Abstract
Calcium silicate hydrate (CSH) plays a crucial role in concrete by controlling its properties and durability. The degradation of CSH often signifies concrete damage. Polydimethylsiloxane (PDMS) is commonly used to protect concrete from sulfate corrosion; however, the comprehensive mechanistic understanding of its protective effects against CSH remains limited. Here, molecular dynamics (MD) simulations were employed to explore atomic-scale interactions between PDMS coatings and CSH in a sulfate-rich environment. Our results reveal that PDMS mitigates sulfate-induced CSH decalcification by forming a positively charged layer, ultimately reducing sulfate bonding by 83.3% compared to the blank group. Molecular structure analysis highlights key hydrogen bonding and calcium–oxygen bonding interactions that are critical for this protection. Higher polymerization stabilizes substrate adsorption, reducing surface diffusion to 33.3% of low-polymerization PDMS, thereby enhancing protection. Additionally, water molecule interactions with the CSH matrix are negatively correlated with the amount of adsorbed sulfate. Simulation results offer valuable insights into the molecular-level dynamic response of the material, contributing to a deeper understanding of the protective mechanisms of PDMS against sulfate-induced CSH degradation in concrete. These findings can guide experimenters and engineers in designing more effective protective coatings for concrete exposed to sulfate-rich environments, thereby laying a foundation for further experimental research and the development of concrete materials with enhanced durability under challenging environmental conditions.
1. Introduction
Concrete is a widely utilized construction material known for its cost-effectiveness, longevity, and durability. However, its porous microstructure and hydrophilic properties make it susceptible to chemical interactions with the surrounding solution environment. This contact is a significant factor contributing to the deterioration of concrete structures [1,2]. In particular, the detection of sulfate erosion at an early stage is challenging in harsh and intricate conditions such as saline soils, marine environments, and subsurface sewage. Sulfate attack initiates gradually and subsequently reacts with hydrates and other cement phases, leading to the formation of expansive corrosion products such as gypsum. This expansion applies pressure on the surrounding matrix, causing extensive concrete fragmentation and posing a prevalent and severe chemical erosion issue in modern times [3,4,5,6].
In recent years, the challenge of mitigating sulfate attack on concrete has garnered significant attention, with a primary focus on enhancing concrete durability [7,8,9,10]. This phenomenon has been closely linked to the intricate transport of water and solutions within concrete, as extensively reported in the literature [11,12,13]. Guided by this advanced insight, the safeguarding of concrete has been effectively stratified into two fundamental domains: modification [14,15] and surface treatment [16,17]. Concrete modification treatments are designed to alter the composition, structure, or characteristics of concrete through techniques such as the addition of admixtures, utilization of specialized formulations, incorporation of chemical additives, or reinforcement with fibers [18,19,20]. These approaches are undertaken to enhance specific properties or fulfill distinct engineering requirements [21,22]. Nonetheless, despite the array of proposed concrete modification methods, their practical application can be restricted by factors including construction feasibility, cost considerations, and long-term performance stability [23,24]. In contrast, surface treatment methods offer a more adaptable and practical avenue for tackling the challenge posed by sulfate attack [25,26,27]. Polydimethylsiloxane (PDMS), lauded for its remarkable hydrophobic properties and unmatched chemical stability, has emerged as a beacon of innovation in the realm of surface protection materials, with its potential applications transcending traditional boundaries. In the context of concrete materials, the exceptional water repellency and enduring structural integrity offered by PDMS present an unparalleled opportunity to revolutionize conventional construction practices [28,29]. Recent developments involving PDMS-based coatings have exhibited notable resistance against various degradation mechanisms, encompassing corrosion, fouling, and chemical attack [30,31]. For instance, Lu et al. [32] successfully formulated a high-performance polymer cement-based coating by innocuously introducing PDMS, which remarkably enhanced hydrophobicity, UV-aging resistance, and resistance to saltwater immersion while maintaining favorable physical properties. Similarly, Gao et al. [33] employed PDMS as a surface functionalizer to prepare an innovative hydrophobic agent, resulting in significant improvements in surface cracking, strength loss, and structural stability of mortar subjected to dry and wet cyclic sulfate attack.
PDMS presents notable advantages, encompassing exceptional flexibility, elasticity, and chemical inertness, making it well-suited for diverse applications. Moreover, its ease of application and room-temperature curing process enhance its appropriateness for our specific requirements [34,35]. In the realm of our research, these properties collectively position PDMS as a superior choice when compared to epoxy coatings or paints [36,37]. It is worth noting that while previous research has demonstrated the efficacy of PDMS coatings in mitigating sulfate-induced concrete degradation, it is crucial to acknowledge that this protective mechanism predominantly operates at the nanoscale level. In recent years, MD simulations have emerged as a valuable tool for investigating the interactions and kinetics of cement hydrates at the nanoscale [38]. CSH holds particular significance among the hydration products of concrete, as it governs the macroscopic properties of concrete materials [39]. In a study by Hou et al. [40], MD simulations were employed to unravel the degradation principles of water and ions concerning the bonding properties and mechanical response at the epoxy/CSH interface. The findings revealed that the presence of aggressive ions exacerbated the detachment between the epoxy and CSH. In another investigation, Cao et al. [41] combined experimental analysis with MD simulations to explore the erosion mechanism of sulfate in CSH. Their simulations were able to dynamically capture the migration and adsorption of sulfate in CSH nanopores, highlighting the substantial decalcification induced by sulfate. The results obtained from the simulations aligned well with the X-Ray diffraction (XRD) hydration product data, providing substantial support for the reliability of molecular dynamics simulations in elucidating the CSH erosion process.
The current understanding of the intricate nanoscale protective mechanism of PDMS against CSH in the challenging presence of sulfate attacks remains notably limited, prompting a pioneering exploration at the forefront of materials research [42,43]. Moreover, the impact of the degree of polymerization of PDMS on its intrinsic protective properties has thus far remained a compelling enigma within the scientific community. Defined as the pivotal determinant of the number of monomer units within the polymer chain, the degree of polymerization intricately governs not only the molecular weight but also the distinctive physical characteristics and structural attributes of the polymer matrix [44,45]. Therefore, this study aims to investigate the atomic-scale interaction between PDMS coatings and CSH in sulfate-rich conditions. To achieve this, PDMS models with varying degrees of polymerization were constructed, allowing the nano-level protective behavior of the coatings against CSH in sulfate environments to be examined. Additionally, we explored the influence of different degrees of polymerization on the performance of PDMS as a protective barrier. The outcomes of this research offer valuable insights into the design of multifunctional PDMS coating systems, enhancing their resistance to sulfate-induced degradation and paving the way for sustainable applications in infrastructure materials.
2. Computational Methods
2.1. Modeling Details
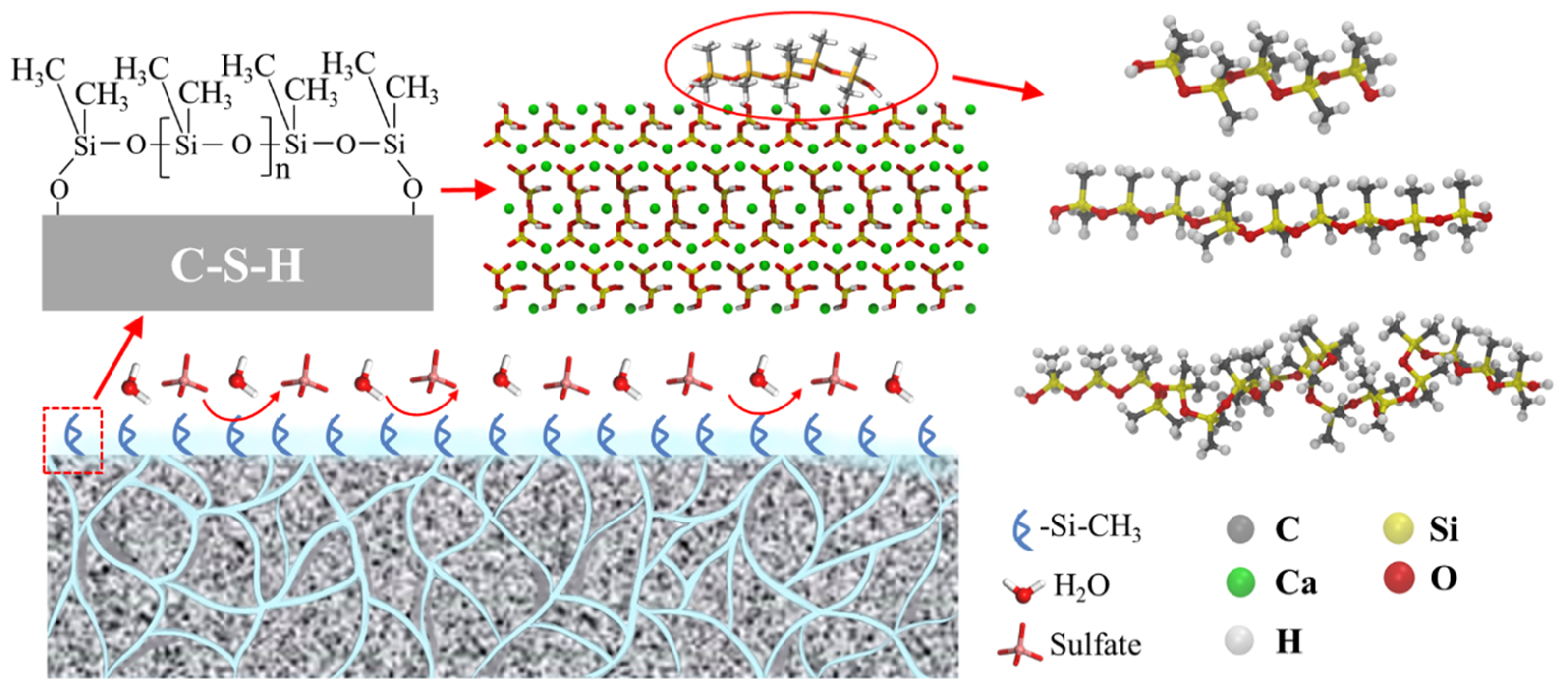
To gain insights into the sulfate resistance of the CSH matrix following the application of a PDMS coating in a sulfate-rich environment, our study leveraged molecular dynamics simulations. Prior research, notably by Wang et al. [46], has substantiated the effective binding of PDMS to cement particles through Si–OH interactions as observed through infrared spectral analysis. Furthermore, the seminal work of Emel Yilgör et al. [47] highlighted the influential role played by the main chain composition (Si-O-Si) of organosilicon polymers in shaping their physicochemical attributes. Notably, it was postulated that high-molecular-weight PDMS might curtail flexibility, particularly at exceedingly low temperatures. In light of these foundational findings, our investigation focused on hydroxylated PDMS variants featuring polymerization degrees of 5, 10, and 20. This meticulous selection allowed us to probe and delineate the protective mechanisms employed by PDMS with varying polymerization degrees against potential matrix degradation. Our approach harnessed molecular dynamics simulations to unravel the intricate interplay between PDMS coatings and the CSH matrix in the face of sulfate exposure. The CSH matrix employed in our simulations followed the tobermorite structure optimized by Pellenq et al. [48,49,50], which has been established as a realistic molecular model for cement hydrates. The specific conformation of CSH was derived by processing an 11 Å tobermorite structure into a three-dimensional configuration with dimensions x = 55.94 Å, y = 44.48 Å, and z = 39.25 Å. To closely mimic experimental conditions, we selected a sulfate concentration of 5%, similar to the study conducted by Yao et al. [51] The water density was set at 1 g/cm3. The entire sulfate erosion solution consisted of 4000 water molecules, 26 sulfate ions, and 52 sodium ions. We employed Packmol (v20.14.3) software [52,53,54] to construct the simulation box, resulting in dimensions of 55.94 Å × 44.48 Å × 200 Å. The extension of 200 Å in the z-direction was implemented to mitigate the effects of periodic boundary conditions applied across the entire simulation in all dimensions. To eliminate interactions resulting from periodic boundaries, we extended the system in the z-direction and added a vacuum layer. Figure 1 illustrates the schematic diagram of the final model used throughout the simulation.
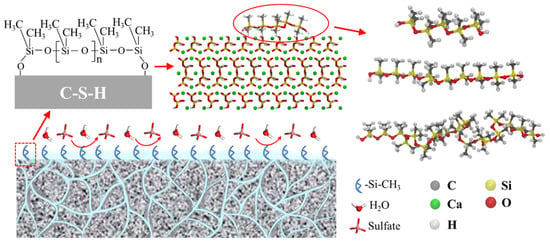
Figure 1.
The schematic diagram of the PDMS/CSH modeling process and its efficacy in protecting the sulfate process.
2.2. Simulation Details
The parameters for the PDMS polymer in the OPLS force field were established based on Ali Ahmad et al.’s investigation [55] of the agglomeration phenomenon of organic molecules during their adsorption and diffusion within cross-linked PDMS membranes. Furthermore, the interactions between PDMS molecules and sulfates were modeled using the optimized potential for liquid-simulated all-atom (OPLS-AA) force field. On the other hand, the CSH matrix was described using the CSH force field, which was derived from the ClayFF force field [56,57,58]. For water molecules present in the entire simulated system, we adopted the SPC model parameters [59,60,61].
All MD simulations were performed using LAMMPS (29Sep2021) software [62,63,64]. The canonical ensemble (NVT) with a simulated temperature of 298 K was employed for all simulation processes [65,66,67,68,69].
The simulation process can be divided into two stages: the pre-adsorption stage and the protection stage. In practical experiments, coatings are typically treated before the matrix is put into service in order to ensure maximum coating protection. Hence, prior to simulating sulfate attack, the PDMS coating on the CSH matrix was pre-treated with four PDMS polymers with a polymerization degree of 5, two PDMS polymers with a polymerization degree of 10, and one PDMS polymer with a polymerization degree of 20. The duration of the adsorption phase was set to 2 ns. After achieving equilibrium, the system was exposed to a sulfate solution; the duration of the sulfate attack simulation was 3 ns. A time step of 1 fs was utilized, and atomic information was sampled every 1000 fs to enable realistic and reliable data for structural dynamic analysis. Snapshots of the simulated trajectories throughout the entire simulation process were generated using VMD (version 1.9.4a53) software [70,71].
