Porous Nickel Electrode for Highly Sensitive Non-Enzyme Electrochemical Glucose Detection
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
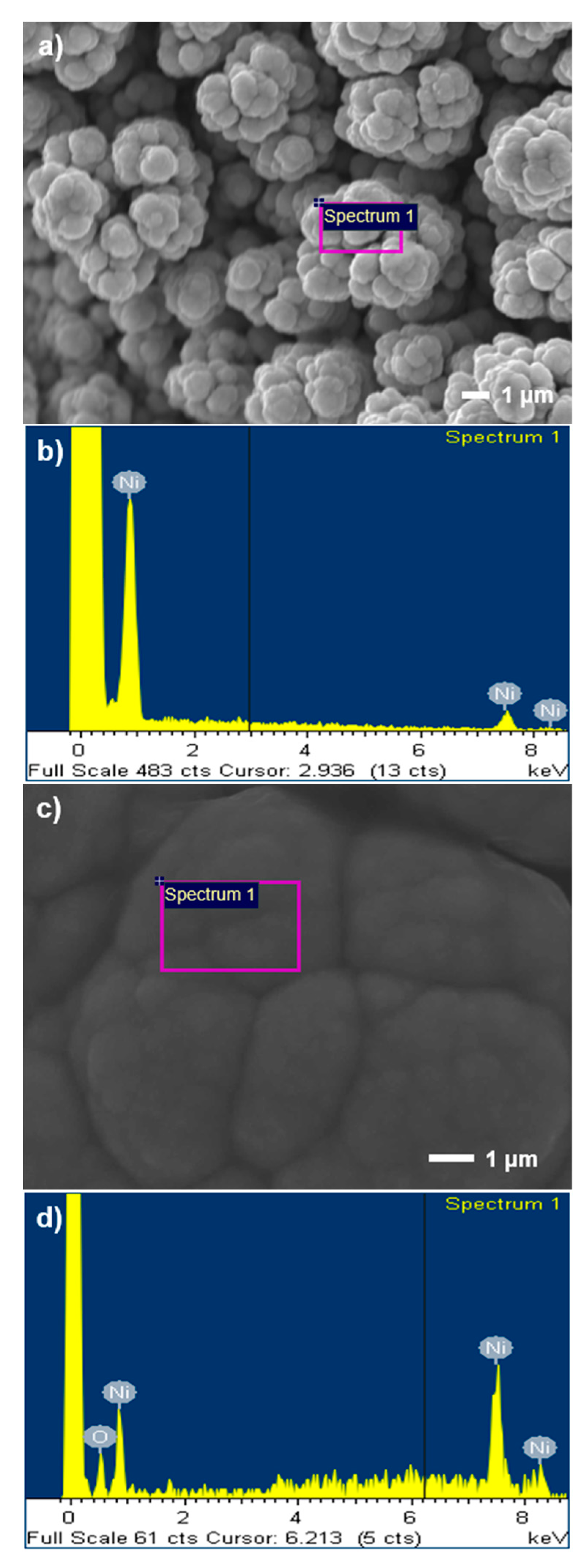
3.1. Surface Morphology and Surface Area
3.2. Effect of Scan Rate on Current-Potential Profile
3.3. Effect of Glucose Concentration on Peak Current and Peak Potential
3.4. Comparison with Other Nickel Electrodes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, D.-W.; Lee, S.; Seo, M.; Chung, T.D. Recent Advances in Electrochemical Non-Enzymatic Glucose Sensors—A Review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Ernst, S.; Heitbaum, J.; Hamann, C.H. The Electrooxidation of Glucose in Phosphate Buffer Solutions: Part I. Reactivity and Kinetics below 350 mV/RHE. J. Electroanal. Chem. Interfacial Electrochem. 1979, 100, 173–183. [Google Scholar] [CrossRef]
- Skou, E. The Electrochemical Oxidation of Glucose on platinum—I. The Oxidation in 1 M H2SO4. Electrochim. Acta 1977, 22, 313–318. [Google Scholar] [CrossRef]
- Hsiao, M.W.; Adžić, R.R.; Yeager, E.B. Electrochemical Oxidation of Glucose on Single Crystal and Polycrystalline Gold Surfaces in Phosphate Buffer. J. Electrochem. Soc. 1996, 143, 759. [Google Scholar] [CrossRef]
- Becerik, I.; Kadirgan, F. The Electrocatalytic Properties of Palladium Electrodes for the Oxidation of D-Glucose in Alkaline Medium. Electrochim. Acta 1992, 37, 2651–2657. [Google Scholar] [CrossRef]
- Guo, M.-M.; Wang, P.-S.; Zhou, C.-H.; Xia, Y.; Huang, W.; Li, Z. An Ultrasensitive Non-Enzymatic Amperometric Glucose Sensor Based on a Cu-Coated Nanoporous Gold Film Involving Co-Mediating. Sens. Actuators B Chem. 2014, 203, 388–395. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, X.; Huang, S. Facile Synthesis of Ni(OH)2 Nanowires on Nickel Foam via One Step Low-Temperature Hydrothermal Route for Non-Enzymatic Glucose Sensor. Mater. Lett. 2017, 198, 19–22. [Google Scholar] [CrossRef]
- Du, Q.; Liao, Y.; Shi, N.; Sun, S.; Liao, X.; Yin, G. Facile Synthesis of Bimetallic Metal–organic Frameworks on Nickel Foam for a High Performance Non-Enzymatic Glucose Sensor. J. Electroanal. Chem. 2022, 904, 1572–6657. [Google Scholar] [CrossRef]
