Preparation and Application of Edible Film Based on Sodium Carboxymethylcellulose-Sodium Alginate Composite Soybean Oil Body
Abstract
:1. Introduction
2. Materials & Methods
2.1. Materials
2.2. Preparation of CA-SOB Films
2.3. Characterization of CA-SOB Films
2.3.1. Thickness
2.3.2. Tensile Strength (TS) and Maximum Elongation at Break (EB)
2.3.3. Color Parameters
2.3.4. Water Vapor Permeability (WVP)
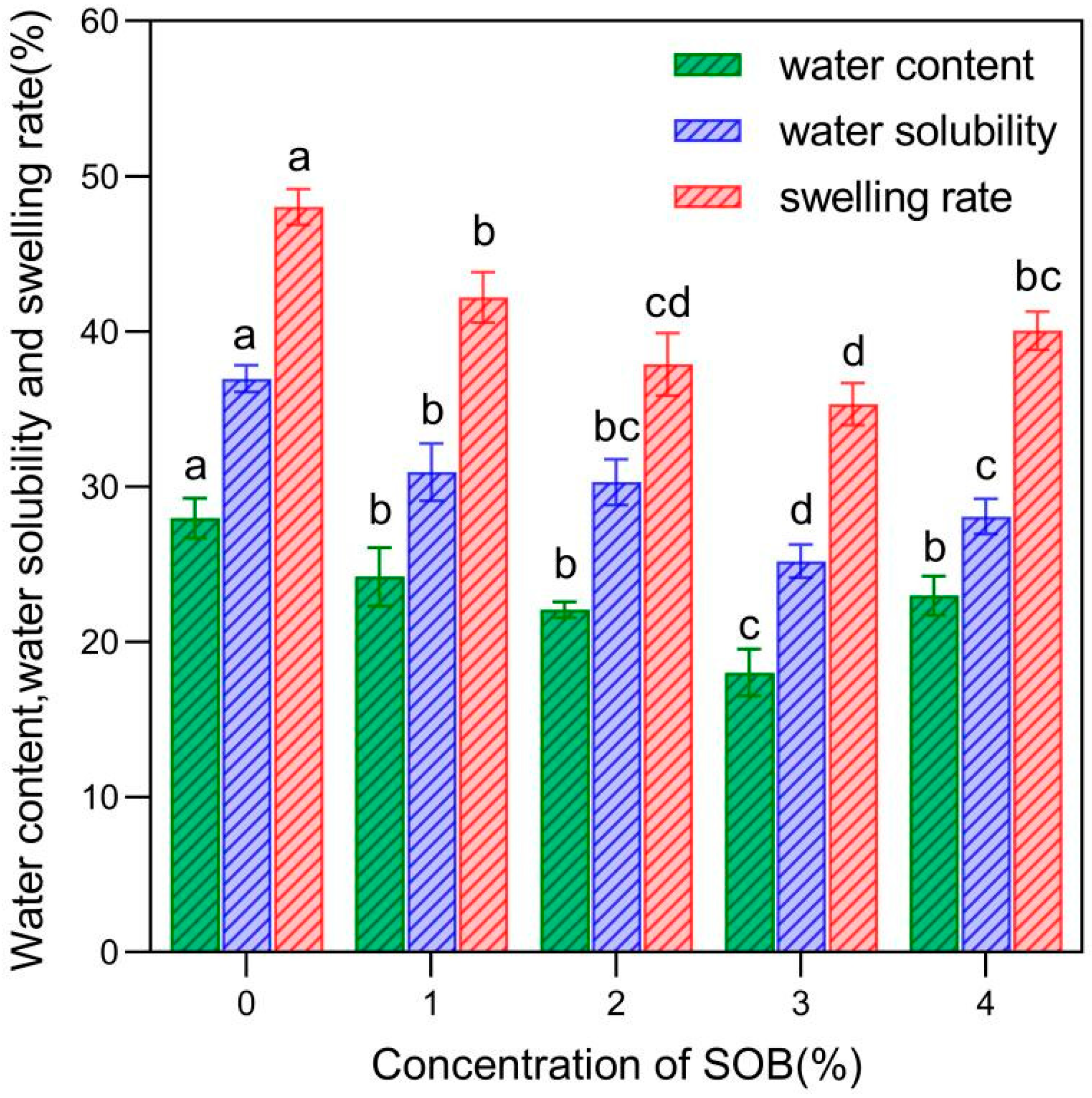
2.3.5. Water Content (WC), Swelling Rate, and Water Solubility (WS)
2.3.6. Fourier Transform Infrared Spectroscopy (FTIR)
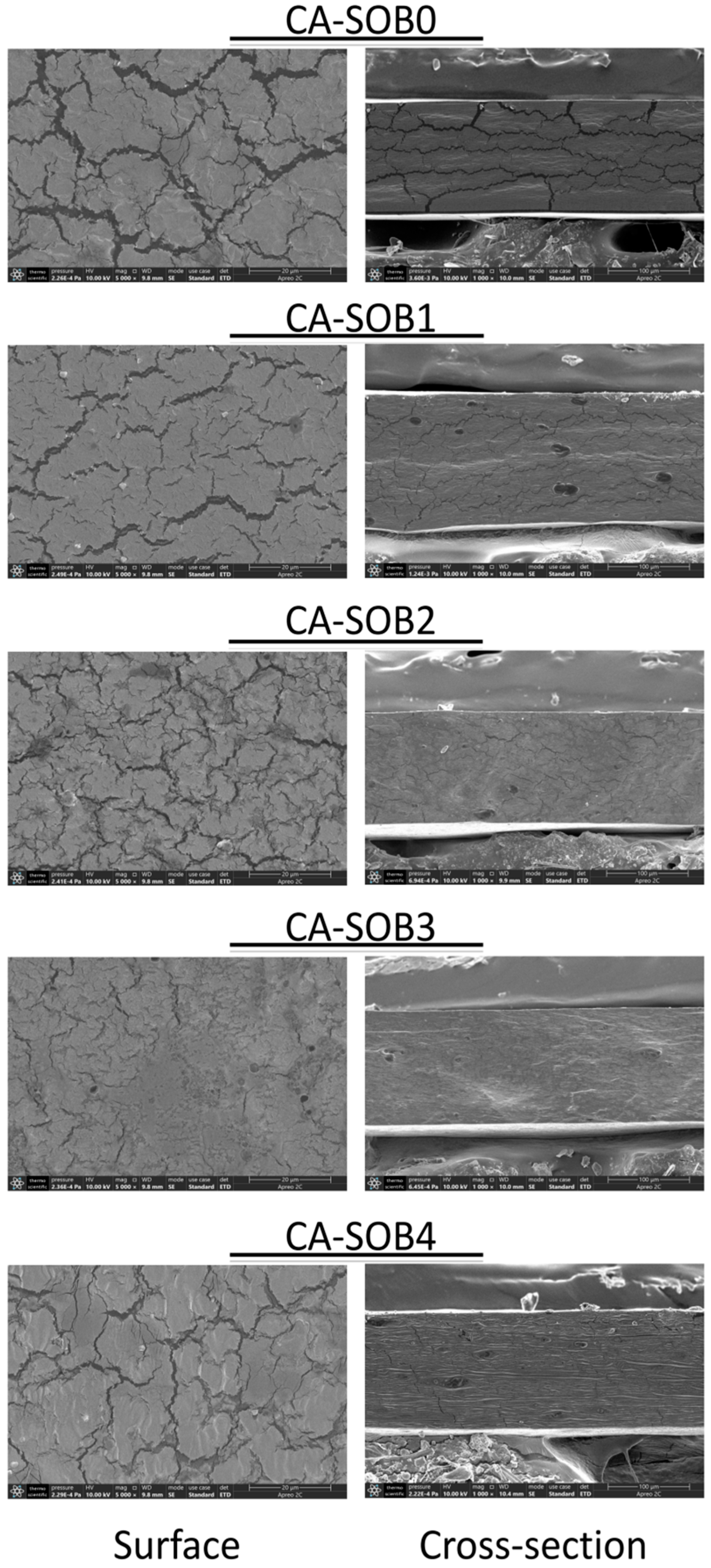
2.3.7. Scanning Electron Microscopy (SEM)
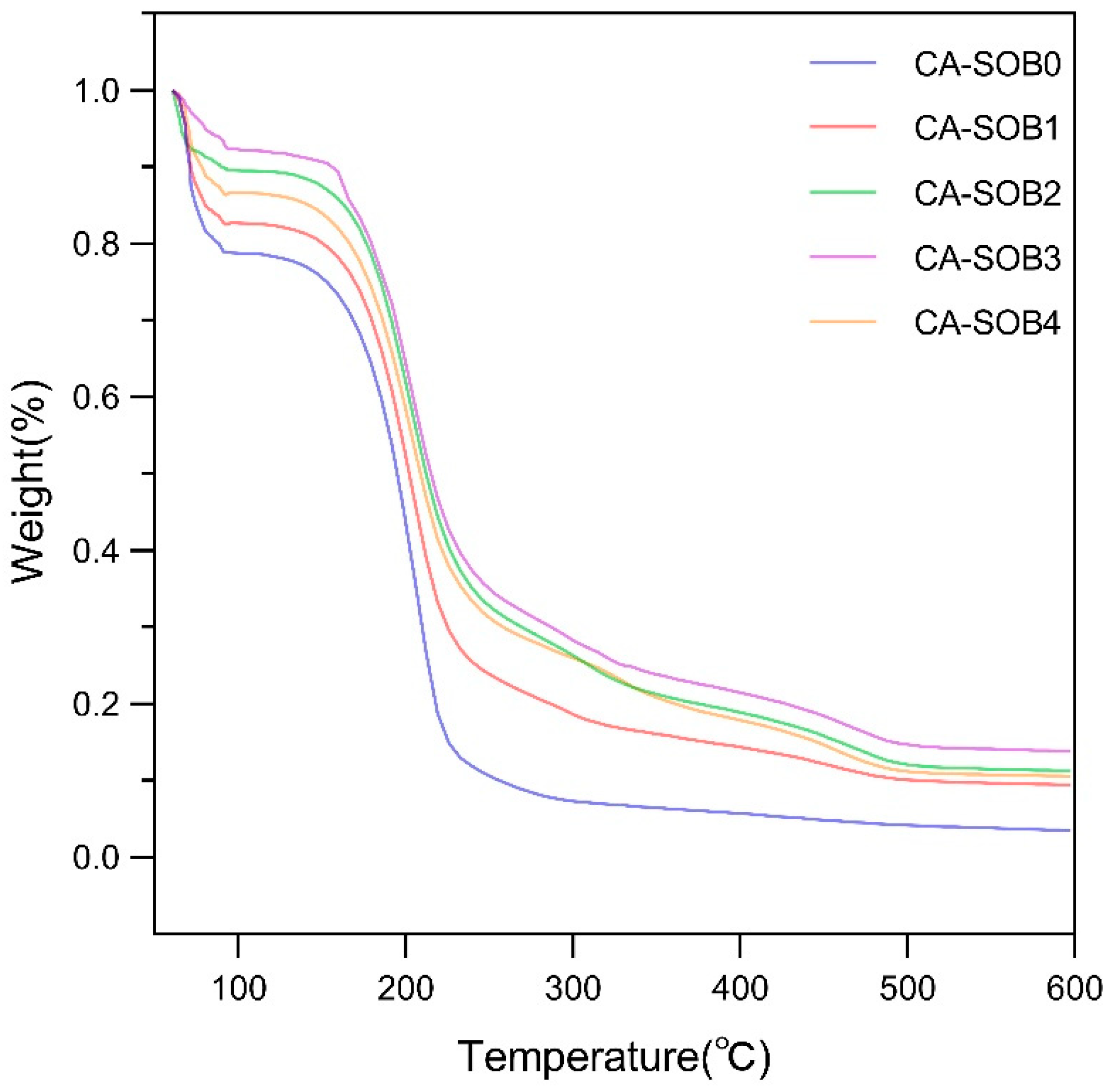
2.3.8. Thermal Gravimetric Analysis (TGA)
2.3.9. X-ray Diffraction Analysis (XRD)
2.3.10. Antioxidant Activity
ABTS Radical Scavenging Rate
DPPH Radical Scavenging Rate
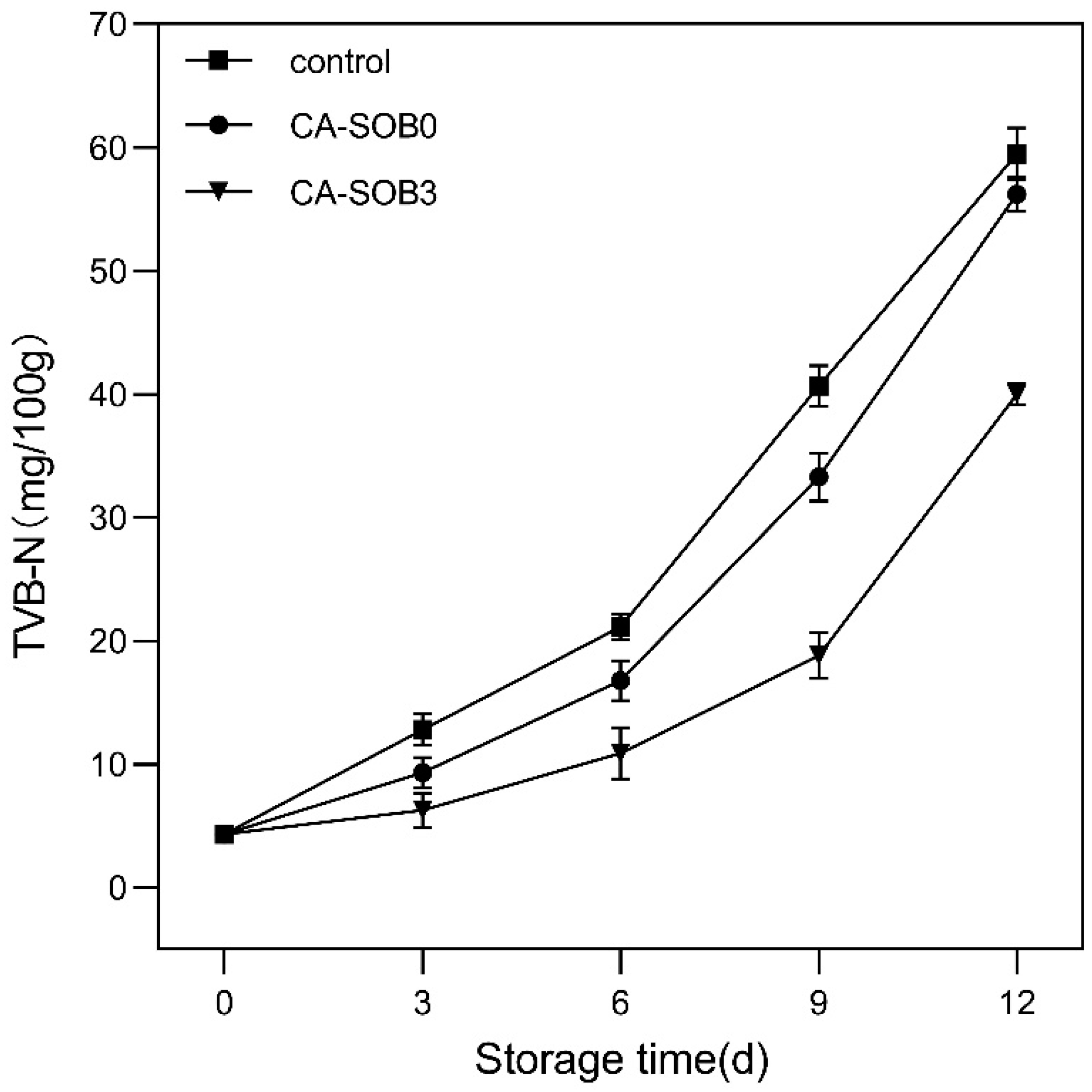
2.3.11. Application of Preservation in Pigeon
2.3.12. Statistical Analysis
3. Results and Discussion
3.1. Mechanical Properties
3.2. Chromaticity Analysis
3.3. Water Content (WC), Swelling Rate, and Water Solubility (WS)
3.4. FTIR Analysis
3.5. SEM Analysis
3.6. TGA
3.7. XRD Analysis
3.8. Antioxidant Activity
3.9. Food Preservation Application Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, M.K.; Shakya, S.; Kumar, P.; Madhavi, J.; Murugai1yan, J.; Rao, M.V.R. Trends in packaging material for food products: Historical background, current scenario, and future prospects. J. Food Sci. Technol. 2021, 58, 4069–4082. [Google Scholar] [CrossRef] [PubMed]
- Long, J.Y.; Zhang, W.Y.; Zhao, M.Z.; Ruan, C.-Q. The reduce of water vapor permeability of polysaccharide-based films in food packaging: A comprehensive review. Carbohydr. Polym. 2023, 321, 121267. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Filho, J.G.d.; Silva, G.d.C.; Oldoni, F.C.A.; Miranda, M.; Florencio, C.; Oliveira, R.M.D.d.; Gomes, M.d.P.; Ferreira, M.D. Edible coating based on carnauba wax nanoemulsion and cymbopogon martinii essential oil on papaya postharvest preservation. Coatings 2022, 12, 1700. [Google Scholar] [CrossRef]
