Investigation of Evolution of Microstructure, Mechanical Properties, and Corrosion Resistance of Novel Al-Mg-Si-Ag Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
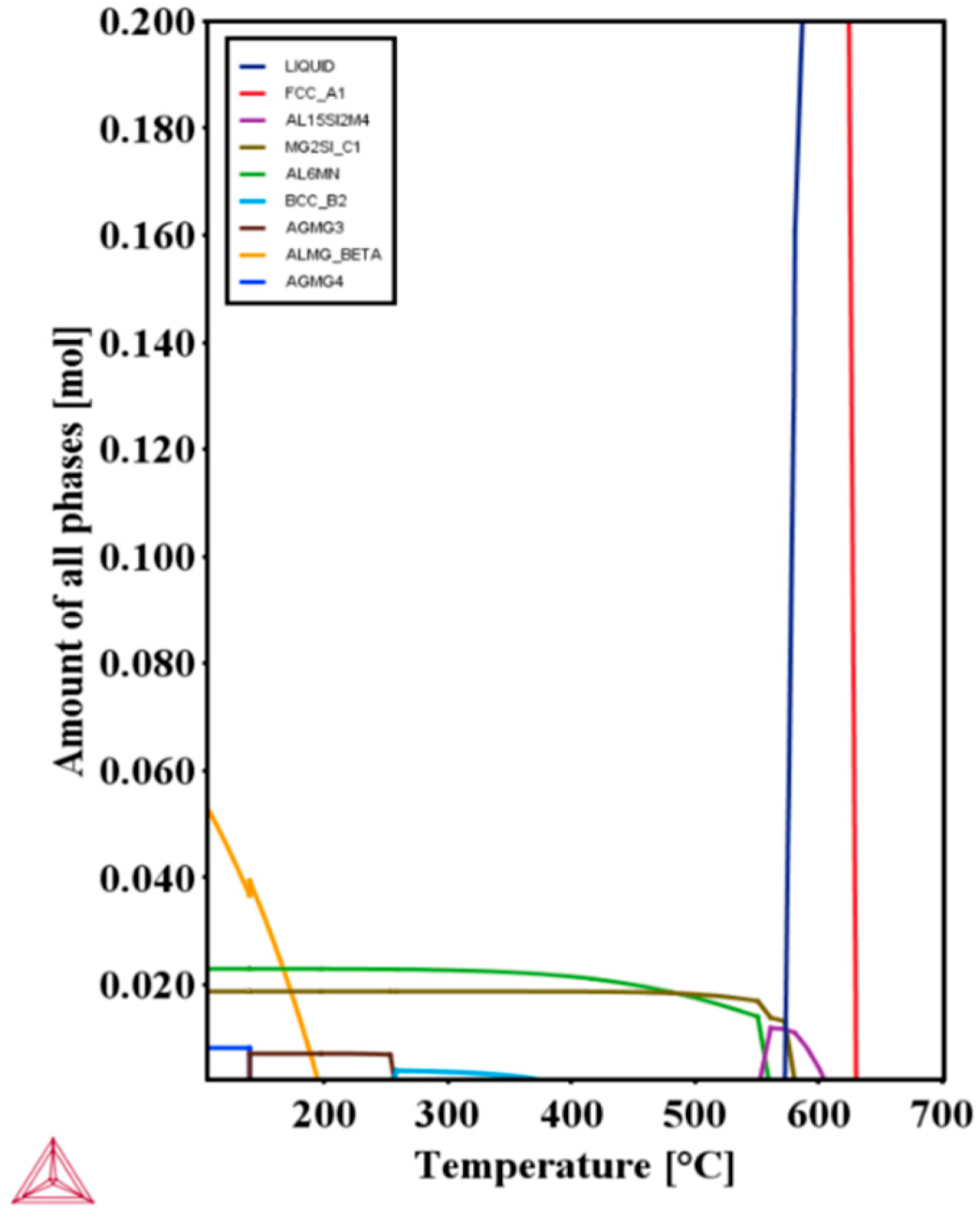
