Oh, D.H. Disinfection efficacy of slightly acidic electrolyzed water combined with chemical treatments on fresh fruits at the industrial scale. Foods 2019, 8, 497. [Google Scholar] [CrossRef]
- Kim, J.G.; Nimitkeatkai, H.; Choi, J.W.; Cheong, S.R. Calcinated calcium and mild heat treatment on storage quality and microbial populations of fresh-cut iceberg lettuce. Hortic. Environ. Biotechnol. 2011, 52, 408–412. [Google Scholar] [CrossRef]
- Mamun, A.A.; Simul, H.A.; Rahman, A.; Gazi, N.N.; Bari, M.L. Prevalence of foodborne pathogens and effectiveness of washing or cooking in reducing micro- biological risk of contaminated red amaranth. Agric. Food Anal. Bacteriol. 2012, 2, 222–231. [Google Scholar]
- Nomoto, Y.; Sawada, S.; Abe, S.; Wakazawa, J.; Kikuchi, M.; Sawai, J. Sorbitol minimizes calcium carbonate scale generation while maintaining the disinfection effect of heated scallop-shell powder for fresh produce. Biocontrol Sci. 2018, 23, 157–165. [Google Scholar] [CrossRef]
- Sawai, J.; Satoh, M.; Horikawa, M.; Shiga, H.; Kojima, H. Heated scallop-shell powder slurry treatment of shredded cabbage. J. Food Prot. 2001, 64, 1579–1583. [Google Scholar] [CrossRef]
- Tsuruma, A.; Nomoto, Y.; Nishio, M.; Ishikawa, M.; Sawai, J. Efficacy of sorbitol-coated heated scallop-shell powder for the antimicrobial treatment of fresh vegetables. Food Control 2020, 110, 106972. [Google Scholar] [CrossRef]
- Bodur, T.; Yaldirak, G.; Kola, O.; Çağri-mehmetoğlu, A. Inhibition of Listeria monocytogenes and Escherichia coli O157: H7 on frankfurters using scallop-shell powder. J. Food Saf. 2010, 30, 740–752. [Google Scholar] [CrossRef]