- Li, S.Y.; Ma, Y.L.; Ji, T.T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Dai, J.W.; Li, S.Q.; Liu, Y.W. Cassava starch/carboxymethylcellulose edible films embedded with lactic acid bacteria to extend the shelf life of banana. Carbohydr. Polym. 2020, 248, 116805. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Peng, S.X.; Shi, C.; Li, C.Z.; Hua, Z.C.; Cui, H.Y. Preparation and characterization of cassava starch/sodium carboxymethyl cellulose edible film incorporating apple polyphenols. Int. J. Biol. Macromol. 2022, 212, 155–164. [Google Scholar] [CrossRef]
- He, X.D.; Zeng, L.Y.; Cheng, X.P.; Yang, C.L.; Chen, J.; Chen, H.M.; Ni, H.L.; Bai, Y.F.; Yu, W.H.; Zhao, K.Q.; et al. Shape memory composite hydrogel based on sodium alginate dual crosslinked network with carboxymethyl cellulose. Eur. Polym. J. 2021, 156, 110592. [Google Scholar] [CrossRef]
- Chi, K.; Wang, H.; Catchmark, J.M. Sustainable starch-based barrier coatings for packaging applications. Food Hydrocoll. 2020, 103, 105696. [Google Scholar] [CrossRef]
- Aghayan, N.S.; Seyfi, J.; Asadollahzadeh, M.J.; Davachi, S.M.; Hasani, M. Developing multicomponent edible films based on chitosan, hybrid of essential oils, and nanofibers: Study on physicochemical and antibacterial properties. Int. J. Biol. Macromol. 2020, 164, 4065–4072. [Google Scholar] [CrossRef]
- Matsakidou, A.; Biliaderis, C.G.; Kiosseoglou, V. Preparation and characterization of composite sodium caseinate edible films incorporating naturally emulsified oil bodies. Food Hydrocoll. 2013, 30, 232–240. [Google Scholar] [CrossRef]
- Şen, A.; Acevedo-Fani, A.; Dave, A.; Ye, A.Q.; Husny, J.; Singh, H. Plant oil bodies and their membrane components: New natural materials for food applications. Crit. Rev. Food Sci. Nutr. 2022, 1–24. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, J.; Zhao, X.; Sun, R.B.; Sun, C.Q.; Hou, D.D.; Zhang, X.W.; Jiang, L.Z.; Hou, J.C.; Jiang, Z.M. Oil bodies extracted from high-oil soybeans (Glycine max) exhibited higher oxidative and physical stability than oil bodies from high-protein soybeans. Food Funct. 2022, 13, 3271–3282. [Google Scholar] [CrossRef]
