Abstract
For decades, a significant amount of research has been conducted on the vitrification of mixtures of all kinds of industrial wastes, especially fly ash, both from thermal power plants and municipal waste incinerators. Although the possibility of creating glass from all types of fly ash has been proven through such research, these studies barely focused on the emission of volatile components that takes place during vitrification processes at high temperatures. This is why, after identifying the types of volatilisation that can occur, we characterised the gasses that are emitted during the vitrification of some types of fly ash and other waste in a laboratory furnace. In order to do so, we analysed the Cl2 and SO2 gasses emitted using the DTA/TG/FTIR techniques, as well as the losses of H2O and CO2. The authors also measured the volatilizations directly from the mouth of the furnace using gas chromatography syringes and analysed the possible emission of dioxins. This study is the first analysis of volatile elements of this kind, after numerous vitrifications in recent decades which ignored the volatilisations that occur when using fly ashes. Although the various types of fly ash used generate emissions of Cl2 and SO2, their use as a by-product on an industrial level could be recommended if previous thermal and washing treatments are conducted. These would minimise the above emissions, enabling the use of said fly ash in the production of glasses for commercial frits, even if an efficient industrial-scale gas cleaning system would apply. Furthermore, an appropriate optimised design of its formulation would make it possible to structurally link some of these gaseous components to the glass structure. These types of results will make it possible to calculate the volatilization when vitrifying certain types of industrial waste on an industrial level, although these studies would require prior assessment in a pilot plant.
1. Introduction
Since the end of last century and in the past two decades, the vitrification processes of industrial waste (especially inorganic waste) have been particularly relevant in both the scientific [1] and technological [2,3,4] literature. However, limited information has been registered on the emissions of volatile components that can take place in these vitrification processes, even though there are prior regulations and knowledge in similar industrial processes, in the production of both flat glass and powdered glass (frit), for adding glazing to floors and ceramic coatings. The study of pollutant emissions into the atmosphere has become an important challenge for official organisms in charge of the implementation of new community policies in favour of protecting the atmosphere, and more specifically to accomplish Directive 96/62/CE regarding the management and evaluation of the quality of the air. In order to successfully perform these directives, it is necessary to undertake many different types of partial studies involving the source of pollution and other factors involved, aiming to control and reduce contamination, in order to ensure a high level of environmental protection.
From a sustainable economy point of view, the main general issue with manufacturing glass [5,6] and frits is having to pay special attention to the emissions of both volatile and particulate components into the atmosphere in the immediate environments [7]. Of course, there are other issues that affect the environment, such as the use chain of raw materials [8], the pollution of process wastewater and both solid waste and effluents in the form of mud and sludge. However, this study focuses on the emission of the most toxic volatile components. The recycling possibilities of industrial waste are shown in Table 1.

Table 1.
Most noteworthy possibilities for recycling industrial.
Of all this recyclable waste, fly ash (FA) from thermal power plants and the incineration processes of municipal solid waste (MSW) has been studied extensively [1,9] and has real technological uses [9,10]. Fly ash is a sub-product or waste that comes from these combustion processes and is usually collected in fabric filters. Its chemical and mineralogical composition depends on the initial mineral content of the combustible substance used, which can be one of many (e.g., coal, fuel, MSW or biomass waste), and it ends up as waste after the combustion [11]. This type of waste is created in large amounts, causing environmental issues regarding its storage in landfills due to the aqueous leaching of its toxic secondary components. This is why, for over five decades, experts have been proposing the vitrification of FA as the most efficient solution for its disposal or recycling, to turn it into a material that takes up less space for its subsequent storage or use as a ‘secondary raw material’ (SRM) for manufacturing construction materials [12].
Technical regulations do not allow the use of vitrified FA from MSW or thermal power plants to manufacture conventional glass products, such as hollow glass or flat glass, due to the significant presence of impurities (e.g., iron oxides), but also because of the indirect social rejection that can take place. Therefore, the solution would be to use it as an SRM in the sector of cementitious material manufacturing and in the ceramic sector for flooring and coatings in frits for glazing, thanks to the high versatility of the products and compositions in which it could be used [12,13].