- Cagri-Mehmetoglu, A. Inhibition of Listeria monocytogenes and Salmonella enteritidis on chicken wings using scallop-shell powder. Poult. Sci. 2011, 90, 2600–2605. [Google Scholar] [CrossRef] [PubMed]
- Ro, E.Y.; Ko, Y.M.; Yoon, K.S. Survival of pathogenic enterohemorrhagic Escherichia coli (EHEC) and control with calcium oxide in frozen meat products. Food Microbiol. 2015, 49, 203–210. [Google Scholar] [CrossRef]
- Ahmed, S.; Akand, N.R.; Islam, M.T.; Mamun, A.; Bari, M.L. Effectiveness of scallop powder ice in reducing bacterial load on fresh whole fish and in the melted ice water. LWT Food Sci. Technol. 2015, 64, 270–274. [Google Scholar] [CrossRef]
- Fransisca, L.; Zhou, B.; Park, H.; Feng, H. The effect of calcinated calcium and chlorine treatments on Escherichia coli O157:H7 87-23 population reduction in radish sprouts. J. Food Sci. 2011, 76, M404–M412. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Matthews, K.R. Efficacy of chlorine and calcinated calcium treatment of alfalfa seeds and sprouts to eliminate Salmonella. Int. J. Food Microbiol. 2003, 87, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y.; Huang, Y.C.; Chiu, S.Y.; Kuo, K.L.; Hwang, P.A. Bacteriostatic effect of a calcined waste clamshell-activated plastic film for food packaging. Materials 2018, 11, 1370. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Begum, A.; Rabbani, K.S.; Bari, L. Low cost and sustainable surface water purification methods using Moringa seeds and scallop powder followed by bio-sand filtration. Water Sci. Technol. Water Supply 2017, 17, 125–137. [Google Scholar] [CrossRef]
