Abstract
Hafnium oxide (HfO2) is widely recognized as one of the most promising high-k dielectric materials due to its remarkable properties such as high permittivity, wide band gap, and excellent thermal and chemical stability. The atomic layer deposition (ALD) of HfO2 has attracted significant attention in recent decades since it enables uniform and conformal deposition of HfO2 thin films on various substrates. In this study, we examined the initial surface reactions of a series of homoleptic hafnium precursors on hydroxylated Si(100) surfaces using density functional theory calculations. Our theoretical findings align with previous experimental studies, indicating that hafnium amides exhibit higher reactivity compared to other precursors such as hafnium alkoxides and hafnium halides in surface reactions. Interestingly, we found that the chemisorption and reactivity of hafnium precursors are considerably affected by their thermal stability and size. For alkoxide precursors, which have similar thermal stabilities, the size of alkoxide ligands is an important factor in determining their reactivity. Conversely, the reactivity of hafnium halides, which have ligands of similar sizes, is primarily governed by their thermal stability. These insights are valuable for understanding the surface reaction mechanisms of precursors on hydroxylated Si(100) surfaces and for designing new materials, particularly heteroleptic precursors, in future research.
1. Introduction
Atomic layer deposition (ALD) is a cutting-edge technology for growing thin films, utilizing self-limiting reactions between reactants and substrate surfaces [1]. This process involves repeating sequences of two half-cycles, consisting of a reactant pulse and a subsequent inactive purging step. The self-limiting nature of ALD facilitates the production of high-quality thin films, allowing precise control over their thickness, conformality, and morphology at the molecular level [2,3]. Given these advantages, it is not surprising that both academia and industry have shown significant interest in ALD over the past few decades [4,5,6,7,8,9]. Nowadays, this technology is utilized in various applications, including microelectronics, solar cells, catalysts, and optical devices [6,7,8].
Recently, the replacement of silicon oxide (SiO2) with high-k dielectric materials in microelectronic technology has gained increasing attention [10,11,12]. Among these materials, hafnium oxide (HfO2) is one of the most promising candidates owing to its superior properties, such as high permittivity, wide band gap, and thermal and chemical stability [13,14]. Significant research efforts have been devoted in recent decades to producing high-performance HfO2 thin films on Si substrate surfaces using ALD technology. Several types of precursors have been explored, including hafnium halides [15,16,17,18,19,20,21,22,23,24], hafnium amides [25,26,27,28,29,30,31,32,33,34,35,36,37,38], hafnium alkoxides [39,40,41], and heteroleptic precursors based on cyclopentadienyl (Cp) [42,43]. Among them, hafnium chloride (HfCl4) and hafnium amides are the most widely used precursors in both academia and industry. HfCl4, known for its thermal stability and ease of use in industrial applications, has the disadvantage of having corrosive byproducts that can damage equipment. In contrast, hafnium amides are considered one of the most promising families of organometallic precursors for HfO2 ALD due to their high reactivity, non-corrosiveness, and suitability for low-temperature processes. Hafnium alkoxide precursors are an interesting alternative, offering moderate reactivity and thermal stability, and are cost-effective and safe for the ALD processes.
Despite numerous experimental studies on the HfO2 ALD, a comprehensive understanding of the process is still limited. This limitation primarily arises from the fact that the performance of HfO2 thin films produced via ALD is influenced by a myriad of experimental factors. In this context, modeling studies have proven to be advantageous. Nowadays, theoretical studies utilizing density functional theory (DFT) are extensively employed in material science and engineering [9,38,44]. DFT simulations provide highly accurate theoretical results with minimal variance in reproducibility. Furthermore, DFT modeling is particularly useful for gaining insights into the reaction mechanism of surface reactions and the chemical and physical properties of systems at the molecular level, which can be challenging in experimental studies.
However, it is surprising that theoretical investigations into the HfO2 ALD process are scarce in the literature, despite the considerable potential of this technique [45,46,47,48,49]. Using a cluster model in which the silicon (Si) surface is represented by SinHm atomic clusters, Zhang and his coworkers [45] explored the reaction mechanism of HfO2 ALD using HfCl4 and water as precursors. Wang and his colleagues [46,47] conducted a DFT study of HfO2 ALD with a tetrakis (ethylmethylamino) hafnium (TEMAH) precursor by simulating the Si surface with a Si9H12 cluster. The cluster model, however, has several limitations in representing surface reactions, primarily because it heavily depends on the cluster size and does not fully simulate the surface environment. Therefore, the application of theoretical investigations using periodic boundary condition DFT methods in the HfO2 ALD process becomes essential, as these methods are likely to produce predictions that more closely align with the experimental outcomes.
In a recent study, we investigated the reaction mechanism of the HfO2 ALD process using TEMAH and water [38], employing DFT calculations. Our research provided deeper insights into the conversion of TEAMH into a Hf atom on the hydroxylated Si(100) surface. To further understand the influence of different ligands on the reactivity of homoleptic hafnium precursors, we performed a systematic study on the chemisorption in HfO2 ALD, using DFT calculations. It is known that chemisorption, usually occurring during the first ligand exchange reaction between the precursors and the surface, is crucial for the efficacy of ALD processes. We analyzed nine homoleptic hafnium precursors, including hafnium alkoxides, hafnium halides, and hafnium amides. Intriguingly, our theoretical findings reveal a correlation between the thermal stability of these precursors and their surface reactivity. Additionally, these computational results shows good agreement with experimental data. Among the nine precursors examined, the energy barriers for the surface reaction of hafnium amides range from 7.1 kJ/mol to 28.4 kJ/mol, significantly lower than those for hafnium alkoxides and hafnium halides. This finding helps explain why the HfO2 ALD process with hafnium amide precursors is feasible at lower temperatures compared to other precursors.
2. Computational Methods
Spin-polarized density functional theory (DFT) calculations were carried out using the project augmented wave (PAW) method [50], as implemented in the Vienna ab initio simulation package (VASP) [51,52,53]. The Perdew–Burke–Ernzerhof (PBE) functional was employed to obtain the exchange and correlation energies within the generalized gradient approximate (GGA) framework [54]. The electronic wave functions were expanded in a plane-wave basis set with a kinetic energy cutoff of 400 eV. The force convergence criteria were set at 0.01 eV/Å for ionic relaxations and 0.05 eV/Å for transition state searches, respectively, while the energy convergence criterion for the electronic self-consistency loop was established at 0.01 meV. The transition states were examined using the climbing-image nudged elastic band (CI-NEB) method [55,56] which involves seven images per search. A (3 × 3) supercell model of the fully hydroxylated Si(100) surface was used. The Si atoms in the bottom layer were passivated by H atoms. The two bottom Si layers and their terminating H atoms were kept fixed, while others were allowed to relax during simulations. A more detailed description of the surface model can be found in our recent report [38]. For the surface calculations, a (3 × 3 × 1) Monkhorst–Pack [57] k-point mesh was applied, with no symmetry constraints and a dipole correction was included. Long-range dispersion interactions were accounted for by incorporating dispersion corrections calculated via the DFT-D3 method [58] into the DFT energies. The images of configurations were generated using VESTA (Version 3, Koichi Momma and Fujio Izumi, Ibaraki City, Japan) [59] and Mercury (Version 3.8, Cambridge Crystallographic Data Center, Cambridge City, UK) [60] visualization software.
