Surface Modification of Fast-Growing Wood with a Titanium-Dioxide-Based Nanocoating to Improve Weathering Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Synthesis of Anatase TiO2-NP Using the Hydrothermal Method
2.3. Characterization of Synthesised TiO2-NPs
2.4. Preparation and Coating Application of Nanocoating Materials
2.5. Colour Change Test
2.6. Surface Roughness Test
2.7. Contact Angle Measurement
2.8. Determination of Equilibrium Contact Angles and Wettability Values
2.9. Photocatalyst Activity Test
2.10. FTIR Spectrometry
2.11. Data Analysis
3. Results
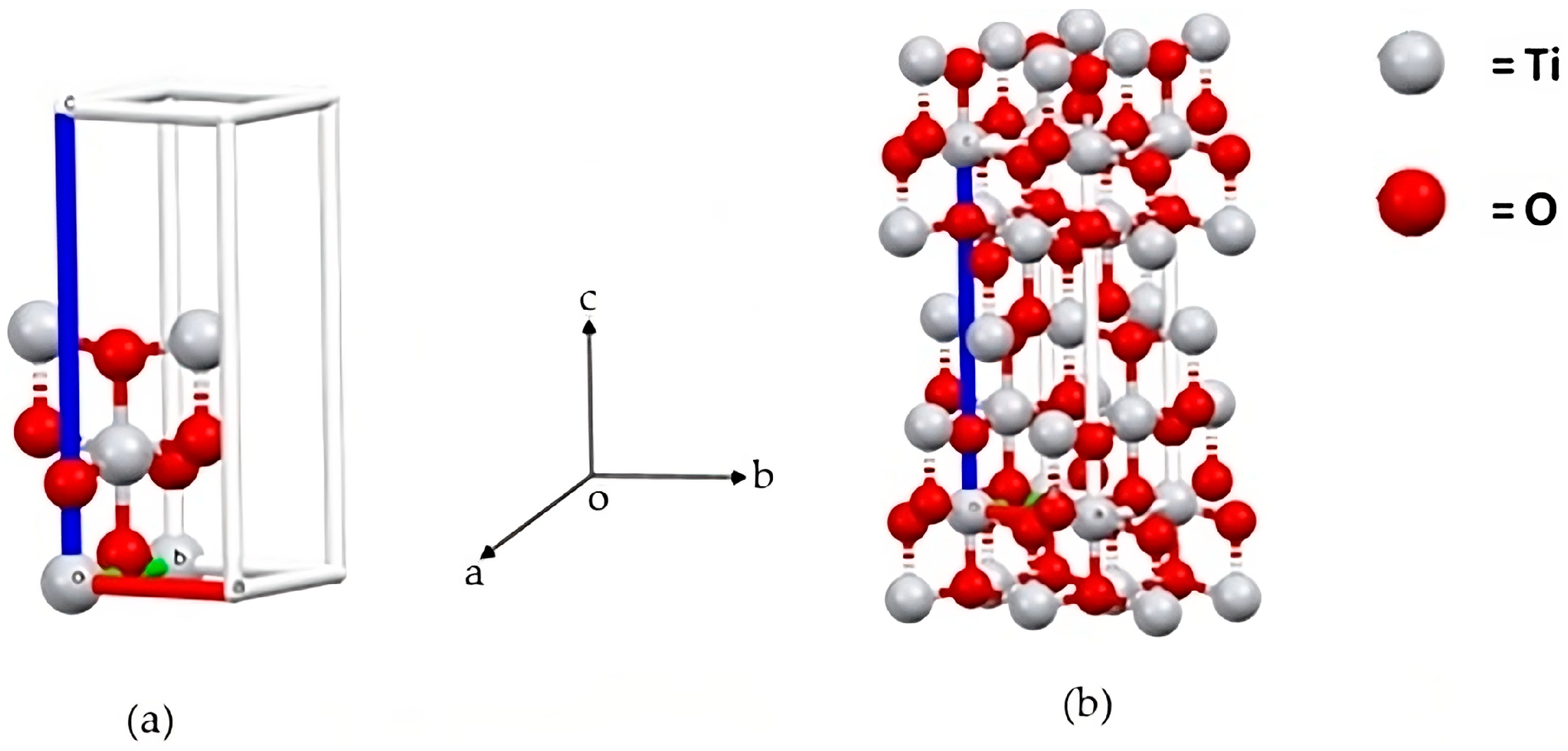
3.1. Synthesised TiO2-NP
3.1.1. Particle Size and Zeta Potential Analysis