- Long, B.; Cao, P.; Zhao, Y.; Fu, Q.; Mo, Y.; Zhai, Y.; Liu, J.; Lyu, X.; Li, T.; Guo, X.; et al. Pt1/Ni6Co1 Layered Double hydroxides/N-Doped Graphene for Electrochemical Non-Enzymatic Glucose Sensing by Synergistic Enhancement of Single Atoms and Doping. Nano Res. 2022, 16, 318–324. [Google Scholar] [CrossRef]
- Sg, M.; Adarakatti, P.S.; Udayakumar, V.; Almalki, A.S.A. Fabrication of Cerium Oxide and β-Ni (OH) 2 Nano Hexagonal Architectures Assembled on Reduced Graphene Oxide for Non-Enzymatic Electrochemical Detection of Glucose. Ionics 2022, 28, 1957–1972. [Google Scholar]
- Shu, H.; Peng, S.; Lai, T.; Cui, X.; Ren, J.; Chen, T. Nickel Foam Electrode Decorated with Fe-CdIn2O4 Nanoparticles as an Effective Electrochemical Sensor for Non-Enzymatic Glucose Detection. J. Electroanal. Chem. 2022, 919, 116524. [Google Scholar] [CrossRef]
- Wahab, M.A.; Hossain, S.M.A.; Masud, M.K.; Park, H.; Ashok, A.; Mustapić, M.; Kim, M.; Patel, D.; Shahbazi, M.; Hossain, M.S.A.; et al. Nanoarchitectured Superparamagnetic Iron Oxide-Doped Mesoporous Carbon Nanozymes for Glucose Sensing. Sens. Actuators B Chem. 2022, 366, 131980. [Google Scholar] [CrossRef]
- Sun, A.; Zheng, J.; Sheng, Q. A Highly Sensitive Non-Enzymatic Glucose Sensor Based on Nickel and Multi-Walled Carbon Nanotubes Nanohybrid Films Fabricated by One-Step Co-Electrodeposition in Ionic Liquids. Electrochim. Acta 2012, 65, 64–69. [Google Scholar] [CrossRef]
- Song, Y.-Y.; Zhang, D.; Gao, W.; Xia, X.-H. Nonenzymatic Glucose Detection by Using a Three-Dimensionally Ordered, Macroporous Platinum Template. Chemistry 2005, 11, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Ci, S.; Huang, T.; Wen, Z.; Cui, S.; Mao, S.; Steeber, D.A.; Chen, J. Nickel Oxide Hollow Microsphere for Non-Enzyme Glucose Detection. Biosens. Bioelectron. 2014, 54, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.-Q.; Wang, M.; Bao, S.-J.; Wang, Y.C.; Gu, S. Analysis of Cobalt Phosphide (CoP) Nanorods Designed for Non-Enzyme Glucose Detection. Analyst 2016, 141, 256–260. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Fung, T.; Zhang, L.P.; Zhang, F.L. Lost Carbonate Sintering Process for Manufacturing Metal Foams. Scr. Mater. 2005, 52, 295–298. [Google Scholar] [CrossRef]
