Preparation and Properties of Hydrophobic and Oleophobic Coating for Inkjet Printing
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Equipment
2.3. Experimental Method
2.3.1. Sample Preparation
2.3.2. Performance Tests and Characterization
3. Results and Discussion
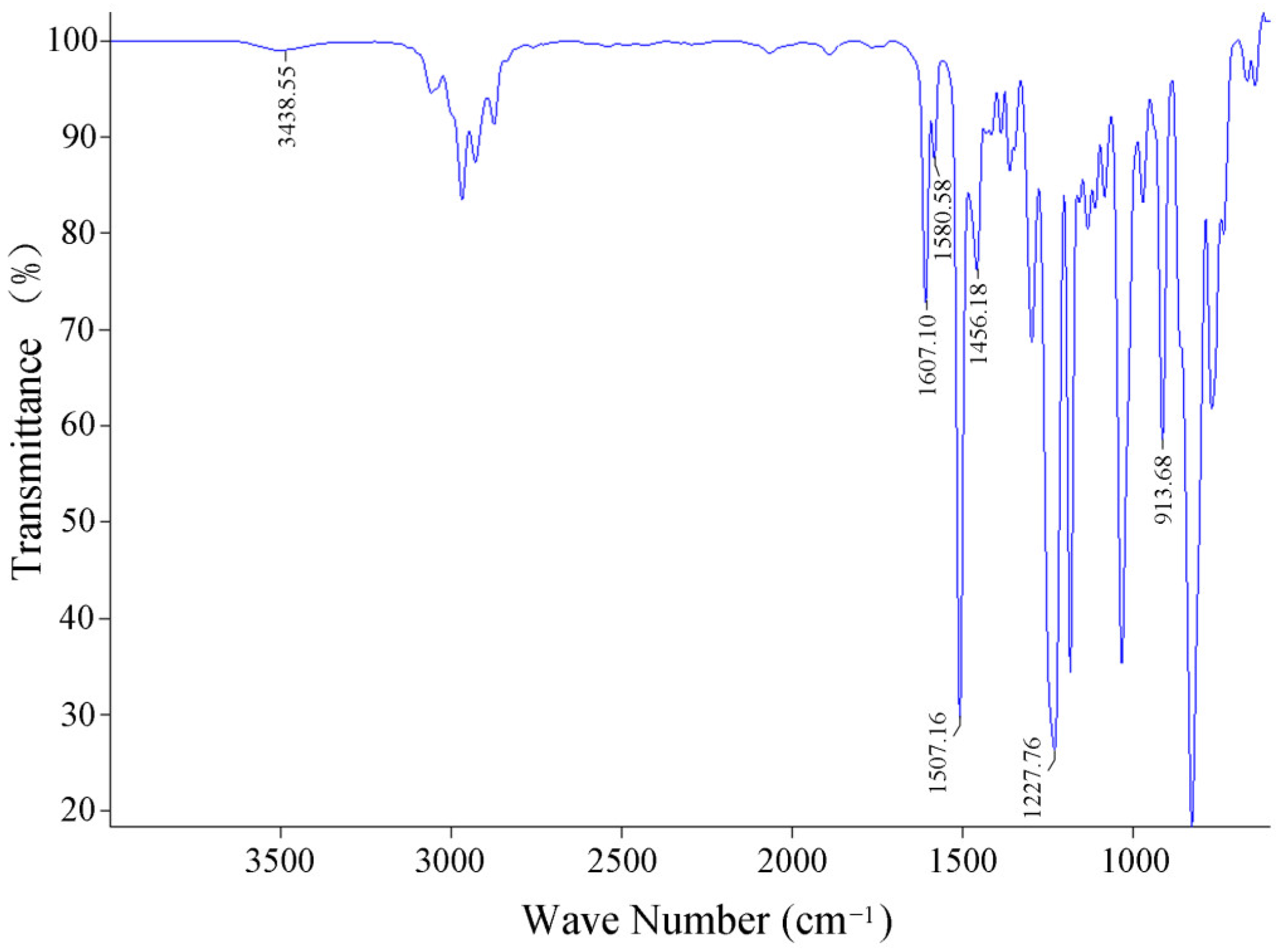
3.1. Infrared Spectrum Analysis
3.2. Effect of Fluorine Monomer Content on the Properties of Coating Prepolymer
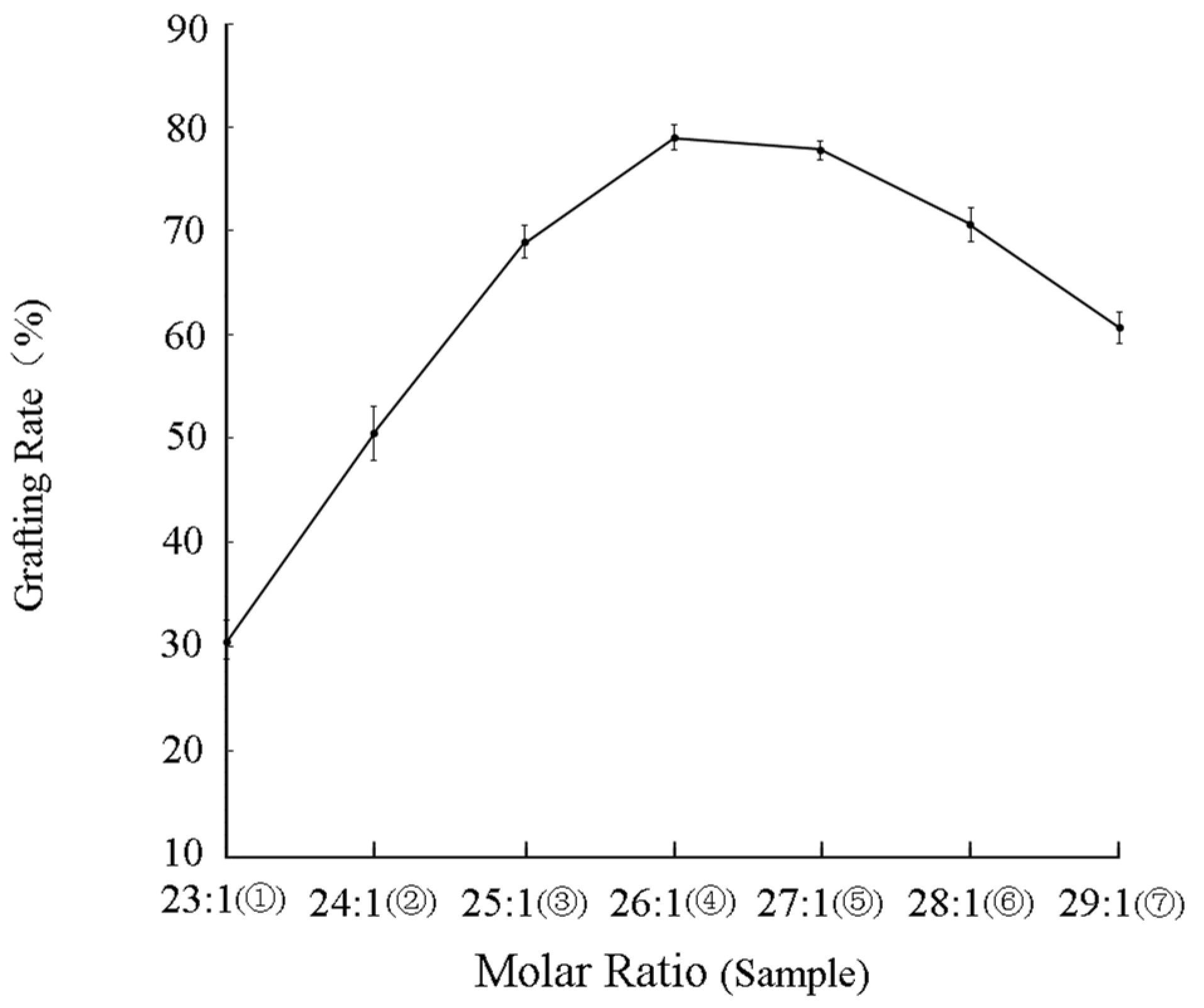
3.2.1. Grafting Rate
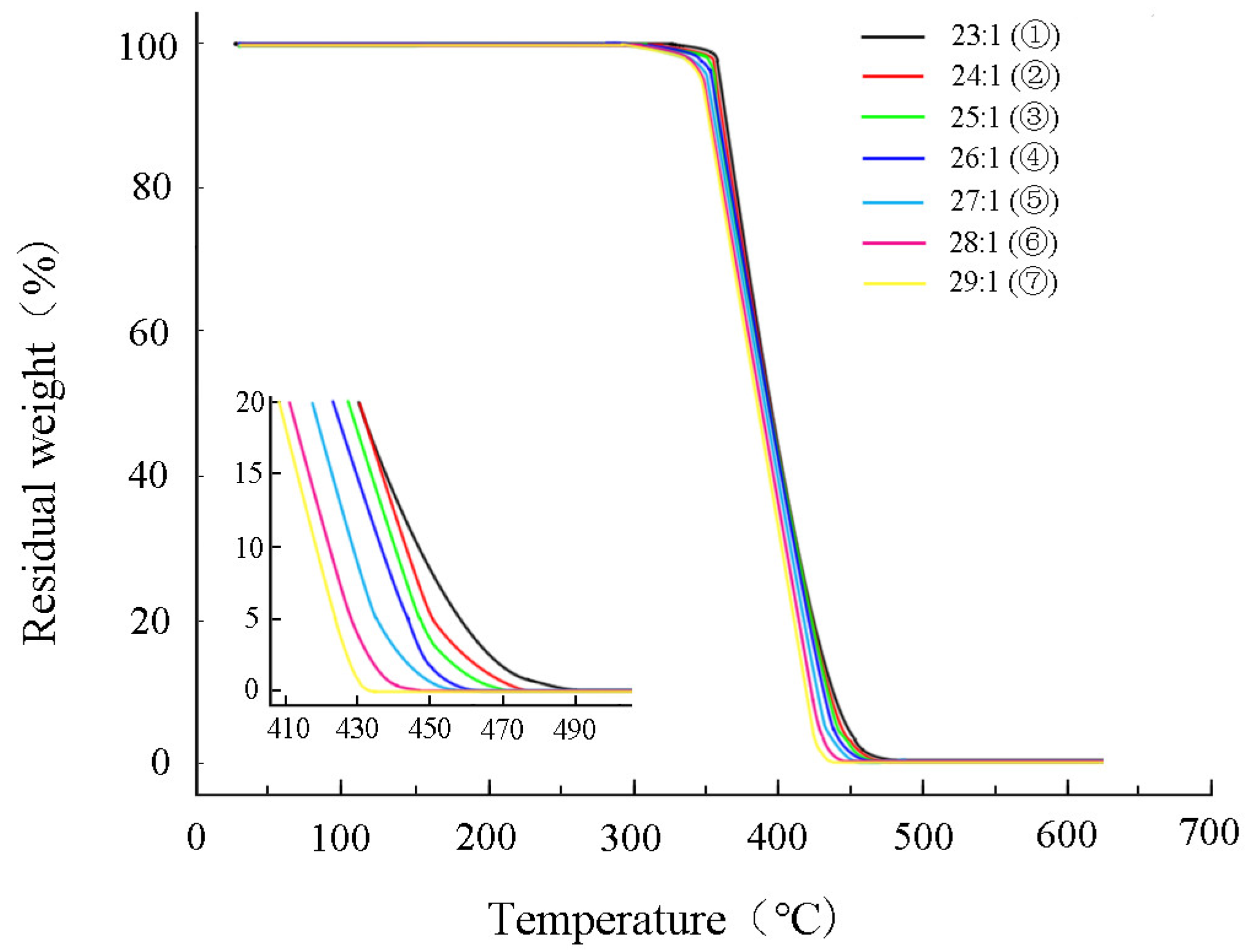
3.2.2. Membrane Thermogravimetric Analysis
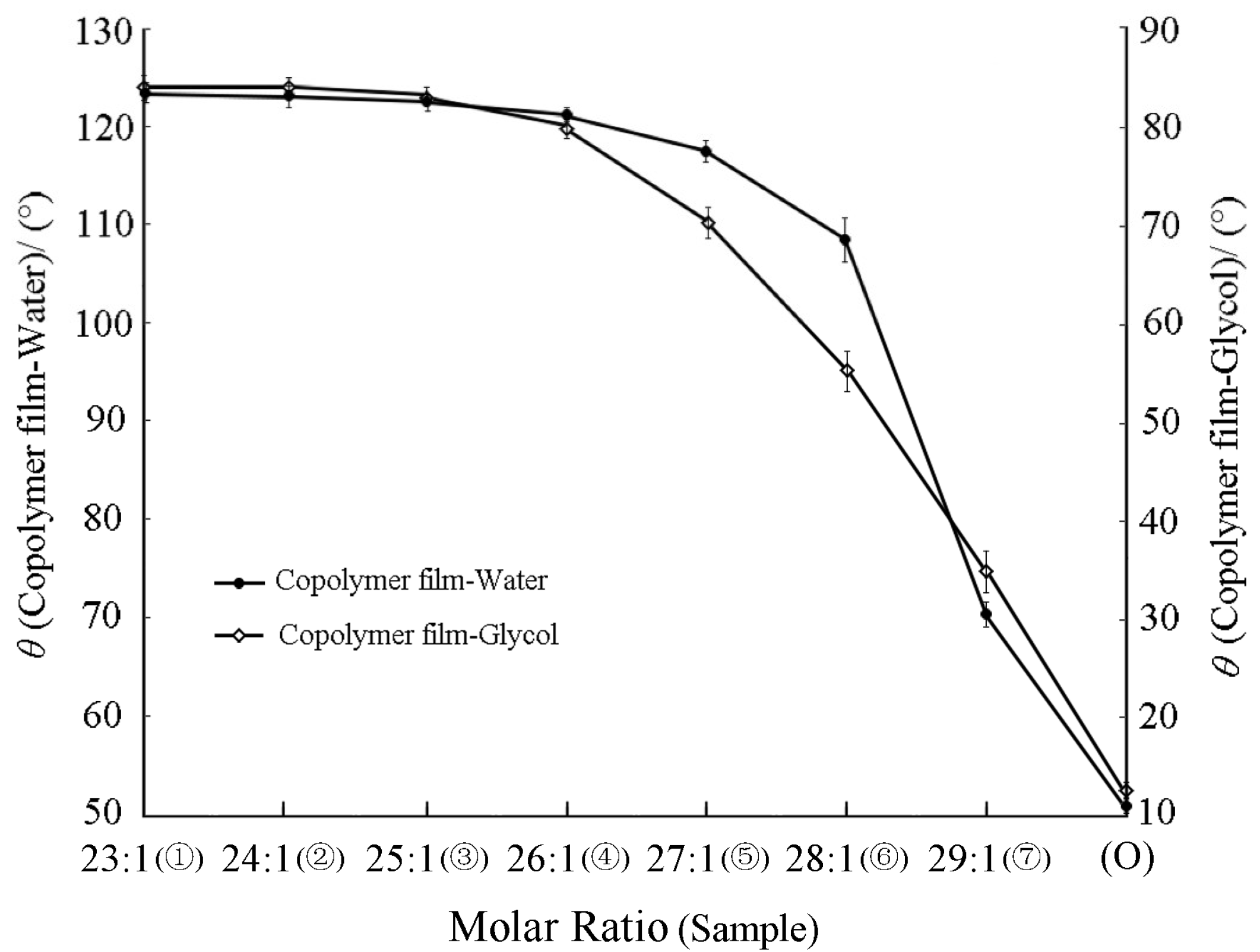
3.2.3. Effect of Fluorine Monomer Content on the Surface Hydrophobicity and Oleophobicity of Coating Film
3.2.4. Effect of Adding Methods of Fluorine Monomer on the Surface Hydrophobicity and Oleophobicity of Coating Film
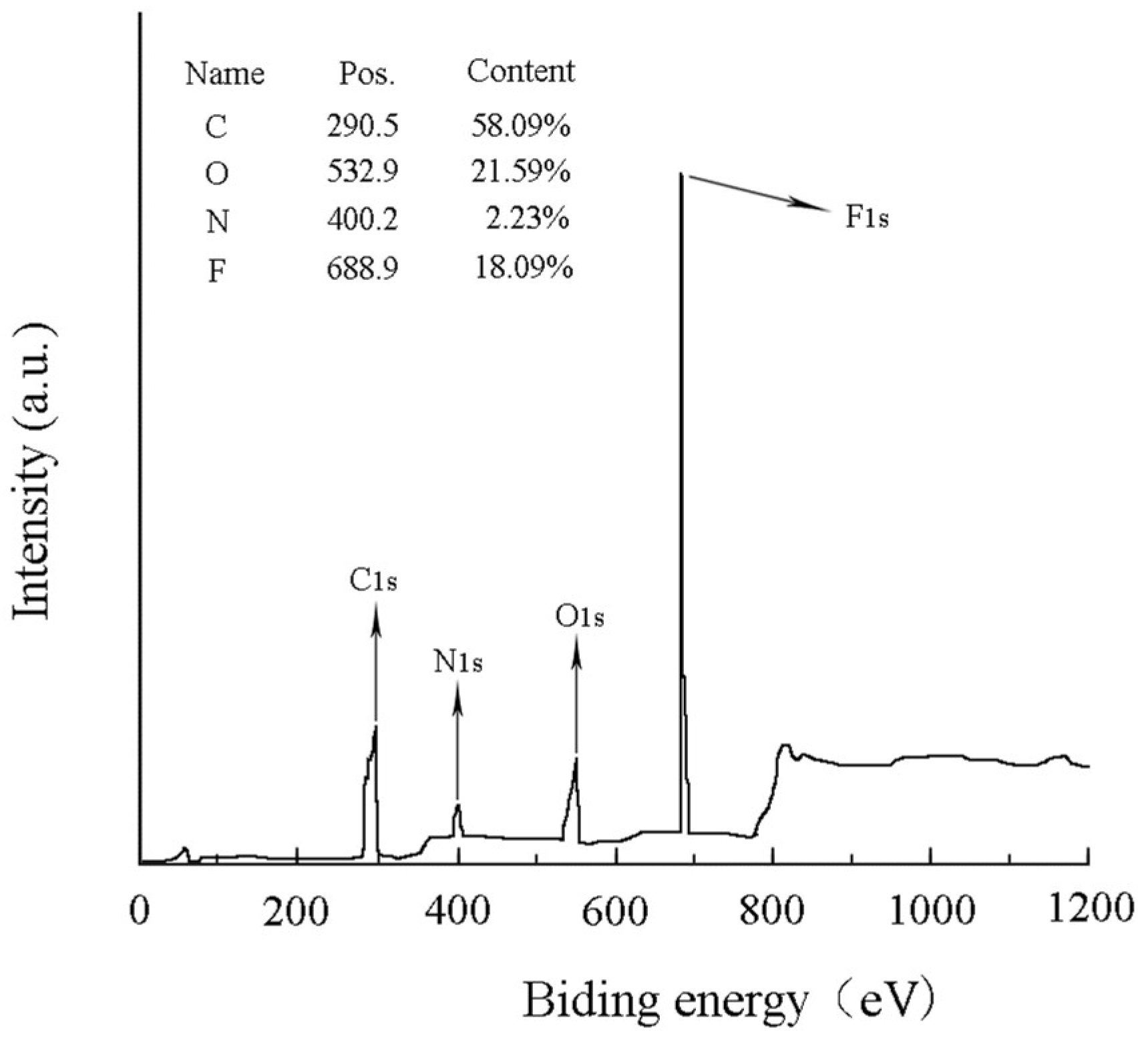
3.3. Chemical Composition Analysis of Membrane Surface
3.4. Effect of Fluorine Monomer on Performance of Coating
3.4.1. Hydrophobic and Oleophobic Performance
3.4.2. Adhesion and Hardness
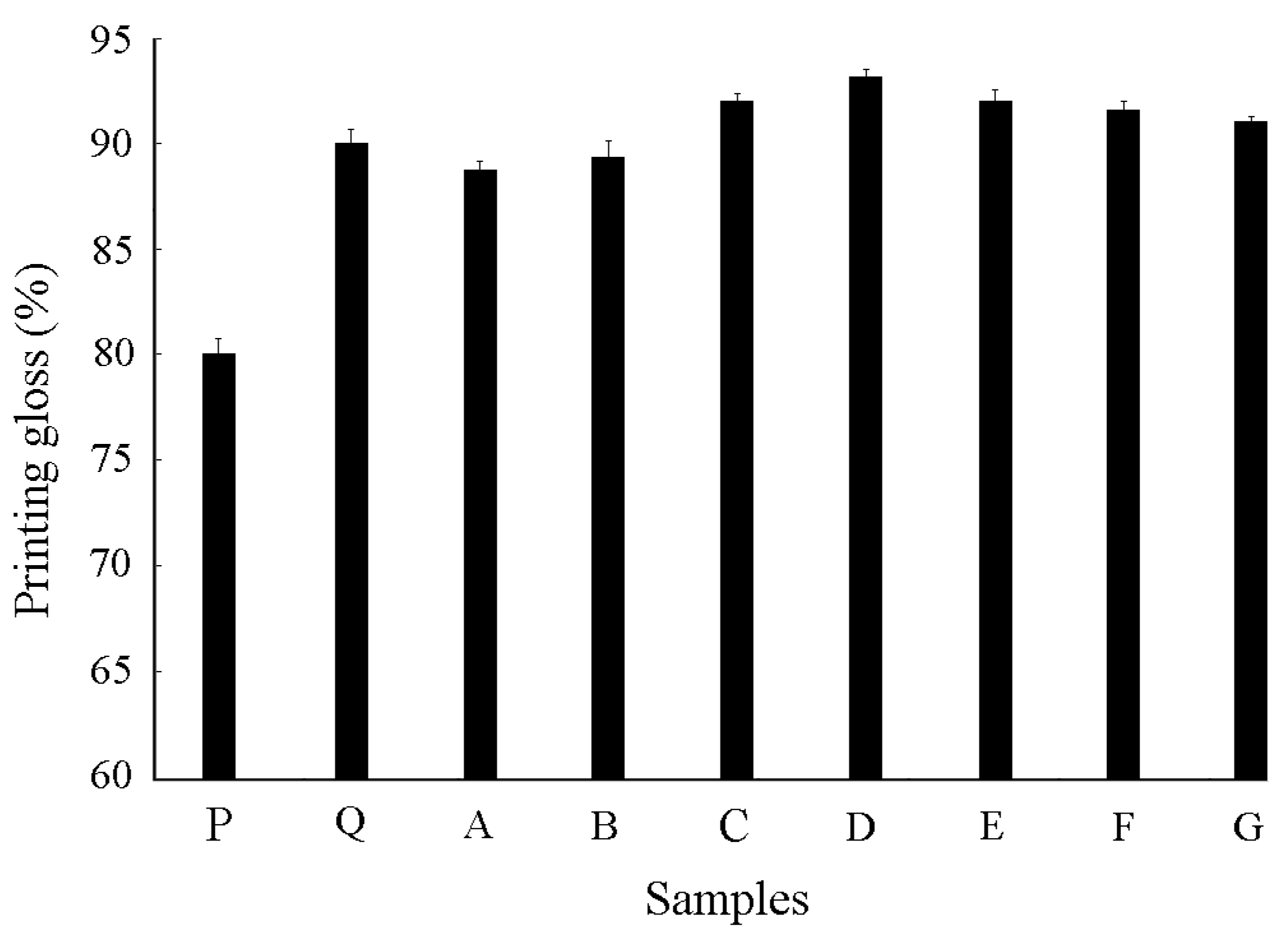