- Nikiforidis, C.V. Structure and functions of oleosomes (oil bodies). Adv. Colloid Interface Sci. 2019, 274, 102039. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Zhong, M.M.; Liao, Y.; Kang, M.X.; Li, Y.; Qi, B.K. Interfacial characteristics of artificial oil body emulsions (O/W) prepared using extrinsic and intrinsic proteins: Inspired by natural oil body. LWT-Food Sci. Technol. 2023, 173, 114270. [Google Scholar] [CrossRef]
- Moieau, R.A. Vegetable Oils in Food Technology-Composition, Properties and Uses, 2nd ed.; Gunstone, F.D., Ed.; Wiley-Blackwell: Oxford, UK, 2011. [Google Scholar]
- Yang, X.; Wu, Y.; Liu, Y.; Ding, X.; Zhang, D.; Zhao, L. Digestive characteristics of oil body extracted from soybean aqueous extract at different pHs. Food Res. Int. 2022, 161, 111828. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Properties of Soybean Oil Bodies and Oleosin Proteins as Edible Films and Coatings. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 2004. [Google Scholar]
- Sharma, S.; Darshan, D.; Singh, N.P.; Shetty, N.P. Development and characterisation of a pectin-based edible film that contains mulberry leaf extract and its bio-active components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, X.N.; Zhu, Y.L.; Zeng, Y.; Fang, C.S.; Liu, Y.; Hu, S.W.; Ge, Y.M.; Jiang, W. Preparation and application of a colorimetric film based on sodium alginate/sodium carboxymethyl cellulose incorporated with rose anthocyanins. Food Chem. 2022, 393, 133342. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, Y.M.; Lv, J.H.; Wu, Y.; Guo, Y.X.; Sun, C.F.; Li, X. Biodegradable composite films based on egg white protein and tea polyphenol: Physicochemical, structural and antibacterial properties. Food Packag. Shelf Life 2023, 38, 101098. [Google Scholar] [CrossRef]