Prior research has paid attention to the emissions of SO2, which, as has been well documented [14], is a suffocating and irritant gas. When it comes into contact with air, it becomes SO3, which, in turn, when in contact with atmospheric humidity, produces sulfuric acid in the form of acid rain. It mainly enters the human body through the airways. Therefore, it is a primary pulmonary irritant and often causes, as well as respiratory disorders, alterations to the sense of taste, headaches and gastric issues.
Chlorine (Cl2) emissions, as well as destroying the ozone layer of the upper layers of the atmosphere, harm the lungs and can cause chemical bronchopneumonia and even acute pulmonary oedema, with a loss of the sense of smell. In concentrations of 0.2 ppm for 30 min, they irritate the eyes and upper airways, and in concentrations of 1 ppm, they cause a burning sensation. In concentrations higher than 1.3–2 ppm, they prevent deep breaths and, after 30–60 min, they cause severe headaches. In concentrations of 15 ppm, there can be deaths from this gas and, in very high concentrations such as 430 ppm, humans die in just around 15 min [14].
Other emissions such as F2 have not been considered in this study, because in our case, the glasses investigated here have not been obtained from raw materials, such as clays or precursors as micaceous minerals, as was the case of the glassy frits investigated from micaceous compositions like miserite, whose fluoride emissions were already analysed in previous years [15].
Vitrification can be achieved from conventional gas furnaces [16] or through plasma arc technology [17,18]. But this type of melting has not been considered here, because the aim of this study involved melting in an electrical laboratory-scale furnace. The main objectives of this paper are (a) to assess, from an environmental point of view, the use of industrial waste from the manufacture of ceramic frits and the impact of the emission of gasses or volatile components from the manufacturing process, and (b) to assess the effect of said emissions on work safety and hygiene, as well as the environmental impact of frits obtained from FA.
2. Materials and Methods
Table 2 shows the mineralogical composition of the original fly ashes and the intensity of the main identification peak via powder X-ray diffraction (XRD) using a BRUKER D8, (Karlsruhe, Germany). The voltage and current employed were 40 kV and 20 mA, respectively. The 2θ scanning interval ranged from 5° to 70°, with an angular step of 0.05° and 3.0 s of counting time per point. The qualitative mineralogical phase analysis was performed with the HighScore Plus 3.0 software (Plus 3.0, PANalytical, Almelo, The Netherlands). The fly ashes from MSWI French incinerators contain chloride compounds, such as KCl, NaCl and CaClOH, as well as an anhydrite sulphate compound (CaSO4). Therefore, when vitrification occurs after the formulation of glass, they will liberate chloride as Cl2 and SO2 in the high-temperature thermal process. Some CO2 also will be liberated, but the purpose of this research was to determine the gases liberated by the chloride and sulphate compounds.

Table 2.
Composition of original fly ashes (French origin, provided by Reyval in L’Alcora, Castellón). XRD intensities of maximum peak for the respective crystalline phases are shown.
Several types of glasses were synthetized in a laboratory electrical Kanthal Super furnace (Kanthal® Super ER, Hallstahammar, Switzerland). In order to formulate these glasses, a mix of FA-1, FA-2, FA-4 and FA-4 (25 wt% each) was made as the only representative sample for mixing with glass cullet (Table 3). The mixing of a total of 50 g, the volume able to fill the refractory (alumina–silica) crucibles used for melting, was carried out in a Turbula (planetary) mixer for one hour. One of the glasses was obtained with a 50–50 mixture (% of the weight) of fly ash + soda lime cullet glass (GLASS 1), and the other with a 75–25 mixture of fly ash + cullet glass (GLASS 2), obtained from the average FA from a French MSW incinerator, in order to vitrify it and then assess the gasses (Cl2 and SO2) emitted throughout the process on a laboratory scale. For these initial formulations, we mixed the FA with the cullet glass, whose oxide composition can be seen in Table 2. Figure 1a shows the four original FA compositions simplified to the CaO-Al2O3-SiO2 ternary diagram. Figure 1b shows the areas where similar wastes are located, compared to other construction materials.

Table 3.
Composition of the cullet glass and FA (% of the weight) determined via XRF (provided by the company Reyval in L’Alcora, Castellón).
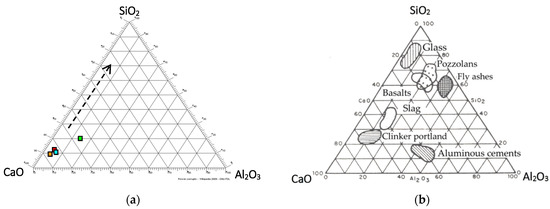
Figure 1.
