The Effects of Quenching with Clay on the Microstructure and Corrosion Performance of Steel Blades
Abstract
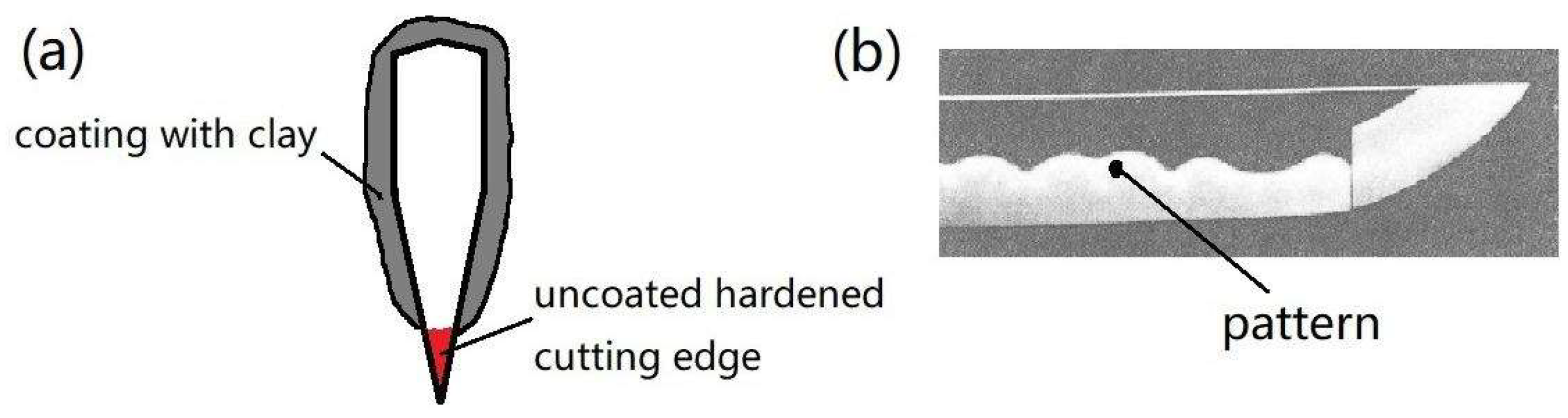
:1. Introduction
2. Materials and Methods
2.1. Materials and Processing
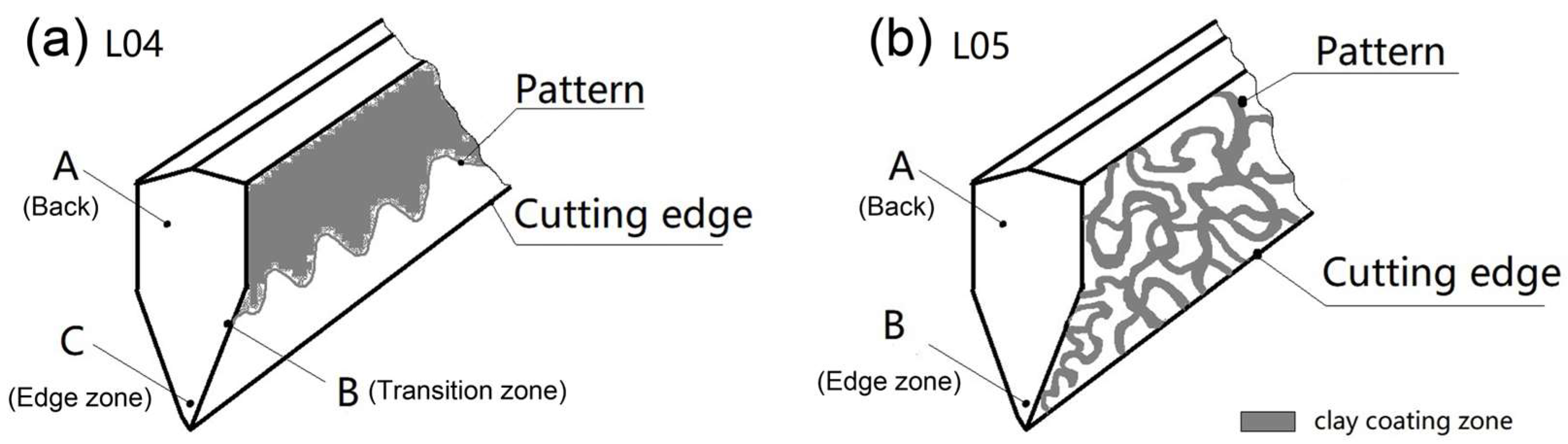
2.2. Sample Preparation
2.3. Characterization of the Microstructure
2.4. Microhardness Tests
2.5. Electrochemical Corrosion Behavior
3. Results
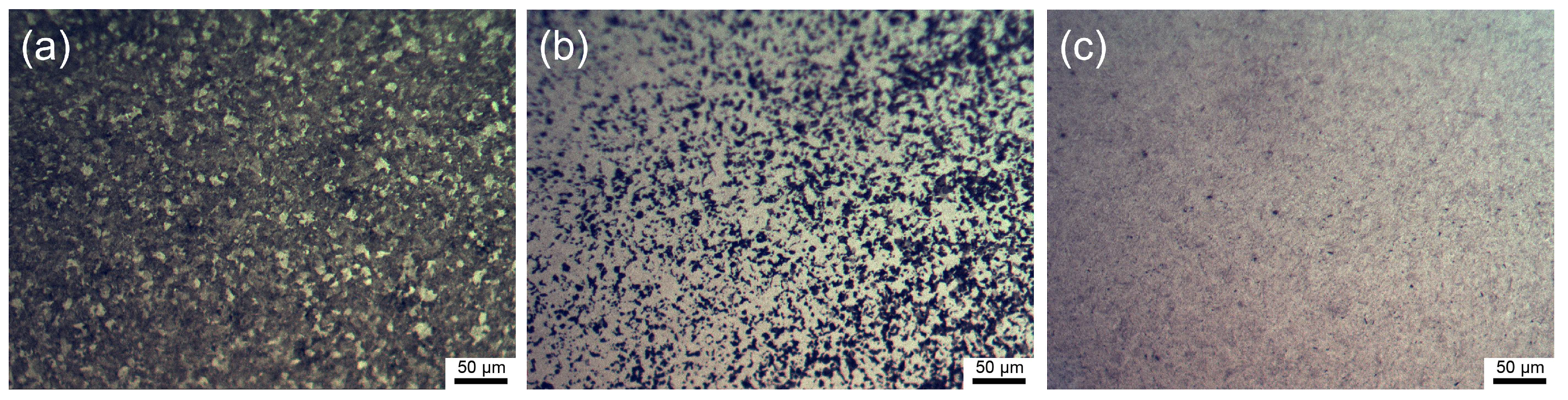
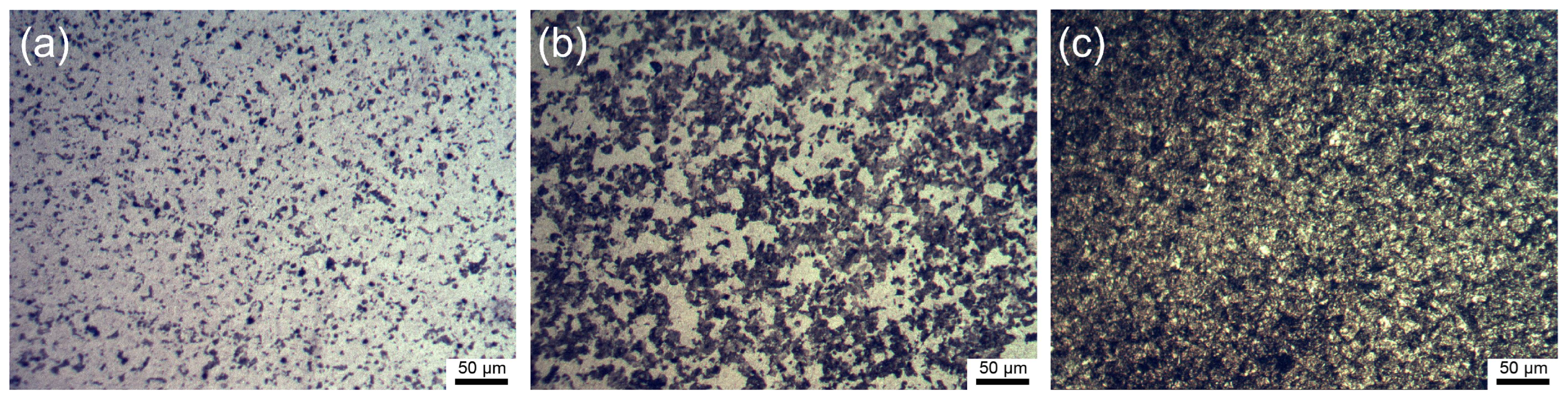
3.1. Characterization of the Microstructure
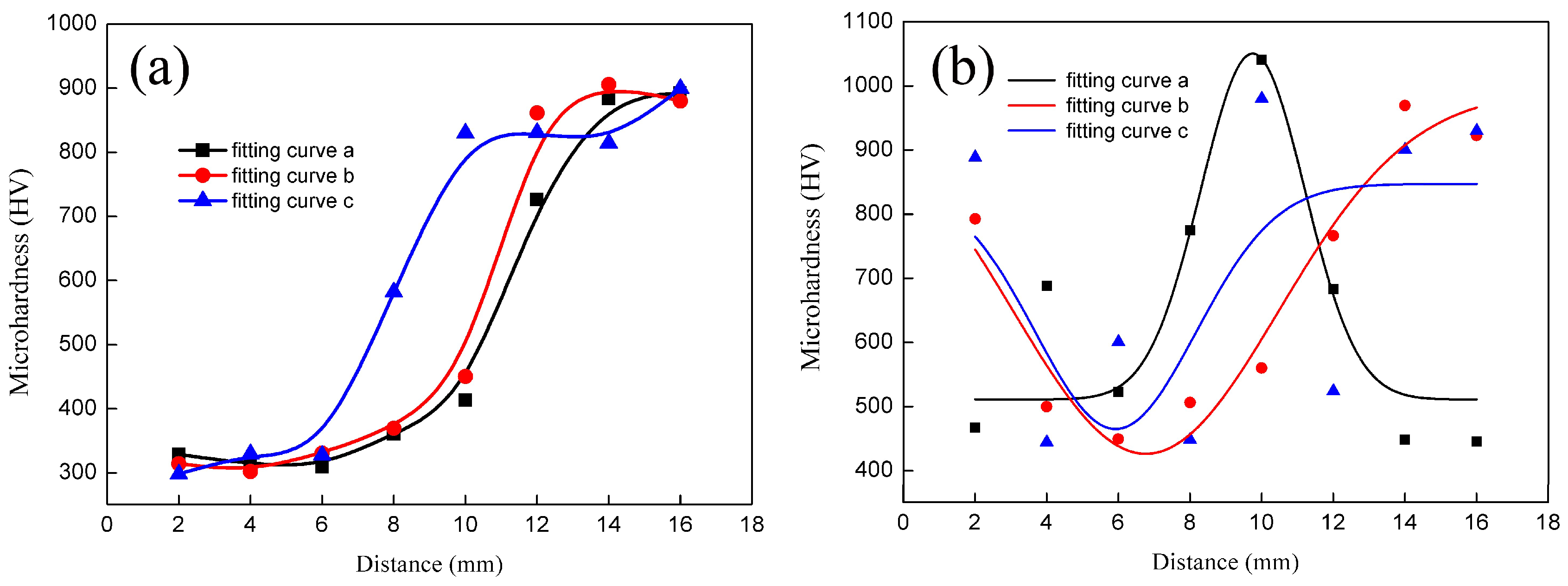
3.2. Microhardness Tests
3.3. Electrochemical Corrosion Behavior
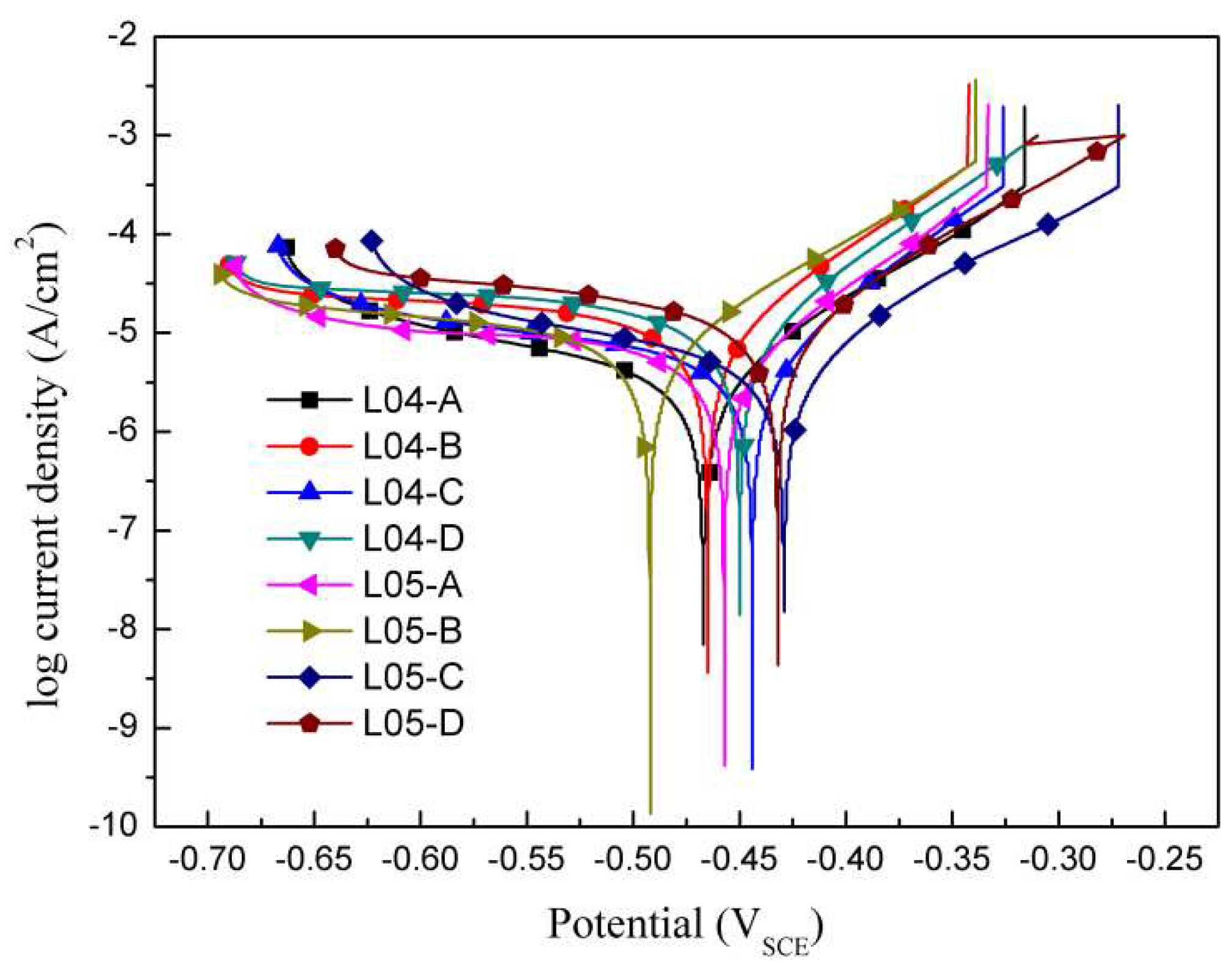
3.3.1. Potentiodynamic Polarization Tests
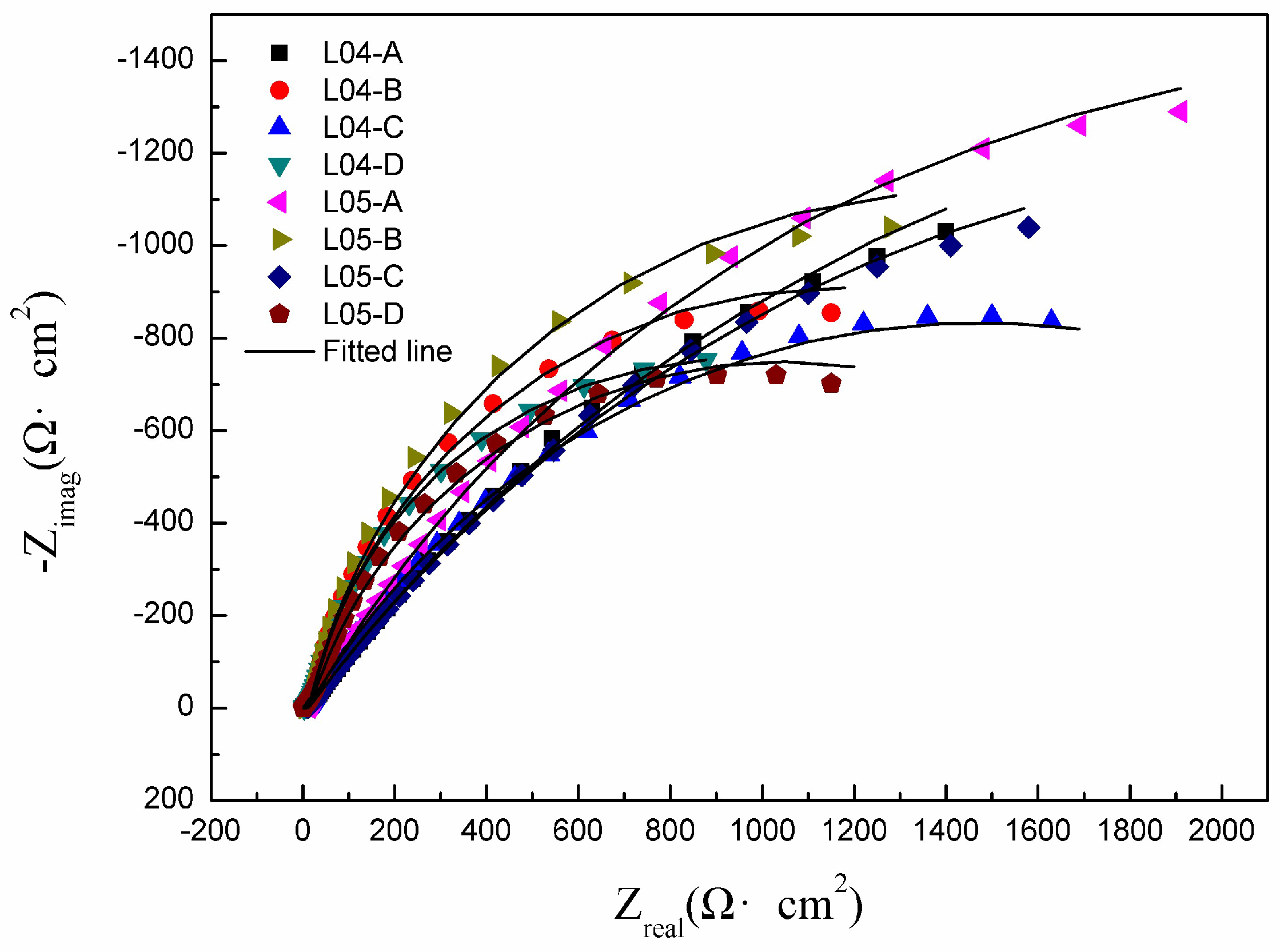
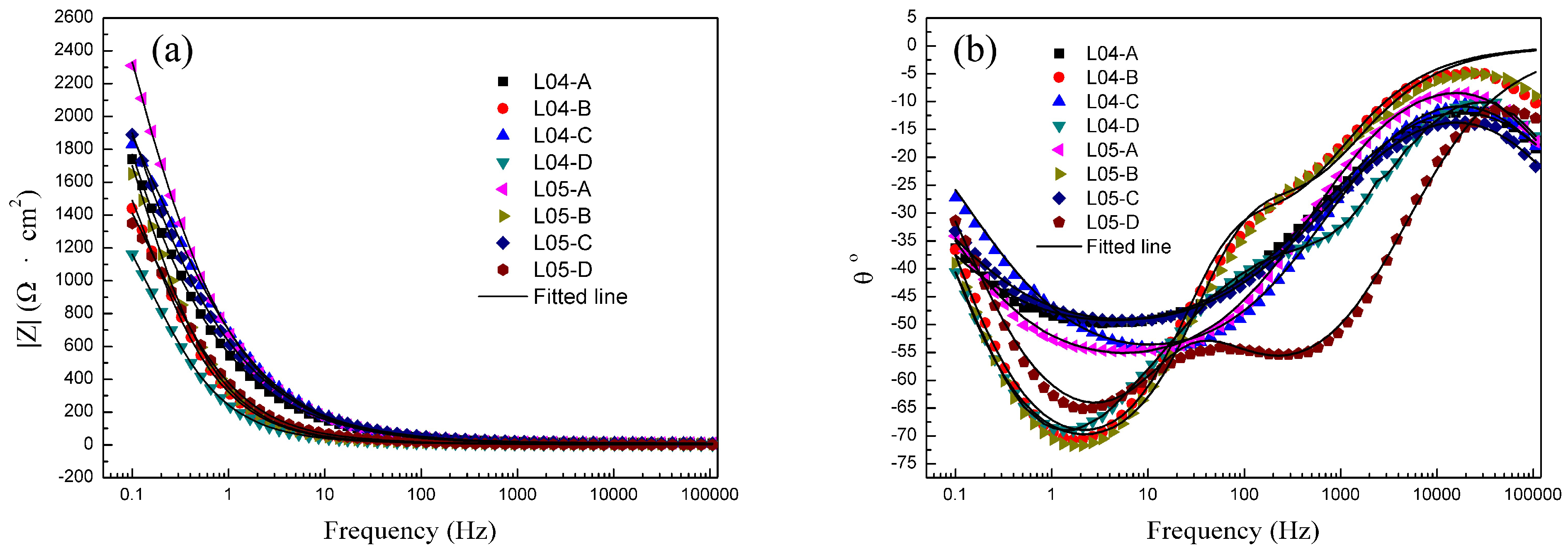
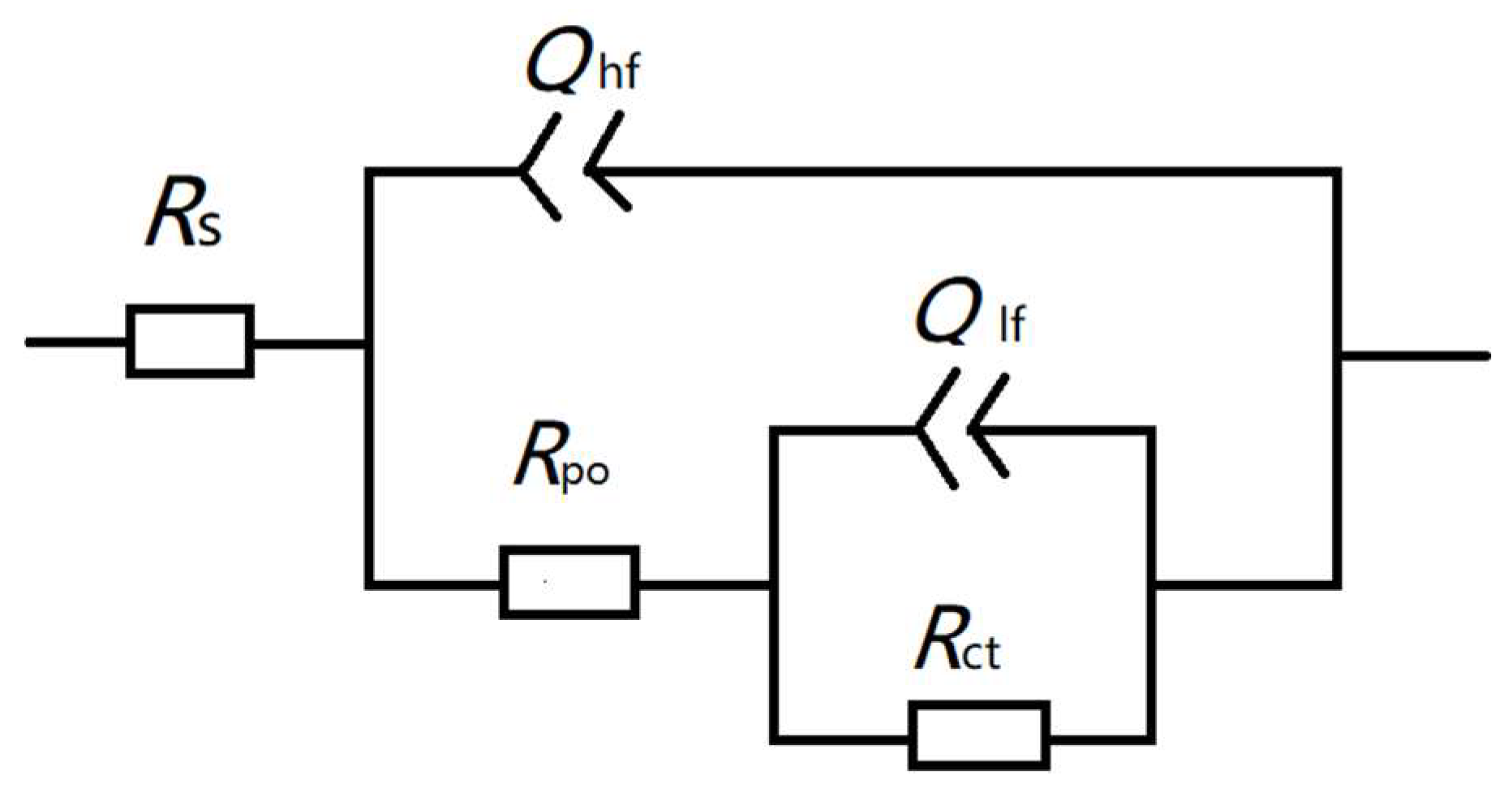
3.3.2. EIS Measurements
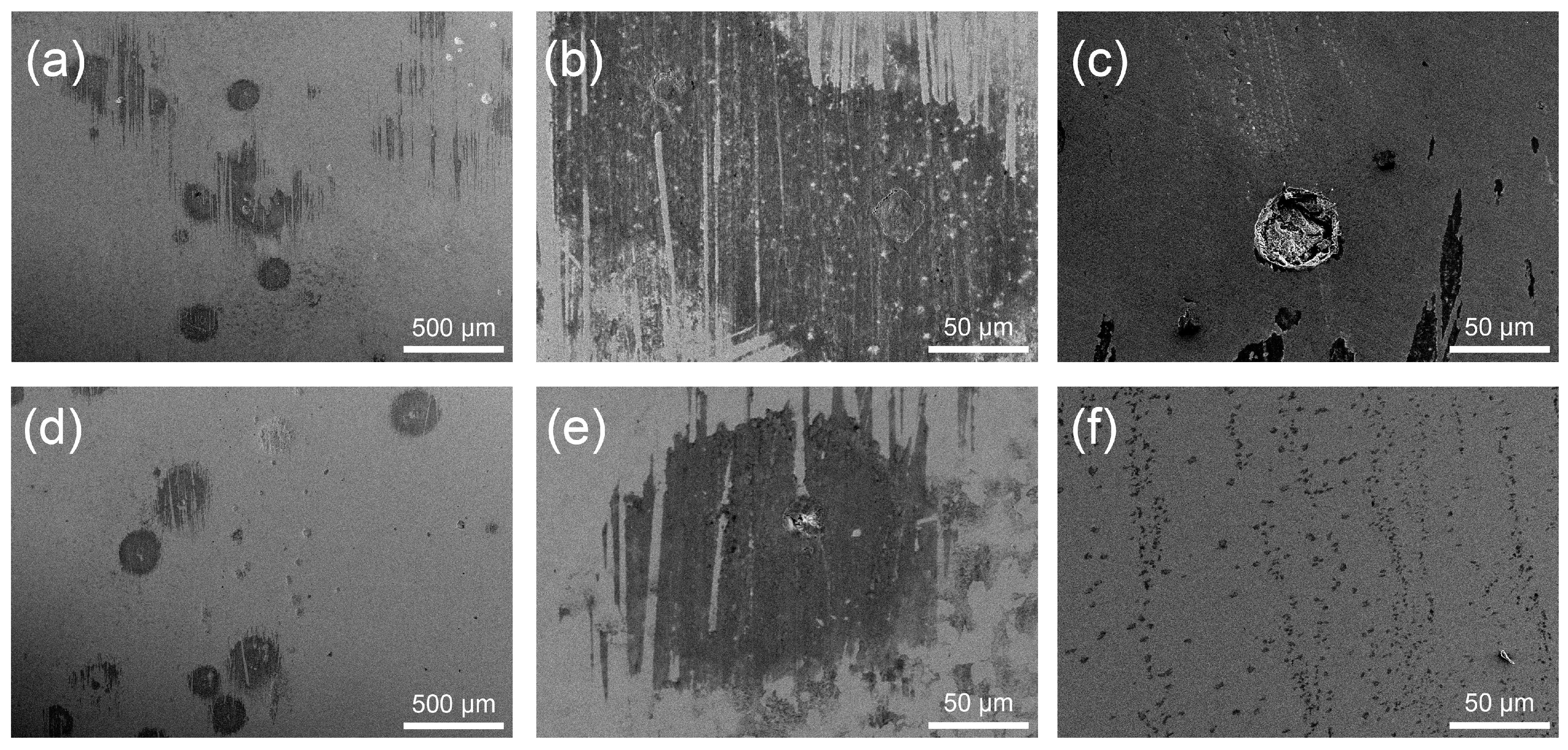
3.3.3. Corrosion Morphologies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mackenzie, D. History of quenching. Int. Heat Treat. Surf. Eng. 2008, 2, 68–73. [Google Scholar] [CrossRef]
- Wadsworth, J.; Lesuer, D. Ancient and modern laminated composite—From the Great Pyramid of Gizeh to Y2K. Mater. Charact. 2000, 45, 289–313. [Google Scholar] [CrossRef]
- Notis, M. The history of the metallographic study of the Japanese sword. Mater. Charact. 2000, 45, 253–258. [Google Scholar] [CrossRef]
- Inoue, T. The Japanese sword the material, manufacturing and computer simulation of quenching process. Mater. Sci. Res. Int. 1997, 4, 193–203. [Google Scholar]