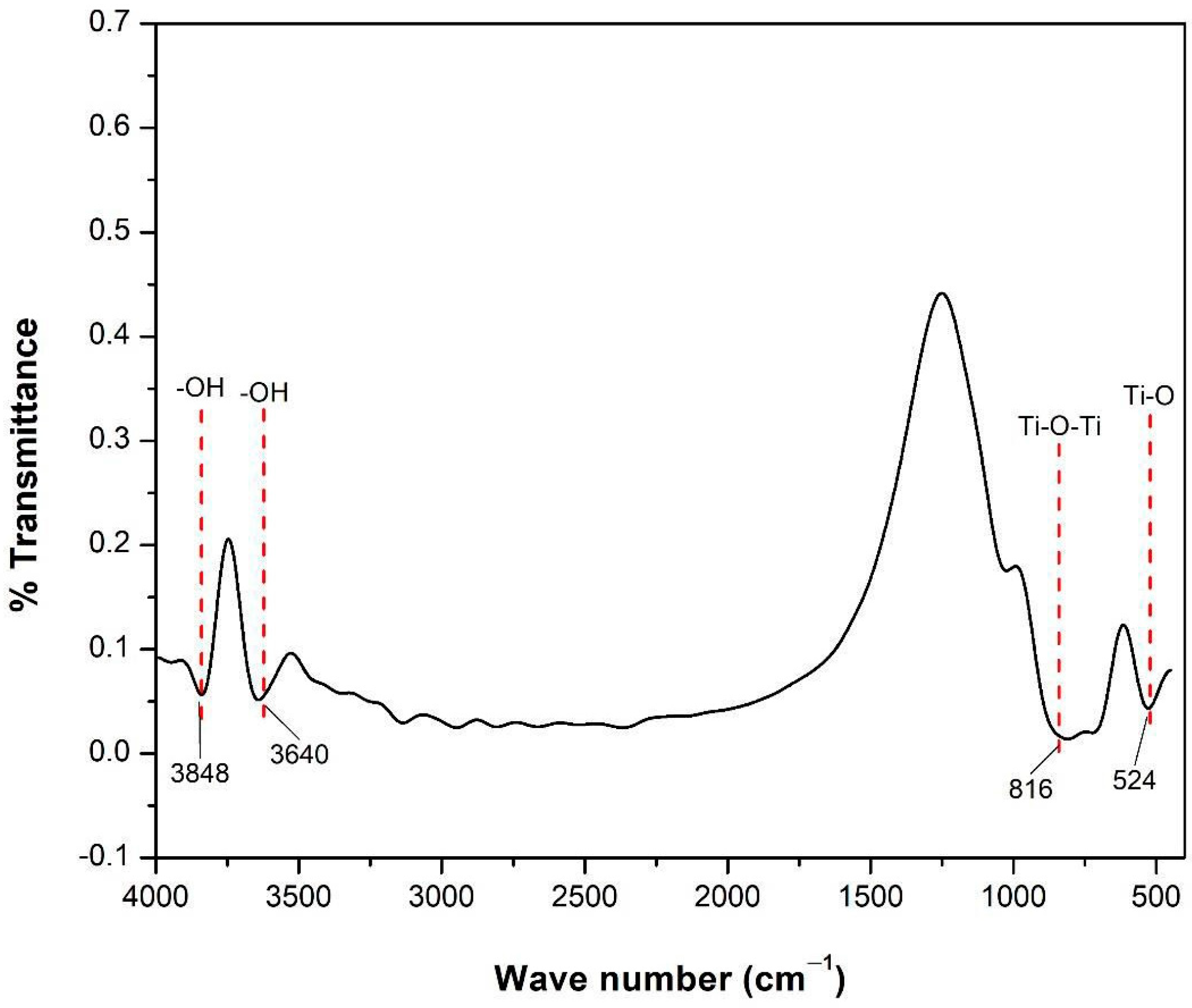
3.1.2. FTIR Result
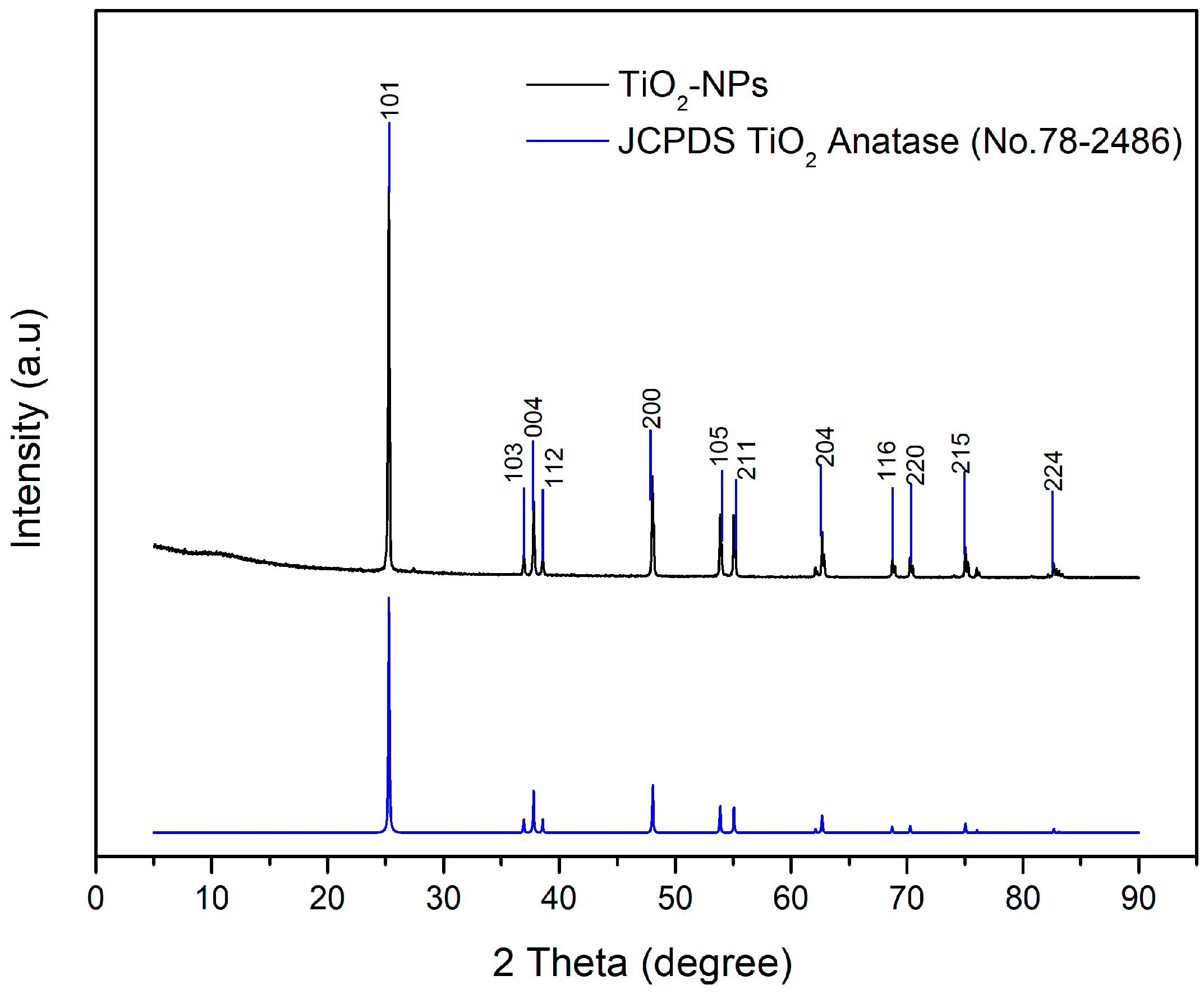
3.1.3. XRD Result
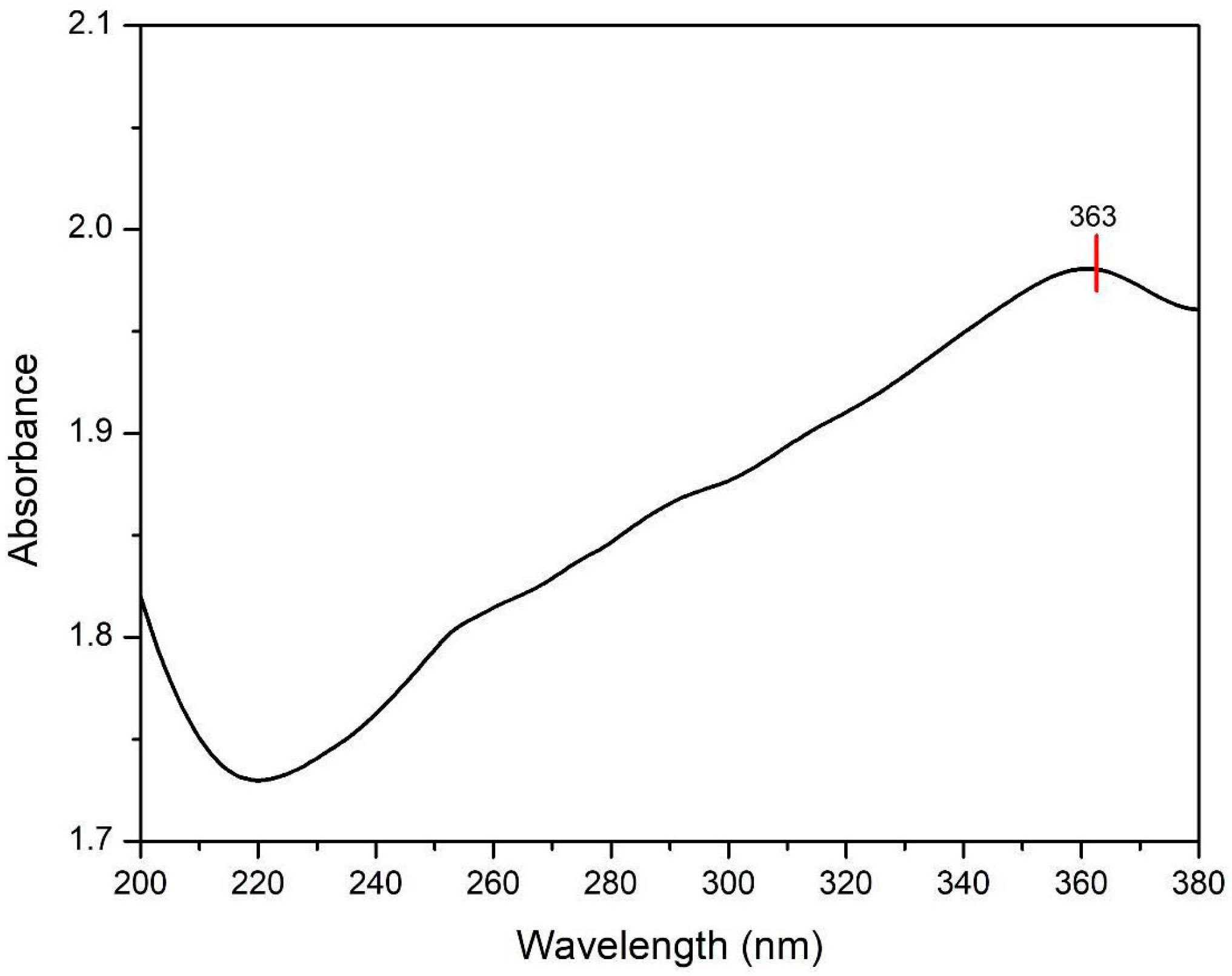
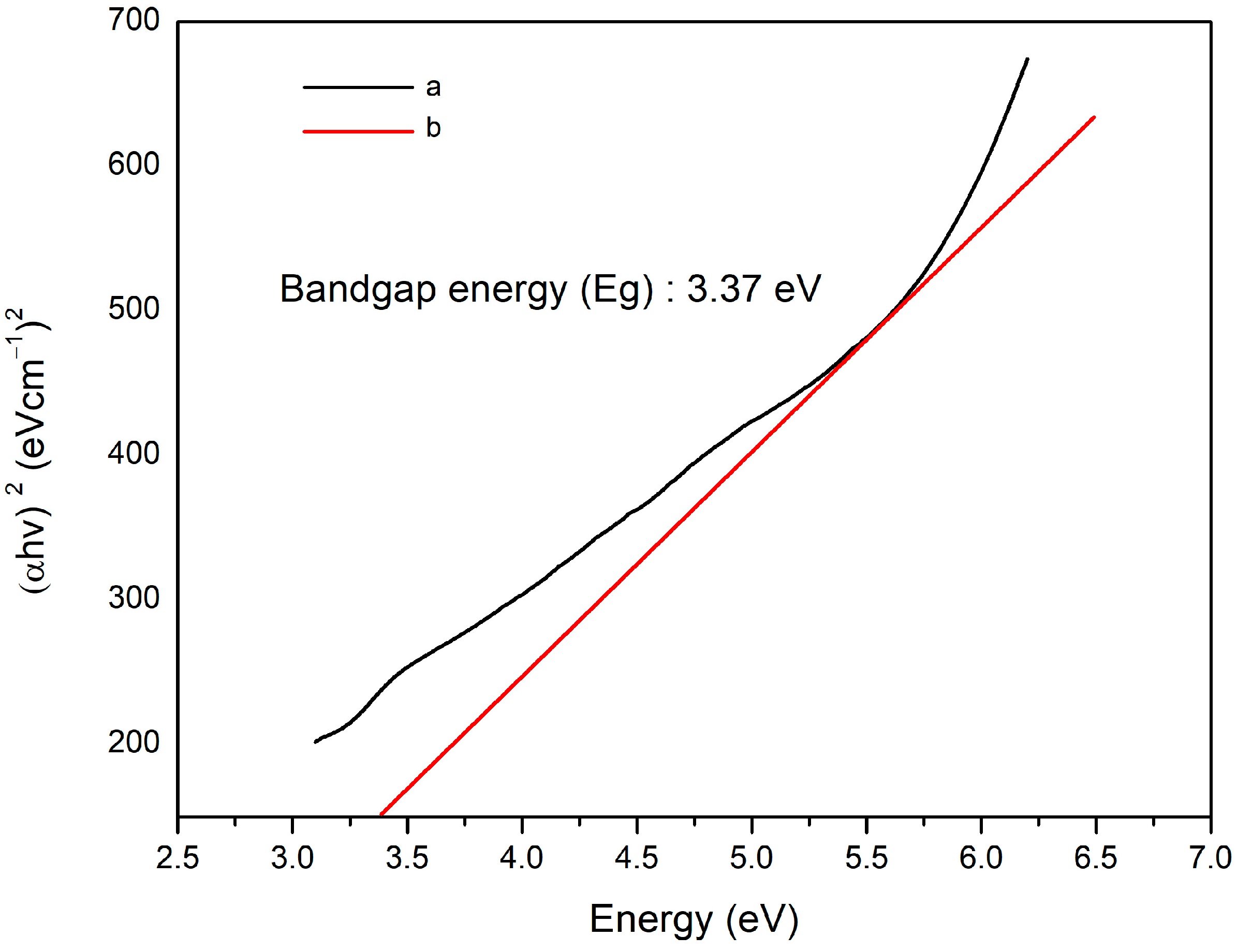
3.1.4. UV-Vis Spectrophotometer Result
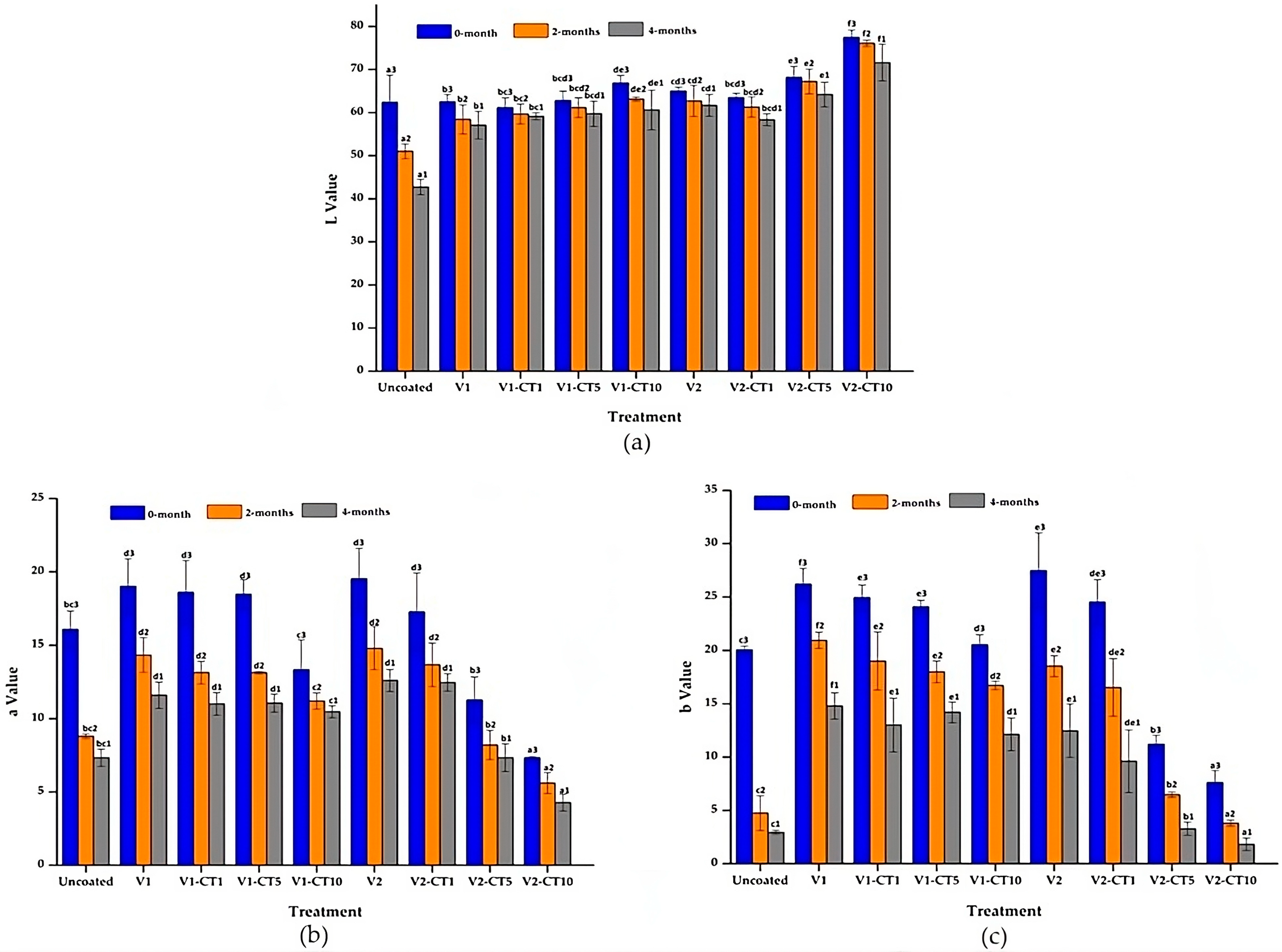
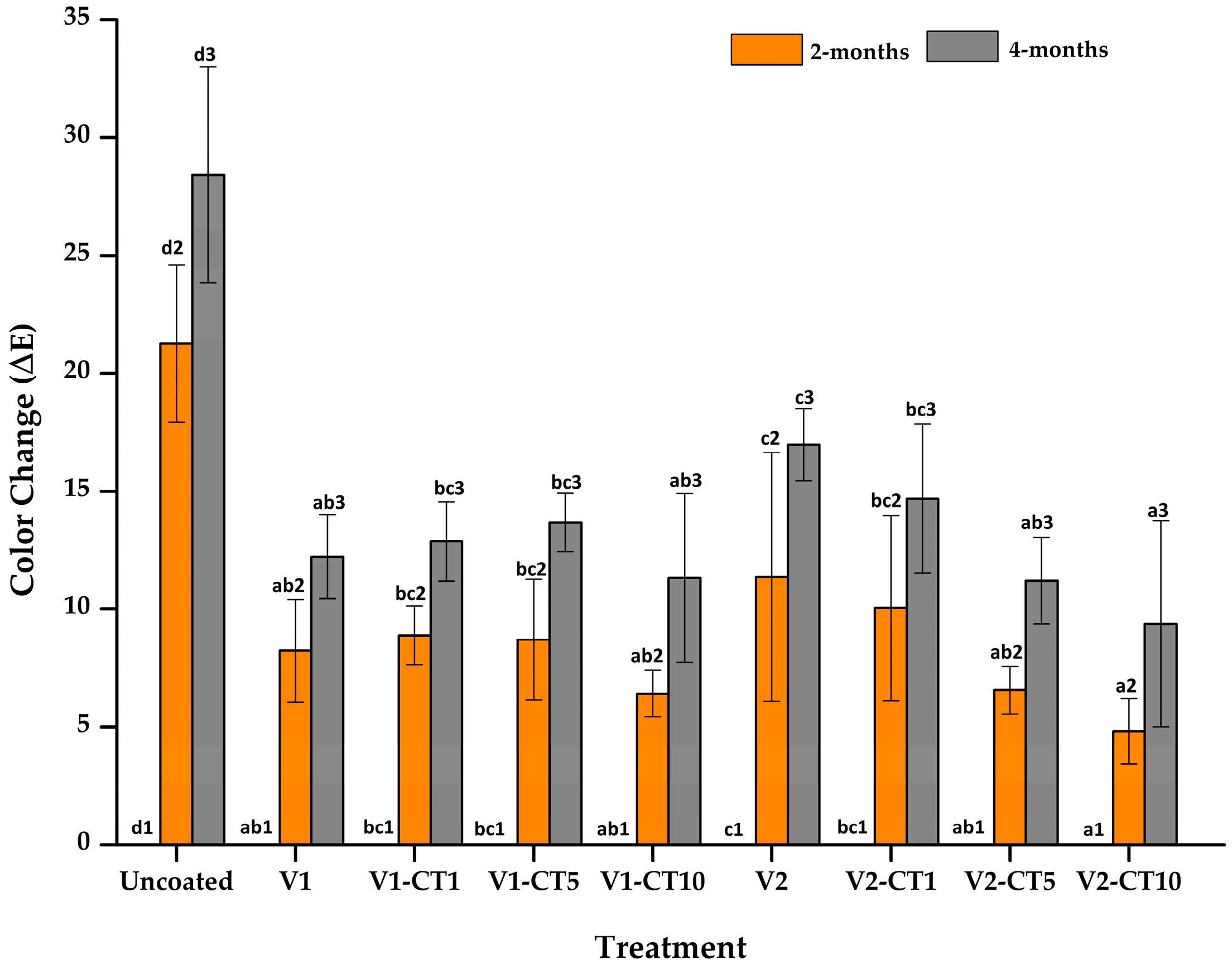
3.2. Colour Changes of Mangium Wood
3.3. Surface Roughness of Mangium Wood
3.4. Equilibrium Contact Angle and Wettability of Mangum Wood
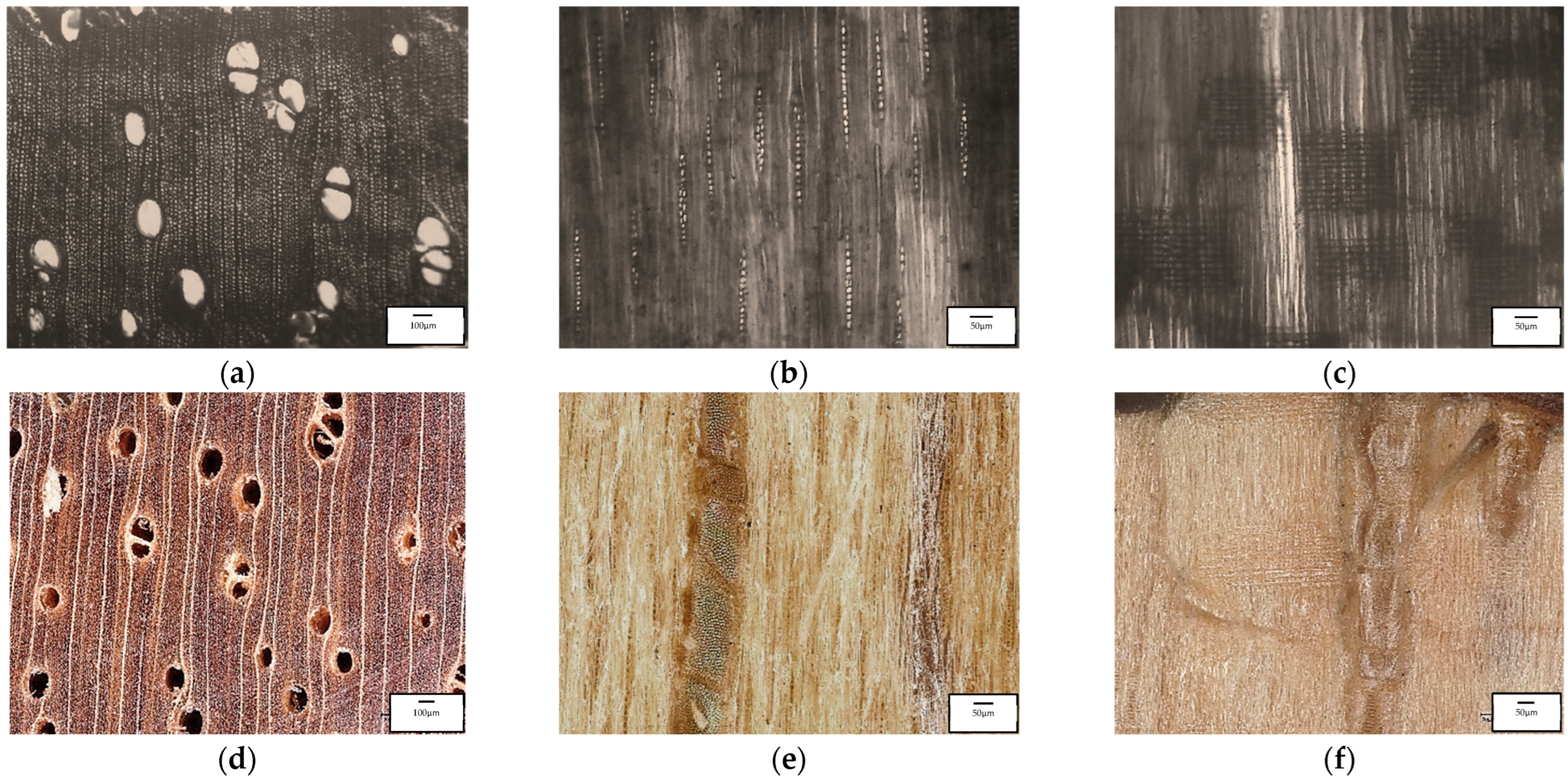
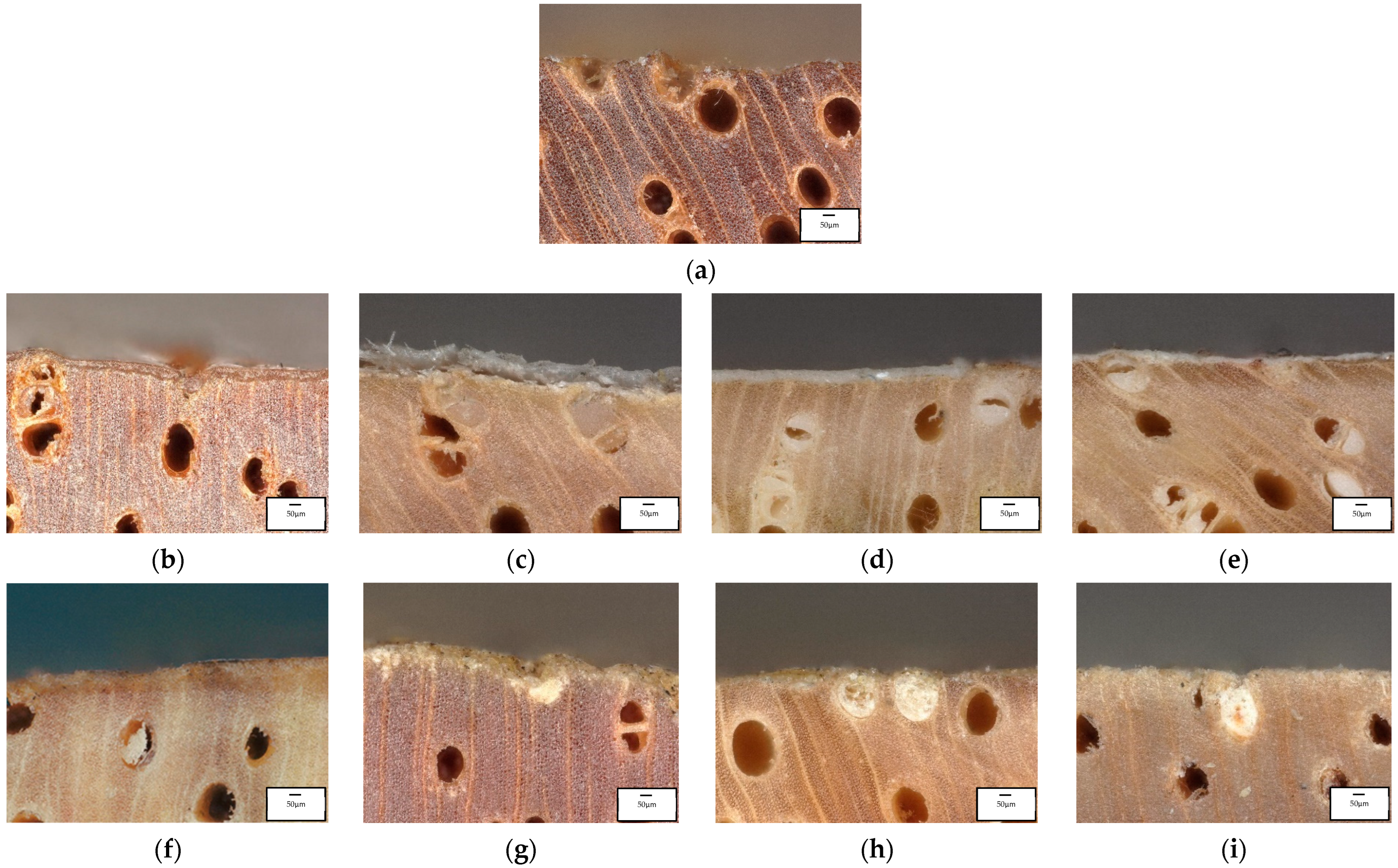
3.5. Microscopic and Macroscopic Mangium Wood
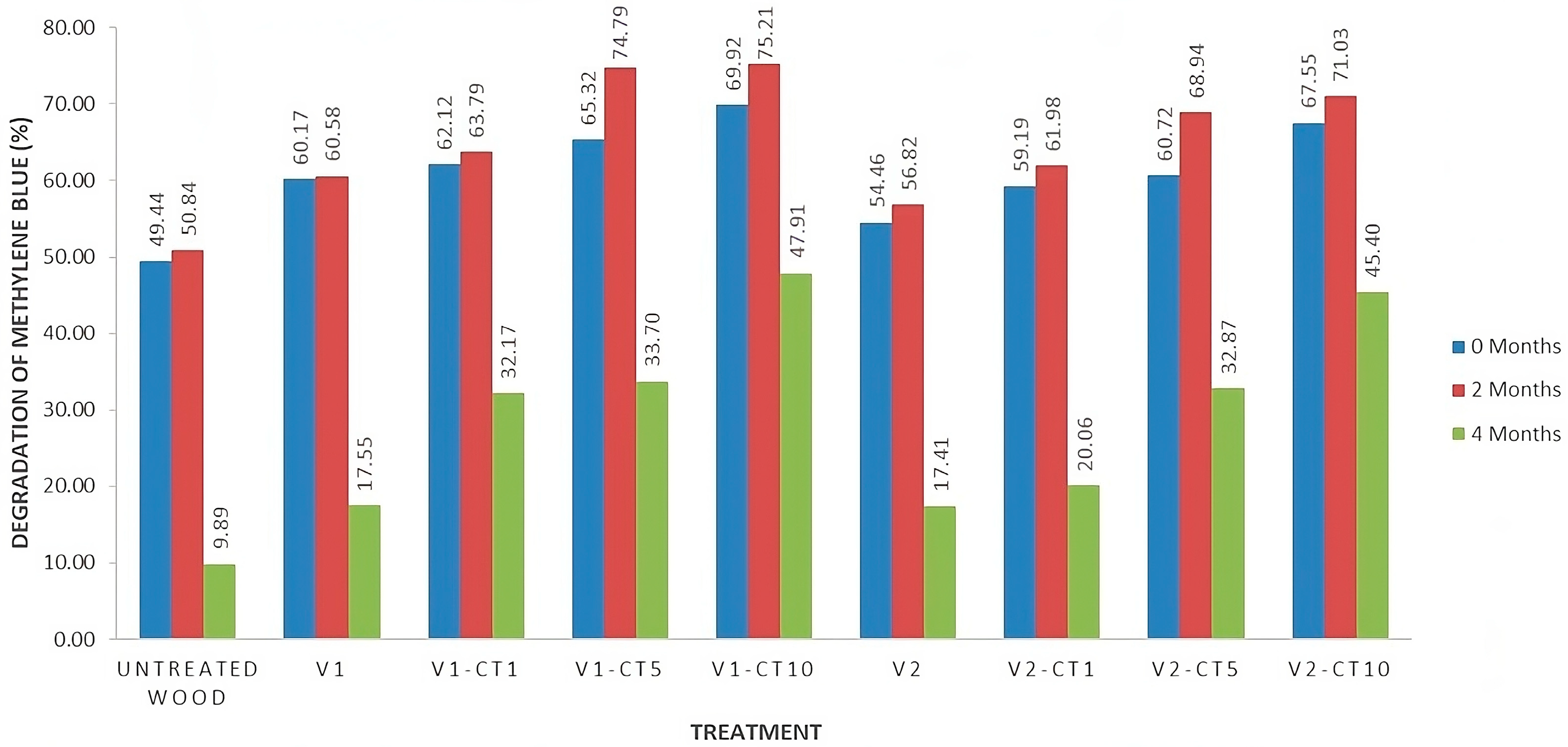
3.6. Degradation Ability of MB Compound
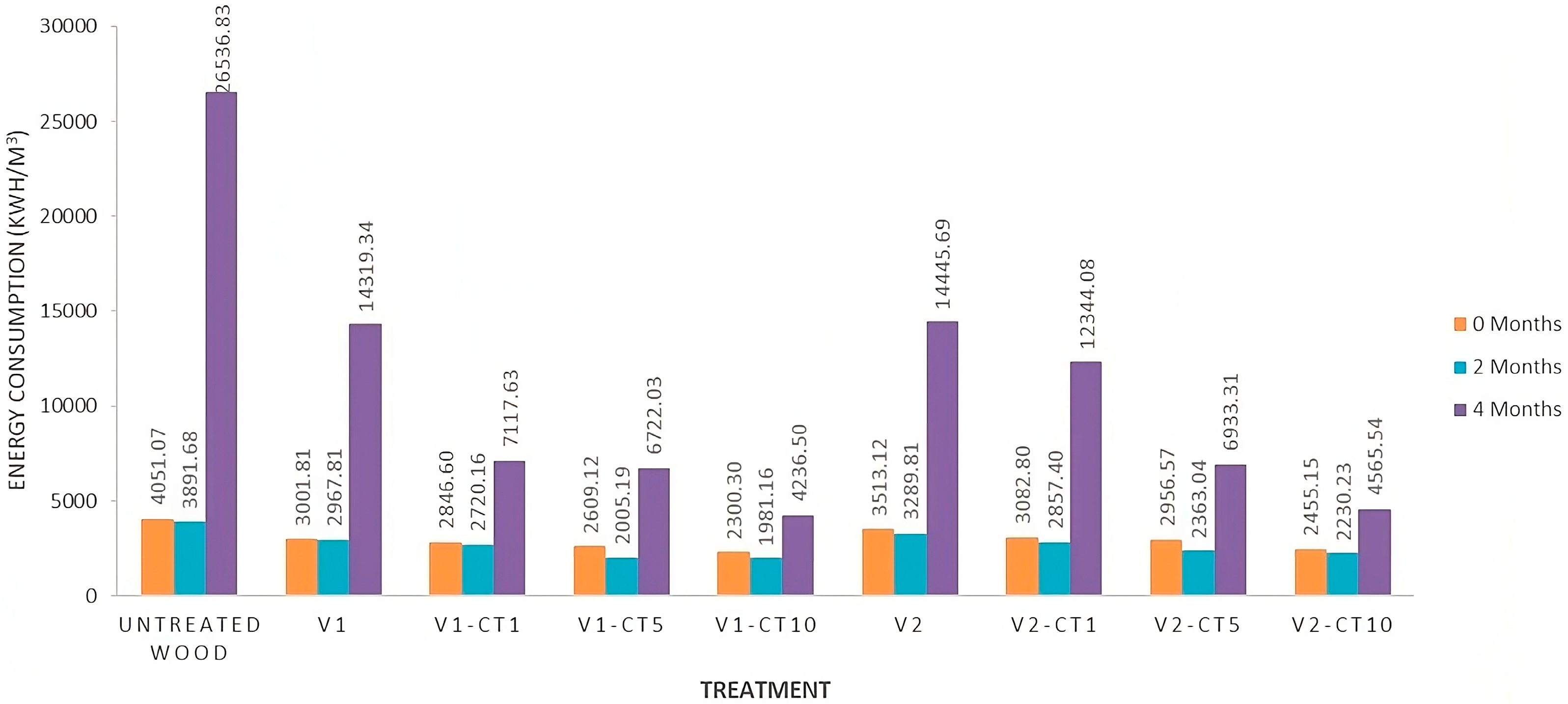
