Abstract
In the present study, the effect of pipe materials on water quality as well as the microbial community was researched with static devices as well as dynamic ones. Five kinds of pipe materials (SP: steel plastic composite pipe, SS: stainless steel pipe, DI: ductile iron pipe, CI: cast iron pipe, GS: galvanized steel pipe) were chosen, and the soaking experiment was carried out with bench-scale devices. To further investigate the performance of pipe materials over a long term, a pilot-scale simulated drinking water distribution system was constructed, and the water quality parameters were monitored for six months. The pipe materials were ranked as SP, DI, and CI by the order of increasing turbidity, CODMn, and NH3-N. Furthermore, the biofilm samples were analyzed via pyrosequencing and COG functional categories. The DI biofilm possessed the highest bacterial diversity with a Shannon index of 3.56, followed by SP (3.14) and CI (0.77). The presence of nitrate-reducing bacteria (NRB), iron-oxidizing bacteria (IOB), iron-reducing bacteria (IRB), and sulfate-reducing bacteria (SRB)was identified, and NRB composed the largest share in all pipe materials (13.0%–17.2%), with other redox bacteria making up a minor proportion (0.02%–1.52%). NRB and IRB inhibited the corrosion process while IOB and SRB enhanced it. Most dominant genera present in samples were derived firstly from soil or active sludge, indicating a turbidity problem due to soil contamination in the distribution network.
1. Introduction
Disinfected water experiences water deterioration in the drinking water distribution systems. The pipe material is considered to be one of the dominant factors in the process, owing to the release of nutrients and complicated physiochemical reactions with microbes [1]. In previous research, the effect of pipe materials on a series of substances has been reported. Particles are quantified and investigated, including corrosion scales [2,3], metal ions [4], organic matters [5], disinfection residuals [6], DBPs [7], nitrogen [8,9], and biofilm.
Biofilm plays a central role in reactions with pipe materials, and thus an increasing enthusiasm for biofilm investigation is found. The biomass quantification [10,11,12], bacterial numeration [6], microbe community structure [13,14], and identification of pathogens [15,16,17] are measured and analyzed. From different pipe materials, significant diversity of bacterial abundance and community distribution is found. Additionally, bacteria crucial in nitrification, corrosion, and chlorine resistance are found. The influence of phenotypic, chemotaxonomic, and phylogenetic characteristics of dominant bacteria on water parameters is not discussed.
A variety of pipe materials has been tested in terms of biofilm formation. In some essays, plastic-based materials (PVC, PE, HDPE) are observed to have better performance [6,18], while in other research, steel/iron-based materials [5] or cement-based materials are recommended. The steel-plastic composite pipe is a kind of lined material, with the combined property of steel, and HDPE. Ductile iron is commonly used in southern China. The effects of these materials on bacteria were rarely discussed before and need further research.
In the last decade, the use of next-generation sequencing has incredibly enhanced the understanding of the microbial ecology of different ecosystems [19]. With the KEGG Ortholog (KO) database of reference genomes, analysis tools such as the PICRUSt are widely applied to predict the functional composition of a metagenome [20]. Meanwhile, more functional genes have been shown to be linked to microbial metabolism [21,22]. To provide information on the microbial controlling strategy, the role of interspecies relationships in different pipe materials still needs further discussion, from the perspective of microbial ecology.
The simulation of distribution systems has been carried out with a bench-top device and a pilot-scale simulated system. The device avoids the uncertain influence of actual pipeline sampling (variation of pipe age, hydraulic condition, and temperature) and makes it convenient to sample and control. The pilot-scale simulated system, compared to a bench-top device, exhibits a better simulation of the real hydraulic condition [23].
In the present study, a bench-scale and a pilot-scale experiment were carried out to give an insight into the biological and chemical process both in a static and a dynamic model. A model pilot-scale distribution system was built for dynamic simulation to achieve better experimental results [24]. With the result of water quality parameters and 16S rRNA-based pyrosequencing, the optimal pipe materials were recommended and confirmed by the improvement of water quality in an engineering project.
2. Materials and Methods
2.1. Static Soaking Experiment
Five kinds of pipe material were soaked in two kinds of water, prepared in the laboratory, and collected from a distribution site, respectively. All pipes were purchased from Chengdu ChuanLi Intelligence Fluid Equipment Co. Ltd. (Chengdu China). The water preparation was based on the methodology of standard for assessment of drinking water distribution equipment and protective materials. The characteristics of the water mixed in the laboratory were determined as turbidity of 0.12 NTU, chlorine residuals of 2 mg/L, and a total number of bacteria of 0 CFU/mL. Correspondingly, the characteristics of the water collected in a real distribution site were determined as turbidity of 0.53 NTU, chlorine residuals of 0.08 mg/L, and a total number of bacteria of 3100 CFU/mL. Heshanqiao note was chosen as it has the most severe problem of water deterioration.
Sixty centimeters in length and 20 mm in diameter, the pipe materials were thoroughly washed out and disinfected with sodium hypochlorite. The pipes were soaked at 25 °C in the constant-temperature incubator. To simulate a real circumstance, the pipes were shaded. Turbidity, BDOC, DOC, and TPC were measured every day for a week, reflecting water quality in different water ages.
2.2. Dynamic Distribution System Setup and Operation
A model pilot-scale distribution system was built in Suzhou, Taihu lake region (Figure 1). This system included three loops, using SS pipe, CI pipe, and DI pipe. The pipes were purchased from Chengdu ChuanLi Intelligence Fluid Equipment Co. Ltd. (Chengdu, China). Every loop was equipped with an inlet valve, flowmeter, and pumps. To simulate the real circumstances in pipelines as precisely as possible, the distribution system was fed with water from the clean water basin in the Xiangcheng water treatment plant consistently. The flow rate was maintained at 5.6 m3/h.

Figure 1.
The simulated drinking water distribution system.
After a stabilizing period of three months, the turbidity, CODMn, and NH3-N were measured every two weeks for eight months. Biofilm samples were collected at the end of the term.
2.3. Pyrosequencing of Bacterial 16S rRNA Genes
Biofilm samples were selected for bacterial community profiling by pyrosequencing, targeting 16S rRNA genes, which was performed at the I-Sanger Bioinformatics Analysis Cloud Platform, the Majorbio (http://www.i-sanger.com/, accessed on 4 March 2022). In total, 40,242, 40,237, and 36,911 pyrosequences were obtained from three biofilm samples, and they were swabbed from DI, CI, and SS. The length of sequences varied between 271 and 499. In total, 334 OTUs were defined based on clustering. Pyrosequences were assigned to 20 phyla, of which proteobacteria were the most abundant.
