Cold Spray Process for Co-Deposition of Copper and Aluminum Particles
Abstract
:1. Introduction
2. Experiment Method
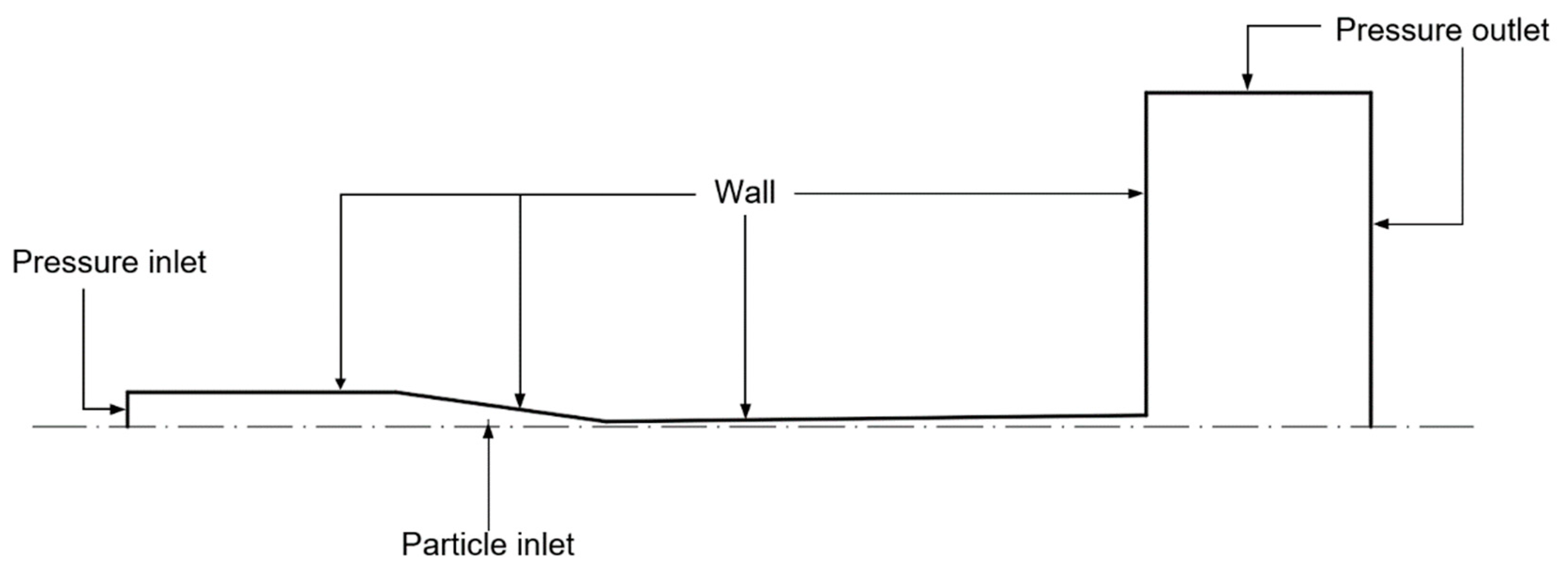
2.1. Cold Spray
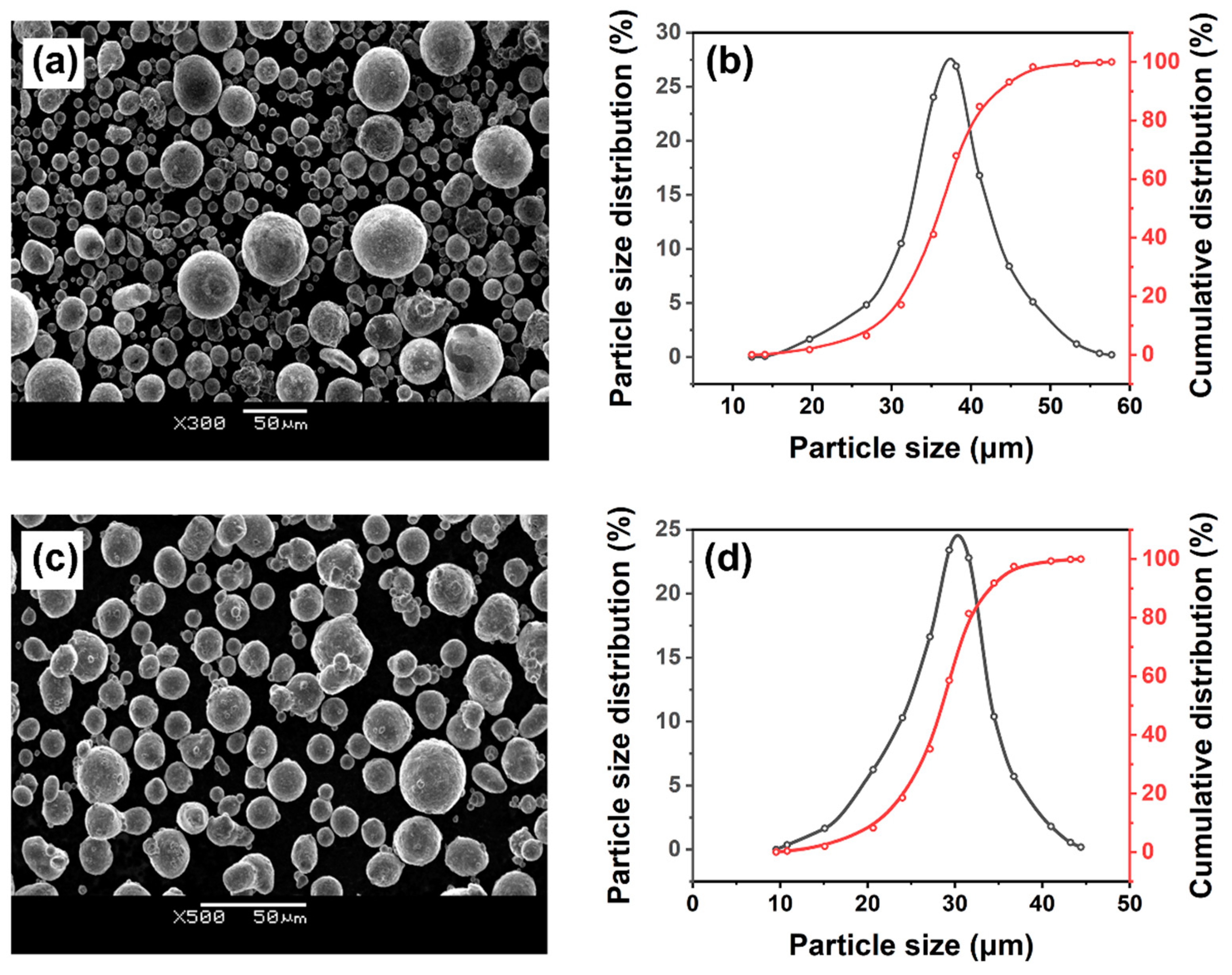
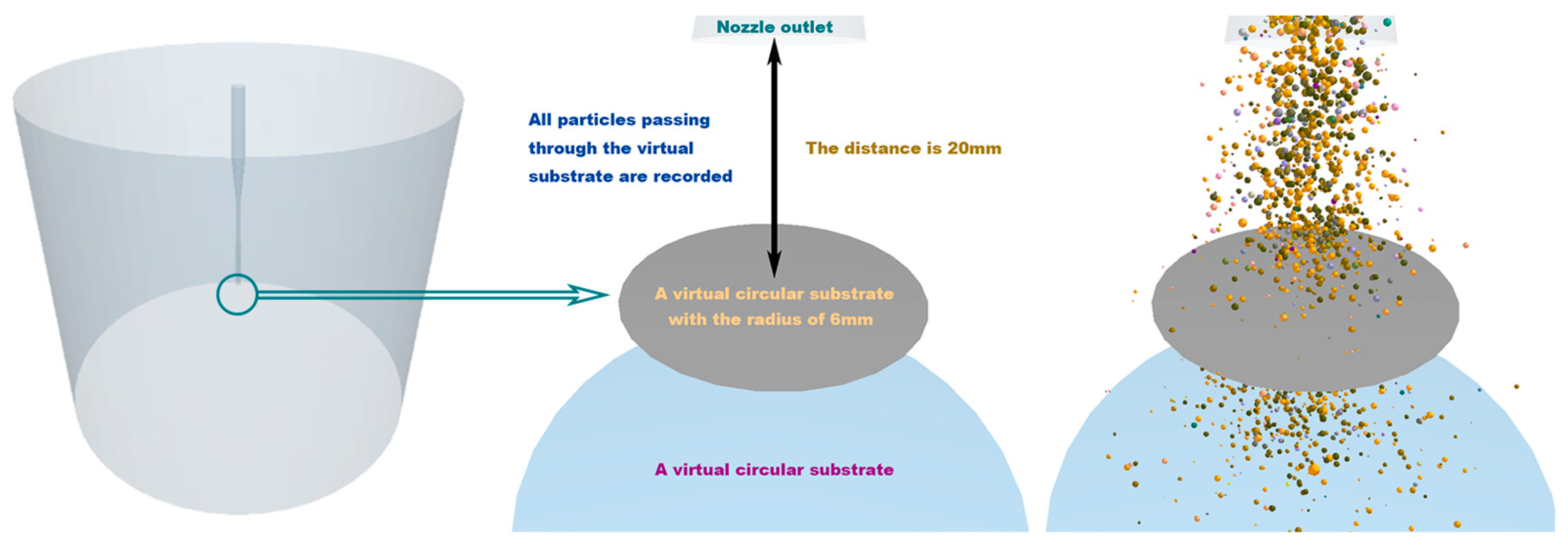
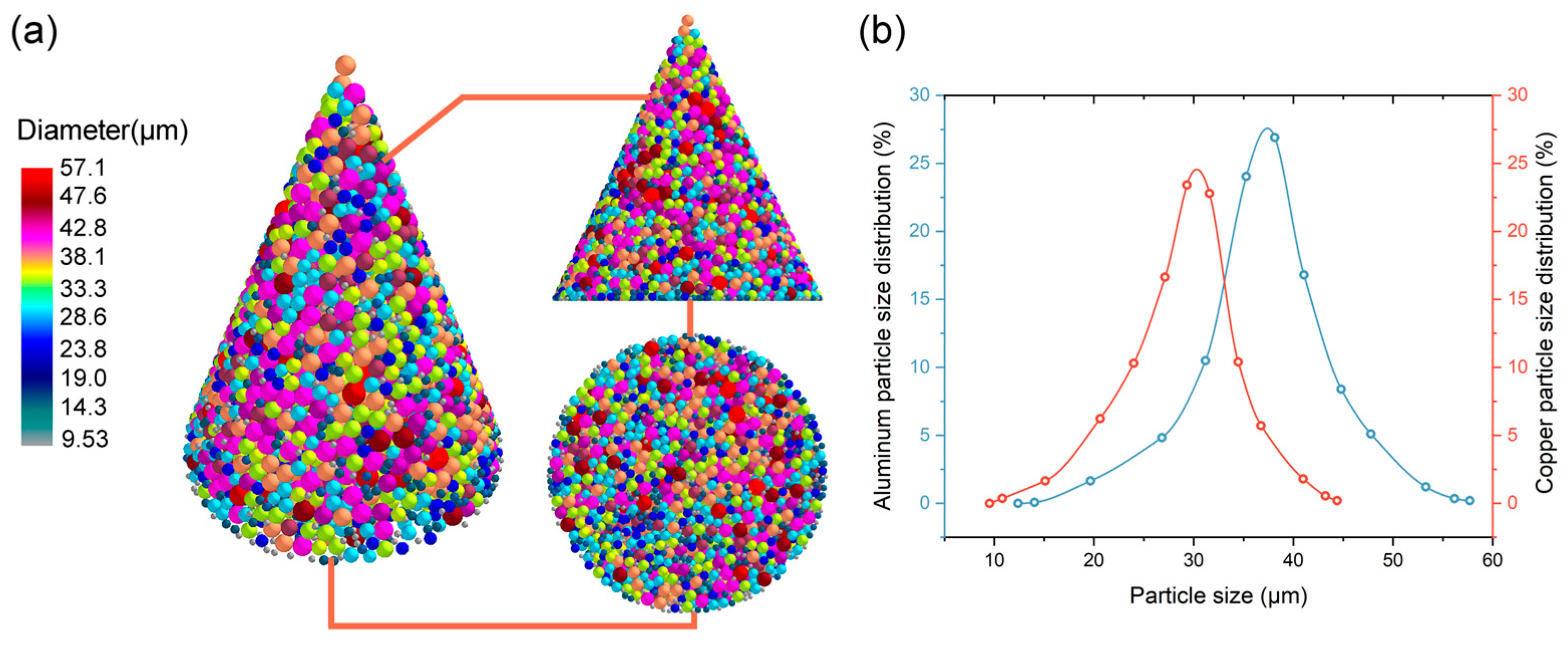