To evaluate the thermal stability of the precursors, their bond dissociation energies (BDEs) were calculated using the isolated molecular models. The BDEs for the hafnium alkoxides (Hf[OR]4) and hafnium halides (HfX4) are defined as the energy required to dissociate a chemical bond between hafnium and alkoxide groups (in hafnium alkoxides) or hafnium and halide groups (in hafnium halides). These processes are represented in Equations (1) and (2).
All electronic structure calculations for the isolated molecular models were performed using Gaussian 16 [61] programs. Geometry optimizations and calculations of harmonic vibrational frequencies for the hafnium precursors, both in neutral and radical states, were fully conducted using the hybrid PBE0 functional [62]. These calculations were coupled with the 6-31G(d,p) basis set [63] for light elements (carbon (C), hydrogen (H), and oxygen (O)), and the def2sv basis set [64] for halogens and hafnium (Hf). As detailed in Table S1, to verify the accuracy of the results obtained with the def2sv basis set, additional calculations were carried out for hafnium halides using larger basis sets. These included aug-cc-pVTZ for fluorine (F) [65] and chlorine (Cl) [66], and aug-cc-pVTZ-PP for Hf [67] and iodine (I) [68,69]. The aug-cc-pVTZ-PP basis sets for Hf and I were sourced from EMSL/PNNL Basis Set Exchange [70,71,72]. Structural images of the precursors were generated using Chemcraft software (Version 1.8, Grigoriy A. Andrienko, https://www.chemcraftprog.com access on 1 December 2023) [73]. (Figure S1)
3. Results and Discussion
3.1. Chemisorption and Surface Reaction of Hafnium Alkoxide Precursors
Metal alkoxide precursors (M[OR]n), where a central metal (M) is directly bonded to alkoxide ligands (-OR) have attracted significant interest in metal oxide ALD research [39,40,41]. These precursors typically exhibit moderate reactivity along with high volatility, thermal stability, and safety. Owing to their relatively high thermal stability and volatility, metal alkoxides containing bulky ligands, such as isopropoxide (M[OiPr]4 and tert-butoxide (M[OtBu]4), are commonly employed as homoleptic alkoxide precursors in previous experimental studies on HfO2 ALD [39,40,41]. Mui and Musgrave carried out a DFT study on the reaction mechanisms of HfO2 ALD using hafnium ethoxide (Hf[OEt]4) and water as reactants, employing an isolated molecular model in gas phase [48]. However, the chemisorption and their structural characteristics on the surface have not been studied in detail since the model did not consider the interaction between the Si surface and reactants. In this work, we performed periodic condition DFT calculations to analyze the chemisorption and surface reaction of a series of hafnium alkoxides, including hafnium methoxide (Hf[OMe]4), hafnium isopropoxide (Hf[OiPr]4) and hafnium tert-butoxide (Hf[OtBu]4) on the hydroxylated Si(100) surface.
3.1.1. Hafnium Methoxide Precursor
As shown in Figure 1, hafnium methoxide (Hf[OMe]4) initially adsorbs onto the hydroxylated Si(100) surface with an adsorption energy of −126.0 kJ/mol. This exothermic chemical adsorption forms a new bond between the Hf atom of Hf[OMe]4 and the O atom of surface hydroxyls (OH) (1a). The bonding length is 2.28 Å, slightly longer than the average Hf-O bond distance (1.95 Å) in the free precursor Hf[OMe]4.
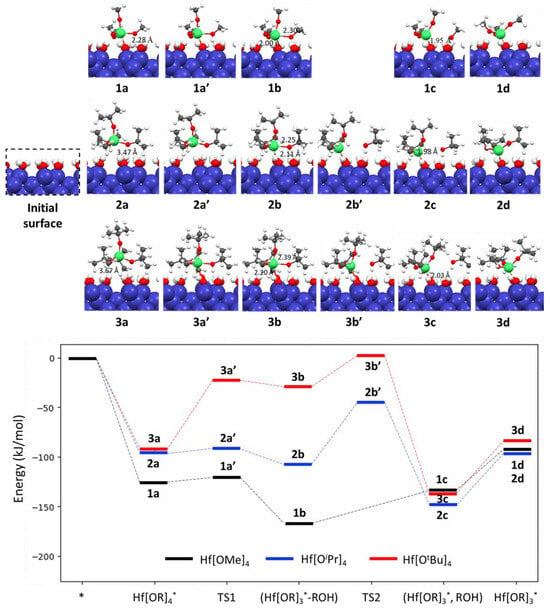
Figure 1.
Reaction energy profiles in kJ/mol for chemisorption and surface reactions of hafnium alkoxides (Hf[OR]4) on the fully hydroxylated Si(100) surface. Blue, red, white, gray and green balls indicate Si, O, H, C, and Hf atoms, respectively.
The first ligand exchange reaction converts the adsorbed Hf[OMe]4 (1a) into an intermediate (1b), via a transition state (1a′), and then into an adsorbed hafnium tri-methoxide (Hf[OMe]3) (1c). This step is an exothermic process with a small activation energy of 5.4 kJ/mol, leading to the formation of the intermediate (1b). The intermediate (1b) is a stable complex in which a methanol molecule remains weakly bonded to the adsorbed Hf[OMe]3, forming a Hf[OMe]3-MeOH complex. The configuration of intermediate (1b), depicted in Figure 1, reveals demonstrated that the MeOH molecule is bonded to the Hf atom in Hf[OMe]3 through an Hf-O bond with a length of 2.30 Å. This bond length is 0.30 Å longer than the distance between the Hf atom in the Hf[OMe]3-MeOH and the O atom of the OH group on the hydroxylated Si(100) surface.
The dissociation of methanol (MeOH) from 1b, leading to the formation of state 1c where one methanol is adsorbed onto the surface, is an endothermic process. This step occurs without a transition state and has a dissociation energy of 33.0 kJ/mol. This energy change reduces the distance between the Hf atom in Hf[OMe]3 and the O atom of the surface OH group from 2.00 Å to 1.95 Å. Subsequently, the methanol molecule desorbs from the Si(100) surface, resulting in the final configuration, 1d, with an endothermic desorption energy of 41.0 kJ/mol. It is noteworthy that this overall surface reaction process involves the breaking of two chemical bonds: Hf-OMe bond in the precursor Hf[OMe]4 and a H-O bond in the surface OH group. As a result, a new Hf-O chemical bond is formed between the Hf atom of the adsorbed Hf[OMe]3 and a surface O atom, with methanol released as a byproduct. Overall, the entire process is exothermic, with a net energy release of −87.5 kJ/mol.