2.4. Data Analysis and Statistics
Kruskal–Wallis ANOVA was used to evaluate the effects of pipe materials on turbidity, BDOC, DOC, and TPC, respectively. Statistical significance was set at p < 0.05. Multiple comparison tests were performed to find out which pair of the five pipe materials were significantly different. Kruskal–Wallis ANOVA and multiple comparison tests were performed with OriginPro 2021.
A redundancy analysis was carried out with Canoco 4.5. Water quality statistics from the pilot-scale experiment and the transformation engineering project were analyzed with the above multivariate statistical analysis tools.
3. Results
3.1. The Difference in Water Quality Due to Leaching Matters from Pipe Materials
In the bench-scale experiment, pipe materials were soaked with water samples from the laboratory and in situ distribution system. During seven days, leaching components as well as bacterial growth were analyzed with indicators involving turbidity, BDOC, DOC, and TPC (Figure 2).
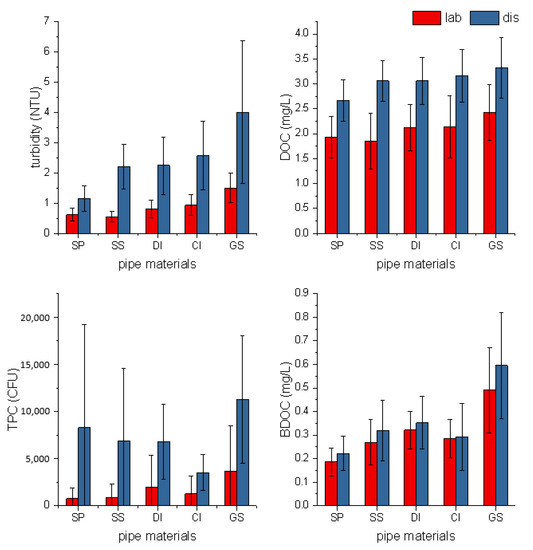
Figure 2.
Comparison of effluent from different pipe materials during a seven-day soaking experiment.
The soaking experiment gave a profile of the soaking water quality indicators related to the leaching process by varying the soaking water. The characteristics of the soaking water, collected from distribution systems or prepared in the laboratory, are listed in Table 1. The initial bacterial concentration was much higher from the distribution system node (3100 CFU/mL), with a relatively lower chlorine residual (0.08 mg/L) and higher turbidity. For metal pipe materials tested (SS, DI, CI, and GS), the leaching levels of metal ions, mainly iron and zinc, are higher when a higher level of chlorine is presented [25,26,27]. Besides the chlorine residuals, the initial bacteria concentration also proceeds the bacterial growth in the pipe, which accelerates the corrosion and subsequent iron release [28].

Table 1.
Characteristics of the soaking water.
The influence of pipe materials on water quality was evaluated by using Kruskal–Wallis ANOVA analysis. As illustrated in Table 2, pipe materials had a significant influence on water quality measured by turbidity, DOC, and BDOC (Figure 3). Considering the relatively short period, changes in the turbidity, DOC, and BDOC were mainly caused by the diffusion process, and the pipe material did play an important role. However, no insignificant influence by TPC was observed. Although there were no obvious differences observed in TPC between different materials, it was not to say the effect of the pipe material on microbes was negligible. The total plate counts were only a reflection of quantities of microbes that could be fostered without the special requirement of nutrition and oxide. It did not reflect the structure of the microbe community, and further investigation should be carried out.

Table 2.
Effects of pipe materials on water quality in water from different sources using Kruskal–Wallis ANOVA. Factors with a p-value smaller than 0.05 are highlighted in bold.
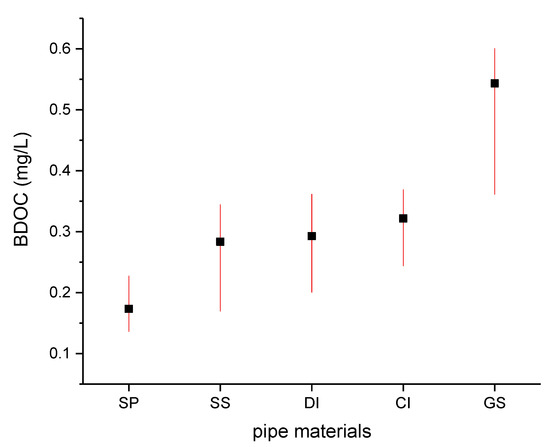
Figure 3.
Multiple comparison test of the BDOC between different kinds of pipe material using water collected from the distribution system node (the type of critical value to use for the multiple comparisons is Kruskal–Wallis test, and the significance level is 0.05).
The SP is a kind of steel pipe lined with HDPE. For organic pipes, residual chlorine, temperature, and time have a positive effect on the released organics [29]. Organic compounds leaching from PE pipes are mostly antioxidants and plasticizers, which serve as nutrients for bacteria. Besides, PE releases biodegradable organic compounds and phosphorus which promote biofilm development on plastic surfaces [30]. Lehtola et al. observed a significant rate of phosphorus that was released from PE pipes into the water for 2–3 weeks, and the biofilm development was influenced by the phosphorus availability in the water systems [31].
3.2. The Difference in Water Quality Due to the Activity of Microorganisms
Redundancy analysis was carried out with Canoco 4.5 to investigate the influence of pipe materials, as well as temperature and chlorine residuals. The water quality was expressed as a linear combination of turbidity, CODMn, and NH3-N. The analysis demonstrated that the water quality was highly correlated with the environmental factors (p-value = 0.0040, Monte Carlo test). Briefly, 18.7% of the information corresponded to the first sequencing axis, and 9.1% corresponded to the second axis.
As illustrated in Figure 4, CODMn corresponded positively with NH3-N, whereas no obvious correlation between CODMn and turbidity was observed, with the angle between the two vectors equal to nearly 90°. Among all the environmental factors, pipe materials (CI) were located the most far away from the coordinate origin, indicating a violent influence on the water quality. In the direction of CODMn and NH3-N, the projection of CI was the longest, followed by DI and SP. The negative projection of SP and DI revealed a relatively good property in maintaining water quality, whereas CI showed an opposite tendency. However, in the direction of turbidity, no obvious difference was confirmed between these three pipe materials.
