Eco-Friendly Sol–Gel Coatings as Microfouling Barrier for Marine Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluation of the Antibacterial Activity of CP and CO
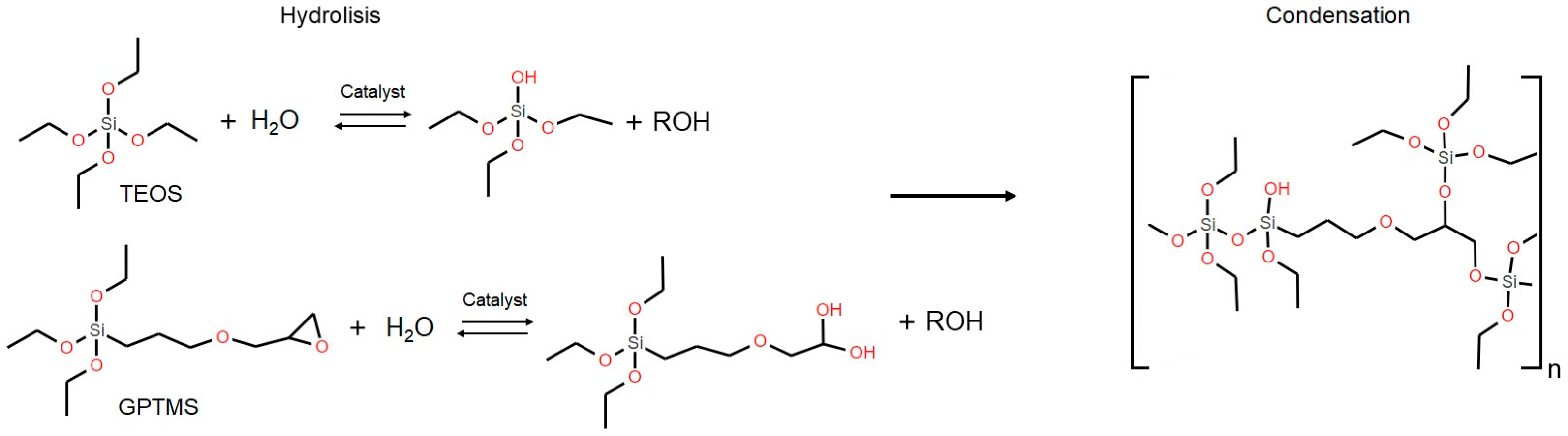
2.2. Preparation of Hybrid Sols with Biocides
2.3. Coating of Nylon Substrates with the Sol/Biocide Formulations
2.4. Characterization of the Coated Substrates
2.5. Evaluation of Antibacterial Activity of Surfaces with the Active Compounds
2.6. Leaching Tests and Quantification