3.4.3. Printing Gloss
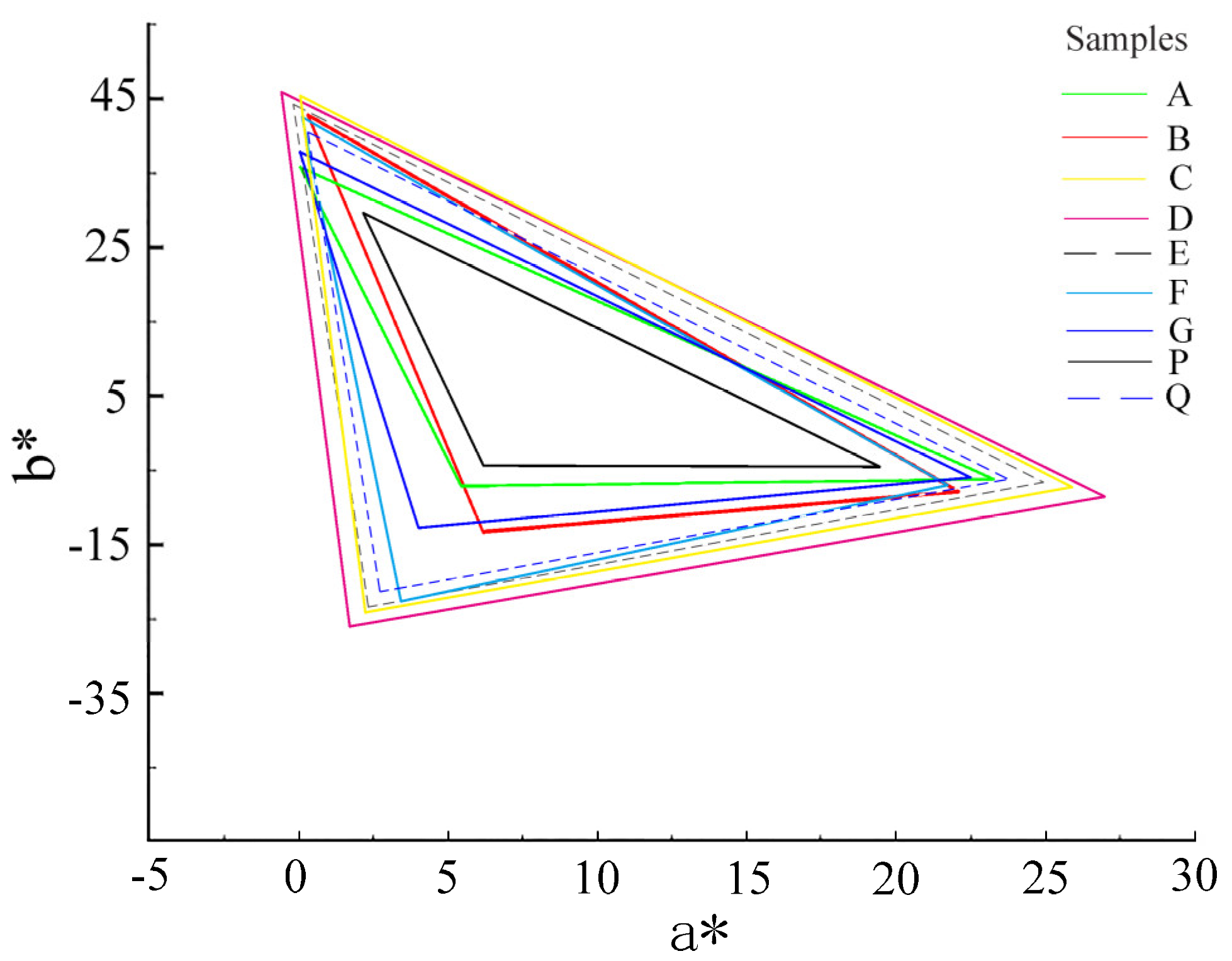
3.4.4. Color Reproducibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, F. Development and Prospect of digital printing in China. Digit. Print. 2018, 11, 18–21. [Google Scholar]
- Chang, X. Development status and Prospect of digital printing in China in 2021. Print. Technol. 2021, 6, 1–5. [Google Scholar]
- Peng, B. Digital printing technology and its application in packaging printing. Int. J. Electr. Eng. Educ. 2021, 3, 1–10. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, T.; Qi, Y. Research on the Quality of Digital Printing. Lect. Notes Electr. Eng. 2021, 754, 284–292. [Google Scholar]
- Li, F. Summarize my secrets of digital printing technology. Digit. Print. 2018, 7, 44–46. [Google Scholar]
- Geng, C. Research on the application of digital printing technology in packaging printing. Packag. World 2022, 1, 46–48. [Google Scholar]
- Long, J. Discussion on the application of digital printing technology in paper packaging printing. China Pap. Newsl. 2021, 6, 95–96. [Google Scholar]
