Abstract
In this paper, ZnCo2O4(Nd–ZnCo2O4) films were prepared using a hydrothermal method and an electrodeposition method. We characterized the samples using X-ray diffraction, scanning electron microscopy, and transmission electron microscopy. The effect of Nd–ZnCo2O4 doping on the stability of the materials was studied. The results show that the Nd−ZnCo2O4 prepared in the experiment presents a porous network structure, no other impurity peaks and impurity elements exist, and the purity is high. In the experiment, the electrochemical properties of Nd−ZnCo2O4 were studied. When the current density is 3 A·g−1, the specific capacitance F·g−1 of the electrode is 1982 F·g−1. When the current density is changed 100 times, and every time it is changed back to 3 A·g−1, the specific capacitance F·g−1 is 1973 F·g−1, which is 99.5% of the initial specific capacitance F·g−1. 1982 F·g−1 indicates that Nd−ZnCo2O4 has good stability and can be reused many times. In the cycle stability performance test of Nd–ZnCo2O4//CNTs devices, when the current density is 3 A·g−1, the specific capacitance is 180 F·g−1. After 10,000 cycle charge discharge tests, the specific capacitance F·g−1 becomes 162 F·g−1, maintaining 90% of the initial specific capacitance. These results show that the electrode material has a long cycle life and good cycle stability.
1. Introduction
With the rapid popularization of smart home appliances, people’s lives are more convenient, and the quality of the environment is reduced due to the impact of human activity and factors in the natural world. This change, in turn, has an adverse impact on human production and life. Therefore, major countries and regions around the world have set the goal of carbon neutrality. Dozens of countries and regions have proposed “zero-carbon” or “carbon-neutral” climate goals, so there is recognition of the need to develop clean, renewable energy. Reducing carbon emissions, solving energy consumption, and environmental pollution have become critical issues that need to be solved urgently. The development of clean energy has achieved a series of results, but the supply pattern of these technologies tends to be intermittent and is also affected by environmental and regional factors. Therefore, we urgently need to develop better energy storage devices. When traditional battery life is exhausted, a large amount of environmentally harmful substances are produced. We investigated methods of effectively degrading organic pollutants in solution, and decided that we want to explore a multifunctional material with good electrochemical performance and photoelectric performance, which can alleviate the problems of environmental pollution and energy shortage to a certain extent. Supercapacitors use an electric double layer effect to store electrical energy, rather than the traditional solid dielectric material used to separate charges. In the electric double layer of the electrode, two different principles are mainly used to store electrical energy. In a variety of energy storage equipment, supercapacitors not only have high power density, but also high safety factors, easy maintenance, and they cause no pollution to the environment. They have received extensive attention in scientific research and industry in recent years [1,2,3].
Bimetallic oxides have higher electronic conductivity and theoretical specific capacitance, and their redox reaction is reversible, showing better electrochemical performance as pseudocapacitors than mono metallic oxides [4,5,6]. We found that among various types of bimetallic oxides, ZnCo2O4 is expected to be used as a supercapacitor active material due to its good electrochemical properties, high conductivity, low cost, and environmental friendliness [7,8], but its capacitor performance and storage capacity still need further improvement. We found that doping-related materials can improve some properties. After doping Nd, the energy density and power of the supercapacitor are improved without affecting the long cycle life and conductive properties, and the supercapacitor has a higher specific capacitance F·g−1 as well as an increased ability to store charges. Wei et al. [9] introduced Ce into the CeCoOx/iron mesh monolithic catalyst, which has a spalling rate of 0.07%. After more than 10,000 charging and discharge cycles in 1 mol·L−1 KOH aqueous solution, the initial capacity of 94% can be maintained, indicating good cycling performance. Shen et al. [10] successfully prepared layered electrode materials with ZnCo2O7 nanorods/nickel foam integration using a hydrothermal synthesis method, with a specific capacitance of 1400 F·g−1. Gao et al. [11] doped cobalt nickel oxide nanocages with Ce and the specific capacitance of the nanomaterial was as high as 1976 F·g−1 at a current density of 1 A·g−1. After 10,000 cycles, the capacitance retention rate of the NiCo2Ox/CeOy/CC device is 91.5%, indicating that the material has good application value in high performance supercapacitors. An article by Dai et al. [12] indicates that NiS2@C, NiS2/NiS@C, and NiS@C use nickel-based metal–organic frameworks as the precursors. The BSH energy storage device assembled with a NiS2/NiS@C cathode and an Fe2O3 anode provides 244 C·g−1 at 1 A·g−1 and 49 Wh·kg−1 at 725 W·kg−1. In conclusion, it is significant for the practical application of flexible energy-storing electronic devices that ZnCo2O4 flexible electrodes can be synthesized using an environmentally friendly and easy-to-operate method.