3. Results and Discussion
3.1. Microscopic Origin of Sulfate Adsorption Trends
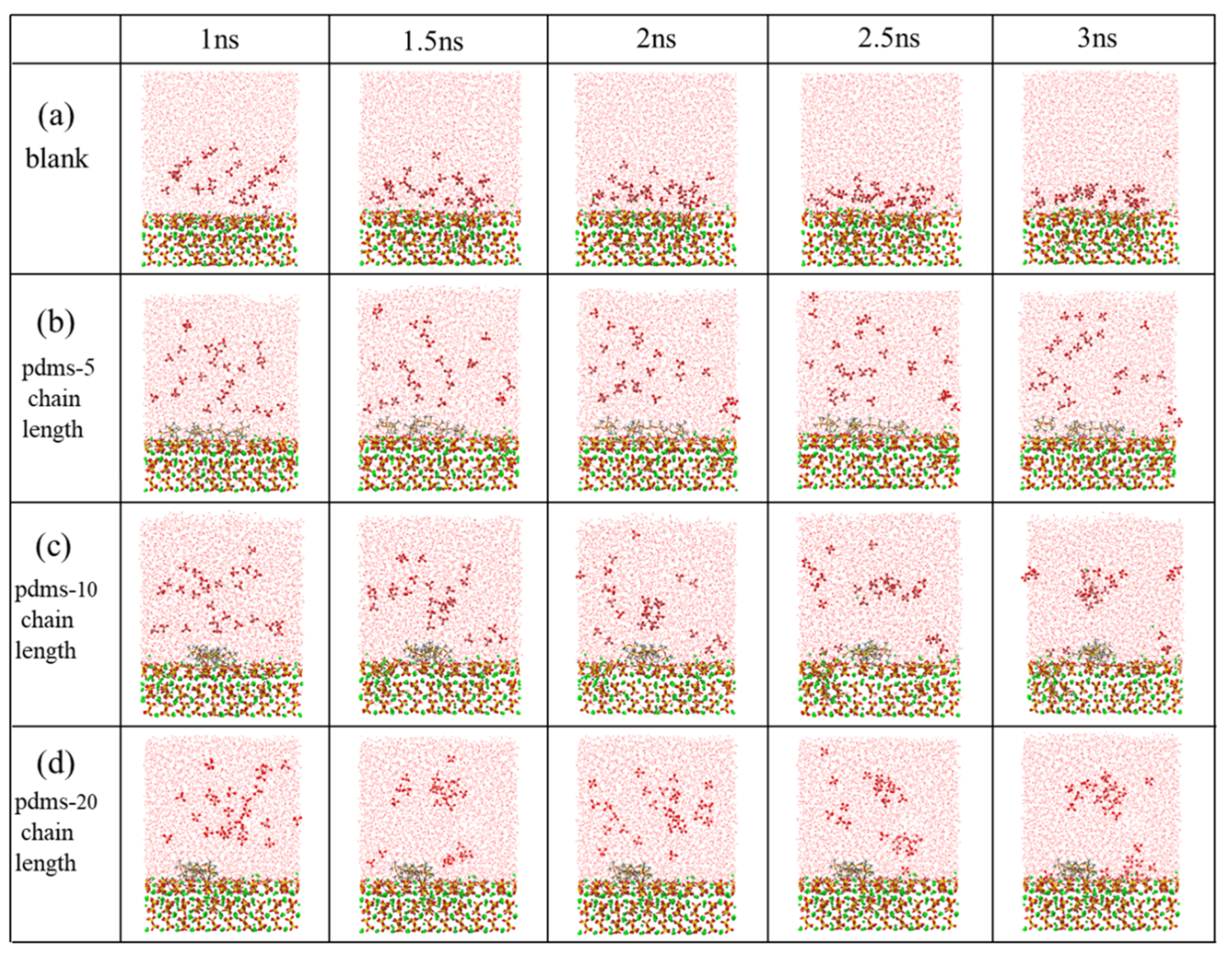
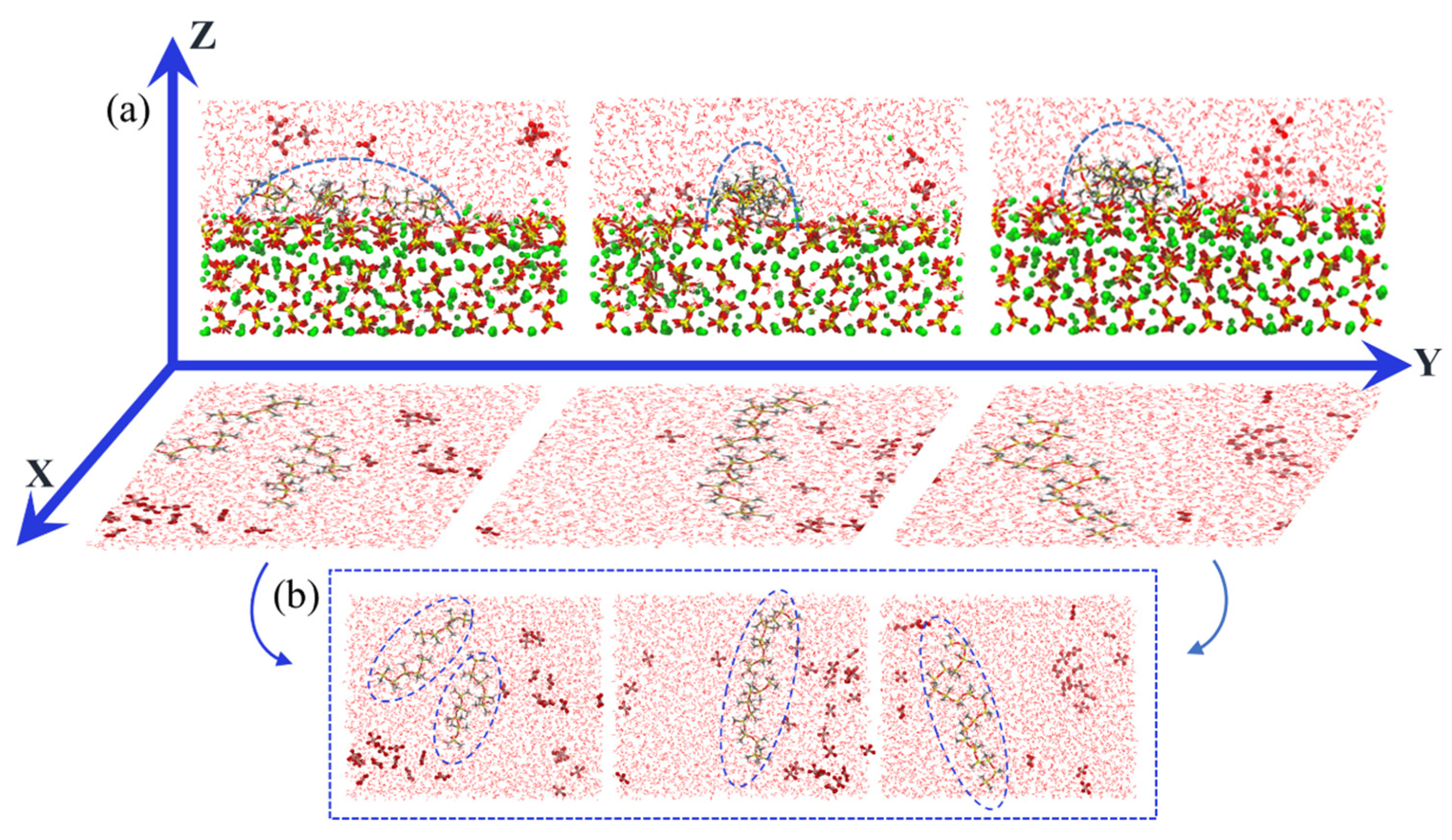
In this study, we assessed the adsorption erosion behavior of sulfate at the interface between the CSH matrix and PDMS coatings with varying polymerization degrees (polymerization degrees of 0, 5, 10, and 20). Figure 2 illustrates snapshots of the system dynamics at different time points (1 ns, 1.5 ns, 2 ns, 2.5 ns, and 3 ns) during the sustained sulfate erosion period. Notably, there were significant differences in the kinetic behavior of sulfate between the PDMS-coated and uncoated groups. In the uncoated group, a majority of sulfates gradually adsorbed onto the CSH surface, resulting in calcium leaching. However, in the PDMS-coated group, we observed that sulfates did not desorb from PDMS. Instead, they tended to adsorb in the regions where PDMS was absent, leading to decalcification in those areas. This observation suggests that the inclusion of PDMS in the system partially impedes the sulfate adsorption process, indicating its protective properties against the matrix.
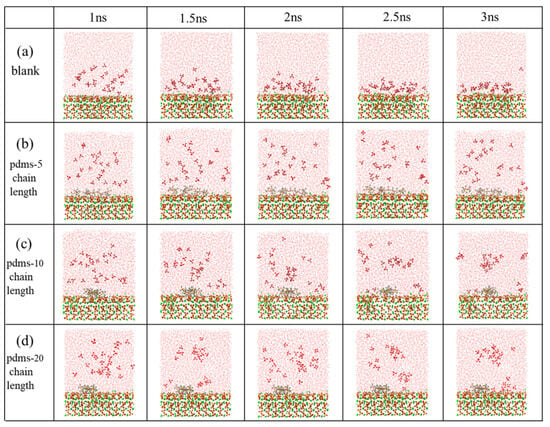
Figure 2.
The adsorption behavior of sulfate on the CSH matrix with PDMS was investigated throughout the simulation considering four different scenarios: (a) blank group, (b) PDMS with a polymerization degree of 5, (c) PDMS with a polymerization degree of 10, and (d) PDMS with a polymerization degree of 20. (Red, yellow, green, white, pink and gray represent O, Si, Ca, H, S and C atoms, respectively).
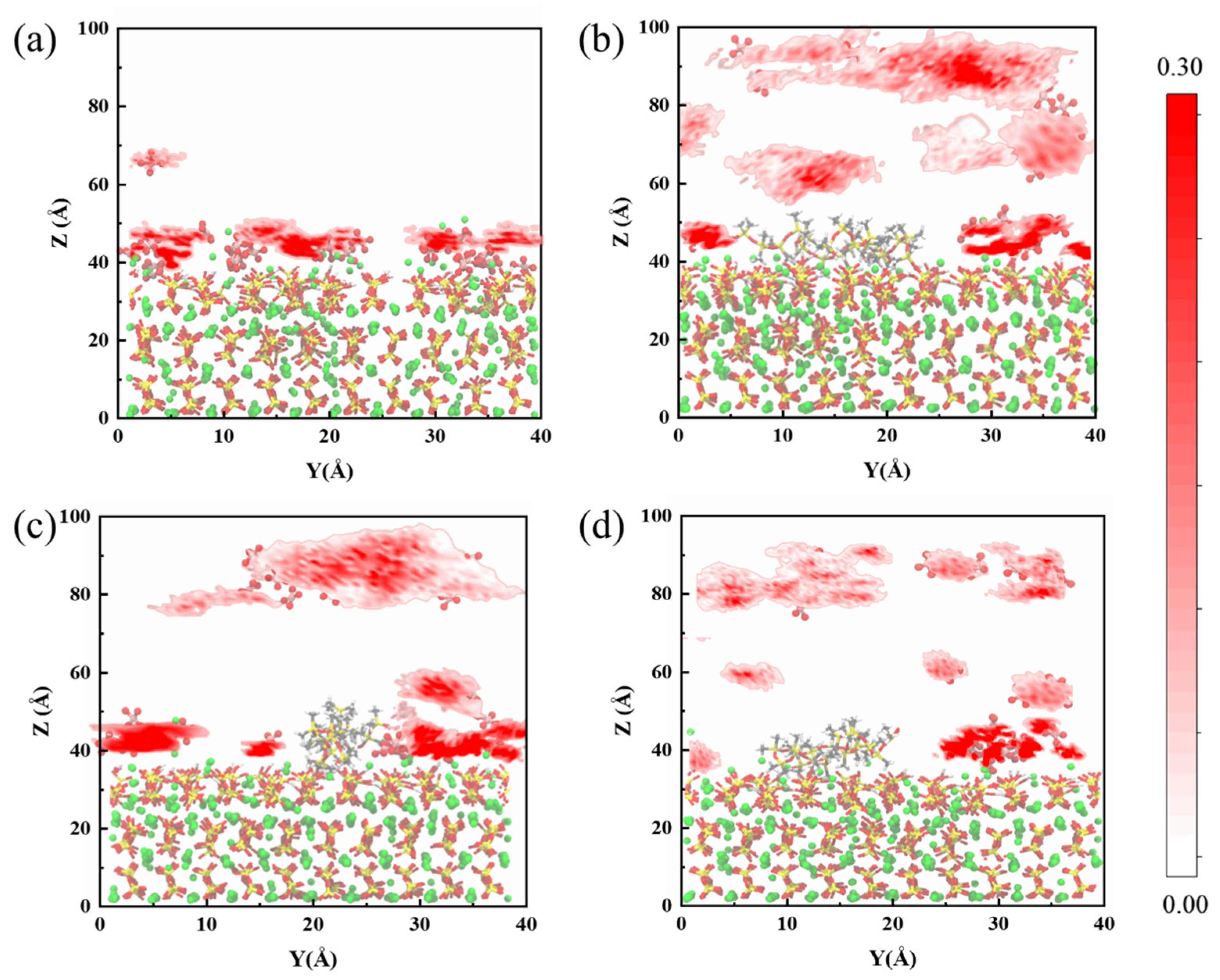
In order to quantitatively analyze the adsorption behavior of sulfate at the CSH interface, we examined the two-dimensional density profiles of sulfate in the Y-direction within the spatial domain, as depicted in Figure 3. Initially, as previously mentioned, in the uncoated group the sulfate remains predominantly adsorbed on the CSH surface at the conclusion of the erosion period, exhibiting limited desorption. This phenomenon results in a region of high sulfate density within the range of 40 Å to 50 Å, which can be considered the primary location of sulfate erosion on the CSH matrix. However, upon the introduction of PDMS, a significant portion of sulfate gradually starts to move away from the CSH surface. Consequently, the high-density region becomes more dispersed and less compact at this stage. Furthermore, we observed that the remaining sulfate on the CSH surface is primarily located in the uncoated PDMS region. The quantity of sulfate adsorbed on the CSH surface is correlated with the positioning of the PDMS coating, suggesting that the manner and extent of PDMS coverage may determine the amount of sulfate adsorption during this process. This observation highlights the importance of the interaction between PDMS and CSH, which is discussed in more detail in Section 3.2.
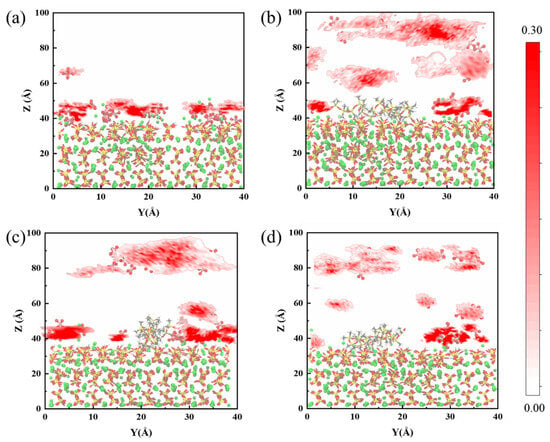
Figure 3.
Schematic diagram illustrating the two-dimensional density profiles of sulfate in the CSH matrix for the blank group and PDMS systems with varying polymerization degrees: (a) blank group, (b) PDMS with a polymerization degree of 5, (c) PDMS with a polymerization degree of 10, and (d) PDMS with a polymerization degree of 20.