- Li, Z. Application of digital printing technology in packaging and printing industry. Packag. World 2022, 2, 1–3. [Google Scholar]
- Martin, G.; Hoath, S.; Hutchings, I. Inkjet printing–the physics of manipulating liquid jets and drops. Eng. Phys. Synerg. Success 2008, 105, 12001–12014. [Google Scholar] [CrossRef]
- Polston, K.; Chapman, L.; Moore, M. Print-on-demand inkjet digital textile printing technology: An initial understanding of user types and skill levels. Int. J. Fash. Des. 2015, 8, 87–96. [Google Scholar] [CrossRef]
- Wen, X.; Chen, H.; Lv, C. Key technologies and implementation of digital inkjet printing. Imaging Sci. Photochem. 2019, 3, 227–233. [Google Scholar]
- Yan, L. Inkjet printing—opportunities and worries under rapid growth. Print. Manag. 2022, 1, 31–32. [Google Scholar]
- Ji, J.; Chen, K. Thoughts on the application of inkjet digital printing technology. Digit. Print. 2018, 4, 22–24. [Google Scholar]
- Xue, P. The far-reaching influence of inkjet printing technology on graphic fast printing industry. Print. Technol. 2021, 6, 6–7. [Google Scholar]
- Song, Y.W.; Fang, K.J.; Zhang, J.B.; Cai, Y.Q.; Hao, L.Y. Inkjet technology and its application in textile printing. J. Text. Res. 2015, 8, 165–172. [Google Scholar]
- Wang, T. The application of production inkjet printer in the field of printing and packaging is higher and higher. Guangdong Print. 2022, 1, 51. [Google Scholar]
- Qing, H. The Latest Trend of UV Inkjet Digital Printing for Flexible Packaging. Mon. Packag. World 2021, 341, 93–97. [Google Scholar]
- Chen, J.; Zhang, K.; Zhang, K.; Yang, L.; Jiang, B. The research progress in recording layer of the inkjet printing materials. J. Appl. Polym. Sci. 2021, 35, 50894. [Google Scholar] [CrossRef]
- Chao, J.; Shi, R.Z.; Guo, Y.L.; Chu, F.Q.; Deng, Q. Printability of Ink for On-Demand Inkjet Printing on Different Paper. Lect. Notes Electr. Eng. 2021, 754, 334–339. [Google Scholar]
- Guo, M.; Ama, H.; Harada, Y.; Nishimura, H. Analysis of Droplet Permeation into Coated Paper for Inkjet Printing. J. Imaging Soc. Jpn. 2021, 6, 580–591. [Google Scholar]
- Ufuk, Y.; Ahmet, T.; Sinan, S. A research of Comparing of the Effects on the Color Properties of Offset, Laser and Inkjet Print Systems on Uncoated Recycled Papers. Erciyes Üniversitesi Fen Bilim. Enstitüsü Fen Bilim. Derg. 2021, 1, 130–136. [Google Scholar]