- Wu, H.J.; Li, T.; Peng, L.; Wang, J.; Lei, Y.X.; Li, S.S.; Li, Q.Y.; Yuan, X.Y.; Zhou, M.; Zhang, Z.Q. Development and characterization of antioxidant composite films based on starch and gelatin incorporating resveratrol fabricated by extrusion compression moulding. Food Hydrocoll. 2023, 139, 108509. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Li, X.T.; Liu, L.; Li, Y.; Qi, B.K.; Jiang, L.Z. Preparation of spray-dried soybean oil body microcapsules using maltodextrin: Effects of dextrose equivalence. LWT-Food Sci. Technol. 2022, 154, 112874. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, X.; Zhao, J.H.; Li, J.W.; Yu, X.Y.; Peng, Y.J.; Wu, J.H. Characterization of ionically crosslinked mango peel pectin-based films: Effect of different cations on the improved properties of film. Food Packag. Shelf Life 2023, 38, 101131. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Hadi, A.; Lutf, Z. Development and characterization of edible film made from mango kernel starch. J. Packag. Technol. Res. 2022, 6, 63–72. [Google Scholar] [CrossRef]
- Gao, H.X.; He, Z.; Sun, Q.; He, Q.; Zeng, W.C. A functional polysaccharide film forming by pectin, chitosan, and tea polyphenols. Carbohydr. Polym. 2019, 215, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, K.A.; Benjakul, S.; Sae-leaw, T. Effect of Chamuang (Garcinia cowa Roxb.) leaf extract on inhibition of melanosis and quality changes of Pacific white shrimp during refrigerated storage. Food Chem. 2019, 270, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Erdem, B.G.; Kaya, S. Characterization and application of novel composite films based on soy protein isolate and sunflower oil produced using freeze drying method. Food Chem. 2022, 366, 130709. [Google Scholar] [CrossRef]
- Galus, S. Functional properties of soy protein isolate edible films as affected by rapeseed oil concentration. Food Hydrocoll. 2018, 85, 233–241. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Liu, X.L.; Liao, W.Y.; Wang, Q.; Xia, W.S. Chitosan/bacterial cellulose films incorporated with tea polyphenol nanoliposomes for silver carp preservation. Carbohydr. Polym. 2022, 297, 120048. [Google Scholar] [CrossRef]