- Shin, H.-C.; Dong, J.; Liu, M. Nanoporous Structures Prepared by an Electrochemical Deposition Process. Adv. Mater. 2003, 15, 1610–1614. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.-Y.; Yang, C.; Xia, X.-H. Hydrogen Bubble Dynamic Template Synthesis of Porous Gold for Nonenzymatic Electrochemical Detection of Glucose. Electrochem. Commun. 2007, 9, 981–988. [Google Scholar] [CrossRef]
- Sciortino, F.; Rydzek, G.; Grasset, F.; Kahn, M.L.; Hill, J.P.; Chevance, S.; Gauffre, F.; Ariga, K. Electro-Click Construction of Hybrid Nanocapsule Films with Triggered Delivery Properties. Phys. Chem. Chem. Phys. 2018, 20, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Sato, T.; Sakamoto, Y.; Tominaka, S.; Miyasaka, K.; Miyamoto, N.; Nemoto, Y.; Terasaki, O.; Yamauchi, Y. Synthesis of Mesoporous Pt Films with Tunable Pore Sizes from Aqueous Surfactant Solutions. Chem. Mater. 2012, 24, 1591–1598. [Google Scholar] [CrossRef]
- Zhu, P.; Zhao, Y. Effects of Electrochemical Reaction and Surface Morphology on Electroactive Surface Area of Porous Copper Manufactured by Lost Carbonate Sintering. RSC Adv. 2017, 7, 26392–26400. [Google Scholar] [CrossRef]
- Diao, K.K.; Xiao, Z.; Zhao, Y.Y. Specific Surface Areas of Porous Cu Manufactured by Lost Carbonate Sintering: Measurements by Quantitative Stereology and Cyclic Voltammetry. Mater. Chem. Phys. 2015, 162, 571–579. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications. Surf. Technol. 1983, 20, 91–92. [Google Scholar]
- Zhu, P.; Zhao, Y. Cyclic Voltammetry Measurements of Electroactive Surface Area of Porous Nickel: Peak Current and Peak Charge Methods and Diffusion Layer Effect. Mater. Chem. Phys. 2019, 233, 60–67. [Google Scholar] [CrossRef]
- Moldenhauer, J.; Meier, M.; Paul, D.W. Rapid and Direct Determination of Diffusion Coefficients Using Microelectrode Arrays. J. Electrochem. Soc. 2016, 163, 672. [Google Scholar] [CrossRef]
- Darayen, J.; Chailapakul, O.; Praserthdam, P.; Panpranot, J.; Tungasmita, D.N.; Boonyongmaneerat, Y. Porous Electrodeposited Cu as a Potential Electrode for Electrochemical Reduction Reactions of CO2. Appl. Sci. 2021, 11, 11104. [Google Scholar] [CrossRef]
