Effect of Anthocyanins on Colorimetric Indicator Film Properties
Abstract
:1. Introduction
2. Barrier Properties
2.1. Water Vapour Permeability (WVP)
2.2. Oxygen Permeability (OP)
2.3. Light Barrier Property
3. Stability
3.1. Color Stability
3.2. Thermal Stability
4. Mechanical Properties
5. Antioxidant and Antibacterial
6. pH-Sensitive
6.1. Sources of Anthocyanins and pH-Sensitive
6.2. Extraction of Anthocyanins and pH-Sensitive
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BPPE | Black Plum Peel Extract |
| WVP | Water Vapour Permeability |
| RCAs | Red Cabbage Anthocyanins |
| OCN | Oxidized-chitin Nanocrystals |
| CS | Chitosan |
| GL | Gelatin |
| SA | Sodium Alginate |
| DFSE | Dragon Fruit Skin Extract With Anthocyanins |
| RAE | Roselle Anthocyanin Extracts |
| PHR film | Polyvinyl Alcohol/Hydroxypropyl Methylcellulose/Roselle Anthocyanins Film |
| PVA | Polyvinyl Alcohol |
| HPMC | Hydroxypropyl Methylcellulose |
| WMP | Watermelon Peel Pectin |
| PCE | Purple Cabbage Extract |
| MBE | Mulberry Extracts |
| KGM | Konjac Glucomannan |
| TS | Tensile Strength |
| EAB | Elongation At Break |
| MC | Methyl Cellulose |
| BW | Black Wolfberry |
| KC | Kadsura Coccinea Extract |
| SA | Sodium Alginate |
| PLA | P olylactic Acid |
| PEG | Polyethylene Glycol |
| CB | Calcium Bentonite |
| MAC | M. Sylvestris Anthocyanins |
| PPE | P urple Potato Extract |
| RE | R oselle |
| PEE | Purple Rice Extract |
| BEE | Black Rice Extract |
| DPPH | 2,2-Diphenyl-1-Picrylhydrazyl |
| CMC | Carboxymethyl Cellulose |
| BCA | Blackcurrant Anthocyanin |
| EVOH | Ethylene Vinyl Alcohol |
| PGA | Anthocyanins of Pomegranate |
| CTA | Anthocyanins of Clitoria Ternatea |
| UAE | Ultrasound-assisted Extraction |
| MAE | Microwave-assisted Extraction |
| SFE | Supercritical Fluid Extraction |
| HPLE | High-pressure Liquid Extraction |
| PEFE | Pulsed Electric Fields |
| PTA | Anthocyanins of Purple Tomato |
| PA-PSPA | Pads of Purple Sweet Potato Anthocyanins |
| HVED | High Voltage Electrical Discharge |
| EAE | Enzyme-assisted Extraction |
| PSRF | Polyvinylidene Fluoride |
References
- Robertson, G.L. Food Packaging: Principles and Practice; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- De Abreu, D.A.P.; Cruz, J.M.; Losada, P.P. Active and Intelligent Packaging for the Food Industry. Food Rev. Int. 2012, 28, 146–187. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Recent advances in the preparation, physical and functional properties, and applications of anthocyanins-based active and intelligent packaging films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Capello, C.; Leandro, G.C.; Gagliardi, T.R.; Valencia, G.A. Intelligent Films from Chitosan and Biohybrids Based on Anthocyanins and Laponite®: Physicochemical Properties and Food Packaging Applications. J. Polym. Environ. 2021, 29, 3988–3999. [Google Scholar] [CrossRef]
- Huimin, Y.; Xingchi, W.; Ruyu, B.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar]
- Mohammadalinejhad, S.; Kurek, M.A. Microencapsulation of Anthocyanins—Critical Review of Techniques and Wall Materials. Appl. Sci. 2021, 11, 3936. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Applications of Chitosan as Food Packaging Materials. In Sustainable Agriculture Reviews 36; Springer: Cham, Switzerland, 2019; pp. 81–123. [Google Scholar]
- Cazón, P.; Vázquez, M. Mechanical and barrier properties of chitosan combined with other components as food packaging film. Environ. Chem. Lett. 2019, 18, 257–267. [Google Scholar] [CrossRef]
- Chen, M.; Yan, T.; Huang, J.; Zhou, Y.; Hu, Y. Fabrication of halochromic smart films by immobilizing red cabbage anthocyanins into chitosan/oxidized-chitin nanocrystals composites for real-time hairtail and shrimp freshness monitoring. Int. J. Biol. Macromol. 2021, 179, 90–100. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, H.; Yuan, M.; Qin, Y.; Chen, H. Effects of anthocyanin-rich Kadsura coccinea extract on the physical, antioxidant, and pH-sensitive properties of biodegradable film. Food Biophys. 2022, 17, 375–385. [Google Scholar] [CrossRef]
- Azlim, N.A.; Mohammadi, N.A.; Oladzadabbasabadi, N.; Ariffin, F.; Ghalambor, P.; Jafarzadeh, S.; Al-Hassan, A.A. Fabrication and characterization of a pH-sensitive intelligent film incorporating dragon fruit skin extract. Food Sci. Nutr. 2022, 10, 597–608. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, J.; Jiang, L.; Zhu, Z.; He, S.; He, S.; Shao, W. Development of intelligent/active food packaging film based on TEMPO-oxidized bacterial cellulose containing thymol and anthocyanin-rich purple potato extract for shelf life extension of shrimp. Food Packag. Shelf Life 2021, 29, 100709. [Google Scholar] [CrossRef]
- Naghdi, S.; Rezaei, M.; Abdollahi, M. A starch-based pH-sensing and ammonia detector film containing betacyanin of paperflower for application in intelligent packaging of fish. Int. J. Biol. Macromol. 2021, 191, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kim, H.-J.; Rhim, J.-W. Effect of blended colorants of anthocyanin and shikonin on carboxymethyl cellulose/agar-based smart packaging film. Int. J. Biol. Macromol. 2021, 183, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Y.; Zhou, Y.; Li, R.; Jiang, Y.; Hossen, A.; Dai, J.; Qin, W.; Liu, Y. Facile fabrication of sandwich-like anthocyanin/chitosan/lemongrass essential oil films via 3D printing for intelligent evaluation of pork freshness. Food Chem. 2021, 370, 131082. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black plum peel extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Chen, M.; Qian, Y.; Hu, Y. Immobilization of roselle anthocyanins into polyvinyl alcohol/hydroxypropyl methylcellulose film matrix: Study on the interaction behavior and mechanism for better shrimp freshness monitoring. Int. J. Biol. Macromol. 2021, 184, 666–677. [Google Scholar] [CrossRef]
- Liu, H.; Shi, C.; Sun, X.; Zhang, J.; Ji, Z. Intelligent colorimetric indicator film based on bacterial cellulose and pelargonidin dye to indicate the freshness of tilapia fillets. Food Packag. Shelf Life 2021, 29, 100712. [Google Scholar] [CrossRef]
- Yan, J.; Cui, R.; Qin, Y.; Li, L.; Yuan, M. A pH indicator film based on chitosan and butterfly pudding extract for monitoring fish freshness. Int. J. Biol. Macromol. 2021, 177, 328–336. [Google Scholar] [CrossRef]