3.7. Evaluation of the Energy Efficiency of Coating Materials in Degrading MB Compounds
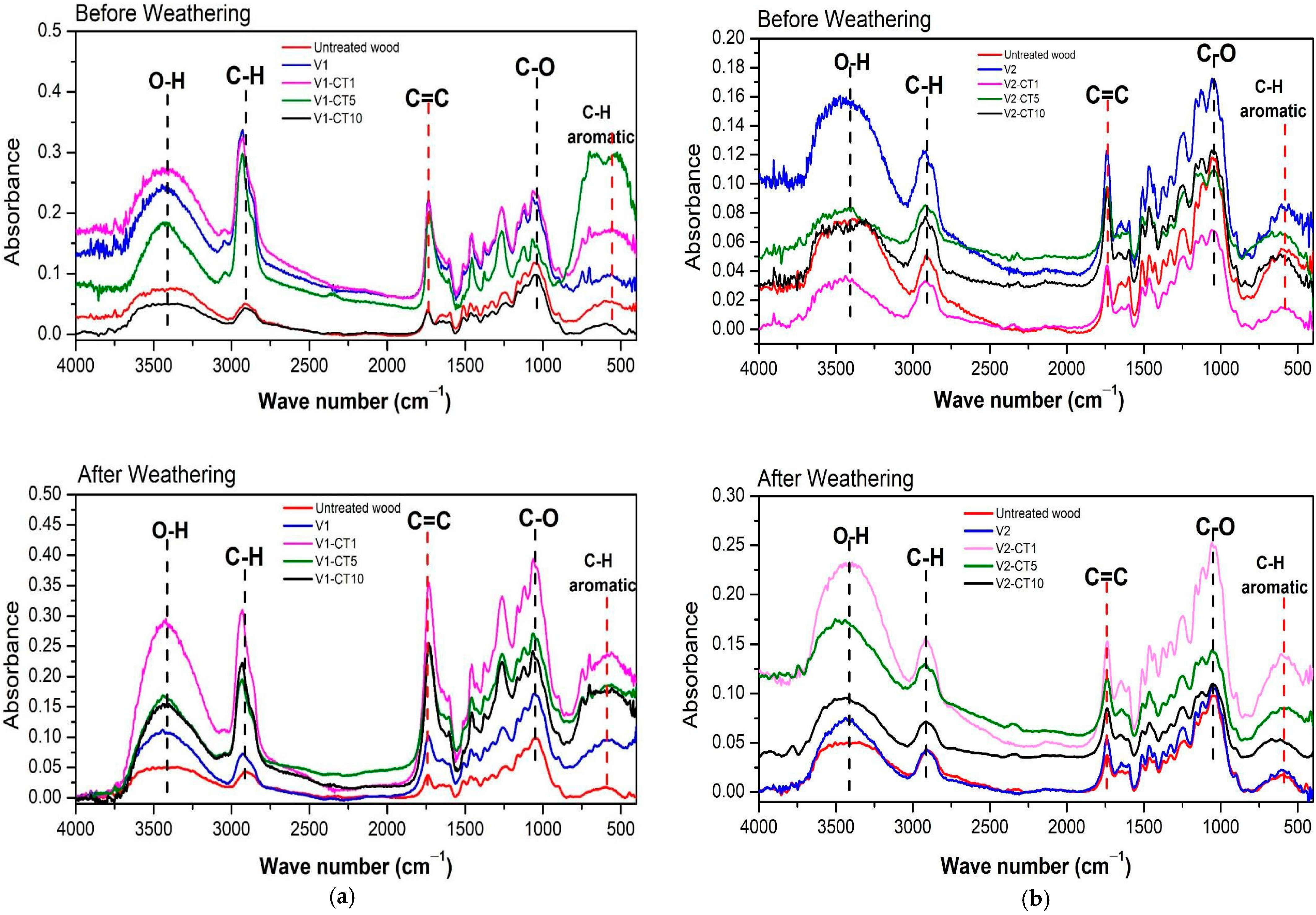
3.8. FTIR Testing and Functional Group Absorption Band Ratio Index Calculation
4. Discussion
4.1. Colour Changes of Mangium Wood
4.2. Surface Roughness of Mangium Wood
4.3. Equilibrium Contact Angle and Wettability of Mangum Wood
4.4. Microscopic and Macroscopic Mangium Wood
4.5. Degradation Ability of the MB Compound
4.6. Evaluation of the Energy Efficiency of Coating Materials in Degrading MB Compounds
4.7. FTIR Testing and Functional Group Absorption Band Ratio Index Calculation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunt, D. Properties of wood in the conservation of historical wooden artifacts. J. Cult. Herit. 