However, the use of ZnCo2O4 as a capacitor material still presents some challenges, including electrochemical performance attenuation, capacitance attenuation, and cycle life limitation problems. The construction of the electrode structure to improve the electrochemical performance of ZnCo2O4 has become a problem to be solved. In recent years, researchers have explored the possibility of doping rare earth elements in electrode materials to improve the electrochemical performance of materials by adjusting oxygen vacancies and using the synergistic reaction of the two. Doped with rare earth elements, the energy density and power density of supercapacitors are increased, and the electrode material shows higher specific capacitance F·g−1 and a stronger charge storage capacity. So, we added the Nd element in many attempts. The materials we prepared have a porous structure, connecting with each other to facilitate the transfer of electrolyte ions and electrons between the material interface and the surface. Nd–ZnCo2O4 prepared in the experiment showed a uniform network structure, no other impurity peaks or impurity elements, and high purity. When the current density of Nd–ZnCo2O4 is 3 A·g−1, the specific capacitance F·g−1 of the electrode is 1962 F·g−1, and the specific capacitance F·g−1 is 99.5% of the initial specific capacitance F·g−1 with 100 changes each time. When the current density of the Nd–ZnCo2O4//CNTs device is 3 A·g−1, the specific capacitance is 180 F·g−1. After 10,000 charging cycles and discharges, the specific capacitance F·g−1 is 90% of the original. It can therefore be found that the cycle life and cycle stability of the electrode material are relatively good.
2. Materials and Methods
2.1. Experimental Instruments
In this experiment, Hitachi’s S4800 electron microscope was used. The magnification ratio is 7~106 times, the field of vision is 6 mm, the acceleration voltage is 0.2~30 kV, the receiving angle is 35°, the pressure range is 10~400 Pa, the maximum height and the maximum diameter of the samples is 100 μm and 200 μm, respectively, and the X-ray parameter is 8.5 mm WD. The JEM–2100F transmission electron microscope from JEOL, Kyoto, Japan, and the XRD 700 X-ray diffractometer of Shimadzu Co., Kyoto, Japan, On this basis, we used analytical instruments from Shanghai Chenhua Instrument Co., Ltd. in the China to analyze the material composition and crystal structure of cobalt molybdate network structure samples The UV-2550 visible spectrophotometer was used to analyze the photocatalytic performance of the samples.
2.2. Preparation of Substrate
As an inert material, carbon cloth has the advantages of low cost, resistance to damage, and conductivity, and has a larger specific surface area than conventional metal plates, which is more convenient for the next reaction. Carbon cloth has excellent electrical conductivity, a surface-active adsorption site, and low impedance, so it is very suitable for various electrochemical tests. The carbon cloth prepared by us has a certain lack of mechanical properties for electrochemical testing, and the texture becomes relatively brittle, but this does not affect the performance of carbon cloth in electrochemical testing.
First, the carbon cloth purchased from Toray, Japan is cut to a size of 1 × 1.5 cm and soaked in concentrated nitric acid for 24 h. The surface of the cloth becomes rough and has hydrophilic properties. It is then washed sequentially with acetone, absolute ethanol, and deionized water. After drying, a clean carbon cloth electrode substrate is obtained.
2.3. Preparation of ZnCo2O4
First, 1 mmol Zn(NO3)2·6H2O, 2 mmol Co(NO3)2·6H2O, 0.074 g NH4F, and 0.3 g urea were dissolved in 50 mL deionized water and stirred for 30 min to form a pink uniform solution, then a small piece of nickel foam was put into the solution. Secondly, we poured the above solution into an 80 mL stainless steel autoclave at 130 °C and heated it for 6 h. When the solution was cooled to room temperature, the product was washed several times with deionized water and ethanol. Finally, the obtained samples were calcined at 350 °C for 2 h, and the final ZnCo2O4 samples were prepared.
2.4. Preparation of Nd–ZnCo2O4 Materials
Firstly, the ZnCo2O4 sample was used as the working electrode, Nd(NO3)2·6H2O as the electrolyte solution, Ag/AgCl as the reference electrode, and Pt sheet as the opposite electrode. Secondly, the CV test was carried out to sweep 3, 9, and 15 cycles, respectively, at a sweep rate of 10 mV·s−1 (electrodeposition process), and the corresponding potential range was from −1.2 V to 0.2 V. After the reaction, the obtained sample was washed three times with deionized water and anhydrous ethanol. Finally, the composite Nd–ZnCo2O4 electrode material was obtained by vacuum drying at 60 °C for 12 h. In addition, the whole preparation process is shown in Figure 1.
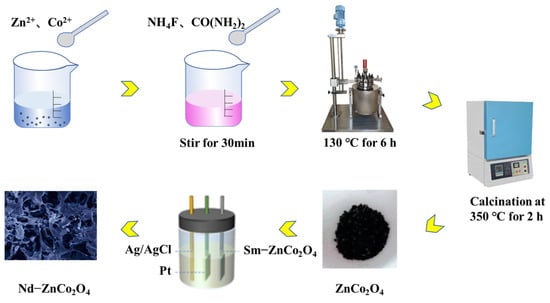
Figure 1.
Preparation process diagram of Nd–ZnCo2O4 electrode material.
2.5. Preparation of Supercapacitor Separators
We first needed to prepare fibrillated tencel fiber, take 30 g of absolutely dry tencelament fiber, dilute it to a concentration of 10%, and then evenly disperse the diluted slurry in the PFI refining tank, set the linear pressure to 1 N/mm, and obtain fibrillated tencelament fibers with different degrees of fibrillation after different refining transfers.