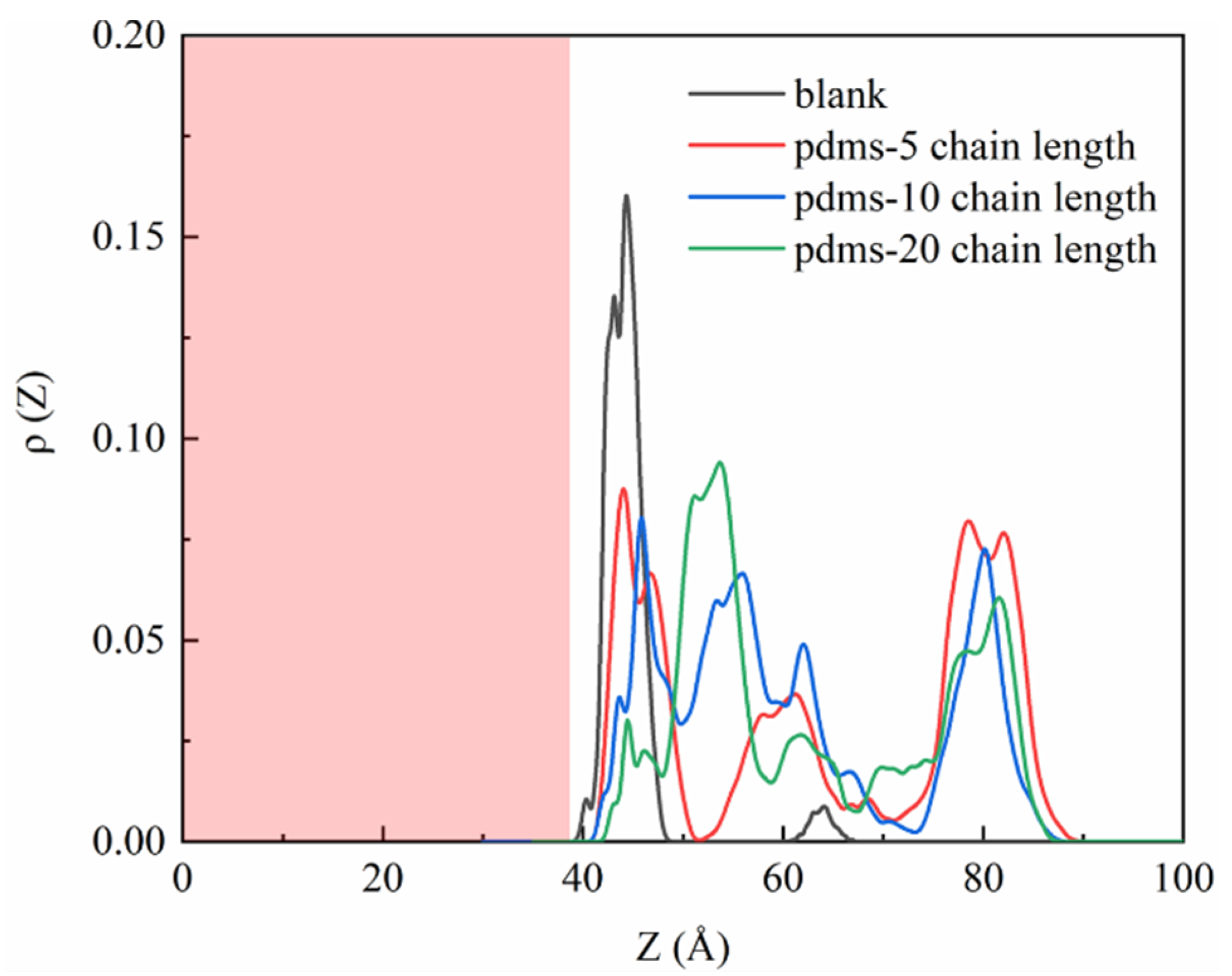
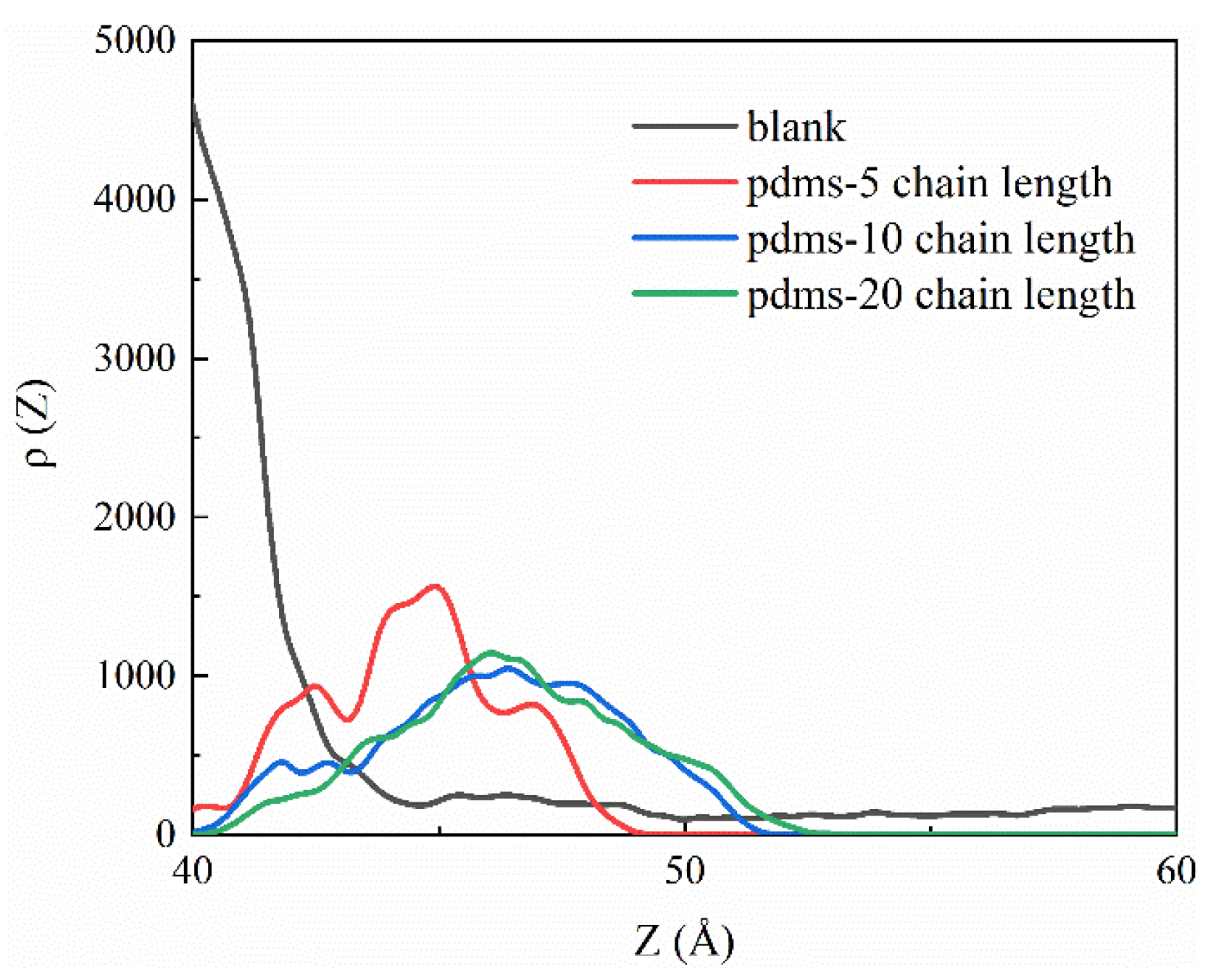
To further analyze the sulfate density profiles, we present the one-dimensional data visualization in Figure 4. The CSH interface line is positioned at approximately 38.96 Å, indicating that the red region corresponds to the matrix component. In the uncoated group, sulfate predominantly accumulates around 42.5 Å, indicated by a prominent peak with a height of 0.16. Only a small fraction is observed at 65 Å, suggesting a strong interaction between sulfate and the CSH matrix. Upon the incorporation of PDMS with varying polymerization degrees, a decrease in the sulfate accumulation peak is observed, although there is a remaining fraction present at 42 Å, corresponding to sulfate adsorbed in the uncoated PDMS region, that aligns with our simulated snapshots. Notably, when the polymerization degree is 20, a significant portion of the sulfate is located far from the CSH surface, indicating that a larger coating area is formed and that the protective effect is enhanced with the increasing polymerization degree.
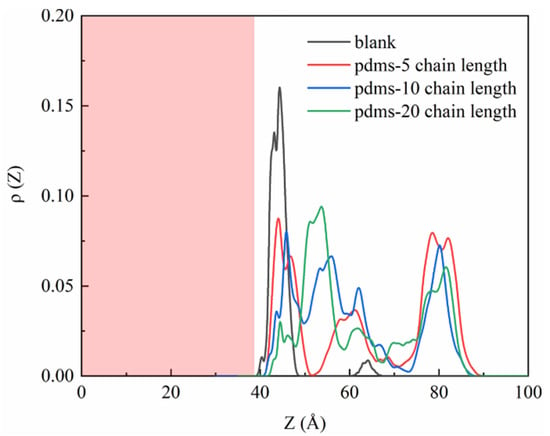
Figure 4.
One-dimensional density profile curves depicting the sulfate profiles in the blank group and PDMS systems with varying polymerization degrees.
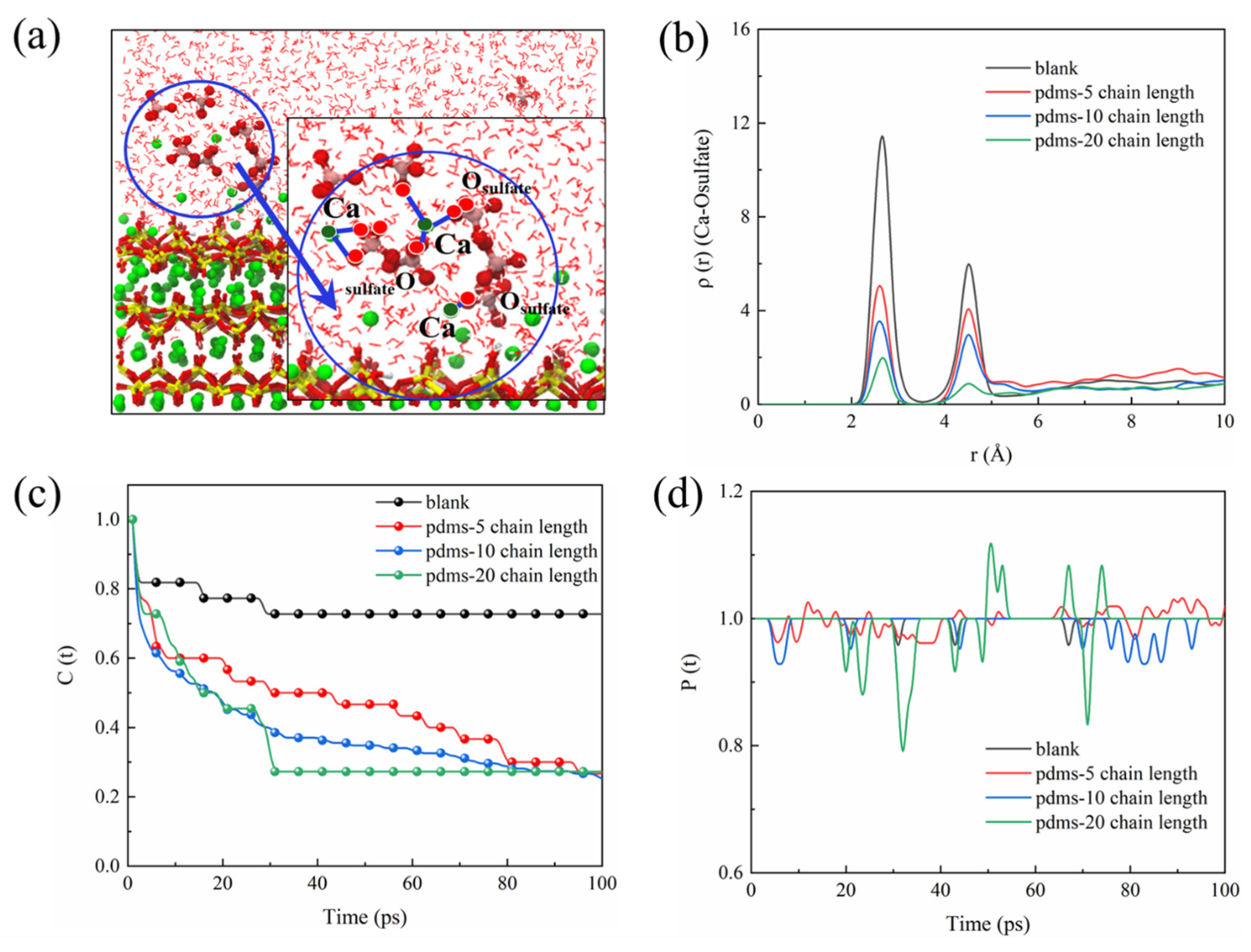
We employed a set of rigorous analyses to gain a comprehensive understanding of the interaction between sulfate and the CSH matrix, including the radial density function (RDF), time correlation function (TCF), and bond time evolution (BTE). The RDF was employed to offer qualitative insights into the spatial distribution of sulfate ions within the CSH matrix. RDF analysis allowed us to discern the density of sulfate ions at varying distances from the CSH matrix, thereby shedding light on the erosion mechanism [72]. To assess the stability of bonds formed between sulfate ions and the CSH matrix, we employed the TCF. A TCF value closer to 1 indicates a more stable bonding interaction, while a value closer to 0 suggests frequent bond breakage. The TCF analysis provides a dynamic perspective on the temporal evolution of bonding interactions [73]. Furthermore, BTE analysis was conducted to capture both bond breaking and re-bonding events. The P(t) parameter in the BTE analysis denotes the ratio of the number of bonds at time t to the initial number of bonds. A P(t) > 1 indicates the formation of new bonds, while P(t) < 1 indicates bond breakage. The BTE analysis allowed us to quantify the dynamic nature of the sulfate–CSH matrix interactions over time [74]. Due to the formation of gypsum during sulfate erosion, the Ca–O bond is presumed to play a significant role in sulfate adsorption. Figure 5a illustrates calcium leaching and sulfate agglomeration, suggesting that the calcium–sulfate interaction in CSH is a crucial factor in sulfate adsorption. Analyzing the RDF (Figure 5b), the blank group exhibits a prominent peak at 2.7 Å for Ca-Osulfate, indicating a bonding interaction between calcium and sulfate. However, with the introduction of PDMS, the peak at 2.7 Å diminishes as the polymerization degree increases, suggesting a weakened interaction between sulfate and CSH in the presence of PDMS coating. TCF and BTE analysis of the Ca-Osulfate bond in the blank group (Figure 5c,d) reveals a highly stable chemical bonding interaction, with the TCF remaining close to 1 over time. In contrast, when PDMS with a polymerization degree of 20 is added, the TCF exhibits a significant decrease to around 0.2. Moreover, the BTE shows noticeable fluctuations, indicating a substantial number of bond generation and breakage. This suggests that PDMS with a polymerization degree of 20 provides enhanced protection against CSH. When PDMS with a polymerization degree of 5 is added, the TCF of the Ca-Osulfate bond decreases to around 0.2 with time, indicating a certain protective effect of PDMS at this stage. The variation in protective performance observed for PDMS with different polymerization degrees is discussed further in Section 3.2.
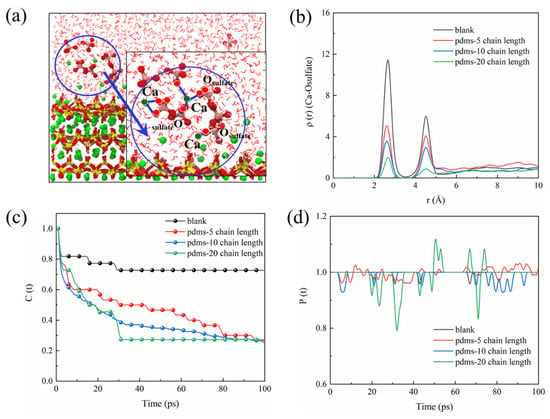
Figure 5.
(a) Schematic representation illustrating the interaction between sulfate and CSH, (b) RDF of Ca–Osulfate, (c) TCF of Ca–Osulfate, and (d) BTE of Ca–Osulfate.