- Agarwal, S.; Hoque, M.; Bandara, N.; Pal, K.; Sarkar, P. Synthesis and characterization of tamarind kernel powder-based antimicrobial edible films loaded with geraniol. Food Packag. Shelf Life 2020, 26, 100562. [Google Scholar] [CrossRef]
- Shao, X.R.; Sun, H.T.; Jiang, R.P.; Yu, Y.X. Physical and antibacterial properties of corn distarch phosphate/carboxymethyl cellulose composite films containing tea. J. Food Process. Preserv. 2020, 44, e14401. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Liu, X.L.; Wang, Q.; Lin, G.M.; Yang, H.B.; Yu, D.W.; Cui, S.W.; Xia, W.S. Antimicrobial and antioxidant films formed by bacterial cellulose, chitosan and tea polyphenol—Shelf life extension of grass carp. Food Packag. Shelf Life 2022, 33, 100866. [Google Scholar] [CrossRef]
- Dinh, T.A.; Le, Y.N.; Nhi, P.N.Q.; Ton-That, P.; Van-Xuan, T.; Ho, T.G.-T.; Nguyen, T.; Phuong, H.H.K. Fabrication of antimicrobial edible films from chitosan incorporated with guava leaf extract. Prog. Org. Coat. 2023, 183, 107772. [Google Scholar] [CrossRef]
- Abdin, M.; El-Beltagy, A.E.; El-sayed, M.E.; Naeem, M.A. Production and Characterization of Sodium Alginate/Gum Arabic Based Films Enriched with Syzygium cumini Seeds Extracts for Food Application. J. Polym. Environ. 2021, 30, 1–12. [Google Scholar] [CrossRef]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel Materials in the Preparation of Edible Films and Coatings—A Review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Liaotrakoon, V.; Raviyan, P. Modifying the properties of whey protein isolate edible film by incorporating palm oil and glycerol. Songklanakarin J. Sci. Technol. 2018, 40, 243–249. [Google Scholar]
- dos Santos Paglione, I.; Galindo, M.V.; de Medeiros, J.A.S.; Yamashita, F.; Alvim, I.D.; Ferreira Grosso, C.R.; Setsuko, S.L.; Shirai, M.A. Comparative study of the properties of soy protein concentrate films containing free and encapsulated oregano essential oil. Food Packag. Shelf Life 2019, 22, 100419. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.N.; Wei, Q.H.; Li, X.P.; Guo, Y.; Zhang, S. Design and fabrication of sodium alginate/carboxymethyl cellulose sodium blend hydrogel for artificial skin. Gels 2021, 7, 115. [Google Scholar] [CrossRef]