- Sani, M.A.; Tavassoli, M.; Salim, S.A.; Azizi-Lalabadi, M.; McClements, D.J. Development of green halochromic smart and active packaging materials: TiO2 nanoparticle- and anthocyanin-loaded gelatin/κ-carrageenan films. Food Hydrocoll. 2021, 124, 107324. [Google Scholar] [CrossRef]
- Kan, J.; Liu, J.; Xu, F.; Yun, D.; Yong, H.; Liu, J. Development of pork and shrimp freshness monitoring labels based on starch/polyvinyl alcohol matrices and anthocyanins from 14 plants: A comparative study. Food Hydrocoll. 2021, 124, 107293. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; McClements, D.J.; Hamishehkar, H. Multifunctional halochromic packaging materials: Saffron petal anthocyanin loaded-chitosan nanofiber/methyl cellulose matrices. Food Hydrocoll. 2020, 111, 106237. [Google Scholar] [CrossRef]
- Freitas, P.A.; Silva, R.R.; de Oliveira, T.V.; Soares, R.R.; Junior, N.S.; Moraes, A.R.; Pires, A.C.d.S.; Soares, N.F. Development and characterization of intelligent cellulose acetate-based films using red cabbage extract for visual detection of volatile bases. LWT 2020, 132, 109780. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Krzyściak, P.; Talaga-Ćwiertnia, K.; Juszczak, L. Intelligent and active furcellaran-gelatin films containing green or pu-erh tea extracts:Characterization, antioxidant and antimicrobial potential. Int. J. Biol. Macromol. 2019, 122, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Han, J.-W.; Ruiz-Garcia, L.; Qian, J.-P.; Yang, X.-T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef]
- Abedi-Firoozjah, R.; Yousefi, S.; Heydari, M.; Seyedfatehi, F.; Jafarzadeh, S.; Mohammadi, R.; Rouhi, M.; Garavand, F. Application of Red Cabbage Anthocyanins as pH-Sensitive Pigments in Smart Food Packaging and Sensors. Polymers 2022, 14, 1629. [Google Scholar] [CrossRef]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.T.; Dias, A.R.G.; da Rosa Zavareze, E. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Guo, Z.; Zuo, H.; Ling, H.; Yu, Q.-L.; Gou, Q.; Yang, L. A novel colorimetric indicator film based on watermelon peel pectin and anthocyanins from purple cabbage for monitoring mutton freshness. Food Chem. 2021, 383, 131915. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Huang, Y.; Li, H.; Chen, L.; Liu, X. Two colorimetric films based on chitin whiskers and sodium alginate/gelatin incorporated with anthocyanins for monitoring food freshness. Food Hydrocoll. 2022, 127, 107517. [Google Scholar] [CrossRef]
- You, P.; Wang, L.; Zhou, N.; Yang, Y.; Pang, J. A pH-intelligent response fish packaging film: Konjac glucomannan/carboxymethyl cellulose/blackcurrant anthocyanin antibacterial composite film. Int. J. Biol. Macromol. 2022, 204, 386–396. [Google Scholar] [CrossRef]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT 2015, 61, 258–262. [Google Scholar] [CrossRef]
- Liang, T.; Sun, G.; Cao, L.; Li, J.; Wang, L. A pH and NH3 sensing intelligent film based on Artemisia sphaerocephala Krasch. gum and red cabbage anthocyanins anchored by carboxymethyl cellulose sodium added as a host complex. Food Hydrocoll. 2018, 87, 858–868. [Google Scholar] [CrossRef]
- Li, S.; Yanglin, W.; Yuan, G.; Sun, Q.; Li, C. Preparation of acetic acid modified roselle Anthocyanidin indicator film and its application in meat freshness evaluation. Packag. Food Mach. 2022, 40, 31–38. [Google Scholar]
- Wang, D.; Lin, L.; Wang, X.; Sun, Z.; Liu, F.; Wang, D. A fast-response acidic and basic vapor visual indicator film based on black wolfberry anthocyanin for monitoring chicken and shrimp freshness. SSRN 2022, 90, 216–224. [Google Scholar] [CrossRef]
- Liu, D.; Cui, Z.; Shang, M.; Zhong, Y. A colorimetric film based on polyvinyl alcohol/sodium carboxymethyl cellulose incorporated with red cabbage anthocyanin for monitoring pork freshness. Food Packag. Shelf Life 2021, 28, 100641. [Google Scholar] [CrossRef]
- Rezaie, A.; Rezaei, M.; Albooftileh, M. Preparation and evaluation some properties pH indicator film based on Arabic gum-carboxy methyl cellulose composite film containing of Violet basil (Ocimum basilicum. L) anthocyanin. Iran. Food Sci. Technol. Res. J. 2021, 17, 527–541. [Google Scholar]
- Liu, L.; Zhang, J.; Zou, X.; Arslan, M.; Shi, J.; Zhai, X.; Xiao, J.; Wang, X.; Huang, X.; Li, Z.; et al. A high-stable and sensitive colorimetric nanofiber sensor based on PCL incorporating anthocyanins for shrimp freshness. Food Chem. 2022, 377, 131909. [Google Scholar] [CrossRef]
- Singh, S.; Nwabor, O.F.; Syukri, D.M.; Voravuthikunchai, S.P. Chitosan-poly(vinyl alcohol) intelligent films fortified with anthocyanins isolated from Clitoria ternatea and Carissa carandas for monitoring beverage freshness. Int. J. Biol. Macromol. 2021, 182, 1015–1025. [Google Scholar] [CrossRef]
- Li, B.; Bao, Y.; Li, J.; Bi, J.; Chen, Q.; Cui, H.; Wang, Y.; Tian, J.; Shu, C.; Wang, Y.; et al. A sub-freshness monitoring chitosan/starch-based colorimetric film for improving color recognition accuracy via controlling the pH value of the film-forming solution. Food Chem. 2022, 388, 132975. [Google Scholar] [CrossRef]
- Fang, S.; Guan, Z.; Su, C.; Zhang, W.; Zhu, J.; Zheng, Y.; Li, H.; Zhao, P.; Liu, X. Accurate fish-freshness prediction label based on red cabbage anthocyanins. Food Control 2022, 138, 109018. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Fernandes, S.C.; Sánchez, M.A.; Labidi, J. Halochromic and antioxidant capacity of smart films of chitosan/chitin nanocrystals with curcuma oil and anthocyanins. Food Hydrocoll. 2021, 123, 107119. [Google Scholar] [CrossRef]
- Mustafa, P.; Niazi, M.B.K.; Jahan, Z.; Rafiq, S.; Ahmad, T.; Sikander, U.; Javaid, F. Improving functional properties of PVA/starch-based films as active and intelligent food packaging by incorporating propolis and anthocyanin. Polym. Polym. Compos. 2020, 29, 1472–1484. [Google Scholar] [CrossRef]
- Qi, D.; Xiao, Y.; Xia, L.; Li, L.; Jiang, S.; Jiang, S.; Wang, H. Antibacterial-antioxidant Colorimetric films Incorporated with nisin and anthocyanins of pomegranate/Clitoria Ternatea. Food Packag. Shelf Life 2022, 33, 100898. [Google Scholar] [CrossRef]
- Boonsiriwit, A.; Itkor, P.; Sirieawphikul, C.; Lee, Y.S. Characterization of Natural Anthocyanin Indicator Based on Cellulose Bio-Composite Film for Monitoring the Freshness of Chicken Tenderloin. Molecules 2022, 27, 2752. [Google Scholar] [CrossRef]