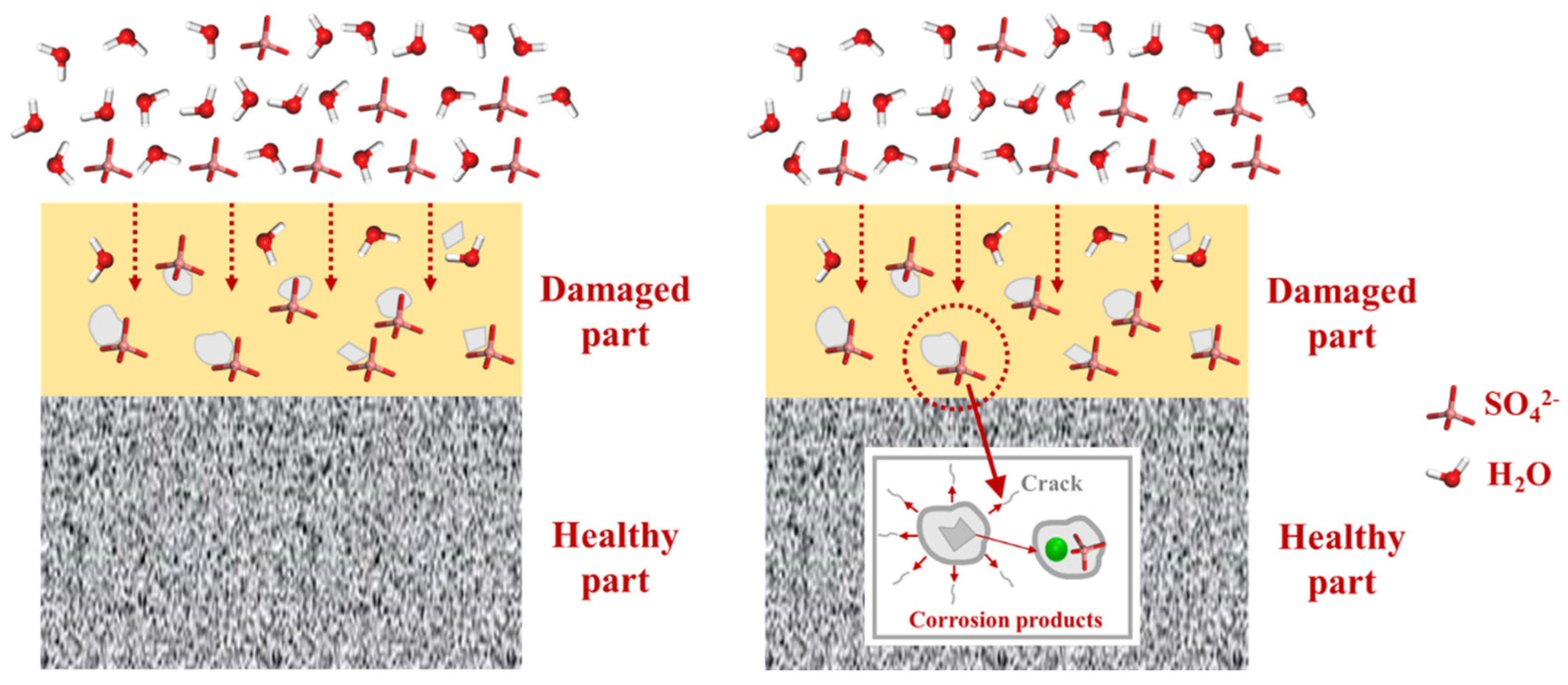
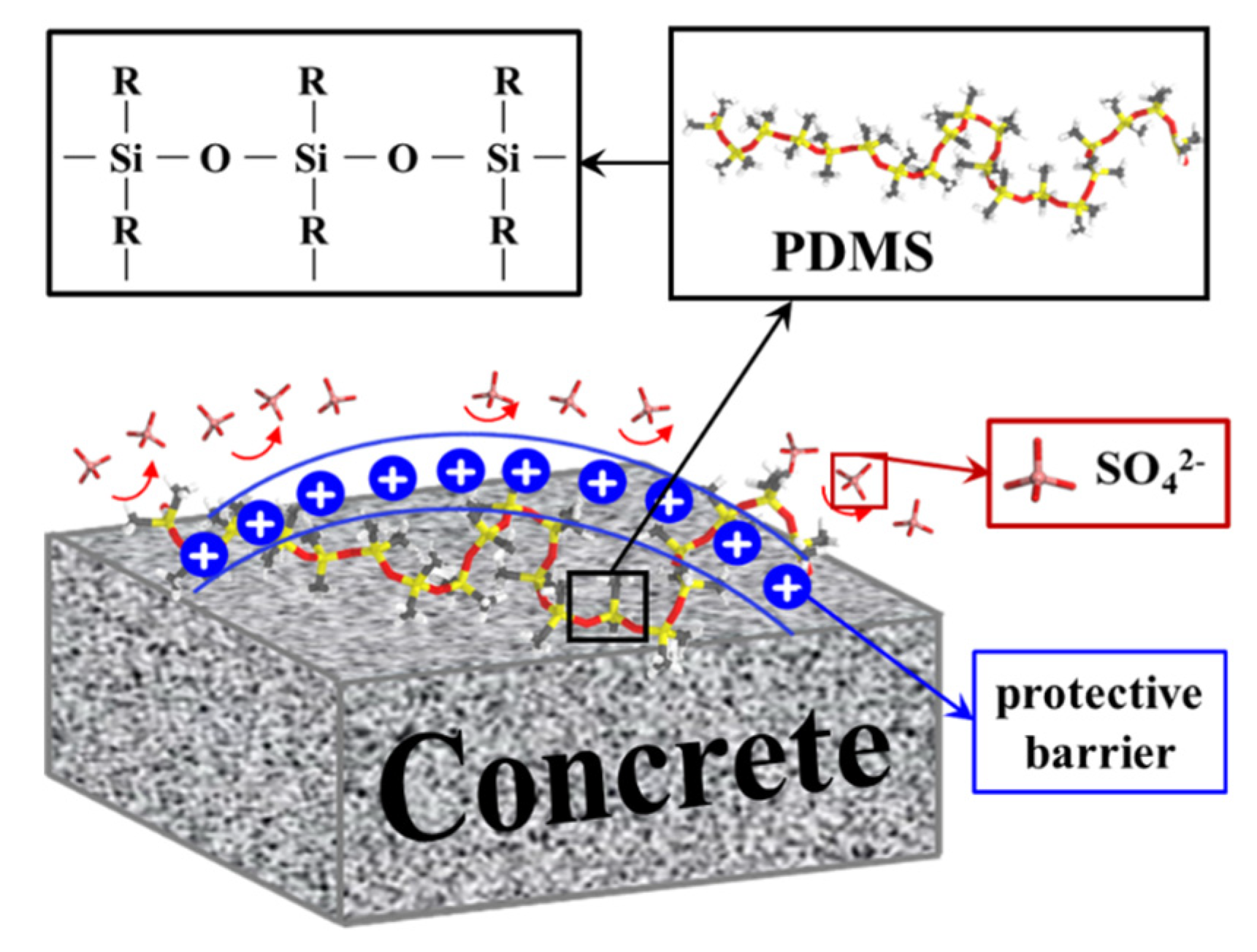
The interaction between CSH and sulfate reveals an important fact: sulfate in the environment possesses a strong negative charge, causing it to preferentially adsorb onto the surface of the CSH matrix and interact strongly with its cation Ca2+, forming a stable bonding interaction known as Ca-Osulfate. As a significant amount of sulfate is adsorbed, the Ca–Osulfate bonds increase, resulting in the formation of Ca–O–S linkages that represent the ionic structure of the Ca–SO4 cluster. This process ultimately leads to the generation of gypsum (CaSO4·2H2O) as a corrosion product. Figure 6 provides a detailed illustration of the sulfate erosion process. The increasing presence of gypsum leads to internal expansion and cracking, resulting in accumulating microstructural deterioration that ultimately impairs the macroscopic properties of the concrete. Building on the preceding discussion, our investigation revealed that the application of PDMS coating led to an impressive 83.3% reduction in sulfate bonding on the substrate surface in comparison to the blank group. This substantial reduction holds the potential to significantly alleviate sulfate-induced decalcification on the CSH surface. In the subsequent sections, we delve into a detailed exploration of this protective phenomenon conferred by PDMS.
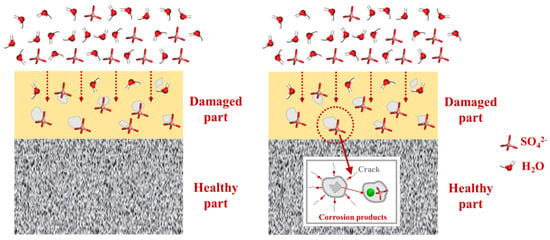
Figure 6.
Schematic diagram illustrating the mechanism of the sulfate erosion process on the matrix.
3.2. Local Structure of PDMS Coating and CSH Interface
In our previous discussion, we observed that the presence of PDMS coating on the CSH matrix impedes sulfate adsorption and disrupts its interaction. In this subsection, our focus is on examining the molecular structure and protective mechanism of PDMS at the CSH interface with different degrees of polymerization.
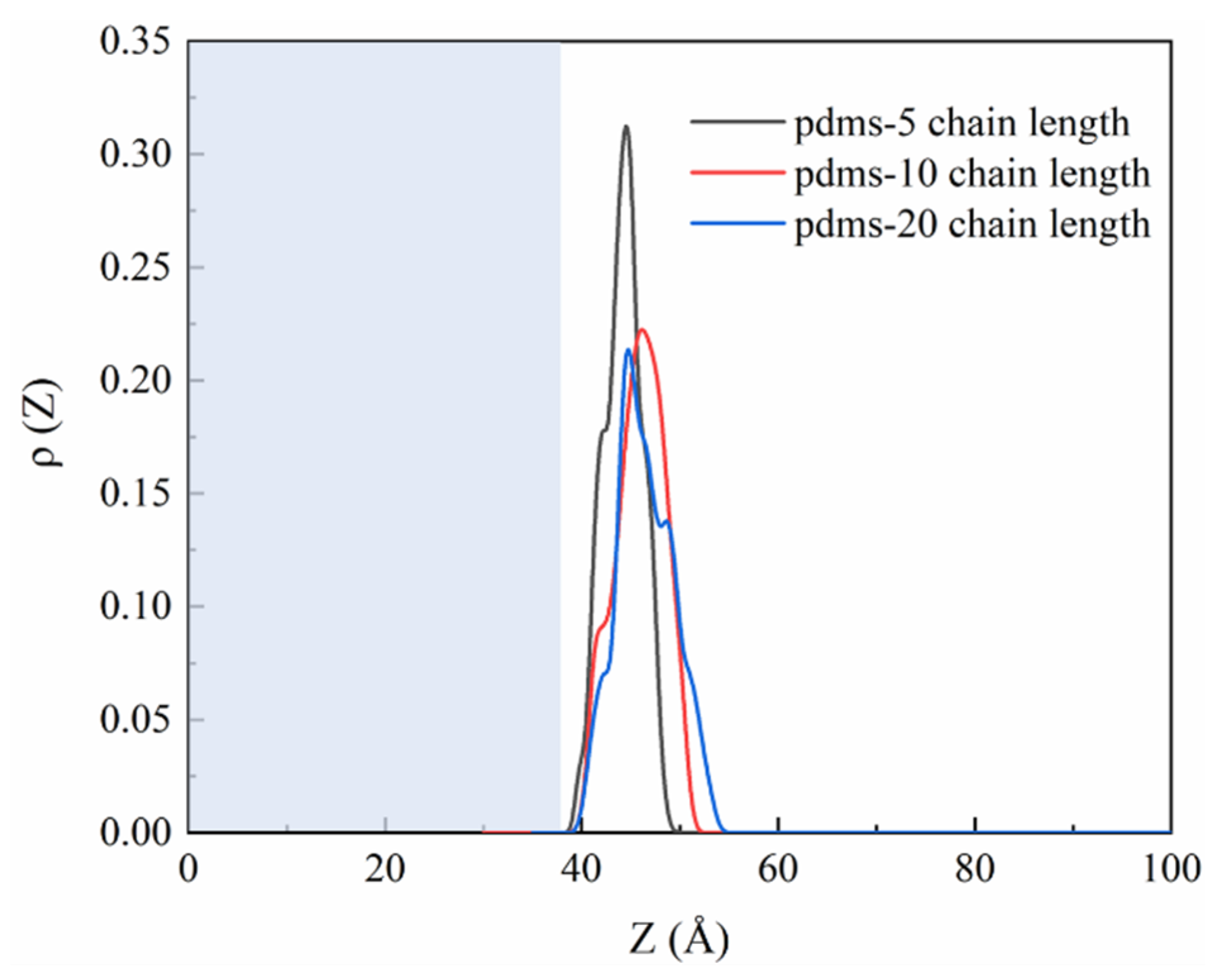
To provide data supporting the PDMS protection phenomenon, we calculated the one-dimensional density profiles of PDMS, as illustrated in Figure 7. The results show that polymerization degrees of 5, 10, and 20 all exhibit peaks in the range of 40 Å to 50 Å, indicating the distribution of PDMS on the CSH surface throughout the simulation period. The blue area in the figure represents the CSH part. The peak for PDMS with a polymerization degree of 5 is higher than for the other two groups, which can be attributed to the initial quantity of PDMS molecules used in the simulation. Initially, to ensure that the number of molecules would not affect the final results and to compare the effects of different polymerization degrees, we placed four PDMS molecules with a polymerization degree of 5, two PDMS molecules with a polymerization degree of 10, and one PDMS molecule with a polymerization degree of 20. Consequently, the peak size of the one-dimensional density profiles follows the order of 5 > 10 > 20 in relation to the polymerization degree, indicating the extent of PDMS coverage on the CSH matrix.
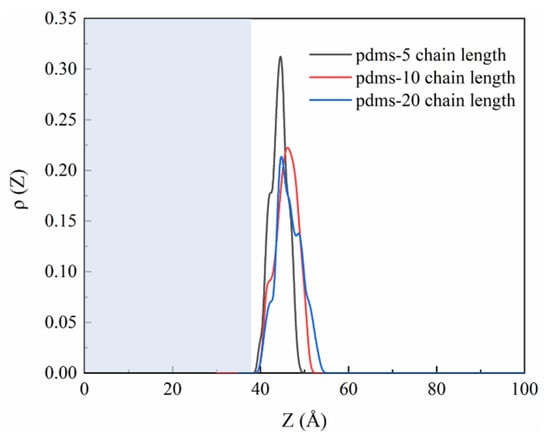
Figure 7.
The one-dimensional density profile curves of PDMS with various polymerization degrees around CSH.