- Guo, Z.G.; Liu, W.M. Biomimic from the superhyhobic plant leaves in nature: Binary structure and unitary structure. Plant Sci. 2007, 6, 1103–1112. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, W.X.; Yan, W.A. Tailoring waterproof and breathable properties of environmentally friendly electrospun fibrous membranes by optimizing porous structure and surface wettability. Compos. Commun. 2019, 15, 40–45. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Gao, X.F.; Jiang, L. Directional adhesion of superhydrophobic butterfly wings. Soft Matter 2007, 2, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Choi, C.K.; Meng, D.D. Superhydrophilic Surfaces for Antifoqging and Antifouling Microfluidic Devices. Slas Technol. Transl. Life Sci. Innov. 2010, 2, 114–119. [Google Scholar] [CrossRef]
- Kiyoharu, T.; Junichi, M.; Atsunori, M. Superhydrophobic Superhydrophilic Micropatterning on Flowerlike Alumina Coating Film by the Sol-Gel Method. Chem. Mater. 2000, 3, 590–592. [Google Scholar]
- Wang, D.; Sun, Q.; Hokkanen, M.; Zhang, C.; Lin, F.Y.; Liu, Q.; Zhu, S.P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]
- Pan, Z.H.; Cheng, F.Q.; Zhao, B.X. Bio-Inspired Polymeric Structures with Special Wettability and Their Applications: An Overview. Polymers 2017, 12, 725. [Google Scholar] [CrossRef]
- Zhao, T.; Feng, Y.; Cao, M.L.; Wang, L.L.; Zhou, Z.Y. P Preparation and properties of super hydrophobic and oleophobic polystyrene. J. Funct. Mater. 2021, 11, 209–214. [Google Scholar]
- Zhou, G.B.; Wen, X.F.; Pi, P.H.; Cai, Z.Q.; Cheng, J.; Yang, Z.R. Study on synthesis of fluorinated acrylate copolymer and surface hydro-oleophobic properties of the copolymer films. Electroplat. Finish. 2010, 8, 50–53. [Google Scholar]
- Kaspar, P.; Sobola, D.; Cástková, K.; Dallaev, R.; Štastná, E.; Sedlák, P.; Knápek, A.; Trcka, T.; Holcman, V. Case Study of Polyvinylidene Fluoride Doping by Carbon Nanotubes. Materials 2021, 14, 1428. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.H.; Zhang, T.Y.; Li, Z.J. Progress of fluorinated acrylate copolymer latexes (I): Polymerization techniques and safety of fluorinated acrylate monomers. China Leather 2009, 9, 52–57. [Google Scholar]