- He, Y.; Li, B.; Du, J.; Cao, S.; Liu, M.; Li, X.; Ren, D.; Wu, X.; Xu, D. Development of pH-responsive absorbent pad based on polyvinyl alcohol/agarose/anthocyanins for meat packaging and freshness indication. Int. J. Biol. Macromol. 2022, 201, 203–215. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Ying, Y.; Hu, L.; Hu, Y. pH-sensitive and antibacterial films developed by incorporating anthocyanins extracted from purple potato or roselle into chitosan/polyvinyl alcohol/nano-ZnO matrix: Comparative study. Int. J. Biol. Macromol. 2021, 178, 104–112. [Google Scholar] [CrossRef]
- Li, Y.; Wu, K.; Wang, B.; Li, X. Colorimetric indicator based on purple tomato anthocyanins and chitosan for application in intelligent packaging. Int. J. Biol. Macromol. 2021, 174, 370–376. [Google Scholar] [CrossRef]
- Gao, R.; Hu, H.; Shi, T.; Bao, Y.; Sun, Q.; Wang, L.; Ren, Y.; Jin, W.; Yuan, L. Incorporation of gelatin and Fe(2+) increases the pH-sensitivity of zein-anthocyanin complex films used for milk spoilage detection. Curr. Res. Food Sci. 2022, 5, 677–686. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, W.; Xia, M.; Zeng, Q.; Cai, Z. Intelligent colorimetric film incorporated with anthocyanins-loaded ovalbumin-propylene glycol alginate nanocomplexes as a stable pH indicator of monitoring pork freshness. Food Chem. 2021, 368, 130825. [Google Scholar] [CrossRef]
- Bao, Y.; Cui, H.; Tian, J.; Ding, Y.; Tian, Q.; Zhang, W.; Wang, M.; Zang, Z.; Sun, X.; Li, D.; et al. Novel pH sensitivity and colorimetry-enhanced anthocyanin indicator films by chondroitin sulfate co-pigmentation for shrimp freshness monitoring. Food Control 2022, 131, 108441. [Google Scholar] [CrossRef]
- Sun, W.; Liu, Y.; Jia, L.; Saldaña, M.D.; Dong, T.; Jin, Y.; Sun, W. A smart nanofibre sensor based on anthocyanin poly l lactic acid for mutton freshness monitoring. Int. J. Food Sci. Technol. 2021, 56, 342–351. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Ribeiro, C.P.P.; Uliana, N.R.; Rodrigues, M.B.C.; da Rosa, C.G.; Ferrareze, J.P.; Veeck, A.P.d.L.; Nunes, M.R. Bioactive and pH-sensitive films based on carboxymethyl cellulose and blackberry (Morus nigra L.) anthocyanin-rich extract: A perspective coating material to improve the shelf life of cherry tomato (Solanum lycopersicum L. var. cerasiforme). Biocatal. Agric. Biotechnol. 2021, 33, 101989. [Google Scholar] [CrossRef]
- Ghorbani, M.; Divsalar, E.; Molaei, R.; Ezati, P.; Moradi, M.; Tajik, H.; Abbaszadeh, M. A halochromic indicator based on polylactic acid and anthocyanins for visual freshness monitoring of minced meat, chicken fillet, shrimp, and fish roe. Innov. Food Sci. Emerg. Technol. 2021, 74, 102864. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Shi, J.; Liu, L.; Zhang, X.; Zou, X.; Xiao, J.; Zhai, X.; Zhang, D.; Li, Y.; et al. A visual bi-layer indicator based on roselle anthocyanins with high hydrophobic property for monitoring griskin freshness. Food Chem. 2021, 355, 129573. [Google Scholar] [CrossRef]
- Wu, L.-T.; Tsai, I.-L.; Ho, Y.-C.; Hang, Y.-H.; Lin, C.; Tsai, M.-L.; Mi, F.-L. Active and intelligent gellan gum-based packaging films for controlling anthocyanins release and monitoring food freshness. Carbohydr. Polym. 2020, 254, 117410. [Google Scholar] [CrossRef]
- Zhou, N.; Wang, L.; You, P.; Wang, L.; Mu, R.; Pang, J. Preparation of pH-sensitive food packaging film based on konjac glucomannan and hydroxypropyl methyl cellulose incorporated with mulberry extract. Int. J. Biol. Macromol. 2021, 172, 515–523. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, X.; Zou, X.; Shi, J.; Huang, X.; Li, Z.; Gong, Y.; Holmes, M.; Povey, M.; Xiao, J. Bilayer pH-sensitive colorimetric films with light-blocking ability and electrochemical writing property: Application in monitoring crucian spoilage in smart packaging. Food Chem. 2021, 336, 127634. [Google Scholar] [CrossRef]
- Duan, M.; Yu, S.; Sun, J.; Jiang, H.; Zhao, J.; Tong, C.; Hu, Y.; Pang, J.; Wu, C. Development and characterization of electrospun nanofibers based on pullulan/chitin nanofibers containing curcumin and anthocyanins for active-intelligent food packaging. Int. J. Biol. Macromol. 2021, 187, 332–340. [Google Scholar] [CrossRef]
- Yan, J.; Cui, R.; Tang, Z.; Wang, Y.; Wang, H.; Qin, Y.; Yuan, M.; Yuan, M. Development of pH-sensitive films based on gelatin/chitosan/nanocellulose and anthocyanins from hawthorn (Crataegus scabrifolia) fruit. J. Food Meas. Charact. 2021, 15, 3901–3911. [Google Scholar] [CrossRef]
- Hashim, S.B.; Tahir, H.E.; Liu, L.; Zhang, J.; Zhai, X.; Mahdi, A.A.; Awad, F.N.; Hassan, M.M.; Xiaobo, Z.; Jiyong, S. Intelligent colorimetric pH sensoring packaging films based on sugarcane wax/agar integrated with butterfly pea flower extract for optical tracking of shrimp freshness. Food Chem. 2022, 373, 131514. [Google Scholar] [CrossRef]
- Tuany, G.H.; Betina, L.A.; Sávio, L.B.; de Souza, C.K. Intelligent pH-sensing film based on jaboticaba peels extract incorporated on a biopolymeric matrix. J. Food Sci. Technol. 2021, 59, 1001–1010. [Google Scholar]
- Anghel, N.; Dinu, M.V.; Zaltariov, M.; Pamfil, D.; Spiridon, I. New cellulose-collagen-alginate materials incorporated with quercetin, anthocyanins and lipoic acid. Int. J. Biol. Macromol. 2021, 181, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Koshy, R.R.; Koshy, J.T.; Mary, S.K.; Sadanandan, S.; Jisha, S.; Pothan, L.A. Preparation of pH sensitive film based on starch/carbon nano dots incorporating anthocyanin for monitoring spoilage of pork. Food Control 2021, 126, 108039. [Google Scholar] [CrossRef]
- Sai-Ut, S.; Suthiluk, P.; Tongdeesoontorn, W.; Rawdkuen, S.; Kaewprachu, P.; Karbowiak, T.; Debeaufort, F.; Degraeve, P. Using Anthocyanin Extracts From Butterfly Pea as pH Indicator for Intelligent Gelatin Film and Methylcellulose Film. Curr. Appl. Sci. Technol. 2021, 21, 652–661. [Google Scholar]
- Qin, Y.; Yun, D.; Xu, F.; Chen, D.; Kan, J.; Liu, J. Smart packaging films based on starch/polyvinyl alcohol and Lycium ruthenicum anthocyanins-loaded nano complexes: Functionality, stability and application. Food Hydrocoll. 2021, 119, 106850. [Google Scholar] [CrossRef]
- Wang, F.; Qiu, L.; Tian, Y. Super Anti-Wetting Colorimetric Starch-Based Film Modified with Poly(dimethylsiloxane) and Micro-/Nano-Starch for Aquatic-Product Freshness Monitoring. Biomacromolecules 2021, 22, 3769–3779. [Google Scholar] [CrossRef]