2.2. Particle Distribution Analysis
3. Numerical Method
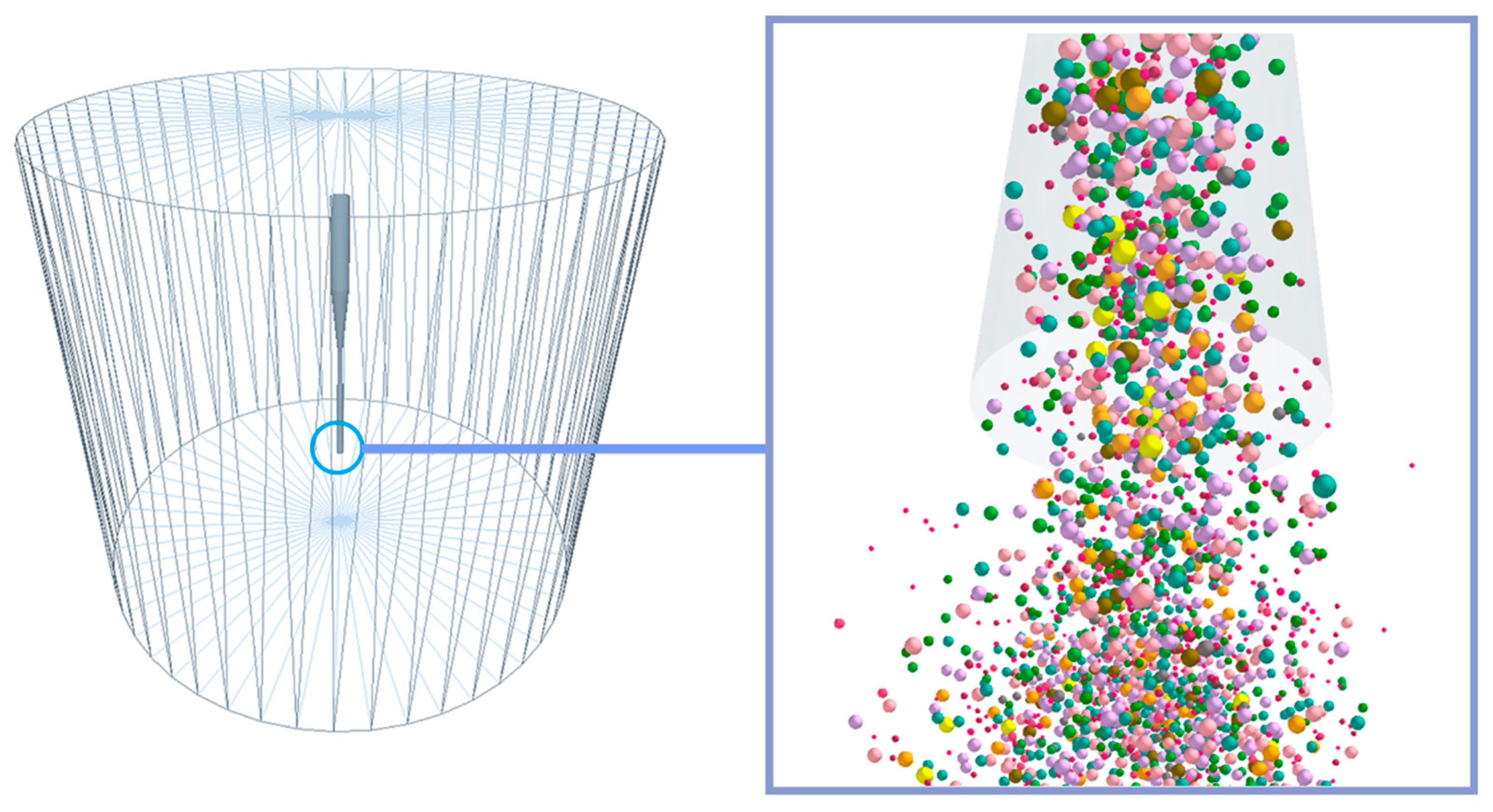
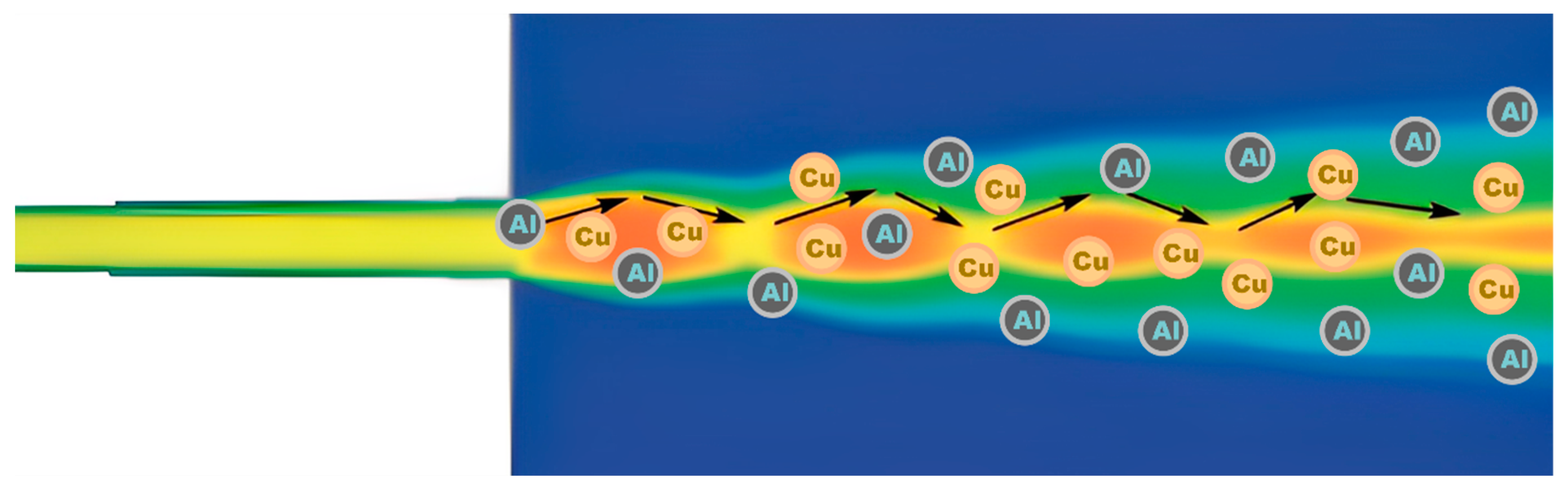
3.1. Coupling Process
3.2. CFD Model
3.2.1. Gas Flow
3.2.2. The Model of CFD
3.3. CFD Model
3.3.1. Particle-Phase Control Equations
3.3.2. Continuous-Phase Control Equation