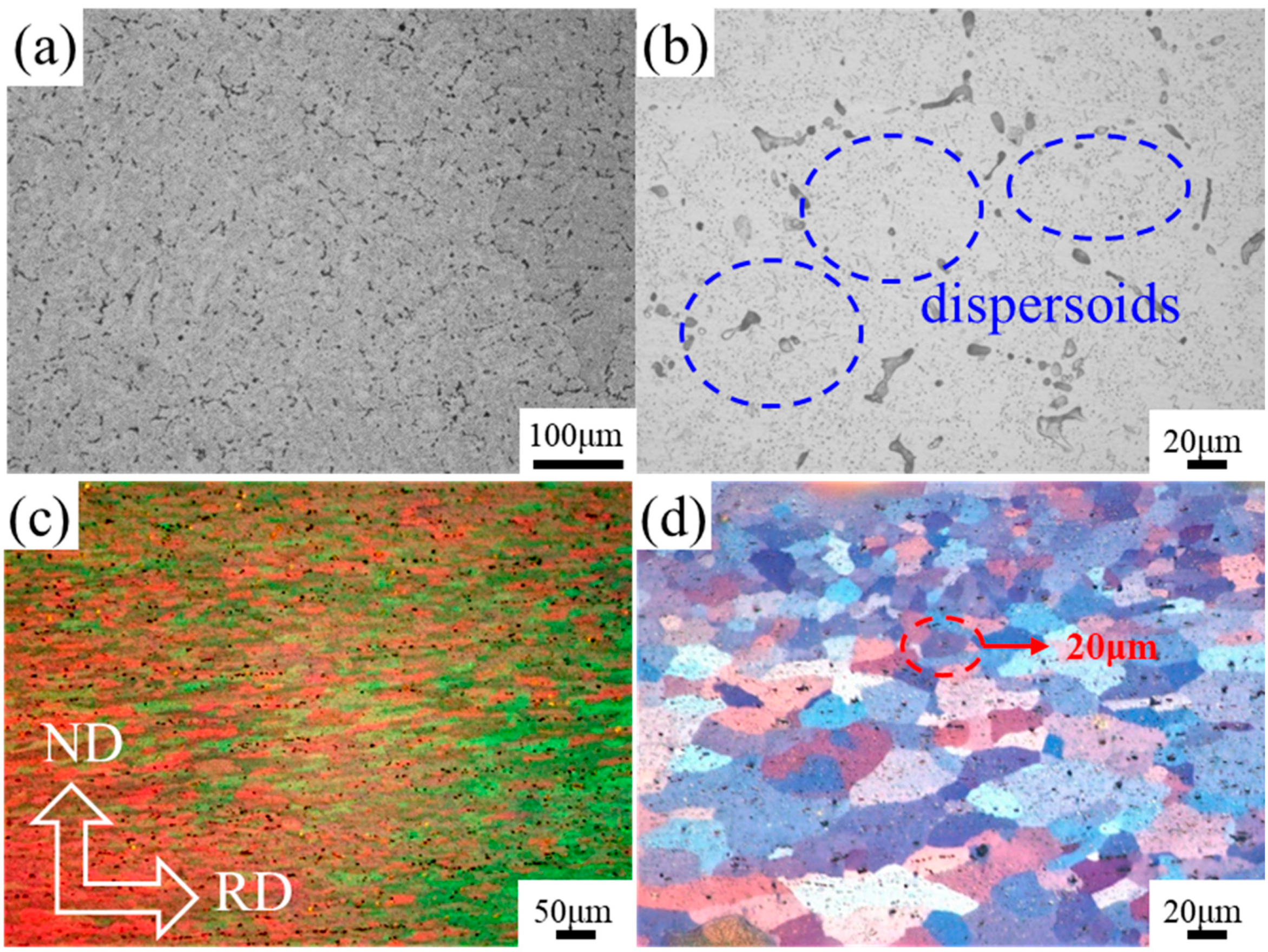
3.1. Evolution of the Microstructure during Processing
3.2. Evolution of the Mechanical Properties and Aging Precipitates
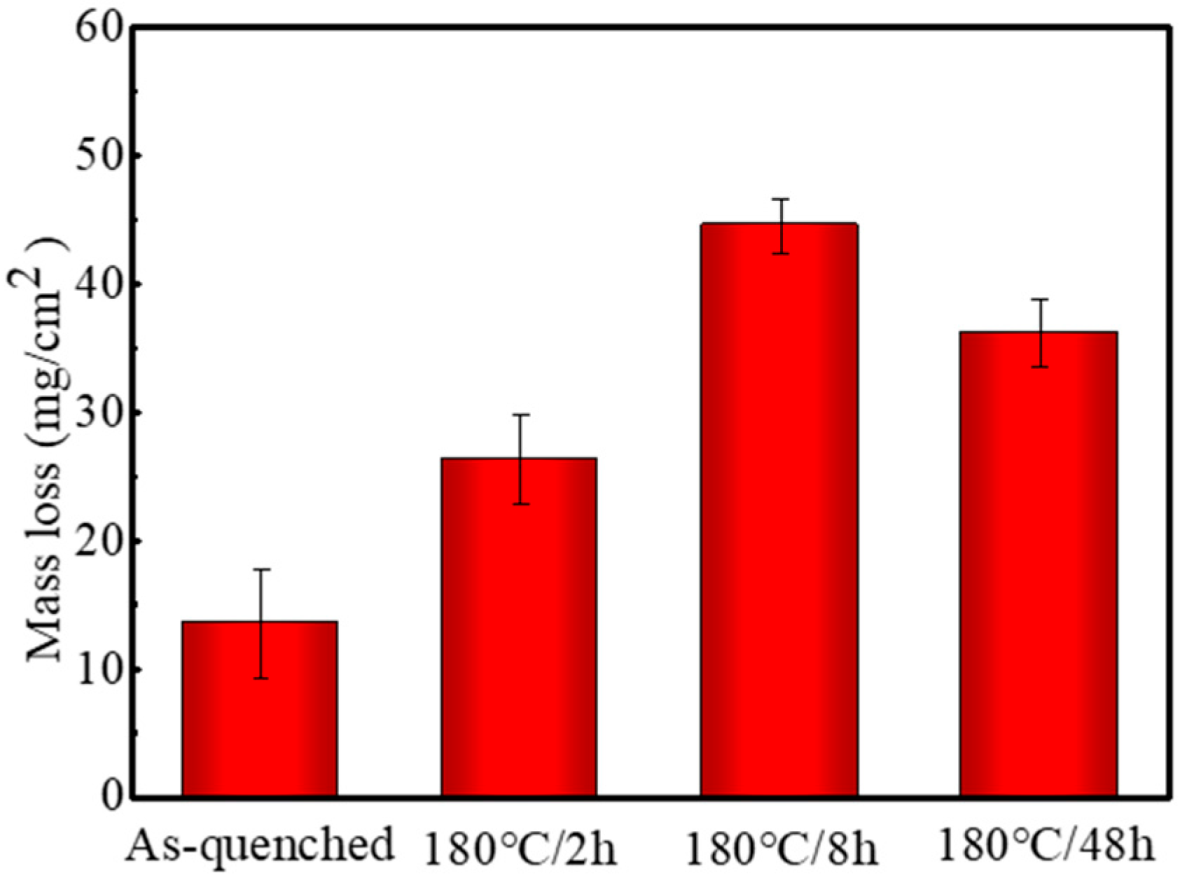
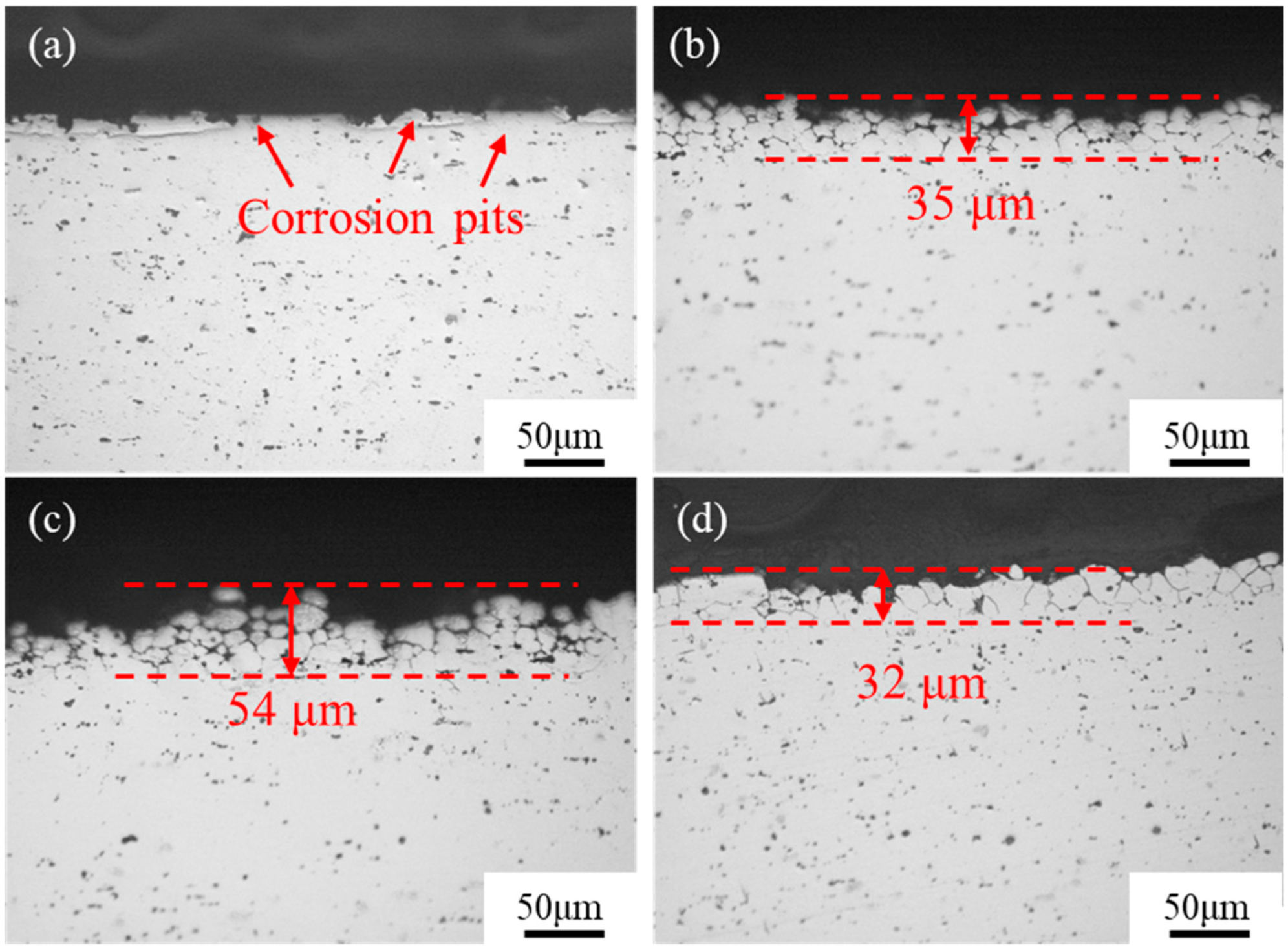