- Harjo, S.; Kawasaki, T.; Grazzi, F.; Shinohara, T.; Tanaka, M. Neutron diffraction study on full-shape Japanese sword. Materialia 2019, 7, 100377. [Google Scholar] [CrossRef]
- Pham, A.; Ohba, T.; Morito, S.; Hayashi, T. Automatic reconstruction approach to characterization of prior-austenite microstructure in various Japanese swords. Mater. Trans. 2015, 56, 1639–1647. [Google Scholar] [CrossRef]
- Yaso, M.; Takaiwa, T.; Minagi, Y.; Kanaizumi, T.; Kubota, K.; Hayashi, T.; Morito, S.; Ohba, T. Study of Japanese sword from a viewpoint of steel strength. J. Alloys Compd. 2013, 577S, S690–S694. [Google Scholar] [CrossRef]
- Tanimura, H. Development of the Japanese sword. JOM 1980, 32, 63–73. [Google Scholar] [CrossRef]
- Inoue, T. Tatara and the Japanese sword: The science and technology. Acta Mech. 2010, 214, 17–30. [Google Scholar] [CrossRef]
- William, D. Materials Science and Engineering: An Introduction, 7th ed.; John Wiley& Sons. Inc.: New York, NY, USA, 2009; pp. 623–637. [Google Scholar]
- Wu, W.; Li, X. Investigation of effect of heat treatment on the Han dynasty steel sword corrosion by electrochemical corrosion testing. Int. J. Electrochem. Sci. 2022, 17, 221268. [Google Scholar] [CrossRef]
- Tian, W.; Chen, F.; Cheng, F.; Li, Z.; Pang, G. Corrosion properties of pure aluminum prepared by spark plasma sintering (SPS) using different grain size of aluminium powders as raw material. Int. J. Electrochem. Sci. 2020, 15, 9120–9134. [Google Scholar] [CrossRef]
