The Effect of Silica Sol on the Stain Resistance of Exterior Wall Latex Coatings through Natural Exposure to Sunlight
Abstract
:1. Introduction
2. Materials and Experimental
3. Results and Discussion
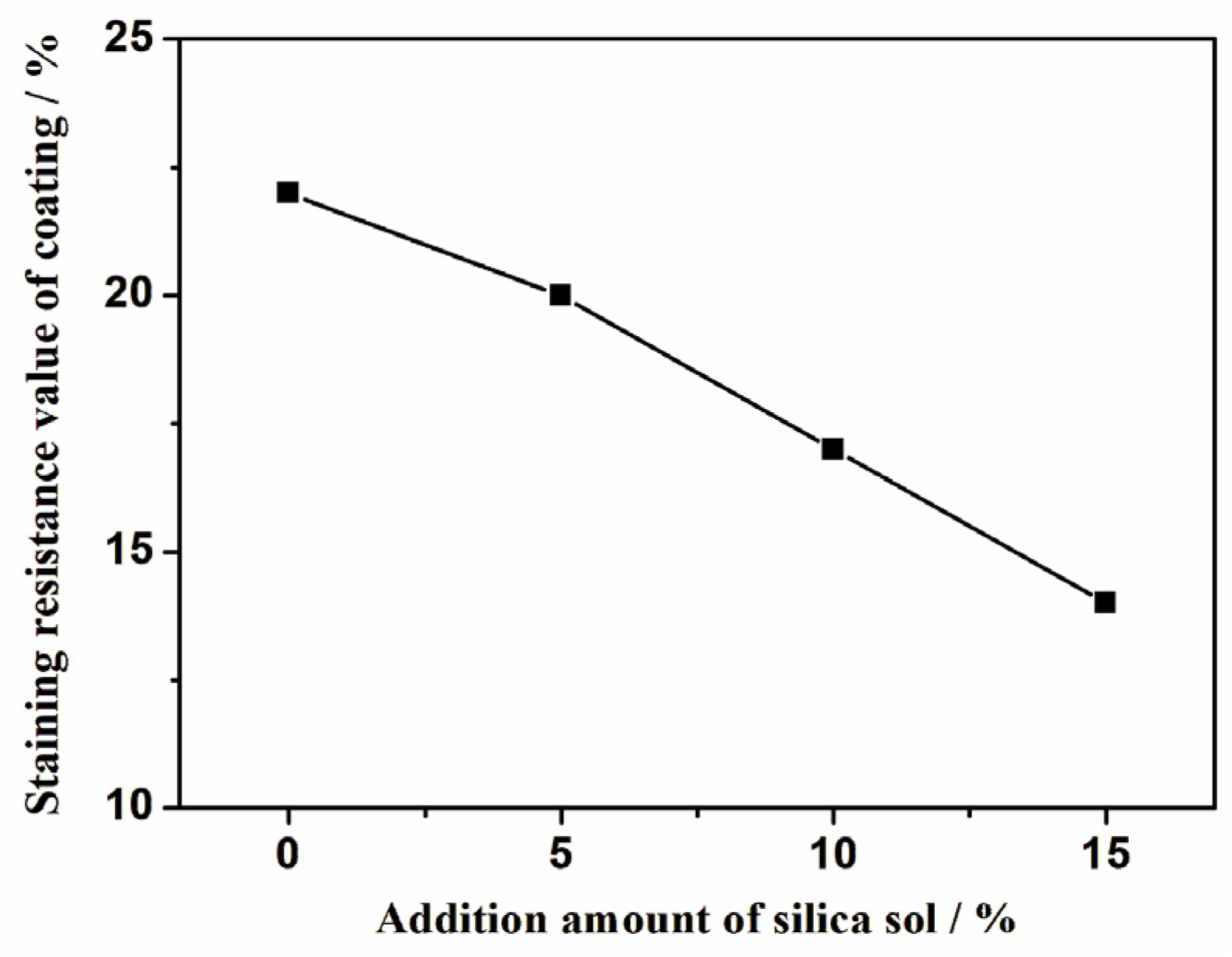
3.1. The Effect of the Amount of Silica Sol Added
3.2. Heat Storage Test
3.3. Natural Exposure to Sunlight Test
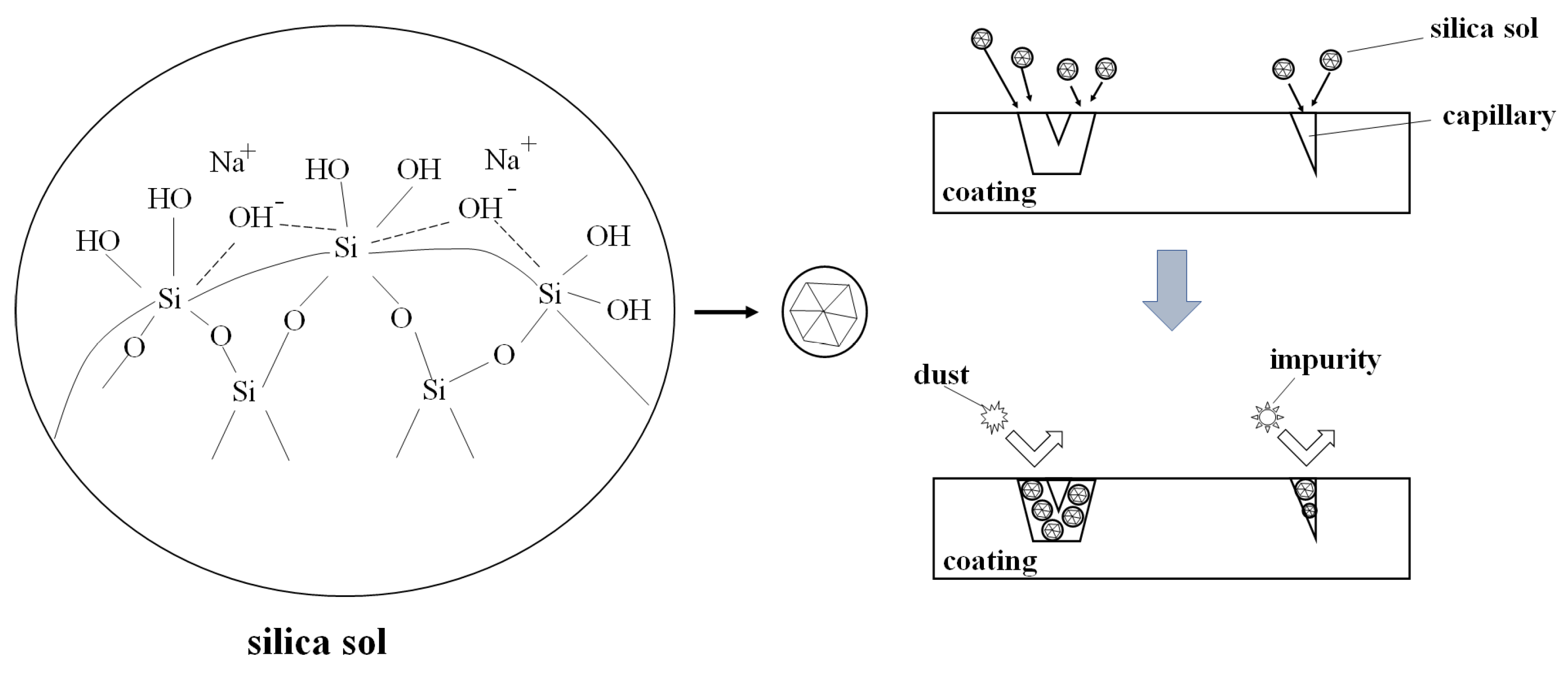
3.4. Staining Resistance Mechanism
4. Conclusions