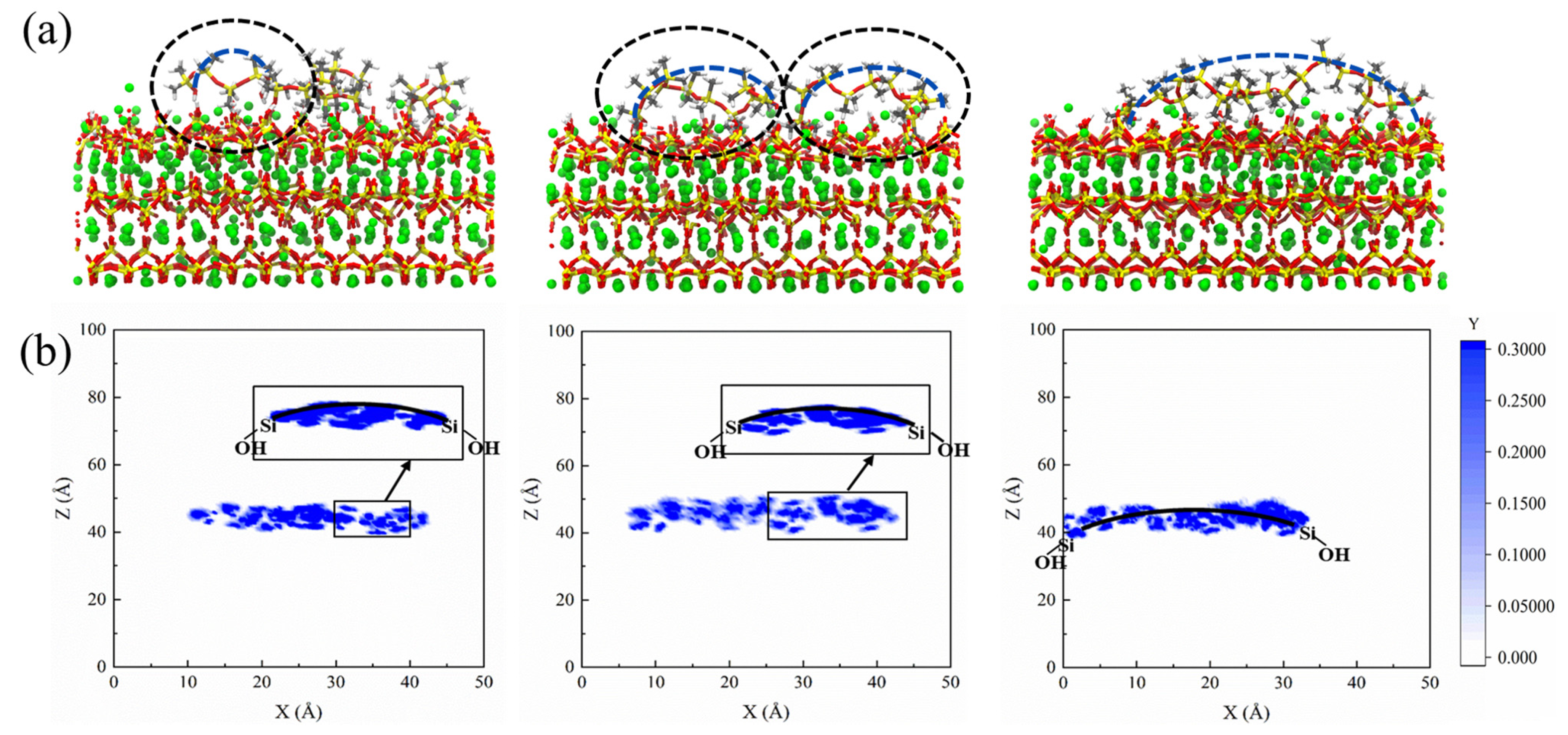
We examined the two-dimensional density profiles of PDMS with different polymerization degrees along the spatial X-direction. Figure 8 reveals that PDMS exhibits strong adsorption on the matrix during sulfate attack, providing a solid foundation for its ability to hinder sulfate. Furthermore, as the polymerization degree increases, PDMS transitions from a tightly bound adsorption on the entire CSH surface to partial desorption in the central region while continuing to maintaining strong adsorption on both sides. This behavior gives rise to a bridge-like distribution pattern in its molecular structure.
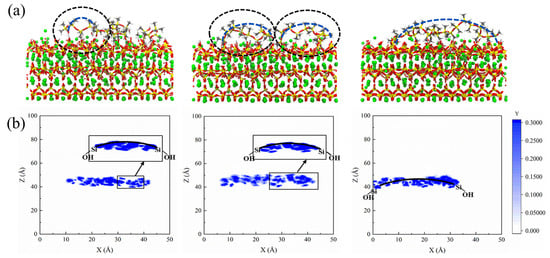
Figure 8.
(a) Adsorption modes of PDMS with varying polymerization degrees within the CSH structure and (b) schematic diagram depicting the two-dimensional density profiles of PDMS with different polymerization degrees; from left to right, the polymerization degrees are 5, 10, and 20 in sequential order.
Continuing our analysis, we further investigated the protective behavior of PDMS on CSH surfaces. In Figure 9, the visualizations of the main view of PDMS and the top view with sulfate distribution confirm that there is no sulfate adsorption within a specific range around PDMS. This finding supports our previous analysis of the sulfate movement trend. Similarly, in the top view, sulfate is unable to displace PDMS from its adsorption sites, which prevents desorption. We attribute this phenomenon to the formation of a protective barrier by the PDMS coating on the CSH surface that effectively hinders sulfate from approaching the surface and adsorbing onto it.
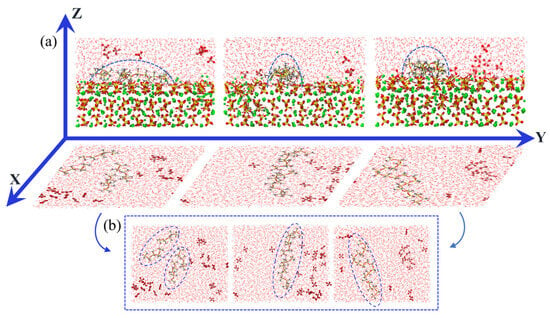
Figure 9.
(a) Schematic diagram illustrating the spatial pattern of PDMS with various polymerization degrees and the distribution of sulfate within the matrix and (b) top view of PDMS systems with varying polymerization degrees; from left to right, the order of representation is 5, 10, and 20.
Based on our previous speculations, we performed calculations on the charge density profiles of PDMS with different degrees of polymerization compared to the blank group, with the aim of providing a deeper understanding of the PDMS protection mechanism. as the results are depicted in Figure 10. Charge density profiles offer a comprehensive and precise means of examining the charge distribution within a system, enabling detailed analysis of the electrostatic interactions and interfacial phenomena at the molecular level [75]. In our study, the choice to employ charge density profiles was rooted in the significance of electrostatic forces and their profound influence on the structural stability and functional properties of the materials under investigation. By leveraging this approach, it is possible to effectively delineate the intricate variations in charge distribution along the interfaces, facilitating a nuanced understanding of the molecular-level interactions between the PDMS coatings and the concrete substrate in the presence of sulfate-rich environments. It is important to note that in our OPLS force field the system itself is electrically neutral; however, when PDMS with various polymerization degrees forms a coating, a difference in charges arises within the simulated system. This difference is primarily attributed to the presence of adsorbed or dissociated species on the CSH surface, which give rise to charged sites capable of electrostatic interactions (either attractive or repulsive) with other groups in the system. These processes occur spontaneously. Notably, we observed that the PDMS-coated system formed a positively charged layer on the CSH surface near 40–50 Å that was not present in the blank group. This positive layer acts as a barrier, impeding the proximity of sulfate ions. Interestingly, we discovered that the positive layer formed by PDMS with a degree of polymerization of 5 was slightly larger than with degrees of polymerization of 10 and 20. This observation is attributable to the fact that we initially placed four groups of PDMS with a degree of polymerization of 5, which was more abundant than for the other two groups. Consequently, these groups randomly coated the CSH surface, resulting in a slightly higher charge density due to the positive layers formed at their respective adsorption sites. The schematic representation in Figure 11 aims to provide a visual interpretation of our proposed mechanism, emphasizing the fundamental concept that PDMS serves as a robust protective shield preventing the detrimental effects of sulfate attack on the concrete matrix. This depiction aligns with our comprehensive findings, and further supports the hypothesis that PDMS coatings play a pivotal role in enhancing the resilience of concrete structures against sulfate-induced degradation.
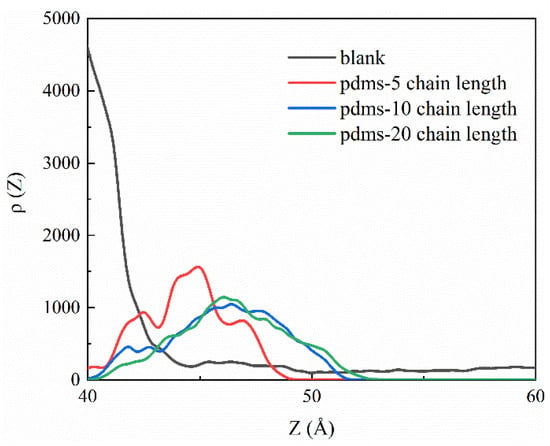
Figure 10.
The charge density profiles as analyzed for both the blank group and PDMS systems with different degrees of polymerization.
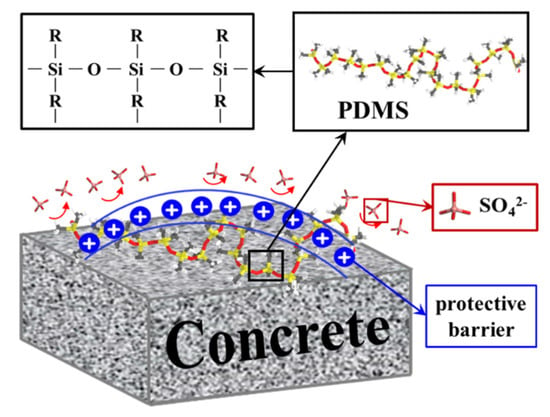
Figure 11.
Schematic diagram of PDMS in matrix protection mechanism.
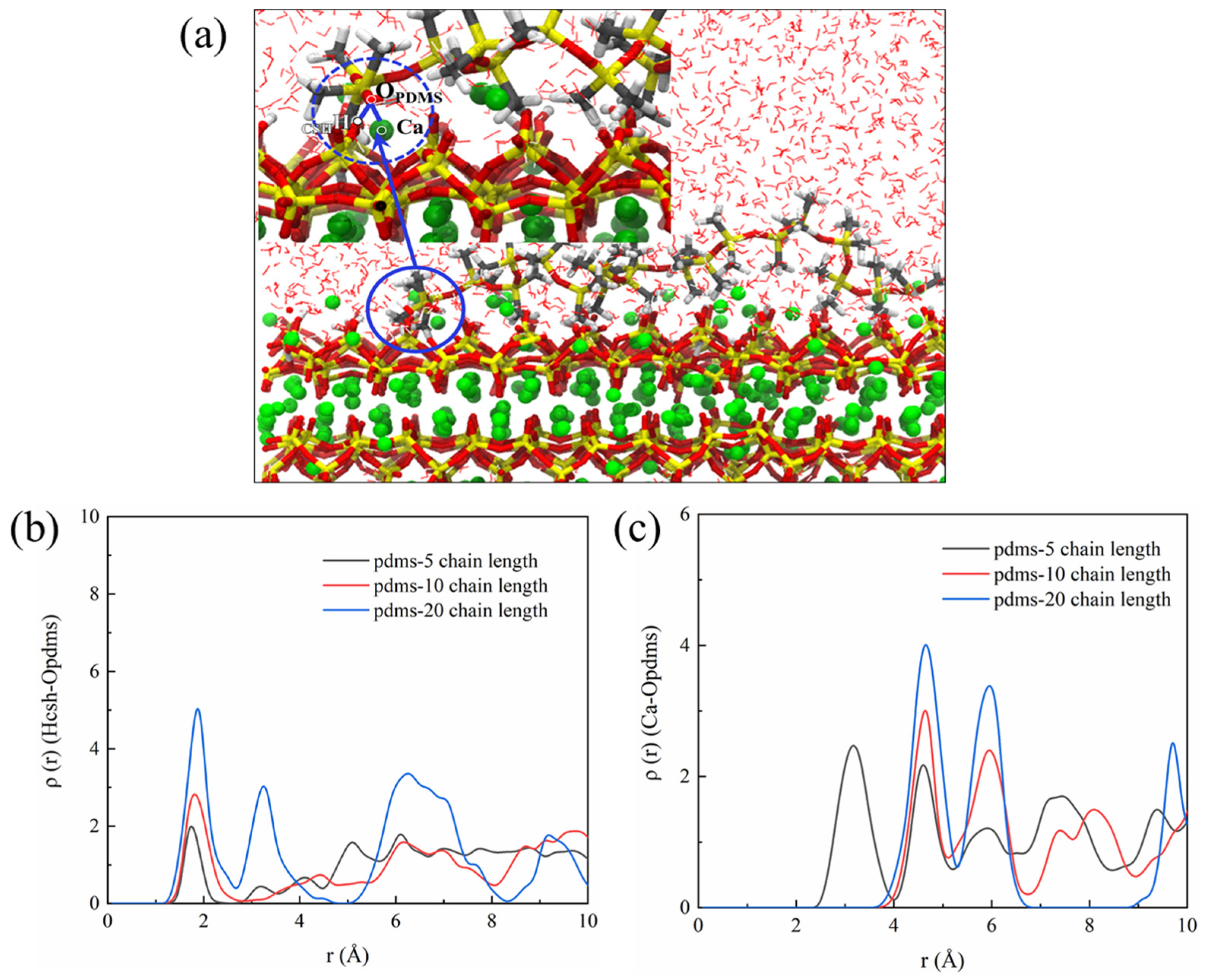
Based on our analysis of the molecular structure of PDMS, we propose that the “bridged” segments are formed due to hydrogen bonding between the hydroxyl groups of PDMS and CSH along with electrostatic interactions. The weakly adsorbed and easily arched portion is primarily influenced by the van der Waals forces. Notably, higher polymerization degrees lead to an increase in the van der Waals forces, enhancing the stability of adsorption and improving the protective effect. Hence, in order to delve into the microscopic origins of PDMS and CSH interactions, we conducted an analysis using RDF and BTE. We hypothesized that as the CSH underwent protonation, hydrogen bonding would be present between the PDMS and CSH, as depicted in Figure 12b. The RDF analysis revealed a distinct peak around 2.0 Å after coating with PDMS. Importantly, this peak became more prominent with higher degrees of polymerization, indicating stronger interaction between the PDMS and CSH. This correlation explains why PDMS with a higher degree of polymerization exhibits a greater protective effect. Additionally, we examined the promotion of Ca–OPDMS bonding, as illustrated in Figure 12c. Notably, PDMS with a polymerization degree of 5 displayed a characteristic peak around 3.2 Å, indicative of the Ca–OPDMS bonding interaction. Although the hydrogen bonding is weaker, in this case it enhances the adsorption of PDMS with a polymerization degree of 5 to the CSH and contributes to the protective effect. Because adsorption between the PDMS and CSH is a prerequisite for its protective effect, this raises questions regarding the differences in protection and the underlying mechanisms presented by PDMS with different degrees of aggregation. These aspects are explored further in the subsequent discussion.
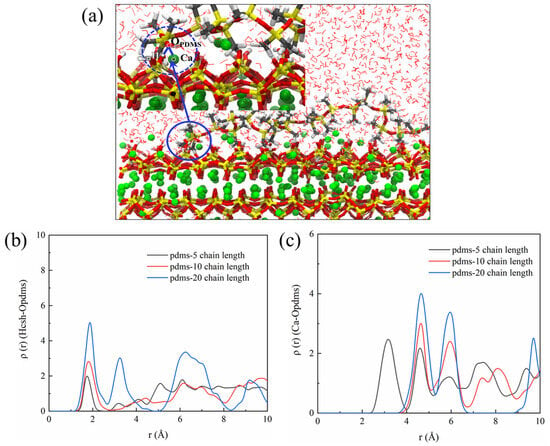
Figure 12.
(a) Schematic representation illustrating the interaction between PDMS and the CSH matrix, (b) RDF of the HCSH–OPDMS bond, and (c) RDF of the Ca–OPDMS bond.