- Loyo, C.; Moreno-Serna, V.; Fuentes, J.; Amigo, N.; Sepúlveda, F.A.; Ortiz, J.A.; Rivas, L.M.; Ulloa, M.T.; Benavente, R.; Zapata, P.A. PLA/CaO nanocomposites with antimicrobial and photodegradation properties. Polym. Degrad. Stab. 2022, 197, 109865. [Google Scholar] [CrossRef]
- Lim, H.; Chae, D.; Son, S.; Ha, J.; Lee, H. CaCO3 micro particle-based radiative cooling device without metal reflector for entire day. Mater. Today Commun. 2022, 32, 103990. [Google Scholar] [CrossRef]
- Egerić, M.; Smičiklas, I.; Mraković, A.; Jović, M.; Šljivić-Ivanović, M.; Antanasijević, D.; Ristić, M. Experimental and theoretical consideration of the factors influencing cationic pollutants retention by seashell waste. J. Chem. Technol. Biotechnol. 2018, 93, 1477–1487. [Google Scholar] [CrossRef]
- Yalcinkaya, F. Experimental study on electrospun polyvinyl butyral nanofibers using a non-solvent system. Fibers Polym. 2015, 16, 2544–2551. [Google Scholar] [CrossRef]
- Baştürk, E.; Çakmakçi, E.; Madakbaş, S.; Kahraman, M.V. Surface and proton conductivity properties of electrospun poly(vinyl butyral)/polyaniline nanofibers. Adv. Polym. Technol. 2018, 37, 1774–1781. [Google Scholar] [CrossRef]
- Swain, B.; Park, J.R.; Lee, C.G. Industrial recycling of end-of-life vehicle windshield glass by mechanical beneficiation and complete recovery of polyvinyl butyral. J. Clean. Prod. 2022, 334, 130192. [Google Scholar] [CrossRef]
- Watanabe, T.; Fujimoto, R.; Kikuchi, M.; Sawai, J.; Yahata, S.; Satoh, T. Antibacterial characteristics of heated scallop-shell nano-particles. Biocontrol Sci. 2014, 19, 93–97. [Google Scholar] [CrossRef]