3.1.2. Hafnium Isopropoxide Precursor
Figure 1 also presents the adsorption configuration of hafnium isopropoxide (Hf[OiPr]4) on a hydroxylated Si(100) surface, along with the transition, intermediate, and final states of the ligand exchange reaction that transforms the adsorbed Hf[OiPr]4 into adsorbed hafnium tri-isopropoxide (Hf[OiPr]3). The steric effects of the bulky isopropoxide groups result in a Hf-O distance of 3.47 Å between the Hf atom of the adsorbed Hf[OiPr]4 (2a) and the O atom of the surface OH. This distance is significantly longer than the 2.28 Å observed for Hf[OMe]4 (1a), leading to physisorption without the formation of a Hf-O bond.
The ligand exchange reaction that converts the adsorbed Hf[OiPr]4 into the final product, adsorbed Hf[OiPr]3, occurs in two-steps. In the first step, the adsorbed Hf[OiPr]4 (2a) readily transforms into a stable intermediate complex (2b), via a transition state (2a′) with a small activation energy of 3.0 kJ/mol. The configuration of complex 2b shows that the Hf-O distance between the O atom of the resulting isopropanol and the Hf atom is 2.25 Å, only 0.14 Å longer than the Hf-O bond length between the Hf atom and the surface oxygen (2.11 Å). In the second step, an exothermic reaction occurs, but with a high activation energy of 63.8 kJ/mol (2b′). The Hf-O bond length between the Hf atom and the surface O atom decreases from 2.11 Å in the intermediate complex 2b to 1.98 Å in the final product (2c), as shown in Figure 1. In the final step, isopropanol is released from the surface, and a covalent bond is formed between the Hf atom of Hf[OiPr]3 and the surface O-atom.
3.1.3. Hafnium Tert-Butoxide Precursor
Similar to the Hf[OiPr]4 precursor, hafnium tert-butoxide (Hf[OtBu]4) features large-sized tert-butoxide ligands, resulting in the physisorption of Hf[OtBu]4 on the hydroxylated Si(100) surface. In the physisorption configuration 3a, shown in Figure 1, the Hf-O distance between the Hf atom of the precursor Hf[OtBu]4 and the O atom of the surface hydroxyl is 3.67 Å, similar to that observed for Hf[OiPr]4.
The transformation of adsorbed Hf[OtBu]4 into hafnium tri-tert-butoxide (Hf[OBu]3) also involves two steps. The first step is the conversion of adsorbed Hf[OBu]4 (3a) into an intermediate (3 b), characterized by a high energy barrier of 70.2 kJ/mol. In this intermediate, the distance between the O atom of tert-butanol and the Hf atom is 2.39 Å, sufficient to break their Hf-O bond. The second step leads to the formation of hafnium tri-tert-butoxide (3c) and primarily involves a configuration rearrangement, with an activation energy of 34.1 kJ/mol. This value is significantly lower than the 63.8 kJ/mol observed in the second step for Hf[OiPr]4. Ultimately, tert-butanol is released from surface and a covalent bond is formed between the Hf atom of hafnium tri-tert-butoxide and a surface O atom.
Our computational results indicate that hafnium methoxide, with its smaller-sized ligands, can form a Hf-O chemical bond between the Hf atom of the precursor and the surface OH group in the first step (as seen in 1a, Figure 1). This leads to a low energy barrier of 5.4 kJ/mol in the subsequent chemisorption steps. In contrast, for other alkoxide precursors with larger ligands, only hydrogen bonds between the alkoxide groups and surface hydroxyl groups were observed, mainly contributing to their adsorption energies on the surfaces. Consequently, their following chemisorption exhibited revealed much higher energy barriers (63.8 kJ/mol for Hf[OiPr]4 and 70.2 kJ/mol for Hf[OtBu]4).
3.2. Chemisorption and Surface Reaction of Hafnium Halide Precursors
Hafnium halides are recognized as highly promising precursors for HfO2 ALD, owing to their notable thermal stability and low impurity levels. Over the past decades, several experimental studies employing these precursors have been conducted. In order to gain a deeper understanding of the reactivity and chemisorption characteristics of hafnium halides on the hydroxylated Si(100) surface, we performed DFT calculations on a series of these compounds, encompassing hafnium iodide (HI4), hafnium chloride (HfCl4), and hafnium fluoride (HfF4).
3.2.1. Hafnium Iodide Precursor
Our calculations indicated that hafnium iodide (HfI4) is adsorbed onto the hydroxylated Si(100) surface and forms a new bond with a surface hydroxyl group, with an adsorption energy of −104.9 kJ/mol. In the preferred adsorption configuration 4a (as shown in Figure 2), hafnium exhibits a stable five-fold coordination state in which it is bound to four I atoms and one O atom of the surface hydroxyl. The ligand exchange reaction results in the formation of the final state, hafnium tri-iodide (HfI3) 4b, and releases one molecule of HI (also shown in Figure 2). In this ligand exchange process, the HI molecule is generated through the interaction between an I-atom from adsorbed HfI4 and a hydrogen atom from the surface OH-group. The Hf-O distance between the Hf atom and the surface oxygen is reduced from 2.23 Å for 4a to 1.89 Å for 4b. This reaction is exothermic, with an energy barrier of 71.4 kJ/mol through a transition state 4a’. Interestingly, the hafnium atom in the state 4b retains a five-fold coordination state in which hafnium is bound to three I atoms, one surface O atom, and one O atom of a neighboring hydroxyl group. Removing free HI from the surface via desorption has a small desorption energy of 23.1 kJ/mol.
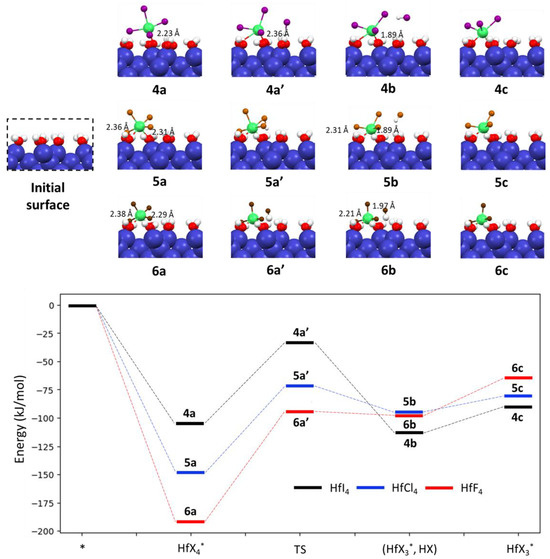
Figure 2.
Reaction energy profiles in kJ/mol for chemisorption and surface reactions of hafnium halides on the fully hydroxylated Si(100) surface. Blue, red, white, gray, green, purple, orange and brown balls indicate Si, O, H, C, Hf, I, Cl, and F atoms, respectively.