- (1)
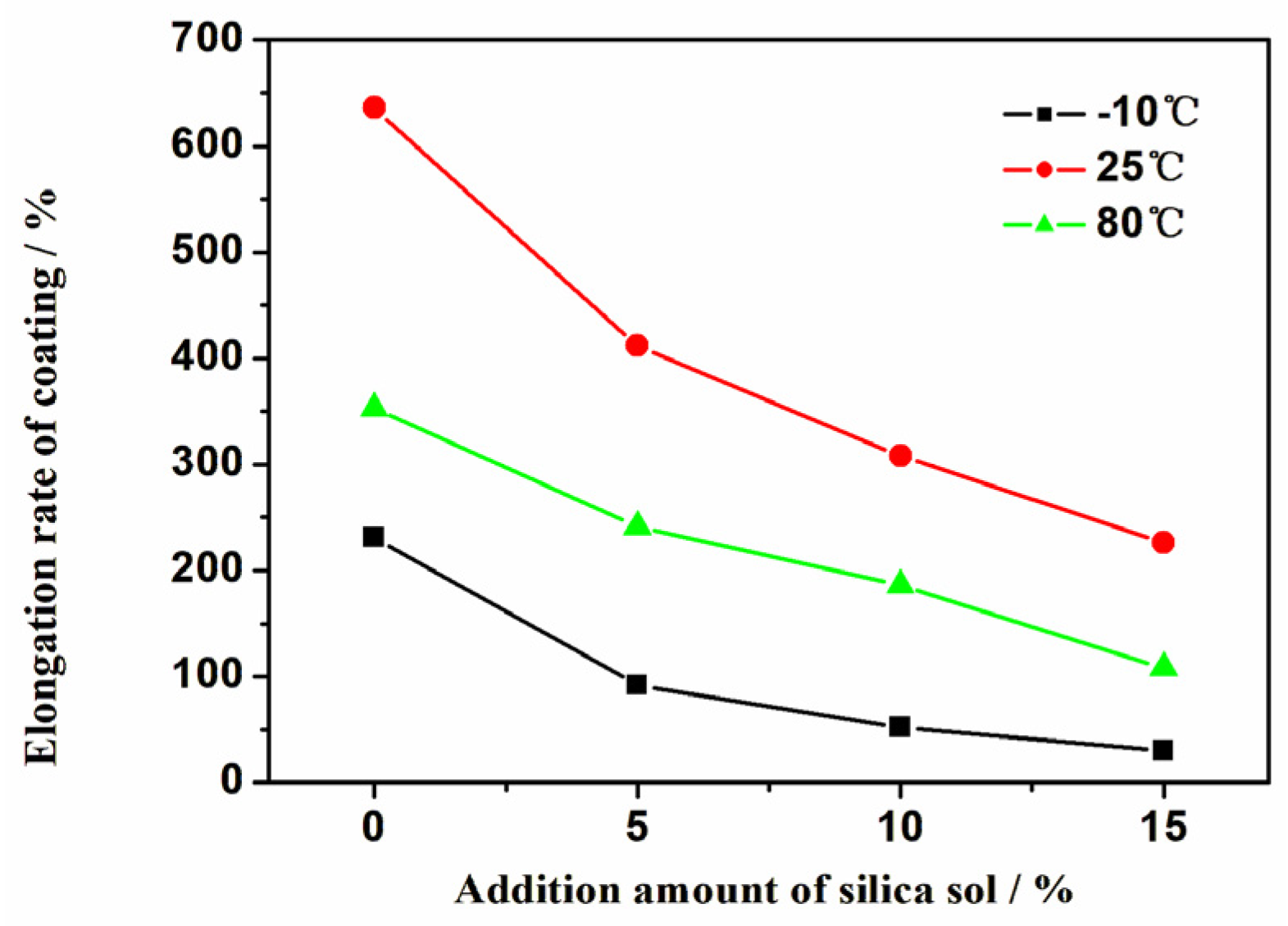
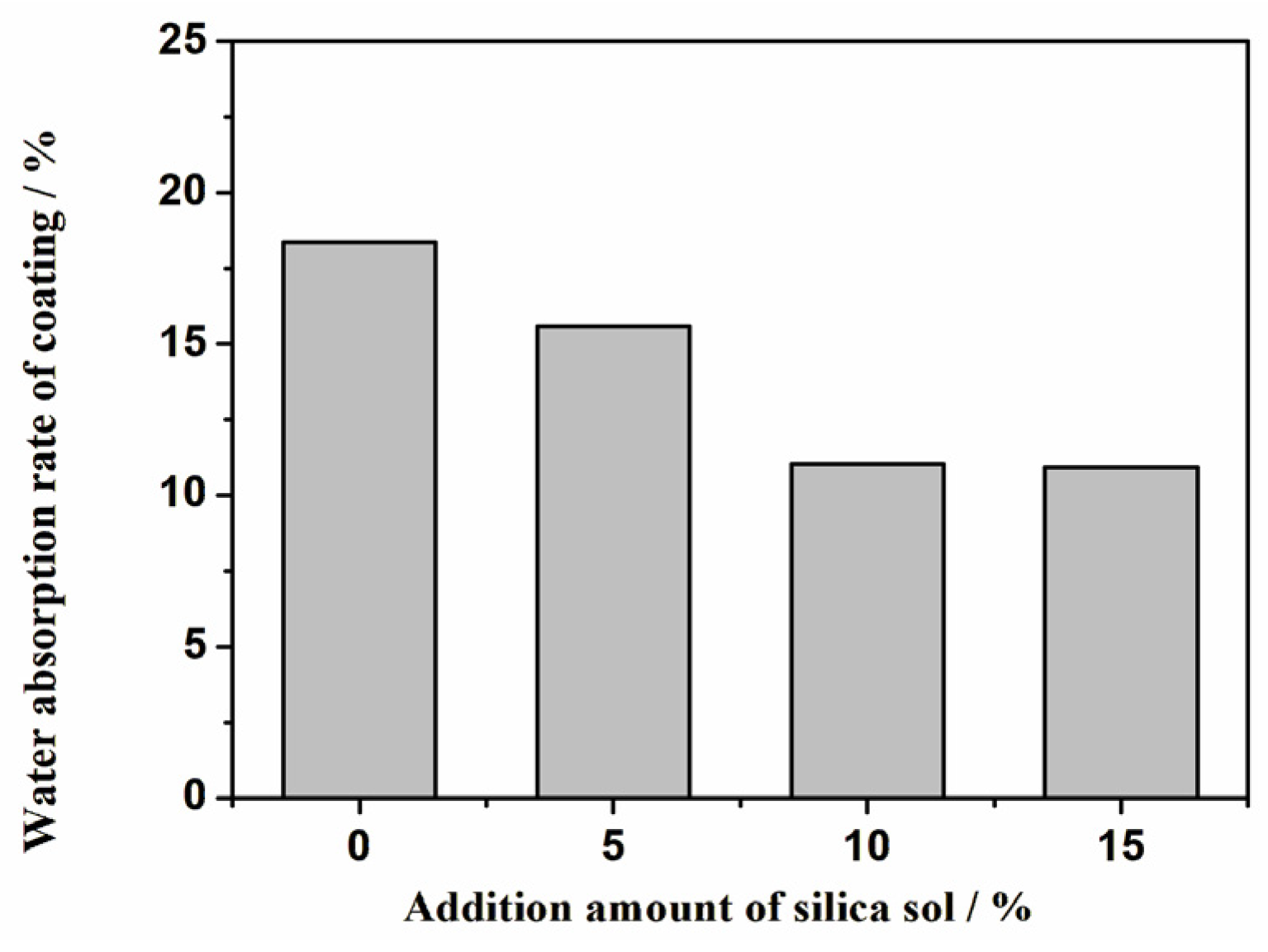
- As the addition amount of silica sol increases, the elongation of the coating significantly decreases, while the water absorption also decreases and the stain resistance is improved.
- (2)
- When the amount of NAOH added is 0.4%–2.4%, the pH value of the coating is controlled between 8.7–10.93 and the thermal storage stability of the coating can meet the requirements.
- (3)
- After 12 months of natural exposure to sunlight, the stain resistance value of the coating with added silica sol was significantly better than that without added silica sol, increasing by 65.8%.
- (4)
- The formation of a network structure of Si O-Si bonds in the silica sol enhances the hardness and rigidity of the coating, while also allowing it to enter the capillary tubes of the coating caused by prolonged exposure to sunlight, avoiding cracking of the coating and preventing the entry of dust and impurities. Therefore, the stain resistance of the coating has been improved.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Improved Outdoor Durability and Environmental Impact with a New Resin for Exterior Coatings; Pitture e Vernici: Milan, Italy, 2022.
- Covestro Unveils New Decovery® Resin for Exterior Coatings; Covestro: Leverkusen, Germany, 2021.