In the preparation of the supercapacitor separation membrane, we took into account that tencel fiber is a regenerated cellulose fiber with a core structure, and it has the characteristics of fabricability. If the tencel fiber is refined, the fiber length distribution in nanometer to micron level of bridled fibers can be obtained. The size of fibrillated tencel fibers has a certain degree of polydispersity, and the apparent morphology of the fibers can be completely observed by preparing them into a paper-based diaphragm. The high beating degree of fibrillation slurry contains more raw fiber, and the diameter of the ordinary mesh is large, which is easy to cause the loss of raw fiber. In order to preserve the raw fiber as much as possible, it is molded using a 500-mesh molding mesh. We weighed 14 g/m2 quantitative fibrillated fibers, dredged 5000 revolutions, poured the slurry into the former, diluted to 10 L, and stirred evenly. After dehydration forming began, we waited for the slurry water filtration to finish, then pressed and dried the wet paper web to obtain fibrillated fiber septa with different pulping revolutions and the same quantification.
2.6. Assembly of Asymmetric Supercapacitors
The supercapacitor is a special capacitor between traditional capacitors and rechargeable batteries, which combines the high-current rapid charge and discharge characteristics of ordinary capacitors with the energy storage characteristics of batteries, filling the gap of energy and specific power between ordinary capacitors and batteries. The basic structure of a supercapacitor consists of two plates with electrodes and a dielectric layer. During charging, the charge is stored on the surface of a conductive polymer or activated carbon on a metal electrode plate, forming an electrode–electrolytic medium-electrode structure. The choice of electrode material affects maximum voltage, capacitance, and durability. The working principle of supercapacitors is based on phenomena such as physical adsorption, electric double layer, and redox reaction. The ability of a capacitor to store electrical energy depends on the surface area of the metal electrode, the ion concentration of the electrolyte, and the thickness of the medium. When voltage is applied to a capacitor, the positive and negative charges separate and store energy on the capacitor plate, rather than by chemical reaction.
According to the energy storage mechanism, supercapacitors can be divided into two categories: electric double-layer capacitors, and Faraday quasi-capacitors. Of the two, the electric double-layer capacitor has two parallel plates with a larger area; the difference is that the distance between the plates is small. These plates are made of metal and immersed in an electrolyte, and are separated by a thin layer called an insulator. When opposite charges form on both sides of the insulator, an electric double layer is formed and the plates are charged. Faraday quasi-capacitors are under potential deposition of electroactive substances on the electrode surface or in the bulk phase, and highly reversible chemical adsorption, desorption or oxidation, reduction reaction, and capacitance related to the electrode charging potential are generated; thereby, energy storage and conversion are realized. Therefore, Faraday quasi-capacitors are used to measure energy storage by analyzing redox reactions in terms of these active electrode materials, while electric double-layer capacitors are used to measure energy storage between the adsorption charges on the surface of the electrodes. In this article, we manufacture a Faraday quasi-capacitor.
Asymmetric supercapacitors are installed with Nd–ZnCo2O4 as the positive electrode and CNTs as the negative electrode, as shown in Figure 2.
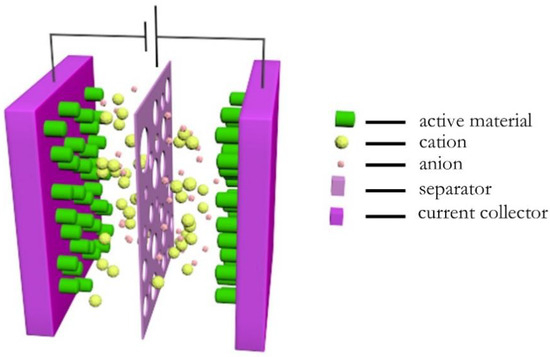
Figure 2.
Device diagram of supercapacitor device.
In order to maintain charge balance, the mass ratio of the positive and negative terminals in the prepared asymmetric supercapacitor is usually determined by the formula as follows:
where Cs (F·g−1) is the specific capacity; i (A·g−1) is the current density; Δt (s) is the discharge time; ΔV (V) is the voltage drop during discharge; m (g) is the mass of the active material; E (Wh·kg−1) is the energy density; P (W·kg−1) is the power density; q (V) is the charge on the plate; m+ (g) is the mass of the positive active material; M− (g) is the mass of the negative active material; ∆V+ (V) is the positive voltage window; and ∆V− (V) is the negative voltage window.
Cs = iΔt/mΔV
E = 0.5CsΔV2/3.6
P = 3600E/Δt
q = CS ∆Vm
3. Results and Discussion
We used the Metrohm PGSTAT101 electrochemical station for a series of tests. The electrochemical workstation occupies an important position in battery testing—it organically combines the potentiostat, the galvanostat, and the electrochemical AC impedance analyzer. It can do conventional tests usually performed by these three basic functions, but can also do programmed tests based on these functions. When performing a test, it can not only detect the basic parameters such as battery voltage, current, capacity, etc., but also the AC impedance parameters reflecting the reaction mechanism of the battery, so as to complete the tracking and analysis of battery parameters in various states.