3.3.3. Continuous-Phase Particle Coupling
4. Results and Discussion
4.1. Numerical Validation of Models
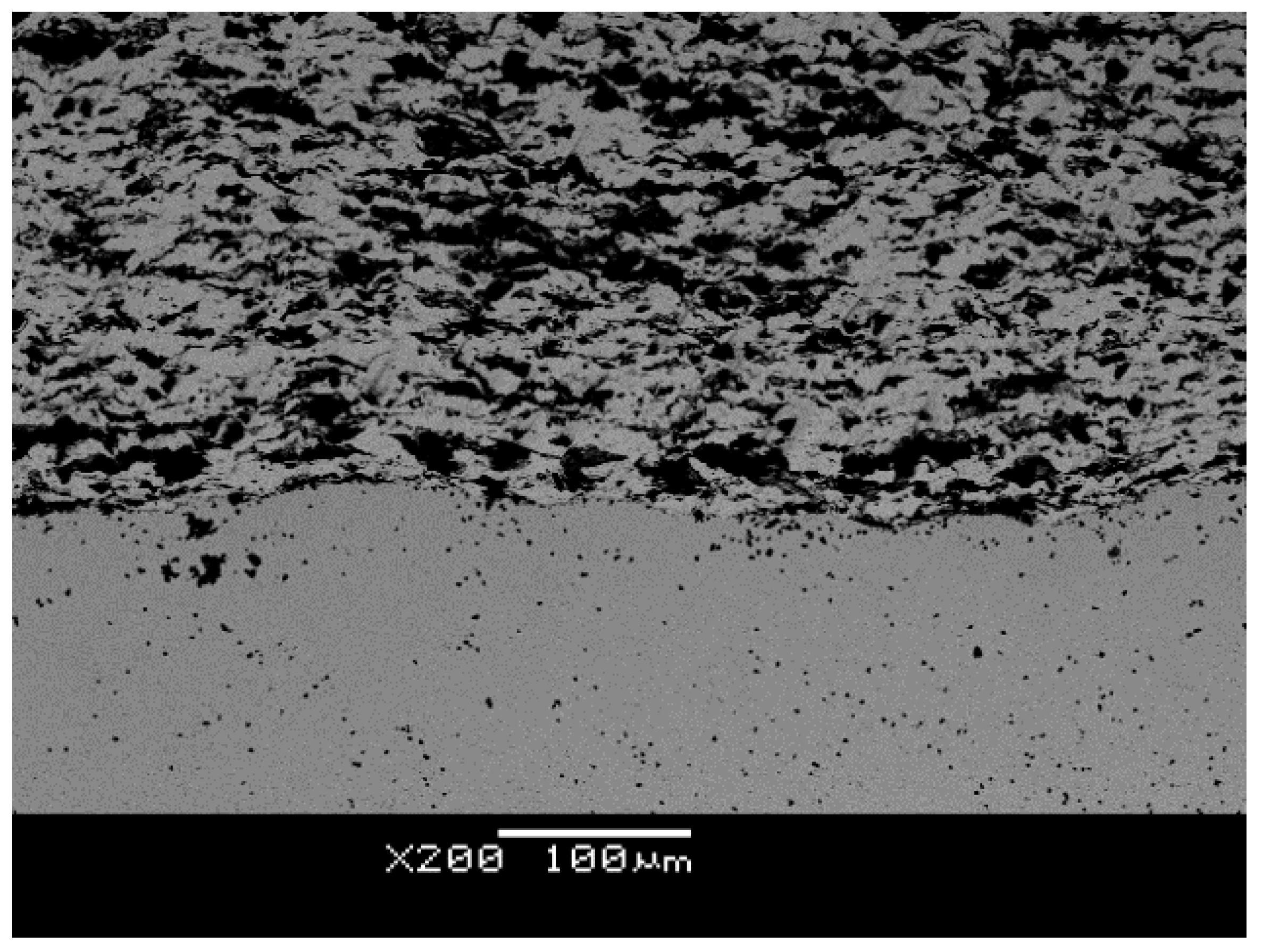
4.1.1. SEM + EDS Verification
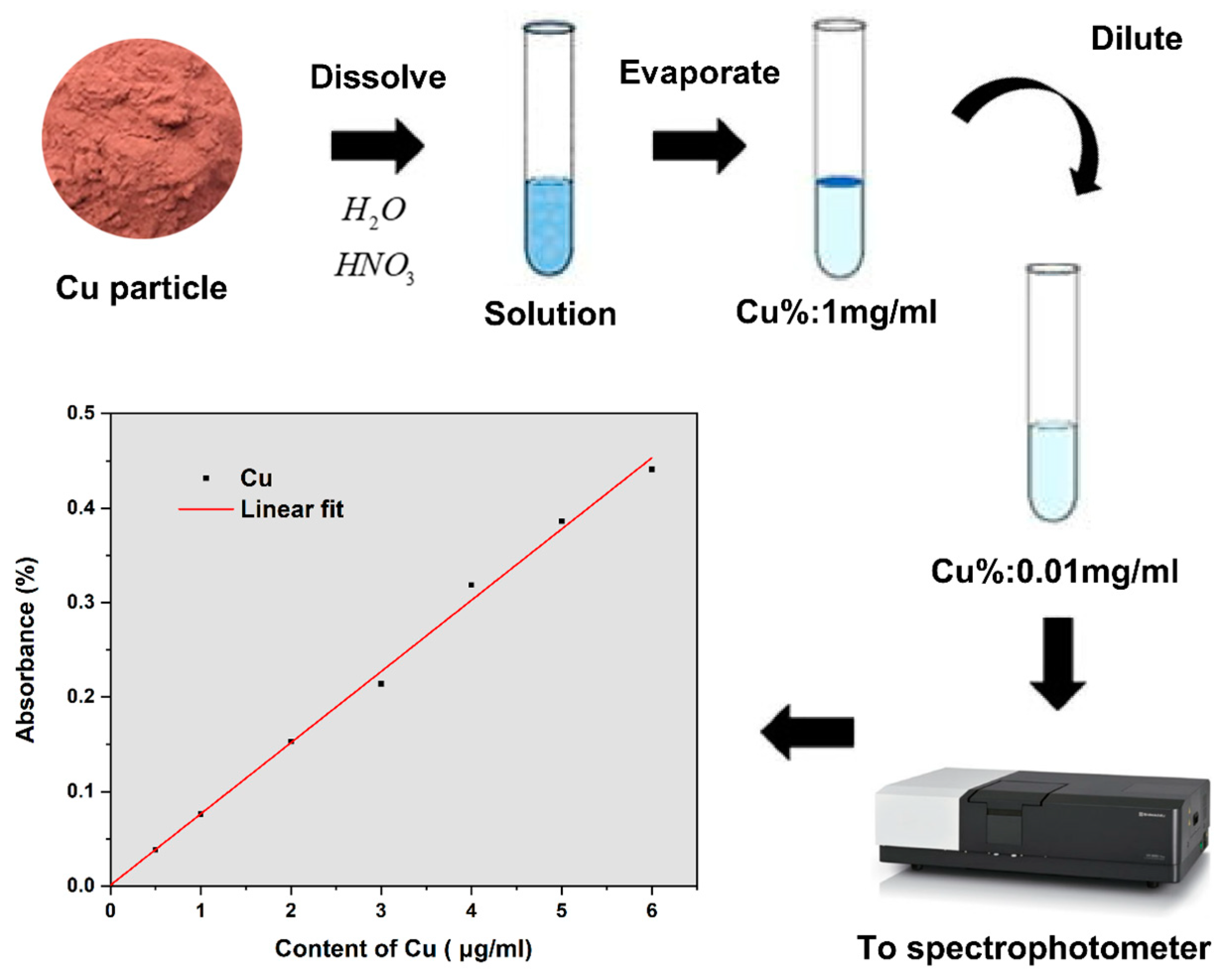
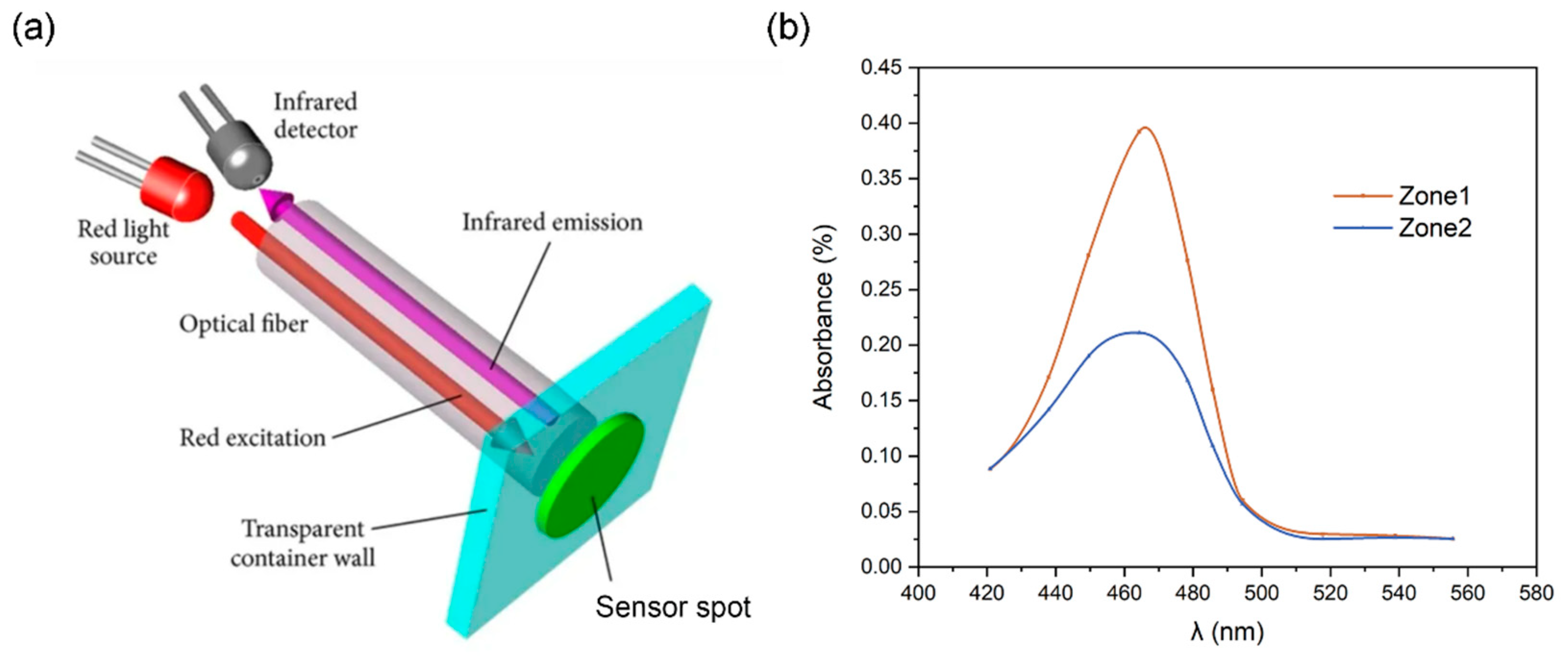
4.1.2. Chemical Composition Verification