- Machotová, J.; Kalendová, A.; Steinerová, D.; Mácová, P.; Šlang, S.; Šňupárek, J.; Vajdák, J. Water-Resistant Latex Coatings: Tuning of Properties by Polymerizable Surfactant, Covalent Crosslinking and Nanostructured ZnO Additive. Coatings 2021, 11, 347. [Google Scholar] [CrossRef]
- Promlok, D.; Sonongbua, K.; Wilepsuwan, M.; Suteewong, T.; Tangboriboonrat, P. Hollow natural rubber latex particles as bio-based alternative white pigment for coating applications. Ind. Crops Prod. 2022, 188, 115593. [Google Scholar] [CrossRef]
- Ranka, A.I.; El-Aasser, M.S.; Vanderhoff, J.W. The influence of colloidal stability on critical pigment volume concentration (cpvc). J. Dispers. Sci. Technol. 2010, 5, 403–420. [Google Scholar] [CrossRef]
- Cherian, Z. Addressing Stain Resistance of Coatings by New Experimental and Modeling Technique; Longdom Publishing: Barcelona, Spain, 2021. [Google Scholar]
- Huang, X.; Wang, C.; Zhu, D. An Experimental Study for the Improvement of the Stain Resistance for Exterior Wall Paints in a Western City in China. Coatings 2021, 11, 220. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, X.; Li, X.; Zhang, J. Deposition of stain resistance coating by non-thermal plasma brush application to dental whitening. Plasma Sci. Technol. 2019, 21, 65501. [Google Scholar] [CrossRef]
- Xiaoning, Z.; Etc, C.W. Preparation of Nitrogen Adulterated Nano-TiO_2 Modified Coatings and Study of Their Antibacterial Performance in Sunlight; China Paint: Hong Kong, China, 2005. [Google Scholar]
- Coupled Effect of Water Absorption and Ion Transport in Hudrated Latex Anti-Corrosion Coatings; Pitture e Vernici: Milan, Italy, 2023.
- Yoshikai, K.; Sueyoshi, A.; Ohsaki, T. Study on the Formation of Bound Rubber and Reinforcing Behavior of Silica Prepared by Sol-Gel Process in the Latex. Gomu 2000, 73, 660–667. [Google Scholar]