- Tan, L.F.; Elaine, E.; Pui, L.P.; Lin, N.K.; Yusof, Y.A. Development of chitosan edible film incorporated with Chrysanthemum morifolium essential oil. Acta Sci. Pol. Technol. Aliment. 2021, 20, 55–66. [Google Scholar] [CrossRef]
- Yong, Y.Y.; Wang, S.C.; Li, L.H.; Li, R.; Ahmad, H.N.; Munawar, N.; Zhu, J. A curcumin-crosslinked bilayer film of soy protein isolate and chitosan with enhanced antibacterial property for beef preservation and freshness monitoring. Int. J. Biol. Macromol. 2023, 247, 125778. [Google Scholar] [CrossRef]
- Wang, X.C.; Yong, H.M.; Gao, L.; Li, L.L.; Jin, M.J.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2018, 89, 56–66. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Pham, B.-T.T.; Le, H.N.; Bach, L.G.; Thuc, C.N.H. Comparative characterization and release study of edible films of chitosan and natural extracts. Food Packag. Shelf Life 2022, 32, 100830. [Google Scholar] [CrossRef]
- Chu, Y.F.; Cheng, W.W.; Feng, X.; Gao, C.C.; Wu, D.; Meng, L.H.; Zhang, Y.; Tang, X.Z. Fabrication, structure and properties of pullulan-based active films incorporated with ultrasound-assisted cinnamon essential oil nanoemulsions. Food Packag. Shelf Life 2020, 25, 100547. [Google Scholar] [CrossRef]
- Wei, L.P.; Zhang, W.L.; Yang, J.L.; Pan, Y.G.; Chen, H.M.; Zhang, Z.K. The application of deep eutectic solvents systems based on choline chloride in the preparation of biodegradable food packaging films. Trends Food Sci. Technol. 2023, 139, 104124. [Google Scholar] [CrossRef]
- Zhao, R.; Guan, W.L.; Zhou, X.M.; Lao, M.J.; Cai, L.Y. The physiochemical and preservation properties of anthocyanidin/chitosan nanocomposite-based edible films containing cinnamon-perilla essential oil pickering nanoemulsions. LWT-Food Sci. Technol. 2022, 153, 112506. [Google Scholar] [CrossRef]