- Di, K.Y.; Lv, S.N.; Cai, P.L.; Chen, X.T.; Xu, J.M.; Xin, J.F. Preparation and properties of fluorinated siloxane modified epoxy resins. Fine Chem. 2021, 4, 774–781. [Google Scholar]



| Molar Ratios of Epoxy Group to DFHMA (Sample) | Adding Methods of DFHMA | Contact Angles (°) | |
|---|---|---|---|
| Water | Glycol | ||
| 23:1 (①) | Continuous dropping | 125 | 86 |
| One-time | 103 | 71 | |
| 24:1 (②) | Continuous dropping | 124 | 85 |
| One-time | 100 | 72 | |
| 25:1 (③) | Continuous dropping | 123 | 83 |
| One-time | 110 | 71 | |
| 26:1 (④) | Continuous dropping | 122 | 81 |
| One-time | 114 | 74 | |
| 27:1 (⑤) | Continuous dropping | 117 | 71 |
| One-time | 110 | 65 | |
| 28:1 (⑥) | Continuous dropping | 108 | 56 |
| One-time | 102 | 50 | |
| 29:1 (⑦) | Continuous dropping | 70 | 35 |
| One-time | 67 | 30 | |
| Mechanical Property | Adhesion (Grade) | Hardness (9B~9H) | |
|---|---|---|---|
| Molar Ratio (Sample) | |||
| Coating Without Fluorine Modification | (O) | 3 | 5H |