- Siramanont, J. Reinforcement of Natural Rubber by Silica Generated from Sol-Gel Process of Silanes in Latex/Jirawan Siramanont; Digital Research Information Center: Bangkok, Thailand, 2005. [Google Scholar]
- Mahadik, S.A.; Kavale, M.S.; Mukherjee, S.K.; Rao, A.V. Transparent Superhydrophobic silica coatings on glass by sol–gel method. Appl. Surf. Sci. 2010, 257, 333–339. [Google Scholar] [CrossRef]
- Trovato, V.; Teblum, E.; Kostikov, Y.; Pedrana, A.; Re, V.; Nessim, G.D.; Rosace, G. Electrically conductive cotton fabric coatings developed by silica sol-gel precursors doped with surfactant-aided dispersion of vertically aligned carbon nanotubes fillers in organic solvent-free aqueous solution–ScienceDirect. J. Colloid Interface Sci. 2020, 586, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Jaglarz, J.; Dulian, P.; Karasiński, P.; Winkowski, P. Scattering Phenomena in Porous Sol-Gel-Derived Silica Films. Coatings 2020, 10, 509. [Google Scholar] [CrossRef]
- Oldani, V.; Sergi, G.; Pirola, C.; Sacchi, B.; Bianchi, C.L. Sol-gel hybrid coatings containing silica and a perfluoropolyether derivative with high resistance and anti-fouling properties in liquid media. J. Fluor. Chem. 2016, 188, 43–49. [Google Scholar] [CrossRef]
- Liang, T.; Yongqiang, X.; Wenjuan, L.I. Preparation of thermal insulation coatings for exterior wall and its application in building energy efficiency. New Build. Mater. 2009, 36, 74–77. [Google Scholar]
- Chi, F.; Zeng, Y.; Liu, C.; Liang, D.; Li, Y.; Xie, R.; Pan, N.; Ding, C. Enhancing mechanical stability of sol-gel silica antireflection coatings via ammonia treatment at low temperature. Results Phys. 2020, 18, 103315. [Google Scholar] [CrossRef]