3.1. SEM and TEM Characterization of Materials
Figure 3a,b show the SEM images of Nd–ZnCo2O4 network nanomaterials at different magnification ratios. It can be seen from the SEM image that the Nd–ZnCo2O4 prepared by electrochemical deposition has a uniform porous network structure. Figure 3c–f show that the prepared material contains elements such as Zn, Co, O, and Nd. The above results show that the prepared material does not contain other impurities.
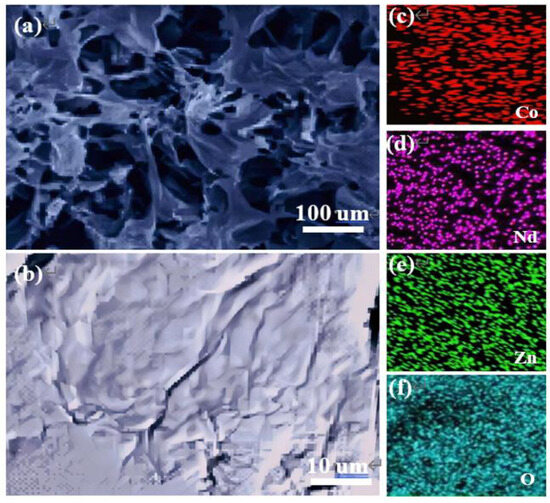
Figure 3.
(a,b) SEM images of ZnCo2O4 nanomaterials; (c–f) SEM maps of Zn, Co, O, and Nd elements.
In order to further observe the microstructures of Nd–ZnCo2O4 nanomaterials, TEM analysis was conducted, and the results are shown in Figure 4. The porous structure can be clearly seen in the inner region of Figure 4a, indicating that the material’s porous structure has been formed. It can be seen from the HRTEM Figure 4b that the crystal plane spacing of the lattice fringe is 0.24 nm and 0.23 nm, corresponding to the (311) and (222) lattice planes of Nd–ZnCo2O4, respectively. The results show that the porous Nd–ZnCo2O4 is a polycrystalline material with good diffraction rings.
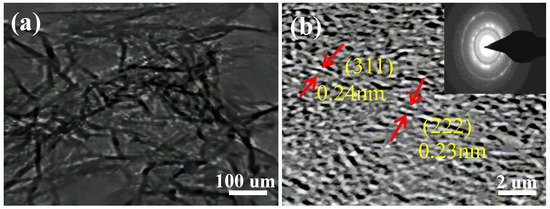
Figure 4.
(a) TEM image of an individual Nd–ZnCo2O4; (b) HRTEM image of the Nd–ZnCo2O4 and corresponding SAED pattern.
3.2. Result from Analysis and Discussion
Figure 5a shows the X-ray diffraction (XRD) results of Nd–ZnCo2O4, which is confirmed to be ZnCo2O4 (JCPDS Card No. 23–1390) [13,14]. There are 110, 220 diffraction peaks in the figure, which are in line with the standard card. Figure 5b shows the EDS test results of the porous Nd–ZnCo2O4 network structure, indicating that the material contains only Zn, Co, O, and Nd elements, and no other impurity elements. All the above results show that Nd element doping is successful and the purity of the prepared samples is very high.
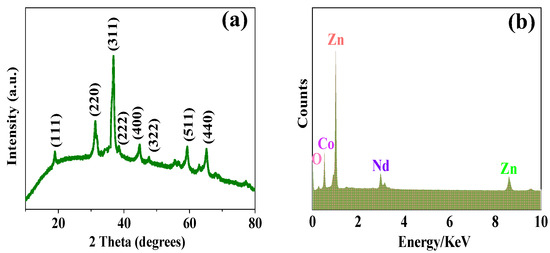
Figure 5.
(a) XRD patterns of porous Nd–ZnCo2O4 nanomaterials; (b) EDS test results of the porous Nd–ZnCo2O4.
Figure 6a shows the cyclic voltammetry curves of nickel foam conductive substrate, ZnCo2O4, and Nd–ZnCo2O4. The scanning speed is 3 mV·s−1. The voltage window is −0.2~0.6 V. As can be seen from Figure 6a, the area of the cyclic voltammetry curve of nickel foam conductive substrate is relatively small, indicating that the specific capacitance F·g−1 of nickel foam conductive substrate in the electrochemical reaction process is small and negligible. It can also be seen from Figure 6a that the curve area surrounded by Nd–ZnCo2O4 is the largest, indicating that the specific capacitance F·g−1 and the charge storage capacity of the material are greatly improved after doping with the Nd element. Figure 6b shows the cyclic voltammetry curves of the porous Nd–ZnCo2O4 network structure at 5, 10, 30, 50, and 80 mV·s−1, with a potential range of −0.2~0.6V. The area enclosed by the voltammetry curve increases proportionally with the increase of the scanning rate. This indicates that the material load transfer is good and the ion diffusivity is obviously pseudo-capacitance behavior.