2012, 13, S10–S15. [Google Scholar] [CrossRef]
- Mazzanti, P.; Togni, M.; Uzielli, L. Drying shrinkage and mechanical properties of poplar wood (Populus alba L.) across the grain. J. Cult. Herit. 2012, 13, S85–S89. [Google Scholar] [CrossRef]
- Marcon, B.; Mazzanti, P.; Uzielli, L.; Cocchi, L.; Dureisseix, D.; Gril, J. Mechanical study of a support system for cupping control of panel paintings combining crossbars and springs. J. Cult. Herit. 2012, 13, S109–S117. [Google Scholar] [CrossRef]
- Pandit, I.K.N.; Kurniawan, D. Wood Anatomy: Wood Structure, Wood as Raw Material and Diagnostic Characteristics of Indonesian Trade Wood; Bogor Agricultural Institute: Bogor, Indonesia, 2008. [Google Scholar]
- Nugroho, W.D.; Marsoem, S.N.; Yasue, K.; Fujiwara, T.; Nakajima, T.; Hayakawa, M.; Nakaba, S.; Yamagishi, Y.; Jin, H.O.; Kubo, T.; et al. Radial variations in the anatomical characteristics and density of the wood of Acacia mangium of five different provenances in Indonesia. J. Wood Sci. 2012, 58, 185–194. [Google Scholar] [CrossRef]
- Rowel, R.M. Handbook of Wood Chemistry and Wood Composites, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-4398-5381-8. [Google Scholar] [CrossRef]
- Feist, W.C. Outdoor Wood Weathering and Protection; Forest Products Laboratory: Madison, WI, USA, 1990.