3. Results
3.1. Evaluation of Antibacterial Activity of CP and CO
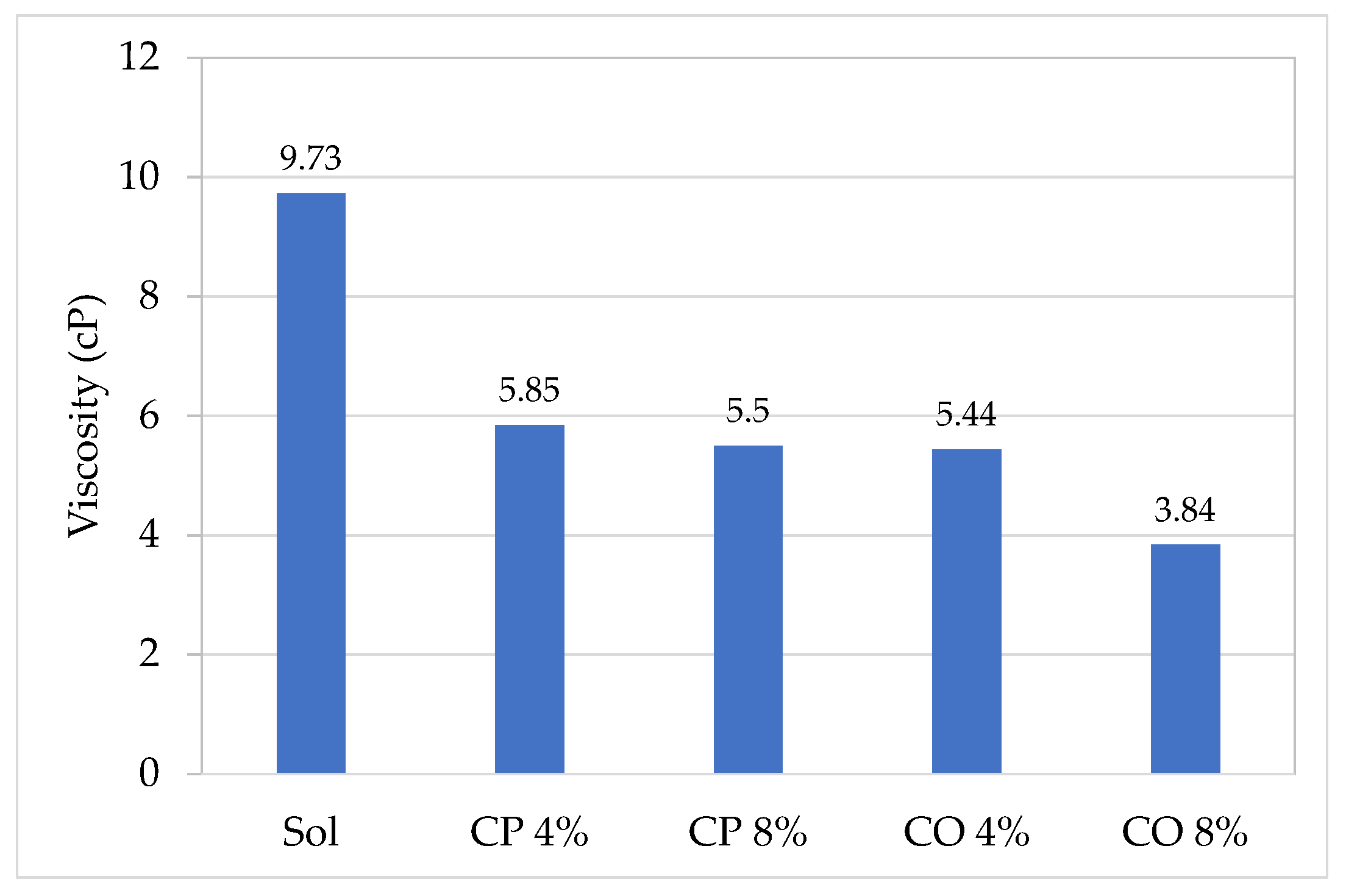
3.2. Preparations of Hybrid Sols and Incorporation of Biocides
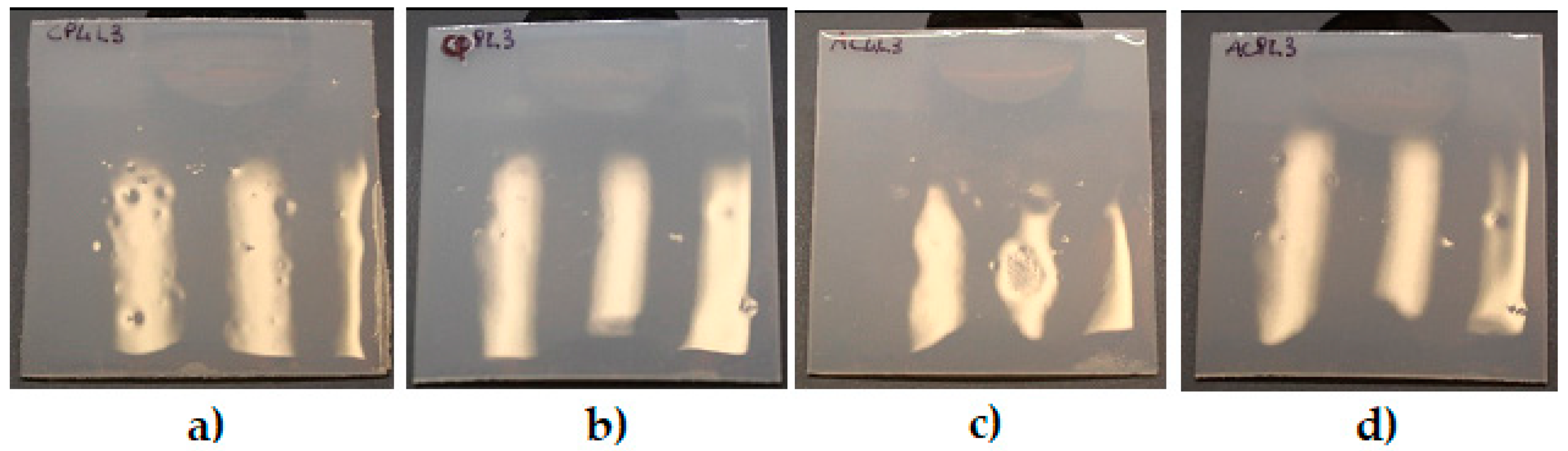
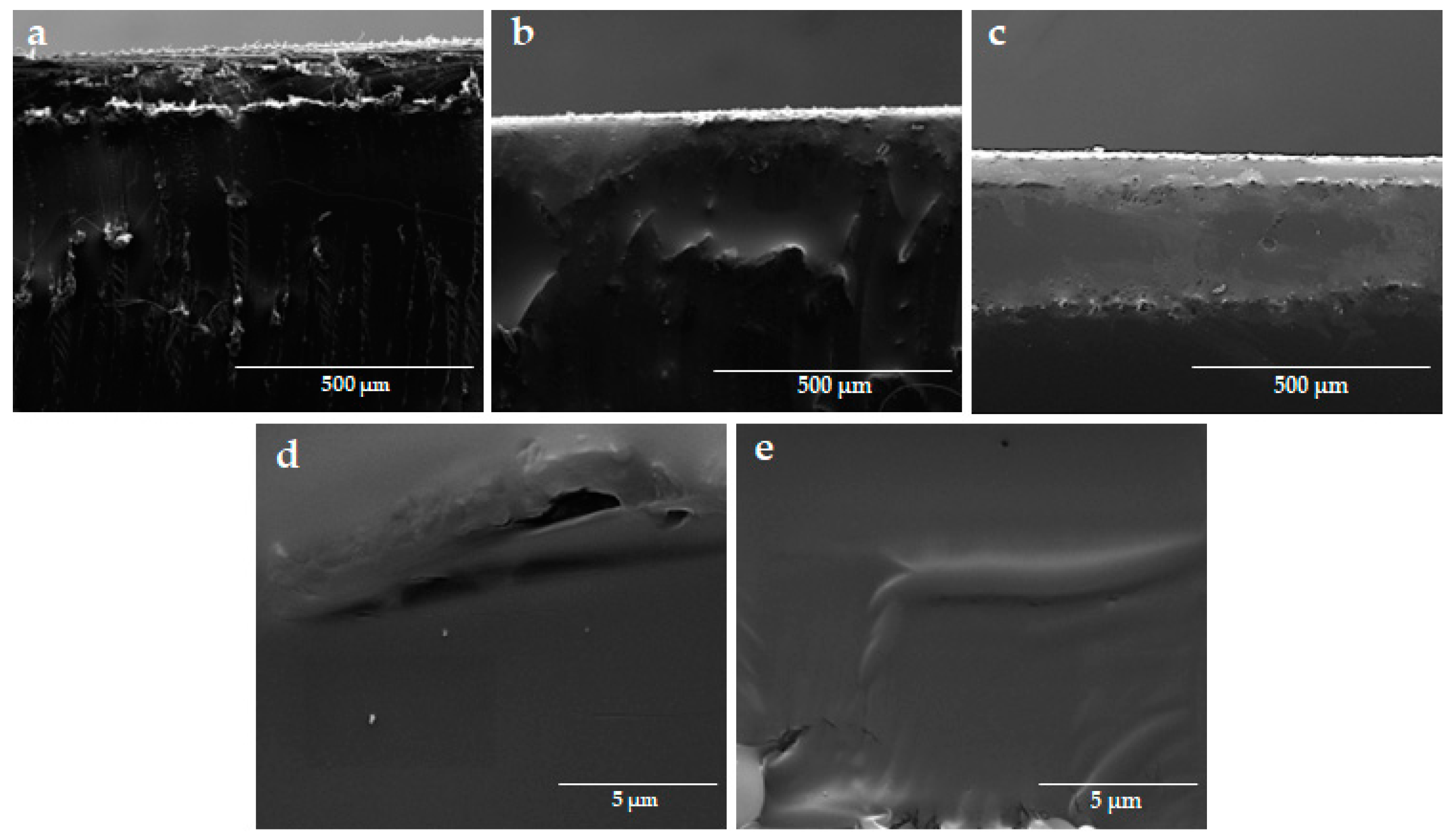
3.3. Scanning Electron Microscopy and Adhesion Test
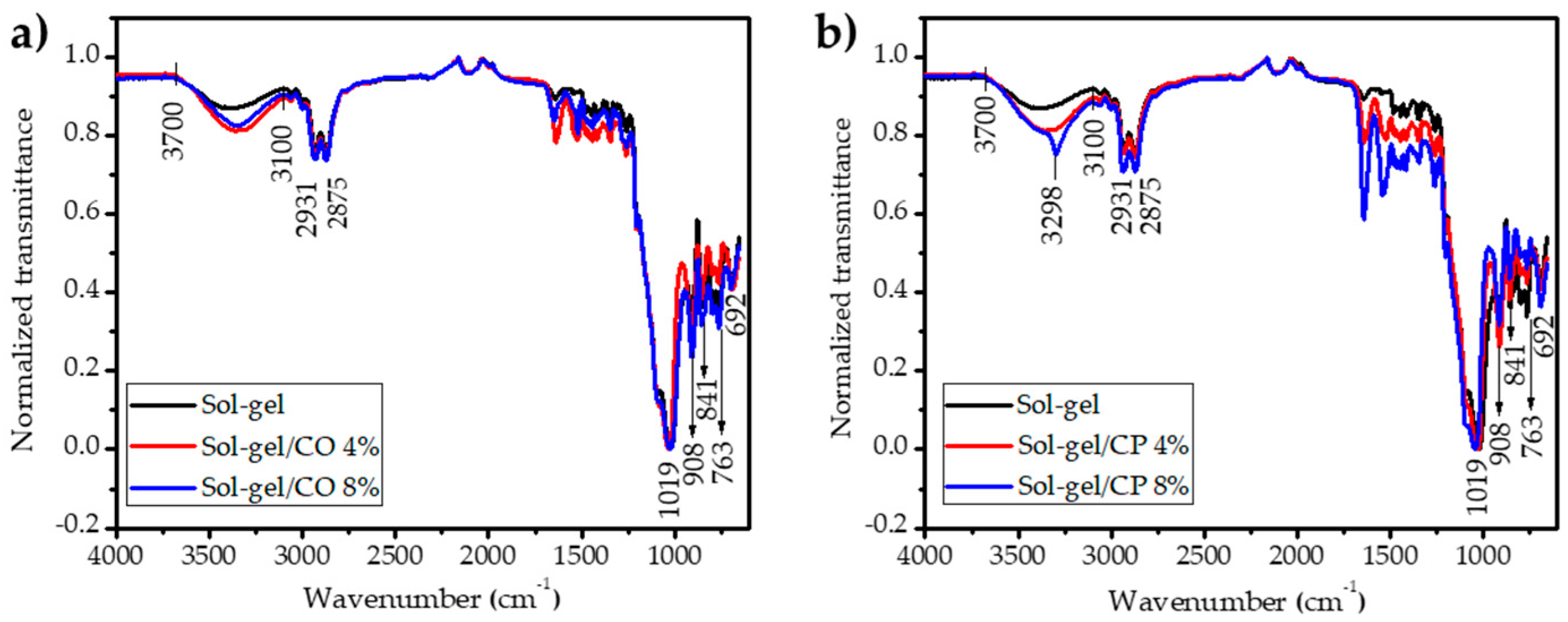
3.4. FTIR Characterization
3.5. Antibacterial Activity of Surfaces with Active Compounds
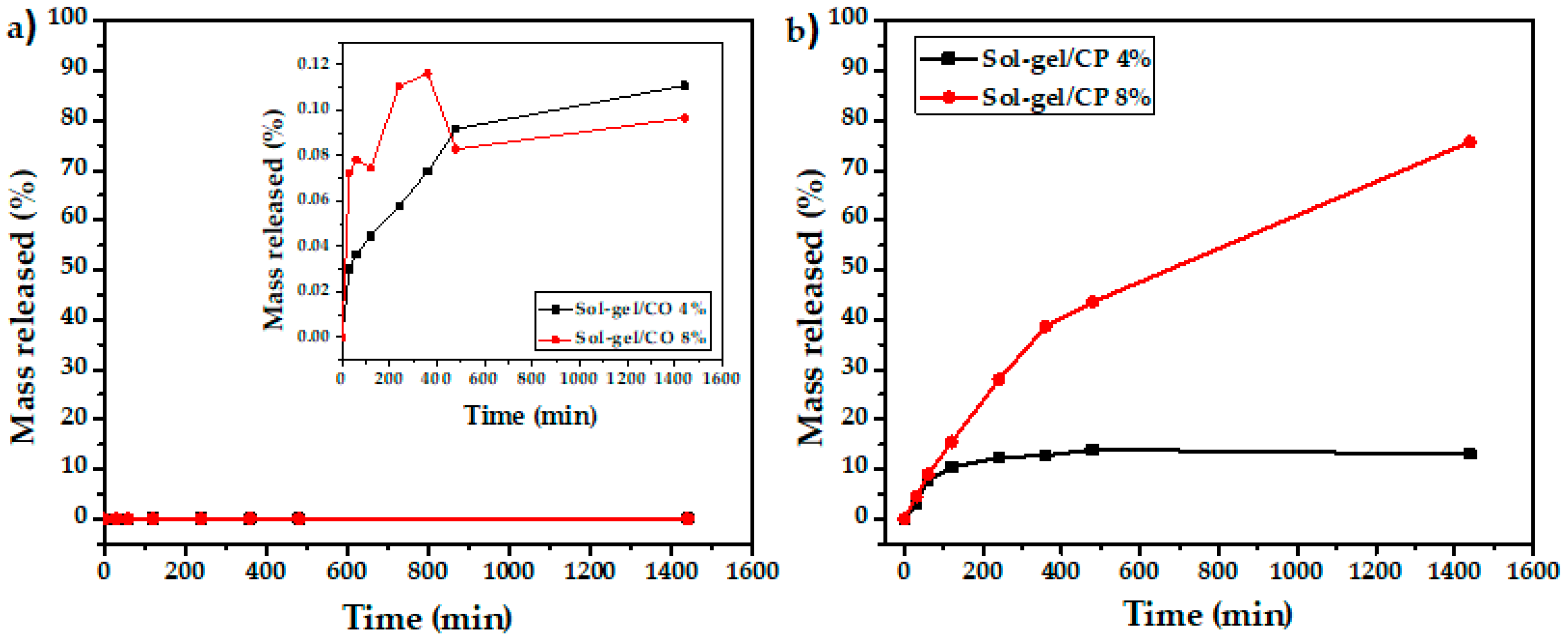
3.6. Leaching of Biocides in Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, L.; Yin, Y.; Bing, W.; Jin, E. Antifouling Technology Trends in Marine Environmental Protection. J. Bionic Eng. 2021, 18, 239–263. [Google Scholar] [CrossRef]
- Richards, C.; Briciu-Burghina, C.; Jacobs, M.R.; Barrett, A.; Regan, F. Assessment of antifouling potential of novel transparent sol gel coatings for application in the marine environment. Molecules 2019, 24, 2983. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, X.; Huang, J.; Li, H. Flame sprayed environmentally friendly high density polyethylene (HDPE)–capsaicin composite coatings for marine antifouling applications. Mater. Lett. 2019, 238, 46–50. [Google Scholar] [CrossRef]
- SUBPESCA Determinación y evaluación de los componentes presentes en las pinturas anti-incrustantes utilizadas en la acuicultura. Chile 2013. Available online: https://www.subpesca.cl/portal/618/articles-81701_documento.pdf (accessed on 5 October 2023).