| Coating with fluorine modification | 23:1 (①) | 3 | 4H |
| 24:1 (②) | 2 | 4H | |
| 25:1 (③) | 1 | 3H | |
| 26:1 (④) | 1 | 3H | |
| 27:1 (⑤) | 1 | 4H | |
| 28:1 (⑥) | 2 | 4H | |
| 29:1 (⑦) | 3 | 5H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, J.; Shi, R.; Chu, F.; Guo, Y.; Deng, Q.; Sun, B. Preparation and Properties of Hydrophobic and Oleophobic Coating for Inkjet Printing. Coatings 2023, 13, 286. https://doi.org/10.3390/coatings13020286
Chao J, Shi R, Chu F, Guo Y, Deng Q, Sun B. Preparation and Properties of Hydrophobic and Oleophobic Coating for Inkjet Printing. Coatings. 2023; 13(2):286. https://doi.org/10.3390/coatings13020286
Chicago/Turabian StyleChao, Jilei, Ruizhi Shi, Fuqiang Chu, Yanling Guo, Qian Deng, and Bing Sun. 2023. "Preparation and Properties of Hydrophobic and Oleophobic Coating for Inkjet Printing" Coatings 13, no. 2: 286. https://doi.org/10.3390/coatings13020286
APA StyleChao, J., Shi, R., Chu, F., Guo, Y., Deng, Q., & Sun, B. (2023). Preparation and Properties of Hydrophobic and Oleophobic Coating for Inkjet Printing. Coatings, 13(2), 286. https://doi.org/10.3390/coatings13020286