- Pu, X.; Zhao, D.; Fu, C.; Chen, Z.; Cao, S.; Wang, C.; Cao, Y. Understanding and Calibration of Charge Storage Mechanism in Cyclic Voltammetry Curves. Angew. Chem. Int. Ed. Engl. 2021, 60, 21310–21318. [Google Scholar] [CrossRef]
- Holman, J.P. Experimental Methods for Engineers; McGraw Hill: New York, NY, USA, 2012. [Google Scholar]
- Ko, C.-Y.; Huang, J.-H.; Raina, S.; Kang, W.P. A High Performance Non-Enzymatic Glucose Sensor Based on Nickel Hydroxide Modified Nitrogen-Incorporated Nanodiamonds. Analyst 2013, 138, 3201–3208. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. About Estimating the Limit of Detection by the Signal to Noise Approach. Pharm. Anal. Acta. 2015, 6, 355. [Google Scholar]
- Wang, Z.; Hu, Y.; Yang, W.; Zhou, M.; Hu, X. Facile One-Step Microwave-Assisted Route towards Ni Nanospheres/reduced Graphene Oxide Hybrids for Non-Enzymatic Glucose Sensing. Sensors 2012, 12, 4860–4869. [Google Scholar] [CrossRef]
- Lu, L.-M.; Zhang, L.; Qu, F.-L.; Lu, H.-X.; Zhang, X.-B.; Wu, Z.-S.; Huan, S.-Y.; Wang, Q.-A.; Shen, G.-L.; Yu, R.-Q. A Nano-Ni Based Ultrasensitive Nonenzymatic Electrochemical Sensor for Glucose: Enhancing Sensitivity through a Nanowire Array Strategy. Biosens. Bioelectron. 2009, 25, 218–223. [Google Scholar] [CrossRef]
- Niu, X.; Lan, M.; Zhao, H.; Chen, C. Highly Sensitive and Selective Nonenzymatic Detection of Glucose Using Three-Dimensional Porous Nickel Nanostructures. Anal. Chem. 2013, 85, 3561–3569. [Google Scholar] [CrossRef]
- Yi, Q.; Huang, W.; Yu, W.; Li, L.; Liu, X. Hydrothermal Synthesis of Titanium-Supported Nickel Nanoflakes for Electrochemical Oxidation of Glucose. Electroanalysis 2008, 20, 2016–2022. [Google Scholar] [CrossRef]
- Cao, F.; Guo, S.; Ma, H.; Shan, D.; Yang, S.; Gong, J. Nickel Oxide Microfibers Immobilized onto Electrode by Electrospinning and Calcination for Nonenzymatic Glucose Sensor and Effect of Calcination Temperature on the Performance. Biosens. Bioelectron. 2011, 26, 2756–2760. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, H.; Hou, H.; You, T. Nonenzymatic Glucose Sensor Based on Renewable Electrospun Ni Nanoparticle-Loaded Carbon Nanofiber Paste Electrode. Biosens. Bioelectron. 2009, 24, 3329–3334. [Google Scholar] [CrossRef]