- Yan, X.; Wang, C.; Liu, X. Research on Weather Resistance and Stain Resistance of Organic-inorganic Composite Coatings for Exterior Wall. In Proceedings of the China Coatings, Shanghai, China, 20–22 November 2013. [Google Scholar]
- Dan, W.; Zhenbo, Q.; Tong, Z.; Liu, L.; Hu, W. Effect of Silica Sol on Corrosion Resistance of Aluminum Phosphate Composite Coatings. Paint. Coat. Ind. 2018, 48, 7–13. [Google Scholar]
- Illescas, J.F.; Mosquera, M.J. Surfactant-Synthesized PDMS/Silica Nanomaterials Improve Robustness and Stain Resistance of Carbonate Stone. J. Phys. Chem. C Nanomater. Interfaces 2011, 115, 14624–14634. [Google Scholar] [CrossRef]
- Kirtay, S. Preparation of hybrid silica sol–gel coatings on mild steel surfaces and evaluation of their corrosion resistance. Prog. Org. Coat. 2014, 77, 1861–1866. [Google Scholar] [CrossRef]
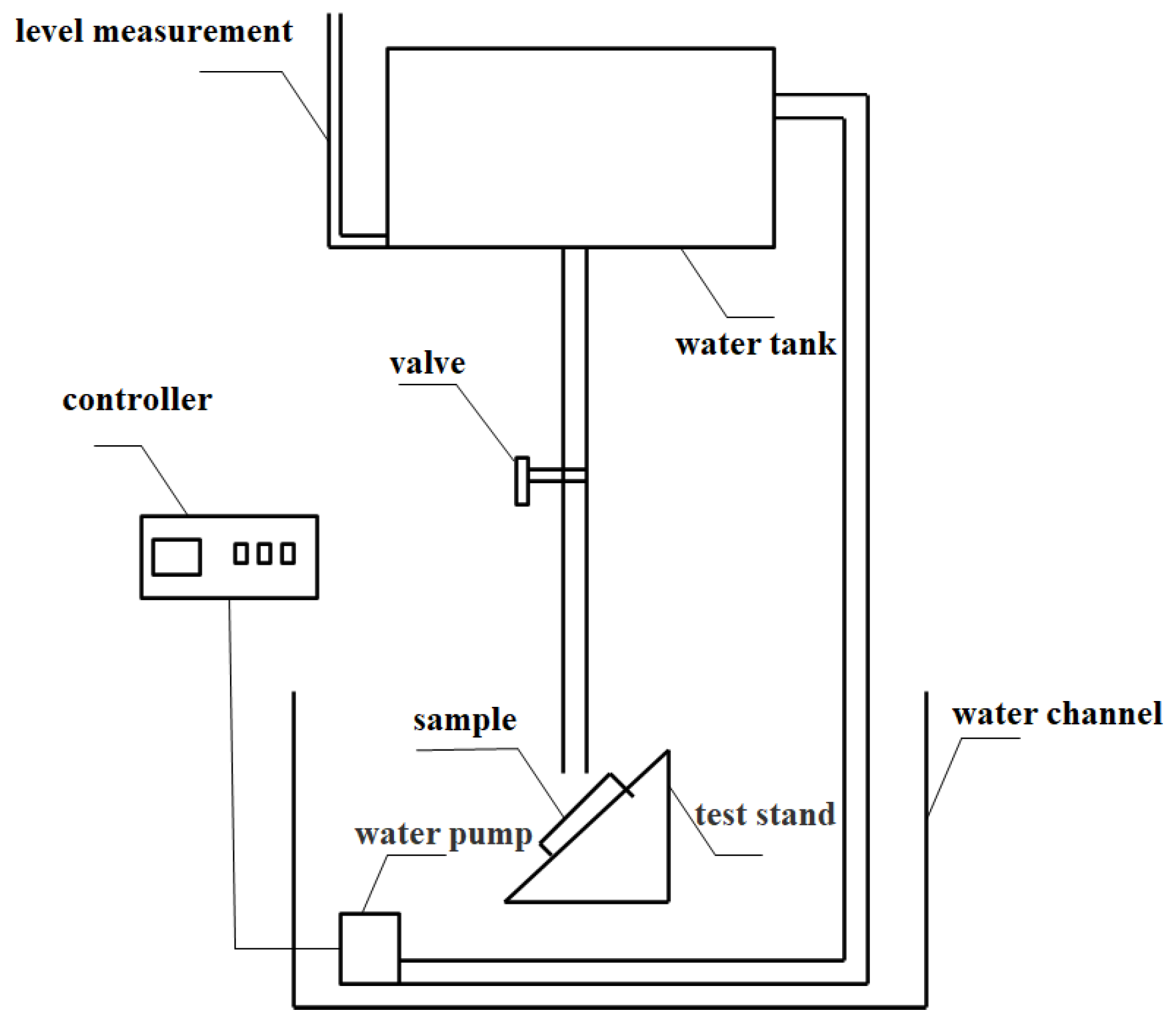
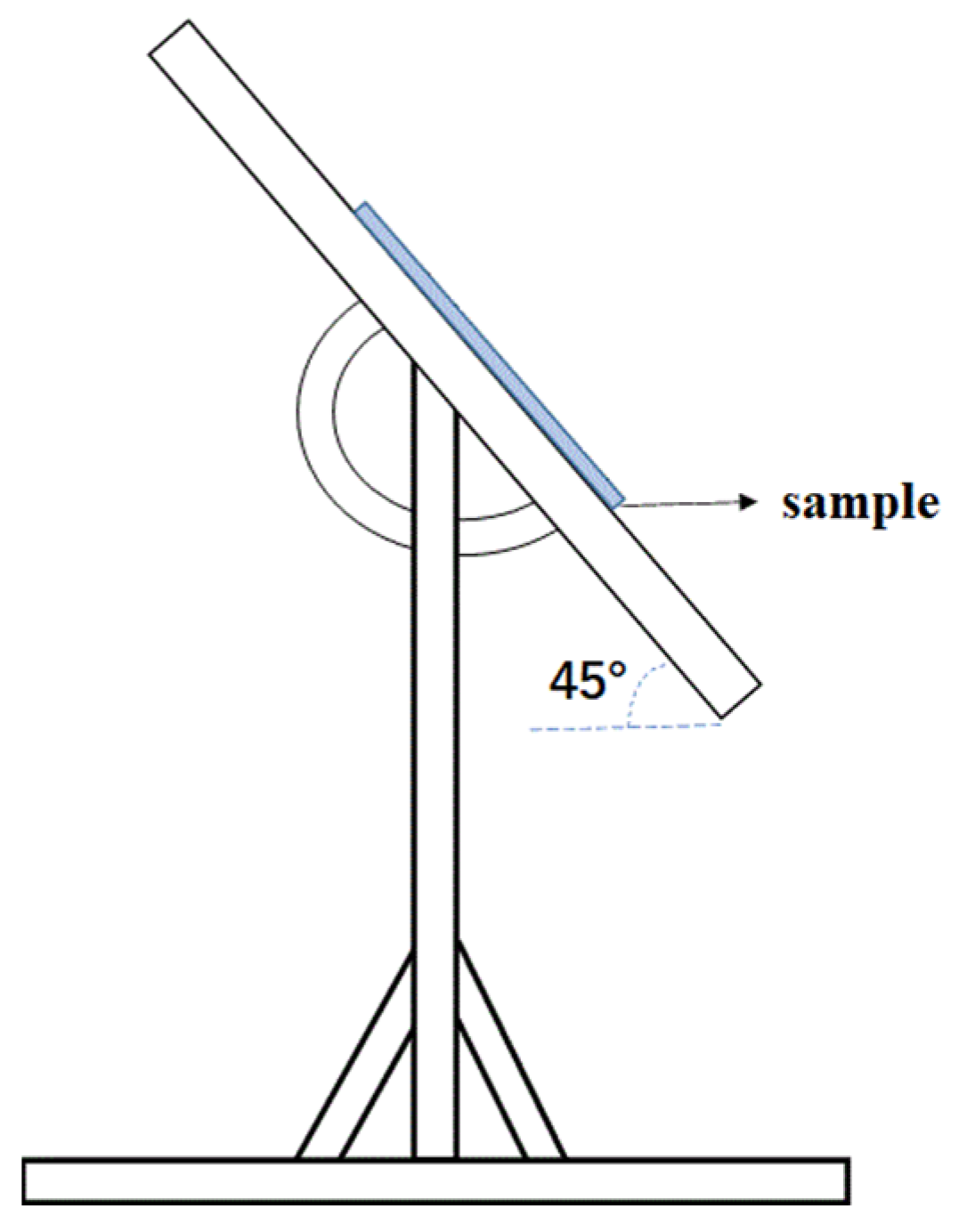

| Raw Material Name | Elastic Emulsion | Dispersant | Defoamer | Wetting Agent | Titanium Dioxide | Heavy Calcium Carbonate | Coalescent | Preservative | Thickener |
|---|---|---|---|---|---|---|---|---|---|
| Mass fraction/% | 30 | 0.5 | 0.3 | 0.1 | 15 | 20 | 0.5 | 0.2 | 0.5 |
| Name | SiO2 Content/% | Stabilizer Type/% | PH Value | Density at 20 °C | Average Particle Size/nm |
|---|---|---|---|---|---|
| Data | 30 | NaO2 ≤ 0.5 | 9–10.5 | 1.21 | 12 |
| 15% NaOH Content/% | 0.2 | 0.4 | 0.8 | 1.6 | 2.4 | 3.2 | |
|---|---|---|---|---|---|---|---|
| Heat Storage Time/Day | |||||||
| 0 | 73.1 ± 0.2 | 82.2 ± 0.1 | 108.9 ± 0.3 | 98.1 ± 0.2 | 97.5 ± 0.1 | 99.8 ± 0.2 | |
| 3 | 91.2 ± 0.1 | 84.4 ± 0.3 | 113.3 ± 0.1 | 99.2 ± 0.2 | 97.4 ± 0.1 | 100.7 ± 0.1 | |
| 7 | 93.2 ± 0.1 | 86.5 ± 0.1 | 114.5 ± 0.1 | 98.5 ± 0.1 | 97.4 ± 0.1 | 100.5 ± 0.1 | |
| 14 | 92.0 ± 0.2 | 85.9 ± 0.2 | 114.3 ± 0.1 | 97.7 ± 0.3 | 96.9 ± 0.1 | 100.6 ± 0.1 | |