- Sani, M.A.; Tavassoli, M.; Hamishehkar, H.; McClements, D.J. Carbohydrate-based films containing pH-sensitive red barberry anthocyanins: Application as biodegradable smart food packaging materials. Carbohydr. Polym. 2021, 255, 117488. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant antiinflammatory activities of anthocyanins their aglycon cyanidin from tart cherries. J. Nat. Prod. 1999, 62, 294–296. [Google Scholar] [CrossRef]
- Huimin, Y.; Jing, L.; Yan, Q.; Bai, R.; Zhang, X.; Liu, J. Antioxidant and pH-sensitive films developed by incorporating purple and black rice extracts into chitosan matrix. Int. J. Biol. Macromol. 2019, 137, 307–316. [Google Scholar]
- Yong, H.; Wang, X.; Zhang, X.; Liu, Y.; Qin, Y.; Liu, J. Effects of anthocyanin-rich purple and black eggplant extracts on the physical, antioxidant and pH-sensitive properties of chitosan film. Food Hydrocoll. 2019, 94, 93–104. [Google Scholar] [CrossRef]
- Su, M.-S.; Silva, J.L. Antioxidant activity, anthocyanins, and phenolics of rabbiteye blueberry (Vaccinium ashei) by-products as affected by fermentation. Food Chem. 2006, 97, 447–451. [Google Scholar] [CrossRef]
- Sun, L.-Q.; Ding, X.-P.; Qi, J.; Yu, H.; He, S.-A.; Zhang, J.; Ge, H.-X.; Yu, B.-Y. Antioxidant anthocyanins screening through spectrum–effect relationships and DPPH-HPLC-DAD analysis on nine cultivars of introduced rabbiteye blueberry in China. Food Chem. 2011, 132, 759–765. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Faseha, A.; Benjakul, S.; Kaewprachu, P. Application of anthocyanin as a color indicator in gelatin films. Food Biosci. 2020, 36, 100603. [Google Scholar] [CrossRef]
- Xin, W.; Tingting, L.; Longfei, F.; Zhang, T. Study on Chitosan based Intelligent Indicator Film Loaded with Blueberry Anthocyanidin to Monitor the Freshness of Clam. Packaging 2023, 44, 10–19. [Google Scholar]
- Shiyang, F.; Qiuxia, Z.; Yichi, Z.; Liu, J.; Hancheng, C.; Xiaoping, F. Indication of milk freshness by ethyl cellulose/blueberry Anthocyanidin soaking label. Packaging 2023, 44, 27–37. [Google Scholar]
| Anthocyanidin | Basic Structure | R1 | R2 | Main Color | Example of Source |
|---|---|---|---|---|---|
| Cyanidin | 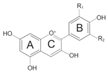 | -OH | -H | Red, orange | Blackberries, blood oranges, plums, strawberries, red cabbage, apricots, haskap berries, red onions |
| Delphinidin | -OH | -OH | Purple, blue | Eggplant, red oranges, pomegranates, black beans, peppers, purple tomatoes | |
| Pelargonidin | -H | -H | Orange | Radish, pomegranates, red-fleshed potatoes, turnips | |
| Malvidin | -OCH3 | -OCH3 | Purple | Bilberries, red wine, blueberries | |
| Peonidin | -OCH3 | -H | Purplish, red | Sweet potatoes, cranberries, grapes, purple corn, mangoes, rice | |
| Petunidin | -OH | -OCH3 | Purple, dark | Blackcurrants, black beans, red berries, purple petals of flowers |
| Mechanical Criteria | Define | Influence Factor |
|---|---|---|
| Tensile strength (TS) | The amount of load or stress that can be handled by a composite film before it stretches and breaks. | The molecular interaction between anthocyanins, and the polymer, type of polymer and concentration of anthocyanins, electrostatic repulsions and hydrogens, the films’s preparation and storage conditions. |
| Elongation at break (EAB) | The optimum potential of the films to resist changes in the film length. |
| Sources | Film Materials | pH Values/Color Change | Products | Effect/Results | Reference |
|---|---|---|---|---|---|
| Black wolfberry | Polyvinyl alcohol (PAV)/methyl cellulose (MC) |  | Shrimp/chicken | Positively affected the hydrogen-bond interactions, stability, tensile strength, breakage elongation and pH sensitivity character of the films. | Wang et al., 2022 [36] |
| In the range of pH = 2–13, as the pH increases, the color of the film changes from red to yellow. Under storage conditions of 4 ° C, the film can react with acidic and alkaline vapors in chickens and shrimp, as well as NH3 as low as 25 ppm, within 10 s. | |||||
| Black wolfberry | Sodium alginate (SA)/gelatin (GE) |  | Milk/pork | The water resistance and thermal stability of the film are enhanced. The membrane exhibits good responsiveness to lactic acid or amine gases. The films were able to detect freshness of milk or pork and showed excellent durability and accuracy in food freshness monitoring. | Zheng et al., 2022 [31] |
| The solution turns red at pH 3, and as the pH increases, the color becomes lighter. At pH WEI 8–10, the solution turns blue-purple, and at pH 11–12, it turns yellow. | |||||
| Clitoria ternatea Linn | Polycaprolactone (PCL) | The film showed visual color changes from pale blue to yellow-green (shrimp spoilage 21 h). Note: PCL polycaprolactone; CA clitoria ternatea Linn anthocyanin | Shrimp | Positively affected the microstructure, thickness, TS, EB, WVP, color stability pH and ammonia sensitivity character of the bilayer PCL/PCL-CA films. | Liu et al., 2022 [39] |
| Clitoria ternatea/Carissa carandas | Chitosan–poly/vinyl alcohol |  | Beverage | Positively affected the stability properties, integration, and pH sensitivity of the film. After 72 h of storage at 25 °C, the color of the coating changed. | Singh et al., 2021 [40] |
| The Carissa ternatea flower extract showed purple-red coloration in acidic pH and greenish-yellow color in alkaline pH. However, the Carissa carandas fruit extract indicated light red and yellow color at acidic and alkaline pH. The color of the membrane mixed with anthocyanins changes significantly at a pH of 2 to 8. | |||||
| Purple cabbage | Watermelon peel pectin (WMP) |  | Mutton | Positively affected the tensile strength, barrier properties, thermal stability, color stability and pH response properties with low PCE content (≤1.5%). Negatively affected elongation at break. | Guo et al., 2022 [30] |
| This movie showcases the color changes of anthocyanins as the freshness of lamb changes, from fresh lamb to spoiled lamb, and the color of the film changes from light purple to light blue. | |||||
| Blackcurrant | Konjac glucomannan (KGM)/carboxymethyl cellulose (CMC) | 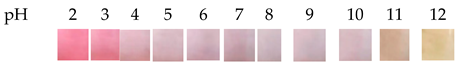 | Fish | Positively affected the barrier properties (water vapor permeability, WVP), thermal stability, antioxidant and antibacterial properties. This will result in a decrease in the strength coefficient. | You et al., 2022 [32] |