- Arboleda-Baena, C.; Osiadacz, N.; Parragué, M.; González, A.E.; Fernández, M.; Finke, G.R.; Navarrete, S.A. Assessing Efficacy of “Eco-Friendly” and Traditional Copper-Based Antifouling Materials in a Highly Wave-Exposed Environment. J. Mar. Sci. Eng. 2023, 11, 217. [Google Scholar] [CrossRef]
- Amara, I.; Miled, W.; Slama, R.B.; Ladhari, N. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Environ. Toxicol. Pharmacol. 2018, 57, 115–130. [Google Scholar] [CrossRef]
- Evans, S.M.; Leksono, T.; McKinnell, P.D. Tributyltin pollution: A diminishing problem following legislation limiting the use of TBT-based anti-fouling paints. Mar. Pollut. Bull. 1995, 30, 14–21. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, L.; Zhang, J.; Habib, S.; Lu, G.; Dai, J.; Liu, X. Bio-based polybenzoxazines coatings for efficient marine antifouling. Prog. Org. Coat. 2023, 174, 107298. [Google Scholar] [CrossRef]
- Sha, J.; Liu, X.; Chen, R.; Yu, J.; Liu, Q.; Liu, J.; Zhu, J.; Liu, P.; Li, R.; Wang, J. Surface hydrolysis-anchored eugenol self-polishing marine antifouling coating. J. Colloid Interface Sci. 2023, 637, 67–75. [Google Scholar] [CrossRef]
- Chiang, H.Y.; Pan, J.; Ma, C.; Qian, P.Y. Combining a bio-based polymer and a natural antifoulant into an eco-friendly antifouling coating. Biofouling 2020, 36, 200–209. [Google Scholar] [CrossRef]
- Siddiquee, S. Recent Advancements on the Role and Analysis of Volatile Compounds (VOCs) from Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780444595768. [Google Scholar]
- Deng, Y.; Xia, L.; Song, G.L.; Zhao, Y.; Zhang, Y.; Xu, Y.; Zheng, D. Development of a curcumin-based antifouling and anticorrosion sustainable polybenzoxazine resin composite coating. Compos. Part B Eng. 2021, 225, 109263. [Google Scholar] [CrossRef]
- Alam, M.; Alandis, N.M.; Ahmad, N.; Husain, F.M. Anticorrosive and antibacterial nanocomposite coating material from sustainable resource. Ind. Crops Prod. 2020, 158, 112955. [Google Scholar] [CrossRef]
- Füchtbauer, S.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Antibacterial properties of capsaicin and its derivatives and their potential to fight antibiotic resistance—A literature survey. Eur. J. Microbiol. Immunol. 2021, 11, 10–17. [Google Scholar] [CrossRef]
- Marini, E.; Magi, G.; Mingoia, M.; Pugnaloni, A.; Facinelli, B. Antimicrobial and anti-virulence activity of capsaicin against erythromycin-resistant, cell-invasive group A streptococci. Front. Microbiol. 2015, 6, 1281. [Google Scholar] [CrossRef]
- Goci, E.; Haloci, E.; Di Stefano, A.; Chiavaroli, A.; Angelini, P.; Miha, A.; Cacciatore, I.; Marinelli, L. Evaluation of in vitro capsaicin release and antimicrobial properties of topical pharmaceutical formulation. Biomolecules 2021, 11, 432. [Google Scholar] [CrossRef]
- Menezes, R.d.P.; Bessa, M.A.d.S.; Siqueira, C.d.P.; Teixeira, S.C.; Ferro, E.A.V.; Martins, M.M.; Cunha, L.C.S.; Martins, C.H.G. Antimicrobial, Antivirulence, and Antiparasitic Potential of Capsicum chinense Jacq. Extracts and Their Isolated Compound Capsaicin. Antibiotics 2022, 11, 1154. [Google Scholar] [CrossRef]
- Wang, F.; Xue, Y.; Fu, L.; Wang, Y.; He, M.; Zhao, L.; Liao, X. Extraction, purification, bioactivity and pharmacological effects of capsaicin: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 5322–5348. [Google Scholar] [CrossRef]
- Hassan, M.; Abdulrazik, G.; El Awady, M.; Hamed, A.; Abdel-Monem, M. Antimicrobial Activity of Capsaicin and Its Derivatives against Klebsiella pneumoniae. Egypt. Acad. J. Biol. Sci. G. Microbiol. 2021, 13, 79–90. [Google Scholar] [CrossRef]
- Morrine, A.O.; Zen-Zi, W.; Weih, G.B.; Grant, A.H.; Kamal, D.; David, J.B. Comparative analysis of capsaicin in twenty nine varieties of unexplored Capsicum and its antimicrobial activity against bacterial and fungal pathogens. J. Med. Plants Res. 2018, 12, 544–556. [Google Scholar] [CrossRef]
- Xing, F.; Cheng, G.; Yi, K. Study on the antimicrobial activities of the capsaicin microcapsules. J. Appl. Polym. Sci. 2006, 102, 1318–1321. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, F.; Xu, W.; Han, X. Enhanced antibacterial performance of gelatin/chitosan film containing capsaicin loaded MOFs for food packaging. Appl. Surf. Sci. 2020, 510, 145418. [Google Scholar] [CrossRef]
- Ayariga, J.A.; Abugri, D.A.; Amrutha, B.; Villafane, R. Capsaicin Potently Blocks Salmonella typhimurium Invasion of Vero Cells. Antibiotics 2022, 11, 666. [Google Scholar] [CrossRef]