(a) Location of the FA compositions in the ternary diagram CaO-Al2O3-SiO2 (red: FA-1; green: FA-2; orange: FA-3 and blue: FA-4); (b) the same ternary structure, but adding the areas where some types of solid waste, basaltic rocks and several other construction materials are located in this ternary diagram.
The methods that are commonly applied to analyse raw materials and their resulting glasses have been used: X-ray diffraction (XRD), X-ray fluorescence (XRF), differential thermal and thermogravimetric analyses (DTA/TGA) and scanning electron microscopy The arrow requires no explanation. It is usually used in this type of charts with microanalysis using energy dispersive spectroscopy (SEM/EDS). The chemical analysis of the main elements was carried out via X-ray fluorescence (XRF) using conventional techniques, with a PHILIPS PW2400 X-ray Spectrometer (Mahwah, NJ, USA). In this research, a DTA/TGA Mettler Toledo, 1 Star System model, Barcelona (Spain) and a SEM/EDX Hitachi S-3000N, Tokyo (Japan), have been used. To assess the gasses emitted, we conducted a thermogravimetric analysis using mass spectrometry (TG/MS) with a PerkinElmer Clarus® 680 C GC/MS (Danbury, CT, USA), heating at a speed of 20 K/min in an Ar atmosphere, and a direct analysis of gasses via chromatography, taken directly through an alumina pipe placed on the lid of the furnace as a chimney [19]. To do so, we used Kitagawa (EURO-Gas Management Services, Brixham, UK) glass tubes.
The furnace used was the Kanthal Super HT (Kanthal® Super ER, Hallstahammar, Switzerland), which has a side door, where the mixtures were vitrified at 1450 °C for 1 h in refractory silico-aluminous crucibles from Lomba-Camiña, Vigo, Spain. We used glass cullet as a fluxing agent and thinner in the formulation of original or initial mixtures due to its additional provision of SiO2.
3. Results and Discussion
As predicted, due to the amount of formers, modifiers and intermediate oxides of the glass structures, following the vitrification process, we obtained transparent glasses that had colours between brown and dark beige, similar to others that had been obtained previously by Barbieri [10]. They flow well due to their low viscosity at 1450 °C through stainless steel metallic moulds. Table 4 shows the results of the chemical analysis carried out by using XRF. They reveal a significant difference between the compositions expected in the initial formulation and the glasses that were ultimately obtained, both for GLASS 1 and GLASS 2. The alumina and silica (expressed in oxides) increased significantly due to the attack or corrosion of the cast components on the silico-aluminous crucibles used. The same occurs with MgO and the other oxides of the formulation, with the differences being due to the adjustment of the compositions due to these increases in SiO2 and Al2O3.

Table 4.
Average analysis (SEM/EDS) of the areas observed at low magnification in glasses obtained from averaged fly ashes used for vitrification. The microanalysis was performed at 30× magnification, so that the entire sample in the sample holder was covered by the scan area.
It can be seen in Table 4 that the initial contents of chlorides and sulphates (expressed in XRF analysis as Cl2 and SO2) decrease notably in the final glasses. Therefore, these differences give us an idea about the severe volatilization rates of these components, representing of 94 and 96% for the Cl2 compared to the theoretical initial mixtures, and a relative volatilization of SO2 between 84 and 94%, for GLASS 1 and GLASS 2, respectively. Representing the actual and simplified composition of these glasses after the vitrification process of the fly ash studied herein, simplifying by using the ternary plot CaO-Al2O3-SiO2 [20,21], makes it possible to calculate their location regarding possible crystallisations. GLASS 1, which has more silica, is in the centre of the crystallisation area of pseudo-wollastonite, and GLASS 2 is at the end of the crystallisation area of alpha dicalcium silicate (alfa-2CaO·SiO2) and close to the area of gehlenite (2CaO·Al2O3·SiO2). This diagram has frequently been used for optimizing the vitrification of fly ashes and for the evaluation of which crystalline phases can be nucleated in the cooling of melt for moulding these glasses, or by quenching in water as powdered frits [22].