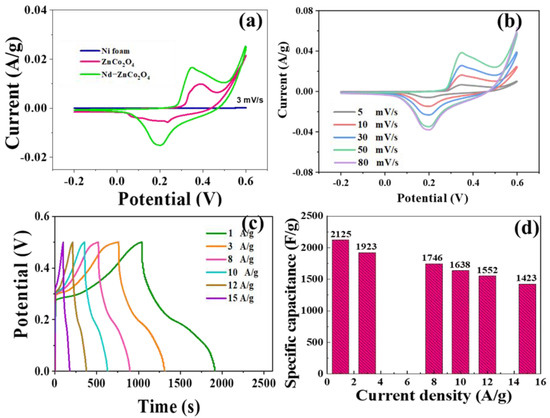
Figure 6.
(a) CV curves of nickel foam with foamed nickel, ZnCo2O4, and Nd–ZnCo2O4 at a scanning rate of 3 mV·s−1; (b) CV curves at different scanning speeds; (c) charge and discharge of Nd–ZnCo2O4 at different current densities; (d) specific capacitance of the material at different current densities.
The three-electrode system is the most commonly used device for electrochemical research. It consists of a working electrode, a counter electrode, and a reference electrode. In this case, the current flows between the counter electrode and the working electrode, and the potential difference between the reference electrode and the working electrode is always controlled. During the electrochemical test, what needs to be studied is a semi-reaction of an electrochemical reaction. If a two-electrode system is used, only the voltage–current curve of the anode and the cathode is obtained; once the current passes through the system, the electrode will be polarized, resulting in a change in potential. Therefore, it is impossible to accurately determine the reaction of the half-reaction under what potential it occurs, but adding a third electrode to the system to form a three-electrode system can measure the electrode reaction we make. When using a three-electrode test system, a uniform polymer layer is plated on a conductive substrate; Nd–ZnCo2O4 is prepared as a working electrode; Ag/Ag+ electrode is used as a reference electrode; and Pt wire is used as a counter electrode. The electrolyte solution is 0.1 M LiClO4 solution. Figure 6c shows the results of charge and discharge tests on Nd–ZnCo2O4 materials at different current densities. The charge–discharge curve in the figure is basically symmetrical. Based on the discharge curve, the specific capacitance of Nd–ZnCo2O4 can be calculated. The results are shown in Figure 6d, indicating excellent electrochemical performance. Under the current density of 1, 3, 8, 10, 12, and 15 A·g−1, the specific capacitance of the device is 2125, 1923, 1746, 1638, 1552, and 1423 F·g−1, respectively. Table 1 shows the electrochemical properties of ZnCo2O4 nanomaterials and their comparison with references. Table 2 shows a comparison of the electrochemical performance of the devices.

Table 1.
Comparison of properties of electrode materials with references.

Table 2.
Electrochemical performance comparison of the devices.
Figure 7a shows the AC impedance of the Nd–ZnCo2O4 electrode after the first and 10,000th cycles. The low-frequency region is a straight line, and the high-frequency region is a small semicircle. There was no significant difference in arc increase in the high-frequency band, and it was maintained well after 10,000 cycles. In the low-frequency region, the slope of the line decreases after 10,000 cycles, which is caused by the loss of some active substances during charging and discharging. Figure 7b shows the cyclic stability performance of the material at different current densities, alternating 100 cycles before returning to the initial current density condition. As can be seen from the figure, when the current density is 3 A·g−1, the specific capacitance F·g−1 of the material is 1982 F·g−1. When the current density is changed 100 times, back to 3 A·g−1, the specific capacitance F·g−1 is 1973 F·g−1, 99.5% of the initial specific capacitance F·g−1 of 1782 F·g−1. It can be found that the specific capacitance F·g−1 attenuation is not large when the current density is changed, indicating that the material has good magnification performance and cycle stability.
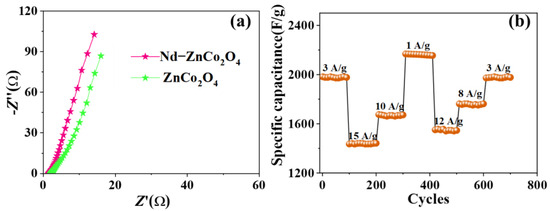
Figure 7.
(a) Nyquist plots of ZnCo2O4, and Nd–ZnCo2O4; (b) rate and cycling properties of porous Nd–ZnCo2O4 nanomaterials at different current densities.
Figure 8 shows the charge–discharge curves of CNTs electrodes at different current densities. The charge–discharge curve of constant current is nonlinear, and the symmetry of the charge–discharge curve is similar to the triangle, indicating that the electrode has good reversibility. When the current density is 1, 3, 8, 10, 12, and 15 A·g−1, the specific capacitance calculated by the discharge curve is 135 F·g−1, 109 F·g−1, 96 F·g−1, 89 F·g−1, 83 F·g−1, and 75.2 F·g−1. According to the above specific capacitance and mass equation, the maximum mass ratio of Nd–ZnCo2O4 to CNTs is about 1:7.