- Jeong, M.S.; Park, J.S.; Song, S.H.; Jang, S.B. Characterization of antibacterial nanoparticles from the scallop, Ptinopecten yessoensis. Biosci. Biotechnol. Biochem. 2007, 71, 2242–2247. [Google Scholar] [CrossRef]
- JIS K 5600-5-4:1999; Testing Methods for Paints—Part 5: Mechanical Property of Film—Section 4: Scratch Hardness (Pencil Method). Japanese Standards Association: Tokyo, Japan, 1999.
- JIS Z2801; Antimicrobial Products-Test for Antimicrobial Activity and Efficacy. Japanese Standards Association: Tokyo, Japan, 2001.
- ISO 21702: 2019; Measurement of Antiviral Activity on the Plastics and Other Nonporous Surfaces. International Standard Organization: London, UK, 2019.
- Druffel, T.; Buazza, O.; Lattis, M.; Farmer, S.; Spencer, M.; Mandzy, N.; Grulke, E.A. The role of nanoparticles in visible transparent nanocomposites. In Nanophotonic Materials V; SPIE: Bellingham, WA, USA, 2008; p. 70300F. [Google Scholar] [CrossRef]
- Isshiki, K.; Suhara, H.; Mizuuchi, K.; Tokuoka, K. Effectiveness of calcium preparation to control microbial growth in food. Nippon. Shokuhin Kogyo Gakkaishi 1994, 41, 135–140. (In Japanese) [Google Scholar] [CrossRef]
- Sawai, J.; Kawada, E.; Kanou, F.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M. Detection of active oxygen generated from ceramic powders having antibacterial activity. J. Chem. Eng. Jpn. 1996, 29, 627–633. [Google Scholar] [CrossRef]
- Sawai, J. Antimicrobial characteristics of heated scallop shell powder and its application. Biocontrol Sci. 2011, 16, 95–102. [Google Scholar] [CrossRef]