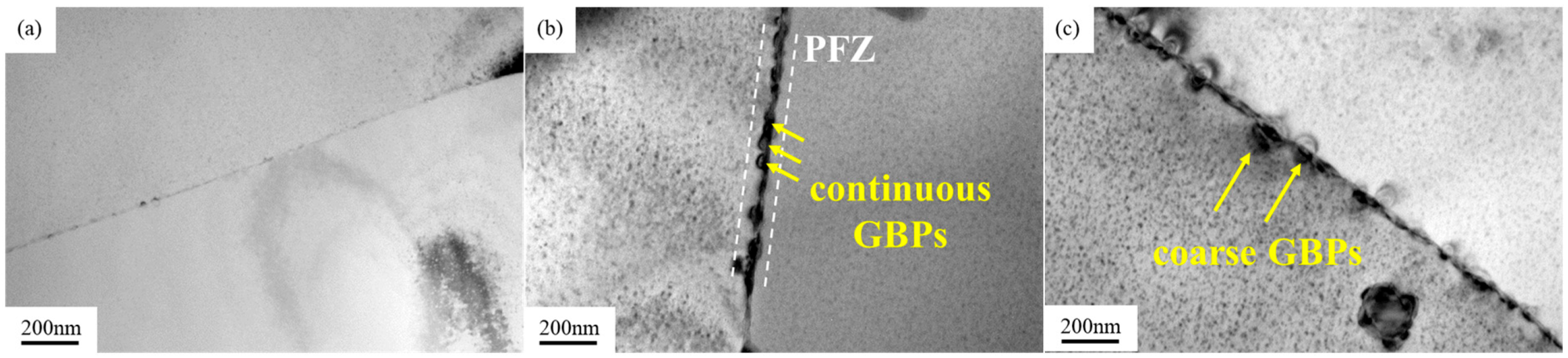
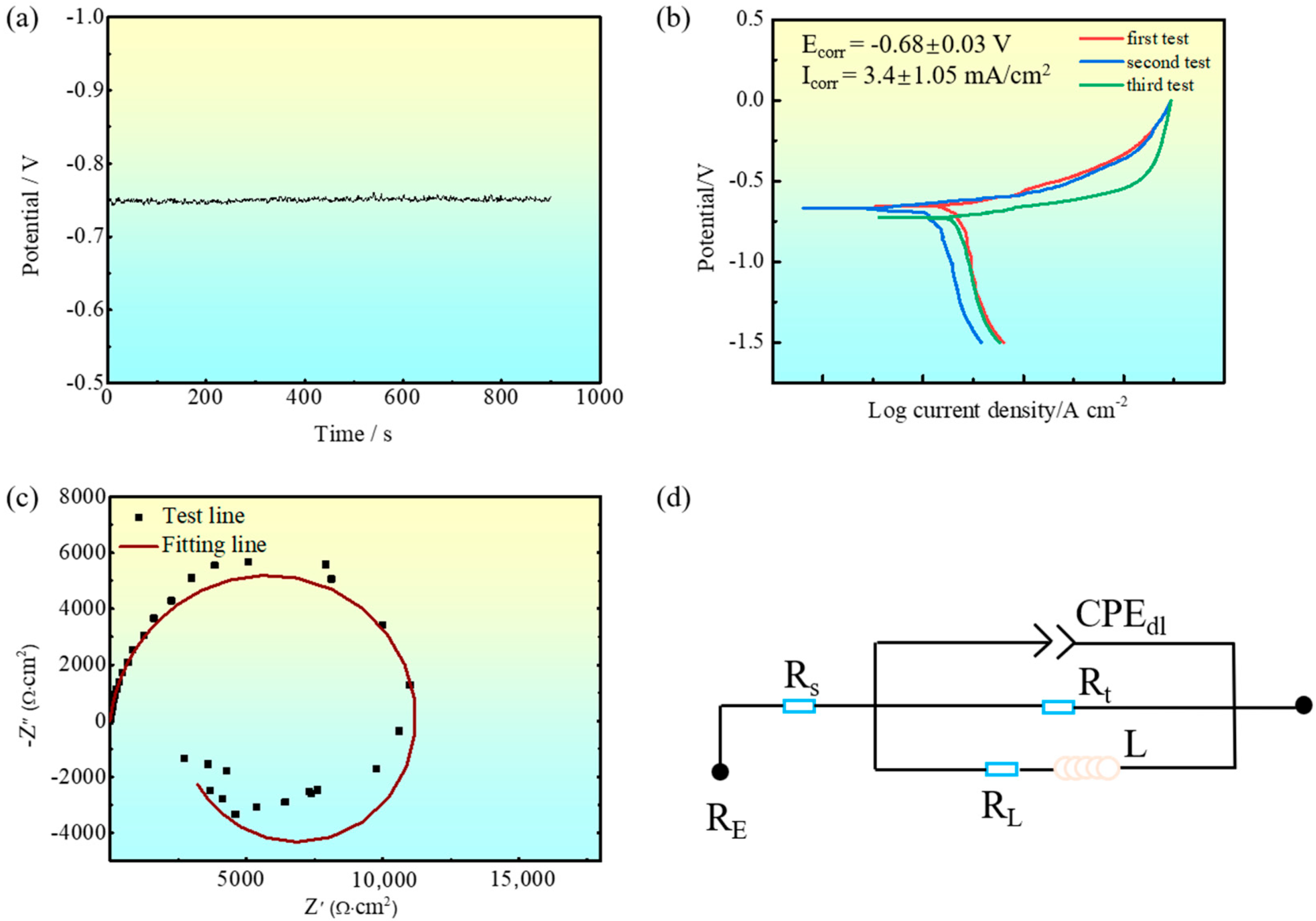
3.3. Evolution of the Intergranular Corrosion Behavior
4. Conclusions
- (1)
- The microstructure of the as-cast alloy consists of the Mg2Si phase and Mg32(Al, Ag)49 phase. For the as-rolled alloy, the grains present a fibrous stripe structure, which transforms to equiaxed grains after solution heat treatment.
- (2)
- During isothermal aging, numerous nanoscale particles are distributed in the matrix uniformly. The precipitates at the peak-aged stage have a BCC structure with the lattice constant of a = 0.33 nm and can be identified as MgAg phase.