- Nie, H.; Yao, Z.; Zhou, X.; Yang, Z.; Huang, S. Nonenzymatic Electrochemical Detection of Glucose Using Well-Distributed Nickel Nanoparticles on Straight Multi-Walled Carbon Nanotubes. Biosens. Bioelectron. 2011, 30, 28–34. [Google Scholar] [CrossRef]
- Lu, W.; Qin, X.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Ni Foam: A Novel Three-Dimensional Porous Sensing Platform for Sensitive and Selective Nonenzymatic Glucose Detection. Analyst 2013, 138, 417–420. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Yu, J.; He, J.; Yang, H.; Ye, Y.; Song, Y. A Green and Simple Strategy to Prepare Graphene Foam-like Three-Dimensional Porous carbon/Ni Nanoparticles for Glucose Sensing. Sens. Actuators B Chem. 2017, 239, 172–179. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Ahmadi, N.; Rezaei, B. Nickel Nanoparticles Supported on Porous Silicon Flour, Application as a Non-Enzymatic Electrochemical Glucose Sensor. Sens. Actuators B Chem. 2017, 239, 807–815. [Google Scholar] [CrossRef]
- Malka, D.; Berkovic, G.; Tischler, Y.; Zalevsky, Z. Super-Resolved Raman Spectra of Toluene and Toluene–Chlorobenzene Mixture. Spectrosc. Lett. 2015, 48, 431–435. [Google Scholar] [CrossRef]
- Malka, D.; Berke, B.A.; Tischler, Y.; Zalevsky, Z. Improving Raman Spectra of Pure Silicon Using Super-Resolved Method. J. Opt. 2019, 21, 075801. [Google Scholar] [CrossRef]
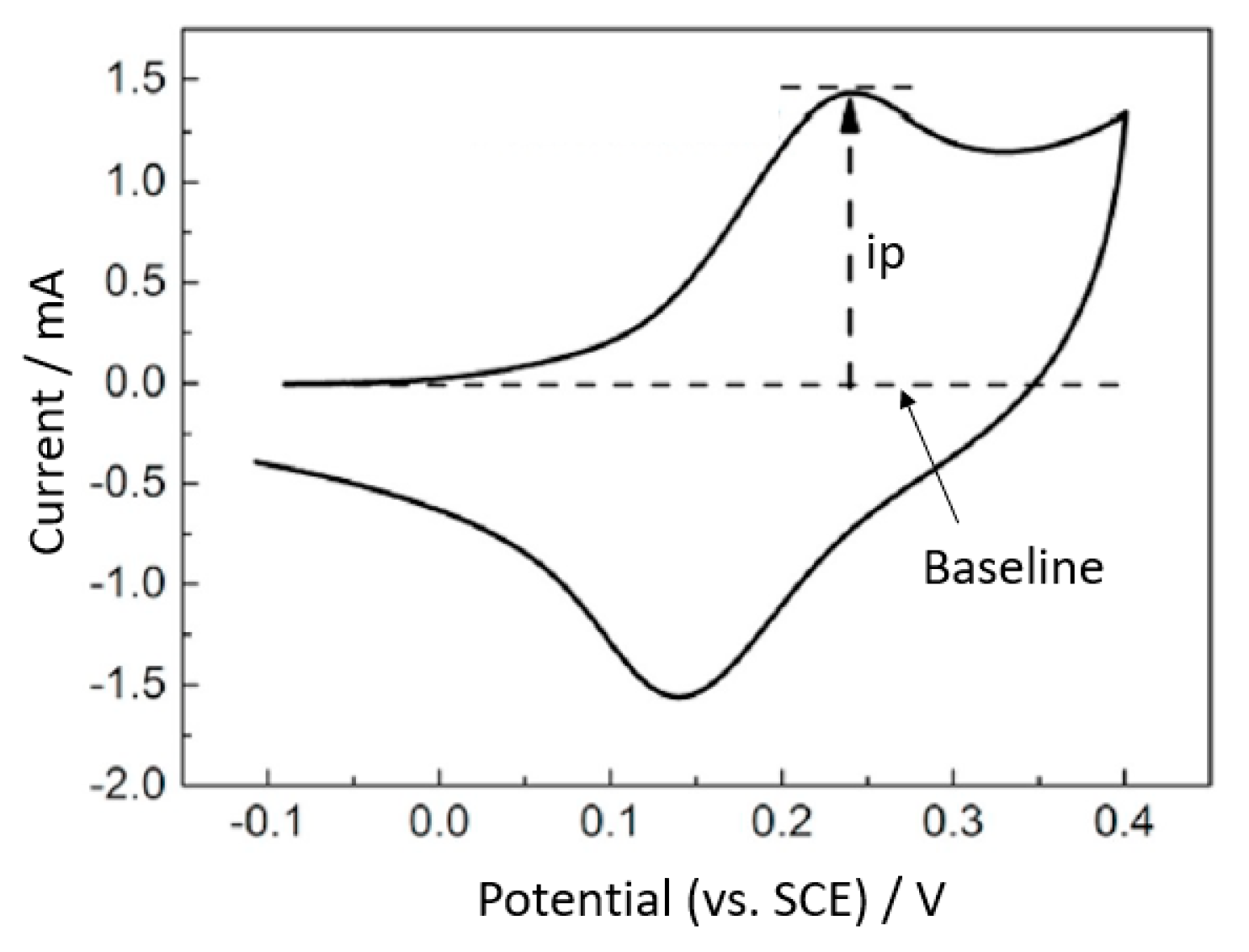





| Scan Rate(V/s) | Plate | LCS | LCS/DHBT | |||
|---|---|---|---|---|---|---|
| Current Density (mA/cm2) | Potential (V) | Current Density (mA/cm2) | Potential (V) | Current Density (mA/cm2) | Potential (V) | |
| 0.025 | 0.314 ± 0.0002 | 0.42 | 1.922 ± 0.009 | 0.50 | 3.428 ± 0.028 | 0.48 |
| 0.05 | 0.546 ± 0.0007 | 0.43 | 2.474 ± 0.014 | 0.52 | 5.09 ± 0.062 | 0.52 |
| 0.1 | 0.885 ± 0.002 | 0.44 | 3.363 ± 0.027 | 0.54 | 7.597 ± 0.137 | 0.58 |
| 0.15 | 1.176 ± 0.003 | 0.45 | 4.013 ± 0.038 | 0.55 | 9.448 ± 0.212 | 0.64 |
| 0.2 | 1.436 ± 0.005 | 0.46 | 4.762 ± 0.054 | 0.57 | 11.22 ± 0.298 | 0.70 |
| 0.25 | 1.644 ± 0.006 | 0.47 | 5.094 ± 0.062 | 0.58 | 12.84 ± 0.398 | 0.73 |
| 0.3 | 1.848 ± 0.008 | 0.48 | 5.569 ± 0.074 | 0.59 | 13.94 ± 0.459 | 0.78 |
| Ni Electrodes | Linear Range (mM) | Slope | Intercept | R2 |