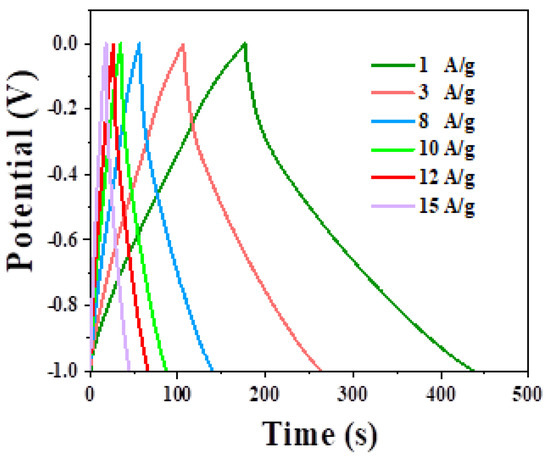
Figure 8.
Charging and discharging curves of CNTs electrodes at different current densities.
Figure 9a shows the CV curves of the Nd–ZnCo2O4 and CNTs electrodes. The potential window of the Nd–ZnCo2O4 electrode was −0.2~0.6 V, and that of the CNTs electrode was −1.0~0 V. The Nd–ZnCo2O4 electrode and the CNTs electrode were made into an asymmetric device, whose voltage range was the difference of potential window between the positive and negative electrodes. Therefore, the theoretical potential window of Nd–ZnCo2O4//CNTs is 1.6 V for an asymmetric device assembled with Nd–ZnCo2O4 electrodes and CNTs electrodes. Cyclic voltammetry curves under different voltage windows are shown in Figure 9b. It is not difficult to see that the shapes of CV curves under different voltage windows are similar, indicating that the device has strong reversibility. The capacity of the device increases with the increase of the potential window. The stable voltage window when the device capacity reaches the maximum value is 0~1.6 V. Figure 9c shows the CV curve of Nd–ZnCo2O4//CNTs devices at a scanning rate of 8~50 mV·s−1 at a high potential window of 0~1.6 V. The area of the closed curve increases with the increase of the scanning rate, and the shape almost does not change, indicating that the device has good stability. Figure 9d shows the charging and discharging curves of Nd–ZnCo2O4//CNTs at different current densities. The figure shows the pseudo-capacitance characteristics of Nd–ZnCo2O4 nanoarray materials, and the results are consistent with the cyclic voltammetry analysis. The curve shows a tendency to approximate symmetry, indicating good electrochemical reversibility. Figure 9e shows the cyclic stability test results of Nd–ZnCo2O4//CNTs devices. When the current density is 3 A·g−1, the specific capacitance is 180 F·g−1, and the specific capacitance F·g−1 is 162 F·g−1 after 10,000 cycles of charge–discharge test; additionally, the cyclic stability can reach 90%. These results show that the electrode material has good cycle stability and good cycle life. Figure 9f shows the comparison between the energy density and the power density of this device and that of other energy storage devices [23,24,25,26]. When the current density is 1 A·g−1, the corresponding maximum energy density is 85.2 Wh·kg−1, and when the potential window is 1.4 V, the power density is 900 W·kg−1. When the current density is 15 A·g−1, the corresponding maximum power density is 16,000 W·kg−1.
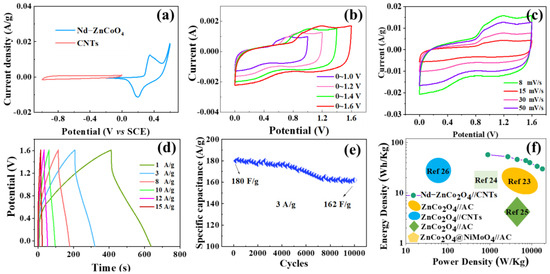
Figure 9.
(a) CV curves of Nd–ZnCo2O4 electrode and CNTs electrode in the three-electrode test system; (b) CV curves of Nd–ZnCo2O4//CNTs at different potential windows; (c) CV curves of Nd–ZnCo2O4//CNTs at different scanning rates in the 0~1.6 V potential window; (d) Charging and discharging curves of Nd–ZnCo2O4//CNTs at different current densities; (e) Cycle performance of 10,000 cycles of charge and discharge at 3 A·g−1 current density; (f) Comparison of energy density and power density between Nd–ZnCo2O4//CNTs device and other devices.
4. Conclusions
In this paper, Nd–ZnCo2O4 films were prepared with a porous mesh structure, high purity, and good cycle stability. The electrochemical properties of Nd–ZnCo2O4 were researched with good capacitive performance. When the current density is 3 A·g−1, the specific capacitance F·g−1 of the electrode is 1982 F·g−1, and the specific capacitance F·g−1 can be maintained at 99.5% when the current density is changed 100 times. In the cycle stability test of Nd–ZnCo2O4//CNTs devices, when the current density is 3 A·g−1, 10,000 cycle charge discharge tests are carried out, and the specific capacitance stability of the devices can reach 90%. The results show that the as-prepared electrode material has good cycle stability and cycle life.