- Liu, X.; Lin, T.; Peng, B.; Wang, X. Antibacterial activity of capsaicin-coated wool fabric. Text. Res. J. 2012, 82, 584–590. [Google Scholar] [CrossRef]
- Zhai, X.; Ju, P.; Guan, F.; Ren, Y.; Liu, X.; Wang, N.; Zhang, Y.; Duan, J.; Wang, C.; Hou, B. Electrodeposition of capsaicin-induced ZnO/Zn nanopillar films for marine antifouling and antimicrobial corrosion. Surf. Coat. Technol. 2020, 397, 125959. [Google Scholar] [CrossRef]
- Wang, W.; Hao, X.; Chen, S.; Yang, Z.; Wang, C.; Yan, R.; Zhang, X.; Liu, H.; Shao, Q.; Guo, Z. pH-responsive Capsaicin@chitosan nanocapsules for antibiofouling in marine applications. Polymer 2018, 158, 223–230. [Google Scholar] [CrossRef]
- Wang, J.; Shi, T.; Yang, X.; Han, W.; Zhou, Y. Environmental risk assessment on capsaicin used as active substance for antifouling system on ships. Chemosphere 2014, 104, 85–90. [Google Scholar] [CrossRef]
- Hao, X.; Chen, S.; Qin, D.; Zhang, M.; Li, W.; Fan, J.; Wang, C.; Dong, M.; Zhang, J.; Cheng, F.; et al. Antifouling and antibacterial behaviors of capsaicin-based pH responsive smart coatings in marine environments. Mater. Sci. Eng. C 2020, 108, 110361. [Google Scholar] [CrossRef]
- Wang, X.; Yu, L.; Liu, Y.; Jiang, X. Synthesis and fouling resistance of capsaicin derivatives containing amide groups. Sci. Total Environ. 2020, 710, 136361. [Google Scholar] [CrossRef]
- Oliveira, I.B.; Beiras, R.; Thomas, K.V.; Suter, M.J.F.; Barroso, C.M. Acute toxicity of tralopyril, capsaicin and triphenylborane pyridine to marine invertebrates. Ecotoxicology 2014, 23, 1336–1344. [Google Scholar] [CrossRef]
- Oliveira, I.B.; Groh, K.J.; Schönenberger, R.; Barroso, C.; Thomas, K.V.; Suter, M.J.F. Toxicity of emerging antifouling biocides to non-target freshwater organisms from three trophic levels. Aquat. Toxicol. 2017, 191, 164–174. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Liu, S.; Li, S. Cinnamon Cassia Oil Emulsions Stabilized by Chitin Nanofibrils: Physicochemical Properties and Antibacterial Activities. J. Agric. Food Chem. 2020, 68, 14620–14631. [Google Scholar] [CrossRef]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63–67. [Google Scholar] [CrossRef]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; St. Rose, T.; Puchalski, K.; Langland, J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement. Med. Ther. 2021, 21, 124. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Silva, K.E.; Croda, J.; Simionatto, S. Antibacterial activity of Cinnamomum cassia L. essential oil in a carbapenem- and polymyxin-resistant Klebsiella aerogenes strain. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200032. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; De Sá Queiroz, J.H.F.; Da Silva, K.E.; De Paula Vasconcelos, P.C.; Croda, J.; Simionatto, S. Synergistic effects of Cinnamomum cassia L. essential oil in combination with polymyxin B against carbapenemase-producing Klebsiella pneumoniae and Serratia marcescens. PLoS ONE 2020, 15, e0236505. [Google Scholar] [CrossRef]
- Utchariyakiat, I.; Surassmo, S.; Jaturanpinyo, M.; Khuntayaporn, P.; Chomnawang, M.T. Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complement. Altern. Med. 2016, 16, 158. [Google Scholar] [CrossRef]
- Kacaniova, M.; Galovicova, L.; Valkova, V.; Tvrda, E.; Terentjeva, M.; Ziarovska, J.; Kunova, S.; Savitskaya, T.; Grinshpan, D.; Stefanikova, J.; et al. Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation. Open Chem. 2021, 19, 214–227. [Google Scholar] [CrossRef]
- Ferreira, L.R.; Rosário, D.K.A.; Silva, P.I.; Carneiro, J.C.S.; Pimentel Filho, N.J.; Bernardes, P.C. Cinnamon essential oil reduces adhesion of food pathogens to polystyrene. Int. Food Res. J. 2019, 26, 1103–1110. [Google Scholar]
- Cox, H.J.; Li, J.; Saini, P.; Paterson, J.R.; Sharples, G.J.; Badyal, J.P.S. Bioinspired and eco-friendly high efficacy cinnamaldehyde antibacterial surfaces. J. Mater. Chem. B 2021, 9, 2918–2930. [Google Scholar] [CrossRef]
- Cuzman, O.A.; Camaiti, M.; Sacchi, B.; Tiano, P. Natural antibiofouling agents as new control method for phototrophic biofilms dwelling on monumental stone surfaces. Int. J. Conserv. Sci. 2011, 2, 3–16. [Google Scholar]
- Mertsch, R.; Meyer, J.; Michael, G.; Rochnia, A.; Schultz, T.; Tscherjaew, J.; European Patent Application. EP 2 281 855 A1, 09/02/2011. Available online: https://patents.google.com/patent/EP2281855A1/en?q=(Antifouling+additive+coating+systems+in+contact+with+water)&oq=Antifouling+additive+for+coating+systems+in+contact+with+water+ (accessed on 5 October 2023).