Therefore, the theoretical location in the equilibrium diagram (Figure 2) of these synthesized glasses makes it possible to predict that, if these glasses that are obtained from fly ash are cooled slowly, they would give way to glass–ceramic materials composed of these crystalline phases. Similarly, when obtaining frits for glazing from these compositions, the glazing could have a ‘latent’ glass crystalline microstructure, as is common in many glazes formulated with glass–ceramic compositions. This type of crystalline phase formation would take place on the surface, leading to matt surfaces or even surfaces with greater microhardness resistance and/or improved abrasive wear due to surface crystallisations.
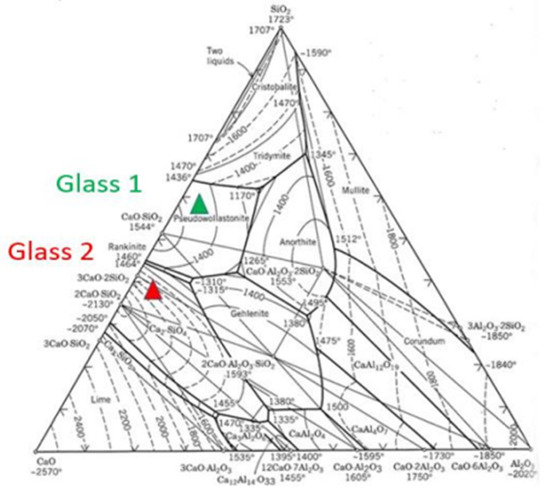
Figure 2.
Equilibrium diagram of the CaO-Al2O3-SiO2 ternary structure (green: GLASS 1, red: GLASS 2) [20,21].
As is usual in the thermal analysis of complex systems (slags, glasses and ceramics), the DTA/TG analysis shows the losses of volatile components [23]. In this case, progressive losses were observed from 100 °C to 1300 °C. Therefore, as is shown in Figure 3, the FA-4 ash, which is representative of three of the types of ashes used here, clearly shows a progressive loss due to the volatilization of these components (Figure 3).

Figure 3.
Results of the TGA and DTA analysis when heating fly ash FA-4 (in this case, the peaks or endo bands are represented upwards, and the exo bands are downwards).
It seems like ash FA-4, which is representative of the types of ash used in the vitrification process in the present research, gives rise to significant volatilizations at 450 °C and 950 °C, which would correspond, respectively, to the losses of chlorine and sulphur dioxide if these ashes were treated thermally without mixing them with any other vitrification component. The small endothermic step at 100 °C corresponds to the water losses from the humidity of samples.
A more accurate analysis, combining the thermal and spectroscopic techniques (TG/MS) on the glasses, confirms that these volatilisations always take place when heating the glasses at higher temperatures, which signals that part of both volatile components of the original ashes are ‘retained’ in the glass structure, becoming part of it. In fact, it has been known for some time [24] that many industrial glasses, and especially amber-type or amber-coloured bottles (hollow glass), have significant amounts of sulphates structurally retained due to their connection to iron oxides, giving rise to bonds to the structure of these types of glass in specific redox conditions. Table 5 shows the temperatures at which these volatilizations take place (after heating the glasses obtained at high temperatures in a TG/MS analysis).

Table 5.
Temperatures at which volatilisations were detected in GLASS 1 and GLASS 2.
Table 6 shows the end results of measuring the volatile elements directly from the mouth or furnace ‘chimney’, as we made a hole to create a ‘chimney’ to insert specific commercial syringes to take samples using gas chromatography [19]. According to the BREF documentation on the emission of volatile elements shown in Table 6, the emissions of chlorine and sulphur surpassed the limits set when considering that this study only deals with fusions on a laboratory scale. According to this regulation, we were unable to establish the loss of other components or particles, as we worked with experimental ‘model glasses’ melted using 30 g of the initial mixtures, without adding boron or fluoride to the raw materials. The same can be said for the metals that appear in this monitoring of emissions for the industrial scale.

Table 6.
Results of the concentration of volatile elements via direct sample retrieval during the vitrification process in the Kanthal Super HT laboratory furnace (method: gas chromatography of samples taken with commercial syringes in the mouth of the furnace during the fusion process).