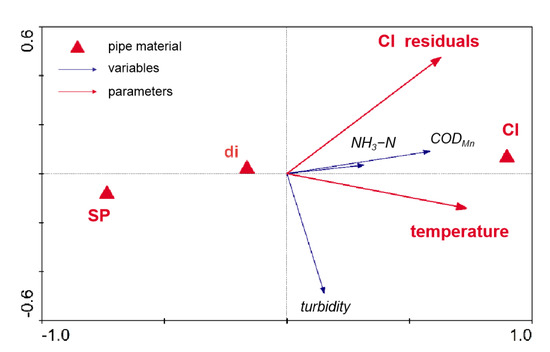
Figure 4.
Redundancy analysis (RDA) ordination triplot of environment factors and water quality indicators over a long period. Red triangles represent three kinds of pipe material; red arrows represent variables: temperature and chlorine residuals; blue arrows represent parameters: turbidity, CODMn, and NH3-N.
Comparing the three pipe materials, the distance between the SP and CI was the longest, indicating a great difference between these two materials. Based on the projection in the direction of CODMn and NH3-N, the property of the pipe materials could be listed in the following order: SP > DI > CI, which was consistent with the outcome of a bench-scale experiment. Higher levels of CODMn were observed in DI and CI, revealing an inclination in biofilm formation and detachment in iron pipes versus plastic-lining materials. A higher level of CODMn correlated with biofilm detachment. EPS was produced by the attached community to provide structure and stability for the biofilm. If the EPS adhesive strength deteriorated or was overcome by external shear forces, biofilm was mobilized into the water, potentially leading to the degradation of the water quality. With carbohydrates as the dominant component in the biofilm, the level of organic matter measured by CODMn is raised as a result of the detachment [32]. Microbes’ preference for iron materials is also confirmed by results in the previous report, with higher TPC and HPC in iron pipes than that in plastic ones [33].
In addition to the influence of pipe materials, the temperature trended to vary in a way with the change of all the water parameters, supporting the assumption that water deteriorates fiercely with the rise of temperature. On the one hand, the temperature is the main reason that causes the change in the diversity of bacteria [34]. On the other hand, biofilm formation and the release of EPS are much slower and much more separated at a lower temperature, which accounts for water deterioration [35].
3.3. Phylogenetic Assessment of Microbial Community
A total of 117,390 pyrosequences were obtained from three biofilm samples, with an average sequence yield of 39,130 ± 1569 for each sample (range 36,911–40,242). Based on the clustering of these sequences at 97% gene similarity, 110, 202, and 205 OTUs were found in the biofilms from the CI pipe, DI pipe, and SP pipe, respectively. The CI biofilm possessed the lowest bacterial diversity with the Shannon index (0.77) and Simpson index (0.66), while no notable differences were confirmed between DI and SP with the Shannon and Simpson indexes (Table 3). In terms of community richness, SP demonstrated the highest community richness expressed by the Chao and Ace indices, followed by DI and CI.

Table 3.
Alpha-diversity indexes of the bacterial.
The community richness of SP (HDPE-lined steel pipe) was the highest, indicating that plastic could stimulate biofilm growth. The mechanism of the stimulation is attributed to the release of biodegradable organic compounds [36] and phosphorus from this material.
The taxonomic distributions of phylogenetic groups for biofilms are listed in Figure 5. The pyrosequences were assigned to 14 phyla, of which Proteobacteria was the most abundant, followed by Bacteroidetes and Firmicutes. On the genus level, the composition of biofilm samples is shown in Figure 5. The SP sample was dominated by several genera including Bradyrhizobium, Sphingobium, and Novosphingobium. The CI sample accommodated two major genera, Brevundimonas, and Brevibacillus, with a minor portion of other genera. On the contrary, the genera in the DI sample exhibited were well distributed among the genera spectra. The diversity of the CI samples was relatively low, which was also confirmed by the Shannon Index.
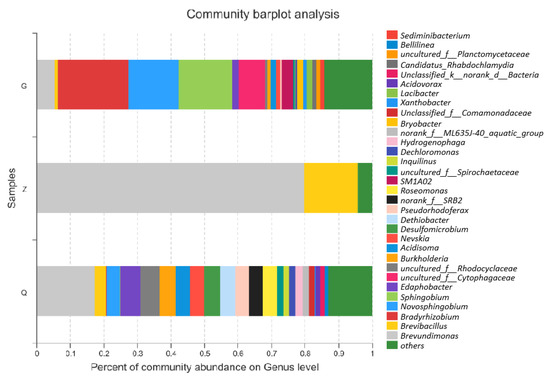
Figure 5.
Taxonomic distribution of phylogenetic groups for biofilms from SP, CI, and DI at the genus level.
The phenotypic, chemotaxonomic, and phylogenetic characteristics of dominant bacteria in SP, DI, and CI are listed in Table 4. In SP, Novosphingobium, Sphingobium, and Bradyrhizobium were found with a higher abundance than the other materials. All three genera are α-proteobacteria, and they share common features: Gram-negative; mesophilic and neutrophilic; chemo-organotrophic; aerobes or facultative anaerobes. Sphingobium can be found in soil, pentachlorophenol-contaminated soil, and clinical specimens. They can degrade a variety of chemicals in the environment, such as aromatic and chloroaromatic compounds, phenols like nonylphenol and pentachlorophenol, herbicides, propionic acid, hexachlorocyclohexane, and polycyclic aromatic hydrocarbons. These compounds exist in SP as a result of organics leaching and provide nutrients for Sphingobium. Closely related to Sphingobium, Novosphingobium is also strictly aerobic and chemo-organotrophic. Especially, it can reduce nitrate to nitrite. Novosphingobium is a genus of Gram-negative bacteria that includes N. taihuense, which can degrade aromatic compounds such as phenol, aniline, nitrobenzene, and phenanthrene [37]. Bradyrhizobium, a genus of slow-growing, root nodule bacteria from leguminous plants, fixes atmospheric nitrogen (dinitrogen) when in the symbiotic state within root nodules [38].

Table 4.
Phenotypic, chemotaxonomic, and phylogenetic characteristics of dominant bacteria in SP, DI, and CI. NA: not acquired.