- Liang, Z.; Jiang, K.; Feng, B.; Lin, S.; Chao, X.; Sui, Q.; Zhang, T. Corrosion evolution of Cu-Pb alloys from the Western Zhou dynasty in simulated archaeological soil environment. J. Electroanal. Chem. 2021, 899, 115688. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, X.; Wu, T.; Liu, M.; Li, C.; Yin, F. Corrosion of X80 steel welded joint under disbonded coating in an acidic soil solution. Int. J. Pres. Ves. Pip. 2021, 194, 104508. [Google Scholar] [CrossRef]
- Tian, W.; Li, Z.; Kang, H.; Cheng, F.; Chen, F.; Pang, G. Passive film properties of bimodal grain size AA7075 aluminium alloy prepared by spark plasma sintering. Materials 2020, 13, 3236. [Google Scholar] [CrossRef]
- Tian, W.; Chen, F.; Li, Z.; Pang, G.; Meng, Y. Corrosion product concentration in a single three-dimensional pit and the associated pitting dynamics. Corros. Sci. 2020, 173, 108775. [Google Scholar] [CrossRef]
- Jafery, K.M.; Embong, Z.; Othman, N.; Yaakob, N.; Shah, M.; Hashim, N. Initial stage of corrosion formation for X70 pipeline external surface in acidic soil (peat) environment. Mater. Today Proc. 2022, 51, 1381–1387. [Google Scholar] [CrossRef]
- Qin, Q.; Wei, B.; Bai, Y.; Fu, Q.; Xu, J.; Sun, C.; Wang, C.; Wang, Z. Effect of alternating current frequency on corrosion behavior of X80 pipeline steel in coastal saline soil. Eng. Fail. Anal. 2021, 120, 105065. [Google Scholar] [CrossRef]