- (3)
- The strength of the alloy increases greatly in the aging process. The UTS and YS of the peak-aged alloy are 342 MPa and 231 MPa, which increase by 18% and 57% to the as-quenched alloy, respectively. However, the peak-aged alloy presents a worse IGC resistance than that of the as-quenched alloy. The continuous distribution of GBPs and the appearance of PFZs are the main reasons for the deterioration of the IGC resistance of the alloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, L.; Li, X.; Wang, X.; Zhang, H.; Cui, J. Effects of homogenization on microstructures and properties of a new type Al-Mg-Mn-Zr-Ti-Er alloy. Mater. Sci. Eng. A 2010, 527, 7510–7518. [Google Scholar] [CrossRef]
- Mofarrehi, M.; Javidani, M.; Chen, X. Effect of Mn content on the hot deformation behavior and microstructure evolution of Al-Mg-Mn 5xxx alloys. Mater. Sci. Eng. A 2022, 845, 143217. [Google Scholar] [CrossRef]
- Li, M.; Pan, Q.; Shi, Y.; Wang, Y. Microstructure dependent fatigue crack growth in Al-Mg-Sc alloy. Mater. Sci. Eng. A 2014, 611, 142–151. [Google Scholar] [CrossRef]
- Yi, G.; Cullen, D.; Littrell, K.; Golumbfskie, W.; Sundberg, E.; Free, M. Characterization of Al-Mg alloy aged at low temperatures. Metall. Mater. Trans. A 2017, 48, 2040–2050. [Google Scholar] [CrossRef]
- Wu, X.; Chen, S.; Lai, B.; Hui, H.; Wen, S.; Gao, K.; Wang, W.; Nie, Z.; Zaluzec, N. Microstructure Characterization of Microalloyed 5xxx Aluminum Alloys with Er and Zr using Analytical Transmission Electron Microscopy and Synchrotron X-ray Fluorescence Microscopy. Microsc. Microanal. 2018, 24, 760–761. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, F.; Wang, S.; Ji, S. The development of low-temperature heat-treatable high-pressure die-cast Al-Mg-Fe-Mn alloys with Zn. J. Mater. Sci. 2021, 56, 11083–11097. [Google Scholar] [CrossRef]
- Mariusz, K.; Tański, T.; Snopiński, P.; Tomiczek, B. Structure and properties of aluminium-magnesium casting alloys after heat treatment. J. Therm. Anal. Calorim. 2017, 127, 299–308. [Google Scholar]
- Lee, B.; Kim, S.; Park, J.; Kim, H.; Lee, J. Role of Mg in simultaneously improving the strength and ductility of Al-Mg alloys. Mater. Sci. Eng. A 2016, 657, 115–122. [Google Scholar] [CrossRef]
- Huskins, E.; Cao, B.; Ramesh, K. Strengthening mechanisms in an Al-Mg alloy. Mater. Sci. Eng. A 2010, 527, 1292–1298. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.; Oberhauser, P.; Uggowitzer, P.; Pogatscher, S. Age-hardening response of AlMgZn alloys with Cu and Ag additions. Acta Mater. 2020, 195, 541–554. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.; Dumitraschkewitz, P.; Mendez-Martin, F.; Tosone, R.; Marchand, D.; Curtin, W.; Uggowitzer, P.; Pogatscher, S. Giant hardening response in AlMgZn(Cu) alloys. Acta Mater. 2021, 206, 116617. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.; Tosone, R.; Uggowitzer, P.; Pogatscher, S. On the potential of aluminum crossover alloys. Prog. Mater. Sci. 2022, 124, 100873. [Google Scholar] [CrossRef]