In DI, a moderate-high level of Dechloromonas, Pseudorhodoferax, Edaphobacter, Phreatobacter, and Simplicispira was identified. Dechloromonas is the predominant chlorate-reducing bacteria in the environment. As a facultative anaerobe, it can oxidize acetate with O2, ClO3−, ClO4−, or NO3− as alternative electron acceptors [46]. Pseudorhodoferax is aerobic and chemo-organotrophic. The species is isolated from activated sludge and soil. Both Pseudorhodoferax and Dechloromonas are located in the β-subclass of proteobacteria. Edaphobacter belongs to Acidobacteria. It is adapted to environments with low carbon concentrations and neutral to slightly acidic conditions, which is consistent with drinking water distribution systems [47]. Phreatobacter is phylum Proteobacteria, Gram-negative, and inhibited by the presence of high concentrations of NaCl. The type species, Phreatobacter oligotrophus, is isolated from the ultrapure water of the water purification system of a power plant [42]. The optimum environmental condition for its growth is 25–37 °C, pH 6–8. Additionally, it does not produce acid production from all carbon sources of the API 50CH test. Simplicispira comprises anaerobes and facultative anaerobes, accustomed to the neutral and moderated environment. The common habitat for Simplicispira is freshwater, wastewater, and Antarctic mosses. It is capable of denitrification [43]. It provides a possible explanation for the negative correlation between DI materials and the concentration of NH3-N in Figure 4.
In CI, Brevundimonas and Brevibacillus were dominant. Brevundimonas, a large portion of all samples, exhibited a dominant position in the distribution system. It belongs to α-proteobacteria. The strains exhibit a limited nutritional spectrum; only DL-P-hydroxybutyrate, pyruvate, L-glutamate, and L-proline are used by 90% or more of the strains as sole carbon and energy sources. Additionally, pantothenate, biotin, and cyanocobalamin are required as growth factors [44]. From the above description, it can be inferred that the nutrition of Brevundimonas is restricted. Thus, the carbon source may come from other organisms’ metabolic products, and a mutually promoted relationship with other microbes exists. Brevibacillus belongs to Firmicutes, strictly aerobic (except for Brevibacillus Zaterospoius). Optimum growth occurs at pH 7.0. The optimum growth temperature of nine species (all species except Brevibacillus themoruber) is 30 °C. Some species are capable of reducing nitrate to nitrite [45].
3.4. Presence of Redox Bacteria and Chlorine-Resistant Bacteria
To further analyze the role of redox bacteria and the metabolic cycle of iron, nitrogen, and sulfate in different pipe materials, the OUT proportions are compared between different pipe materials in Figure 6. In all kinds of pipes, the presence of NRB, IOB, IRB, and SRB was identified, whereas nearly none of the AOB, NOB, and SOB were found. NRB composed the largest share in all pipe materials (13.0–17.2%), with other redox bacteria making up a minor proportion (0.02–1.52%). The highest abundance of NRB was presented in SP, followed by CI and DI. In terms of IOB, IRB, and SRB, the highest abundance was presented in DI, and extremely few IOBs were identified in CI.
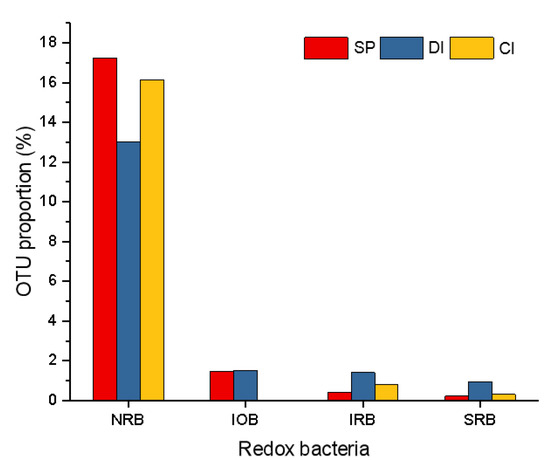
Figure 6.
OUT proportions of redox bacteria in different pipe materials. Abbreviations are used for NRB, IOB, IRB, and SRB [48,49,50].
Owing to the chlorine residuals in the influent, chlorine-resistant bacteria were identified in all the pipe materials. The abundance of chlorine-resistant bacteria was 0.57%, 0.55%, and 0.49% for SP, CI, and DI, respectively. SP had the largest proportion, but the distinction was not conspicuous. Research indicated that multispecies biofilms are generally more resistant to disinfection than single-species biofilms [51]. Therefore, more chlorine-resistant bacteria can be attributed to a higher community richness of SP.
As seen from Table 5, chlorine-resistant bacteria existed in all pipe materials, and the proportion in SP was the highest. Therefore, SP favored microorganisms with tolerance to chlorine. Correspondingly, the COG categories including cell wall/membrane/envelope biogenesis and defense mechanisms had the biggest share in SP. The reaction site of disinfection was reported to be the cell wall [52]. Additionally, the number of induced membrane-associated genes increases with increasing chlorine doses [53].

Table 5.
Proportion of Chlorine-resistant bacteria genus.
3.5. Effect of Pipe Materials on COG Functional Categories
An analysis of genes in COG functional categories was carried out to compare the proteomic variation in different pipe materials (Table 6). Generally, the occupation of COG groups does not diversify drastically from each other, representing a high degree of similarity in protein function. The proportion of unknown functional genes in the three samples was the highest, which were 10.66% (DI), 11.72% (CI), and 9.48% (SP), respectively, indicating that some functions of these samples had not been successfully annotated by the COG database, so more annotation information was necessary. Except for unknown function genes and general function prediction-only genes, amino acid transport and metabolism genes accounted for the highest proportion, which were 8.31% (DI), 7.23% (CI), and 7.84% (SP), respectively.

Table 6.
Comparison of COG functional categories percentage for SP, CI, and DI.
Nevertheless, more distinctions were found between CI and the others. In CI, amino acid transport and metabolism genes, energy production and conversion genes, and carbohydrate transport and metabolism genes accounted for the lowest proportion. Meanwhile, CI, with the highest proportion of cell motility genes and relatively poor water quality, suggested that the correlation between water quality deterioration and cell motility genes is due to cell detachment.
4. Discussion
Different concentrations of organic matter are presented in the effluent of different pipe materials. Variation in ammonium concentration in different pipe materials is attributed to the microorganism’s metabolic process for nitrogen. The ammonium ion is partially oxidized via nitrite to nitrate in the drinking water distribution systems. Additionally, the nitrate generated by nitrification is partly utilized by nitrate-reducing (and denitrifying) bacteria [62]. More ammonium is affirmed in CI, suggesting the absence of nitrifying bacteria. For SP, ammonia nitrogen was rarely monitored in bulk water. It demonstrated a good performance of SP in water quality maintenance.