- Darmawan, W.; Rahayu, I.S.; Padlinurjaji, I.M.; Pandit, K.N. Woodworking: Supporting Sciences and Process Technology; IPB Press: Bogor, Indonesia, 2011. [Google Scholar]
- Hoadley, R.B. Understanding Wood: A Craftsman’s Guide to Wood Technology; The Taunton Press: Newtown, CT, USA, 2000. [Google Scholar]
- Fufa, S.M.; Jelle, B.P.; Hovde, P.J.; Rorvik, P.M. Coated wooden claddings and the influence of nanoparticles on the weathering performance. Prog. Org. Coat. 2012, 75, 72–78. [Google Scholar] [CrossRef]
- Tu, K.; Kong, L.; Wang, X.; Liu, J. Semitransparent, durable superhydrophobic polydimethylsiloxane/SiO2 nanocomposite coatings on varnished wood. Holzforschung 2016, 70, 1039–1045. [Google Scholar] [CrossRef]
- Guo, H.; Fuchs, P.; Cabane, E.; Michen, B.; Hagendorfer, H.; Romanyuk, Y.E.; Burgert, I. UV-protection of wood surfaces by controlled morphology fine-tuning of ZnO nanostructures. Holzforschung 2016, 70, 699–708. [Google Scholar] [CrossRef]
- Salla, J.; Pandey, K.K.; Srinivas, K. Improvement of UV resistance of wood surfaces by using ZnO nanoparticles. Polym. Degrad. Stab. 2012, 97, 592–596. [Google Scholar] [CrossRef]
- Auffan, M.; Masion, A.; Labille, J.; Diot, M.A.; Liu, W.; Olivi, L.; Proux, O.; Ziarelli, F.; Chaurand, P.; Geantet, C.; et al. Long-term aging of a CeO2 based nanocomposite used for wood protection. Environ. Pollut. 2014, 188, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Veronovski, N.; Verhovšek, D.; Godnjavec, J. The influence of surface-treated nano-TiO2 (rutile) incorporation in water-based acrylic coatings on wood protection. Wood Sci. Technol. 2013, 47, 317–328. [Google Scholar] [CrossRef]
- Wang, B.; Feng, M.; Zhan, H. Improvement of wood properties by impregnation with TiO2 via ultrasonic-assisted sol-gel process. RSC Adv. 2014, 4, 56355–56360. [Google Scholar] [CrossRef]
- Chen, X.D.; Wang, Z.; Liao, Z.F.; Mai, Y.L.; Zhang, M.Q. Roles of anatase and rutile TiO2 nanoparticles in photooxidation of polyurethane. Polym. Test. 2007, 26, 202–208. [Google Scholar] [CrossRef]
- Kim, T.K.; Lee, M.N.; Lee, S.H.; Park, Y.C.; Jung, C.K.; Boo, J.H. Development of surface coating technology of TiO2 powder and improvement of photocatalytic activity by surface modification. Thin Solid Film. 2005, 475, 171–177. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, H.; Liu, Y.; Li, J.; Lu, Y.; Hunt, J.F. Improvement of water resistance and dimensional stability of wood through titanium dioxide coating. Holzforschung 2010, 64, 757–761. [Google Scholar] [CrossRef]
- Rahayu, I.; Darmawan, W.; Nawawi, D.S.; Prihatini, E.; Ismail, R.; Laksono, G.D. Physical Properties of Fast-Growing Wood-Polymer Nano Composite Synthesized through TiO2 Nanoparticle Impregnation. Polymers 2022, 14, 4463. [Google Scholar] [CrossRef] [PubMed]
- Mahltig, B.; Swaboda, C.; Roessler, A.; Böttcher, H. Functionalising wood by nanosol application. J. Mater. Chem. 2008, 18, 3180–3192. [Google Scholar] [CrossRef]
- Rassam, G.; Abdi, Y.; Abdi, A. Deposition of TiO2 nano-particles on wood surfaces for UV and moisture protection. J. Exp. Nanosci. 2012, 7, 468–476. [Google Scholar] [CrossRef]
- Schmalzl, K.J.; Evans, P.D. Wood surface protection with some titanium, zirconium and manganese compounds. Polym. Degrad. Stab. 2003, 82, 409–419. [Google Scholar] [CrossRef]
- Tshabalala, M.A.; Libert, R.; Schaller, C.M. Photostability and moisture uptake properties of wood veneers coated with a combination of thin sol-gel films and light stabilizers. Holzforschung 2011, 65, 215–220. [Google Scholar] [CrossRef]
- Meng, L.Y.; Wang, B.; Ma, M.G.; Lin, K.L. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater. Today Chem. 2016, 1–2, 63–83. [Google Scholar] [CrossRef]
- Liu, S.X.; Chen, X.Y.; Chen, X. A TiO2/AC composite photocatalyst with high activity and easy separation prepared by a hydrothermal method. J. Hazard. Mater. 2007, 143, 257–263. [Google Scholar] [CrossRef]
- Park, Y. A new paradigm shift for the green synthesis of antibacterial silver nanoparticles utilizing plant extracts. Toxicol. Res. 2014, 30, 169–178. [Google Scholar] [CrossRef]
- Karthikeyan, K.T.; Nithya, A.; Jothivenkatachalam, K. Photocatalytic and antimicrobial activities of chitosan-TiO2 nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Oktaviani, Y.; Muttaqin, A. Pengaruh Temperatur Hidrotermal Terhadap Konduktivitas Listrik Zeolit Sintetis Dari Abu Dasar Batubara Dengan Metode Alkali Hidrotermal. J. Fis. Unand 2015, 4, 358–364. [Google Scholar]
- Famia, A.M.; Muldarisnur, M. Pengaruh Temperatur Sintesis Hidrotermal Terhadap Diameter Nanopartikel Seng Oksida. J. Fis. Unand 2019, 8, 127–132. [Google Scholar] [CrossRef]
- Sapputra, G.P.A.; Noerochim, L. Pengaruh Waktu Hidrotermal pada Proses Sintesis Lithium Mangan Oksida Spinel (LiMn2O4) Nanopartikel terhadap Efisiensi Adsorpsi dan Desorpsi Ion Lithium dari Lumpur Sidoarjo. J. Tek. Pomits 2014, 3, 163–167. [Google Scholar]
- Aneesh, P.M.; Vanaja, K.A.; Jayaraj, M.K. Synthesis of ZnO nanoparticles by hydrothermal method. Nanophotonic Mater. IV 2007, 6639, 66390J. [Google Scholar] [CrossRef]
- Shahat, A.M.; El-Hossary, F.M.; Ghitas, A.; Abd El-Rahman, A.M.; Ebnalwaled, A.A. Low-temperature Hydrothermal Synthesis of Titanium Dioxide Nanoparticles for Photocatalytic Applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1171, 012008. [Google Scholar] [CrossRef]
- Gerasimov, A.; Eremina, O.; Cherkasova, M.; Dmitriev, S. Application of particle-size analysis in various industries. J. Phys. Conf. Ser. 2021, 1728, 12003. [Google Scholar] [CrossRef]
- Bagheri, S.; Shameli, K.; Abd Hamid, S.B. Synthesis and characterization of anatase titanium dioxide nanoparticles using egg white solution via Sol-Gel method. J. Chem. 2013, 2013, 6–11. [Google Scholar] [CrossRef]
- Hong, S.; Lee, S.; Jung, H.J.; Yu, Y.; Shin, J.; Kwon, K.-Y.; Choi, M. Simple Preparation of Anatase TiO2 Nanoparticles via Pulsed Laser Ablation in Liquid. Bull. Korean Chem. Soc. 2013, 34, 279–282. [Google Scholar] [CrossRef]
- ISO 4287-1997; Geometrical Product Specifications (GPS) Surface Texture: Profile Method. Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 1997.
- Hunter Lab. Color Scale. 2008. Available online: https://www.hunterlab.com (accessed on 13 August 2021).
- Shi, S.Q.; Gardner, D.J. Dynamic adhesive wettability of wood. Wood Fiber Sci. 2001, 33, 58–68. [Google Scholar]
- Alsubaie, N.; Alshamrani, R.; Domyati, D.; Alahmadi, N.; Bannani, F. Methylene Blue Dye Adsorption onto Polyoxometalate Ionic Liquid Supported on Bentonite: Kinetic, Equilibrium and Thermodynamic Studies. Open J. Phys. Chem. 2021, 11, 106–127. [Google Scholar] [CrossRef]
- Ghavi, A.; Bagherian, G.; Vahidian, H. Investigating the Synergistic Effect of UV/PS/TiO2 and UV/PI/TiO2 Processes on Paraquat Herbicide Degradation in Media Aqueous: Statistical Optimization, Kinetic Study, and Estimation of Electrical Energy Consumption. 2021. Available online: https://www.researchsquare.com/article/rs-277799/v1 (accessed on 6 September 2023).