3.2.2. Hafnium Chloride Precursor
It is interesting to show that the precursor HfCl4 is adsorbed onto the hydroxylated Si(100) surface with a large adsorption energy of 147.6 kJ/mol, resulting in a six-fold coordinate state for the Hf-atom (as depicted in image 5a, Figure 2). The adsorption energy of HfCl4 on the hydroxylated Si surface was reported to be 73.1 kJ/mol by Zhang et al. [45], in which HfCl4 only interacts with one OH group. This difference can be understood by the fact that the authors used a cluster model Si9O3H12-OH in which only one OH group was available. The Hf-O distances between the Hf atom and the O atoms of the surface hydroxyl groups were 2.31 Å and 2.36 Å.
The adsorption configuration 5a undergoes a ligand exchange reaction, resulting in the formation of hafnium tri-chloride (HfCl4) 5b through a transition state 5a’ (as shown in Figure 2). The activation energy of this reaction is 76.1 kJ/mol, which is comparable to the value of 71.4 kJ/mol found for HfI4. The Hf-O bonding length between the Hf atom and the surface O atom is reduced from 2.31 Å for 5a to 1.89 Å for 5b, while a small change of 0.05 Å is observed for the Hf-O distance between the Hf atom and the neighboring OH group of 5a and 5b (from 2.36 Å to 2.31 Å). Free HCl is removed from surface via desorption which is 14.1 kJ/mol endothermic.
3.2.3. Hafnium Fluoride Precursor
Similar to the hafnium chloride precursor, hafnium fluoride HfF4 is adsorbed on the hydroxylated Si(100) surface and forms two chemical bonds with two surface OH groups (image 6a, Figure 2).The adsorption energy is −192.1 kJ/mol, which is considerably higher that of HfCl4 and HfI4. The adsorbed HfF4 6a is converted into the state 6b through a transition state 6a′, with a high energy barrier of 97.4 kJ/mol. In the configuration 6b, the Hf-O bond between the Hf atom and the surface O atom is reduced by 0.32 Å compared to the initial configuration 6a and is 1.97 Å in length.
At final step, free HF is released from surface with desorption energy of 30.1 kJ/mol. The product HfF3* is directly bound to surface O-atom which is formed by exchange-ligand process between HfF4* and surface OH-groups.
3.3. Chemisorption and Surface Reaction of Hafnium Amide Precursors
Hafnium amides are another family of hafnium precursors that are widely used in the HfO2 ALD process. Hafnium amides are known to have lower thermal stabilities, but higher reactivities, compared to hafnium halides, making them suitable for use in thermal ALD processes at low temperature.
In this section, a series of hafnium amides, including tetrakis(dimethylamino) hafnium TDMAH (Hf[NMe2]4), tetrakis(ethylmethylamino) hafnium TEMAH (Hf[NEtMe]4), and tetrakis(diethylamino) hafnium TDEAH (Hf[NEt2]4), were investigated (Me and Et stand for -CH3 and -C2H5, respectively). The adsorption configurations, transition and final states, and desorption configurations of these hafnium amides on the hydroxylated Si(100) surface along with their energy profile are given in Figure 3.
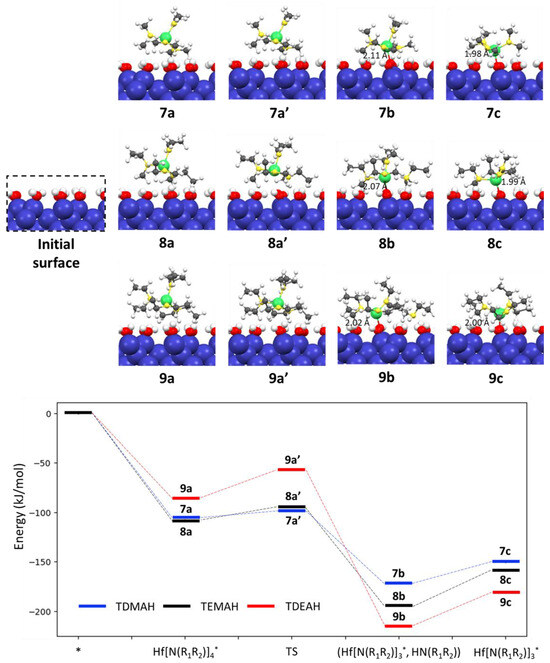
Figure 3.
Reaction energy profiles in kJ/mol for chemisorption and surface reactions of hafnium amides on the fully hydroxylated Si(100) surface. Blue, red, white, gray, green and yellow balls indicate Si, O, H, C, Hf, and N atoms, respectively.
3.3.1. Tetrakis (Dimethylamino) Hafnium (TDMAH)
Our calculations demonstrate that TDMAH (Hf[NMe2]4) initially adsorbs onto the hydroxylated Si surface, forming the adsorption configuration 7a with an adsorption energy of −104.9 kJ/mol. The Hf-O distance between the Hf atom and the surface O atoms in 7a varies in the range from 3.51 to 3.67 Å. Subsequently, a ligand exchange reaction occurs between one of the ligands of TDMAH and nearby surface OH groups, resulting in the formation of the product 7b (tri(dimethylamino)hafnium) (Hf[NMe2]3) through a transition state 7a′. This reaction exhibits an exceptionally low energy barrier of only 7.1 kJ/mol and is thermodynamically favorable, with an exothermic energy of −66.1 kJ/mol. The Hf atom in 7b retains a four-fold coordination state, binding with three remaining amino ligands and one surface O atom. After the desorption of free organic molecules HNMe2 from 7b, the configuration of 7c undergoes slight changes in the Hf-O bonding length in 7d measuring 1.98 Å, slightly shorter than the 2.11 Å in 7b.
3.3.2. Tetrakis (Ethylmethylamino) Hafnium (TEMAH)
Our predictions, as depicted in Figure 3, also indicate relatively small differences in the adsorption configurations, of TEAMH (Hf[NEtMe]4) and TDMAH precursors on the Si(100) surface. Due to the large size of the ligand -NEtMe, TEMAH only experiences physical adsorption on the OH-terminated Si(100) surface, resulting in configuration 8a, with an adsorption energy of 107.5 kJ/mol. Chemisorption is achieved through a subsequent ligand exchange reaction between TEMAH and neighboring surface OH groups. The adsorbed TEAMH* 8a is converted into adsorbed tri(ethylmethylamino) hafnium 8b via a transition state 8a’ with a small activation energy of 12.8 kJ/mol. This exothermic reaction has a reaction energy of −86.9 kJ/mol. The Hf-O bonding lengths of the chemisorption configurations 8b and 8c are 2.07 Å and 1.99 Å, respectively, which are almost the same as those of 2.11 Å and 1.98 Å in 7b and 7c. At final step, molecule HNEtMe (HN[CH3][C2H5]) is released from surface with desorption energy of 35.4 kJ/mol
Being different from our predictions, by employing a cluster model in which the Si surface is substituted by a Si9H12 cluster, Wang et al. [46] demonstrated that TEAMH undergoes direct conversion into a chemisorption state, characterized by an adsorption energy of 1.42 eV. Notably, no initial adsorption state was identified in this model. The observed disparity can be attributed to the substantial size of TEAMH, whereas the cluster surface model, containing only two OH groups, is relatively diminutive. Consequently, the interaction between the surface and TEAMH was not adequately described.