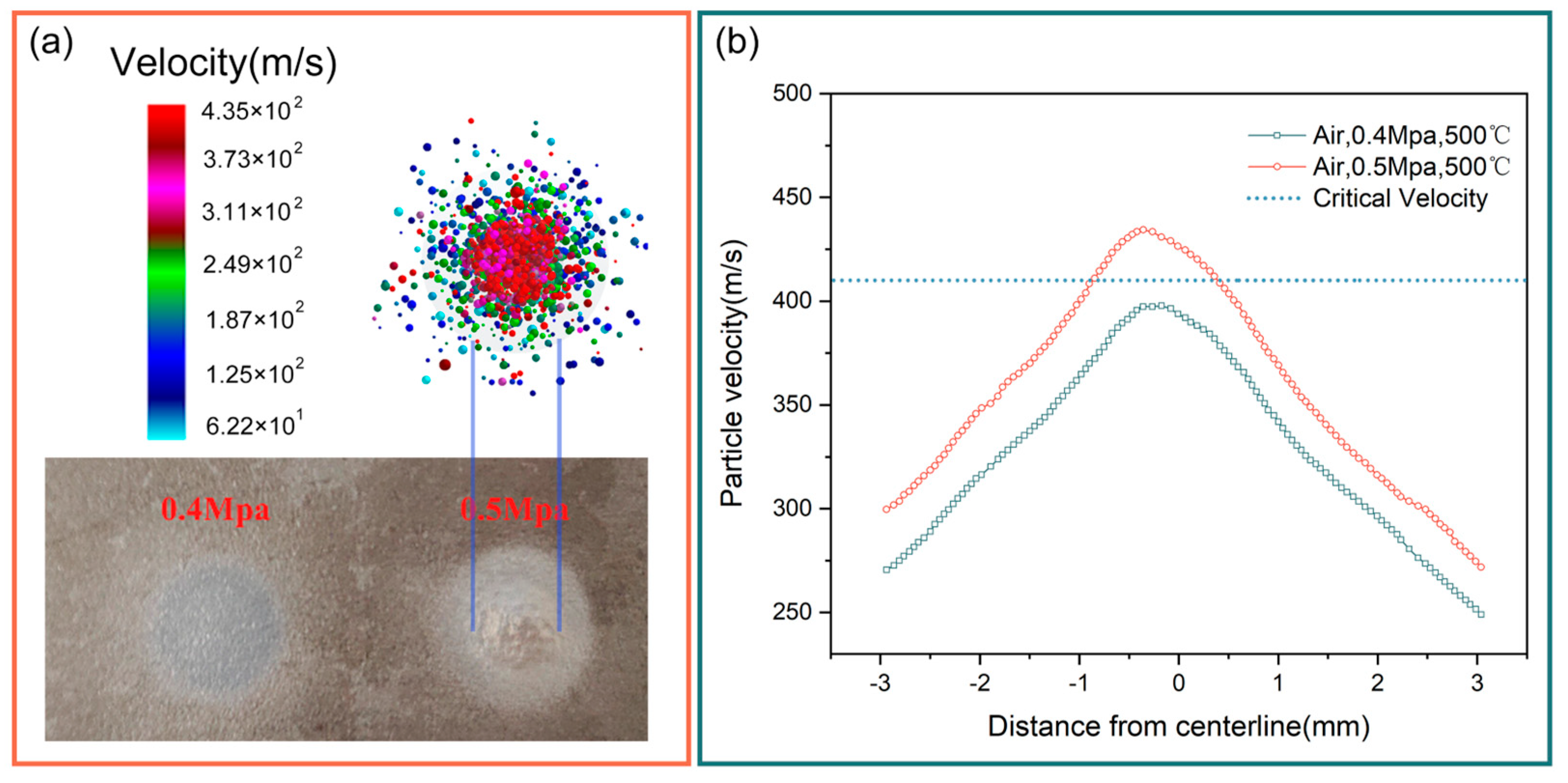
4.1.3. Critical Velocity Verification
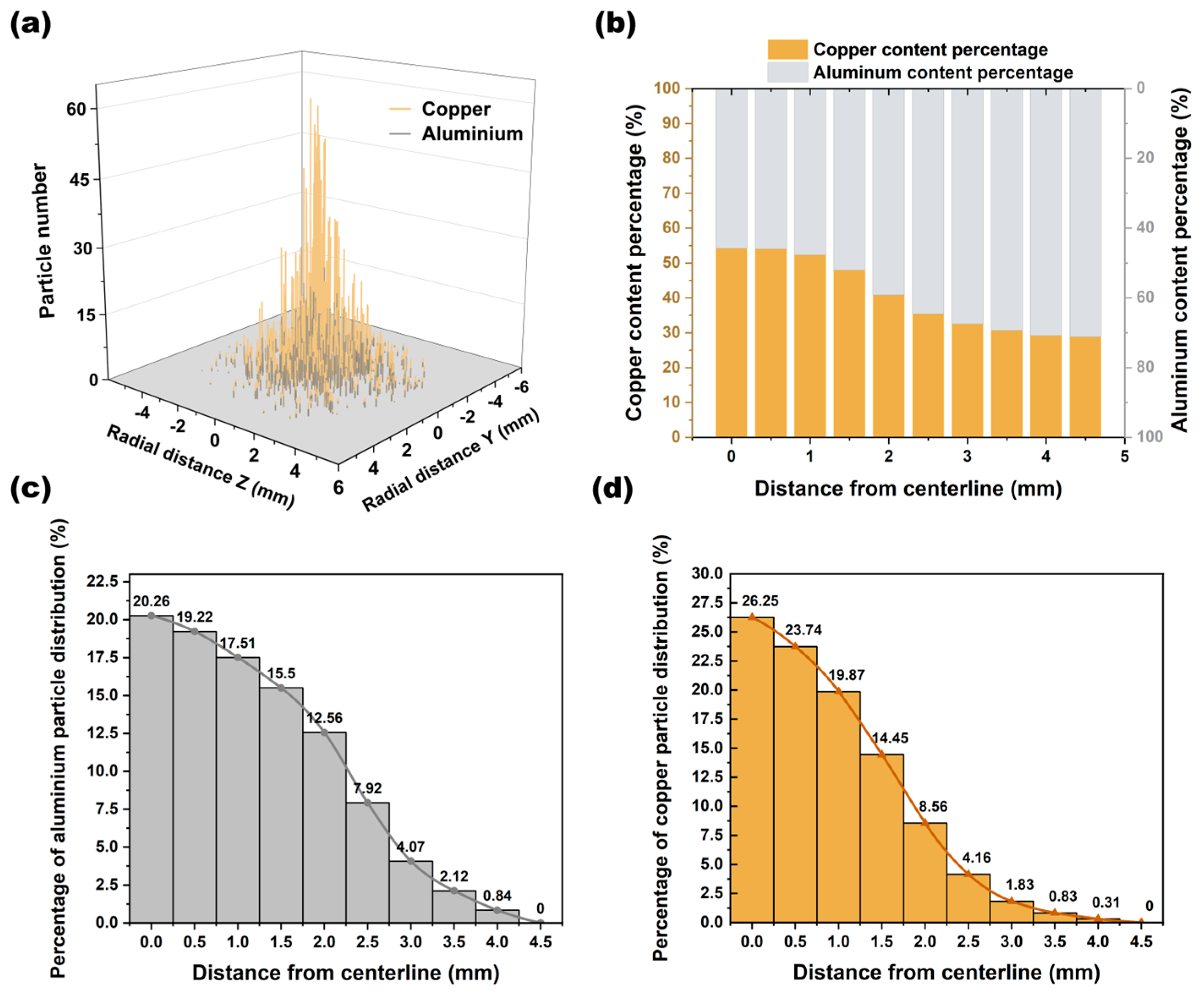
4.2. Particle Distribution
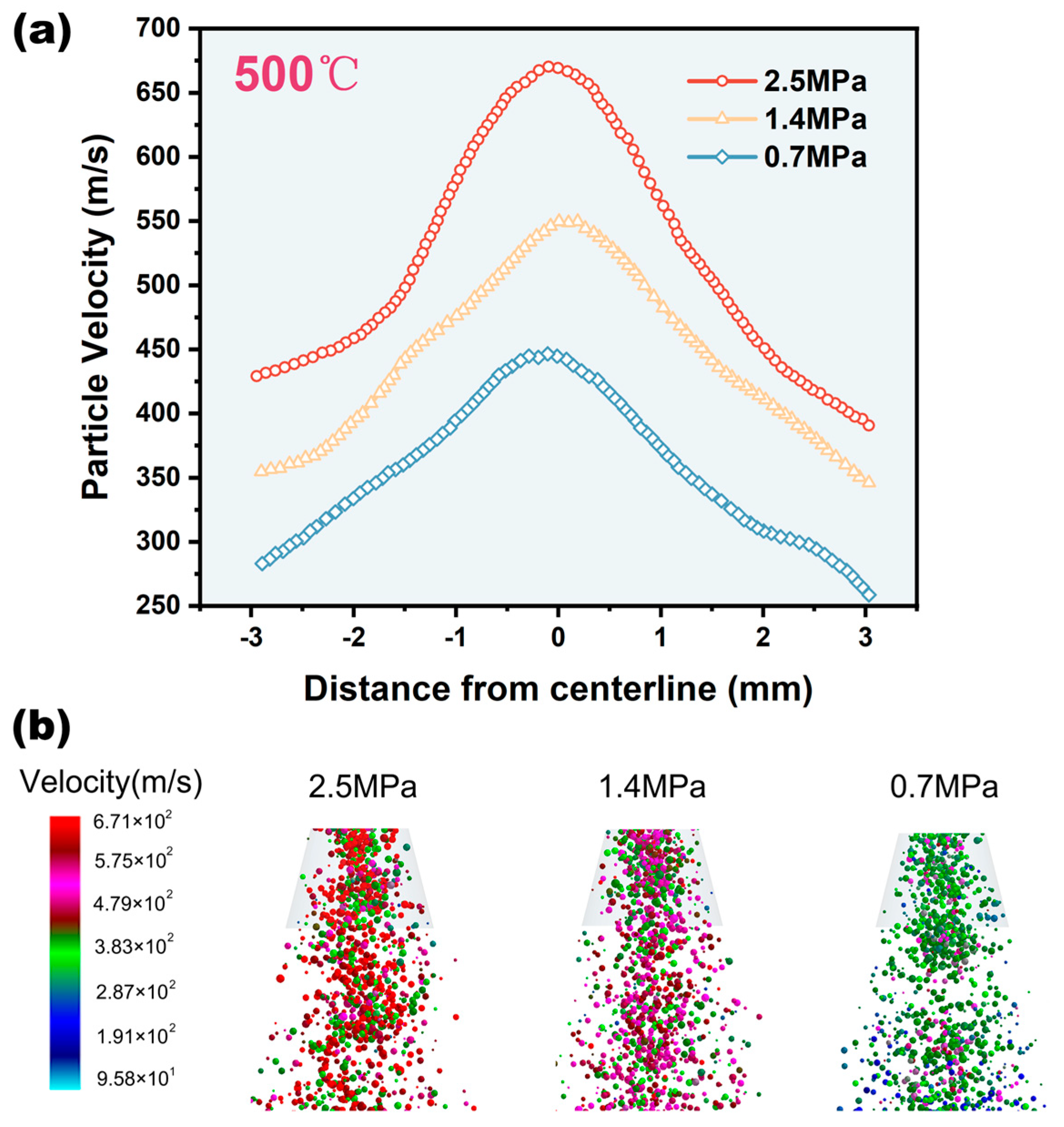
4.3. Influence of Inlet Pressure on Mixed Particles
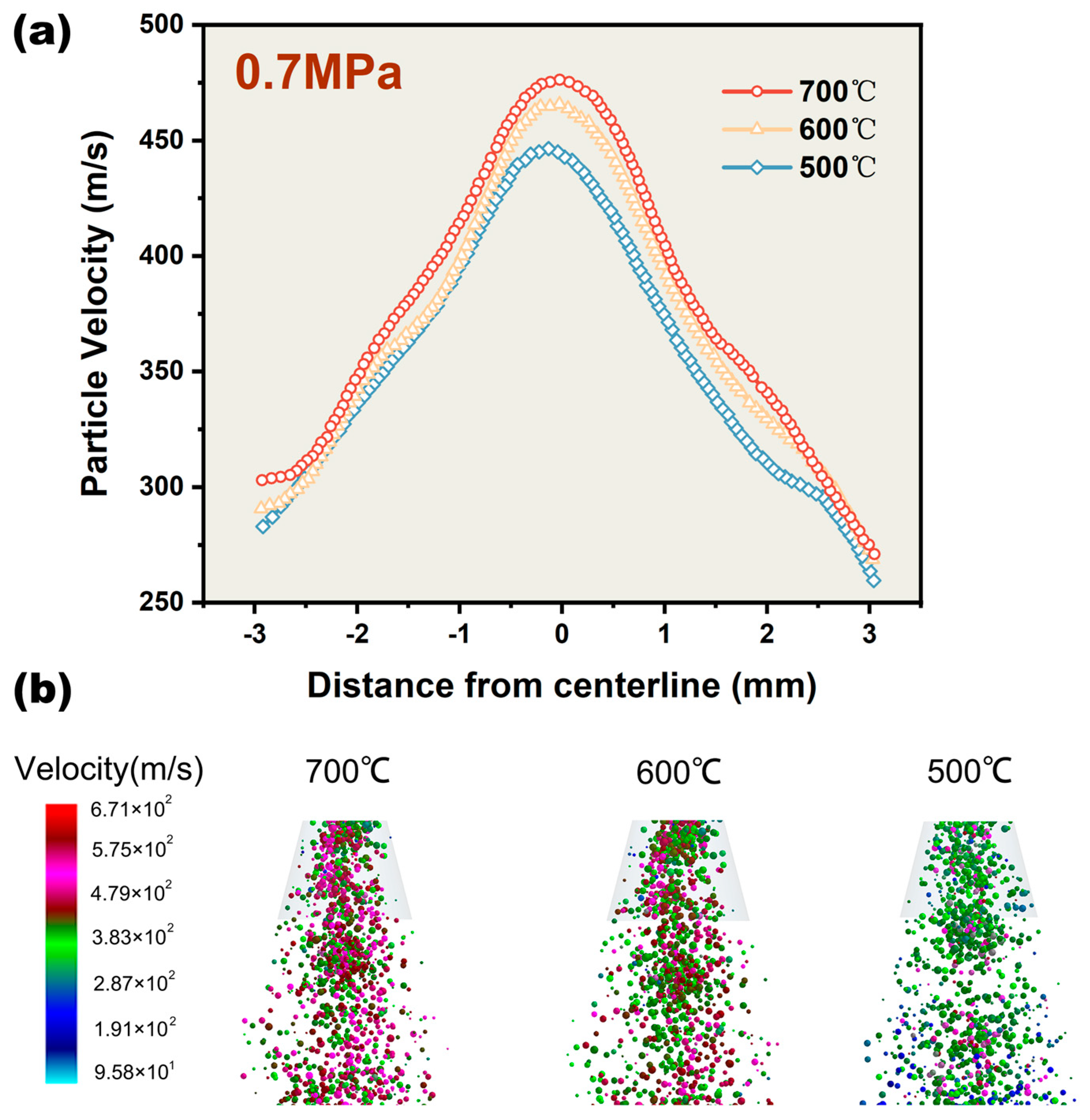