- Wei, B.; Xu, J.; Cheng, Y.; Sun, C.; Yu, C.; Wang, Z. Effect of uniaxial elastic stress on corrosion of X80 pipeline steel in an acidic soil solution containing sulfate-reducing bacteria trapped under disbonded coating. Corros. Sci. 2021, 193, 109893. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, B.; Yuan, Y.; Yin, S.; Guo, S. Effect of annealing temperature on microstructure and corrosion behavior of CoCrFeMnNi high-entropy alloy in alkaline soil simulation solution. Mater. Chem. Phys. 2022, 279, 125725. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Zhang, S.; Shi, Z.; Wei, C.; Ma, M.; Liu, R. Effect of Cu content on the corrosion behavior of Ti-based bulk amorphous alloys in HCl solution. Mater. Lett. 2023, 337, 133742. [Google Scholar] [CrossRef]
- Liu, C.; Gao, Y.; Chong, K.; Guo, F.; Wu, D.; Zou, Y. Effect of Nb content on the microstructure and corrosion resistance of FeCoCrNiNbx high-entropy alloys in chloride ion environment. J. Alloys Compd. 2023, 935, 168013. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Z.; Wang, X.; Zhang, S.; Zheng, Y. Effect of thermally induced B2 phase on the corrosion behavior of an Al0.3CoCrFeNi high entropy alloy. J. Alloys Compd. 2022, 903, 163886. [Google Scholar] [CrossRef]
- Ahmadi, M.; Mirzaee, O.; Azadi, M.; Biukani, H. Effects of various double-quenching treatments on the microstructure and corrosion properties of D2 tool steel. Int. J. Pres. Ves. Pip. 2023, 202, 104915. [Google Scholar] [CrossRef]
- Khan, M.; Sarkar, A.; Mehtani, H.; Raut, P.; Prakash, A.; Prasad, M.; Samajdar, I.; Parida, S. Microstructure and aqueous corrosion in carbon steel: An emerging correlation. Mater. Chem. Phys. 2022, 290, 126623. [Google Scholar] [CrossRef]