- Liu, J.; Sun, J.; Duan, J.; Dong, X.; Wang, X.; Liu, C.; Hou, B. Capsaicin-Modified Fluorosilicone Based Acrylate Coating for Marine Anti-Biofouling. Coatings 2022, 12, 988. [Google Scholar] [CrossRef]
- Deva Krupa, A.N.; Vimala, R. AgNPs doped TEOS sol–gel coatings to prevent the adhesion of marine fouling organisms. IET Nanobiotechnology 2018, 12, 99–105. [Google Scholar] [CrossRef]
- Krupa, A.N.D.; Vimala, R. Evaluation of tetraethoxysilane (TEOS) sol-gel coatings, modified with green synthesized zinc oxide nanoparticles for combating microfouling. Mater. Sci. Eng. C 2016, 61, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Su, X.; Hao, D.; Yang, M.; Gui, T.; Cong, W.; Jiang, W.; Ge, X.; Guo, X. One-pot method for preparation of capsaicin-containing double-network hydrogels for marine antifouling. RSC Adv. 2022, 12, 15613–15622. [Google Scholar] [CrossRef]
- Wanka, R.; Koc, J.; Clarke, J.; Hunsucker, K.Z.; Swain, G.W.; Aldred, N.; Finlay, J.A.; Clare, A.S.; Rosenhahn, A. Sol-Gel-Based Hybrid Materials as Antifouling and Fouling-Release Coatings for Marine Applications. ACS Appl. Mater. Interfaces 2020, 12, 53286–53296. [Google Scholar] [CrossRef]
- Krzak, J.; Szczurek, A.; Babiarczuk, B.; Gąsiorek, J. Sol-Gel Surface Functionalization Regardless of Form and Type of Substrate; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128213810. [Google Scholar]
- Rahimi, H.; Mozaffarinia, R.; Hojjati Najafabadi, A.; Shoja Razavi, R.; Paimozd, E. Optimization of process factors for the synthesis of advanced chrome-free nanocomposite sol-gel coatings for corrosion protection of marine aluminum alloy AA5083 by design of experiment. Prog. Org. Coat. 2013, 76, 307–317. [Google Scholar] [CrossRef]
- Kiomarsipour, N.; Eshaghi, A.; Ramazani, M.; Zabolian, H.; Abbasi-Firouzjah, M. Preparation and evaluation of high-transparent scratch-resistant thin films on plasma treated polycarbonate substrate. Arab. J. Chem. 2023, 16, 104667. [Google Scholar] [CrossRef]
- Su, C.; Hong, B.Y.; Tseng, C.M. Sol-gel preparation and photocatalysis of titanium dioxide. Catal. Today 2004, 96, 119–126. [Google Scholar] [CrossRef]
- Neto, E.P.F.; Simões, M.B.; Noveletto, J.C.; Yabarrena, J.M.S.C.; Ullah, S.; Filho, U.P.R. The effect of ormosil matrix composition and alkaline earth metal doping on the photochromic response of ormosil-phosphotungstate films. J. Braz. Chem. Soc. 2015, 26, 2598–2606. [Google Scholar] [CrossRef]
- Monteiro, D.A.; Gozzi, G.; Chinaglia, D.L.; Oliveira, O.N.; de Vicente, F.S. Proton conduction mechanisms in GPTMS/TEOS-derived organic/silica hybrid films prepared by sol-gel process. Synth. Met. 2020, 267, 116448. [Google Scholar] [CrossRef]
- Ashraf, P.M.; Edwin, L. Nano copper oxide incorporated polyethylene glycol hydrogel: An efficient antifouling coating for cage fishing net. Int. Biodeterior. Biodegrad. 2016, 115, 39–48. [Google Scholar] [CrossRef]
- Canada, P.; Pereira, A.; Nogueira, N.; Png-Gonzalez, L.; Andrade, C.; Xavier, R. Analysis of bacterial microbiome associated with nylon and copper nets in an aquaculture context. Aquaculture 2020, 516, 734540. [Google Scholar] [CrossRef]
- González, E.P.; Hurtado, C.F.; Gace, L.; Augsburger, A. Economic Impacts of Using Copper Alloy Mesh in Trout Aquaculture: Chilean Example. Aquac. Econ. Manag. 2013, 17, 71–86. [Google Scholar] [CrossRef]
- Dong, S.; You, X.; Hu, F. Effects of wave forces on knotless polyethylene and chain-link wire netting panels for marine aquaculture cages. Ocean Eng. 2020, 207, 107368. [Google Scholar] [CrossRef]
- Nelo, M.; Myllymäki, S.; Juuti, J.; Uusimäki, A.; Jantunen, H. Cobalt Nanoparticle Inks for Printed High Frequency Applications on Polycarbonate. J. Electron. Mater. 2015, 44, 4884–4890. [Google Scholar] [CrossRef]
- Properties, M.; Mucosa, H.O. Technical Report, 3rd Resear; Pulse Lab Jakarta: Central Jakarta, Indonesia, 2017; pp. 22–25. [Google Scholar]
- Wiegand, C.; Völpel, A.; Ewald, A.; Remesch, M.; Kuever, J.; Bauer, J.; Griesheim, S.; Hauser, C.; Thielmann, J.; Tonndorf-Martini, S.; et al. Critical physiological factors influencing the outcome of antimicrobial testing according to ISO 22196/JIS Z 2801. PLoS ONE 2018, 13, e0194339. [Google Scholar] [CrossRef]
- SERNAPESCA Sernapesca al Dia, Seiscientas Certificaciones de Desembarque en Quince Días. Available online: http://www.sernapesca.cl/noticias/region-del-biobio#:~:text=En%20cuanto%20a%20la%20acuicultura,alevinaje%20y%20smolts%20(cr%C3%ADas) (accessed on 5 October 2023).