|---|---|---|---|---|
| Ni plate | 0–1 | 0.63 | 1.13 | 0.953 |
| 2–15 | 0.34 | 1.88 | 0.992 | |
| LCS porous Ni | 0–1 | 1.19 | 1.50 | 0.987 |
| 1–6 | 0.67 | 2.09 | 0.995 | |
| 4–15 | 0.46 | 3.16 | 0.993 | |
| LCS/DHBT porous Ni | 0–1 | 2.34 | 3.92 | 0.970 |
| 1–6 | 1.14 | 4.81 | 0.996 | |
| 4–12 | 0.94 | 5.88 | 0.997 | |
| 12–15 | 0.33 | 13 | 0.953 |
| Material | Linear Range (mM) | LOD (μM) | Sensitivity (μA/cm2mM) | Reference |
|---|---|---|---|---|
| Ni nanospheres/RGO | 0.001–0.11 | N/A | 813 | [32] |
| Ordered Ni nanowire arrays | 0.0005–7 | 0.1 | 1043 | [33] |
| 3D porous Ni networks | 0.0005–4 | 0.007 | 2900 | [34] |
| Ni-MWCNT | 3.2–17.5 | 0.98 | 67.19 | [13] |
| Ni nanoflake/Ti plate | 0.05–0.6 | 1.2 | 7320 | [35] |
| NiO microfiber/FTO | 0.001–0.27 | 0.033 | 1785.41 | [36] |
| Ni nanoparticle/C fiber mixture | Up to 2.55 | 1 | 420.4 | [37] |
| Ni nanoparticle | 0.001–1 | 0.05 | 1438 | [38] |
| Ni foam | 0.05–7.35 | 2.2 | N/A | [39] |
| Ni nanoparticle on porous GF | 0.015–6.45 | 4.8 | 207.3 | [40] |
| Ni nanoparticle on porous Si | 0.002–5 | 0.2 | N/A | [41] |
| Ni plate | 0–1 | 0.49 | 1724 | This work |
| 2–15 | 7.13 | 568 | ||
| LCS porous nickel | 0–1 | 0.53 | 2651 | This work |
| 1–6 | 2.15 | 1374 | ||
| 4–15 | 4.29 | 839 | ||
| LCS/DHBT porous nickel | 0–1 | 0.43 | 5775 | This work |
| 1–6 | 1.83 | 2781 | ||
| 4–12 | 2.91 | 1686 | ||
| 12–15 | 0.66 | 1297 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhao, Y. Porous Nickel Electrode for Highly Sensitive Non-Enzyme Electrochemical Glucose Detection. Coatings 2023, 13, 290. https://doi.org/10.3390/coatings13020290
Wang Z, Zhao Y. Porous Nickel Electrode for Highly Sensitive Non-Enzyme Electrochemical Glucose Detection. Coatings. 2023; 13(2):290. https://doi.org/10.3390/coatings13020290
Chicago/Turabian StyleWang, Zejun, and Yuyuan Zhao. 2023. "Porous Nickel Electrode for Highly Sensitive Non-Enzyme Electrochemical Glucose Detection" Coatings 13, no. 2: 290. https://doi.org/10.3390/coatings13020290
APA StyleWang, Z., & Zhao, Y. (2023). Porous Nickel Electrode for Highly Sensitive Non-Enzyme Electrochemical Glucose Detection. Coatings, 13(2), 290. https://doi.org/10.3390/coatings13020290