- Makhatova, A.; Ulykbanova, G.; Sadyk, S.; Sarsenbay, K.; Atabaev, T.S.; Inglezakis, V.J.; Poulopoulos, S.G. Degradation and mineralization of 4-tert-butylphenol in water using Fe-doped TiO2 catalysts. Sci. Rep. 2019, 9, 19284. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.S.; Jelle, B.; Gao, T. Application of ATR-FTIR Spectroscopy to Compare the Cell Materials of Wood Decay Fungi with Wood Mould Fungi. Int. J. Spectrosc. 2015, 2015, 521938. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Z.; Guo, H.; Yang, H.; Zhang, G.; Ji, S.; Zeng, T. Low-Temperature Synthesis of Anatase TiO2 Nanoparticles with Tunable Surface Charges for Enhancing Photocatalytic Activity. PLoS ONE 2014, 9, e114638. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chandra, R., Gupta, N., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 43–58. ISBN 978-0-08-100557-6. [Google Scholar]
- Jasmani, L.; Rusli, R.; Khadiran, T.; Jalil, R.; Adnan, S. Application of Nanotechnology in Wood-Based Products Industry: A Review. Nanoscale Res. Lett. 2020, 15, 207. [Google Scholar] [CrossRef]
- Sharfudeen, B.F.J.M.; Latheef, A.F.A.; Ambrose, R.V. Synthesis and characterization of TiO2 nanoparticles and investigation of antimicrobial activities against human pathogens. J. Pharm. Sci. Res. 2017, 9, 1604–1608. [Google Scholar]
- Hartland, G.V. Nanoparticle crystallinity: Is perfect better? Nat. Mater. 2007, 6, 716–718. [Google Scholar] [CrossRef]
- Li, Z.; Shen, W.; He, W.; Zu, X. Effect of Fe-doped TiO2 nanoparticle derived from modified hydrothermal process on the photocatalytic degradation performance on methylene blue. J. Hazard. Mater. 2008, 155, 590–594. [Google Scholar] [CrossRef]
- Rathod, P.B.; Waghuley, S.A. Synthesis and UV-Vis spectroscopic study of TiO2 nanoparticles. Int. J. Nanomanuf. 2015, 11, 185–193. [Google Scholar] [CrossRef]
- Zanatta, A.R. Revisiting the optical bandgap of semiconductors and the proposal of a unified methodology to its determination. Sci. Rep. 2019, 9, 11225. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Altomare, A.; Corriero, N.; Cuocci, C.; Falcicchio, A.; Moliterni, A.; Rizzi, R. QUALX2.0: A qualitative phase analysis software using the freely available database POW-COD. J. Appl. Crystallogr. 2015, 48, 598–603. [Google Scholar] [CrossRef]
- Opoku, F.; Govender, K.K.; van Sittert, C.G.C.E.; Govender, P.P. Understanding the synergistic effects, optical and electronic properties of ternary Fe/C/S-doped TiO2 anatase within the DFT + U approach. Int. J. Quantum Chem. 2018, 118, e25505. [Google Scholar] [CrossRef]
- Krisdianto, K.; Satiti, E.R.; Supriadi, A. Perubahan Warna Dan Lapisan Finishing Lima Jenis Kayu Akibat Pencuacaan. J. Penelit. Has. Hutan 2018, 36, 205–210. [Google Scholar] [CrossRef]
- Turkoglu, T.; Baysal, E.; Kureli, I.; Toker, H.; Ergun, M.E. The effects of natural weathering on hardness and gloss of impregnated and varnished Scots pine and Oriental beech wood. Wood Res. 2015, 60, 833–844. [Google Scholar]
- Lu, X.; Hu, Y. Layer-by-layer deposition of TiO2 nanoparticles in the wood surface and its superhydrophobic performance. BioResources 2016, 11, 4605–4620. [Google Scholar] [CrossRef]
- Čolović, B.; Kisić, D.; Jokanović, B.; Rakočević, Z.; Nasov, I.; Petkoska, A.T.; Jokanović, V. Wetting properties of titanium oxides, oxynitrides and nitrides obtained by DC and pulsed magnetron sputtering and cathodic arc evaporation. Mater. Sci. Pol. 2019, 37, 173–181. [Google Scholar] [CrossRef]
- Martha, R.; Dirna, F.C.; Hasanusi, A.; Rahayu, I.S.; Darmawan, W. Surface free energy of 10 tropical woods species and their acrylic paint wettability. J. Adhes. Sci. Technol. 2020, 34, 167–177. [Google Scholar] [CrossRef]
- Darmawan, W.; Nandika, D.; Noviyanti, E.; Alipraja, I.; Lumongga, D.; Gardner, D.; Gérardin, P. Wettability and bonding quality of exterior coatings on jabon and sengon wood surfaces. J. Coat. Technol. Res. 2018, 15, 95–104. [Google Scholar] [CrossRef]
- Rowel, R.M. Handbook of Wood Chemistry and Wood Composites, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780429209000. [Google Scholar] [CrossRef]
- Svora, P.; Svorová Pawełkowicz, S.; Ecorchard, P.; Plocek, J.; Schieberová, A.; Prošek, Z.; Ptáček, P.; Pošta, J.; Targowski, P.; Kuklík, P.; et al. Study of Interactions between Titanium Dioxide Coating and Wood Cell Wall Ultrastructure. Nanomaterials 2022, 12, 2678. [Google Scholar] [CrossRef] [PubMed]
- Baeissa, E.S. Photocatalytic degradation of methylene blue dye under visible light irradiation using In/ZnO nanocomposite. Front. Nanosci. Nanotechnol. 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Akkus, C.; Ozturk, A.; Kalem, V.; Park, J. Influence of TiO2 Content on the Photocatalytic Activity of TiO2-B2O3 Glasses Prepared by the Sol-Gel Process. In Proceedings of the 18th Metallurgy and Materials Congress, Istanbul, Turkey, 29 September–1 October 2016. [Google Scholar]
- Wang, C.; Bai, H.; Kang, X. Optimization Study on Synergistic System of Photocatalytic Degradation of AR 26 and UV-LED Heat Dissipation. Catalysts 2023, 13, 669. [Google Scholar] [CrossRef]
- Li, F.; Kong, W.; Zhao, X.; Pan, Y. Multifunctional TiO2-Based Superoleophobic/Superhydrophilic Coating for Oil–Water Separation and Oil Purification. ACS Appl. Mater. Interfaces 2020, 12, 18074–18083. [Google Scholar] [CrossRef]
- Varma, K.S.; Tayade, R.J.; Shah, K.J.; Joshi, P.A.; Shukla, A.D.; Gandhi, V.G. Photocatalytic degradation of pharmaceutical and pesticide compounds (PPCs) using doped TiO2 nanomaterials: A review. Water-Energy Nexus 2020, 3, 46–61. [Google Scholar] [CrossRef]
- Raheb, I.; Manlla, M.S. Kinetic and thermodynamic studies of the degradation of methylene blue by photo-Fenton reaction. Heliyon 2021, 7, e07427. [Google Scholar] [CrossRef]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; abdel nazeer, A.; Amin, M.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 7104. [Google Scholar] [CrossRef]
- Milani Moghaddam, H.; Nasirian, S. Dependence of activation energy and lattice strain on TiO2 nanoparticles. J. Exp. Nanosci. 2012, 1, 201–212. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Pánek, M.; Oberhofnerová, E.; Zeidler, A.; Šedivka, P. Efficacy of Hydrophobic Coatings in Protecting Oak Wood Surfaces during Accelerated Weathering. Coatings 2017, 7, 172. [Google Scholar] [CrossRef]
- Wohlert, M.; Benselfelt, T.; Wågberg, L.; Furó, I.; Berglund, L.A.; Wohlert, J. Cellulose and the role of hydrogen bonds: Not in charge of everything. Cellulose 2022, 29, 1–23. [Google Scholar] [CrossRef]
- Butylina, S.; Hyvärinen, M.; Kärki, T. A study of surface changes of wood-polypropylene composites as the result of exterior weathering. Polym. Degrad. Stab. 2012, 97, 337–345. [Google Scholar] [CrossRef]
- Baysal, E. Surface characteristics of CCA treated scots pine after accelerated weathering. Wood Res. 2012, 57, 375–382. [Google Scholar]
- Baysal, E.; Dizman Tomak, E.; Ozbey, M.; Altin, E. Surface properties of impregnated and varnished Scots pine wood after accelerated weathering. Color. Technol. 2014, 130, 140–146. [Google Scholar] [CrossRef]
- Temiz, A.; Yildiz, U.C.; Aydin, I.; Eikenes, M.; Alfredsen, G.; Çolakoglu, G. Surface roughness and color characteristics of wood treated with preservatives after accelerated weathering test. Appl. Surf. Sci. 2005, 250, 35–42. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Militz, H.; Mai, C. Natural weathering of scots pine (Pinus sylvestris L.) boards modified with functionalised commercial silicone emulsions. BioResources 2009, 4, 659–673. [Google Scholar] [CrossRef]
- Muflihati; Nawawi, D.S.; Rahayu, I.S.; Syafii, W. Perubahan Warna Kayu Jabon Terwarnai Ekstrak Kulit Kayu Samak (Syzygium inophyllum). J. Ilmu Teknol. Kayu Trop. 2014, 12, 14–15. [Google Scholar]
- Temiz, A.; Terziev, N.; Eikenes, M.; Hafren, J. Effect of accelerated weathering on surface chemistry of modified wood. Appl. Surf. Sci. 2007, 253, 5355–5362. [Google Scholar] [CrossRef]
- Deka, M.; Petric, M. Modified and Non-Modified Wood During Artificial Light Exposure. BioResources 2008, 3, 346–362. [Google Scholar] [CrossRef]
- Hansmann, C.; Deka, M.; Wimmer, R.; Gindl, W. Artificial weathering of wood surfaces modified by melamine formaldehyde resins. Holz Als Roh Werkst. 2006, 64, 198–203. [Google Scholar] [CrossRef]
- Ozgenc, O.; Hiziroglu, S.; Yildiz, U.C. Weathering properties of wood species treated with different coating applications. BioResources 2012, 7, 4875–4888. [Google Scholar] [CrossRef]