- Tian, W.; Chen, F.; Li, Z.; Pang, G.; Li, Y. Stress corrosion cracking of 05Cr17Ni4Cu4Nb and 1Cr12Ni3Mo2VN martensitic stainless steels under constant load. Int. J. Electrochem. Sci. 2020, 15, 6572–6581. [Google Scholar] [CrossRef]
- Kang, L.; Zeng, Q.; Wang, B.; Zeng, J.; Liao, B.; Wu, H.; Cheng, Z.; Guo, X. Facile fabrication of multi superlyophobic nano soil coated-mesh surface with excellent corrosion resistance for efficient immiscible liquids separation. Sep. Purif. Technol. 2022, 284, 120266. [Google Scholar] [CrossRef]
- Azoor, R.; Deo, R.; Birbilis, N.; Kodikara, J. On the optimum soil moisture for underground corrosion in different soil types. Corros. Sci. 2019, 159, 108116. [Google Scholar] [CrossRef]
- Rossi, S.; Pinamonti, M.; Calovi, M. Influence of soil chemical characteristics on corrosion behaviour of galvanized steel. Case Stud. Constr. Mat. 2022, 17, e01257. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, Z.; Liu, W.; Zhang, D.; Zhao, H.; Wang, L.; Li, X. Superhydrophobic oligoaniline-containing electroactive silica coating as pre-process coating for corrosion protection of carbon steel. Chem. Eng. J. 2018, 348, 940–951. [Google Scholar] [CrossRef]
- Yin, X.; Mu, P.; Wang, Q.; Li, J. Superhydrophobic ZIF-8-based dual-layer coating for enhanced corrosion protection of Mg alloy. ACS Appl. Mater. Interfaces 2020, 12, 35453–35463. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Wang, X.; Yuan, S.; Lin, D.; Zhu, Y.; Wang, H. Synthesis of hydrophobic fluoro-substituted polyaniline filler for the long-term anti-corrosion performance enhancement of epoxy coatings. Corros. Sci. 2021, 178, 109094–109104. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y. Corrosion of initial pits on abandoned X52 pipeline steel in a simulated soil solution containing sulfate-reducing bacteria. J. Mater. Res. Technol. 2020, 9, 7180–7189. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, B.; Liu, Z.; Du, C.; Shou, B. Local chemistry–electrochemistry and stress corrosion susceptibility of X80 steel below disbonded coating in acidic soil environment under cathodic protection. Constr. Build. Mater. 2020, 243, 118203. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, C.; Zhang, Y.; Liu, H.; Liu, H.; Liu, H. Early corrosion behavior of X80 pipeline steel in a simulated soil solution containing Desulfovibrio desulfuricans. Bioelectrochemistry 2021, 141, 107880. [Google Scholar] [CrossRef]
- Su, P.; Wu, X.; Guo, Y.; Jiang, Z. Effects of cathode current density on structure and corrosion resistance of plasma electrolytic oxidation coatings formed on ZK60 Mg alloy. J. Alloys Compd. 2009, 475, 773–777. [Google Scholar] [CrossRef]
- Osório, W.; Peixoto, L.; Garcia, L.; Garcia, A. Electrochemical corrosion response of a low carbon heat treated steel in a NaCl solution. Mater. Corros. 2009, 60, 804–812. [Google Scholar] [CrossRef]
- Qin, J.; Li, Z.; Ma, M.; Yi, D.; Wang, B. Diversity of intergranular corrosion and stress corrosion cracking for 5083 Al alloy with different grain sizes. Trans. Nonferrous Met. Soc. China 2022, 32, 765–777. [Google Scholar] [CrossRef]