3.3.3. Tetrakis (Diethylamino) Hafnium (TDEAH)
Similar to the cases of the TDMAH (Hf[NEt2]4) and TEMAH precursors, TDEAH can also undergo a ligand exchange reaction to form chemisorption on the OH-terminated Si(100) surface with a relatively small energy barrier. The adsorption energy of the initially physically adsorbed configuration 9a of TDEAH on the Si surface is −85.7 kJ/mol, which is slightly smaller than the values of −104.9 kJ/mol and −107.5 kJ/mol for TDMAH and TEMAH, respectively. This can be attributed to the fact that TDEAH is bulkier than TDMAH and TEMAH, making it more difficult to approach the Si surface. The LER that converts 9a to the product 9b through a transition state 9a′ has an activation energy of 28.4 kJ/mol, which is also slightly higher than that observed for TDMAH and TEMAH. However, the chemisorption configurations 9b and 9c exhibit bonding characteristics similar to those of TDMAH and TEMAH. The Hf-O bonding lengths in 9b and 9c are 2.02 Å and 2.00 Å, respectively. The molecule HNEt2 is desorbed from the surface with desorption energy of 34.4 kJ/mol.
In general, we observed that the difference in energy barriers of LERs for the three hafnium amide precursors is quite small. The activation energy of the LER converting TDMAH into tri(dimethylamino) hafnium is 7.1 kJ/mol, which is slightly lower than the value of 12.8 kJ/mol for TEMAH and 28.4 kJ/mol for TDEAH. These theoretical predictions align well with the experimental results previously reported by Hausmann et al. [74], where the temperature required for hafnium amide precursors to achieve an exactly saturating dose is 75 °C for TDMAH, 115 °C for TEMAH, and 130 °C for TDEAH
3.4. Effect of Thermal Stability and Size of Precursors on Their Reactivates
The precursors utilized in the ALD process play a pivotal role in influencing both the performance of the ALD process and the quality of the deposited films. Previous research has highlighted the significance of precursor reactivity on the substrate surface as one of the most critical factors in determining the ALD temperature window and the growth rate of the deposited materials. Precursors with higher reactivity toward the surface substrate will necessitate lower process temperatures in comparison to those with lower reactivities.
Our calculations, summarized in Table 1, show that hafnium amide precursors are more reactive than hafnium alkoxide and hafnium halide precursors for HfO2 ALD process on hydroxylated Si(100) surface. The activation energies of the LERs between hafnium amides and surface OH groups of Si(100) surface range from 7.1 to 28.4 kJ/mol, which are much lower than those of hafnium halides (from 71.4 kJ/mol to 97.4 kJ/mol) and hafnium alkoxides (33.0 kJ/mol to 70.2 kJ/mol). These results are consistent with experimental findings showing that the ALD process using hafnium amide precursors only requires low processing temperatures, even below 100 °C. The temperature window of the ALD process using hafnium halides and hafnium alkoxides is in the range of 250–480 °C [3].

Table 1.
The adsorption energies (∆Eads.), activation energies (∆Eact1. for first reaction step (a to b) of all precursors and ∆Ereact2. for second reaction step (b to c) of hafnium alkoxides), and reaction energies (∆Ereact1. for first reaction step (a to b) of all precursors and ∆Ereact2. for second reaction step (b to c) of hafnium alkoxides) of hafnium precursors and desorption energy (∆Edes.) of byproduct (ROH for hafnium alkoxides, HX for hafnium halides, and HN(R1R2) for hafnium amides) in kJ/mol units on the hydroxylation Si(100) surface.
Interestingly, our above predictions also demonstrate that while the reactivities of the above amide precursors are similar, significant differences were found for the alkoxide and halide precursors. The energy barrier of ligand-exchange reaction during the chemisorption of hafnium methoxide (Hf[OMe]4) is only 33.0 kJ/mol (1b → 1c), which is much lower than the corresponding values of 63.8 kJ/mol for hafnium isopropoxide (Hf[OiPr]4) and 70.2 kJ/mol for hafnium tert-butoxide (Hf[OtBu]4). This prediction agrees well with the early experimental results showing that the temperature window of the ALD process using hafnium isopropoxide is 250–300 °C, which is about 100 °C lower than that of the process using hafnium tert-butoxide [3]. In other way, we found that the activation energies of LERs for HfCl4 and HfI4 are 76.1 kJ/mol and 71.4 kJ/mol, respectively, which are lower than the value of 97.4 kJ/mol for hafnium fluoride (HfF4).
These findings raise a question regarding whether the size and thermal stability of hafnium precursors have an impact on their reactivity and subsequent chemisorption onto the Si(100) surface. In the case of hafnium alkoxides, it is evident that hafnium methoxide exhibits higher reactivity with the OH-terminated Si(100) surface compared to hafnium isopropoxide and hafnium tert-butoxide, primarily due to the smaller size of the methoxide ligands. However, for hafnium halides, the effect of ligand size is negligible, likely due to the approximate size of halogen atoms. Thus we suppose that the thermal stability of precursors can considerably impact on their reactivities.
In order to further understand the effect of the thermal stability of precursors on their reactivity, their bond dissociation energies (BDEs) were calculated by using the isolated molecular model. BDEs, referring to the energy required to break a specific bond in a molecule and form two separate radical species, can be used as a factor of the thermal stability of a compound. By comparing the BDEs of different compounds, one can assess the relative stability of these compounds. Although a BDE value is a thermodynamic quantity, it can be used to predict reaction kinetics [75,76]. In previous studies, the BDE values were also used to gain a greater insight into the reaction mechanisms and characteristic dominant pathways of different types of reactions [77,78].
The molecular geometries of hafnium alkoxides and hafnium halides at a neutral state were optimized by using PBE0 functional and are depicted in Figure S1, while their average BDEs are given in Table S1 (Supplementary Information). It can be seen that there are negligible differences in the BDEs of alkoxide precursors. The BDE of Hf[OMe]4, Hf[OiPr]4, and Hf[OtBu]4 is 448 kJ/mol, 451 kJ/mol, and 454 kJ/mol, respectively. This means that the energy barrier of reaction of hafnium alkoxides on the surface is mainly effected by the size of alkoxide ligands rather than their thermal stability.