| The color of the film is red when the pH is 2–3, pink when the pH is 4–8, and yellow-green when the pH is 9–13. | |||||
| Lonicera caerulea L. | Potato starch (PS)/chitosan (CH) |  | Shrimp | Positively affected the tensile strength, water solubility, and sensitive color responsiveness. | Li et al., 2022 [41] |
| Within the pH range of 2–6, the color of the film gradually changes from orange-red to colorless and is almost colorless at a pH value of 6. Within the pH range of 6–7, the color of the film changes to brown. At pH 7–12, the color of the film changes from brown to deep purple. This film effectively indicates the freshness of the shrimp. | |||||
| Red cabbage | Carboxymethyl/chitosan/oxidized sodium alginate (CMCS/OSA) |  | Fish | Positively affected the UV–vis light transmittance property and pH sensitivity. The sensing label can be integrated into smartphones for effective and rapid determination of the freshness of fish. | Fang et al., 2022 [42] |
| The solution turns red at pH 3, pink at pH 4–6, blue at pH 7–11, and yellow at pH 12. Tags allow us to quickly obtain freshness information | |||||
| Red cabbage | Nanocrystals with curcuma oil |  | - | Positively affected the mechanical properties, hydrophobicity, water solubility, moisture content, antioxidant, and pH sensitive of this film with alpha-chitin nanocrystals. The films were at the same time antioxidant, and sensitive to color change when exposed to ammonia gas and different pH solutions | Fernández-Marín et al., 2022 [43] |
| Red cabbage | Polyvinyl alcohol/sodium carboxymethyl cellulose | 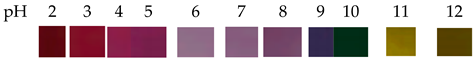 | Pork | Positively affected the spatial structure, elongation at break (EAB), and water solubility (WS) of the film. Negatively affected the crystallinity, tensile strength (TS), and swelling index (SI) of the film. The film undergoes a color change from red to blue-green when it deteriorates, and can be used to monitor the freshness of pork. | Liu et al., 2021 [37] |
| The color of RCA solutions was orange-red when pH was less than 3 and turned purple gradually at pH 4− 5. When the solutions were basic, the color changed from blue to green and, finally, to blue. | |||||
| Red cabbage | Chitosan/oxidized-chitin nanocrystals |  | Fish/shrimp |
The permeability, mechanical properties, and UV barrier of the film are enhanced. This film is sensitive to changes and can quickly and clearly identify changes in product quality. This intelligent system is assembled from non-toxic and biodegradable components and has a wide range of applications, such as seafood. | Chen et al., 2021 [10] |
| The color of RCAs solutions exhibited changes from rose-red to purple (pH 3.0–6.0) and blue to blue-green (pH 7.0–10.0), as well as a sudden color change from purple to blue (pH 6.0–7.0). | |||||
| Red cabbage | Polyvinyl alcohol/starch/glutaraldehyde/propolis |  | Milk | Positively affected the mechanical strength, physical properties, antibacterial activity and compatibility of the films. The films were capable of inhibiting and alerting food spoilage. | Mustafa et al., 2021 [44] |
| In the solution of pH 1–14, the film changes obviously with pH. | |||||
| Pomegranate/Clitoria ternatea | EVOH/nisin-(PGA/CTA) | Photographs of EVOH/nisin-(PGA/CTA)3 for freshness monitoring (a) and freshness retaining plus monitoring (b) The film was distinguishable at pH 2–12 film and was sensitive to the pH stimuli of volatile ammonia and acetic acid. | Shrimp | Positively affected the pH-sensitive, distinguishable, antioxidant activity, and antibacterial activity of the film. This film allows manufacturers and consumers to clearly obtain freshness information, and can also extend the shelf life of shrimp meat stored at 4 °C. | Qi et al., 2022 [45] |
| Roselle | Hydroxypropyl methylcellulose (HPMC)/microcrystalline cellulose (MCC) | At pH 1–4, red gradually decreases with increasing pH, becoming light coral red at pH 5–6, magenta at pH 7–8, brownish red at pH 9, gray at pH 10, brown at pH 11, and yellow at pH 12. | Chicken | There is a positive impact on ammonia exposure sensitivity and changes in chicken fillet quality. | Boonsiriwit et al., 2022 [46] |
| Saffron or red barberry | Gelatin/κ-carrageenan | 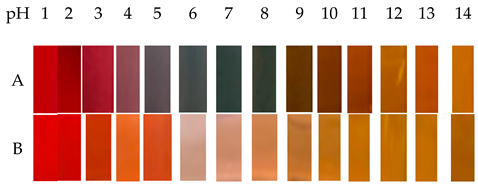 | Fish | Positively affected the mechanical, moisture resistance, bacteriostatic properties, inhibiting oxidative reactions and is biodegradable. | Alizadeh et al., 2022 [21] |
| A: saffron petal B: red berries The color of the saffron petal anthocyanin solution changes from red under acidic conditions to blue/purple/gray under neutral conditions, and to green/yellow under alkaline conditions (alkaline pH). The color of anthocyanins in red berries appears red under acidic conditions, pale peach in neutral solutions, and yellow in alkaline solutions. | |||||
| Saffron petal | Chitosan nanofibers/methyl cellulose | T At a pH of 1–14, the membrane changes from red/pink to purple, and from green to yellow. As the concentration of ammonia vapor increases, the membrane changes from purple to green/yellow. | Lamb | The tensile strength, shading performance, antibacterial activity and antioxidant activity of the film against Staphylococcus aureus and Staphylococcus aureus have all been enhanced. The strength coefficient and thermal performance of the film have decreased. | Alizadeh et al., 2021 [23] |
| Purple sweet potato | Polyvinyl alcohol/agarose |  | Meat | The shelf life has also been extended. But it has adverse effects on mechanical properties, water solubility, and swelling rate | He et al., 2022 [47] |
| The solutions appear to be red at pH 3, pink at pH 3–6, purple at pH 7, blue-purple at pH 8–10. | |||||
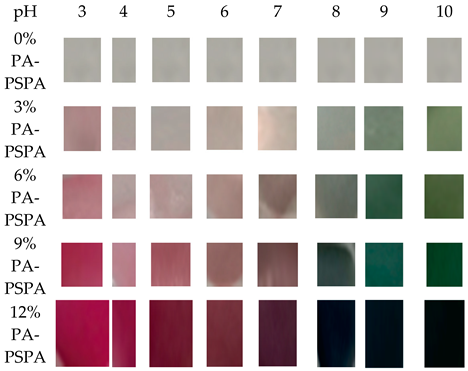 | |||||
| PA-PSPA (pads of purple sweet potato anthocyanins) 0% was transparent under all pH environments, while the addition of PSPA made the pads’ color change from pink to purple and then to blue-green when the pH changed from 3 to 10. | |||||
| Purple potato | TEMPO-oxidized bacterial cellulose |  | Shrimp | Positively affected the thermal stability, UV protection, and water vapor barrier properties of the film. Negatively affected the tensile strength, elongation at break and thermal properties of the film. | Wen et al., 2021 [13] |
| The solutions appear to be red at pH 2–5, purple at pH 6–7, blue at pH 8–11, and yellow at pH 12–13. | |||||