- Wang, S.; Lamborn, L.; Chen, W. Pre-cyclic-loading-enhanced Stage-1b stress corrosion crack growth of pipeline steels. Corros. Sci. 2022, 208, 110693. [Google Scholar] [CrossRef]



| C | Si | Mn | P | S | Loss |
|---|---|---|---|---|---|
| 0.98 | 0.3 | 0.2 | 0.03 | 0.02 | 98.47 |
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | Loss |
|---|---|---|---|---|---|---|---|
| 70.6 | 12.5 | 3.8 | 5.6 | 2.2 | 2.9 | 1.8 | 0.6 |
| Na2B4O7 | Chloride | Sulfate | Carbonate |
|---|---|---|---|
| 95.1 | 0.03 | <0.2 | 0.1 |
| Fe | Cu | S | Zn | O | Sn |
|---|---|---|---|---|---|
| 99.99 | 0.003 | 0.003 | 0.001 | 0.002 | 0.001 |
| Fixed Carbon | Ash Content | Volatiles |
|---|---|---|
| 85.65 | 13.06 | 1.29 |
| Samples | ba (mV∙dec−1) | bc (mV∙dec−1) | icorr (μA·cm−2) | Ecorr (VSCE) | Corrosion Rate (μm/a) | Rp (Ω·cm2) |
|---|---|---|---|---|---|---|
| L04-A | 56 ± 7 | 236 ± 40 | 2.93 ± 0.42 | −0.46 | 0.068 ± 0.01 | 6716 |
| L04-B | 57 ± 6 | 302 ± 45 | 8.73 ± 1.23 | −0.46 | 0.204 ± 0.03 | 2388 |
| L04-C | 46 ± 4 | 231 ± 32 | 3.67 ± 0.52 | −0.44 | 0.086 ± 0.01 | 4544 |
| L04-D | 53 ± 5 | 339 ± 40 | 10.44 ± 1.56 | −0.45 | 0.245 ± 0.03 | 1908 |
| L05-A | 59 ± 7 | 234 ± 28 | 2.48 ± 0.35 | −0.45 | 0.062 ± 0.01 | 8260 |
| L05-B | 65 ± 8 | 194 ± 25 | 5.12 ± 0.72 | −0.49 | 0.120 ± 0.01 | 4134 |
| L05-C | 54 ± 7 | 165 ± 16 | 3.01 ± 0.43 | −0.42 | 0.070 ± 0.01 | 5876 |
| L05-D | 56 ± 8 | 195 ± 29 | 8.08 ± 1.15 | −0.43 | 0.189 ± 0.02 | 2341 |