Moreover, the values presented in Table S1 demonstrate that the bond dissociation energies (BDEs) of HfI4, HfCl4, and HfF4 are 359 kJ/mol, 487 kJ/mol, and 633 kJ/mol at the PBE0/def2sv level of theory (these BDE values are 349 kJ/mol, 475 kJ/mol, and 628 kJ/mol at the PBE0/aug-cc-pVTZ-PP), respectively. Notably, these values align in the same order as their energy barriers of ligand-exchange reactions during chemisorption on the Si(100) surface (HfI4 < HfCl4 < HfF4). Among the hafnium halides, HfF4 exhibits the highest thermal stability, with a remarkably high BDE of 628 kJ/mol, and it displays the highest energy barrier of 97.4 kJ/mol for chemisorption. These results suggest that, for hafnium halides with approximately similar ligand sizes, a higher thermal stability of the precursors leads to an elevated energy barrier for ligand exchange reactions on the surface. These predictions also align with previous experimental findings that reported a lower reactivity of HfCl4 toward the Si(100) surface compared to HfI4 [20].
4. Conclusions
In this report, we have investigated the surface reactions of a series of homoleptic hafnium precursors on the hydroxylated Si(100) surface. We have explored nine homoleptic hafnium precursors, including Hf[OMe]4, Hf[OiPr]4, Hf[OtBu]4, HfI4, HfCl4, HfF4, TDMAH, TEAMH, and TDEAH. Our theoretical predictions are consistent with previously reported experimental studies, which suggest that hafnium amides are more reactive than other types of precursors. In contrast, the hafnium halides exhibit high energy barriers, whereas the hafnium alkoxides demonstrate moderate reactivity on the hydroxylated Si(100) surface.
Among alkoxide precursors, Hf[OMe]4 exhibits the lowest energy barrier, while Hf[OtBu]4 has the highest activation energy. In case of hafnium halides, HfF4 exhibits the highest energy barrier in the first ligand exchange reaction. For hafnium amide precursors, the activation energy of TDMAH is slightly lower than compared to the other amides.
Our investigation indicate that the chemisorption and reactivity of the hafnium precursors are considerably affected by their thermal stabilities and sizes. For hafnium alkoxides, which have similar thermal stability, the size of alkoxide ligands is crucial for their chemisorption on the surface. Alternatively, for hafnium halides, whose sizes of ligands are similar, their thermal stabilities play an key role in their reactivities on the surface.
These insights not only shed light on the relationship between precursor reactivity and factors like size and thermal stability but also provide guidance for designing new heteroleptic precursors. Further investigation into heteroleptic precursors is anticipated to yield significant advancements in enhancing the performance of HfO2 thin films in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/2079-6412/13/12/2094/s1. Figure S1: The optimized geometries of hafnium alkoxides and hafnium halides obtained at PBE0 functional; Table S1: The average bond dissociation energies (BDEs) of the hafnium precursors obtained at PBE0 functional along with 6-31G(d,p) basis set for C, H, O and def2sv basis set for F, Cl, I and Hf. The values in brackets are obtained by using aug-cc-pVTZ (for F and Cl) and aug-cc-pVTZ-PP (for Hf and I) basis sets.
Author Contributions
Conceptualization, T.B.T., J.L. and H.S.; methodology, T.B.T. and H.S.; validation, T.B.T., J.L. and H.S.; formal analysis, T.B.T., J.L. and H.S.; investigation, T.B.T., J.L. and H.S.; writing—original draft preparation, T.B.T.; writing—review and editing, T.B.T., J.L. and H.S.; supervision, J.L. and H.S.; funding acquisition, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Republic of Korea Grant funded by the Korean Government (MOE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the readability of Table 1. This change does not affect the scientific content of the article.
References
- Suntola, T. Atomic layer epitaxy. Mater. Sci. Rep. 1989, 4, 261–312. [Google Scholar] [CrossRef]
- Suntola, T.; Hyvarinen, J. Atomic layer epitaxy. Annu. Rev. Mater. Sci. 1985, 15, 177–195. [Google Scholar] [CrossRef]
- Puurunen, R.L. A short history of atomic layer deposition: Tuomo Suntola’s atomic layer epitaxy. Chem. Vap. Depos. 2014, 20, 332–344. [Google Scholar] [CrossRef]
- Tai, T.B.; Cao, L.; Mattelaer, F.; Rampelberg, G.; Hashemi, F.S.M.; Dendooven, J.; van Ommen, J.R.; Detavernier, C.; Reyniers, M.-F. Atomic Layer Deposition of Al2O3 Using Aluminum Triisopropoxide (ATIP): A Combined Experimental and Theoretical Study. J. Phys. Chem. C 2019, 123, 485–494. [Google Scholar] [CrossRef]
- Shtepliuk, I.; Yakimova, R. Special Issue “Fundamentals and Recent Advances in Epitaxial Graphene on SiC”. Appl. Sci. 2021, 11, 3381. [Google Scholar] [CrossRef]
- Elliott, S.D.; Dey, G.; Maimaiti, Y.; Ablat, H.; Filatova, E.A.; Fomengia, G.N. Modeling mechanism and growth reactions for new nanofabrication processes by atomic layer deposition. Adv. Mater. 2015, 28, 5367–5380. [Google Scholar] [CrossRef] [PubMed]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Mallick, B.C.; Hsieh, C.-T.; Yin, K.-M.; Gandomi, Y.A.; Huang, K.-T. Review—On Atomic Layer Deposition: Current Progress and Future Challenges. ECS J. Solid State Sci. Technol. 2019, 8, N55–N78. [Google Scholar] [CrossRef]
- Shahmohammadi, M.; Mukherjee, R.; Sukotjo, C.; Diwekar, U.M.; Takoudis, C.G. Recent Advances in Theoretical Development of Thermal Atomic Layer Deposition: A Review. Nanomaterials 2022, 12, 831. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskela, M.; Ritala, M.; Puurunen, R.L. Crystallinity of Inorganic Films Grown by Atomic Layer Deposition: Overview and General Trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- Dingemans, G.; Kessels, W.M.M. Status and prospects of Al2O3-based surface passivation schemes for silicon solar cells. J. Vac. Sci. Technol. A 2012, 30, 040802. [Google Scholar] [CrossRef]