A total of 0.744 mg/m3 of Cl2 emissions was detected via chromatography (Table 6), following the sample collection methodology shown in the non-published Rincón video [16], a figure that is within the 0.1–20 mg/Nm3 range allowed for this type of emission, and which is shown in Table 6. Cl2 emissions are conditioned based on the presence of chlorine in the raw materials. The emissions are not related to the studied temperature interval, depending only on the initial contents. Furthermore, whole chlorine is emitted, as there is no intermediate mineral phase able to delay the process. Similarly, the emitted SO2 value is 786.01 mg/m3 (Table 6), which is within the 50–4000 mg/Nm3 range allowed by the BREF regulation (Table 7). Lastly, when taking as a reference the limit values for kg/t melted, and considering that, in each crucible of the laboratory furnace, we melted around 30 g of the mixture, we also obtain values that are within the lower limit for emissions laid out by the BREF regulation [25]. Sulphur emissions are conditioned based on the mineralogical composition and, to a lesser extent, the firing temperature.

Table 7.
Emissions according to the BREF regulation [24].
4. Conclusions
This study has experimentally shown that the laboratory vitrification of fly ash can make it possible to obtain useful frits for the flooring and ceramic coating industries. Although different types of fly ash are already used extensively in cement plants (where their level of production and trading has been established for several decades), the abovementioned industries would be ideal to recycle fly ash as a secondary raw material. The glass (frits) obtained are environmentally stable and translucent when moulded into blocks or bars, although slightly coloured due to the percentage of FeO and Fe2O3 from the fly ash from the incineration processes of MSW. This study is the first time that an analysis of volatile elements of this kind has been performed, after numerous vitrifications in recent decades which ignored the volatilisations that occur when using fly ash waste.
Although the various types of fly ash used herein for the production of glass on a laboratory scale generate emissions of Cl2 and SO2, their use as a secondary raw material on an industrial level could be recommended if previous thermal and washing treatments are conducted. These would minimise the above emissions, enabling the use of said fly ash in the production of glasses for commercial frits, even if an efficient industrial-scale gas cleaning system would apply. Furthermore, an appropriate optimised design of its formulation would make it possible to structurally link some of these gaseous components to the glass structure.
Author Contributions
Conceptualisation, J.M.R. and M.M.J.V.; methodology, M.B.A.-C. and M.Á.M.; formal analysis, P.C.; investigation, J.M.R. and M.M.J.V.; resources, J.M.R.; writing—original draft preparation, J.M.R., M.B.A.-C. and M.M.J.V.; writing—review and editing, J.M.R., M.B.A.-C. and M.M.J.V.; supervision, J.M.R. and M.M.J.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request from the authors.
Acknowledgments
We wish to thank the company REYVAL in L’Alcora, which made funding this research possible, and Mettler-Toledo (Hammer and Reisen, Switzerland) for helping with the TG/DSC 1-MS. We also wish to acknowledge Juan Rubio of the ICV-CSIC for the TG/FTIR measurements, the technical services of the UC-LM for the FRX and SEM/EDS analyses, and Leticia Moya of INTERLAB, Barcelona, for the gas sample collection for chromatography. Lastly, we would particularly like to thank Beatriz Rincón-Mora, for her help with the experimental work.
Conflicts of Interest
Author M.A. Montealegre was employed by the company Ikergune A.I.E.-Grupo Inzu. All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Rincón, J.M.; Romero, M.; Boccaccini, A.R. Microstructural characterisation of a glass and a glass-ceramic obtained from municipal incinerator fly ash. J. Mater. Sci. 1999, 34, 4413–4423. [Google Scholar] [CrossRef]