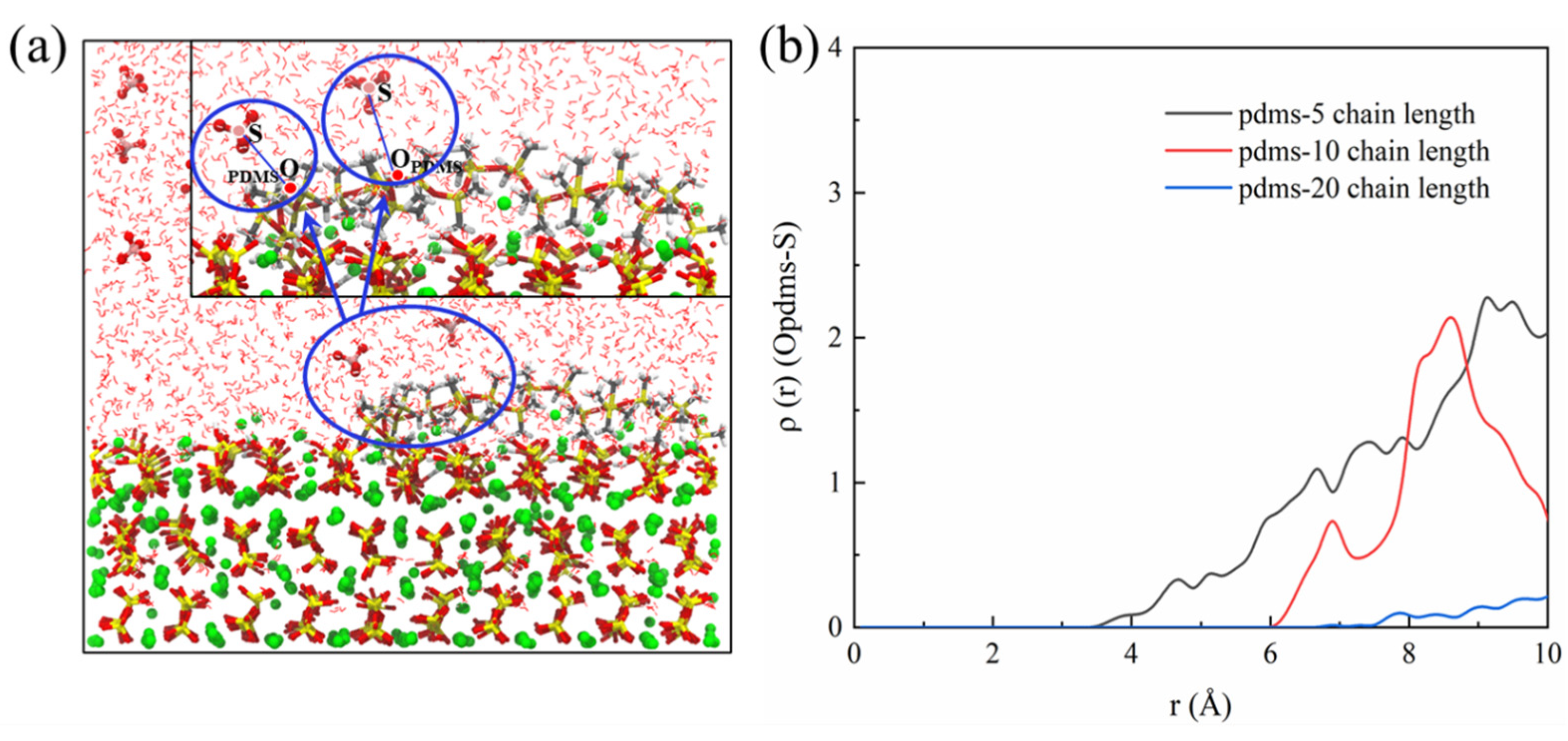
In our exploration, we discerned notable distinctions in the protective capabilities exhibited by the three distinct degrees of polymerization within the PDMS molecules concerning the CSH matrix. Specifically, within the schematic representation depicting PDMS interactions with sulfate ions within the system across varying degrees of polymerization, as depicted in Figure 13, intriguing patterns emerge. For PDMS with a polymerization degree of 5, a subtle yet discernible peak in the RDF can be observed at approximately 4.5 Å, signifying interaction with sulfate ions. As the polymerization degree increases, a noteworthy trend becomes apparent in that the interaction strength diminishes when moving away from this peak. Remarkably, at a polymerization degree of 20 this interaction appears to approach negation. This compelling observation underscores a discernible positive correlation between the degree of polymerization within PDMS and its efficacy in providing protective attributes.
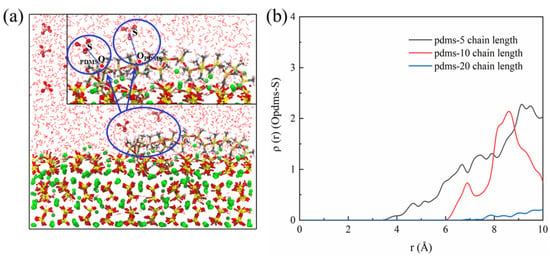
Figure 13.
(a) Schematic representation illustrating the interaction between PDMS and sulfate and (b) RDF of the OPDMS–S bond.
The Mean Square Displacement (MSD) serves as a crucial parameter for the evaluation of PDMS atom adsorption dynamics onto the CSH surface. MSD(t) is mathematically defined in Equation (1) as follows.
MSD(t) = (|ri(t) − ri(0)|2)
Here, ri(0) represents the initial position of atom i, while ri(t) signifies the position of atom i at time t and MSD(t) effectively characterizes the deviation of an atom from its initial spatial coordinates as a function of time.
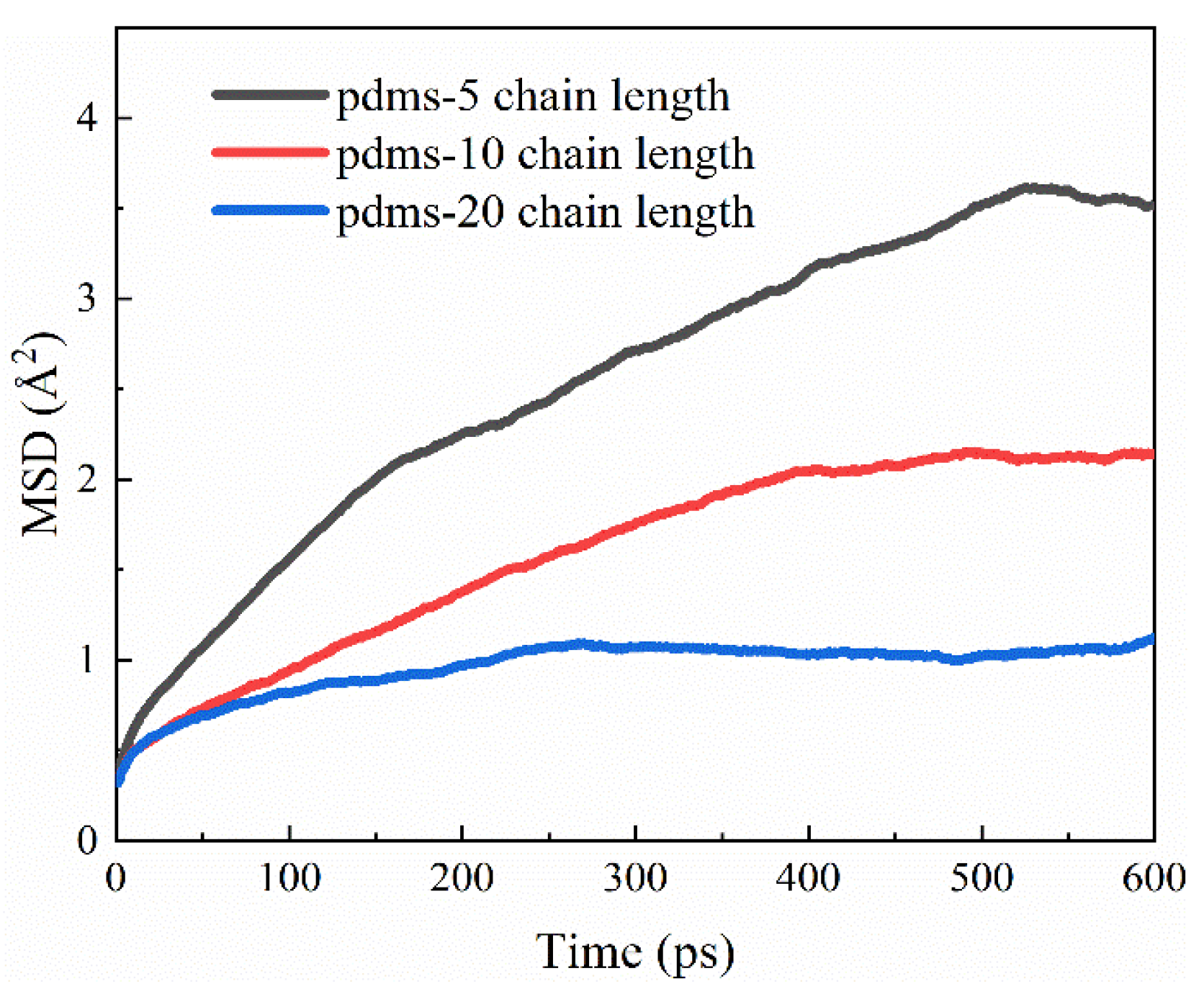
Drawing upon the aforementioned disparities in the protective attributes exhibited by PDMS molecules of varying polymerization degrees, we conducted a comprehensive analysis of atomic mobility. The temporal analysis of the CSH surface, depicted in Figure 14, unveils intriguing insights. Notably, the mobility of PDMS on the CSH surface follows a descending order corresponding to the degree of polymerization: polymerization 5 > polymerization 10 > polymerization 20. Furthermore, it is noteworthy that the diffusion rate on the surface of PDMS with a high degree of polymerization decreases to 33.3% in comparison to the PDMS groups with a lower degree of polymerization. This observation underscores a compelling relationship; as the degree of polymerization increases, PDMS exhibits enhanced stability when adhering to the CSH surface. This increased stability in turn fortifies the structural integrity of the CSH surface, resulting in heightened adhesion. Consequently, this heightened adhesion acts as a formidable barrier, impeding the diffusion of PDMS and thereby amplifying its protective prowess.
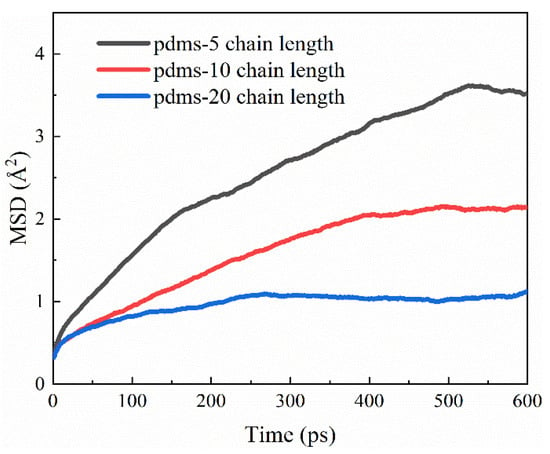
Figure 14.
The MSD evolution for PDMS with different degrees of polymerization on CSH surfaces.
3.3. Trend of Water Molecule Movement
Because the entire simulation takes place in an aqueous solution environment, the motion of the species involved cannot be examined without considering the influence of water molecules. Thus, we analyzed the factors that affect the adsorption behavior of silane and sulfate by studying the motion trends and interactions of water molecules. Specifically, we quantitatively evaluated the order parameter of water molecules (Sm) in order to assess their motion trends in PDMS systems with varying polymerization degrees, as depicted in Figure 15a. The Sm value is calculated in Equation (2) as follows [76].
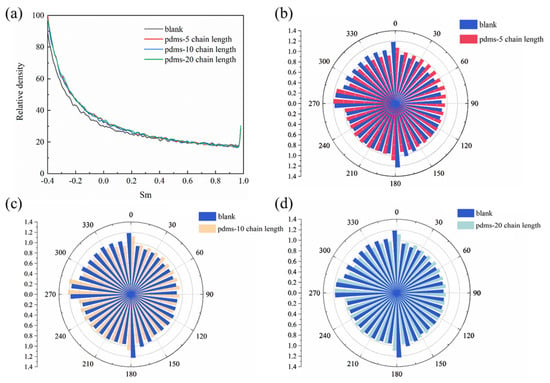
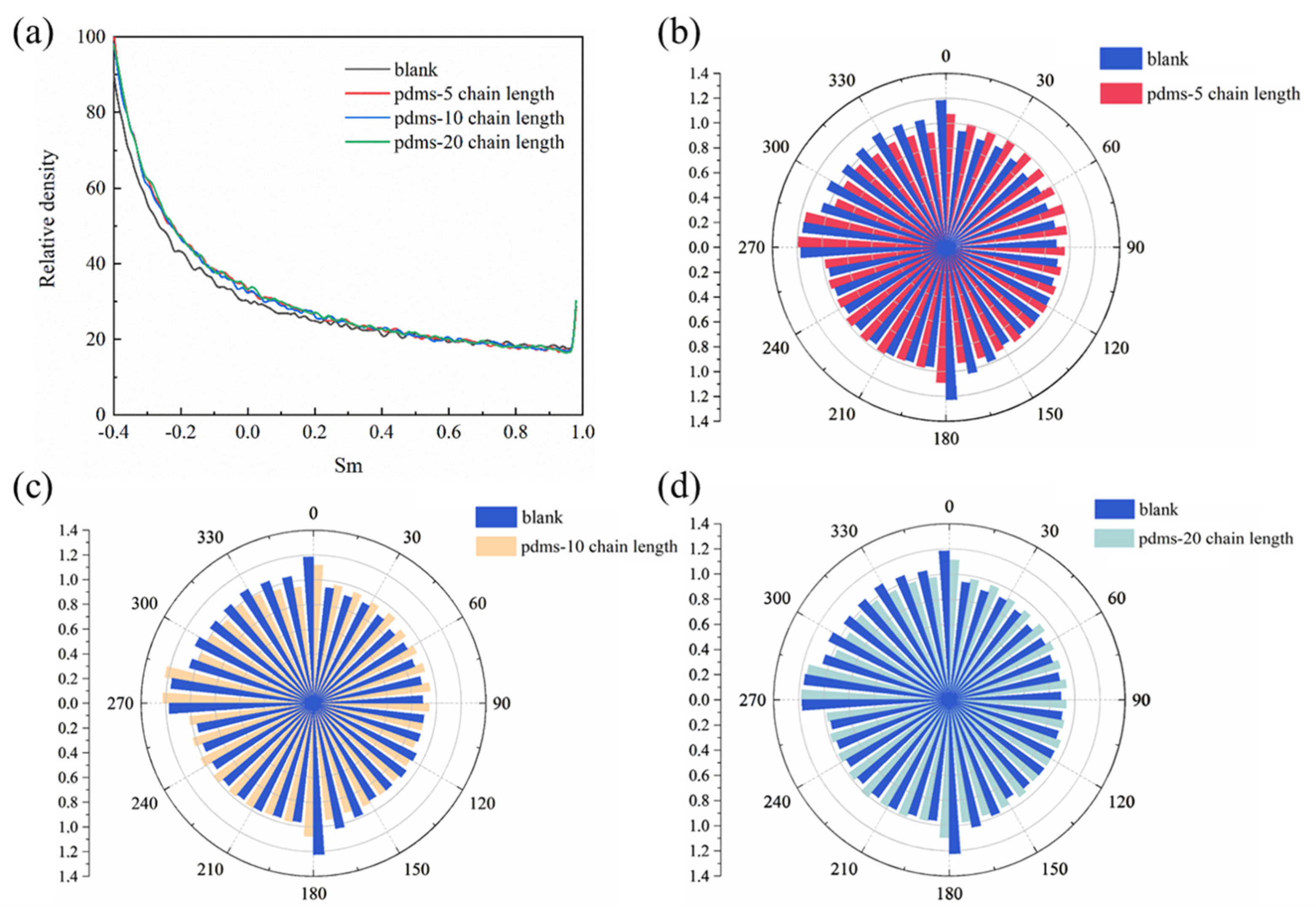
Figure 15.
(a) The curves of water molecule order parameters of the four systems and (b–d) schematic diagrams of the dipole direction distribution of water molecules in the blank group and PDMS groups with different polymerization degrees.