- Hou, S.; Liu, P.; Zhang, D.; Zhang, J.; Zhuang, L. Precipitation hardening behavior and microstructure evolution of Al-5.1Mg-0.15Cu alloy with 3.0Zn (wt%) addition. J. Mater. Sci. 2018, 53, 3846–3861. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, D.; Ding, Q.; Zhang, J.; Zhuang, L. Solute clustering and precipitation of Al-5.1Mg-0.15Cu-xZn alloy. Mater. Sci. Eng. A 2019, 759, 465–478. [Google Scholar] [CrossRef]
- Ji, S.; Yang, W.; Gao, F.; Watson, D.; Fan, Z. Effect of iron on the microstructure and mechanical property of Al-Mg-Si-Mn and Al-Mg-Si diecast alloys. Mater. Sci. Eng. A 2013, 564, 130–139. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, D.; Liu, H.; Zhuang, L.; Zhang, J. Precipitation hardening and intergranular corrosion behavior of novel Al-Mg-Zn(-Cu) alloys. J. Alloys Compd. 2021, 853, 157199. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, H.; Wu, Z.; Shen, X.; Wang, P.; Li, B.; Cui, J.; Nagaumi, H. An atomic-resolution investigation of precipitation evolution in Al-Mg-Ag alloys. Mater. Lett. 2019, 248, 231–235. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, H.; Wu, Z.; Wang, D.; Li, B.; Cui, J. Effects of Ag on the age hardening response and intergranular corrosion resistance of Al-Mg alloys. Mater. Charact. 2019, 147, 84–92. [Google Scholar] [CrossRef]
- Djema, O.; Bouabdallah, M.; Badji, R.; Saadi, A.; Kherrouba, N.; Sahli, A. Isothermal and non-isothermal precipitation kinetics in Al-Mg-Si-(Ag) alloy. Mater. Chem. Phys. 2020, 240, 122073. [Google Scholar] [CrossRef]
- Weng, Y.; Jia, Z.; Ding, L.; Pan, Y.; Liu, Y.; Liu, Q. Effect of Ag and Cu additions on natural aging and precipitation hardening behavior in Al-Mg-Si alloys. J. Alloys Compd. 2017, 695, 2444–2452. [Google Scholar] [CrossRef]
- ASTM G67-04; Standard Test Method for Determining the Susceptibility to Intergranular Corrosion of 5xxx Series Aluminum Alloys by Mass Loss after Exposure to Nitric Acid (NAMLT Test). ASTM: West Conshohocken, PA, USA, 2004.
- Chen, J.; Cheng, X.; Ding, L.; Weng, Y.; Yin, J.; Yao, H.; Yu, H. Effect of multi-stage aging on the precipitation strengthening and mechanical properties for an Al-Mg-Si-Ag alloy. Mater. Charact. 2022, 190, 112004. [Google Scholar] [CrossRef]