Nitrates may also undergo denitrification in steady water, i.e., they would support bacterial existence in the distribution system. Such an environment forms favorable conditions for sulfate-reducing bacteria and, in all of the steady water samples, we have easily (but qualitatively) detected sulfides [63].
4.1. Bacteria’s Common Requirement of Habitat from Different Pipe Materials
In this work, Gram-negative, chemo-organotrophic, mesophilic and neutrophilic, and aerobic bacteria were found to be the major groups in the distribution system. Most of them belonged to α- and β-Proteobacteria, which was similar to previous work [64]. Some dominant genera were first derived from soil or active sludge, which agreed with the turbidity problems observed due to soil contamination in the distribution network. While other dominant genera were first derived from pure water, the oligotrophic nutrient-deprived habitat suggested an adaption to environments with low carbon concentrations.
4.2. Nutrients in the Effluent as a Result of Biofilm Detachment
In the pilot scale experiment, the maximum nitrogen and organic matter were observed in CI. It suggested the most serious deterioration problem in CI and may prosper the bacteria regrowth downstream.
The organic matter in effluent mainly comes from the detachment of biofilm and EPS by hydraulic shear force. In CI, the COG functional group controlling cell motility exhibited the highest abundance in accordance with the cod concentration results.
Except for biofilm detachment, the metabolites in the respiration process also enter the effluent. Thus, the nutrients’ concentration is also related to metabolic intensity. In CI, the abundance of two functional groups was the lowest: amino acid and carbohydrate, whose transport and metabolism resulted in the release of organic matter into the bulk water instead of being utilized by microorganisms.
4.3. Corrosion Bacteria in Different Pipe Materials
For iron pipes, corrosion plays an important role in water deterioration. The process is related to several bacteria capable of utilizing iron, nitrogen, and sulfate.
In SP, the dominant genus Dechloromonas and Bradyrhizobium inhibited the corrosion process, in accordance with a better effluent water quality. In previous research, Dechloromonas was reported to cast the greatest corrosion inhibition by inducing the redox cycling of iron to enhance the precipitation of iron oxides and the formation of Fe3O4 [15,48]. Rhizobia Bradyrhizobium-producing siderophores have a weaker corrosion-inhibition effect by capturing iron to inhibit the dissolution of iron and iron corrosion [1,48].
In DI, the highest abundance of IRB, IOB, and SRB was identified, indicating the existence of corrosion. IRB can reduce ferric iron to ferrous iron, which was reported to have the ability to inhibit iron corrosion [65]. Additionally, the EPS at the metal surface can impede the dissolution of ferrous iron corrosion products [66]. IRB can also favor the formation of Fe3O4 [67]. Conversely, the presence of SRB and IOB in biofilms can speed up iron corrosion. The IOB contributes to the formation of a-FeOOH. Beneath the iron-rich tubercles produced by IOB, SRB may increase rapidly. SRB is usually associated with anaerobic iron corrosion by producing hydrogen sulfide (H2S) as a corrosive agent. The interaction between SRB/IOB can accelerate the iron corrosion process [68]. These bacteria play a collaborative as well as an opposite role in the corrosion process (Figure 7).
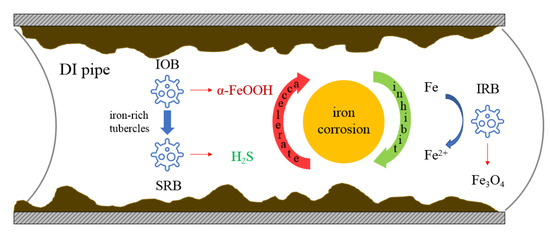
Figure 7.
The relationship between bacteria (IOB, SRB, and IRB) and DI pipe corrosion.
5. Conclusions
The pipe materials had a salient effect on water quality and microbial community. In the static soaking experiment, the pipe material apparently affected the water quality determined by turbidity, DOC, and BDOC, while having no significant effect on TPC. In the dynamic simulation of the distribution system, the performance of the pipe materials was ordered as SP, DI, and CI with respect to water quality maintenance. The presence of NRB, IOB, IRB, and SRB was identified, and NRB composed the largest share in all pipe materials (13.0%–17.2%), with other redox bacteria composing a minor proportion (0.02%–1.52%). In DI, the highest abundance of IRB, IOB, and SRB was identified, indicating the existence of corrosion. SP favored chlorine-resistant bacteria, and the COG categories including cell wall/membrane/envelope biogenesis and defense mechanisms had the biggest share. CI, with the highest proportion of cell motility genes, suggested that the correlation between water quality deterioration and cell motility genes is due to cell detachment. The turbidity problems caused by soil contamination in the distribution network led to the most dominant genus being derived firstly from soil or active sludge.