- Giannazzo, F.; Schilirò, E.; Nigro, R.L.; Roccaforte, F.; Yakimova, R. Atomic Layer Deposition of High-k Insulators on Epitaxial Graphene: A Review. Appl. Sci. 2020, 10, 2440. [Google Scholar] [CrossRef]
- Lee, J.-C.; Oh, S.-J.; Cho, M.; Hwang, C.S.; Jung, R. Chemical structure of the interface in ultrathin HfO2/Si films. Appl. Phys. Lett. 2004, 84, 1305–1307. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.-B.; Maeng, W.-J. Applications of atomic layer deposition to nanofabrication and emerging nanodevices. Thin Solid Films 2009, 517, 2563–2580. [Google Scholar] [CrossRef]
- Sandell, A.; Karlsson, P.; Richter, J.; Blomquist, J.; Uvdal, P. Surface chemistry of HfI4 on Si(100)-(2 × 1) studied by core level photoelectron spectroscopy. Surf. Sci. 2007, 601, 917–923. [Google Scholar] [CrossRef]
- Fedorenko, Y.; Swerts, J.; Maes, J.W.; Tois, E.; Haukka, S.; Wang, C.-G.; Wilk, G.; Delabie, A.; Deweerd, W.; De Gendt, S. Atomic Layer Deposition of Hafnium Silicate from HfCl4, SiCl4, and H2O. Electrochem. Solid-State Lett. 2007, 10, H149–H152. [Google Scholar] [CrossRef]
- Takahashi, N.; Nonobe, S.; Nakamura, T. Growth of HfO2 films using an alternate reaction of HfCl4 and O2 under atmospheric pressure. J. Solid State Chem. 2004, 177, 3944–3948. [Google Scholar] [CrossRef]
- Kukli, K.; Ritala, M.; Sajavaara, T.; Keinonen, J.; Leskela, M. Comparison of hafnium oxide films grown by atomic layer deposition from iodide and chloride precursors. Thin Solid Films 2002, 416, 72–79. [Google Scholar] [CrossRef]
- Nonobe, S.; Takahashi, N.; Nakamura, T. Preparation of HfO2 nano-films by atomic layer deposition using HfCl4 and O2 under atmospheric pressure. Solid State Sci. 2004, 6, 1217–1219. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Kikas, A.; Käämbre, T.; Rammula, R.; Ritslaid, P.; Uustare, T.; Sammelselg, V. Effects of precursors on nucleation in atomic layer deposition of HfO2. Appl. Surf. Sci. 2004, 230, 292–300. [Google Scholar] [CrossRef]
- Boher, P.; Defranoux, C.; Heinrich, P.; Wolstenholme, J.; Bender, H. VUV spectroscopic ellipsometry applied to the characterization of high-k dielectrics. Mater. Sci. Eng. B 2004, 109, 64–68. [Google Scholar] [CrossRef]
- Kang, S.-W.; Rhee, S.-W.; George, S.M. Infrared spectroscopic study of atomic layer deposition mechanism for hafnium silicate thin films using HfCl2[N(SiMe3)2]2 and H2O. J. Vac. Sci. Technol. A 2004, 22, 2392–2397. [Google Scholar] [CrossRef][Green Version]
- Vitchev, R.; Pireaux, J.; Conard, T.; Bender, H.; Wolstenholme, J.; Defranoux, C. X-ray photoelectron spectroscopy characterisation of high-k dielectric Al2O3 and HfO2 layers deposited on SiO2/Si surface. Appl. Surf. Sci. 2004, 235, 21–25. [Google Scholar] [CrossRef]
- Kim, H.; Saraswat, K.C.; McIntyre, P.C. Comparative Study on Electrical and Microstructural Characteristics of ZrO2 and HfO2 Grown by Atomic Layer Deposition. J. Mater. Res. 2005, 20, 3125–3132. [Google Scholar] [CrossRef]
- Triyoso, D.H.; Hegde, R.I.; Gregory, R. Impact of Deposition Processes on Properties of Atomic-Layer-Deposited Hafnium Zirconate High-k Dielectrics. Electrochem. Solid-State Lett. 2007, 10, H354. [Google Scholar] [CrossRef]
- Swerts, J.; Peys, N.; Nyns, L.; Delabie, A.; Franquet, A.; Maes, J.W.; Van Elshocht, S.; De Gendt, S. Impact of Precursor Chemistry and Process Conditions on the Scalability of ALD HfO2 Gate Dielectrics. J. Electrochem. Soc. 2010, 157, G26–G31. [Google Scholar] [CrossRef]
- Liu, X.; Ramanathan, S.; Longdergan, A.; Srivastava, A.; Lee, E.; Seidel, T.E.; Barton, J.T.; Pang, D.; Gordon, R.G. ALD of Hafnium Oxide Thin Films from Tetrakis(ethylmethylamino)hafnium and Ozone. J. Electrochem. Soc. 2005, 152, G213. [Google Scholar] [CrossRef]
- Jin, H.; Oh, S.K.; Kang, H.J.; Cho, M.-H. Band gap and band offsets for ultrathin (HfO2)x(SiO2)1−x dielectric films on Si(100). Appl. Phys. Lett. 2006, 89, 122901. [Google Scholar] [CrossRef]
- Kim, Y.; Roh, Y.; Yoo, J.-B.; Kim, H. Characteristics of atomic layer deposition grown HfO2 films after exposure to plasma treatments. Thin Solid Films 2007, 515, 2984–2989. [Google Scholar] [CrossRef]
- Kirsch, P.D.; Quevedo-Lopez, M.A.; Li, H.-J.; Senzaki, Y.; Peterson, J.J.; Song, S.C.; Krishnan, S.A.; Moumen, N.; Barnett, J.; Bersuker, G.; et al. Nucleation and growth study of atomic layer deposited HfO2 gate dielectrics resulting in improved scaling and electron mobility. J. Appl. Phys. 2006, 99, 023508. [Google Scholar] [CrossRef]
- Park, P.K.; Roh, J.-S.; Choi, B.H.; Kang, S.-W. Interfacial Layer Properties of HfO2 Films Formed by Plasma-Enhanced Atomic Layer Deposition on Silicon. Electrochem. Solid-State Lett. 2006, 9, F34–F37. [Google Scholar] [CrossRef]
- Rose, M.; Bartha, J.; Endler, I. Temperature dependence of the sticking coefficient in atomic layer deposition. Appl. Surf. Sci. 2010, 256, 3778–3782. [Google Scholar] [CrossRef]
- Senzaki, Y.; Park, S.; Chatham, H.; Bartholomew, L.; Nieveen, W. Atomic layer deposition of hafnium oxide and hafnium silicate thin films using liquid precursors and ozone. J. Vac. Sci. Technol. A 2004, 22, 1175–1181. [Google Scholar] [CrossRef]
- Son, S.Y.; Kumar, P.; Cho, H.; Min, K.J.; Kang, C.J.; Singh, R.K. An evaluation of thermal stability of TiB2 metal gate on Hf silicate for p-channel metal oxide semiconductor application. Appl. Phys. Lett. 2008, 92, 172106. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, M.; Ho, M.-T.; Wielunski, L.S.; Chabal, Y.J. Infrared characterization of hafnium oxide grown by atomic layer deposition using ozone as the oxygen precursor. Appl. Phys. Lett. 2007, 90, 022906. [Google Scholar] [CrossRef]
- Won, S.-J.; Kim, J.-Y.; Choi, G.-J.; Heo, J.; Hwang, C.S.; Kim, H.J. The formation of an almost full atomic monolayer via surface modification by N2O-plasma in atomic layer deposition of ZrO2 thin films. Chem. Mater. 2009, 21, 4374–4379. [Google Scholar] [CrossRef]
- Kamiyama, S.; Miura, T.; Nara, Y. Electrical properties of ultrathin HfO2 films for replacement metal gate transistors, fabricated by atomic layer deposition using Hf(N(CH3)(C2H5))4 and O3. Appl. Phys. Lett. 2005, 87, 132904. [Google Scholar] [CrossRef]