- Hayashi, K.; Hirano, M.; Matsuishi, S.; Hosono, H. Microporous crystal 12CaO·7Al2O3 encaging abundant O− radicals. J. Am. Chem. Soc. 2002, 124, 738–739. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Hata, Y.; Hiruma, S.; Takayama, T.; Nakamura, S.; Sato, Y.; Ando, N.; Fukuda, K.; Murakami, K.; Yokoe, H. Safety of concentrated bioshell calcium oxide water application for surface and skin disinfections against pathogenic microbes. Molecules 2020, 25, 4502. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, Y.; Takada, D.; Namai, S.; Sawai, J.; Kikuchi, M.; Hotta, M. Antimicrobial characteristics of heated eggshell powder. Biocontrol Sci. 2015, 20, 239–246. [Google Scholar] [CrossRef]
- Yasue, S.; Sawai, J.; Kikuchi, M.; Nakakuki, T.; Sano, K.; Kikuchi, T. Sporicidal characteristics of heated dolomite powder against Bacillus subtilis spores. Biocontrol Sci. 2014, 19, 113–119. [Google Scholar] [CrossRef]
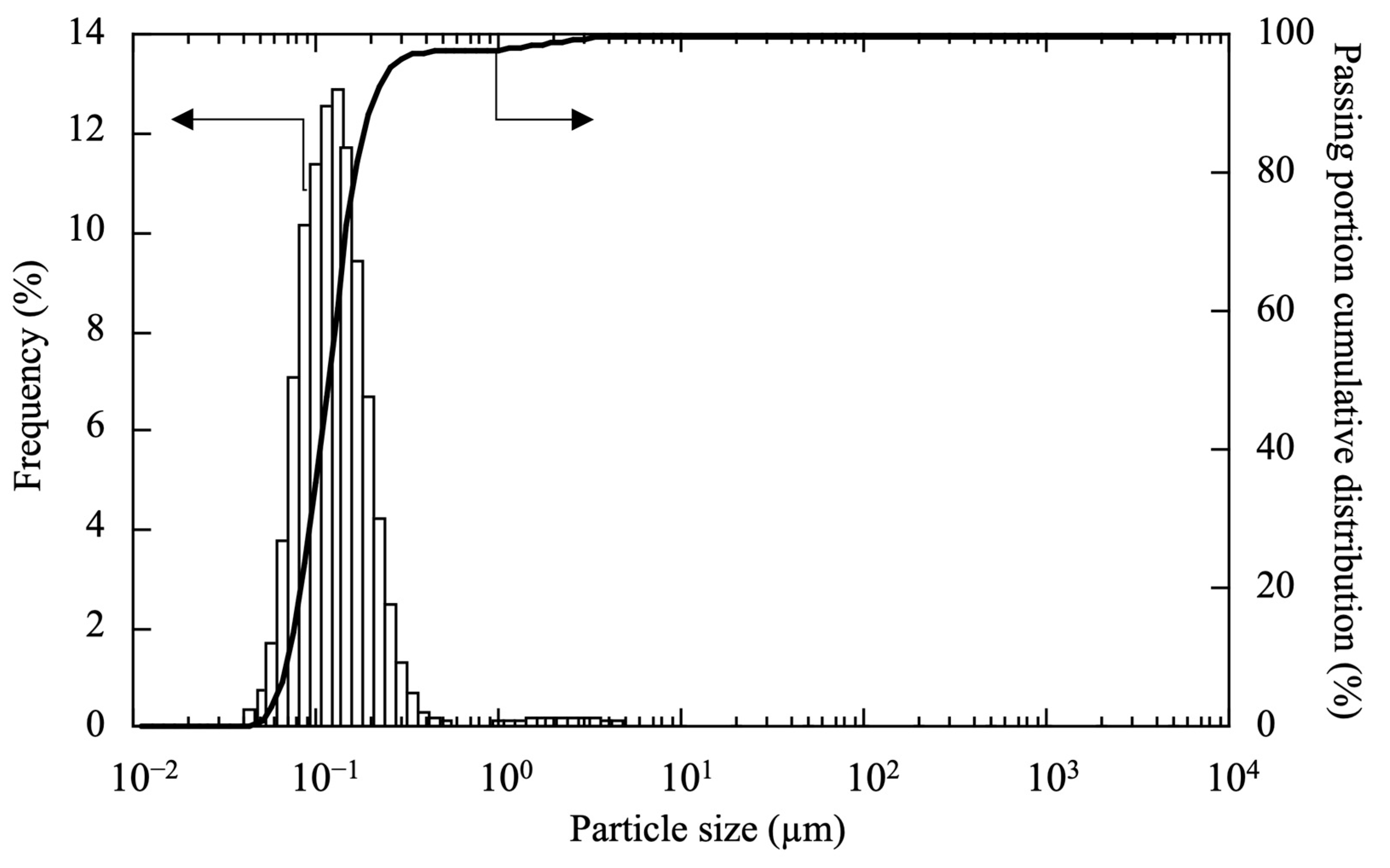
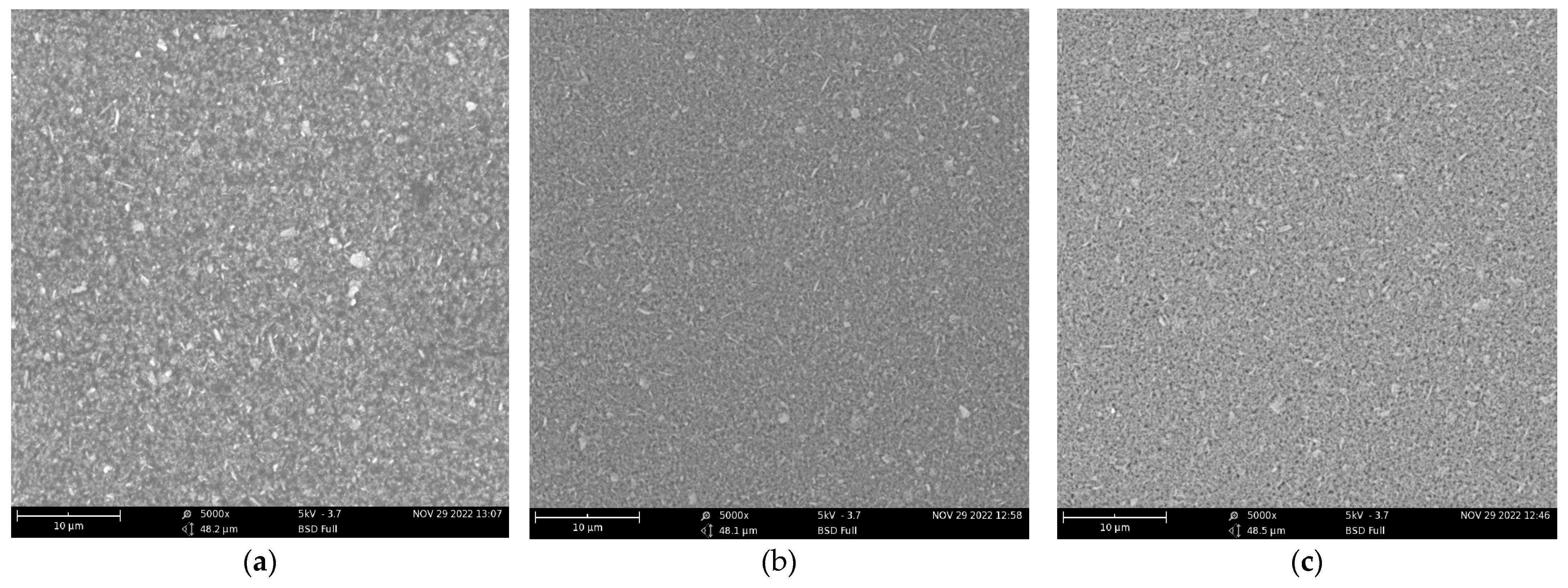
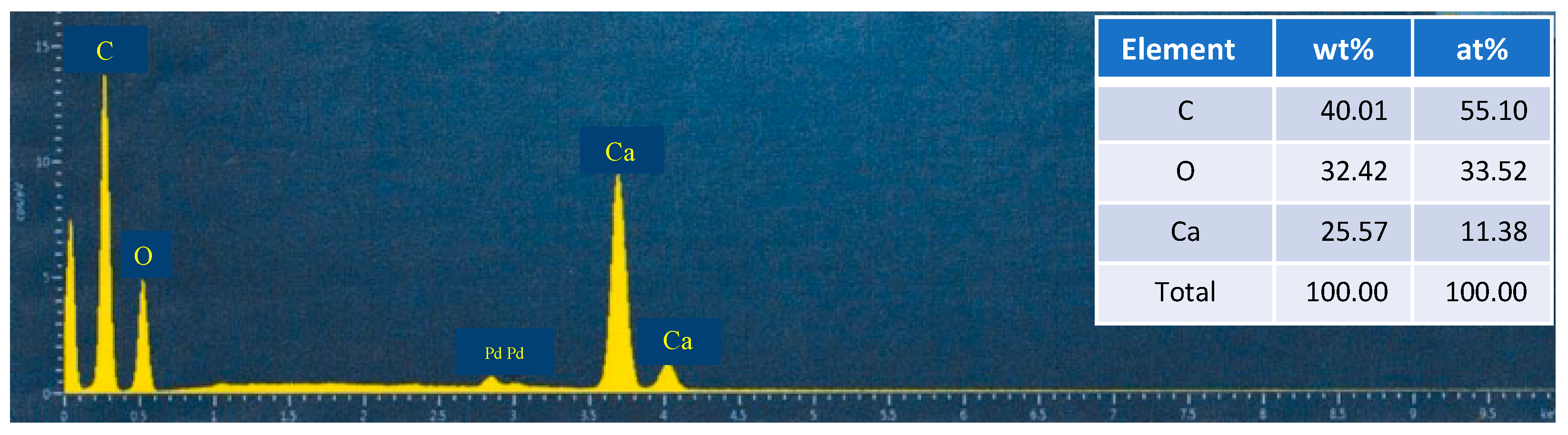
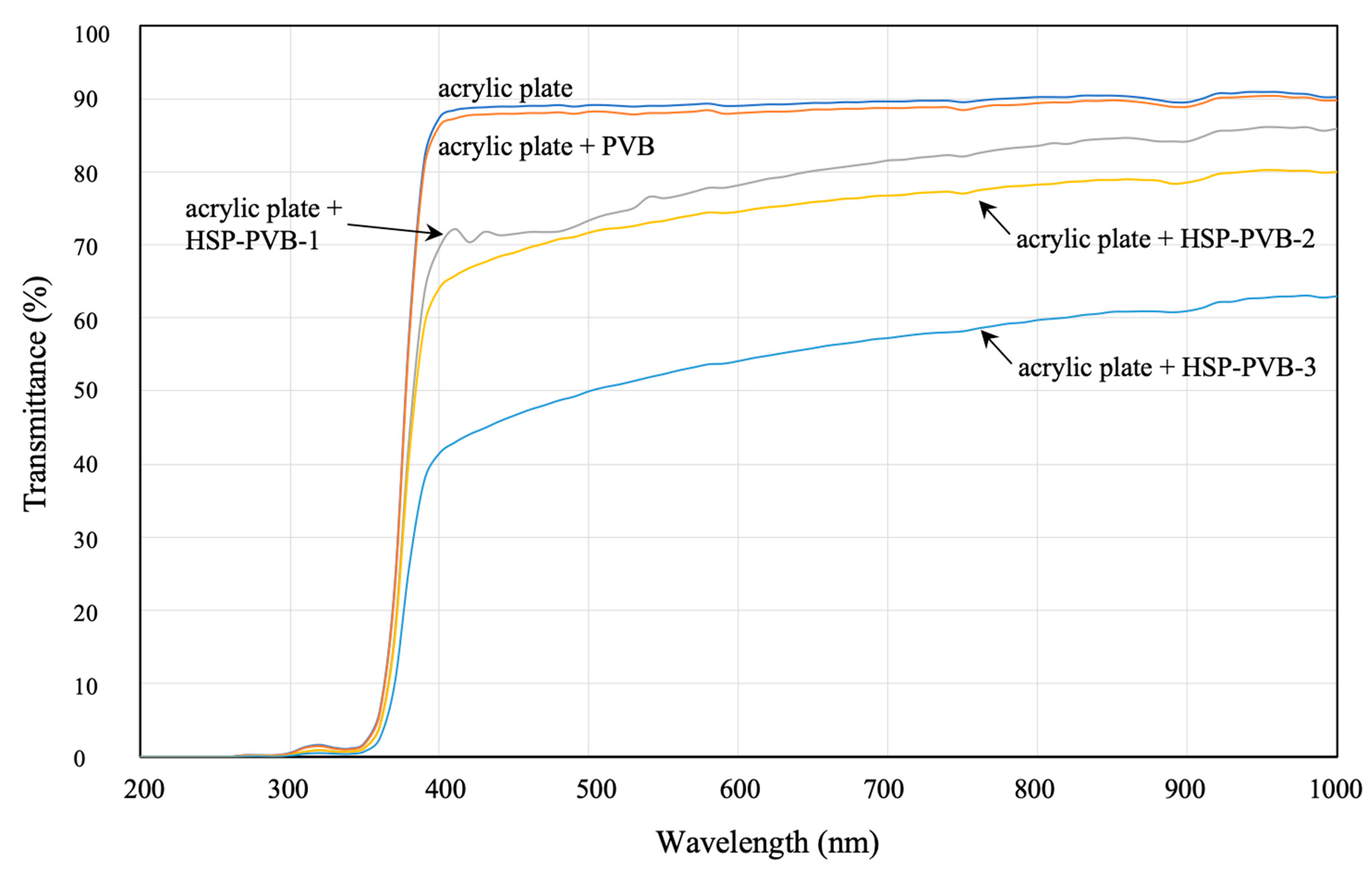

| Test Sample | Scallop HSP | Ethanol Solvent | Dispersing Agent | PVB | Total |
|---|---|---|---|---|---|
| HSP-PVB-1 | 7.8 | 78.1 | 2.3 | 11.7 | 100 |
| HSP-PVB-2 | 8.1 | 81.3 | 2.4 | 8.1 | 100 |
| HSP-PVB-3 | 8.5 | 84.7 | 2.5 | 4.2 | 100 |
| Test Sample | Amount of Coating (g/m2) | Contact Angel (°) * | Hardness |
|---|---|---|---|
| PVB | 4.4 ± 0.6 | 25.3 ± 0.6 | HB |
| HSP-PVB-1 | 6.4 ± 1.6 | 25.3 ± 1.5 | <6B |
| HSP-PVB-2 | 9.6 ± 0.3 | 20.0 ± 2.6 | 2B |
| HSP-PVB-3 | 11.8 ±0.3 | 15.3 ± 2.5 | 4B |