Author Contributions
Conceptualization, S.S.; methodology, S.S. and H.Y.; validation, H.Y. and W.Z. (Wenying Zhou); formal analysis, S.S.; investigation, S.S., H.Y. and G.L.; resources, W.Z. (Wei Zhang); data curation, S.S., Y.Z. and W.Z. (Wenying Zhou); writing—original draft preparation, S.S. and W.Z. (Wenying Zhou); writing—review and editing, S.S. and Y.Z.; visualization, G.L. and W.Z. (Wei Zhang); supervision, W.L. and W.Z. (Wei Zhang); project administration, Y.Z. and W.L.; funding acquisition, W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the Study on the Cross Regional Joint Prevention and Control Mechanism and Strategy of Water quality and biosafety risks in the upper reaches of the Yangtze River (2021-YB-CQ-3), and the comparative study on corrosion characteristics of pipes and microbial safety of water quality in water supply systems (kh0040020191200).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature and Acronyms
DBP: disinfection byproduct; SP: steel plastic composite pipe; SS: stainless steel pipe; DI: ductile iron pipe; CI: cast iron pipe; GS: galvanized steel pipe; COG: clusters of orthologous genes; NRB: nitrate-reducing bacteria; IOB: iron-oxidizing bacteria; IRB: iron-reducing bacteria; SRB: sulfate-reducing bacteria; PVC: polyvinyl chloride; PE: polyethylene; HDPE: high-density polyethylene; BDOC: biodegradable dissolved organic carbon; DOC: dissolved organic carbon; TPC: total plate counts; OTU: operational taxonomic unit; EPS: extracellular polymeric substances; HPC: heterotrophic plate count; AOB: ammonia-oxidizing bacteria
References
- Concha, C.; Doerner, P. The impact of the rhizobia-legume symbiosis on host root system architecture. J. Exp. Bot. 2020, 71, 3902–3921. [Google Scholar] [CrossRef]
- Li, M.J.; Liu, Z.W.; Chen, Y.C.; Hai, Y. Characteristics of iron corrosion scales and water quality variations in drinking water distribution systems of different pipe materials. Water Res. 2016, 106, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Perrin, Y.; Bouchon, D.; Delafont, V.; Moulin, L.; Hechard, Y. Microbiome of drinking water: A full-scale spatio-temporal study to monitor water quality in the Paris distribution system. Water Res. 2019, 149, 375–385. [Google Scholar] [CrossRef]
- Zhang, S.; Tian, Y.; Guo, Y.; Shan, J.; Liu, R. Manganese release from corrosion products of cast iron pipes in drinking water distribution systems: Effect of water temperature, pH, alkalinity, SO42− concentration and disinfectants. Chemosphere 2021, 262, 127904. [Google Scholar] [CrossRef] [PubMed]
- Rand, J.L.; Sharafimasooleh, M.; Walsh, M.E. Effect of water hardness and pipe material on enhanced disinfection with UV light and chlorine. J. Water Supply Res. Technol. Aqua 2013, 62, 426–432. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Kang, M.; Chen, C.; Song, Y.; Li, H. Effects of chlorination/chlorine dioxide disinfection on biofilm bacterial community and corrosion process in a reclaimed water distribution system. Chemosphere 2019, 215, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Abokifa, A.A.; Yang, Y.J.; Lo, C.S.; Biswas, P. Investigating the role of biofilms in trihalomethane formation in water distribution systems with a multicomponent model. Water Res. 2016, 104, 208–219. [Google Scholar] [CrossRef]
- Ding, S.; Deng, Y.; Bond, T.; Fang, C.; Cao, Z.; Chu, W. Disinfection byproduct formation during drinking water treatment and distribution: A review of unintended effects of engineering agents and materials. Water Res. 2019, 160, 313–329. [Google Scholar] [CrossRef]
- Zhang, H.; Andrews, S.A. Effects of pipe materials, orthophosphate, and flow conditions on chloramine decay and NDMA formation in modified pipe loops. J. Water Supply Res. Technol. Aqua 2013, 62, 107–119. [Google Scholar] [CrossRef]
- Gamri, S.; Soric, A.; Tomas, S.; Molle, B.; Roche, N. Effects of pipe materials on biofouling under controlled hydrodynamic conditions. J. Water Reuse Desalination 2016, 6, 167–174. [Google Scholar] [CrossRef]
- Learbuch, K.L.G.; Smidt, H.; van der Wielen, P. Water and biofilm in drinking water distribution systems in the Netherlands. Sci. Total Environ. 2022, 831, 154940. [Google Scholar] [CrossRef] [PubMed]
- Spark, A.; Wang, K.; Cole, I.; Law, D.; Ward, L. Microbiologically influenced corrosion: A review of the studies conducted on buried pipelines. Corros. Rev. 2020, 38, 231–262. [Google Scholar] [CrossRef]
- Rozej, A.; Cydzik-Kwiatkowska, A.; Kowalska, B.; Kowalski, D. Structure and microbial diversity of biofilms on different pipe materials of a model drinking water distribution systems. World J. Microbiol. Biotechnol. 2015, 31, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, C.; Shi, B. The control of red water occurrence and opportunistic pathogens risks in drinking water distribution systems: A review. J. Environ. Sci. 2021, 110, 92–98. [Google Scholar] [CrossRef]
- Fu, Y.; Peng, H.; Liu, J.; Nguyen, T.H.; Hashmi, M.Z.; Shen, C. Occurrence and quantification of culturable and viable but non-culturable (VBNC) pathogens in biofilm on different pipes from a metropolitan drinking water distribution system. Sci. Total Environ. 2021, 764, 142851. [Google Scholar] [CrossRef]
- Leslie, E.; Hinds, J.; Hai, F.I. Causes, Factors, and Control Measures of Opportunistic Premise Plumbing Pathogens—A Critical Review. Appl. Sci. 2021, 11, 4474. [Google Scholar] [CrossRef]
- Zhu, Z.B.; Wu, C.G.; Zhong, D.; Yuan, Y.X.; Shan, L.L.; Zhang, J. Effects of Pipe Materials on Chlorine-resistant Biofilm Formation under Long-term High Chlorine Level. Appl. Biochem. Biotechnol. 2014, 173, 1564–1578. [Google Scholar] [CrossRef]
- Niquette, P.; Servais, P.; Savoir, R. Impacts of pipe materials on densities of fixed bacterial biomass in a drinking water distribution system. Water Res. 2000, 34, 1952–1956. [Google Scholar] [CrossRef]