- Tai, T.B.; Son, J.; Shin, H. A theoretical study of the atomic layer deposition of HfO2 on Si(1 0 0) surfaces using tetrakis(ethylmethylamino) hafnium and water. Appl. Surf. Sci. 2023, 612, 155702. [Google Scholar] [CrossRef]
- Kim, J.C.; Heo, J.S.; Cho, Y.S.; Moon, S.H. Atomic layer deposition of an HfO2 thin film using Hf(O-iPr)4. Thin Solid Films 2009, 517, 5695–5699. [Google Scholar] [CrossRef]
- Kukli, K.; Ritala, M.; Leskelä, M.; Sajavaara, T.; Keinonen, J.; Jones, A.; Roberts, J. Atomic layer deposition of hafnium dioxide films using hafnium bis(2-butanolate)bis(1-methoxy-2-methyl-2-propanolate) and water. Chem. Vap. Depos. 2003, 9, 315–320. [Google Scholar] [CrossRef]
- Rauwel, E.; Clavel, G.; Willinger, M.; Rauwel, P.; Pinna, N. Non-aqueous routes to metal oxide thin films by atomic layer deposition. Angew. Chem. 2008, 120, 3648–3651. [Google Scholar] [CrossRef]
- Niinistö, J.; Putkonen, M.; Niinistö, L.; Stoll, S.L.; Kukli, K.; Sajavaara, T.; Ritala, M.; Leskelä, M. Controlled growth of HfO2 thin films by atomic layer deposition from cyclopentadienyl-type precursor and water. J. Mater. Chem. 2005, 15, 2271–2275. [Google Scholar] [CrossRef]
- Dezelah, C.L.; Niinistö, J.; Kukli, K.; Munnik, F.; Lu, J.; Ritala, M.; Leskelä, M.; Niinistö, L. The atomic layer deposition of HfO2 and ZrO2 using advanced metallocene precursors and H2O as the oxygen source. Chem. Vap. Depos. 2008, 14, 358–365. [Google Scholar] [CrossRef]
- Sibanda, D.; Oyinbo, S.T.; Jen, T.-C. A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics. Nanotechnol. Rev. 2022, 11, 1332–1363. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.-T.; Zhang, D.W. Denssity functional theoretical study of initial stage of HfO2 atomic layer deposition on hydroxylated SiO2 surface. J. Mol. Struct. Theochem 2007, 803, 23–28. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Q.-Q.; Xu, M.; Ding, S.-J.; Zhang, D.W.; Wang, L.-K. Atomic Layer Deposition of Hafnium Oxide from Tetrakis(ethylmethylamino)hafnium and Water Precursors. J. Phys. Chem. C 2007, 111, 6495–6499. [Google Scholar] [CrossRef]
- Cortez-Valadez, M.; Fierro, C.; Farias-Mancilla, J.; Vargas-Ortiz, A.; Flores-Acosta, M.; Ramírez-Bon, R.; Enriquez-Carrejo, J.; Soubervielle-Montalvo, C.; Mani-Gonzalez, P. Comparison of HfCl4, HfI4, TEMA-Hf, and TDMA-Hf as precursors in early growing stages of HfO2 films deposited by ALD: A DFT study. Chem. Phys. 2016, 472, 81–88. [Google Scholar] [CrossRef]
- Mui, C.; Musgarve, C.B. Atomic layer deposition of HfO2 using alkoxides as precursors. J. Phys. Chem. B 2004, 108, 15150–15164. [Google Scholar] [CrossRef]
- D’Acunto, G.; Tsyshevsky, R.; Shayesteh, P.; Gallet, J.J.; Bournel, F.; Rochet, F.; Pinsard, I.; Timm, R.; Head, A.R.; Kuklja, M.; et al. Biomolecular reaction mechanism in the amido complex-based atomic layer deposition of HfO2. Chem. Mater. 2003, 35, 529–538. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Frisch, M.J. (Ed.) Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron Affinities of the First-Row Atoms Revisited. Systematic Basis Sets and Wave Functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H., Jr. Gaussian Basis Sets for Use in Correlated Molecular Calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Figgen, D.; Peterson, K.A.; Dolg, M.; Stoll, H. Energy-consistent pseudopotentials and correlation consistent basis sets for the 5d elements Hf–Pt. J. Chem. Phys. 2009, 130, 164108. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.A.; Figgen, D.; Goll, E.; Stoll, H.; Dolg, M. Systematically convergent basis sets with relativistic pseudopotentials. II. Small-core pseudopotentials and correlation consistent basis sets for the post-d group 16–18 elements. J. Chem. Phys. 2003, 119, 11113–11123. [Google Scholar] [CrossRef]
- Peterson, K.A.; Shepler, B.C.; Figgen, D.; Stoll, H. On the Spectroscopic and Thermochemical Properties of ClO, BrO, IO, and Their Anions. J. Phys. Chem. A 2006, 110, 13877–13883. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.T.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef]
- Feller, D. The role of databases in support of computational chemistry calculations. J. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Schuchardt, K.L.; Didier, B.T.; Elsethagen, T.; Sun, L.; Gurumoorthi, V.; Chase, J.; Li, J.; Windus, T.L. Basis Set Exchange: A Community Database for Computational Sciences. J. Chem. Inf. Model. 2007, 47, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Chemcraft—Graphical Software for Visualization of Quantum Chemistry Computations. Version 1.8, Build 682. Available online: http://www.chemcraftprog.com (accessed on 1 December 2023).
- Hausmann, D.M.; Kim, E.; Becker, J.; Gordon, R.G. Atomic layer deposition of hafnium and zirconium oxides using metal amide precursors. Chem. Mater. 2002, 14, 4350–4358. [Google Scholar] [CrossRef]
- John, P.C.S.; Guan, Y.; Kim, Y.; Kim, S.; Paton, R.S. Prediction of organic homolytic bond dissociation enthalpies at near chemical accuracy with sub-second computational cost. Nat. Commun. 2020, 11, 2328. [Google Scholar] [CrossRef] [PubMed]
- Gani, T.Z.H.; Kulik, H.J. Understanding and breaking scaling relations in single-site catalysis: Methane to methanol conversion by FeIV═O. ACS Catal. 2018, 8, 975–986. [Google Scholar] [CrossRef]
- Kim, S.; Fioroni, G.M.; Park, J.-W.; Robichaud, D.J.; Das, D.D.; John, P.C.S.; Lu, T.; McEnally, C.S.; Pfefferle, L.D.; Paton, R.S.; et al. Experimental and theoretical insight into the soot tendencies of the methylcyclohexene isomers. Proc. Combust. Inst. 2019, 37, 1083–1090. [Google Scholar] [CrossRef]
- Gallegos, L.C.; Luchini, G.; John, P.C.S.; Kim, S.; Paton, R.S. Importance of engineered and learned molecular representations in predicting organic reactivity, selectivity, and chemical properties. Acc. Chem. Res. 2021, 54, 827–836. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).