In this equation, β represents the angle between the two orientations of the water molecule’s dipole. The Sm value, ranging from −0.5 to 1, serves as a measure of the water molecule’s order. A value of Sm close to 1 indicates a higher degree of order, implying that the water molecules have a more intricate structure, a greater propensity for hydrogen bonding, and an overall more organized arrangement. On the other hand, a value of Sm closer to −0.5 suggests a lower degree of order, indicating less structured water molecules with a reduced tendency to form hydrogen bonds [77,78,79,80].
Here, we calculated the order parameters for a total of 4000 water molecules in the entire system. In the equilibrium state, we observed that the higher-order water remains relatively unchanged with the introduction of PDMS coatings with different degrees of polymerization. However, for lower-order water we found that all three PDMS-coated systems exhibited slightly higher values compared to the blank group. This suggests that the interaction with the solution environment may be more active in the absence of PDMS. The hydrophobic nature of PDMS itself hinders the tendency of water molecules to interact. It is important to note that the distinction between higher-order and lower-order water is not always clear, and may depend on various factors. Therefore, we further analyzed the systems using the dipole direction distribution of water molecules, as depicted in Figure 15b,c. The dipole moments of water molecules arise from the separation of positive and negative charges due to the difference in electronegativity between oxygen and hydrogen atoms. In our previous discussion, we observed weak differences between the blank group and the PDMS-coated group in terms of lower-order water. Moreover, the dipole moment distribution of water molecules revealed a similar arrangement in the presence or absence of PDMS coatings. In other words, coating with PDMS has a minimal impact on the motion trends of most water molecules in the solution at this stage. Of course, these observations require further explanation through the interactions between the corresponding interfaces.
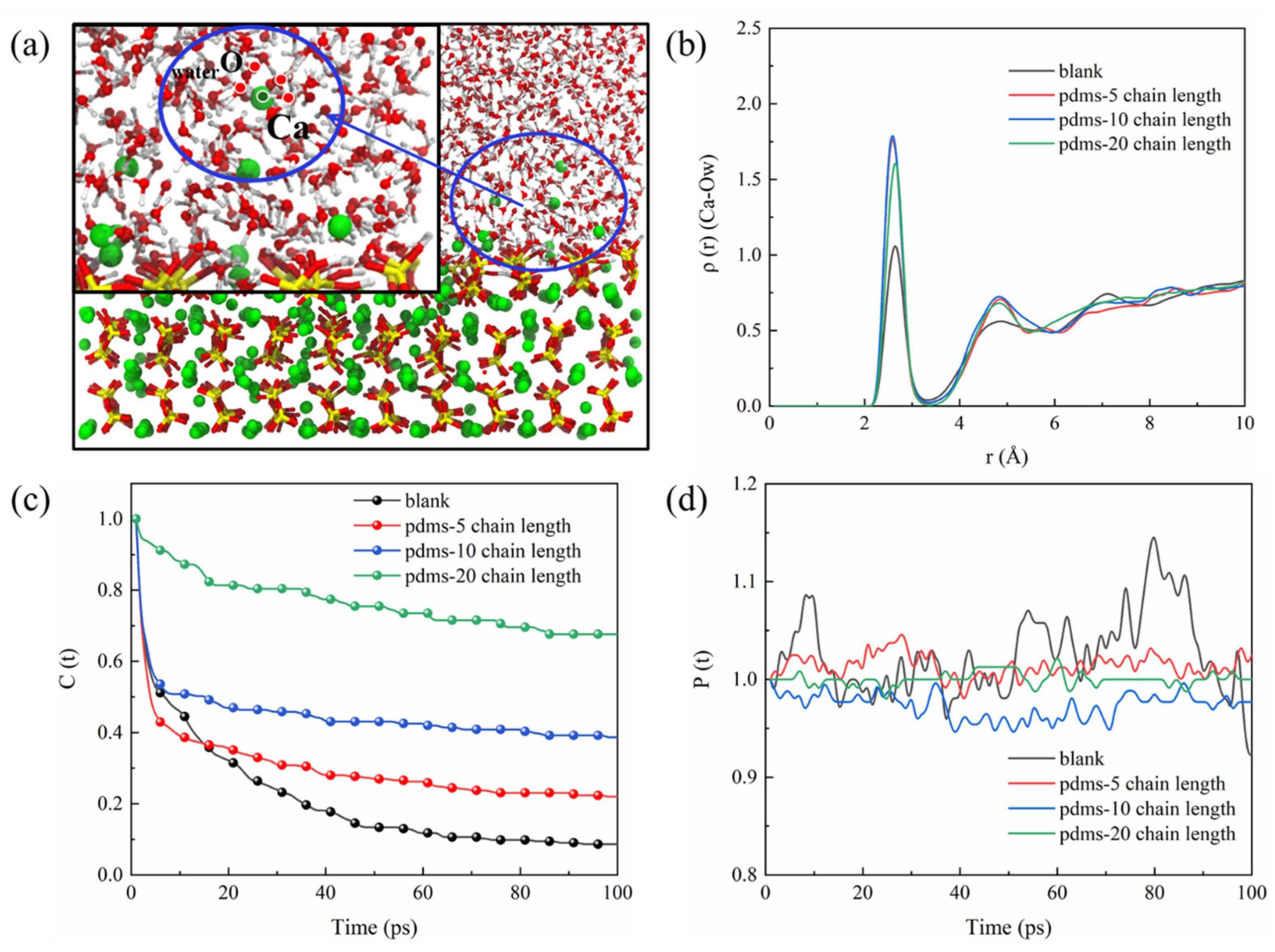
In our previous discussion, we observed that the PDMS coating impedes the bonding of sulfate to the CSH matrix. We speculated that water molecules play a role in these behaviors. As depicted in Figure 16b, the RDF of Ca–Ow for all four systems exhibits a characteristic peak around 2.5 Å, with the peak in the blank group slightly lower than in the PDMS-coated groups. This is attributed to the fact that in the blank group, without the hindrance of PDMS, sulfate adsorption promotes the interaction of water molecules with the CSH matrix. Following PDMS coating, water molecules drive sulfate to the uncoated region due to the hindrance posed by the positive layer formed by the PDMS. This adsorption necessitates the involvement of numerous water molecules, resulting in stronger interaction with the CSH matrix. The TCF of water molecules (Ow) in the solution with Ca in CSH is calculated in Figure 16c, and the trend shows the fastest decrease in TCF in the blank group. This change is associated with sulfate adsorption, as the formation of Ca–Osulfate bonds leads to the breakage of a significant number of Ca–Ow bonds. In contrast, the TCF of the Ca–Ow bond remains relatively stable in the PDMS group with a polymerization degree of 20. This demonstrates the protective effect of PDMS, reducing the interfacial adsorption of sulfate and increasing the opportunity for water molecules to bond with CSH. The fluctuation of the BTE of the Ca–Ow bond (as depicted in Figure 16d) reflects this behavior as well. The blank group exhibits significant fluctuations, indicating the generation and breakage of a large number of Ca–Ow bonds. Conversely, the BTE fluctuation of the Ca–Ow bond in the PDMS group with a polymerization degree of 20 is minimal, indicating stability. Thus, the adsorption process of sulfate is intricately linked to the movement of water molecules in the solution, and the interaction between water molecules and the CSH matrix tends to have a negative correlation with the amount of sulfate adsorption.
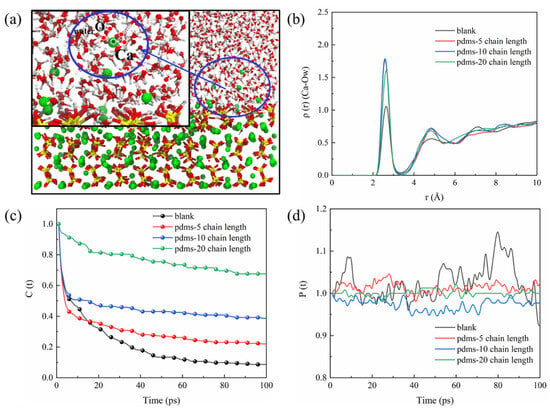
Figure 16.
(a) Schematic diagram of the interaction between the solution environment and the matrix, (b) RDF of Ca–Ow, (c) TCF of Ca–Ow, and (d) BTE of Ca–Ow.
4. Conclusions
In this investigation, we have employed MD simulations to delve into the adsorption behavior of diverse species at the CSH interface when coated with PDMS featuring varying polymerization degrees within a sulfate-rich environment. Our results demonstrate that the sulfate environment instigates the decalcification of CSH substrates, while PDMS coating mitigates this degradation. Additionally, the degree of polymerization profoundly influences the molecular weight, physical properties, and structural characteristics of PDMS. The presence of water in the solution exerts a discernible impact on the sulfate degradation process. Our observations lead to the following key conclusions.
(1) The sulfate-induced decalcification of the CSH substrate primarily occurs through the binding of SO42− ions with surface calcium, leading to the formation of Ca–Osulfate bonds and subsequent Ca–O–S links. This process ultimately yields the corrosive product gypsum (CaSO4·2H2O). The introduction of PDMS coatings resulted in a remarkable 83.3% reduction in sulfate bonding on the substrate surface. This substantial reduction effectively mitigates sulfate-induced decalcification of the CSH surface.
(2) The protective efficacy of PDMS on CSH arises from its robust adhesion to the substrate, which is facilitated by strong hydrogen bonds and Ca–Opdms interactions between PDMS and the substrate. These interactions establish a distinctive positively charged layer on the substrate surface that proficiently impedes sulfate ion penetration. Remarkably, the migration rates of PDMS on the CSH surface follow a descending order in terms of polymerization degree, as follows: polymerization 5 > polymerization 10 > polymerization 20. The diffusion rate on the surface of PDMS with a higher polymerization degree can be reduced to 33.3% compared to the PDMS group with a lower polymerization degree, implying that higher polymerization enhances the stability of the skeleton’s adsorption on the substrate surface, thereby reinforcing its protective effect.
(3) Compared with the blank group, the water molecule order parameter and dipole direction distribution in the PDMS system exhibit minimal changes. However, the interaction between water molecules and the substrate significantly influences the adsorption behavior of sulfate. In other words, the sulfate corrosion process is intricately linked to the movement of water molecules in the solution, showcasing a negative correlation with the amount of sulfate adsorbed.
Author Contributions
Software, D.H.; Data curation, Y.D., J.H., Y.G., M.H. and Z.L.; Writing—review & editing, J.J., S.L. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
National key research and development project (2022YFE0133800), Financial support from the National Natural Science Foundation of China (Grant No. 51978355, No. 52078260), the Natural Science Foundation of Shandong Province (ZR2021QD009), Jiangsu Key Laboratory of Civil Engineering Materials Open Foundation (CM 2016-06), Cooperative Innovation Center of Engineering Construction and Safety in Shandong Blue Economic Zone, and Shandong Province Marine Environment Concrete Material Corrosion Control and Monitoring Research Innovation Team.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Department of Civil Engineering, Qingdao University of Technology; Engineering Research Center of Concrete Technology Under Marine Environment, Ministry of Education, Qingdao University of Technology; State Key Laboratory of Hydraulic Engineering Simulation and Safety, Tianjin University; Collaborative Innovation Center of Engineering Construction and Safety in Shandong Blue Economic Zone are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Das, J.K.; Pradhan, B. Significance of chloride salt type and sulfate salt on chloride transport mechanism of concrete in the presence of corrosion inhibiting admixtures. Constr. Build. Mater. 2023, 387, 131658. [Google Scholar] [CrossRef]
- Brekailo, F.; Pereira, E.; Medeiros-Junior, R.A. Migration test as an accelerated methodology in concrete for evaluating sulfate attack by Na2SO4 and MgSO4. Constr. Build. Mater. 2023, 388, 131681. [Google Scholar] [CrossRef]
- Liu, D.; Cao, K.; Tang, Y.; Zhong, A.; Jian, Y.; Gong, C.; Meng, X. Ultrasonic and X-CT measurement methods for concrete deterioration of segmental lining under wetting-drying cycles and sulfate attack. Measurement 2022, 204, 111983. [Google Scholar] [CrossRef]
- Men, B.; Qin, Y.; Zhang, X.; Wu, J.; Liang, D.; Li, M.; Zhou, H. Investigation on the change of shear strength of concrete with cold joint under the action of sulfate dry–wet cycles. J. Build. Eng. 2023, 73, 106770. [Google Scholar] [CrossRef]
- Wang, K.; Cheng, Y.; Yang, L.; Sun, B.; Zhang, P.; Guo, J. Effects and interactions of scouring abrasion and external sulfate attack on deterioration of lining concrete: Experiments and preliminary modeling. Constr. Build. Mater. 2023, 393, 132077. [Google Scholar] [CrossRef]
- Yin, G.J.; Shan, Z.Q.; Miao, L.; Tang, Y.J.; Zuo, X.B.; Wen, X.D. Finite element analysis on the diffusion-reaction-damage behavior in concrete subjected to sodium sulfate attack. Eng. Fail. Anal. 2022, 137, 106278. [Google Scholar] [CrossRef]
- Bahraq, A.A.; Al-Osta, M.A.; Al-Amoudi, O.S.B.; Saleh, T.A.; Obot, I.B. Atomistic simulation of polymer-cement interactions: Progress and research challenges. Constr. Build. Mater. 2022, 327, 126881. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Ren, Q.; Zheng, Q.; Jiang, Z. Durability of concrete coupled with life cycle assessment: Review and perspective. Cem. Concr. Compos. 2023, 139, 105041. [Google Scholar] [CrossRef]
- Ikumi, T.; Segura, I. Numerical assessment of external sulfate attack in concrete structures. A Rev. Cem. Concr. Res. 2019, 121, 91–105. [Google Scholar] [CrossRef]
- Kim, K.; Hyung, C.; Ingole, P.G.; Kim, J.; Lee, H. Preparation, characterization, and performance evaluation of coated PES polymer materials fabricated via dry/wet phase inversion technique. J. Appl. Polym. Sci. 2013, 131, 39711. [Google Scholar] [CrossRef]
- Wang, K.; Guo, J.; Wu, H.; Yang, L. Influence of dry-wet ratio on properties and microstructure of concrete under sulfate attack. Constr. Build. Mater. 2020, 263, 120635. [Google Scholar] [CrossRef]
- Wang, K.; Guo, J.; Yang, L. Effect of dry–wet ratio on sulfate transport-reaction mechanism in concrete. Constr. Build. Mater. 2021, 302, 124418. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Yang, J.; Zou, Y.; Wang, Z. Understanding of the deterioration characteristic of concrete exposed to external sulfate attack: Insight into mesoscopic pore structures. Constr. Build. Mater. 2020, 260, 119932. [Google Scholar] [CrossRef]
- Nagrockienė, D.; Girskas, G.; Skripkiūnas, G. Properties of concrete modified with mineral additives. Constr. Build. Mater. 2017, 135, 37–42. [Google Scholar] [CrossRef]
- Xu, S.; Xie, N.; Cheng, X.; Huang, S.; Feng, L.; Hou, P.; Zhu, Y. Environmental resistance of cement concrete modified with low dosage nano particles. Constr. Build. Mater. 2018, 164, 535–553. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.C.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Suleiman, A.R.; Soliman, A.M.; Nehdi, M.L. Effect of surface treatment on durability of concrete exposed to physical sulfate attack. Constr. Build. Mater. 2014, 73, 674–681. [Google Scholar] [CrossRef]
- Makul, N. Modern sustainable Cem. Concr. Compos.: Review of current status, challenges and guidelines. Sustain. Mater. Technol. 2020, 25, e00155. [Google Scholar] [CrossRef]
- Makul, N. Advanced smart concrete—A review of current progress, benefits and challenges. J. Clean Prod. 2020, 274, 122899. [Google Scholar] [CrossRef]
- Li, S.; Hu, M.; Chen, X.; Sui, S.; Jin, L.; Geng, Y.; Jiang, J.; Liu, A. The performance and functionalization of modified cementitious materials via nano titanium-dioxide: A review. Case Stud. Constr. Mater. 2023, 19, e02414. [Google Scholar] [CrossRef]
- Ban, C.C.; Khalaf, M.A.; Ramli, M.; Ahmed, N.M.; Ahmad, M.S.; Ahmed Ali, A.M.; Dawood, E.T.; Ameri, F. Modern heavyweight concrete shielding: Principles, industrial applications and future challenges; review. J. Build. Eng. 2021, 39, 102290. [Google Scholar] [CrossRef]