4.4. Influence of Temperature on Mixed Particles
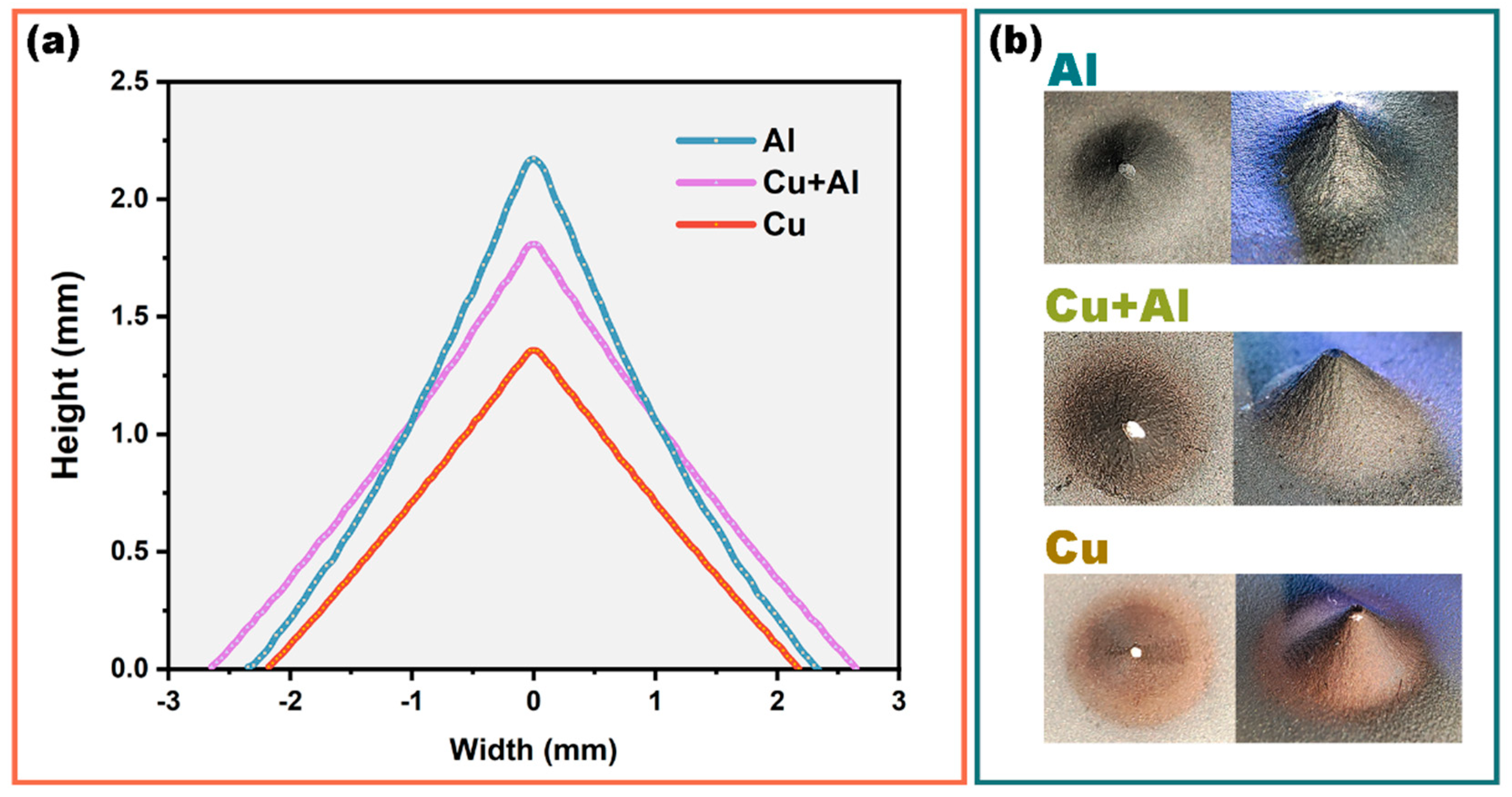
4.5. Deposition Profile and Efficiency
5. Conclusions
- (1)
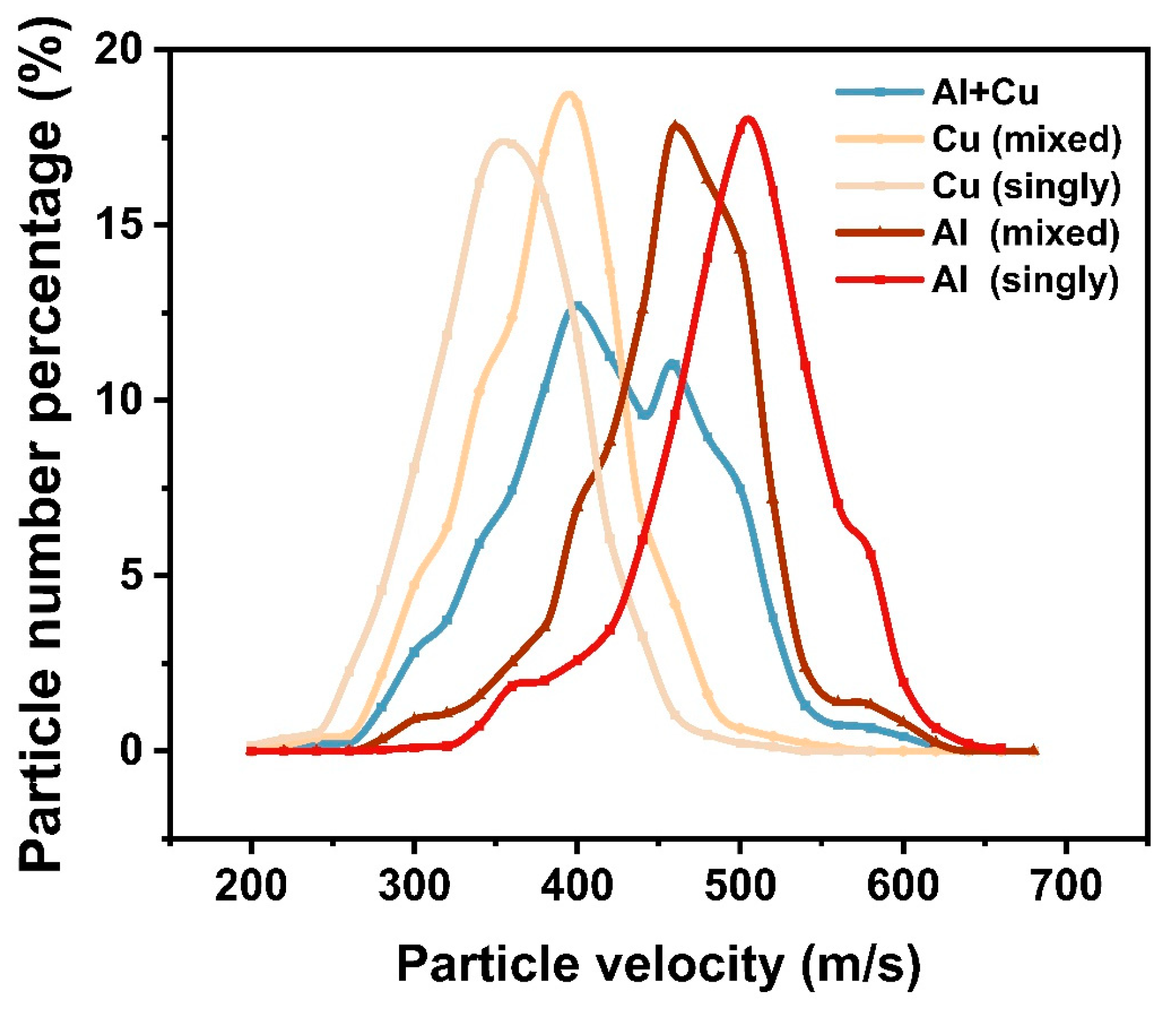
- A large number of particle collisions were observed during spraying, and the mutual collisions of copper and aluminum particles increased the number of copper particles exceeding the critical velocity in the mixed powder by 24.2%. This indicates that when the deposition efficiency of one kind of powder is unsatisfactory, it can be improved by mixing it with another kind of powder with a lower density for spraying.