- Xu, Y.X.; Liu, X.L.; Jiang, Q.X.; Yu, D.W.; Xu, Y.S.; Wang, B.; Xia, W.S. Development and properties of bacterial cellulose, curcumin, and chitosan composite biodegradable films for active packaging materials. Carbohydr. Polym. 2021, 260, 117778. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.E.; Abdel Aziz, M.S.; Sabaa, M.W. Novel biodegradable and antibacterial edible films based on alginate and chitosan biguanidine hydrochloride. Int. J. Biol. Macromol. 2018, 116, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Du, J.; Zhou, H.M.; Zhou, S.Y.; Lv, Y.N.; Cheng, Y.; Tao, Y.H.; Lu, J.; Wang, H.S. Biodegradable intelligent film for food preservation and real-time visual detection of food freshness. Food Hydrocoll. 2022, 129, 107665. [Google Scholar] [CrossRef]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- Zaaboul, F.; Zhao, Q.L.; Xu, Y.J.; Liu, Y.F. Soybean oil bodies: A review on composition, properties, food applications, and future research aspects. Food Hydrocoll. 2022, 124, 107296. [Google Scholar] [CrossRef]
- Yang, J.; Vardar, U.S.; Boom, R.M.; Bitter, J.H.; Nikiforidis, C.V. Extraction of oleosome and protein mixtures from sunflower seeds. Food Hydrocoll. 2023, 145, 109078. [Google Scholar] [CrossRef]
- Patricia, C.; Manuel, V. Improving bacterial cellulose films by ex-situ and in-situ modifications: A review. Food Hydrocoll. 2021, 113, 106514. [Google Scholar] [CrossRef]
- Luo, J.J.; Zuo, D.Q.; Deng, Z.H.; Ji, A.P.; Xia, G.F. Effects of heat treatment and tea polyphenols on the structure and properties of polyvinyl alcohol nanofiber films for food packaging. Coatings 2020, 10, 49. [Google Scholar] [CrossRef]
- Wu, C.H.; Sun, J.H.; Zheng, P.Y.; Kang, X.; Chen, M.Y.; Li, Y.Z.; Ge, Y.J.; Hu, Y.Q.; Pang, J. Preparation of an intelligent film based on chitosan/oxidized chitin nanocrystals incorporating black rice bran anthocyanins for seafood spoilage monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.D.; Hao, X.; Liu, G.R.; Yue, Y.; Duan, J.J. A novel composite edible film fabricated by incorporating W/O/W emulsion into a chitosan film to improve the protection of fresh fish meat. Food Chem. 2022, 385, 132647. [Google Scholar] [CrossRef] [PubMed]