- Oliveira, A.M.d.; Cascudo, O. Effect of mineral additions incorporated in concrete on thermodynamic and kinetic parameters of chloride-induced reinforcement corrosion. Constr. Build. Mater. 2018, 192, 467–477. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.; Tam, V.W.Y.; Tan, J.; Shen, A.; Zhang, C.; Zhang, J. Feasibility of recycled aggregates modified with a compound method involving sodium silicate and silane as permeable concrete aggregates. Constr. Build. Mater. 2022, 361, 129747. [Google Scholar] [CrossRef]
- Ma, M.; Tam, V.W.Y.; Le, K.N.; Osei-Kyei, R. Factors affecting the price of recycled concrete: A critical review. J. Build. Eng. 2022, 46, 103743. [Google Scholar] [CrossRef]
- Sadati, S.; Arezoumandi, M.; Shekarchi, M. Long-term performance of concrete surface coatings in soil exposure of marine environments. Constr. Build. Mater. 2015, 94, 656–663. [Google Scholar] [CrossRef]
- Habibnejad Korayem, A.; Ghoddousi, P.; Shirzadi Javid, A.A.; Oraie, M.A.; Ashegh, H. Graphene oxide for surface treatment of concrete: A novel method to protect concrete. Constr. Build. Mater. 2020, 243, 118229. [Google Scholar] [CrossRef]
- Shen, L.; Jiang, H.; Wang, T.; Chen, K.; Zhang, H. Performance of silane -based surface treatments for protecting degraded historic concrete. Prog. Org. Coat. 2019, 129, 209–216. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, L.; Shu, X.; Yang, Y.; She, W.; Ran, Q. A review on recent advances in the fabrication and evaluation of superhydrophobic concrete. Compos. B Eng. 2022, 237, 109867. [Google Scholar] [CrossRef]
- Zhang, Z.; Ge, B.; Men, X.; Li, Y. Mechanically durable, superhydrophobic coatings prepared by dual-layer method for anti-corrosion and self-cleaning. Colloid Surf. A-Physicochem. Eng. Asp. 2016, 490, 182–188. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, V.; Negi, S.; Kar, S. A systematic review on polymer-based superhydrophobic coating for preventing biofouling menace. J. Coat. Technol. Res. 2023, 20, 1499–1512. [Google Scholar] [CrossRef]
- Nwuzor, I.C.; Idumah, C.I.; Nwanonenyi, S.C.; Ezeani, O.E. Emerging trends in self-polishing anti-fouling coatings for marine environment. Saf. Extreme Environ. 2021, 3, 9–25. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, P.; Liang, C.; Liu, L.; Qin, Z.; Wang, S.; Hou, P.; Lu, L. Utilization of Polydimethylsiloxane (PDMS) in polymer cement-based coating to improve marine environment service performance. Constr. Build. Mater. 2023, 367, 130359. [Google Scholar] [CrossRef]
- Gao, D.; Yang, L.; Pang, Y.; Li, Z.; Tang, Q. Effects of a novel hydrophobic admixture on the sulfate attack resistance of the mortar in the wet-dry cycling environment. Constr. Build. Mater. 2022, 344, 128148. [Google Scholar] [CrossRef]
- Jiang, Z.; Diggle, B.; Tan, M.L.; Viktorova, J.; Bennett, C.W.; Connal, L.A. Extrusion 3D Printing of Polymeric Materials with Advanced Properties. Adv. Sci. 2020, 7, 2001379. [Google Scholar] [CrossRef]
- Chen, C.; Li, Z.; Wang, Y.; Zhang, Z.; Ren, C. A Study on the 3D Deformation Behavior of Porous PDMS Flexible Electronic Composite Films Stretched under Different Temperatures. Materials 2023, 16, 6586. [Google Scholar] [CrossRef]
- Selim, M.S.; Elmarakbi, A.; Azzam, A.M.; Shenashen, M.A.; El-Saeed, A.M.; El-Safty, S.A. Eco-friendly design of superhydrophobic nano-magnetite/silicone composites for marine foul-release paints. Prog. Org. Coat. 2018, 116, 21–34. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Rajan, R.; Pancrecious, J.K.; Rajan, T.P.D.; Pai, B.C. Developments in smart anticorrosive coatings with multifunctional characteristics. Prog. Org. Coat. 2017, 111, 294–314. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Zhou, X.; Wang, P.; Zhang, Y.; Wang, X.; Zhang, X.; Hou, D.; Wang, M. Atomistic Insights into the Deposition of Corrosion Products on the Surfaces of Steels and Passivation Films. Langmuir 2023, 39, 6812–6822. [Google Scholar] [CrossRef]
- Honorio, T.; Carasek, H.; Cascudo, O. Water self-diffusion in C-S-H: Effect of confinement and temperature studied by molecular dynamics. Cem. Concr. Res. 2022, 155, 106775. [Google Scholar] [CrossRef]
- Hou, D.; Yang, Q.; Wang, P.; Jin, Z.; Wang, M.; Zhang, Y.; Wang, X. Unraveling disadhesion mechanism of epoxy/CSH interface under aggressive conditions. Cem. Concr. Res. 2021, 146, 106489. [Google Scholar] [CrossRef]
- Cao, K.; Wu, D.; Chen, K.; Mao, N. Erosion experiments and molecular dynamics simulations of hydrated calcium silicate under the action of sulfate. J. Non-Cryst. Solids 2023, 613, 122362. [Google Scholar] [CrossRef]
- Jahandari, S.; Tao, Z.; Alim, M.A.; Li, W. Integral waterproof concrete: A comprehensive review. J. Build. Eng. 2023, 78, 107718. [Google Scholar] [CrossRef]
- Yao, H.; Xie, Z.; Huang, C.; Yuan, Q.; Yu, Z. Recent progress of hydrophobic cement-based materials: Preparation, characterization and properties. Constr. Build. Mater. 2021, 299, 124255. [Google Scholar] [CrossRef]
- Sebakhy, K.O.; Gavrilov, M.; Valade, D.; Jia, Z.; Monteiro, M.J. Nanoparticles of well-defined 4-arm stars made using nanoreactors in water. Macromol. Rapid Commun. 2014, 35, 193–197. [Google Scholar] [CrossRef]
- Graur, V.; Mukherjee, A.; Sebakhy, K.O.; Bose, R.K. Initiated Chemical Vapor Deposition (iCVD) of Bio-Based Poly(tulipalin A) Coatings: Structure and Material Properties. Polymers 2022, 14, 3993. [Google Scholar] [CrossRef]
- Wang, F.; Lei, S.; Ou, J.; Li, W. Effect of PDMS on the waterproofing performance and corrosion resistance of cement mortar. Appl. Surf. Sci. 2020, 507, 145016. [Google Scholar] [CrossRef]
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: Synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Abdolhosseini Qomi, M.J.; Krakowiak, K.J.; Bauchy, M.; Stewart, K.L.; Shahsavari, R.; Jagannathan, D.; Brommer, D.B.; Baronnet, A.; Buehler, M.J.; Yip, S.; et al. Combinatorial molecular optimization of cement hydrates. Nat. Commun. 2014, 5, 4960. [Google Scholar] [CrossRef]
- Du, J.; Bu, Y.; Shen, Z. Interfacial properties and nanostructural characteristics of epoxy resin in cement matrix. Constr. Build. Mater. 2018, 164, 103–112. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohamed, A.K.; Geissbühler, D.; Manzano, H.; Jamil, T.; Shahsavari, R.; Kalinichev, A.G.; Galmarini, S.; Tao, L.; Heinz, H.; et al. A force field database for cementitious materials including validations, applications and opportunities. Cem. Concr. Res. 2017, 102, 68–89. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, C.; Liu, H.; Zhang, W.; Hu, T. Deterioration mechanism understanding of recycled powder concrete under coupled sulfate attack and freeze–thaw cycles. Constr. Build. Mater. 2023, 388, 131718. [Google Scholar] [CrossRef]
- Allen, B.M.; Predecki, P.K.; Kumosa, M. Integrating open-source software applications to build molecular dynamics systems. J. Comput. Chem. 2014, 35, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Andrade, R.; Birgin, E.G.; Martinez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, S.; Wang, M.; Hou, D.; Hu, J.; Zhang, J.; Geng, Y.; Xie, H.; Hu, M.; Liu, Z. Acid Radical Tolerance of Silane Coatings on Calcium Silicate Hydrate Surfaces in Aggressive Environments: The Role of Nitrate/Sulfate Ratio. Langmuir 2023, 39, 11304–11316. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Li, S.H.; Zhao, Z.P. Insight of organic molecule dissolution and diffusion in cross-linked polydimethylsiloxane using molecular simulation. J. Membr. Sci. 2021, 620, 118863. [Google Scholar] [CrossRef]
- Abdel-Azeim, S. Revisiting OPLS-AA Force Field for the Simulation of Anionic Surfactants in Concentrated Electrolyte Solutions. J. Chem. Theory Comput. 2020, 16, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Heinz, H.; Ramezani-Dakhel, H. Simulations of inorganic-bioorganic interfaces to discover new materials: Insights, comparisons to experiment, challenges, and opportunities. Chem. Soc. Rev. 2016, 45, 412–448. [Google Scholar] [CrossRef]
- Beckstein, O.; Fourrier, A.; Iorga, B.I. Prediction of hydration free energies for the SAMPL4 diverse set of compounds using molecular dynamics simulations with the OPLS-AA force field. J. Comput. Aided Mol. Des. 2014, 28, 265–276. [Google Scholar] [CrossRef]
- Åqvist, J.; Wennerström, P.; Nervall, M.; Bjelic, S.; Brandsdal, B.O. Molecular dynamics simulations of water and biomolecules with a Monte Carlo constant pressure algorithm. Chem. Phys. Lett. 2004, 384, 288–294. [Google Scholar] [CrossRef]
- Fuhrmans, M.; Sanders, B.P.; Marrink, S.-J.; de Vries, A.H. Effects of bundling on the properties of the SPC water model. Theor. Chem. Acc. 2009, 125, 335–344. [Google Scholar] [CrossRef]
- Zielkiewicz, J. Structural properties of water: Comparison of the SPC, SPCE, TIP4P, and TIP5P models of water. J. Chem. Phys. 2005, 123, 104501. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in ‘t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Chavez Thielemann, H.; Cardellini, A.; Fasano, M.; Bergamasco, L.; Alberghini, M.; Ciorra, G.; Chiavazzo, E.; Asinari, P. From GROMACS to LAMMPS: GRO2LAM: A converter for molecular dynamics software. J. Mol. Model 2019, 25, 147. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.T.; Bartels, G.; Campañá, C.; Denniston, C.; Müser, M.H. Implementation of Green’s function molecular dynamics: An extension to LAMMPS. Comput. Phys. Commun. 2009, 180, 1004–1010. [Google Scholar] [CrossRef]
- Chempath, S.; Clark, L.A.; Snurr, R.Q. Two general methods for grand canonical ensemble simulation of molecules with internal flexibility. J. Chem. Phys. 2003, 118, 1562607. [Google Scholar] [CrossRef]
- Eslami, H.; Muller-Plathe, F. Molecular dynamics simulation in the grand canonical ensemble. J. Comput. Chem. 2007, 28, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Hafner, R.; Guevara-Carrion, G.; Vrabec, J.; Klein, P. Sampling the Bulk Viscosity of Water with Molecular Dynamics Simulation in the Canonical Ensemble. J. Phys. Chem. B 2022, 126, 10172–10184. [Google Scholar] [CrossRef]
- Mark, P.; Nilsson, L. Structure and dynamics of liquid water with different long-range interaction truncation and temperature control methods in molecular dynamics simulations. J. Comput. Chem. 2002, 23, 1211–1219. [Google Scholar] [CrossRef]
- Nielsen, S.O. Nested sampling in the canonical ensemble: Direct calculation of the partition function from NVT trajectories. J. Chem. Phys. 2013, 139, 124104. [Google Scholar] [CrossRef]
- Likhachev, I.V.; Balabaev, N.K.; Galzitskaya, O.V. Available Instruments for Analyzing Molecular Dynamics Trajectories. Open. Biochem. J. 2016, 10, 1–11. [Google Scholar] [CrossRef]
- Scheurer, M.; Rodenkirch, P.; Siggel, M.; Bernardi, R.C.; Schulten, K.; Tajkhorshid, E.; Rudack, T. PyContact: Rapid, Customizable, and Visual Analysis of Noncovalent Interactions in MD Simulations. Biophys. J. 2018, 114, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Yu, S.; Hou, D.; Li, Z.; Sun, H.; Wang, P.; Wang, M. Study on the mechanical properties, gelling products and alkalization process of alkali-activated metakaolin: From experiment to molecular dynamics simulation. J. Build. Eng. 2023, 79, 107705. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, H.; Wang, P.; Hou, D.; Wang, M.; Yin, B.; Li, S. Molecular dynamics simulation study on the hydrophobic mechanism of ettringite nanoporous channels modified by silane and silane/graphene oxide. Appl. Surf. Sci. 2023, 623, 156975. [Google Scholar] [CrossRef]
- Czarnik, P.; Dziarmaga, J. Projected entangled pair states at finite temperature: Iterative self-consistent bond renormalization for exact imaginary time evolution. Phys. Rev. B 2015, 92, 035120. [Google Scholar] [CrossRef]
- Hartkamp, R.; Biance, A.-L.; Fu, L.; Dufrêche, J.-F.; Bonhomme, O.; Joly, L. Measuring surface charge: Why experimental characterization and molecular modeling should be coupled. Curr. Opin. Colloid Interface Sci. 2018, 37, 101–114. [Google Scholar] [CrossRef]
- Zang, Y.; Yang, Q.; Wang, P.; Wang, X.; Hou, D.; Zhao, T.; Chen, J. Molecular dynamics simulation of calcium silicate hydrate/tannic acid interfacial interactions at different temperatures: Configuration, structure and dynamic. Constr. Build. Mater. 2022, 326, 126820. [Google Scholar] [CrossRef]
- Duboue-Dijon, E.; Laage, D. Characterization of the Local Structure in Liquid Water by Various Order Parameters. J. Phys. Chem. B 2015, 119, 8406–8418. [Google Scholar] [CrossRef]
- Foroutan, M.; Darvishi, M.; Fatemi, S.M. Structural and dynamical characterization of water on the Au (100) and graphene surfaces: A molecular dynamics simulation approach. Phys. Rev. E 2017, 96, 033312. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, Y.; Jeong, S.; Kumar, A.; Jho, Y. Two Local States of Ambient Water. J. Korean Phys. Soc. 2020, 76, 1–7. [Google Scholar] [CrossRef]
- Jedlovszky, P.; Vincze, Á.; Horvai, G. New insight into the orientational order of water molecules at the water/1,2-dichloroethane interface: A Monte Carlo simulation study. J. Chem. Phys. 2002, 117, 2271–2280. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).