- Pandey, K.K. Study of the effect of photo-irradiation on the surface chemistry of wood. Polym. Degrad. Stab. 2005, 90, 9–20. [Google Scholar] [CrossRef]
- Vlad Cristea, M.; Riedl, B.; Blanchet, P. Enhancing the performance of exterior waterborne coatings for wood by inorganic nanosized UV absorbers. Prog. Org. Coat. 2010, 69, 432–441. [Google Scholar] [CrossRef]
- Nejad, M.; Ung, T.; Cooper, P. Protocol comparison: Laboratory versus natural weathering tests for performance evaluation of coatings on preservative-treated wood. For. Prod. J. 2012, 62, 177–183. [Google Scholar] [CrossRef]
- Schaller, C.; Rogez, D.; Braig, A. Hydroxyphenyl-s-triazines: Advanced multipurpose UV-absorbers for coatings. J. Coat. Technol. Res. 2008, 5, 25–31. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Chang, H.; Liu, J. Sol-gel deposition of TiO2 nanocoatings on wood surfaces with enhanced hydrophobicity and photostability. Wood Fiber Sci. 2014, 46, 109–117. [Google Scholar]
- Moya, R.; Rodríguez-Zúñiga, A.; Vega-Baudrit, J.; Puente-Urbina, A. Effects of adding TiO2 nanoparticles to a water-based varnish for wood applied to nine tropical woods of Costa Rica exposed to natural and accelerated weathering. J. Coat. Technol. Res. 2017, 14, 141–152. [Google Scholar] [CrossRef]
- Pfeffer, A.; Mai, C.; Militz, H. Weathering characteristics of wood treated with water glass, siloxane or DMDHEU. Eur. J. Wood Wood Prod. 2012, 70, 165–176. [Google Scholar] [CrossRef]
- Acosta, A.P.; Zanatta, P.; Gallio, E.; Freire, N.; Lazarotto, M.; Jardim, P.L.G.; Gatto, D.A.; Moreira, M.L. TiO2-Modified Pinus Elliottii: A Pine Wood Presenting Increased Resistance to Water and Ultraviolet Radiation. Maderas Cienc. Tecnol. 2021, 23, 1–12. [Google Scholar] [CrossRef]
- Mahović Poljaček, S.; Tomašegović, T.; Leskovšek, M.; Stanković Elesini, U. Effect of SiO2 and TiO2 nanoparticles on the performance of uv visible fluorescent coatings. Coatings 2021, 11, 928. [Google Scholar] [CrossRef]
- Jnido, G.; Ohms, G.; Viöl, W. Deposition of TiO2 thin films on wood substrate by an air atmospheric pressure plasma jet. Coatings 2019, 9, 441. [Google Scholar] [CrossRef]
- Buyuksari, U.; Avci, E.; Ayrilmis, N.; Akkilic, H. Effect of pine cone ratio on the wettability and surface roughness of particleboard. BioResources 2010, 5, 1824–1833. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Candan, Z.; Akbulut, T.; Balkiz, O.D. Effect of sanding on surface properties of medium density fiberboard. Drv. Ind. 2010, 61, 175–181. [Google Scholar]
- Kishino, M.; Nakano, T. Artificial weathering of tropical woods. Part 2: Color change. Holzforschung 2004, 58, 558–565. [Google Scholar] [CrossRef]
- Unsal, O.; Candan, Z.; Korkut, S. Wettability and roughness characteristics of modified wood boards using a hot-press. Ind. Crop. Prod. 2011, 34, 1455–1457. [Google Scholar] [CrossRef]
- Monni, J.; Alvila, L.; Pakkanen, T.T. Structural and physical changes in phenol-formaldehyde resol resin, as a function of the degree of condensation of the resol solution. Ind. Eng. Chem. Res. 2007, 46, 6916–6924. [Google Scholar] [CrossRef]
- Lopes, J.d.O.; Garcia, R.A.; do Nascimento, A.M. Wettability of the surface of heat-treated juvenile teak wood assessed by drop shape analyzer. Maderas Cienc. Tecnol. 2018, 20, 249–256. [Google Scholar] [CrossRef]
- Piao, C.; Winandy, J.E.; Shupe, T.F. From Hydrophilicity To Hydrophobicity: A Critical Review: Part I. Wettability and Surface Behavior. Wood Fiber Sci. 2010, 42, 490–510. [Google Scholar]
- Van Chu, T.; Van Chuong, P.; Tuong, V.M. Wettability of Wood Pressure-treated with TiO2 Gel under Hydrothermal Conditions. BioResources 2014, 9, 2396–2404. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Imshinetskiy, I.M.; Nadaraia, K.V.; Gnedenkov, A.S.; Suchkov, S.N.; Opra, D.P.; Pustovalov, E.V.; Yu Ustinov, A.; Sinebryukhov, S.L.; Gnedenkov, S.V. Effect of TiO2 nanoparticles on the photocatalytic properties of PEO coatings on Mg alloy. J. Magnes. Alloys 2023, 11, 735–752. [Google Scholar] [CrossRef]
- Ogata, K.; Fujii, T.; Abe, H.; Baas, P. Identification of the Timbers of Southeast Asia and the Western Pacific; Kaiseisha Press: Tsukuba, Japan, 2008; Volume 62, p. 765. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Li, K.; Zeng, Y.; Zhao, W.; Zhang, T.; Zhan, Y.; Xie, R.; Leung, D.Y.C.; Huang, H. Titanium oxide based photocatalytic materials development and their role of in the air pollutants degradation: Overview and forecast. Environ. Int. 2019, 125, 200–228. [Google Scholar] [CrossRef] [PubMed]
- Sivaramalingam, A.; Thankaraj Salammal, S.; Soosaimanickam, A.; Sakthivel, T.; Paul David, S.; Sambandam, B. Role of TiO2 in Highly Efficient Solar Cells BT—Metal, Metal-Oxides and Metal Sulfides for Batteries, Fuel Cells, Solar Cells, Photocatalysis and Health Sensors; Rajendran, S., Karimi-Maleh, H., Qin, J., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 147–168. ISBN 978-3-030-63791-0. [Google Scholar]
- Udrea, M.; Ion, R.-M. Modelling of Methylene Blue Dye Adsorption on Beech and Fir Wood Sawdust as Adsorbent Support Materials. J. Sci. Arts 2019, 19, 675–686. [Google Scholar]
- Vidholdová, Z.; Slabejová, G.; Šmidriaková, M. Quality of Oil- and Wax-Based Surface Finishes on Thermally Modified Oak Wood. Coatings 2021, 11, 143. [Google Scholar] [CrossRef]
- Lou, F.; Qian, X.; Jin, Y.; Zhou, M. Characterisation of water-soluble TiO2 and its photocatalytic activity under visible light. Mater. Res. Innov. 2015, 19, S8-693–S8-696. [Google Scholar] [CrossRef]
- Hart, H.; Hadad, C.M.; Craine, L.E.; Hart, D.J. Organic Chemistry: A Short Course; Cengage Learning: Boston, MA, USA, 2011; ISBN 9781133172833. [Google Scholar]
- Vartanian, E.; Barres, O.; Roque, C. FTIR spectroscopy of woods: A new approach to study the weathering of the carving face of a sculpture. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 136PC, 1255–1259. [Google Scholar] [CrossRef]
- Hazarika, A.; Maji, T.K. Properties of softwood polymer composites impregnated with nanoparticles and melamine formaldehyde furfuryl alcohol copolymer. Polym. Eng. Sci. 2014, 54, 1019–1029. [Google Scholar] [CrossRef]




| Colour Change (ΔE) | Effect |
|---|---|
| <2.0 | Not visible |
| 2.0–1.0 | Very low |
| 1.0–3.0 | Low |
| 3.0–6.0 | Moderate |
| >6.0 | High |
| Treatment | 0 Months | 2 Months | 4 Months | |||
|---|---|---|---|---|---|---|
| Θe | K | θe | K | Θe | K | |
| Uncoated | 51.1 ± 0.4a3 | 1.2 ± 0.0e1 | 19.4 ± 0.6a2 | 1.5 ± 0.0e2 | 16.3 ± 0.6a1 | 1.6 ± 0.0e3 |
| V1 | 72.2 ± 0.2d3 | 0.3 ± 0.0b1 | 60.2 ± 0.0d2 | 0.3 ± 0.0b2 | 54.5 ± 5.9d1 | 0.5 ± 0.1b3 |
| V1-CT1 | 71.0 ± 0.4c3 | 0.3 ± 0.1b1 | 31.9 ± 0.6c2 | 0.4 ± 0.0b2 | 29.2 ± 0.6c1 | 0.6 ± 0.03b3 |
| V1-CT5 | 70.3 ± 0.1c3 | 0.4 ± 0.0c1 | 29.9 ± 7.4c2 | 0.5 ± 0.3c2 | 27.8 ± 2.0c1 | 0.7 ± 0.0c3 |
| V1-CT10 | 65.4 ± 0.3b3 | 0.5 ± 0.0d1 | 22.1± 0.51b2 | 0.5 ± 0.2d2 | 22.4 ± 4.4b1 | 0.9 ± 0.0d3 |
| V2 | 81.7 ± 0.2g3 | 0.0 ± 0.0a1 | 73.9 ± 0.7g2 | 0.0 ± 0.0a2 | 73.7 ± 1.1g1 | 0.0 ± 0.0a3 |
| V2-CT1 | 78.9 ± 0.0f3 | 0.0 ± 0.0a1 | 72.4 ± 0.7f2 | 0.0 ± 0.0a2 | 68.7 ± 0.1f1 | 0.0 ± 0.0a3 |
| V2-CT5 | 76.1 ± 1.2e3 | 0.0 ± 0.0a1 | 67.5 ± 3.6e2 | 0.0 ± 0.0a2 | 64.0 ± 0.7e1 | 0.0 ± 0.0a3 |
| V2-CT10 | 73.4 ± 0.5e3 | 0.0 ± 0.0a1 | 65.5 ± 0.3e2 | 0.0 ± 0.0a2 | 60.8 ± 0.0e1 | 0.0 ± 0.0a3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahayu, I.; Darmawan, W.; Nawawi, D.S.; Prihatini, E.; Ismail, R.; Laksono, G.D.; Martha, R. Surface Modification of Fast-Growing Wood with a Titanium-Dioxide-Based Nanocoating to Improve Weathering Resistance. Coatings 2023, 13, 1924. https://doi.org/10.3390/coatings13111924
Rahayu I, Darmawan W, Nawawi DS, Prihatini E, Ismail R, Laksono GD, Martha R. Surface Modification of Fast-Growing Wood with a Titanium-Dioxide-Based Nanocoating to Improve Weathering Resistance. Coatings. 2023; 13(11):1924. https://doi.org/10.3390/coatings13111924
Chicago/Turabian StyleRahayu, Istie, Wayan Darmawan, Deded Sarip Nawawi, Esti Prihatini, Rohmat Ismail, Gilang Dwi Laksono, and Resa Martha. 2023. "Surface Modification of Fast-Growing Wood with a Titanium-Dioxide-Based Nanocoating to Improve Weathering Resistance" Coatings 13, no. 11: 1924. https://doi.org/10.3390/coatings13111924
APA StyleRahayu, I., Darmawan, W., Nawawi, D. S., Prihatini, E., Ismail, R., Laksono, G. D., & Martha, R. (2023). Surface Modification of Fast-Growing Wood with a Titanium-Dioxide-Based Nanocoating to Improve Weathering Resistance. Coatings, 13(11), 1924. https://doi.org/10.3390/coatings13111924