- Edwards, G.; Stiller, K.; Dunlop, G.; Couper, M. The precipitation sequence in Al-Mg-Si alloys. Acta. Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Weng, Y.; Jia, Z.; Ding, L.; Liu, M.; Wu, X.; Liu, Q. Combined effect of pre-aging and Ag/Cu addition on the natural aging and bake hardening in Al-Mg-Si alloys. Prog. Nat. Sci. Mater. 2018, 28, 363–370. [Google Scholar] [CrossRef]
- Pirhayati, P.; Jamshidi Aval, H.; Loureiro, A. Characterization of microstructure, corrosion, and tribological properties of a multilayered friction surfaced Al-Mg-Si-Ag alloy. Arch. Civ. Mech. Eng. 2022, 22, 176. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, D.; Pan, Y.; Ding, Q.; Long, W.; Zhang, J.; Zhuang, L. Dependence of microstructure, mechanical properties, and inter-granular corrosion behavior of Al-5.1Mg-3.0Zn-0.15Cu alloys with high temperature pre-treatment. Mater. Charact. 2020, 168, 110512. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, D.; Zhuang, L.; Zhang, J. Intergranular corrosion resistance of Zn modified 5xxx series Al alloy during retrogression and re-aging treatment. Mater. Charact. 2018, 144, 264–273. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, H.; Li, S.; Chen, R.; Nan, Y.; Li, L.; Wang, P.; Li, B.; Cui, J.; Nagaumi, H. Evolution of microstructure, mechanical properties and corrosion behavior of Al-4Mg-2Zn-0.3Ag (wt.%) alloy processed by T6 or thermomechanical treatment. Corros. Sci. 2021, 188, 109511. [Google Scholar] [CrossRef]
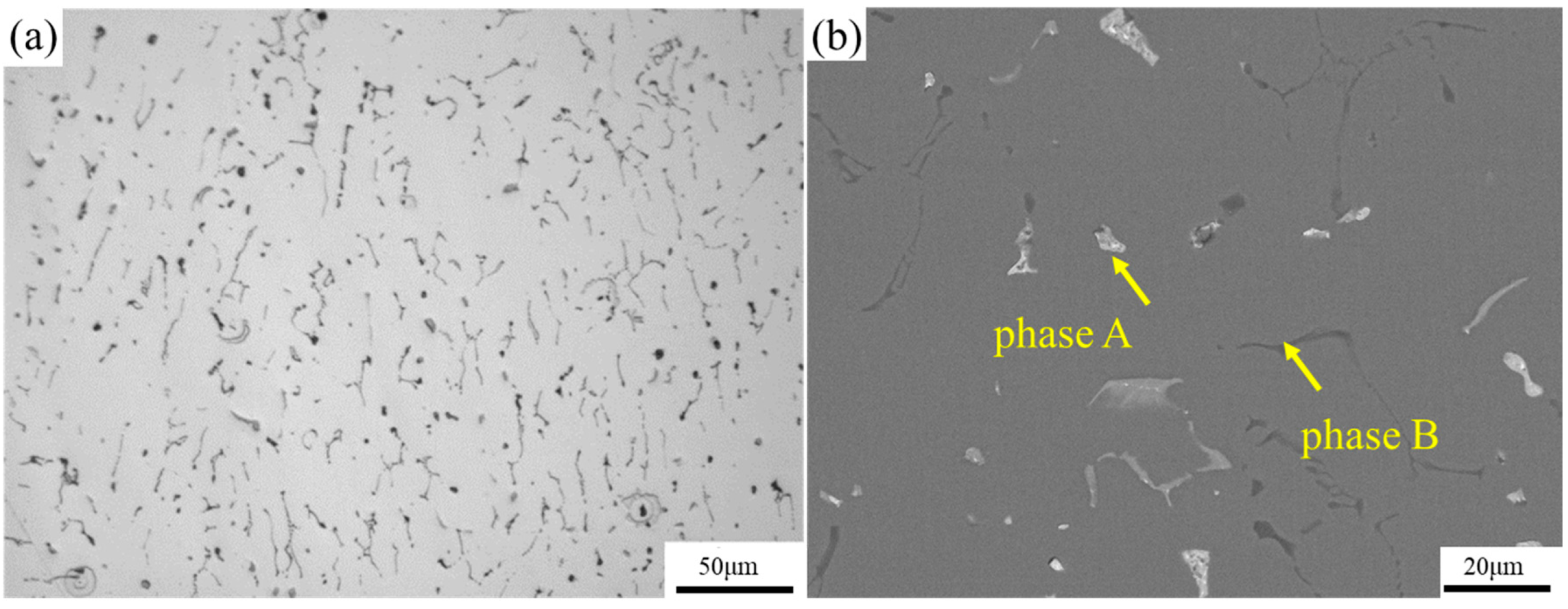





| Phase | Mg | Al | Si | Cr | Mn | Fe | Zr | Ag |
|---|---|---|---|---|---|---|---|---|
| A | 3.31 | 36.33 | 1.48 | 0.06 | 0.07 | 0.12 | 0.11 | 58.52 |
| B | 9.29 | 69.62 | 19.86 | 0.01 | 0.51 | 0.15 | 0.00 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Wu, Y.; Wang, H.; Guo, C. Investigation of Evolution of Microstructure, Mechanical Properties, and Corrosion Resistance of Novel Al-Mg-Si-Ag Alloy. Coatings 2023, 13, 2012. https://doi.org/10.3390/coatings13122012
Liu T, Wu Y, Wang H, Guo C. Investigation of Evolution of Microstructure, Mechanical Properties, and Corrosion Resistance of Novel Al-Mg-Si-Ag Alloy. Coatings. 2023; 13(12):2012. https://doi.org/10.3390/coatings13122012
Chicago/Turabian StyleLiu, Tao, Yanli Wu, Huan Wang, and Cheng Guo. 2023. "Investigation of Evolution of Microstructure, Mechanical Properties, and Corrosion Resistance of Novel Al-Mg-Si-Ag Alloy" Coatings 13, no. 12: 2012. https://doi.org/10.3390/coatings13122012
APA StyleLiu, T., Wu, Y., Wang, H., & Guo, C. (2023). Investigation of Evolution of Microstructure, Mechanical Properties, and Corrosion Resistance of Novel Al-Mg-Si-Ag Alloy. Coatings, 13(12), 2012. https://doi.org/10.3390/coatings13122012