Author Contributions
X.Y. (Xinrui Yue) carried out the experiments and wrote the manuscript, and J.W. designed this experiment and wrote the manuscript and other analyses. H.W. and T.H. carried out the characterization tests, analyzed, wrote the results, and revised the manuscript. J.H., Y.L., T.M. and X.Y. (Xiaolin Yi) analyzed the characterization tests, and wrote and revised the manuscript. J.W., X.Y. (Xiaolin Yi), H.W., T.H. and J.H. analyzed and discussed the results. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was supported by the Young scientific research item of Harbin university of Commerce (18XN034), the National Natural Science Foundation of China (No.52002099), and the Foundation of State Key Laboratory of High-Efficiency Utilization of Coal and Green Chemical Engineering (Grant No. 2022–K74), Harbin University of Commerce College Students’Innovation and Entrepreneurial Training Plan, Program Provincial Project (No. S202310240094).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lin, J.; Yao, L.; Li, Z.; Zhang, P.; Zhong, W.; Yuan, Q.; Deng, L. Hybrid hollow spheres of carbon@CoxNi1−xMoO4 as advanced electrodes for high performance asymmetric supercapacitors. Nanoscale 2019, 11, 3281–3291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, X.; Wang, H.; Teo, S.H.; Ma, T. Free-standing NiCo2S4@VS2 nanoneedle array composite electrode for high performance asymmetric supercapacitor application. J. Alloys Compd. 2018, 771, 274–280. [Google Scholar] [CrossRef]
- Shkir, M.; Alshahrani, T. Impact of Nd doping in Bi2S3 thin films coated by nebulizer spray pyrolysis technique for photodetector applications. Opt. Mater. 2023, 140, 113837. [Google Scholar] [CrossRef]
- Zardkhoshoui, A.M.; Davarani, S.S.H. Construction of complex copper-cobalt selenide hollow structures as an attractive battery-type electrode material for hybrid supercapacitors. Chem. Eng. J. 2020, 402, 126241. [Google Scholar] [CrossRef]
- Zardkhoshoui, A.M.; Davarani, S.S.H. Formation of graphene-wrapped multi shelled NiGa2O4 hollow spheres and graphene-wrapped yolk-shell NiFe2O4 hollow spheres derived from metal–organic frameworks for high-performance hybrid supercapacitors. Nanoscale 2020, 12, 1643–1656. [Google Scholar] [CrossRef]
- Zardkhoshoui, A.M.; Ashtiani, M.M.; Sarparast, M.; Davarani, S.S.H. Enhanced the energy density of supercapacitors via rose-like nanoporous ZnGa2S4 hollow spheres cathode and yolk-shell FeP hollow spheres anode. J. Power Sources 2020, 450, 227691. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, G.; Yu, W.; Liu, D.; Liu, Y.; Li, L.; Huang, Q.; Tong, Z. Electrospun carbon nanofibers coated with urchin-like ZnCo2O4 nanosheets as a flexible electrode material. J. Mater. Chem. A 2016, 4, 5958–5964. [Google Scholar] [CrossRef]
- Venkatachalam, V.; Alsalme, A.; Alswieleh, A.; Jayavel, R. Double hydroxide mediated synthesis of nanostructured ZnCo2O4 as high performance electrode material for supercapacitor applications. Chem. Eng. J. 2023, 321, 474–483. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Z.; Gao, Y.; Wang, Q. The influence of Ce doping on catalytic oxidation of toluene over Co3O4/iron mesh monolithic catalyst. Catal. Today 2023, 418, 114107. [Google Scholar] [CrossRef]
- Liu, B.; Liu, B.; Wang, Q.; Wang, X.; Xiang, Q.; Chen, D.; Shen, G. New Energy Storage Option: Toward ZnCo2O4 Nanorods/Nickel Foam Architectures for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 10011–10017. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Shen, H.; Wu, Z.; Song, J.; Yu, J.; Liu, C.; Jing, H.; Zhao, P.; Lei, W.; et al. Facial design and synthesis of Ce doped Co–Ni oxide nanocages with cubic structure for high-performance asymmetric supercapacitors. Appl. Surf. Sci. 2023, 615, 156132. [Google Scholar] [CrossRef]
- Dai, H.; Zhao, Y.; Zhang, Z.; Yang, J.; Liu, S.; Zhou, J.; Sun, G. Ostwald ripening and sulfur escaping enabled chrysanthemum-like architectures composed of NiS2/NiS@ C heterostructured petals with enhanced charge storage capacity and rate capability. J. Electroanal. Chem. 2022, 921, 116671. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Chen, K.; Liu, T.; Wang, Q. Boosting the photoelectrochemical water oxidation performance of bismuth vanadate by ZnCo2O4 nanoparticles. Chin. Chem. Lett. 2022, 33, 2060–2064. [Google Scholar] [CrossRef]