| Purple potato/Roselle | Chitosan/polyvinyl alcohol/nano-ZnO |  | Shrimp |
The mechanical resistance and pH sensitivity of the membrane are enhanced. This reduces the water content and flexibility of the film. The degree of shrimp spoilage can be determined by the color of the film. When the film changes from purple to light green, the shrimp has already spoilt. | Liu et al., 2021 [48] |
|
Purple potato extract (PPE) Roselle (RE) The color ranges of PPE were red > pink > purple > blue > kelly > yellow from acidic to alkaline buffer solutions. In contrast, the color of RE was much darker than that of PPE in the same buffer solution, presenting red > gray > puce > green with an increase in alkalinity. | |||||
| Purple tomato | Chitosan (CS) |  | Milk/fish | Positively affected the elongation at breaking and swelling index and pH sensitivity of the film. The film became darker and was distinguishable with the increasing pH from 3–11, for juice stored at 25 °C after 72 h. | Li et al., 2021 [49] |
| The change in color of the CS/30%PTA film was from fuchsia (pH = 3) → deep purple (pH = 5) → dark blue (pH = 7) → green (pH = 9) → yellow-green (pH = 11). In the range of pH = 3–11, the color of the film darkens with the increase in pH value, and the color change of CS/10% PTA film is the most obvious. | |||||
| Kadsura coccinea | Chitosan (CH), gelatin (GL), and sodium alginate (SA) |  | Meat/sea food | Positively affected mechanical property, antioxidant capacity, moisture content, and thermal behavior. Will reduce water vapor barrier performance and UV–visible light transmittance | Yan et al., 2022 [11] |
| The solutions appear to be red at pH 1–4, pink at pH 5, gray at pH 6–8, light gray at pH 9, and yellow at pH 10–14. | |||||
| Hylocereus polyrhizus | Gelatin | The films appear to be red at pH 4, colorless at pH 7, and blue at pH 9. | Food | Positively affected the moisture content, elongation at break, and color variability. Negatively affected the thickness, water vapor permeability, and light transmittance of the films. This film can visually determine whether the pH has changed through color, which can be used by consumers and food manufacturers to determine the freshness of food. | Azlim et al., 2022 [12] |
| Mulberry | Chitosan/lemongrass | Changed from red to gray-blue. Drying and color changes in DFBG3 films after immersion in different pH buffer solutions | Pork | Positively affected the sensitivity. This film, combined with a mobile phone analysis system, can be used to determine the freshness of pork. | Li et al., 2022 [16] |
| Blueberry | Gelatin and Fe(2+) |  | Milk | The color change of the indicator film to pH changes is significant, and the color response sensitivity increases. This film can be used to detect the freshness of milk. The color of fresh milk is purple-black, stale milk is purple, and spoiled milk will turn purple-red. | Gao et al., 2022 [50] |
| As the pH increases, the color of the solution gradually becomes lighter than red. At a pH of 4–8, the color is similar and freshness cannot be determined. When the pH is in the range of 9–11, the color of the solution gradually deepens from light purple | |||||
| Blueberry | Polyvinyl alcohol/glycerol | 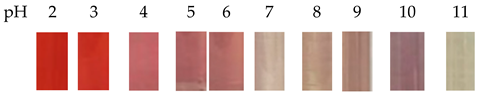 | Pork | The stability and barrier properties of the film are enhanced, but it has a negative impact on the crystallinity of the polyvinyl alcohol film. Fresh pork appears purple-red, and after spoilage, it turns dark blue. This film can be used to detect the freshness of pork products. | Zhang et al., 2022 [51] |
| The solutions appear to be red at pH 2–3, pink at pH 4–6, colorless at pH 7, blue-purple at pH 8–10, and yellow-green at pH 11. | |||||
| Blueberry | Potato starch (PS)/chondroitin sulfate (CS) |  | Shrimp | It has a positive impact on the mechanical properties, pH value, and ammonia responsiveness of the film. It has a negative impact on the water solubility of the film. | Bao et al., 2022 [52] |
| A: blueberry anthocyanin B: with the addition of Chondroitin sulfate The blueberry anthocyanin solution appeared pink at pH 2.0–3.0, which gradually decreased in intensity with pH value increasing to 6.0. When it came to pH 7.0, the BA solution showed a color trend of grey-pink to grey-blue, and the intensity gradually increased in the range of pH 7.0–11.0, and, finally, grey-brown at pH 12.0. The addition of CS enhanced the color intensity of BA solution on the basis of the same color series. | |||||
| Blueberry | Polylactic acid | Due to the ammonia concentration getting higher and higher, the pink color of the sensor gradually becomes lighter, and, eventually, the color disappears. | Mutton | The detection limit is 37 ppm. This sensor can effectively monitor the freshness of the lamb in real time, and the color changes presented are easy to observe with the naked eye, and this sensor can be reused many times. | Sun et al., 2021 [53] |
| Blackberry | Carboxymethyl cellulose |  | Cherry/tomato | Positively affected the water solubility, UV-blocking property (below 15%), and water solubility (WS) of the film. Negatively affected the crystallinity, tensile strength (TS), and swelling index (SI) of the film. The water solubility, UV barrier, and water solubility of the film are enhanced, but the crystallinity, tensile strength, and swelling index of the film decrease. This film can release biologically active antioxidant compounds, thereby extending the shelf life of cherry tomatoes. Due to changes in pH during spoilage, color changes can be used to detect whether they have deteriorated. | Sganzerla et al., 2021 [54] |
| In an acid medium, the extracted color behaved as pink, in the neutral medium the pink color became stronger, and in a basic medium, the yellowish-green was the predominant color. | |||||
| Pelargonidin | Bacterial cellulose (BC) |  | Tilapia fillets | Positively affected the mechanical properties of the film and color difference. Negatively affected the light transmittance of the film. This film can be used for intelligent packaging of fish and can detect the freshness of fish in real time. | Liu et al., 2021 [19] |
| When pH 3 changes to pH 10, the color of the Pg solution and Pg-BC film changes from red to blue. | |||||