- (2)
- Mixed-metal spraying increased the disorder in the CSP. Due to the different densities of the copper and aluminum particles, when the powder feed rate increased, the collisions between particles were inevitable. When copper and aluminum particles collided in the direction perpendicular to the jet, the displacement of aluminum particles was more than three times that of copper particles. These collisions made aluminum particles more divergent. Secondly, the existence of the flow field itself made copper and aluminum particles show different divergence and convergence states.
- (3)
- Increasing the temperature and inlet pressure could improve the deposition efficiency of particles, and CFD-DEM revealed the principle more comprehensively. Increasing the inlet pressure could speed up particle velocity, while increasing temperature could reduce particle critical velocity.
- (4)
- In the case of mixed spraying, as the copper particle content in the aluminum powder increased, the height of the deposit decreased, and due to particle collision increases and fluid action, the area deposited on the substrate increased. However, when all or most of the sprayed particles were copper particles, collisions decreased and the fluid effects on particles tended to be similar, and the area deposited on the substrate decreased.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Assadi, H.; Kreye, H.P.D.; Gärtner, F.; Klassen, T. Cold spraying—A materials perspective. Acta Mater. 2016, 116, 382–407. [Google Scholar] [CrossRef]
- Chavan, N.M.; Kiran, B.; Jyothirmayi, A.; Phani, P.S.; Sundararajan, G. The Corrosion Behavior of Cold Sprayed Zinc Coatings on Mild Steel Substrate. J. Therm. Spray Technol. 2013, 22, 463–470. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Bakhsheshi-Rad, H.R.; Saberi, A.; Razzaghi, M.; Kasar, A.K.; Ramakrishna, S.; Menezes, P.L.; Misra, M.; Ismail, A.F.; Sharif, S.; et al. Surface modification of magnesium alloys using thermal and solid-state cold spray processes: Challenges and latest progresses. J. Magnes. Alloys 2022, 10, 2025–2061. [Google Scholar] [CrossRef]
- Rokni, M.R.; Nutt, S.R.; Widener, C.A.; Champagne, V.K.; Hrabe, R.H. Review of Relationship Between Particle Deformation, Coating Microstructure, and Properties in High-Pressure Cold Spray. J. Therm. Spray Technol. 2017, 26, 1308–1355. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Z.; Zhao, J.; Wang, B.; Cai, Y. Numerical analysis of residual stresses induced by cold spray fabricating cBN-reinforced Ni matrix composites. Surf. Coat. Technol. 2023, 467, 129672. [Google Scholar] [CrossRef]
- Cui, L.; Fang, K.; Cao, J.; Hao, E.; Zhu, J.; Liu, G.; Hao, J. A practical method to improve mechanical and electrical properties of ADS copper prepared by cold spray additive manufacturing through powder pretreatment. J. Alloys Compd. 2023, 965, 171319. [Google Scholar] [CrossRef]
- Huang, C.; List, A.; Wiehler, L.; Schulze, M.; Gärtner, F.; Klassen, T. Cold spray deposition of graded Al-SiC composites. Addit. Manuf. 2022, 59, 103116. [Google Scholar] [CrossRef]
- Seng, D.H.L.; Zhang, Z.; Zhang, Z.-Q.; Meng, T.L.; Teo, S.L.; Tan, B.H.; Loi, Q.; Pan, J.; Ba, T. Impact of spray angle and particle velocity in cold sprayed IN718 coatings. Surf. Coat. Technol. 2023, 466, 129623. [Google Scholar] [CrossRef]
- Zhao, Z.B.; Gillispie, B.A.; Smith, J.R. Coating deposition by the kinetic spray process. Surf. Coat. Technol. 2006, 200, 4746–4754. [Google Scholar] [CrossRef]
- Price, T.S.; Shipway, P.H.; McCartney, D.G.; Calla, E.; Zhang, D. A Method for Characterizing the Degree of Inter-particle Bond Formation in Cold Sprayed Coatings. J. Therm. Spray Technol. 2007, 16, 566–570. [Google Scholar] [CrossRef]
- Wang, H.; Li, C.-J.; Yang, G.; Li, C.-X. Cold spraying of Fe/Al powder mixture: Coating characteristics and influence of heat treatment on the phase structure. Appl. Surf. Sci. 2008, 255, 2538–2544. [Google Scholar] [CrossRef]
- Novoselova, T.; Fox, P.; Morgan, R.; O’Neill, W. Experimental study of titanium/aluminium deposits produced by cold gas dynamic spray. Surf. Coat. Technol. 2006, 200, 2775–2783. [Google Scholar] [CrossRef]
- Lee, H.Y.; Jung, S.H.; Lee, S.Y.; Ko, K.H. Alloying of cold-sprayed Al–Ni composite coatings by post-annealing. Appl. Surf. Sci. 2007, 253, 3496–3502. [Google Scholar] [CrossRef]
- Kang, H.-K.; Kang, S.B. Tungsten/copper composite deposits produced by a cold spray. Scr. Mater. 2003, 49, 1169–1174. [Google Scholar] [CrossRef]
- Aydin, H.; Alomair, M.; Wong, W.; Vo, P.; Yue, S. Cold Sprayability of Mixed Commercial Purity Ti Plus Ti6Al4V Metal Powders. J. Therm. Spray Technol. 2017, 26, 360–370. [Google Scholar] [CrossRef]
- Che, H.; Chu, X.; Vo, P.; Yue, S. Cold spray of mixed metal powders on carbon fibre reinforced polymers. Surf. Coat. Technol. 2017, 329, 232–243. [Google Scholar] [CrossRef]
- Chu, X.; Che, H.; Teng, C.; Vo, P.; Yue, S. A multiple particle arrangement model to understand cold spray characteristics of bimodal size 316L/Fe powder mixtures. Surf. Coat. Technol. 2020, 381, 125137. [Google Scholar] [CrossRef]
- Karimi, M.; Fartaj, A.; Rankin, G.; Vanderzwet, D.; Birtch, W.; Villafuerte, J. Numerical simulation of the cold gas dynamic spray process. J. Therm. Spray Technol. 2006, 15, 518–523. [Google Scholar] [CrossRef]
- Liebersbach, P.; Foelsche, A.; Champagne, V.K.; Siopis, M.; Nardi, A.; Schmidt, D.P. CFD Simulations of Feeder Tube Pressure Oscillations and Prediction of Clogging in Cold Spray Nozzles. J. Therm. Spray Technol. 2020, 29, 400–412. [Google Scholar] [CrossRef]
- Faizan-Ur-Rab, M.; Zahiri, S.H.; Masood, S.H.; Phan, T.D.; Jahedi, M.; Nagarajah, R. Application of a holistic 3D model to estimate state of cold spray titanium particles. Mater. Des. 2016, 89, 1227–1241. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, X.; Zhang, M.; Tian, X.; Li, D. Integral numerical modeling of the deposition profile of a cold spraying process as an additive manufacturing technology. Prog. Addit. Manuf. 2019, 4, 357–370. [Google Scholar] [CrossRef]
- Leitz, K.H.; O’Sullivan, M.; Plankensteiner, A.; Lichtenegger, T.; Pirker, S.; Kestler, H.; Sigl, L.S. CFDEM modelling of particle heating and acceleration in cold spraying. Int. J. Refract. Met. Hard Mater. 2018, 73, 192–198. [Google Scholar] [CrossRef]
- Krull, F.; Hesse, R.; Breuninger, P.; Antonyuk, S. Impact behaviour of microparticles with microstructured surfaces: Experimental study and DEM simulation. Chem. Eng. Res. Des. 2018, 135, 175–184. [Google Scholar] [CrossRef]
- Liang, S.; Wang, Y.; Normand, B.; Xie, Y.; Tang, J.; Zhang, H.; Lin, B.; Zheng, H. Numerical and Experimental Investigations of Cold-Sprayed Basalt Fiber-Reinforced Metal Matrix Composite Coating. Materials 2023, 16, 1862. [Google Scholar] [CrossRef]
- Song, X.; Ng, K.L.; Chea, J.M.-K.; Sun, W.; Tan, A.W.-Y.; Zhai, W.; Li, F.; Marinescu, I.; Liu, E. Coupled Eulerian-Lagrangian (CEL) simulation of multiple particle impact during Metal Cold Spray process for coating porosity prediction. Surf. Coat. Technol. 2020, 385, 125433. [Google Scholar] [CrossRef]