| Samples | Y0 (S·sn·cm−2) | n | Rhf (Ω·cm2) | Rct (Ω·cm2) |
|---|---|---|---|---|
| L04-A | 5.64 × 10−4 | 0.9102 | 11 ± 1.55 | 4526 ± 601 |
| L04-B | 3.14 × 10−4 | 0.8947 | 19 ± 2.69 | 2312 ± 332 |
| L04-C | 3.69 × 10−4 | 0.8931 | 16 ± 2.05 | 3037 ± 458 |
| L04-D | 1.56 × 10−4 | 0.8567 | 34 ± 4.89 | 1988 ± 268 |
| L05-A | 3.91 × 10−4 | 0.9223 | 17 ± 2.35 | 4808 ± 676 |
| L05-B | 3.31 × 10−4 | 0.8918 | 14 ± 1.85 | 2837 ± 371 |
| L05-C | 4.86 × 10−4 | 0.9108 | 12 ± 1.68 | 4732 ± 689 |
| L05-D | 5.28 × 10−4 | 0.8648 | 24 ± 3.26 | 2018 ± 273 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Bu, C.; Li, S.; Du, S.; Chen, Q.; Li, X. The Effects of Quenching with Clay on the Microstructure and Corrosion Performance of Steel Blades. Coatings 2023, 13, 1904. https://doi.org/10.3390/coatings13111904
Wu W, Bu C, Li S, Du S, Chen Q, Li X. The Effects of Quenching with Clay on the Microstructure and Corrosion Performance of Steel Blades. Coatings. 2023; 13(11):1904. https://doi.org/10.3390/coatings13111904
Chicago/Turabian StyleWu, Wei, Chaoqun Bu, Shuoyang Li, Shunhua Du, Qian Chen, and Xiaocen Li. 2023. "The Effects of Quenching with Clay on the Microstructure and Corrosion Performance of Steel Blades" Coatings 13, no. 11: 1904. https://doi.org/10.3390/coatings13111904
APA StyleWu, W., Bu, C., Li, S., Du, S., Chen, Q., & Li, X. (2023). The Effects of Quenching with Clay on the Microstructure and Corrosion Performance of Steel Blades. Coatings, 13(11), 1904. https://doi.org/10.3390/coatings13111904