| Films | Thickness (mm) | TS (MPa) | EB (%) | WVP × 10−10 (g/(m·s·Pa)) |
|---|---|---|---|---|
| CA-SOB0 | 0.209 ± 0.022 a | 2.307 ± 0.017 e | 41.6 ± 2.60 d | 6.249 ± 0.121 a |
| CA-SOB1 | 0.230 ± 0.018 b | 2.605 ± 0.026 d | 58.6 ± 1.50 c | 5.643 ± 0.079 b |
| CA-SOB2 | 0.263 ± 0.020 b | 3.105 ± 0.032 b | 73.0 ± 0.70 b | 5.305 ± 0.146 c |
| CA-SOB3 | 0.269 ± 0.011 a | 3.661 ± 0.067 a | 78.6 ± 3.10 a | 4.881 ± 0.130 d |
| CA-SOB4 | 0.270 ± 0.009 a | 2.710 ± 0.033 c | 70.5 ± 2.00 b | 4.577 ± 0.173 e |
| Fimls | L* | a* | b* | ΔE |
|---|---|---|---|---|
| CA-SOB0 | 70.18 ± 1.21 a | 1.01 ± 3.57 e | 1.79 ± 0.63 c | 2.79 ± 2.54 c |
| CA-SOB1 | 69.36 ± 0.58 ab | 1.05 ± 0.101 d | 1.94 ± 2.43 c | 4.61 ± 2.10 bc |
| CA-SOB2 | 69.20 ± 1.91 ab | 1.63 ± 1.07 c | 2.73 ± 1.11 bc | 5.52 ± 1.80 abc |
| CA-SOB3 | 69.06 ± 1.53 b | 2.59 ± 0.06 b | 3.96 ± 1.86 b | 8.00 ± 0.49 ab |
| CA-SOB4 | 68.78 ± 0.03 ab | 2.68 ± 0.61 a | 6.38 ± 0.02 a | 8.60 ± 0.05 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Wang, L.; Chen, H.; Yin, G. Preparation and Application of Edible Film Based on Sodium Carboxymethylcellulose-Sodium Alginate Composite Soybean Oil Body. Coatings 2023, 13, 1716. https://doi.org/10.3390/coatings13101716
Sun J, Wang L, Chen H, Yin G. Preparation and Application of Edible Film Based on Sodium Carboxymethylcellulose-Sodium Alginate Composite Soybean Oil Body. Coatings. 2023; 13(10):1716. https://doi.org/10.3390/coatings13101716
Chicago/Turabian StyleSun, Jie, Luyang Wang, Han Chen, and Guoyou Yin. 2023. "Preparation and Application of Edible Film Based on Sodium Carboxymethylcellulose-Sodium Alginate Composite Soybean Oil Body" Coatings 13, no. 10: 1716. https://doi.org/10.3390/coatings13101716
APA StyleSun, J., Wang, L., Chen, H., & Yin, G. (2023). Preparation and Application of Edible Film Based on Sodium Carboxymethylcellulose-Sodium Alginate Composite Soybean Oil Body. Coatings, 13(10), 1716. https://doi.org/10.3390/coatings13101716