- Lanka, D.; Damodaram, R.; Sivaprasad, K.; Prashanth, K.G. Microstructural and mechanical behaviour of friction welded SS316L components fabricated by selective laser melting. Mater. Today Commun. 2023, 37, 107430. [Google Scholar] [CrossRef]
- Samareh, B.; Dolatabadi, A. A three-dimensional analysis of the cold spray process: The effects of substrate location and shape. J. Therm. Spray Technol. 2007, 16, 634–642. [Google Scholar] [CrossRef]
- Schmidt, T.; Gärtner, F.; Assadi, H.; Kreye, H. Development of a generalized parameter window for cold spray deposition. Acta Mater. 2006, 54, 729–742. [Google Scholar] [CrossRef]
- Pawar, S.K.; Abrahams, R.H.M.; Deen, N.G.; Padding, J.T.; van der Gulik, G.J.; Jongsma, A.; Innings, F.; Kuipers, J.A.M. An Experimental Study Of Dynamic Jet Behaviour In A Scaled Cold Flow Spray Dryer Model Using Piv. Can. J. Chem. Eng. 2014, 92, 2013–2020. [Google Scholar] [CrossRef]
- Faizan-Ur-Rab, M.; Zahiri, S.H.; Masood, S.H.; Jahedi, M.; Nagarajah, R. PIV Validation of 3D Multicomponent Model for Cold Spray Within Nitrogen and Helium Supersonic Flow Field. J. Therm. Spray Technol. 2017, 26, 941–957. [Google Scholar] [CrossRef]
- Prairie, M.W.; Frisbie, S.H.; Rao, K.K.; Saksri, A.H.; Parbat, S.; Mitchell, E.J. An accurate, precise, and affordable light emitting diode spectrophotometer for drinking water and other testing with limited resources. PLoS ONE 2020, 15, e0226761. [Google Scholar] [CrossRef]
- Kowalski, K.; Błasiak, P.; Pietrowicz, S. A novel cold spray process flow technique—A numerical investigation. Int. J. Heat Mass Transf. 2024, 218, 124817. [Google Scholar] [CrossRef]
- Li, J.J.; Deng, B.Q.; Zhang, B.; Shen, X.Z.; Kim, C.N. CFD simulation of an unbaffled stirred tank reactor driven by a magnetic rod: Assessment of turbulence models. Water Sci. Technol. 2015, 72, 1308–1318. [Google Scholar] [CrossRef]
- Huang, G.; Gu, D.; Li, X.; Xing, L.; Wang, H. Numerical simulation on syphonage effect of laval nozzle for low pressure cold spray system. J. Mater. Process. Technol. 2014, 214, 2497–2504. [Google Scholar] [CrossRef]
- Rab, M.F.U.; Zahiri, S.; Masood, S.H.; Jahedi, M.; Nagarajah, R. Development of 3D Multicomponent Model for Cold Spray Process Using Nitrogen and Air. Coatings 2015, 5, 688–708. [Google Scholar] [CrossRef]
- Rankin, G.; Jodoin, B. Shock-wave induced spraying: Gas and particle flow and coating analysis. Surf. Coat. Technol. 2012, 207, 435–442. [Google Scholar]
- Cleary, P.W.; Serizawa, Y. A coupled discrete droplet and SPH model for predicting spray impingement onto surfaces and into fluid pools. Appl. Math. Model. 2019, 69, 301–329. [Google Scholar] [CrossRef]
- Di Renzo, A.; Di Maio, F.P. Comparison of contact-force models for the simulation of collisions in DEM-based granular flow codes. Chem. Eng. Sci. 2004, 59, 525–541. [Google Scholar] [CrossRef]
- Yamasaki, M.; Sasaki, Y. Determining Young’s modulus of timber on the basis of a strength database and stress wave propagation velocity I: An estimation method for Young’s modulus employing Monte Carlo simulation. J. Wood Sci. 2010, 56, 269–275. [Google Scholar] [CrossRef]
- Moridi, A.; Hassani-Gangaraj, S.M.; Guagliano, M. A hybrid approach to determine critical and erosion velocities in the cold spray process. Appl. Surf. Sci. 2013, 273, 617–624. [Google Scholar] [CrossRef]
- Assadi, H.; Gärtner, F.; Stoltenhoff, T.; Kreye, H. Bonding mechanism in cold gas spraying. Acta Mater. 2003, 51, 4379–4394. [Google Scholar] [CrossRef]
- Schmidt, T.; Assadi, H.; Gartner, F.; Richter, H.; Stoltenhoff, T.; Kreye, H.; Klassen, T. From Particle Acceleration to Impact and Bonding in Cold Spraying. J. Therm. Spray Technol. 2009, 18, 794–808. [Google Scholar] [CrossRef]
- Hassani-Gangaraj, M.; Veysset, D.; Nelson, K.A.; Schuh, C.A. In-situ observations of single micro-particle impact bonding. Scr. Mater. 2018, 145, 9–13. [Google Scholar] [CrossRef]
- Breault, R.W.; Rowan, S.L.; Monazam, E.; Stewart, K.T. Lateral particle size segregation in a riser under core annular flow conditions due to the Saffman lift force. Powder Technol. 2016, 299, 119–126. [Google Scholar] [CrossRef]
- Tabbara, H.; Gu, S.; McCartney, D.G.; Price, T.S.; Shipway, P.H. Study on Process Optimization of Cold Gas Spraying. J. Therm. Spray Technol. 2011, 20, 608–620. [Google Scholar] [CrossRef]
- Lee, M.W.; Park, J.J.; Kim, D.Y.; Yoon, S.S.; Kim, H.Y.; Kim, D.H.; James, S.C.; Chandra, S.; Coyle, T.; Ryu, J.H.; et al. Optimization of supersonic nozzle flow for titanium dioxide thin-film coating by aerosol deposition. J. Aerosol Sci. 2011, 42, 771–780. [Google Scholar] [CrossRef]
- Zahiri, S.H.; Yang, W.; Jahedi, M. Characterization of Cold Spray Titanium Supersonic Jet. J. Therm. Spray Technol. 2009, 18, 110–117. [Google Scholar] [CrossRef]
- Yokoyama, K.; Watanabe, M.; Kuroda, S.; Gotoh, Y.; Schmidt, T.D.; Gärtner, F. Simulation of Solid Particle Impact Behavior for Spray Processes. Mater. Trans. 2006, 47, 1697–1702. [Google Scholar] [CrossRef]









| Pressure, MPa | Temperature, K | |
|---|---|---|
| Inlet | 0.7 | 773.15 |
| Wall | … | Adiabatic |
| Outlet | 0.101 | 293.15 |
| Geometric Parameter | Length, mm |
|---|---|
| Length of diverging section | 141 |
| Nozzle inlet diameter | 13 |
| Nozzle exit diameter | 3 |
| Length of converging section | 55 |
| Throat diameter | 1.35 |
| Particle Material | Poisson’s Ratio | Solids Density | Modulus of Rigidity | Young’s Modulus |
|---|---|---|---|---|
| Cu | 0.34 | 8960 kg/m3 | 4.63 × 1010 Pa | 1.241 × 1011 Pa |
| Al | 0.33 | 2710 kg/m3 | 2.62 × 1010 Pa | 6.969 × 1010 Pa |
| Results | Region 1 | Region 2 |
|---|---|---|
| Absorbance | 0.3981 | 0.2110 |
| Content of copper (μg/mL) | 5.2709 | 2.7873 |
| Experimentally measured mass percentage (%) | 54.175 ± 3.274% (95%) | 30.046 ± 2.892% (95%) |
| Numerical predations of mass percentage (%) | 54.261 | 32.592 |
| Sample | Powder Weight (g) | Deposition Weight (g) | Deposition Efficiency (%) |
|---|---|---|---|
| Cu | 3.368 | 0.739 | 21.932 |
| Al | 0.996 | 0.309 | 31.053 |
| Cu + Al | 2.244 | 0.713 | 31.774 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Li, H.; Zhang, L.; Xu, Y. Cold Spray Process for Co-Deposition of Copper and Aluminum Particles. Coatings 2023, 13, 1953. https://doi.org/10.3390/coatings13111953
Hu S, Li H, Zhang L, Xu Y. Cold Spray Process for Co-Deposition of Copper and Aluminum Particles. Coatings. 2023; 13(11):1953. https://doi.org/10.3390/coatings13111953
Chicago/Turabian StyleHu, Shijie, Hongjun Li, Liying Zhang, and Yuzhen Xu. 2023. "Cold Spray Process for Co-Deposition of Copper and Aluminum Particles" Coatings 13, no. 11: 1953. https://doi.org/10.3390/coatings13111953
APA StyleHu, S., Li, H., Zhang, L., & Xu, Y. (2023). Cold Spray Process for Co-Deposition of Copper and Aluminum Particles. Coatings, 13(11), 1953. https://doi.org/10.3390/coatings13111953