| Bacteria/Virus | Test Sample * | R Values | |||
|---|---|---|---|---|---|
| 5 min | 1 h | 3 h | 24 h | ||
| Escherichia coli NBRC 3301 | PVB | - | - | - | 1.8 |
| HSP-PVB-1 | 3.0 | - | - | >5.1 | |
| HSP-PVB-2 | 3.0 | - | - | >5.1 | |
| HSP-PVB-3 | 3.1 | 3.4 | >3.8 | >4.5 | |
| Staphylococcus aureus NBRC 13276 | PVB | - | - | - | 0 |
| HSP-PVB-1 | 0.5 | - | - | >3.1 | |
| HSP-PVB-2 | 1.4 | - | - | >4.2 | |
| HSP-PVB-3 | 2.2 | 2.8 | >3.1 | >4.4 | |
| Influenza A virus (H3N2) ATCC VR-1679 | HSP-PVB-2 | - | - | - | >4.4 |
| Feline calicivirus ATCC VR-782 | HSP-PVB-2 | - | - | - | >4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akasaka, R.; Osawa, A.; Wada, R.; Sawai, J.; Nakagawa, Y. Antimicrobial Activity and Transparency of Polyvinyl Butyral Paint Containing Heated Scallop-Shell Powder. Coatings 2023, 13, 364. https://doi.org/10.3390/coatings13020364
Akasaka R, Osawa A, Wada R, Sawai J, Nakagawa Y. Antimicrobial Activity and Transparency of Polyvinyl Butyral Paint Containing Heated Scallop-Shell Powder. Coatings. 2023; 13(2):364. https://doi.org/10.3390/coatings13020364
Chicago/Turabian StyleAkasaka, Ryunosuke, Ayako Osawa, Risei Wada, Jun Sawai, and Yoshitaka Nakagawa. 2023. "Antimicrobial Activity and Transparency of Polyvinyl Butyral Paint Containing Heated Scallop-Shell Powder" Coatings 13, no. 2: 364. https://doi.org/10.3390/coatings13020364
APA StyleAkasaka, R., Osawa, A., Wada, R., Sawai, J., & Nakagawa, Y. (2023). Antimicrobial Activity and Transparency of Polyvinyl Butyral Paint Containing Heated Scallop-Shell Powder. Coatings, 13(2), 364. https://doi.org/10.3390/coatings13020364