- Douterelo, I.; Boxall, J.B.; Deines, P.; Sekar, R.; Fish, K.E.; Biggs, C.A. Methodological approaches for studying the microbial ecology of drinking water distribution systems. Water Res. 2014, 65, 134–156. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, C.J.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Blanco-Picazo, P.; Gomez-Gomez, C.; Tormo, M.; Ramos-Barbero, M.D.; Rodriguez-Rubio, L.; Muniesa, M. Prevalence of bacterial genes in the phage fraction of food viromes. Food Res. Int. 2022, 156, 111342. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Li, T.; Zhu, L.; Wang, Q.; Liang, X.; Li, Y.; Wang, X.; Zhao, S.; Li, L.; Li, Y. Obese Individuals with and without Phlegm-Dampness Constitution Show Different Gut Microbial Composition Associated with Risk of Metabolic Disorders. Front. Cell. Infect. Microbiol. 2022, 12, 859708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wu, X.; Zhang, T.; Cen, C.; Mao, R.; Pan, R. Pilot investigation on biostability of drinking water distribution systems under water source switching. Appl. Microbiol. Biotechnol. 2022, 106, 5273–5286. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.B.; Simoes, M.; Simoes, L.C. An overview on the reactors to study drinking water biofilms. Water Res. 2014, 62, 63–87. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Ledingham, G.J.; Catalano, J.G.; Giammar, D.E. Effects of Cu(II) and Zn(II) on PbO2 Reductive Dissolution under Drinking Water Conditions: Short-term Inhibition and Long-term Enhancement. Environ. Sci. Technol. 2021, 55, 14397–14406. [Google Scholar] [CrossRef]
- Tian, Y.; Li, J.; Jia, S.; Zhao, W. Co-release potential and human health risk of heavy metals from galvanized steel pipe scales under stagnation conditions of drinking water. Chemosphere 2021, 267, 129270. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Liu, D.; Wang, J.; Zhang, X.; Chen, C. Early period corrosion and scaling characteristics of ductile iron pipe for ground water supply with sodium hypochlorite disinfection. Water Res. 2020, 176, 115742. [Google Scholar] [CrossRef]
- Jang, H.J.; Choi, Y.J.; Ka, J.O. Effects of diverse water pipe materials on bacterial communities and water quality in the annular reactor. J. Microbiol. Biotechnol. 2011, 21, 115. [Google Scholar] [CrossRef]
- Liu, J.; Peng, H.; Tan, S.; Wu, J.; Bastani, H.; Li, C. Influence factors of organic compounds leaching from PE pipes and the potential toxic effects on E. coli and rat C6 glioma cell. Water Sci. Technol.-Water Supply 2016, 16, 402–409. [Google Scholar] [CrossRef]
- Lequette, K.; Ait-Mouheb, N.; Wery, N. Drip irrigation biofouling with treated wastewater: Bacterial selection revealed by high-throughput sequencing. Biofouling 2019, 35, 217–229. [Google Scholar] [CrossRef]
- Lehtola, M.J.; Laxander, M.; Miettinen, I.T.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. The effects of changing water flow velocity on the formation of biofilms and water quality in pilot distribution system consisting of copper or polyethylene pipes. Water Res. 2006, 40, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Douterelo, I.; Sharpe, R.L.; Husband, S.; Fish, K.E.; Boxall, J.B. Understanding microbial ecology to improve management of drinking water distribution systems. WIREs Water 2018, 6, e01325. [Google Scholar] [CrossRef]
- Ren, H.; Wang, W.; Liu, Y.; Liu, S.; Lou, L.; Cheng, D.; He, X.; Zhou, X.; Qiu, S.; Fu, L.; et al. Pyrosequencing analysis of bacterial communities in biofilms from different pipe materials in a city drinking water distribution system of East China. Appl. Microbiol. Biotechnol. 2015, 99, 10713–10724. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Tian, Y.M.; Liu, H.N.; Zhang, B.; Wu, Q.; Guo, H.N. Research on Microbial Diversity of Pipeline Biofilms in Water Distribution System. J. Pure Appl. Microbiol. 2013, 7, 425–433. [Google Scholar]
- Makris, K.C.; Andra, S.S.; Botsaris, G. Pipe Scales and Biofilms in Drinking-Water Distribution Systems: Undermining Finished Water Quality. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1477–1523. [Google Scholar] [CrossRef]
- Siedlecka, A.; Wolf-Baca, M.; Piekarska, K. Microbial communities of biofilms developed in a chlorinated drinking water distribution system: A field study of antibiotic resistance and biodiversity. Sci. Total Environ. 2021, 774, 145113. [Google Scholar] [CrossRef]
- Takeuchi, M.; Hamana, K.; Hiraishi, A. Proposal of the genus Sphingomonas sensu stricto and three new genera, Sphingobium, Novosphingobium and Sphingopyxis, on the basis of phylogenetic and chemotaxonomic analyses. Int. J. Syst. Evol. Microbiol. 2001, 51, 1405–1417. [Google Scholar] [CrossRef]
- Jordan, D. Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a genus of slow-growing, root nodule bacteria from leguminous plants. Int. J. Syst. Evol. Microbiol. 1982, 32, 136–139. [Google Scholar] [CrossRef]
- Achenbach, L.A.; Michaelidou, U.; Bruce, R.A.; Fryman, J.; Coates, J.D. Dechloromonas agitata gen. nov., sp. nov. and Dechlorosoma suillum gen. nov., sp. nov., two novel environmentally dominant (per)chlorate-reducing bacteria and their phylogenetic position. Int. J. Syst. Evol. Microbiol. 2001, 51, 527–533. [Google Scholar] [CrossRef]
- Bruland, N.; Bathe, S.; Willems, A.; Steinbüchel, A. Pseudorhodoferax soli gen. nov., sp. nov. and Pseudorhodoferax caeni sp. nov., two members of the class Betaproteobacteria belonging to the family Comamonadaceae. Int. J. Syst. Evol. Microbiol. 2009, 59, 2702–2707. [Google Scholar] [CrossRef]
- Koch, I.H.; Gich, F.; Dunfield, P.F.; Overmann, J. Edaphobacter modestus gen. nov., sp. nov., and Edaphobacter aggregans sp. nov., acidobacteria isolated from alpine and forest soils. Int. J. Syst. Evol. Microbiol. 2008, 58, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Tóth, E.M.; Vengring, A.; Homonnay, Z.G.; Keki, Z.; Spröer, C.; Borsodi, A.K.; Márialigeti, K.; Schumann, P. Phreatobacter oligotrophus gen. nov., sp. nov., an alphaproteobacterium isolated from ultrapure water of the water purification system of a power plant. Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 3, 839. [Google Scholar] [CrossRef] [PubMed]
- Grabovich, M.; Gavrish, E.; Kuever, J.; Lysenko, A.M.; Podkopaeva, D.; Dubinina, G. Proposal of Giesbergeria voronezhensis gen. nov., sp. nov. and G. kuznetsovii sp. nov. and reclassification of [Aquaspirillum] anulus, [A.] sinuosum and [A.] giesbergeri as Giesbergeria anulus comb. nov., G. sinuosa comb. nov. and G. giesbergeri comb. nov., and [Aquaspirillum] metamorphum and [A.] psychrophilum as Simplicispira metamorpha gen. nov., comb. nov. and S. psychrophila comb. nov. Int. J. Syst. Evol. Microbiol. 2006, 56, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Segers, P.; Vancanneyt, M.; Pot, B.; Torck, U.; Hoste, B.; Dewettinck, D.; Falsen, E.; Kersters, K.; De Vos, P. Classification of Pseudomonas diminuta Leifson and Hugh 1954 and Pseudomonas vesicularis Büsing, Döll, and Freytag 1953 in Brevundimonas gen. nov. as Brevundimonas diminuta comb. nov. and Brevundimonas vesicularis comb. nov., Respectively. Int. J. Syst. Bacteriol. 1994, 44, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Shida, O.; Takagi, H.; Kadowaki, K.; Komagata, K. Proposal for two new genera, Brevibacillus gen. nov. and Aneurinibacillus gen. nov. Int. J. Syst. Bacteriol. 1996, 46, 939. [Google Scholar] [CrossRef] [PubMed]