- Sanito, R.C.; Bernuy-Zurnaeta, M.; You, S.-J.; Wang, Y.-F. A review on vitrification technologies of hazardous waste. J. Environ. Manag. 2022, 316, 115243. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Heo, J. Vitrification of fly ash from municipal solid waste incinerator. J. Hazard. Mater. 2002, 91, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Bingham, P.A.; Hand, R.J. Vitrification of toxic wastes: A brief review. Adv. Appl. Ceram. 2006, 105, 21–31. [Google Scholar] [CrossRef]
- Geiger, G. Environmental and Health Issues in the Glass Industry. Ceram. Bull. 1995, 71, 193–199. [Google Scholar]
- Vinós, J.A. La fabricación del vidrio y la protección del medio ambiente. Bol. Soc. Esp. Ceram. Vidr. 1988, 27, 283–289. [Google Scholar]
- Jordan, M.M.; Boix, A.; Mateu, J.; Sanfeliu, T. Estudio de los niveles de partículas y dióxido de azufre en un área industrial ceramica. Tec. Cerámica 1997, 268, 1003–1007. [Google Scholar]
- Sánchez-Muñoz, L.; Carda, J.B. Materias Primas y Aditivos. Tomo 2.2. Enciclopedia de la Cerámica; Faenza Editrice Ibérica: Castellón, Spain, 2003. [Google Scholar]
- Lam, C.H.K.; Ip, A.W.M.; Barford, J.P.; McKay, G. Use of Incineration MSW Ash: A Review. Sustainability 2010, 2, 1943–1968. [Google Scholar] [CrossRef]
- Barbieri, L.; Karamanov, A.; Corradi, A.; Lancellotti, I.; Pelino, M.; Rincón, J.M. Structure, chemical durability, and crystallization behavior of incinerator-based glassy systems. J. Non-Cryst. Solids 2008, 354, 521–528. [Google Scholar] [CrossRef]
- Rawlings, R.D.; Wu, J.P.; Boccaccini, A.R. Glass-ceramics: Their production from wastes—A Review. J. Mater. Sci. 2006, 41, 733–761. [Google Scholar] [CrossRef]
- Alonso, M.C.; Luxan, M.P. Aplicaciones de las Cenizas Volantes en el Campo de la Construcción; IETcc-CSIC: Madrid, Spain, 1995; 115p. [Google Scholar]
- Barraco, F.; Demichelis, F.; Sharifikolouei, E.; Ferraris, M.; Fino, D.; Tommasi, T. Life cycle assessment for the production of MSWI fly-ash bases porous glass-ceramics: Scenarios based on the contribution of silica sources, methane aided, and energy recoveries. Waste Manag. 2023, 157, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Ladrón de Guevara, J.; Moya, V. Toxicología Médica (Clínica e industrial); McGraw-Hill Interamericana de España: Madrid, Spain, 1995. [Google Scholar]
- Rincón, J.M.; Casasola, R. TEM Replica of a Fluoride-Miserite Glass-ceramic Glaze Microstructure. MTAEC9 2015, 49, 229–233. [Google Scholar] [CrossRef]
- Ito, T. Vitrification Fly Ash by Swirling-Flow Furnace. Waste Manag. 1996, 16, 453–460. [Google Scholar] [CrossRef]
- Karoly, Z.; Mohai, I.; Tóth, M.; Wéber, F. Production of glass-ceramics from fly-ash using arc plasma. J. Eur. Ceram. Soc. 2007, 27, 1721–1725. [Google Scholar] [CrossRef]
- Qi, C.; Lin, X.; Zhao, R.; Wang, Z.; Wang, G.; Liu, M.; Zhu, Z.; Niu, C. Composition modification and plasma vitrification of bottom ash from industrial hazardous waste incineration. J. Non-Cryst. Solids 2023, 619, 122579. [Google Scholar] [CrossRef]
- Rincon, J.M.; University Miguel Hernández, Elche, Alicante, Spain. Personal Video Communication, Video over Sampling of Gases, 2010. Private File. (In Spanish).
- Gentile, A.L.; Foster, W.R.; The American Ceramic Society, Columbus, OH, USA. Phase Diagrams for Ceramists. Private communication, 1961.
- Gentile, A.L.; Foster, W.R. Calcium hexaluminate and its stability relations in the system CaO-Al2O3-SiO2. J. Am. Ceram. Soc. 1963, 46, 74–76. [Google Scholar] [CrossRef]
- Sharifikolouen, E.; Baino, F.; Salvo, M.; Tommasi, T.; Pirone, R.; Fino, D.; Ferraris, M. Vitrification of municipal solid wste incineration fly ash: An approach to find the successful batch compositions. Ceram. Int. 2021, 47, 7738–7744. [Google Scholar] [CrossRef]
- Benavidez, E. (Ed.) Thermal Analysis Applied to Complex Systems: Slags, Glasses and Ceramics; Transworld Research Network: Trivandrum, Kerala, India, 2013; pp. 33–71. [Google Scholar]
- Fernández-Navarro, J.M. El Vidrio, 3rd ed.; CSIC: Madrid, Spain, 2003. [Google Scholar]
- Escribano, P.; Carda, J.B.; Cordoncillo, E. Esmaltes y Pigmentos Cerámicos; Faenza Editrice Ibérica: Castellón, Spain, 2001. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).