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- Hołowacz, I.; Podbielska, H.; Bauer, J.; Ulatowska-Jarża, A. Viscosity, surface tension and refractive index of tetraethylorthosilicate-based sol-gel materials depending on ethanol content. Opt. Appl. 2005, 35, 691–699. [Google Scholar]
- Fidalgo, A.; Ilharco, L.M. The defect structure of sol-gel-derived silica/polytetrahydrofuran hybrid films by FTIR. J. Non. Cryst. Solids 2001, 283, 144–154. [Google Scholar] [CrossRef]
- Merino, E.; Durán, A.; Ceré, S.; Castro, Y. Hybrid Epoxy-Alkyl Sol—Gel Coatings Reinforced with SiO2 Mg Alloy. Gels 2022, 8, 242. [Google Scholar] [CrossRef]
- Tiringer, U.; Milošev, I.; Durán, A.; Castro, Y. Hybrid sol–gel coatings based on GPTMS/TEOS containing colloidal SiO2 and cerium nitrate for increasing corrosion protection of aluminium alloy 7075-T6. J. Sol-Gel Sci. Technol. 2018, 85, 546–557. [Google Scholar] [CrossRef]
- The OSHA Hazard Communication Standard 29 CFR 1910.1200 Safety Data Sheet. Mater. Saf. Data Sheet 2012, 4, 1–6.


| Label | Description |
|---|---|
| 5B | No sector of the grid is removed |
| 4B | The affected area is <5% |
| 3B | The affected area > than 5% but <15% |
| 2B | The affected area is >15% but <35% |
| 1B | The affected area is >35% but <65% |
| 0B | The affected area is >65% |
| Bacteria Strain | Gram | Minimum Inhibitory Concentration (mg/L) | |
|---|---|---|---|
| CP | CO | ||
| C53 | + | 23 | 23 |
| S56 | − | 12 | 58 |
| C52 | − | 12 | 23 |
| S53 | − | 23 | 58 |
| T41 | − | 12 | 58 |
| Sample | Microbial Counting | R% |
|---|---|---|
| Control | 4.00 × 106 | --- |
| Sol–gel/CP 4% | <1 | 99.9 |
| Sol–gel/CP 8% | 7.33 × 103 | 99.8 |
| Sol–gel/CO 4% | 8.83 × 105 | 77.9 |
| Sol–gel/CO 8% | 3.50 × 106 | 12.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz Jalaff, L.; Ortega Cancino, E.; Altavilla, M.; Vargas Hurtado, K.; Nolan Mella, N.; Faccini, M. Eco-Friendly Sol–Gel Coatings as Microfouling Barrier for Marine Applications. Coatings 2023, 13, 1755. https://doi.org/10.3390/coatings13101755
Diaz Jalaff L, Ortega Cancino E, Altavilla M, Vargas Hurtado K, Nolan Mella N, Faccini M. Eco-Friendly Sol–Gel Coatings as Microfouling Barrier for Marine Applications. Coatings. 2023; 13(10):1755. https://doi.org/10.3390/coatings13101755
Chicago/Turabian StyleDiaz Jalaff, Leslie, Eduardo Ortega Cancino, Manuela Altavilla, Karla Vargas Hurtado, Nicolas Nolan Mella, and Mirko Faccini. 2023. "Eco-Friendly Sol–Gel Coatings as Microfouling Barrier for Marine Applications" Coatings 13, no. 10: 1755. https://doi.org/10.3390/coatings13101755
APA StyleDiaz Jalaff, L., Ortega Cancino, E., Altavilla, M., Vargas Hurtado, K., Nolan Mella, N., & Faccini, M. (2023). Eco-Friendly Sol–Gel Coatings as Microfouling Barrier for Marine Applications. Coatings, 13(10), 1755. https://doi.org/10.3390/coatings13101755