- Khan, M.I.; Muhammad, N.; Tariq, M.; Nishan, U.; Razaq, A.; Saleh, T.A.; Rahim, A. Nonenzymatic electrochemical dopamine sensing probe based on hexagonal shape zinc-doped cobalt oxide (Zn-Co2O4) nanostructure. Microchim. Acta 2021, 189, 37. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Wang, X.; Chen, G.; Chen, D.; Zhou, C.; Shen, G. Hierarchical three-dimensional ZnCo2O4 nanowire arrays/carbon cloth anodes for a novel class of high-performance flexible lithium-ion batteries. Nano Lett. 2012, 12, 3005–3011. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Tingwu, Z.; Xu, N.; Tianrui, W.; Meilian, Z.; Yupeng, S. Construction of hierarchical ZnCo2O4@CoSe core-shell nanosheets on Ni foam for high performance supercapacitor. Ionics 2021, 27, 5251–5261. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Wu, H.B.; Madhavi, S.; Lou, X.W. Controlled Growth of NiMoO4 Nanosheet and Nanorod Arrays on Various Conductive Substrates as Advanced Electrodes for Asymmetric Supercapacitors. Adv. Energy Mater. 2015, 5, 1401172. [Google Scholar] [CrossRef]
- Javed, M.S.; Hussain, I.; Batool, S.; Siyal, S.H.; Najam, T.; Shah, S.S.A.; Imran, M.; Assiri, M.M.; Hussain, S. Energy storage properties of hydrothermally processed ultrathin 2D binder-free ZnCo2O4 nanosheets. Nanotechnology 2021, 32, 385402. [Google Scholar] [CrossRef]
- Wei, X.; Wu, H.; Li, L. 3D N–doped carbon continuous network supported P-doped ZnCo2O4 nanosheets with rich oxygen vacancies for high performance asymmetric pseudocapacitor. J. Alloys Compd. 2020, 861, 158544. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, L.; Sun, L.; Liu, Y.; Jiao, L. Facile synthesis of hierarchical porous ZnCo2O4 microspheres for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 982–985. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, X.; Li, C.; Li, Y.; Chen, X.; Gao, X.; Chen, C.; Guang, Z.; Liu, P. Construction of hydrangea-like ZnCo2O4/Ni3V2O8 hierarchical nanostructures for asymmetric all solid state supercapacitors. Ceram. Int. 2019, 45, 15451–15457. [Google Scholar] [CrossRef]
- Wang, H.; Cai, W.; He, L.; Zhu, M.; Wang, Y. Anchoring ternary NiCoMn–S ultrathin nanosheets on porous ZnCo2O4 nanowires to form core-shell composites for high-performance asymmetric supercapacitor. J. Alloys Compd. 2021, 870, 159347. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, X.; Chen, H.; Tao, K.; Hu, Y.; Han, L. Zeolitic imidazolate framework derived ZnCo2O4 hollow tubular nanofibers for long-life supercapacitors. RSC Adv. 2020, 10, 13922–13928. [Google Scholar] [CrossRef]
- Amiri, M.; Moosavifard, S.E.; Hosseiny Davarani, S.S.; Shamsipur, M. Novel Rugby-Ball-like FeCoCuS2 Triple-Shelled Hollow Nanostructures with Enhanced Performance for Supercapattery. Energy Fuels 2021, 35, 15108–15117. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; He, P.; Zhang, F.; Tang, J.; Guo, Z.; Que, R. Facile synthesis of MnO2@NiCo2O4 core-shell nanowires as good performance asymmetric supercapacitor. J. Mater. Sci. Mater. Electron. 2020, 31, 1355–1366. [Google Scholar] [CrossRef]
- Wu, S.; Yang, X.; Cui, T.; Feng, Q.; Zhou, S.; Xu, X.; Zhao, H.; Wu, L.; He, Y.; Yang, Q. Tubular-like NiS/Mo2S3 microspheres as electrode material for high-energy and long life asymmetric supercapacitors. Colloids Surf. A 2021, 628, 127332. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Anand, S.; Shalini, K.; Ul-Islam, M.; Yang, D.J.; Choudhury, A. MnMoO4 nanorods-encapsulated carbon nanofibers hybrid mat as binder-free electrode for flexible asymmetric supercapacitors. Mater. Sci. Semicond. Process. 2021, 136, 106176. [Google Scholar] [CrossRef]
- Hou, J.F.; Gao, J.F.; Kong, L.B. Interfacial Engineering in Crystalline Cobalt Tungstate/Amorphous Cobalt Boride Heterogeneous Nanostructures for Enhanced Electrochemical Performances. ACS Appl. Energy Mater. 2020, 3, 11470–11479. [Google Scholar] [CrossRef]
- Govindan, R.; Hong, X.-J.; Sathishkumar, P.; Cai, Y.P.; Gu, F.L. Construction of metal-organic framework-derived CeO2/C integrated MoS2 hybrid for high-performance asymmetric supercapacitor. Electrochim. Acta 2020, 353, 136502. [Google Scholar] [CrossRef]
- Sun, J.; Li, S.; Han, X.; Liao, F.; Zhang, Y.; Gao, L.; Chen, H.; Xu, C. Rapid hydrothermal synthesis of snowflake-like ZnCo2O4/ZnO mesoporous microstructures with excellent electrochemical performances. Ceram. Int. 2019, 45, 12243–12250. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).