| Violet basil | Arabic gum–Carboxymethyl cellulose | Exposing the indicator film to ammonia gas can cause the color to change from red to yellow. The color change of phthalocyanine solution also changes from red to yellow. | --- | Positively affected the WVP and antioxidant activity of the film. The water contact angle, elongation at break, and thermal performance of the membrane will decrease. | Rezaie et al., 2021 [38] |
| Malva sylvestris | Polylactic acid (PLA)/polyethylene glycol (PEG)/calcium bentonite (CB) |  | Minced meat/chicken/fillet, shrimp |
The PLA/PEG/CB Malva indicator can distinguish fresh, stale, and spoiled shrimp and fish roes from color changes, as well as fresh and spoiled ground beef and chicken fillets (at 4 ° C for 10 days). The main reason for color changes is due to changes in the total volatile alkaline nitrogen of food samples. The PLA/PEG/CB Malva indicator has satisfactory applications in monitoring the freshness of various protein foods. | Ghorbani et al., 2021 [55] |
| A: Color variations of MAC. As the pH value rose from 2 to 12, visual color changes were detected with colors ranging from pink to blue, which was perceptible to the naked eye at pH 6–9. B: Color variations of PLA/PGE/CB-Malva indicator. The indicator turned to pink at pH 2, and the intensity of this color diminished as the pH increased to 6. A purple color was observed at pH 6–7, which shifted to green as the pH increased (pH 8–9). Ultimately, the most intense green color occurred at pH 11. | |||||
| Butterfly pudding | Polymeric chitosan (CH) |  | Fish | Positively affected the swelling property, microstructure, moisture content, and mechanical property of the film. The swelling property, microstructure, moisture content and mechanical properties of the films were enhanced. The transmittance of the film decreases. In the application of fish preservation, when the quality of fish changes, the film changes from purple blue to dark green, and the change is obvious. The detection limit is 37 ppm. The sensor can be reused. The sensor can be used to monitor the freshness of mutton in real time. When the freshness changes, the color of the membrane will change easily recognized by the naked eye. | Yan et al., 2021 [20] |
| The solutions appear to be red at pH 1, purple at pH 2–5, blue at pH 6–13, and yellow at pH 14. | |||||
| Bougainvillea glabra | Potato starch |  | Fish | Positively affected the surface hydrophobicity, pH sensitivity, and ammonia sensitivity. Negatively affected the water vapor barrier capacity, and mechanical strength of the film. The film could be a novel intelligent label for application in food packaging. | Naghdi et al., 2021 [14] |
| The solutions appear to be purple at pH 2, pink at pH 2–11, and yellow at pH 12–13. | |||||
| Roselle | Polyvinylidene fluoride (PVDF) | 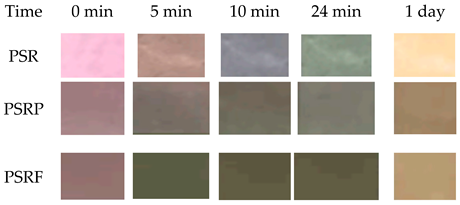 | Griskin | Positively affected the physical properties, microstructure barrier property for moisture and pH sensitivity of the film. The film showed visible color changes to ammonia gas and had a good correlation between TVB-N, pH, and color change of the indicator. The film could be used as an indicator for distinguishing griskin freshness/spoilage process. | Zhang et al., 2021 [56] |
| Clitoria ternatea | Gellan gum/soy protein |  | Shrimp | Positively affected the stability, hydrophobicity, water vapor permeability, swelling capacity, elongation at break, pH-sensitive, antimicrobial activity and antioxidant activity of the film. Negatively affected the tensile stress of the film. The increase in volatile basic nitrogen content is an important feature of shrimp meat spoilage, which will lead to the change of film color. | Wu et al., 2021 [57] |
| The anthocyanins pigment from C. ternatea petals (CT anthocyanins) were brownish yellow at pH higher than 11.0, green at pH 10.0–11.0, blue-green at pH 7.0–9.0, blue at pH 5.0–6.0, violet at pH 3.0–5.0, and red at pH values lower than 3. | |||||
| Mulberry | Konjac glucomannan/hydroxypropyl methyl cellulose |  | Fish |
Positively affected the color stability and pH sensitivity of this film. As the freshness of fresh fish changes, the color of KH-MBE film changes from purple to gray, and then from gray to yellow. Among them, the color stability of KH-MBE-20% film is the best. | Zhou et al., 2021 [58] |
| Within the pH range of 2–12, color changes can be clearly observed. | |||||
| Mulberry fruits | Gelatin (GN)/ZnO nanoparticles/gellan gum (GG) |  | Fish | Positively affected the stability properties, pH sensitivity and NH3 sensitivity of the film. The electrochemical writing ability of the bilayer membrane was also identified. The deterioration of the crucible can cause significant color changes in the thin film with electrochemical writing patterns. | Yang et al., 2021 [59] |
| When the pH value is 11–12, the MBA solution is orange; when the pH value is increased from 7 to 10, the color of the MBA solution gradually changes from light green to yellow-green; when the pH value is 2–6, the MBA solution shows an obvious color change, from light pink to colorless. | |||||
|
Anthocyanins purchased from Xian Huilin Biological Technology Co., Ltd. | Pullulan/chitin nanofibers (PCN) |  | Fish | Positively affected the elongation at break (Eb), pH-sensitive, antimicrobial activity and antioxidant activity of the film. Negatively affected the tensile strength (TS), thermal stability between 250 °C and 400 °C of the film. Electrospun PCN/Cr/ath nanofiber film has broad development prospects in intelligent food packaging. | Duan et al., 2021 [60] |
| The PCN/CR/ATH nanofibers exhibited more noticeable color changes. | |||||
| Hawthorn fruit (Crataegus scabrifolia) | Gelatin/chitosan/nanocellulose |  | Shrimp | Positively affected the pH sensitivity character of the films. When the colors are red and purple, the sample is fresh, light gray when not fresh, and turns yellow-green after complete deterioration. Therefore, under the condition of 4 ± 1 °C, the film can be used to indicate changes in food quality. | Yan et al., 2021 [61] |
| The solutions were red, pink, blue and yellow at pH 1–5, 6–9, 7–11, and 12. | |||||
| Roselle | Polyvinyl alcohol (PVA)/hydroxypropyl methylcellulose (HPMC) |  | Shrimp |
The film thickness is 15.90 ± 0.14~23.20 ± 3.35 μm. The tensile strength was 45.66 ± 1.07~56.98 ± 0.24 MPa, the antioxidant activity increased by 83.18%, the antibacterial activity against Escherichia coli increased by 146.91%, and the antibacterial activity against Staphylococcus aureus increased by 59.18%. The light transmittance and hydrophobicity of the film are reduced, so the film is used in the case of large visible light color changes. | Huang et al., 2021 [18] |