- Anupama, V.N.; Prajeesh, P.V.; Anju, S.; Priya, P.; Krishnakumar, B. Diversity of bacteria, archaea and protozoa in a perchlorate treating bioreactor. Microbiol. Res. 2015, 177, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Kose Mutlu, B.; Ozgun, H.; Ersahin, M.E.; Kaya, R.; Eliduzgun, S.; Altinbas, M.; Kinaci, C.; Koyuncu, I. Impact of salinity on the population dynamics of microorganisms in a membrane bioreactor treating produced water. Sci. Total Environ. 2019, 646, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, H.; Li, X.; Hu, C.; Yang, M.; Qu, J. Characterization of biofilm and corrosion of cast iron pipes in drinking water distribution system with UV/Cl2 disinfection. Water Res. 2014, 60, 174–181. [Google Scholar] [CrossRef]
- Sun, H.; Shi, B.; Lytte, D.A.; Bai, Y.; Wang, D. Formation and release behavior of iron corrosion products under the influence of bacterial communities in a simulated water distribution system. Environ. Sci. Process. Impacts 2014, 16, 576–585. [Google Scholar] [CrossRef]
- Wang, H.; Hu, C.; Zhang, L.; Li, X.; Zhang, Y.; Yang, M. Effects of microbial redox cycling of iron on cast iron pipe corrosion in drinking water distribution systems. Water Res. 2014, 65, 362–370. [Google Scholar] [CrossRef]
- Berry, D.; Xi, C.W.; Raskin, L. Microbial ecology of drinking water distribution systems. Curr. Opin. Biotechnol. 2006, 17, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.P.; Feng, H.Z.; Wang, Q.; Quan, S.W.; Yu, X.Q.; Tao, X.; Wang, Y.; Guo, D.D.; Peng, L.; Feng, H.Y.; et al. Proteomic analysis reveals the mechanism of different environmental stress-induced tolerance of Pseudomonas aeruginosa to monochloramine disinfection. J. Hazard. Mater. 2021, 417, 126082. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Mao, Y.; Wang, Z.; Zhang, T. Diversity and functions of bacterial community in drinking water biofilms revealed by high-throughput sequencing. Sci. Rep. 2015, 5, 10044. [Google Scholar] [CrossRef]
- Hinenoya, A.; Awasthi, S.P.; Yasuda, N.; Shima, A.; Morino, H.; Koizumi, T.; Fukuda, T.; Shibata, T.; Yamasaki, S. Chlorine dioxide is a superior disinfectant against multi-drug resistant Staphylococcus aureus, Pseudomonas aeruginosa and Acinetobacter baumannii. Jpn. Med. J. 2015, 68, 276–279. [Google Scholar]
- Nam, H.; Seo, H.S.; Bang, J.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Efficacy of gaseous chlorine dioxide in inactivating Bacillus cereus spores attached to and in a biofilm on stainless steel. Int. J. Food Microbiol. 2014, 188, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, P.; Epalle, T.; Allegra, S.; Girardot, F.; Garraud, O.; Riffard, S. Monitoring of Legionella pneumophila viability after chlorine dioxide treatment using flow cytometry. Res. Microbiol. 2015, 166, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Furuhata, K.; Koike, K.A. Isolation of Methylobacterium spp. from drinking tank-water and resistance of isolates to chlorine. Nihon Kōshū Eisei Zasshi Jpn. J. Public Health 1993, 40, 1047–1053. [Google Scholar]
- Wang, H.; Edwards, M.; Falkinham, J.O.; Pruden, A. Molecular Survey of the Occurrence of Legionella spp., Mycobacterium spp., Pseudomonas aeruginosa, and Amoeba Hosts in Two Chloraminated Drinking Water Distribution Systems. Appl. Environ. Microbiol. 2012, 78, 6285–6294. [Google Scholar] [CrossRef]
- Taylor, R.H.; Falkinham, J.O.; Norton, C.D.; LeChevallier, M.W. Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl. Environ. Microbiol. 2000, 66, 1702–1705. [Google Scholar] [CrossRef]
- Shrivastava, R.; Upreti, R.K.; Jain, S.R.; Prasad, K.N.; Seth, P.K.; Chaturvedi, U.C. Suboptimal chlorine treatment of drinking water leads to selection of multidrug-resistant Pseudomonas aeruginosa. Ecotoxicol. Environ. Saf. 2004, 58, 277–283. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.; Cui, L.; Zhang, M.; Wang, B. Characterization and identification of a chlorine-resistant bacterium, Sphingomonas TS001, from a model drinking water distribution system. Sci. Total Environ. 2013, 458–460, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Al-Ajeel, S.; Spasov, E.; Sauder, L.A.; McKnight, M.M.; Neufeld, J.D. Ammonia-oxidizing archaea and complete ammonia-oxidizing Nitrospira in water treatment systems. Water Res. X 2022, 15, 100131. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, J.; Raczyk-Stanislawiak, U.; Swietlik, J.; Olejnik, A.; Sroka, M.J. Corrosion in a distribution system: Steady water and its composition. Water Res. 2010, 44, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Corona, Y.; Perez, J.G.J.; Smith, C. Bacterial Contamination of Drinking Water in Guadalajara, Mexico. Int. J. Environ. Res. Public Health 2018, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Chang, W.; Cui, T.; Wang, J.; Qian, H.; Ma, L.; Hao, X.; Zhang, D. Microbiologically influenced corrosion inhibition mechanisms in corrosion protection: A review. Bioelectrochemistry 2021, 141, 107883. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Pan, L.; Chen, R.; Shi, B.; Wang, H.; He, S. Effects of disinfectants and particles on the occurrence of different microorganisms in drinking water distribution systems. Environ. Sci. Water Res. Technol. 2021, 7, 983–992. [Google Scholar] [CrossRef]
- Wang, H.; Hu, C.; Yin, L.; Zhang, S.; Liu, L. Characterization of chemical composition and bacterial community of corrosion scales in different drinking water distribution systems. Environ. Sci. Water Res. Technol. 2017, 3, 147–155. [Google Scholar] [CrossRef]
- Chen, S.; Deng, H.; Liu, G.; Zhang, D. Corrosion of Q235 Carbon Steel in Seawater Containing Mariprofundus ferrooxydans and Thalassospira sp. Front. Microbiol. 2019, 10, 936. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).