| 30 | 95.4 ± 0.1 | 87.7 ± 0.1 | 113.7 ± 0.2 | 97.1 ± 0.5 | 96.9 ± 0.1 | 100.0 ± 0.2 | |
| 15% NaOH Content/% | 0.2 | 0.4 | 0.8 | 1.6 | 2.4 | 3.2 | |
|---|---|---|---|---|---|---|---|
| Heat Storage Time/Day | |||||||
| 0 | 8.39 ± 0.21 | 8.7 ± 0.18 | 9.02 ± 0.17 | 9.79 ± 0.19 | 10.93 ± 0.26 | 11.85 ± 0.24 | |
| 3 | 8.08 ± 0.07 | 8.39 ± 0.24 | 8.73 ± 0.10 | 9.48 ± 0.14 | 10.36 ± 0.15 | 10.98 ± 0.20 | |
| 7 | 8.15 ± 0.04 | 8.33 ± 0.12 | 8.65 ± 0.08 | 9.32 ± 0.12 | 10.19 ± 0.14 | 10.68 ± 0.17 | |
| 14 | 8.05 ± 0.06 | 8.29 ± 0.12 | 8.47 ± 0.11 | 9.19 ± 0.07 | 9.97 ± 0.09 | 10.45 ± 0.17 | |
| 30 | 7.95 ± 0.05 | 8.17 ± 0.17 | 8.31 ± 0.13 | 9.11 ± 0.04 | 9.80 ± 0.07 | 10.25 ± 0.12 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.-J.; Li, C.-D.; Peng, J.; Luo, J.-H.; Hong, Y. The Effect of Silica Sol on the Stain Resistance of Exterior Wall Latex Coatings through Natural Exposure to Sunlight. Coatings 2023, 13, 2013. https://doi.org/10.3390/coatings13122013
Dong L-J, Li C-D, Peng J, Luo J-H, Hong Y. The Effect of Silica Sol on the Stain Resistance of Exterior Wall Latex Coatings through Natural Exposure to Sunlight. Coatings. 2023; 13(12):2013. https://doi.org/10.3390/coatings13122013
Chicago/Turabian StyleDong, Lian-Jie, Cheng-Di Li, Jia Peng, Jia-Hong Luo, and Yun Hong. 2023. "The Effect of Silica Sol on the Stain Resistance of Exterior Wall Latex Coatings through Natural Exposure to Sunlight" Coatings 13, no. 12: 2013. https://doi.org/10.3390/coatings13122013
APA StyleDong, L.-J., Li, C.-D., Peng, J., Luo, J.-H., & Hong, Y. (2023). The Effect of Silica Sol on the Stain Resistance of Exterior Wall Latex Coatings through Natural Exposure to Sunlight. Coatings, 13(12), 2013. https://doi.org/10.3390/coatings13122013