| Roselle anthocyanin extract (RAE) was added to hpmc-pva solution. With the increase in pH value, the color of the film changed from red to green. | |||||
| Butterfly pea flower | Sugarcane wax/agar |  | Shrimp | Packaging film of intelligent colorimetric pH sensor based on shrimp freshness optical tracking with butterfly pea anthocyanin extract. | Hashim et al., 2021 [62] |
| The BF anthocyanin extract was red in acidic solution (pH 2) and transcended purple (pH 3.0). At pH 4.0 the solution was violet, blue at pH 5–6, sky blue at pH 7, bluish-green at pH 8, greenish-blue at pH 9 and, lastly, deep green at pH 10–12. | |||||
| Butterfly pea (Clitoria ternatea) flower | Carboxymethyl cellulose (CMC)/agar | 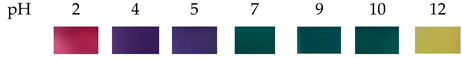 |
The mechanical strength, UV resistance, antibacterial activity, and antioxidant activity of the film have all been enhanced. Reduced water barrier performance. The enhanced physical and functional properties of color indicator films based on CMC/agar make them possible for active and intelligent food packaging applications. | Roy et al., 2021 [15] | |
| The anthocyanin solution showed blue to pink, green, and yellow colors in the acidic (pH 2), neutral (pH 7), and alkaline (pH 12) conditions. | |||||
| Eggplant (Solanum melongena) peel | Chitosan |  | Meat | The freshness of meat at different temperatures was monitored by chitosan film containing BH (-20, 4, and 20 ° C), and the freshness was judged by the change of film color caused by the change of total volatile basic nitrogen produced in meat during storage. | Cristiane et al. [5] |
| In the range of pH 1–3, the color changes from red to pink, purple when the pH exceeds 4, and gradually turns blue as the pH increases. When the pH reaches 12 or even exceeds 12, it appears yellow. | |||||
| Jaboticaba peels | Starch/glycerol |  | Milk | Positively affected the thermal stability and WS of the films. This film also exhibits excellent performance in simulating alcoholic and fatty water-based foods. Negatively affected the MC and WVP properties of the film. | Tuany et al., 2021 [63] |
| The solutions were pink, red, and yellow at pH 1, 3, and 5–11. | |||||
| Saffron petal | Chitosan nanofibers/methyl cellulose |  | Lamb |
It has a positive effect on the tensile strength of the film, the antibacterial activity against Escherichia coli and Staphylococcus aureus and the ability to scavenge DPPH free radicals. The shading performance is reduced. The film can be used as an intelligent packaging material for mutton during storage. | Mahmood et al., 2021 [23] |
| Red/pink (pH1–4); violet/gray (pH 5–6); green (pH 7–9); and, yellow-green/yellow (pH 10–14). | |||||
| Hibiscus sabdariffa flowers | Cellulose/collagen/sodium alginate | -- | -- | Positively affected the compressive strength, elastic modulus and antioxidant of the films. | Anghel et al. [64] |
| Clitoria ternatea flower | Starch/carbon nano |  | Pork |
Positively affected the mechanical, barrier, thermal and antioxidant properties
of this film. As the freshness decreases, the color of the film changes from purple to green. | Koshy et al., 2021 [65] |
| The color is red at pH 1–3, purple at pH 1–3, blue at pH 6–7, green at pH 8–9, colorless at pH 10–11 (10–11), and yellow at pH 11–12 | |||||
| Butterfly Pea | Gelatin/methylcellulose | 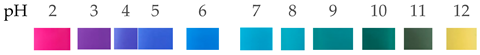 | --- | The addition of BPE has a positive impact on the pH sensitivity, water solubility, mechanical properties, and water vapor permeability of methylcellulose-based films. | Sai-Ut et al., 2021 [66] |
| The butterfly pea extract’s (BPE) original color at pH 6 was purple and then turned violet when the pH of the solution was lower than 4.0. The color of BPE solutions turned blue, dark green, and green-yellow when the pH of the solution was 7.0–8.0, 9.0–10, and 12.0, respectively. The BPE solution’s colors had a remarkable change at pH 2.0 to pink and 12.0 to green-yellow. | |||||
| Lycium ruthenicum | Starch/polyvinyl alcohol |  | Fish |
Film with free anthocyanins had higher light blocking and antioxidant properties. Film with nano-encapsulated anthocyanins had higher moisture-blocking properties. Encapsulation increased the stability of anthocyanins in the films. The freshness of bass fillets was indicated by the films with anthocyanins. | Qin et al., 2021 [67] |
| The deterioration of bass fillets can be observed through significant changes in color. | |||||
| Vitis vinifera | Nano-starch/poly(dimethylsiloxane) | Starch film (SF); poly(dimethylsiloxane)(PDMS) | Shrimp |
The anti-wetting, optical barrier, and mechanical properties of the film have been enhanced. This membrane will not be damaged by water and can be used to monitor the freshness of aquatic products and foods with high water content. | Wang et al., 2021 [68] |
| Red barberry | Chitin nanofiber (CNF) and methylcellulose (MC) |  | Fish | Positively affected the mechanical properties, moisture resistance, UV–vis screening properties, antioxidant and antimicrobial activity of the film. The film could change color from pink to yellow with increasing ammonia vapor concentration. The film could monitor the freshness/spoilage of a model food. | Sani et al., 2021 [69] |
| The color changed from reddish/crimson (in acidic pHs) to pale pink (in neutral pHs) to yellow (in alkali pHs) as the pH was raised from 1 to 14. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wang, W.; Wang, W.; Zhang, J. Effect of Anthocyanins on Colorimetric Indicator Film Properties. Coatings 2023, 13, 1682. https://doi.org/10.3390/coatings13101682
Chen L, Wang W, Wang W, Zhang J. Effect of Anthocyanins on Colorimetric Indicator Film Properties. Coatings. 2023; 13(10):1682. https://doi.org/10.3390/coatings13101682
Chicago/Turabian StyleChen, Lin, Wenli Wang, Wei Wang, and Jiamin Zhang. 2023. "Effect of Anthocyanins on Colorimetric Indicator Film Properties" Coatings 13, no. 10: 1682. https://doi.org/10.3390/coatings13101682
APA StyleChen, L., Wang, W., Wang, W., & Zhang, J. (2023). Effect of Anthocyanins on Colorimetric Indicator Film Properties. Coatings, 13(10), 1682. https://doi.org/10.3390/coatings13101682






